Abstract
Objective
Hemodialysis (HD) patients have high protein and energy requirements, and protein-energy wasting is common and associated with poor outcomes. Eating during dialysis may improve nutritional status by counteracting the catabolic effects of hemodialysis treatment; but, eating during HD may be discouraged due to concerns of postprandial hypotension. However, little data is available to support this practice. In this study, we hypothesized that high protein meals during HD does not lead to symptomatic intradialytic hypotension events.
Design
A 9-week, non-randomized, parallel-arm study.
Setting
A single in-center HD clinic.
Subjects
18 HD patients from two shifts completed the study. Patients were 62±16 years-old in age with dialysis vintage 3.4±2.6 years.
Intervention
The intervention group (n=9) received meals of ~30g protein and ~1/3 daily recommended intakes of sodium, potassium, phosphorus, and fluid for hemodialysis patients during dialysis for 25 consecutive HD sessions. The control group (n=9) completed all aspects of the study including a visit by study personnel but were not given meals. The 25 consecutive sessions prior to the start of the intervention/control phase were used as a baseline comparison for each patient.
Main Outcome Measure
Symptomatic hypotension event frequency.
Results
In the intervention arm, there were 19 symptomatic hypotension events in 5 patients pre-study and 18 events in 6 patients during the study. In the control arm, there were 16 events in 7 patients pre-study and 13 events in 7 patients during the study. Change in the frequency of symptomatic hypotension events from pre-study to during study was not different between groups (P=0.71). There was no effect of meals on nutritional status, but patients reported positive attitudes towards receiving meals during dialysis.
Conclusion
High-protein meals during HD did not increase symptomatic hypotension events. Larger, longer-term studies are needed to confirm these results and evaluate whether high-protein meals on dialysis benefit nutritional status and clinical outcomes.
Keywords: Hemodialysis, nutrition, intradialytic hypotension, high-protein meals, end stage renal disease
Introduction
End stage renal disease (ESRD) is a major public health concern in the United States (US).1 Most ESRD patients undergo hemodialysis (HD) as treatment and these patients have increased protein (1.2 g/kg/day) and energy (30–35 kcal/kg/day) requirements but also diets restricted in phosphorus, sodium, potassium, and/or fluid.2–4 These dietary restrictions make it difficult for patients to meet their energy and protein requirements, a problem which may be compounded by a multitude of other issues including post-dialysis fatigue, nausea and vomiting, poor appetite, dysgeusia, lack of dietary knowledge, or lack of access to food. Thus, the average protein and energy intake of HD patients are estimated to be below requirements at 0.8–1.0 g/kg/d and 20–25 kcal/kg/d, and protein-energy wasting (PEW) is commonly observed in this population.5–9 Additionally, Burrowes et al.10 found that HD patients have significantly lower dietary energy and protein intake on dialysis treatment days compared to non-dialysis treatment days, which contributes to PEW along with the catabolic effects of the dialysis treatment and disease itself. This is concerning because PEW is associated with diminished quality of life and increased mortality rate.11
One intervention that has gained recent interest and support is providing nutrition through food or supplements during HD sessions.4,6,12,13 A recently published consensus statement from the International Society of Renal Nutrition and Metabolism,14 cites the need for larger randomized controlled trials, but suggests that providing nutrition during hemodialysis may be beneficial in patients without contraindications. In many industrialized countries including most European and Southeast Asian countries, eating food and/or providing nutritional supplements during dialysis treatment is allowed and often encouraged and there is evidence to suggest that these practices are associated with improved dietary intake, nutritional status, and survival.6,15 Protein turnover studies have shown that intradialytic nutrition provided via small meals,16 parenteral nutrition,17 or oral nutritional supplements18 counteracts the catabolic effects of HD treatment. Struijk-Wielinga et al.19 found that providing high protein meals to HD patients during dialysis improved intakes of both protein and energy. Further, Rhee et al.20 showed greater improvements in serum albumin while maintaining serum phosphate levels between 3.5–5.5 mg/dL in HD patients given high-protein meals with a phosphate binder for eight weeks during dialysis compared with patients provided low protein meals and no phosphate binder. Additionally, retrospective analyses from three major HD providers have found improved survival in malnourished patients receiving intradialyic oral nutrition supplementation.15,21,22
Despite the potential benefits to quality of life and clinical outcomes in HD patients, eating during HD is commonly prohibited or discouraged in US dialysis centers due to a variety of perceived health risks, particularly concern over increased intradialytic postprandial hypotension.12,23–25 Patients with intradialytic hypotension may experience an abrupt fall in blood pressure, symptoms such as cramping, headaches, nausea, and vomiting, and require medical intervention such as decreasing temperature of dialysate, saline infusion and discontinuation of ultrafiltration which reduces the effectiveness of the dialysis session.2 The prevalence of intradialytic hypotension ranges from 5 to 40% and early small studies suggest that eating during HD increases the incidence of hypotension in HD patients.26–29 In a study of nine HD patients, Sherman et al.28 showed that patients had greater incidence of symptomatic hypotension during dialysis sessions when they were given meals versus sessions where they were fasted. Similarly, Zoccali et al.29 showed an accelerated fall in blood pressure (BP) in a study of 13 patients and increased incidence of symptomatic hypotension in patients given snacks during treatment. However, these studies had differences in treatment parameters and meal composition that may have increased the likelihood of incidence of symptomatic hypotension.30,31 Due to the relative paucity of studies investigating effects of meals given during dialysis, we conducted a pilot study to evaluate the effect of high-protein meals provided during dialysis to HD patients on the primary outcome of frequency of symptomatic hypotensive events and secondary outcomes related to nutritional status, quality of life, and acceptability of meals during dialysis.
Methods
Study Design
This was a pilot/feasibility study of non-randomized, parallel-arm design. A convenience sample of 19 maintenance HD patients at a local dialysis center (U.S. Renal Care, Lafayette, IN) were recruited and enrolled in this study. The inclusion criteria were: 1) any gender; 2) any race; 3) age 18 years or older; and 4) receiving maintenance HD. The exclusion criteria were: NPO requiring exclusive tube feeding or parenteral feeding, or dysphagia that could not be accommodated by texture modification of the meals (no enrolled patients required texture modification due to dysphagia). Patients were not selected for on the basis of serum albumin levels, PEW, or proneness to intradialytic hypotension. Monday, Wednesday, Friday second shift patients (N=10) were allocated to receive high-protein, renal-appropriate meals during dialysis session. Tuesday, Thursday, Saturday second shift patients (N=9) were allocated to be in the control group, and were asked to participate in all aspects of the study protocol, excluding meals, including an “attention” control. Data collection on blood pressure (BP) and pre- and post-dialysis weight in kilograms (kg) included 25 consecutive dialysis sessions of retrospective medical record data immediately prior to the beginning of the study and 25 consecutive dialysis sessions during the intervention/control study period. The study protocol was approved by the Purdue University Institutional Review Board, and U.S. Renal Care approved of the study to be conducted in its center.
Meal Intervention
Meals were given approximately one hour after the start of the dialysis session. These were lunch meals as determined by the shift time. Several meal options with consistent nutrient content were designed by a research dietitian and were available for patients to choose from based on personal preferences. These included (main items): tuna bowtie salad, chicken salad plate, beef wrap with potato salad, chicken breast sandwich, turkey salad, chicken salad sandwich, vegan bowtie salad, and chicken lettuce salad. The target nutrient levels for the meals compared with KDOQI guidelines are shown in Table 1.
Table 1.
Meal Targeted and Actual Nutrient Content, Compared with KDOQI Guidelines
| Nutrient | KDOQI Guidelines2 |
Targeted Meal Content |
Actual Content ± SD |
Kcal from Macronutrients (% of Total Kilocalories) |
|---|---|---|---|---|
| Energy (kcal) | 35 kcal/kg/d | 800 (~1/3 daily requirement) | 715± 34 | - |
| Protein (g) | 1.2 g/kg/d | 30 | 30 ± 1 | 119 (16.5%) |
| Fat (g) | - | - | 31 ± 8 | 278 (39 %) |
| Carbohydrate (g) | - | - | 81 ± 21 | 323 (45%) |
| Sodium (mg) | 2000–3000 mg/d | <600–700 | 620 ± 84 | - |
| Potassium (mg) | 2000–3000 mg/d | <600–700 | 595 ± 93 | - |
| Phosphorus (mg) | 800–1000 mg/d | <250–350 | 312 ± 32 | - |
| Fluid (mL) | Fluid output + 1000 mL/d | 240 | 240 | - |
Starting Wednesday of the first week of the intervention, patients in the intervention group received lunch during the dialysis session. Study staff recorded the time when meal was delivered and returned along with the amount of food consumed using subjective assessment in percentage (0, 10, 25, 50, 75, 90, and 100%). Subjects in the control group were also visited each day, greeted by a study volunteer and given a small treat of hard candies, as an “attention” control.
Symptomatic Hypotensive Events and Other Blood Pressure Outcomes
BP measurements were recorded automatically every 30 minutes as part of routine care during the dialysis session. The primary outcome was symptomatic hypotensive events. A study physician (nephrologist) reviewed blood pressures of each dialysis run after completion of the study. A systolic BP drop of more than 20 mm of Hg from the start of dialysis was defined as numerical BP decrease.32 Each time a numerical decrease was detected, the physician determined if there were instances of any of the following to categorize whether or not that dialysis run was associated with a “symptomatic hypotensive” event: 1) a temporary or permanent stop in ultrafiltration 2) subject complaints of lightheadedness, nausea, dizziness or cramping, 3) cessation of the dialysis treatment itself or 4) administration of intravenous saline in an effort to increase blood pressure. Additionally, SBP and mean arterial pressure (MAP) were analyzed over the course of each treatment. From these values the average highest systolic blood pressure (SBP), average lowest SBP, maximum value of the highest SBP, and minimum value of the lowest SBP out of 25 dialysis sessions were determined from data collected from the dialysis treatment records. MAP from the lowest and highest BP for each patient during each dialysis session were calculated using the equation: . Body weight was measured as part of routine care pre- and post-dialysis and recorded in the medical record by dialysis staff. Interdialytic weight gain (IDWG) was calculated as the weight gained from the end of the previous dialysis session to the beginning of the next dialysis session.
Biochemical Measurements
Monthly routine renal laboratory tests, which include serum albumin, blood urea nitrogen, serum creatinine, glucose, serum calcium, potassium, sodium, phosphate, chloride, and carbon dioxide, were drawn on either Wednesday or Thursday of the first week of the month depending on the patient’s shift. Adjusted calcium in mg/d was automatically calculated using this equation: [0.8 × (4.0 − patient's albumin (g/dL))] + serum calcium (mg/dL), if patient’s serum albumin level was below 4.0 g/dL. Glucose was only included in the monthly routine renal laboratory tests if a patient had diabetes. The monthly laboratory tests prior to the beginning of the study which served as baseline, laboratory tests closest to midpoint of study (week 4), and closest to the end of study (week 9) were used and the routine laboratory data was obtained from the electronic medical record.
Patient Attitudes and Perceptions Regarding Nutrition and Meals on Dialysis
An End-of-Study Questionnaire was administered at week 9 to assess patients’ attitudes and perceptions of nutrition and receiving meals on dialysis. When patients were asked question 1: “how easy do you feel it is for you to eat nutritiously or follow a renal diet?”, they were asked to rate their response using a 5-point Likert scale where 1=very difficult, 2=somewhat difficult, 3=neither difficult nor easy, 4=somewhat easy, and 5=very easy. When patients were asked question 2 and 3: “how interested would you be in receiving nutritious meals during dialysis?” and “how interested would you be in a meal delivery service (to your home)?”, they were asked to rate their response using a 5-point Likert scale where 1=not at all interested, 2=somewhat disinterested, 3=neither interested nor disinterested, 4=somewhat interested, and 5=very interested. For question 4: “how important are price, taste, convenience, and nutrition for you in deciding what to eat?”, patients were asked to rate their response using a 5-point Likert scale where 1=very unimportant, 2=somewhat unimportant, 3=neither important nor unimportant, 4=somewhat important, and 5=very important.
Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the Indiana Clinical and Translational Science Institute.33 Statistical Analysis Software (SAS), version 9.3 (SAS Institute Inc. Cary, NC) was used to perform all statistical testing. The difference in the frequency of symptomatic hypotension events within groups for 2-month pre-study and during study (25 dialysis sessions each), was determined by Wilcoxon signed-rank test, and between group differences were determined by Wilcoxon rank-sum test. Other outcomes were assessed using repeated measures ANOVA with fixed effects for group, time, and group × time interaction. These analyses were limited to BP measurements that occurred in the first 240 minutes of treatment. Significance was set at α = 0.05.
Results
Baseline Results and Patient Demographics
One patient in the intervention arm withdrew from the study due to discontinuation of dialysis. Thus, nine patients per group completed the study and are included in the analyses. Patients were 62 ± 16 years old, 55% female, and had been on dialysis for 3.4 ± 2.6 years; baseline characteristics between the two study arms were generally similar, but those in the intervention group tended to be older with evidence of lower protein intake (evidenced by lower nPCR, BUN, and phosphate and higher carbon dioxide) (Table 2). Mean serum albumin was similar between groups (3.8 g/dL (range: 3.0–4.3 g/dL) and 3.9 g/dL (range: 3.7–4.5 g/dL) in the intervention and control groups, respectively; median 3.8 g/dL for both groups). Only three patients in each group were below the median (intervention group: n=1, 3.0 g/dL; n = 2, 3.6 g/dL; control group: n = 3, 3.7 g/dL). All other patients were at or above the median of 3.8 g/dL at baseline. Thus the majority of the sample were relatively adequate for serum albumin at baseline. A total of ten patients were hospitalized throughout the study (N=5 in each group) and reported to the IRB, but the reasons for hospitalization were deemed unrelated to patients’ participation in the study. The meals were generally well tolerated. Out of the 25 meals, mild symptoms included one patient in the intervention group who reported nausea and vomiting before three separate dialysis sessions, which led them to decline meals twice. One patient vomited during a meal reportedly due to illness, and another patient vomited during a meal reportedly due to the consistency of the food. The average nutrient content of all meals provided are shown in Table 1. This includes calories from beverages provided during the meal. Overall, nutrient content of the meals was within the targeted values. For meal consumption, at least 50% and 75% of the main items were consumed in 79% and 63% of 225 meals provided, respectively.
Table 2.
Baseline Characteristics of Participants†
| Intervention Group (N=9) |
Control Group (N=9) |
P-value | |
|---|---|---|---|
| Age (Years) | 66.8 ± 11.4 | 58.1 ± 19.2 | 0.26 |
|
| |||
| Female (#/total) | 5/9 | 5/9 | - |
|
| |||
| Pre-Dialysis Weight (kg) | 87 ± 21 | 85 ± 22 | 0.86 |
|
| |||
| Post-Dialysis Weight kg) | 85 ± 21 | 83 ± 22 | 0.83 |
|
| |||
| IDWG (kg) | 1.3 ± 2.0 | 1.4 ± 3.1 | 0.89 |
|
| |||
| BMI (kg/m2) | 32.2 ± 10.2 | 31.1 ± 8.0 | 0.47 |
|
| |||
| BMI Categories (#) | |||
| Underweight | 0 | 1 | - |
| Healthy | 2 | 1 | - |
| Overweight | 2 | 1 | - |
| Obese Class I | 2 | 4 | - |
| Obese Class II | 3 | 2 | - |
|
| |||
| Etiology (#) | |||
| Diabetes | 4 | 5 | - |
| Hypertension | 1 | 1 | - |
| Others | 2 | 1 | - |
| Unspecified | 2 | 2 | - |
|
| |||
| Time on Dialysis (Years) | 3.1 ± 2.0 | 3.6 ± 3.3 | 0.68 |
|
| |||
| eGFR (mL/min/1.73m2) | 7.9 ± 2.9 | 8.1 ± 3.7 | 0.89 |
|
| |||
| Kt/V | 1.56 ± 0.28 | 1.64 ± 0.19 | 0.54 |
|
| |||
| nPCR (g/kg) | 0.92 ± 0.24 | 1.15 ± 0.29 | 0.09 |
|
| |||
| Adjusted Calcium (mg/dL) | 9.14 ± 0.53 | 9.43 ± 0.82 | 0.39 |
|
| |||
| Albumin (g/dL) | 3.8 ± 0.3 | 3.9 ± 0.3 | 0.259 |
|
| |||
| BUN (mg/dL) | 45 ± 14 | 61 ± 16 | 0.032* |
|
| |||
| Carbon Dioxide (mEq/L) | 27 ± 2 | 25 ± 1 | 0.044* |
|
| |||
| Chloride (mEq/L) | 99 ± 2 | 99 ± 2 | 0.55 |
|
| |||
| Creatinine (mg/dL) | 6.7 ± 1.4 | 7.3 ± 3.1 | 0.59 |
|
| |||
| Iron Saturation (%) | 30 ± 13 | 27 ± 12 | 0.67 |
|
| |||
| Phosphate (mg/dL) | 5.0 ± 1.0 | 6.7 ± 1.9 | 0.04* |
|
| |||
| Potassium (mEq/L) | 4.4 ± 0.4 | 4.6 ± 0.6 | 0.40 |
|
| |||
| Sodium (mEq/L) | 139 ± 2 | 139 ± 2 | 0.84 |
|
| |||
| TIBC (mcg/dL) | 217 ± 23 | 237 ± 40 | 0.21 |
|
| |||
| Total Iron (mcg/dL) | 65 ± 30 | 66 ± 38 | 0.96 |
|
| |||
| Total Protein (g/dL) | 6.7 ± 0.5 | 6.8 ± 0.6 | 0.69 |
Data are represented as mean ± standard deviation (SD).
BMI, body mass index; BUN, blood urea nitrogen; IDWG: intradialytic weight gain; eGFR, estimated glomerular filtration rate; nPCR, normalized protein catabolic rate; TIBC, total iron binding capacity.
P < 0.05.
Symptomatic Hypotensive Events and Secondary Blood Pressure Outcomes
In the intervention group, there were 19 symptomatic hypotension events in 5 patients over 25 dialysis sessions per patient in the pre-study period and 18 symptomatic hypotension events in 6 patients over 25 dialysis sessions per patient during the study period. The difference in symptomatic hypotension event frequency from pre-study to during study in the intervention group was not statistically significant (P=0.89)(Table 3). In the control group, there were 16 symptomatic hypotension events in 7 patients pre-study and 13 symptomatic hypotension in 7 patients during study. The difference in symptomatic hypotension event frequency from pre-study to during study in the control group was not statistically significant (P=0.71)(Table 3). Change in the frequency of symptomatic hypotension events from pre-study to during study was not different between the intervention and control groups (P=0.56). Two subjects in the intervention group stand out as having different symptomatic hypotensive events pre- versus during study: Subject 2 had 7 events pre-study and 0 during study, and Subject 6 had 0 events pre-study and 5 during study. For subject 2, this decline in events was also accompanied by lower average IDWG (1.1 kg pre-study and 0.6 kg during study). For subject 6, this increase in events may be related to meals, but there was no consistent pattern of when these events occurred in relation to when the meal was eaten.
Table 3.
Number of Symptomatic Hypotension Events by Group
| Intervention Group | |||
| Subject | Pre-Study (Out of 25 sessions per patient) | During Study (Out of 25 sessions per patient) | Change |
| 1 | 5 | 3 | −2 |
| 2 | 7 | 0 | −7 |
| 3 | 2 | 1 | −1 |
| 4 | 0 | 1 | 1 |
| 5 | 1 | 3 | 2 |
| 6 | 0 | 5 | 5 |
| 7 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| 9 | 4 | 5 | 1 |
| Total | 19 | 18† | −1‡ |
| Control Group | |||
| Subject | Pre-Study (Out of 25 sessions per patient) | During Study (Out of 25 sessions per patient) | Change |
| 10 | 1 | 2 | 1 |
| 11 | 1 | 1 | 0 |
| 12 | 0 | 1 | 1 |
| 13 | 4 | 3 | −1 |
| 14 | 3 | 1 | −2 |
| 15 | 2 | 3 | 1 |
| 16 | 1 | 0 | −1 |
| 17 | 4 | 2 | −2 |
| 18 | 0 | 0 | 0 |
| Total | 16 | 13† | −3‡ |
The frequency (f) of symptomatic hypotension events in the intervention group (f = 18) during study was not significantly different than during the pre-study period (f = 19) (P=0.89). The frequency of events in the control group during study (f = 13) was not significantly different than during the pre-study period (f = 16) (P=0.71).
The change in frequency (Δf) from pre-study to during study was not significantly different between the intervention group (Δf = −1) and the control group (Δf = −3) (P=0.56).
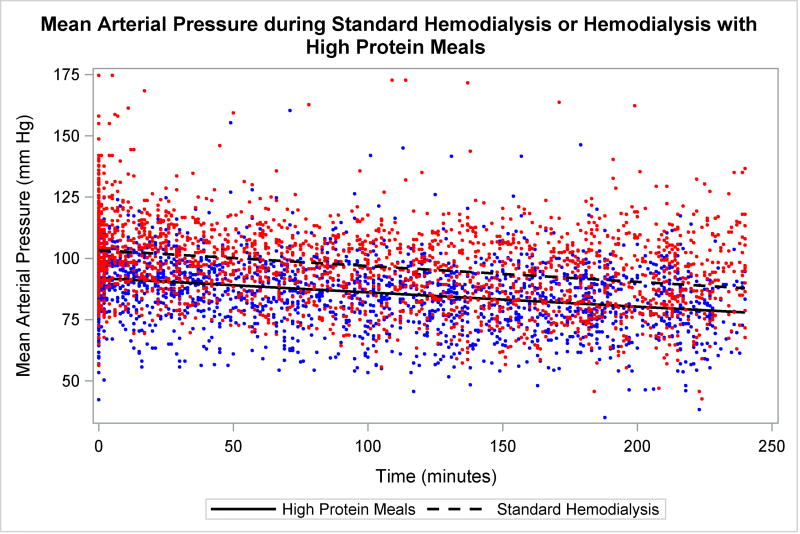
There was no difference in the change in SBP or MAP between the 25 pre-treatment and 25 treatments in which patients received meals (p>0.05 for all). However, there was a main effect of time for all (p<0.05). When comparing the 25 sessions in which patients received meals to the 25 active control sessions in the control group there was similarly no difference in the change in BP (p>0.05 for all) and a main effect of time (p<0.05). However, there was a main effect of group for SBP (p=0.013) and MAP (p=0.037, Figure 1). Furthermore, there were no significant interactions for other significant measures of SBP and MAP such as the lowest and highest treatment BPs (Table S1).
Figure 1. Blood pressure over treatment time.
Changes in mean arterial blood pressure during standard hemodialysis (open circles) and standard hemodialysis in which patients consumed high protein meals (closed circles). No significant interactions between groups (p>0.05). However, main effect of time and group (p<0.05). Abbreviations: mm, millimeters, Hg, mercury.
Biochemical Outcomes
Several significant group or time effects were observed for biochemical measures, but the absence of any significant group × time interactions indicate a lack of intervention effect (Table 4).
Table 4.
Monthly Laboratory Values and Body Weights at Baseline, Mid-Study, and End-of-Study by Group†
| Intervention | Control | ANOVA P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | Mid | End | BL | Mid | End | Group Effect |
Time Effect |
Inter- action Effect |
|
| Pre-Dialysis Weight (kg) | 87.2 ± 21.3 | 88.1 ± 21.5 | 87.4 ± 21.4 | 85.4 ± 21.7 | 85.4 ± 21.5 | 85.1 ± 21.5 | <0.0001* | 0.32 | 0.40 |
| Post-Dialysis Weight (kg) | 85.3 ± 21.3 | 85.6 ± 21.4 | 85.0 ± 20.6 | 83.0 ± 21.5 | 83.3 ± 21.6 | 82.8 ± 20.8 | <0.0001* | 0.34 | 0.99 |
| IDWG | 1.3 ± 2.0 | 2.2 ± 1.1 | 1.8 ± 1.1 | 1.4 ± 3.1 | 2.6 ± 1.4 | 2.4 ± 2.1 | 0.40 | 0.21 | 0.91 |
| Kt/V | 1.56 ± 0.28 | 1.48 ± 0.34 | 1.56 ± 0.34 | 1.64 ± 0.19 | 1.57 ± 0.28 | 1.55 ± 0.29 | 0.35 | 0.51 | 0.70 |
| Treatment duration (min) | 213 ± 48 | 210 ± 51 | 221 ± 31 | 225 ± 21 | 221 ± 22 | 231 ± 31 | 0.02* | 0.36 | 0.71 |
| Serum Albumin (g/dL) | 3.8 ± 0.3 | 3.8 ± 0.3 | 3.7 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.2 | 3.8 ± 0.3 | 0.01* | 0.59 | 0.82 |
| Total Protein (g/dL) | 6.7 ± 0.5 | 6.8 ± 0.6 | 6.7 ± 0.6 | 6.8 ± 0.6 | 6.8 ± 0.6 | 6.7 ± 0.5 | 0.45 | 0.73 | 0.45 |
| Adjusted Calcium (mg/dL) | 9.1 ± 0.5 | 9.2 ± 0.8 | 9.3 ± 0.4 | 9.4 ± 0.8 | 9.4 ± 0.9 | 9.3 ± 0.6 | 0.017* | 0.88 | 0.76 |
| BUN (mg/dL) | 45 ± 14 | 44 ± 15 | 49 ± 16 | 61 ± 16 | 57 ± 17 | 59 ± 13 | <0.0001* | 0.54 | 0.32 |
| Carbon Dioxide (mEq/L) | 27 ± 2 | 25 ± 1 | 25 ± 1 | 25 ± 1 | 24 ± 2 | 25 ± 2 | 0.005* | 0.04* | 0.58 |
| Chloride (mEq/L) | 99 ± 2 | 98 ± 3 | 100 ± 2 | 99 ± 2 | 98 ± 3 | 98 ± 3 | 0.20 | 0.38 | 0.19 |
| Cr (mg/dL) | 6.7 ± 1.4 | 6.8 ± 1.5 | 6.8 ± 1.1 | 7.3 ± 3.1 | 6.9 ± 2.4 | 7.8 ± 4.4 | 0.014* | 0.27 | 0.23 |
| Iron Sat. (%) | 30 ± 13 | 28 ± 10 | 29 ± 10 | 27 ± 13 | 30 ± 15 | 30 ± 9 | 0.43 | 0.45 | 0.19 |
| nPCR (g/kg) | 0.92 ± 0.24 | 0.83 ± 0.21 | 0.94 ± 0.29 | 1.15 ± 0.29 | 1.08 ± 0.33 | 1.12 ± 0.25 | <0.0001* | 0.50 | 0.35 |
| Phosphate (mg/dL) | 5.0 ± 1.0 | 5.2 ± 0.4 | 5.2 ± 0.7 | 6.7 ± 1.9 | 6.4 ± 1.5 | 7.0 ± 1.7 | <0.0001* | 0.59 | 0.53 |
| K (mEq/L) | 4.4 ± 0.4 | 4.5 ± 0.4 | 4.6 ± 0.5 | 4.6 ± 0.6 | 4.7 ± 0.7 | 4.7 ± 0.4 | 0.07 | 0.82 | 0.887 |
| Sodium (mEq/L) | 139 ± 2 | 137 ± 3 | 138 ± 2 | 139 ± 2 | 138 ± 3 | 137 ± 3 | 0.635 | 0.047* | 0.233 |
| TIBC (mcg/dL) | 217 ± 23 | 224 ± 18 | 235 ± 25 | 237 ± 40 | 248 ± 36 | 245 ± 38 | <0.0001* | 0.007* | 0.44 |
| Total Iron (mcg/dL) | 65 ± 30 | 62 ± 19 | 66 ± 19 | 66 ± 38 | 74 ± 38 | 72 ± 24 | 0.01* | 0.14 | 0.234 |
Data are presented as mean ± SD. BL, baseline; BUN, blood urea nitrogen; Cr, creatinine; IDWG: intradialytic weight gain; K, potassium; MP, midpoint; nPCR, normalized protein catabolic rate; N/A: not available; PO4, phosphate; TIBC, total iron binding capacity.
P < 0.05.
Pre-Dialysis Weight, Post-Dialysis Weight, and Intradialytic Weight Gain
Significant group differences were observed for pre-dialysis weight and post-dialysis weight, where the control group patients weighed less for both measures compared with the intervention group patients (Table 4). However, there was no group × time interaction, indicating no effect of intervention. There were no significant group, time, or interaction effects for IDWG from baseline, mid-study, to end-of-study (Table 4). However, paired t-tests comparing the average IDWG from the 25 pre-study dialysis sessions and 25 during study dialysis sessions in the same patients revealed a significant difference for the intervention (P=0.016). The intervention arm had lower average IDWG during study (1.8±0.9 kg) compared with pre-study (2.6±1.5 kg) while the control group had no difference in average IDWG pre-study versus during study (2.3±1.2 kg and 2.3±1.6 kg, respectively).
Dialysis Attendance
In the intervention group, the mean number of absences was 3.1±5.2 sessions in the pre-study period and 0.9±1.1 sessions during study. In the control group, the mean number of absences was 1.3±1.6 sessions in the pre-study period and 1.8±2.6 sessions during study. The difference in the number of absence from pre-study to during study was not statistically significant (P=0.25). Overall, providing high-protein meals during dialysis did not significantly affect the number of absences (P=0.38) as a significant change in absences was also not observed in the control group (P=0.69).
Patient Attitudes and Perceptions Regarding Nutrition and Meals on Dialysis
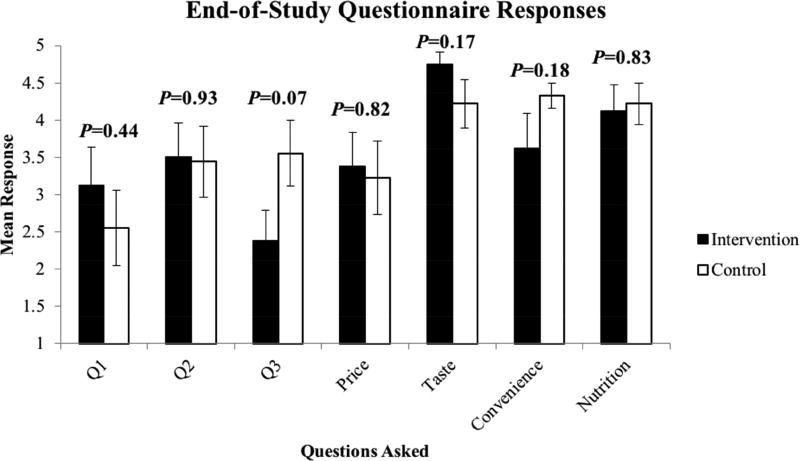
Seventeen patients completed the End-of-Study Questionnaire (one patient in the intervention arm declined to complete it). Overall, patients in both arms reported positive attitudes toward receiving nutritious meals during dialysis and no differences in responses were found between arms (Figure 2). When patients were asked “how easy do you feel it is for you to eat nutritiously or follow a renal diet?”, only 35% responded with “somewhat easy” or “very easy”. When patients were asked “how interested would you be in receiving nutritious meals during dialysis?”, 71% responded with “somewhat interested” or “very interested”. When patients were asked “how interested would you be in a meal delivery service (to your home)”, 47% responded with “somewhat interested” or “very interested”. Taste, convenience, price, and nutrition of meals were rated as somewhat to very important factors by 88%, 76%, 65%, and 78% of patients, respectively.
Figure 2. Patient Attitudes on Nutrition and Meals during Dialysis at the End of the 9-Week Study.
Error bars represent SEM. P-values are for differences between groups. Q1 = “how easy do you feel it is for you to eat nutritiously or follow a renal diet?”; Q2 = “how interested would you be in receiving nutritious meals during dialysis?”; Q3 = “how interested would you be in a meal delivery service (to your home); “How important is price/taste/convenience/nutrition.
Discussion
PEW is related to increased mortality in HD patients.11 To avoid PEW and its related consequences, meeting nutrient recommendations set by KDOQI may be beneficial. Studies suggest that providing either oral nutritional supplements or renal-appropriate meals during dialysis may be effective at helping HD patients to meet nutrient needs, but some studies question the safety of this approach related primarily to postprandial hypotension.28,34 Therefore, our study investigated the effect of high-protein, renal-appropriate meals given during dialysis in HD patients on blood pressure outcomes. We hypothesized that patients in the intervention group, who received high-protein, renal-appropriate meals during dialysis would 1) not have increased number of symptomatic hypotension events on dialysis, and 2) have better nutritional status, electrolyte balance, dialysis attendance, and fluid control compared with the control group. Our results support our main hypothesis that providing high-protein meals during dialysis does not affect the frequency of symptomatic hypotension events in HD patients. Further, neither arm had any significant difference in SBP or MAP during the study compared with the pre-study period. There were, however, overall group differences in BP measures: the control group had significantly higher BP than the intervention group. But, the patients who participated in our study appeared to be relatively hemodynamically stable as indicated by symptomatic hypotension present in only about 8% of treatments during the 25 dialysis sessions pre-study in all patients (35 out of 450 treatments).
In contrast to our findings, Sherman et al.28 conducted a study observing the effect of standard meals (two slices of white bread, two ounces of low sodium turkey breast, one teaspoon of regular mayonnaise, a slice of pound cake, and four ounces of cranberry juice cocktail) given during dialysis on BP and symptomatic hypotension of nondiabetic HD patients. They found an increased frequency of symptomatic hypotension events with meals: 2 out of 63 sessions during control compared with 13 out of 62 sessions with feeding, and in 1 out of 9 patients during control and 5 out of 9 patients during feeding. Similarly, Zoccali et al.29 found an increase in symptomatic hypotension requiring saline solution (23 treatments in 10 patients) when patients were served a large snack, which was composed of white bread, sirloin steak, and water or fruit juice, as compared to standard treatment (12 treatments in 6 patients) (P<0.025).
One explanation for the differences in symptoms between our trial and previous trials are differences in postprandial hemodynamics. Both Sherman and Zoccali found reductions in BP following a meal during HD.28,29 However, previous trials have found mixed results related to this practice with some trials finding a reduction28,29,35–37 and others finding no reduction in BP following eating during HD.38–40 Although our study was not powered to find differences in BP, our findings appear to support the latter group of trials as we found no difference in the change in BP following a high-protein meal consumed during HD. Many factors may explain the equivocal findings of these studies including patient selection (e.g., underlying cardiovascular function, hemodynamic stability, etc.), treatment factors (e.g., ultrafiltration, dialysate composition (including many early studies utilizing acetate dialysate and non high-flux dialyzers), dialysate temperature, etc.), and meal composition (e.g., meal size, temperature, and frequent inclusion of simple carbohydrates in meals, etc.).25–27,30,41–43 Given the discrepancy in hemodynamic findings between studies, additional studies with larger sample size and longer duration may be necessary to more definitively assess the effect of meals on dialysis on BP in HD patients. Furthermore, future studies should investigate patient, treatment, and meal factors that might influence the safety and effectiveness of this practice.
Serum albumin did not change over time in either the intervention or control group. However, average baseline values were ~3.8 g/dL, indicating this group of patients were not at a threshold baseline level where an effect of intervention might be achievable. Additionally, inflammation markers were not available, which would be valuable information in interpreting albumin, a negative acute-phase protein. Tomayko et al.44 showed no significant effect of a high protein oral nutrition supplement on serum C-reactive protein nor serum albumin, but showed reduced serum interleukin-6 and improved physical function with supplementation. Future studies should consider 1) patients’ baseline serum albumin level, as higher level may attenuate the potential effect of high-protein meals, 2) the presence of inflammation, and 3) move beyond the flawed nutritional markers of serum albumin and transthyretin (prealbumin) to more direct assessments of nutritional status such as nutrition-focused physical exam.45
Overall, no improvements with the intervention in renal laboratory values and dialysis attendance were found throughout the study. There was a significant decrease in IDWG in the intervention group during the study compared with pre-study, but not when comparing study baseline to mid-point or end-of-study. Because these two approaches to analyzing the IDWG data produced different results, these data should be interpreted with caution. If IDWG was truly decreased by the meal intervention, this may indicate patients given meals during dialysis maintained better dietary compliance particularly in regard to sodium and fluid intake or glycemic control. However, this hypothesis requires further confirmation.
There are limitations to this study due to its study design as a pilot/feasibility study. First, the small sample size of patients with relatively high baseline serum albumin levels likely limited our ability to examine the effects of high-protein meals during dialysis on nutritional status. Indeed, others have shown significant improvements in serum albumin with intradialytic nutrition in a similar length of time with the same amount46 or even less47 protein provided when patients had lower serum albumin levels at baseline. A newly published48 retrospective analysis of a pilot program that provided intradialytic oral nutritional supplements to in-center hemodialysis patients with baseline serum albumin ≤ 3.5 g/dL at 408 dialysis centers in the US showed that supplements were associated with improved survival after 8 months, improved dialysis session attendance, and nutritional markers of nPCR and postydialysis body weight. However, the supplemented patients actually had significantly lower serum albumin compared to the controls, but the authors note that this may have been due to survivor bias.
Baseline characteristics of patients in our study were also not evenly distributed due to the non-randomized study design. Particularly, the intervention group had lower baseline BP measures, BUN, nPCR, and phosphate, and higher carbon dioxide, though some of these did not reach statistical significance with the small group sizes. The intervention group also tended to be older. Thus, future studies to assess the efficacy of high-protein meals for improvement of nutritional status are needed, should be randomized-controlled, of larger sample size, and should select for patients with sufficiently low baseline serum albumin levels. Despite these limitations, this study had several strengths. These included the collection of data over 25 dialysis sessions in each patient during the study, and 25 pre-study dialysis sessions in each patient for baseline comparison; provision of nutritionally-adequate high-protein meals designed to meet KDOQI nutrient guidelines; and an assessment of patient attitudes towards receiving meals during HD.
Practical Application
These pilot data suggest that meals during HD do not increase the frequency of symptomatic hypotension events. Additionally, patients generally had positive attitudes towards receiving meals and such meals could help educate patients about appropriate food selection. However, changes in nutritional status indicators were not observed. Larger, longer-term, randomized-controlled studies, with patients selected for hypoalbuminemia at baseline are needed to confirm these results along with effects on nutritional and clinical outcomes in HD patients. Nevertheless, our data do not support the current practice of restricting eating meals during HD. Our conclusions are in agreement with the new consensus statement on eating during HD from the International Society of Renal Nutrition and Metabolism.14
Supplementary Material
Acknowledgments
The authors thank the dialysis center patients for their participation and the providers and staff for their gracious assistance and accommodation; Ann Wilcox, MS, RD, Jill Wanchisn, MS, RD, CSR, and student research assistants from Purdue University Department of Nutrition Science, Jessica Isaacs, Hannah Shelton, and Janice Tien, for their assistance in meal preparation and delivery at the dialysis center.
Support and Financial Disclosure: This study was funded through an Indiana CTSI Project Development Team Grant (NIH UL1TR00118) to KMHG; KMHG is supported through NIH K01 DK102864 and has no relevant financial conflicts of interest; RNM is supported through K23 DK102824 and has no relevant financial conflicts of interest. SMM is supported through NIH (AR072581, DK100306, AR070175 and a VA Merit Award) and has no relevant financial conflicts of interest, MSC, BMK, GNW, ERS, and AJW have no relevant financial relationships to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mun Sun Choi, Interdepartmental Nutrition Program, Purdue University, West Lafayette, IN.
Brandon Kistler, Department of Nutrition and Health Science, Ball State University, Muncie, IN.
Gretchen N. Wiese, Department of Nutrition Science, Purdue University, West Lafayette, IN.
Elizabeth R. Stremke, Department of Nutrition Science, Purdue University, West Lafayette, IN.
Amy J. Wright, Indiana Clinical and Translational Science Institute, Department of Nutrition Science, Purdue University, West Lafayette, IN.
Ranjani N. Moorthi, Department of Medicine/Division of Nephrology, Indiana University School of Medicine, Indianapolis, IN.
Sharon M. Moe, Department of Medicine/Division of Nephrology, Indiana University School of Medicine, and the Roudebush Veterans Administration Medical Center, Indianapolis, IN.
Kathleen M. Hill Gallant, Department of Nutrition Science, Purdue University; Adjunct Assistant Research Professor, Department of Medicine, Indiana University School of Medicine.
References
- 1.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 3.Iliescu EA, Coo H, McMurray MH, et al. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003;18(1):126–132. doi: 10.1093/ndt/18.1.126. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Fouque D. Nutritional Management of Chronic Kidney Disease. N Engl J Med. 2017;377(18):1765–1776. doi: 10.1056/NEJMra1700312. [DOI] [PubMed] [Google Scholar]
- 5.Butt S, Leon JB, David CL, Chang H, Sidhu S, Sehgal AR. The prevalence and nutritional implications of fast food consumption among patients receiving hemodialysis. J Ren Nutr. 2007;17(4):264–268. doi: 10.1053/j.jrn.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Ikizler TA. Let them eat during dialysis: an overlooked opportunity to improve outcomes in maintenance hemodialysis patients. J Ren Nutr. 2013;23(3):157– 163. doi: 10.1053/j.jrn.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38(6):1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97(6):1163–1177. doi: 10.3945/ajcn.112.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinaberger CS, Greenland S, Kopple JD, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88(6):1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrowes JD, Larive B, Cockram DB, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: cross-sectional results from the HEMO study. J Ren Nutr. 2003;13(3):191–198. doi: 10.1016/s1051-2276(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 11.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 12.Benner D, Burgess M, Stasios M, et al. In-Center Nutrition Practices of Clinics within a Large Hemodialysis Provider in the United States. Clin J Am Soc Nephrol. 2016;11(5):770–775. doi: 10.2215/CJN.09270915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Cano NJ, Budde K, et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7(7):369–384. doi: 10.1038/nrneph.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistler BM, Benner D, Burrowes JD, et al. Eating During Hemodialysis Treatment: A Consensus Statement From the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28(1):4–12. doi: 10.1053/j.jrn.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lacson E, Jr, Wang W, Zebrowski B, Wingard R, Hakim RM. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis. 2012;60(4):591–600. doi: 10.1053/j.ajkd.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Veeneman JM, Kingma HA, Boer TS, et al. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2003;284(5):E954–965. doi: 10.1152/ajpendo.00264.2002. [DOI] [PubMed] [Google Scholar]
- 17.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17(11):3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 18.Sundell MB, Cavanaugh KL, Wu P, Shintani A, Hakim RM, Ikizler TA. Oral protein supplementation alone improves anabolism in a dose-dependent manner in chronic hemodialysis patients. J Ren Nutr. 2009;19(5):412–421. doi: 10.1053/j.jrn.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struijk-Wielinga GIMR, Neelemaat F, Ter Wee PM, Weijs PJ. Providing In-Between Meals During Dialysis Treatment Contributes to an Adequate Protein And Energy Intake in Hemodialysis Patients: A Non-Randomized Intervention Study. M J Nutr. 2016;1(1):1–8. [Google Scholar]
- 20.Rhee CM, You AS, Koontz Parsons T, et al. Effect of high-protein meals during hemodialysis combined with lanthanum carbonate in hypoalbuminemic dialysis patients: findings from the FrEDI randomized controlled trial. Nephrol Dial Transplant. 2017;32(7):1233–1243. doi: 10.1093/ndt/gfw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benner D, Brunelli SM, Brosch B, Wheeler J, Nissenson AR. Effects of Oral Nutritional Supplements on Mortality, Missed Dialysis Treatments, and Nutritional Markers in Hemodialysis Patients. J Ren Nutr. 2017 doi: 10.1053/j.jrn.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Weiner DE, Tighiouart H, Ladik V, Meyer KB, Zager PG, Johnson DS. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis. 2014;63(2):276–285. doi: 10.1053/j.ajkd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Foley RN, Hakim RM. Why is the mortality of dialysis patients in the United States much higher than the rest of the world? J Am Soc Nephrol. 2009;20(7):1432–1435. doi: 10.1681/ASN.2009030282. [DOI] [PubMed] [Google Scholar]
- 24.Kistler B, Benner D, Burgess M, Stasios M, Kalantar-Zadeh K, Wilund KR. To eat or not to eat-international experiences with eating during hemodialysis treatment. J Ren Nutr. 2014;24(6):349–352. doi: 10.1053/j.jrn.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kistler BM, Fitschen PJ, Ikizler TA, Wilund KR. Rethinking the restriction on nutrition during hemodialysis treatment. J Ren Nutr. 2015;25(2):81–87. doi: 10.1053/j.jrn.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Davenport A. Intradialytic complications during hemodialysis. Hemodial Int. 2006;10(2):162–167. doi: 10.1111/j.1542-4758.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73(6):759–764. doi: 10.1038/sj.ki.5002745. [DOI] [PubMed] [Google Scholar]
- 28.Sherman RA, Torres F, Cody RP. Postprandial blood pressure changes during hemodialysis. Am J Kidney Dis. 1988;12(1):37–39. doi: 10.1016/s0272-6386(88)80069-6. [DOI] [PubMed] [Google Scholar]
- 29.Zoccali C, Mallamaci F, Ciccarelli M, Maggiore Q. Postprandial alterations in arterial pressure control during hemodialysis in uremic patients. Clin Nephrol. 1989;31(6):323–326. [PubMed] [Google Scholar]
- 30.Jansen RW, Peeters TL, Van Lier HJ, Hoefnagels WH. The effect of oral glucose, protein, fat and water loading on blood pressure and the gastrointestinal peptides VIP and somatostatin in hypertensive elderly subjects. Eur J Clin Invest. 1990;20(2):192–198. doi: 10.1111/j.1365-2362.1990.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 31.Liang CS, Lowenstein JM. Metabolic control of the circulation. Effects of acetate and pyruvate. J Clin Invest. 1978;62(5):1029–1038. doi: 10.1172/JCI109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337–414. [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strong J, Burgett M, Buss ML, Carver M, Kwankin S, Walker D. Effects of calorie and fluid intake on adverse events during hemodialysis. J Ren Nutr. 2001;11(2):97–100. doi: 10.1016/s1051-2276(01)51664-7. [DOI] [PubMed] [Google Scholar]
- 35.Barakat MM, Nawab ZM, Yu AW, Lau AH, Ing TS, Daugirdas JT. Hemodynamic effects of intradialytic food ingestion and the effects of caffeine. J Am Soc Nephrol. 1993;3(11):1813–1818. doi: 10.1681/ASN.V3111813. [DOI] [PubMed] [Google Scholar]
- 36.Kara B, Acikel C. The effect of intradialytic food intake on the urea reduction ratio and single-pool Kt/V values in patients followed-up at a hemodialysis center. Turk J Med Sci. 2010;40:91–97. [Google Scholar]
- 37.Sivalingam M, Banerjee A, Nevett G, Farrington K. Haemodynamic effects of food intake during haemodialysis. Blood Purif. 2008;26(2):157–162. doi: 10.1159/000114094. [DOI] [PubMed] [Google Scholar]
- 38.Benaroia M, Iliescu EA. Oral intake during hemodialysis: is there an association with intradialytic hypotension? Hemodial Int. 2008;12(1):62–65. doi: 10.1111/j.1542-4758.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- 39.Muller-Deile J, Lichtinghagen R, Haller H, Schmitt R. Online Kt/V monitoring in haemodialysis by UV absorbance: variations during intra-dialytic meals. Blood Purif. 2014;37(2):113–118. doi: 10.1159/000358212. [DOI] [PubMed] [Google Scholar]
- 40.Shibagaki Y, Takaichi K. Significant reduction of the large-vessel blood volume by food intake during hemodialysis. Clin Nephrol. 1998;49(1):49–54. [PubMed] [Google Scholar]
- 41.Kuipers HM, Jansen RW, Peeters TL, Hoefnagels WH. The influence of food temperature on postprandial blood pressure reduction and its relation to substance-P in healthy elderly subjects. J Am Geriatr Soc. 1991;39(2):181–184. doi: 10.1111/j.1532-5415.1991.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 42.Luciano GL, Brennan MJ, Rothberg MB. Postprandial hypotension. Am J Med. 2010;123(3):281, e281–286. doi: 10.1016/j.amjmed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Puvi-Rajasingham S, Mathias CJ. Effect of meal size on post-prandial blood pressure and on postural hypotension in primary autonomic failure. Clin Auton Res. 1996;6(2):111–114. doi: 10.1007/BF02291232. [DOI] [PubMed] [Google Scholar]
- 44.Tomayko EJ, Kistler BM, Fitschen PJ, Wilund KR. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J Ren Nutr. 2015;25(3):276–283. doi: 10.1053/j.jrn.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Malone A, Hamilton C. The Academy of Nutrition and Dietetics/the American Society for Parenteral and Enteral Nutrition consensus malnutrition characteristics: application in practice. Nutr Clin Pract. 2013;28(6):639–650. doi: 10.1177/0884533613508435. [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Braglia A, Chow J, et al. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr. 2005;15(3):318–331. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Caglar K, Fedje L, Dimmitt R, Hakim RM, Shyr Y, Ikizler TA. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002;62(3):1054–1059. doi: 10.1046/j.1523-1755.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Benner D, Brunelli SM, Brosch B, Wheeler J, Nissenson AR. Effects of Oral Nutritional Supplements on Mortality, Missed Dialysis Treatments, and Nutritional Markers in Hemodialysis Patients. J Ren Nutr. 2018;28(3):191–196. doi: 10.1053/j.jrn.2017.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.