Abstract
The advent of next-generation sequencing (NGS) in recent years has led to a rapid discovery of novel or rare genetic variants in human kidney cell genes, which is transforming the risk assessment, diagnosis, and treatment of kidney disease. Mutations may lead to protein misfolding, disruption of protein trafficking and endoplasmic reticulum (ER) retention. An imbalance between the load of misfolded proteins and the folding capacity of the ER causes ER stress and unfolded protein response. Mutations in nephrin (NPHS1), podocin (NPHS2), laminin β2 (LAMB2) and α-actinin-4 (ACTN4) have been shown to induce ER stress in HEK293 cells and podocytes in hereditary nephrotic syndromes; various founder mutations in collagen IV α chains (COL4A) have been demonstrated to activate podocyte ER stress in collagen IV nephropathies; and mutations in uromodulin (UMOD) have been reported to trigger tubular ER stress in autosomal dominant tubulointerstitial kidney disease. Meanwhile, ER resident protein SEC63 may modify disease severity in autosomal dominant polycystic kidney disease. These findings underscore the importance of ER stress in the pathogenesis of monogenic kidney disease. Recently, we have identified mesencephalic astrocyte-derived neurotrophic factor (MANF) and cysteine-rich with EGF-like domains 2 (CRELD2) as urinary ER stress biomarkers in ER stress-mediated kidney diseases.
Keywords: ER stress, Nephrotic syndrome, Alport syndrome, ADTKD, Gene mutation, Biomarker
Introduction
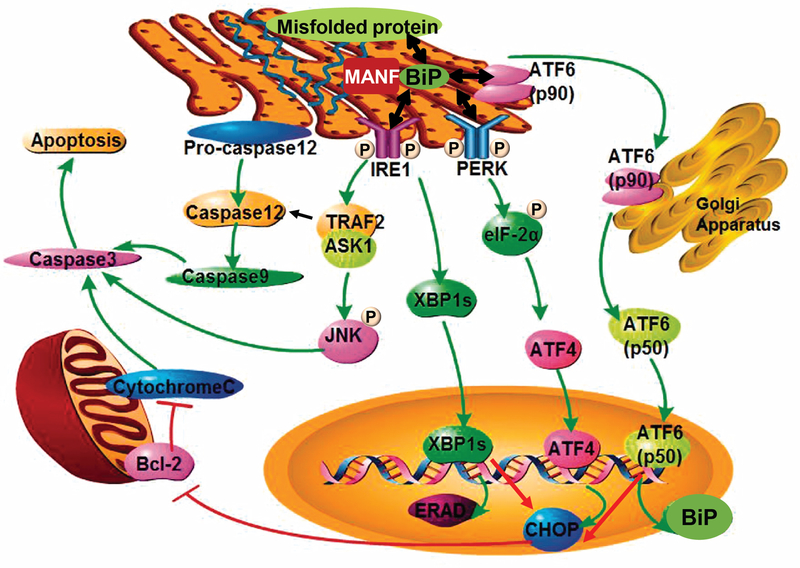
The endoplasmic reticulum (ER) is an essential organelle for folding, post-translational modifications, and trafficking of secreted and membrane proteins, and thus it is responsible for maintaining protein homeostasis (proteostasis) in all eukaryotic cells [1]. Environmental and genetic factors can disrupt normal protein folding processes, and thus cause accumulation of unfolded and misfolded proteins in the ER lumen, resulting in ER stress and activation of the unfolded protein response (UPR) (Figure 1) [2]. The UPR aims to restore ER homeostasis and prevent cell death through the induction of ER chaperones, reduction of protein synthesis, and degradation of misfolded proteins using three major ER-resident transducers: inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6) (Figure 1) [2, 3].
Figure 1: ER stress and the UPR pathway.
Aberrant protein conformations are a major cause of ER disease. A mismatch between the load of unfolded and misfolded proteins and the folding capacity of the ER leads to ER stress. The ER responds to stress by activating intracellular signaling pathways, collectively known as the UPR, which provides a response that aligns cellular physiology to the demands imposed by ER stress. Three different classes of ER stress transducers including IRE1, PERK, and ATF6 have been identified. These proximal sensors are integral membrane proteins, having a domain in the ER lumen, which senses ER stress, and a cytosolic domain, which activates downstream signaling effectors. Three distinct signaling branches IRE1-Xbp1s or IRE1-TRAF2-JNK/caspase 12, Perk-eIF2α-ATF4 and p90ATF6-p50ATF6, are subsequently activated. Cells rendered dysfunctional due to severe or chronic ER stress are eliminated from the organism by ER stress-specific apoptosis, mediated by ER-resident caspase 12, JNK or CHOP. MANF binds to BiP inside the ER, and the interaction is calcium-dependent. The image is modified from Xu et al. [55] with permission.
As illustrated in Figure 1, under ER stress conditions, misfolded and unfolded proteins can dissociate the ER chaperone protein BiP (immunoglobulin heavy chain-binding protein) from these three ER transducers, resulting in their activation and initiation of the UPR [1, 4]. Bifunctional protein kinase/endoribonuclease IRE1 induces unconventional splicing of X-box binding protein (XBP1) mRNA into XBP1s mRNA, encoding a potent transcriptional activator XBP1s that upregulates the expression of ER chaperones and genes related to ER-associated protein degradation (ERAD). IRE1 also recruits tumor necrosis factor receptor-associated factor 2 (TRAF2) and activates pro-apoptotic Jun N-terminal kinase (JNK) or caspase 12 signaling, leading to apoptosis [3–5]. PERK phosphorylates the α-subunit of eukaryotic translation initiation factor 2 (eIF2α). Phosphorylation of eIF2α leads to general inhibition of protein translation and selective upregulation of ATF4, a transcription factor inducing the expression of ER chaperones and genes that are related to autophagy and oxidative response. ATF4 also activates the expression of pro-apoptotic CCAAT/enhancer-binding protein homologous protein (CHOP). ATF6 translocates to the Golgi, where it is cleaved by site-1 protease and site-2 protease. The active form of ATF6 (p50ATF6) then moves into the nucleus, where it acts as a transcription factor to induce synthesis of ER chaperones.
The UPR is fundamentally a cell-protective response, but cells rendered dysfunctional due to severe or prolonged ER stress are eliminated from the organism by ER stress-specific apoptosis, mediated by caspase 12, JNK and CHOP [6, 7]. Genetic mutations may alter the amino acid sequence of proteins and result in misfolding of proteins. The misfolded proteins can accumulate or aggregate in the ER, leading to chronic ER stress. Recent studies have demonstrated that ER stress-mediated cellular dysfunction and apoptosis induced by genetic mutations plays a causative role in the pathogenesis of genetic disorders, including neurodegenerative disease as well as monogenic diabetes and kidney disease [2, 3, 5, 6]. This review summarizes the importance of ER stress in a variety of monogenic kidney diseases, as well as discusses the discovery of urinary ER stress biomarkers in kidney diseases.
Podocyte ER stress and hereditary nephrotic syndromes
Primary nephrotic syndrome (NS), characterized by heavy proteinuria and hypoalbuminemia, is accompanied by increased risk of infection, venous thromboembolism, and progression to end-stage renal disease (ESRD). NS is often a life-threatening condition when occurring in the first year of life. Almost 100% of patients with congenital onset and 44% with infantile onset of NS have gene mutations with the overall mutation detection rate being as high as 52% in steroid-resistant pediatric NS patients [8]. Focal segmental glomerulosclerosis (FSGS) is the most prevalent NS and glomerular disease leading to kidney failure.
Seminal advances in human genetics in past decades have identified NS as a primary podocytopathy with more than 30 mutated podocyte genes discovered in human NS/FSGS patients [9]. These mutated genes can be divided into the following categories: (a) slit diaphragm-associated molecules, including nephrin (NPHS1) and podocin (NPHS2) [10], (b) podocyte cytoskeleton related molecules such as α-actinin-4 (ACTN4) [11], (c) podocyte transcription factors, and (d) adhesion and extracellular matrix molecules such as laminin β2 (LAMB2).
Emerging evidence has shown that podocyte ER stress and dysfunction due to genetic factors plays an important role in the pathogenesis of NS/FSGS. In cell culture studies, Liu L. et al. show that a large number of nephrin missense mutants, which cause autosomal recessive congenital NS of the Finnish type, are trapped inside the ER, most likely due to misfolding [12]. In addition, treatment with a chemical chaperone sodium 4-phenylbutyrate restores impaired trafficking of some disease-causing missense mutants and rescues these mutants from ER to the plasma membrane [13]. Similarly, Ohashi T. et al. report that R138Q mutation of podocin, one of the most common missense mutations in NPHS2 that is causally linked to the autosomal recessive type of steroid-resistant NS, is retained in the ER. Moreover, chemical chaperones glycerol, trimethylamine-N-oxide, and dimethyl sulfoxide (DMSO) elicit a cellular redistribution of R138Q podocin to the plasma membrane [14].
In mouse models, we have developed a podocyte ER stress-induced NS mouse model, which recapitulates Pierson syndrome patients carrying C321R-LAMB2 mutation. Pierson syndrome (OMIM 609049), caused by LAMB2 mutations, is characterized by NS and extrarenal ocular and neurologic manifestations [15–17]. Laminin, type IV collagen, nidogen, and sulfated proteoglycans comprise the glomerular basement membrane (GBM) [18] that is assembled by podocytes and glomerular endothelial cells [19]. Laminins are heterotrimeric glycoproteins containing one α, one β, and one γ chain. The major laminin heterotrimer in the mature GBM is laminin α5β2γ1, or LM-521 [20]. Laminin trimerization occurs in the ER [21]. Once trimers are secreted into the extracellular space, they polymerize to form the supramolecular laminin network [22, 23]. By utilizing a knockout/transgenic strategy, we have demonstrated that podocyte ER stress induced by the C321R misfolded protein activates the ER stress-specific apoptotic signal CHOP, and causes mild podocyte injury before significant proteinuria in vivo [24]. Meanwhile, in a mouse model of FSGS, Cybulsky et al. show that podocyte-specific expression of K256E α-actinin-4 transgene induces podocyte ER stress, which is associated with ubiquitination of the mutant protein and impairment of the ubiquitin-proteasome system [25].
Podocyte ER stress and collagen IV nephropathies
Type IV collagen has six chains, α1 to α6, encoded by three pairs of genes on chromosomes 2, 13, and X. Each chain has three domains: a short 7S domain at the N-terminus, a long, interrupted collagenous domain in the middle, and a noncollagenous domain at the C-terminus. The six distinct α chains are arranged into three different triple helical heterotrimeric protomers: (α1)2α2, α3α4α5 and (α5)2α6, which take place in the ER. Secreted protomers polymerize to create collagen networks [26]. At early stages of glomerulogenesis, the (α1)2α2 network is a component of GBM, Bowman’s capsule and mesangial matrix. During normal glomerulogenesis, most of the (α1)2α2 network is replaced by α3α4α5 in the GBM and by (α5)2α6 in Bowman’s capsule, with (α1)2α2 remaining in the subendothelial region of the GBM and in the mesangial matrix [27, 28]. Experiments in mice showed that podocytes, but not endothelial cells, synthesize the α3α4α5 network [29].
Alport syndrome (AS) and thin basement membrane nephropathy (TBMN) are collagen IV nephropathies and characterized by structural abnormalities in the GBM. Although both conditions typically present with hematuria, AS is associated with proteinuria, progressive renal failure and extrarenal syndromes. In contrast, TBMN is characterized by isolated persistent or recurrent hematuria, and generally never progresses toward ESRD. The hallmark of TBMN is diffuse attenuation of the GBM, which also resembles the ultrastructural changes of early AS patients or Alport carriers. AS and TBMN are also genetically heterogeneous diseases. 85% of AS patients have the X-linked form due to mutations in COL4A5, whereas both autosomal AS and TBMN occur due to mutations in COL4A3/4 genes.
G1332E-COL4A3 mutation is known to be pathogenic in AS and endemic in Cyprus. When WT and mutant Col4α3 chains are overexpressed in human undifferentiated podocytes, microarray analysis shows that the overexpression of WT or mutant COL4A3 chains differentially activates the UPR pathway [30]. Similarly, in a knock-in mouse model carrying the G1332E-COL4A3 mutation, which resembles AS, the UPR pathway is activated in the mutant glomeruli [30]. Moreover, in kidney biopsies from patients with TBMN carrying a heterozygous G1334E-COL4A3 mutation, BiP expression is increased in the glomeruli of these patients compared with expression from controls [30]. These results suggest that podocyte ER stress arising from the mutant collagen IV chains contributes to the pathogenesis of AS and TBMN.
Even more interesting, collagen (COL4A) mutations have been found to be the most frequent mutations underlying adult FSGS patients [31]. Recent studies have suggested that AS, TBMN and FSGS are a spectrum of renal pathologies. In a study of 57 Greek-Cypriot families presenting glomerular microscopic hematuria (classical AS is excluded), with or without proteinuria or chronic kidney function decline, 8 heterozygous causative mutations in COL4A3/A4 genes are identified in 87 patients of 16 families (28.1%). Among these 16 families, eight non-related families feature the founder mutation G1334E-COL4A3. Kidney biopsies, which are available in 7 families, show dual diagnosis of TBMN and FSGS in 6 families and FSGS in one family. Overexpression of some mutations, including G871C, another founder mutation in the Cypriot population that predisposes to severe chronic kidney disease/ESRD, and G484R, induces the UPR in human podocytes [32].
Tubular ER stress and autosomal dominant tubulointerstitial kidney disease (ADTKD)
ADTKD is a monogenic form of renal tubulointerstitial fibrosis leading to chronic kidney disease. It represents as many as 25% of patients with inherited kidney disease, after exclusion of polycystic kidney disease and AS [33]. ADTKD is caused by mutations in UMOD, MUC1, REN and HNF1B. Multiple names have been used in the past, including uromodulin kidney disease, familial juvenile hyperuricemic nephropathy, medullary cystic kidney disease type 1 and type 2. In 2015, KDIGO proposed a new terminology ADTKD for this group of diseases [34]. ADTKD-UMOD is characterized by hyperuricemia, gout, alterations in urinary concentration, and progressive loss of kidney function [34, 35]. Proteinuria is typically mild or absent. ADTKD-UMOD is a phenotypically heterogeneous disorder manifested by variable age of disease onset, disease severity, and rate of disease progression among affected individuals within and between families, with patients reaching ESRD between the ages of 25 and 70 years or older [34].
Uromodulin (Tamm-Horsfall protein) is exclusively expressed in the thick ascending limb (TAL) of Henle’s loop. Human mature uromodulin, mainly localized at the apical plasma membrane of TAL cells [36], contains a signal peptide, three EGF-like domains, a central domain of unknown function, a zona pellucida domain, and a glycosylphosphatidylinositol (GPI) –anchoring site [35]. It is co-translationally inserted in the ER where GPI anchoring, formation of intramolecular disulfide bonds, and N-glycosylation take place [35]. Uromodulin has extremely high cysteine content and extensive disulfide bond formation resulting in extremely slow transit through the ER. Once uromodulin reaches the plasma membrane, proteolytic cleavage by a protease hepsin generates polymerization-competent monomers that are assembled into polymeric filaments and released to the urine [37]. Currently more than 120 UMOD mutations have been identified (http://www.ukdcure.org/), and most of them are missense mutations [35]. These UMOD mutations are clustered (94%) in exons 3 and 4 encoding for the N-terminal half of the protein, and ~ 50% of known UMOD mutations affect cysteine [35].
In vitro and in vivo studies have shown that UMOD mutations can cause protein misfolding, ER retention and ER stress activation in TAL cells [38–42]. By utilizing CRISPR (clustered regularly interspaced short palindromic repeats)-generated Umod C147W knock-in mice, which resemble human patients carrying the UMOD C148W mutation, it has been shown that tubular ER stress induced by the C147W mutant uromodulin activates tubular apoptosis and inhibits autophagy. In addition, TRIB3 may act as an intrinsic ER stress-mediated cell death mediator to sensitize UMOD-producing tubular cells to TNF (tumor necrosis factor) and TRAIL (TNF-related apoptosis-inducing ligand)-induced apoptosis. Most importantly, when the mutant mice are treated with the soluble recombinant fusion protein TNF receptor:Fc, a TNFα signaling antagonizer, ER stress-mediated inflammation, apoptosis and fibrosis are attenuated with improvement in renal function [43].
SEC63 and autosomal dominant polycystic kidney disease (ADPKD)
ADPKD is caused by mutations in either PKD1 or PKD2, which encodes the integral membrane proteins polycystin-1 (PC1) and PC2, respectively. It has been shown that ER-resident proteins SEC63 and XBP1 can modify polycystic disease severity [44]. Mutations in SEC63 lead to isolated autosomal dominant polycystic liver disease, and organ-specific inactivation of Sec63 in mice produces cysts in both the liver and kidneys as the result of reduced PC1.
ER protein folding, modifications, and quality control are governed by several chaperone systems, which include HSP70 (BiP)/HSP40 (DnaJ proteins), HSP90, calnexin/calreticulin, and protein disulfide isomerases. SEC63 is a member of the HSP40 protein family, and it is associated with the SEC61 translocon complex that serves as a channel through which nascent polypeptides are co-translationally imported into the ER lumen for folding, modification, and subsequent trafficking. SEC63 contains a DnaJ domain, which is conserved in HSP40 proteins that act as cochaperones [45]. Using murine genetic models, it has been demonstrated that SEC63 deficiency selectively activates the IRE1α-XBP1 branch of UPR, which is a compensatory mechanism, and activation of XBP1 can enhance functional PC1 biogenesis and ameliorate cystic disease in a murine model with reduced PC1 function [44]. Furthermore, compound inactivation of both SEC63 and XBP1 exacerbates the polycystic kidney phenotype in mice by suppressing cleavage at the G protein–coupled receptor proteolysis site in PC1, which is critical in PC1 maturation. In summary, these findings show that SEC63 function regulates IRE1α/XBP1 activation, and activation of XBP1 can protect against polycystic disease in the setting of impaired biogenesis of PC1 [44].
Discovery of urinary ER stress biomarkers in ER stress-mediated kidney disease
As ER stress has emerged as a signaling platform underlying the pathogenesis of various kidney diseases, there is an urgent need to develop ER stress biomarkers in the incipient stages of ER stress-induced kidney disease, when a kidney biopsy is not yet clinically indicated, for early therapeutic intervention. Recently, we have identified mesencephalic astrocyte-derived neurotrophic factor (MANF) and cysteine-rich with EGF-like domains 2 (CRELD2) as urinary ER stress biomarkers [46, 47].
MANF as a urine ER stress biomarker in mouse models
MANF, also known as arginine-rich mutated in early tumors, or ARMET, was first discovered by Dr. Commissiong’s group in 2003 as a new dopaminergic neurotrophic factor in astrocyte-conditioned medium [48]. MANF cDNA is encoded by a 4.3 kb gene with 4 exons and located on human chromosome 3 [48]. MANF is localized to the ER lumen, and ER stress-induced transcriptional upregulation of MANF is driven by an ER stress response element (ERSE)-II in the MANF promoter [49, 50]. ERSE-II (ACGTGGNCCAAT) contains two transcriptional factor recognition sequences: ACGTGG is recognized by ATF6 or XBP1, whereas CCAAT is recognized by nuclear transcriptional factor Y. It has been shown that both binding sites are required for the MANF induction by ER stress [49]. Meanwhile, MANF secretion is partly regulated by BiP via calcium-dependent interaction of MANF and BiP in the ER (Figure 1) and the BiP-MANF complex levels decrease in response to ER calcium depletion.
We have shown that in our podocyte ER stress-induced hereditary NS mouse model in which podocyte ER stress is activated by C321R-LAMB2, MANF is induced and secreted by ER-stressed podocytes at early stage of proteinuria [24, 46]. Most importantly, MANF is easily detected in urine specimens from Tg-C321R mutants at the incipient stage of NS, but not from the controls. In addition, urinary MANF excretion increases during disease progression in the mutants [46].
Similarly, in the acute kidney injury mouse model triggered by an ER stressor tunicamycin or ischemia/reperfusion, significant upregulation of MANF at both transcriptional and translational levels is observed in the ER-stressed renal tubules before obvious renal histopathologic changes or elevation of serum creatinine (Cr) occur. Moreover, urinary MANF excretion concurrent with tubular cell ER stress precedes histologic or clinical manifestations of acute tubular injury [46].
CRELD2 as a urine ER stress biomarker in both mouse models and human diseases
CRELD2 was first identified as a novel ER stress-inducible gene through RNA analysis of Neuro2a mouse neuroblastoma cells treated with thapsigargin, which depletes ER calcium through inhibition of ER calcium uptake by ER calcium ATPase [51]. It is a ~50 kDa secretory glycoprotein that predominantly localizes to the ER and Golgi apparatus [51, 52]. Its promoter region, which is well conserved among various species, contains a typical ERSE (CGTGG-N9-ATTGG) that is positively regulated by the ER stress master regulator ATF6 [51]. It has also been reported that four C-terminal amino acids (REDL) play a crucial role in CRELD2 secretion and that BiP and MANF significantly enhance its secretion [52, 53]. However, very few studies have been carried out to characterize the intrinsic induction and secretion of CRELD2 in vitro and in vivo.
When mouse primary podocytes are treated for 24 h with tunicamycin, which activates ER stress by blocking N-linked glycosylation in the ER [54], or thapsigargin, both ER stressors induce expression of CRELD2, which is barely detectable in vehicle-treated cells. In addition, both ER stressors increase CRELD2 secretion into the culture medium by mouse podocytes, whereas in the absence of ER stress, there is little CRELD2 secretion [47]. These data suggest that upregulation and secretion of CRELD2 induced by ER stress is not a cell-type-specific response. Furthermore, in our podocyte ER stress-induced NS mouse model carrying the C321R-LAMB2 mutation in podocytes, CRELD2 are upregulated at both transcriptional and translational levels in the mutant podocytes compared with control podocytes. Most importantly, CRELD2 is easily detected in unprocessed urine specimens from C321R mutants, but not from control littermates, at the early stage of NS [47].
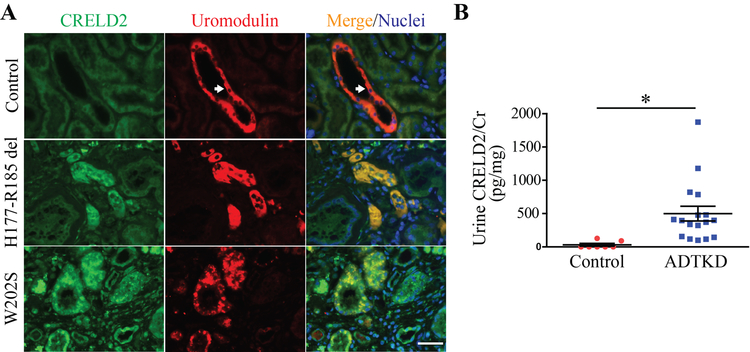
We also investigated CRELD2 as a urinary biomarker for detecting tubular ER stress in ADTKD-UMOD, a prototypical tubular ER stress disease [47]. Co-immunofluorescence (IF) staining of CRELD2 and uromodulin in human kidney biopsies showed that native uromodulin is enriched at the apical membrane of TAL cells (arrows, Figure 2A). In sharp contrast, mutant H177-R185del uromodulin exhibits diffuse expression in the cytoplasm of TAL tubules and mutant W202S uromodulin displays a punctate perinuclear distribution within TAL cells (Figure 2A). Intracellular and intraluminal protein aggregates are also noted in kidney biopsies harboring both mutations, reminiscent of defective intracellular trafficking and ER retention of uromodulin mutants (Figure 2A). Moreover, CRELD2 is markedly enhanced and completely co-localized with mutant uromodulin in TAL cells (Figure 2A). Finally, CRELD2 is easily detected in unconcentrated urine from human ADTKD-UMOD patients, whereas urinary CRELD2 excretion is absent from all tested genetically unaffected controls by ELISA assay (Figure 2B). These results demonstrate the superb ability of CRELD2 to discriminate between controls and ADTKD patients with tubular ER stress [47].
Figure 2: Detection of CRELD2 in the urine from human ADTKD-UMOD patients.
(A) Representative IF images of human renal biopsies obtained from patients with p.H177-R185del or p.W202S UMOD mutation and from normal kidneys, stained for CRELD2 (green) and uromodulin (red) with a nuclear counterstain (Hoechst 33342, blue). Scale bar: 40 μm. (B) Dot plot representation of urine CRELD2/Cr values measured by ELISA in 17 ADTKD-UMOD patients harboring various UMOD mutations, including H177-R185del, C106F, D172H, V93-G97delinsAASC, R178P and G103C, as well as in 7 genetically unaffected controls. Data represent mean ± SEM, *P<0.05 by t test. Figure 2 was originally published in JCI Insight [47].
Our identification of CRELD2 as a sensitive, mechanistic ER stress biomarker for ADTKD-UMOD is important to research in this area, as it will provide a useful tool for clinical trials, overcoming the challenge posed by the slow rates of rise in serum Cr in these patients, which is the major reason why currently clinical trials in ADTKD-UMOD cannot be performed.
Future directions
Accumulating evidence has highlighted the important role of ER stress and disrupted proteostasis in the pathogenesis of various monogenic glomerular and tubular diseases. However, mechanism-based therapies targeting at specific ER stress response elicited by individual mutations are still lacking. Thus, there is emergent need to conduct large-scale drug screening of chemical compound libraries, which is based on high-throughput functional assays, to identify lead compounds. By utilizing the newly developed CRISPR/Cas9 genome-editing system which enables targeted modification of endogenous genomic sequences with high efficiency, more knock-in mouse models carrying different ER stress-inducing mutations will be generated as pre-clinical models for drug testing. In addition, human induced pluripotent stem cells (hiPSCs) directly derived from patients will be established for disease modeling, mechanistic investigation and future drug discovery. These novel technologies will greatly facilitate the implementation of precision medicine in the translational studies of ER stress-mediated monogenic kidney diseases and may lead to development of highly targeted ER stress modulators for individual mutations.
Acknowledgements
Y.M.C. is supported by NIH grants R01 DK105056, R03DK106451 and K08DK089015, Halpin Foundation-American Society of Nephrology Research Grant, Faculty Scholar Award (MD-FR-2013–336) from the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, Clinical Scientist Development Award (2015100) from the Doris Duke Charitable Foundation, Career Development Award from the Nephrotic Syndrome Study Network (NEPTUNE), Early Career Development Award from the Central Society for Clinical and Translational Research (CSCTR), and Renal Translational Innovation Grant from Washington University Division of Nephrology. Y.M.C. is a member of Washington University Diabetes Research Center (supported by NIH P30 DK020579), Washington University Musculoskeletal Research Center (supported by NIH P30AR057235), and Washington University Institute of Clinical and Translational Sciences (UL1 TR000448).
Footnotes
Conflicts of interest
A patent application entitled “Mesencephalic astrocyte-derived neurotrophic factor (MANF) as a urine biomarker for endoplasmic reticulum (ER) stress-related kidney disease, methods and uses therefor” has been filed by Y.M. Chen and Washington University Office of Technology Management (Serial No. 14730465, filed on June 4, 2015). Another patent application entitled “Methods of detecting biomarkers of endoplasmic reticulum (ER) stress-associated kidney diseases” has been filed by Y.M. Chen and Y. Kim and Washington University Office of Technology Management (Serial No. 15664476, filed on July 31, 2017).
References
- 1.Nikesitch N, Lee JM, Ling S, Roberts TL (2018) Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clinical & translational immunology 7:e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cybulsky AV (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13:681–696. [DOI] [PubMed] [Google Scholar]
- 3.Oslowski CM, Urano F (2011) Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490:71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burman A, Tanjore H, Blackwell TS (2018) Endoplasmic reticulum stress in pulmonary fibrosis. Matrix biology : journal of the International Society for Matrix Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nature reviews Neuroscience 15:233–249. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura M (2008) Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol 295:F323–334. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. [DOI] [PubMed] [Google Scholar]
- 8.Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F (2007) Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119:e907–919. [DOI] [PubMed] [Google Scholar]
- 9.Schell C, Huber TB (2012) New players in the pathogenesis of focal segmental glomerulosclerosis. Nephrol Dial Transplant 27:3406–3412. [DOI] [PubMed] [Google Scholar]
- 10.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Done SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, Tryggvason K (2001) Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 10:2637–2644. [DOI] [PubMed] [Google Scholar]
- 13.Liu XL, Done SC, Yan K, Kilpelainen P, Pikkarainen T, Tryggvason K (2004) Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol 15:1731–1738. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi T, Uchida K, Uchida S, Sasaki S, Nihei H (2003) Intracellular mislocalization of mutant podocin and correction by chemical chaperones. Histochem Cell Biol 119:257–264. [DOI] [PubMed] [Google Scholar]
- 15.Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dotsch J, Reis A, Muntefering H, Neumann LM (2004) Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A 130A:138–145. [DOI] [PubMed] [Google Scholar]
- 16.Zenker M, Pierson M, Jonveaux P, Reis A (2005) Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A 138:73–74. [DOI] [PubMed] [Google Scholar]
- 17.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M (2010) Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat 31:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Fassler R, Hohenester E (2004) Laminin: the crux of basement membrane assembly. J Cell Biol 164:959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamson DR (1985) Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol 100:1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD (2005) A simplified laminin nomenclature. Matrix Biol 24:326–332. [DOI] [PubMed] [Google Scholar]
- 21.Miner JH (2005) Building the glomerulus: a matricentric view. J Am Soc Nephrol 16:857–861. [DOI] [PubMed] [Google Scholar]
- 22.Yurchenco PD, Cheng YS (1993) Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem 268:17286–17299. [PubMed] [Google Scholar]
- 23.Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD (1997) Self-assembly of laminin isoforms. J Biol Chem 272:31525–31532. [DOI] [PubMed] [Google Scholar]
- 24.Chen YM, Zhou Y, Go G, Marmerstein JT, Kikkawa Y, Miner JH (2013) Laminin beta2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J Am Soc Nephrol 24:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cybulsky AV, Takano T, Papillon J, Bijian K, Guillemette J, Kennedy CR (2009) Glomerular epithelial cell injury associated with mutant alpha-actinin-4. Am J Physiol Renal Physiol 297:F987–995. [DOI] [PubMed] [Google Scholar]
- 26.Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG (2006) Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem 281:38117–38121. [DOI] [PubMed] [Google Scholar]
- 27.Miner JH, Sanes JR (1994) Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (2003) Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348:2543–2556. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL (2009) Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieri M, Stefanou C, Zaravinos A, Erguler K, Stylianou K, Lapathitis G, Karaiskos C, Savva I, Paraskeva R, Dweep H, Sticht C, Anastasiadou N, Zouvani I, Goumenos D, Felekkis K, Saleem M, Voskarides K, Gretz N, Deltas C (2014) Evidence for Activation of the Unfolded Protein Response in Collagen IV Nephropathies. J Am Soc Nephrol 25:260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gast C, Pengelly RJ, Lyon M, Bunyan DJ, Seaby EG, Graham N, Venkat-Raman G, Ennis S (2015) Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 32.Papazachariou L, Demosthenous P, Pieri M, Papagregoriou G, Savva I, Stavrou C, Zavros M, Athanasiou Y, Ioannou K, Patsias C, Panagides A, Potamitis C, Demetriou K, Prikis M, Hadjigavriel M, Kkolou M, Loukaidou P, Pastelli A, Michael A, Lazarou A, Arsali M, Damianou L, Goutziamani I, Soloukides A, Yioukas L, Elia A, Zouvani I, Polycarpou P, Pierides A, Voskarides K, Deltas C (2014) Frequency of COL4A3/COL4A4 mutations amongst families segregating glomerular microscopic hematuria and evidence for activation of the unfolded protein response. Focal and segmental glomerulosclerosis is a frequent development during ageing. PLoS One 9:e115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gast C, Marinaki T, Arenas-Hernandez M, Campbell S, Venkat-Raman G (2015) Genetic Testing Reveals Increased Prevalence of Uromodulin Associated Kidney Disease. Nephrol Dial Transpl 30. [Google Scholar]
- 34.Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O (2015) Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management-A KDIGO consensus report. Kidney International 88:676–683. [DOI] [PubMed] [Google Scholar]
- 35.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O (2011) The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney International 80:338–347. [DOI] [PubMed] [Google Scholar]
- 36.Pennica D, Kohr WJ, Kuang WJ, Glaister D, Aggarwal BB, Chen EY, Goeddel DV (1987) Identification of Human Uromodulin as the Tamm-Horsfall Urinary Glycoprotein. Science 236:83–88. [DOI] [PubMed] [Google Scholar]
- 37.Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng JH, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu QY, Jovine L, Rampoldi L (2015) The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP, Devuyst O, Rampoldi L (2010) A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Human Molecular Genetics 19:2998–3010. [DOI] [PubMed] [Google Scholar]
- 39.Bernascone I, Vavassori S, Di Pentima A, Santambrogio S, Lamorte G, Amoroso A, Scolari F, Ghiggeri GM, Casari G, Polishchuk R, Rampoldi L (2006) Defective intracellular trafficking of uromodulin mutant isoforms. Traffic 7:1567–1579. [DOI] [PubMed] [Google Scholar]
- 40.Kemter E, Prueckl P, Sklenak S, Rathkolb B, Habermann FA, Hans W, Gailus-Durner V, Fuchs H, de Angelis MH, Wolf E, Aigner B, Wanke R (2013) Type of uromodulin mutation and allelic status influence onset and severity of uromodulin-associated kidney disease in mice. Human Molecular Genetics 22:4148–4163. [DOI] [PubMed] [Google Scholar]
- 41.Rampoldi L, Caridi G, Santon D, Boaretto F, Bernascone I, Lamorte G, Tardanico R, Dagnino M, Colussi G, Scolari F, Ghiggeri GM, Amoroso A, Casari G (2003) Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Human Molecular Genetics 12:3369–3384. [DOI] [PubMed] [Google Scholar]
- 42.Vylet’al P, Kublova M, Kalbacova M, Hodanova K, Baresova V, Stiburkova B, Sikora J, Hulkova H, Zivny J, Majewski J, Simmonds A, Fryns JP, Venkat-Raman G, Elleder M, Kmoch S (2006) Alterations of uromodulin biology: A common denominator of the genetically heterogeneous FJHN/MCKD syndrome. Kidney International 70:1155–1169. [DOI] [PubMed] [Google Scholar]
- 43.Johnson BG, Dang LT, Marsh G, Roach AM, Levine ZG, Monti A, Reyon D, Feigenbaum L, Duffield JS (2017) Uromodulin p.Cys147Trp mutation drives kidney disease by activating ER stress and apoptosis. J Clin Invest 127:3954–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fedeles SV, So JS, Shrikhande A, Lee SH, Gallagher AR, Barkauskas CE, Somlo S, Lee AH (2015) Sec63 and Xbp1 regulate IRE1alpha activity and polycystic disease severity. J Clin Invest 125:1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann R, Muller L, Wullich B (2006) Protein transport into the endoplasmic reticulum: mechanisms and pathologies. Trends in molecular medicine 12:567–573. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y, Lee H, Manson SR, Lindahl M, Evans B, Miner JH, Urano F, Chen YM (2016) Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Urine Biomarker for Endoplasmic Reticulum Stress-Related Kidney Diseases. Journal of the American Society of Nephrology : JASN 27:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y, Park SJ, Manson SR, Molina CA, Kidd K, Thiessen-Philbrook H, Perry RJ, Liapis H, Kmoch S, Parikh CR, Bleyer AJ, Chen YM (2017) Elevated urinary CRELD2 is associated with endoplasmic reticulum stress-mediated kidney disease. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20:173–188. [DOI] [PubMed] [Google Scholar]
- 49.Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K (2007) ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct 32:41–50. [DOI] [PubMed] [Google Scholar]
- 50.Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC (2008) Mesencephalic Astrocyte-Derived Neurotrophic Factor Is an Ischemia-Inducible Secreted Endoplasmic Reticulum Stress Response Protein in the Heart. Circ Res 103:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh-hashi K, Koga H, Ikeda S, Shimada K, Hirata Y, Kiuchi K (2009) CRELD2 is a novel endoplasmic reticulum stress-inducible gene. Biochem Biophys Res Commun 387:504–510. [DOI] [PubMed] [Google Scholar]
- 52.Oh-hashi K, Kunieda R, Hirata Y, Kiuchi K (2011) Biosynthesis and secretion of mouse cysteine-rich with EGF-like domains 2. FEBS Lett 585:2481–2487. [DOI] [PubMed] [Google Scholar]
- 53.Oh-hashi K, Norisada J, Hirata Y, Kiuchi K (2015) Characterization of the Role of MANF in Regulating the Secretion of CRELD2. Biol Pharm Bull 38:722–731. [DOI] [PubMed] [Google Scholar]
- 54.Olden K, Pratt RM, Jaworski C, Yamada KM (1979) Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. P Natl Acad Sci USA 76:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, Guo M, Jiang W, Dong H, Han Y, An XF, Zhang J (2016) Endoplasmic reticulum stress and its effects on renal tubular cells apoptosis in ischemic acute kidney injury. Renal failure 38:831–837. [DOI] [PubMed] [Google Scholar]