Abstract
Background:
Platelets store large amounts of serotonin that they release during thrombus formation or acute inflammation. This facilitates hemostasis and modulates the inflammatory response.
Methods:
Infarct size, heart function and inflammatory cell composition were analyzed in mouse models of myocardial reperfusion injury with genetic and pharmacologic depletion of platelet serotonin. These studies were complemented by in vitro serotonin stimulation assays of platelets and leukocytes in mice and men and by measuring plasma serotonin levels and leukocyte activation in patients with acute coronary syndrome.
Results:
Platelet-derived serotonin induced neutrophil degranulation with release of myeloperoxidase (MPO) and hydrogen peroxide (H2O2) and increased expression of membrane-bound leukocyte adhesion molecule CD11b, leading to enhanced inflammation in the infarct area, and reduced myocardial salvage. In patients hospitalized with acute coronary syndrome, plasmatic serotonin levels correlated with CD11b expression on neutrophils and MPO plasma levels. Long-term serotonin reuptake inhibition (SRI) – reported to protect patients with depression from cardiovascular events – resulted in the depletion of platelet serotonin stores in mice.These mice displayed a reduction in neutrophil degranulation and preserved cardiac function. In line, patients with depression using SRI, presented with suppressed levels of CD11b surface expression on neutrophils and lower MPO levels in blood.
Conclusions:
Taken together, we identify serotonin as a potent therapeutic target in neutrophil-dependent thromboinflammation during myocardial reperfusion injury.
Keywords: Serotonin, ischemia reperfusion injury, inflammation, reactive oxygen species, platelet, degranulation, integrins, neutrophils
Introduction
Immediate restoration of blood flow by coronary intervention improves the survival of patients with myocardial infarction (MI). Targeting the deleterious processes during subsequent reperfusion injury holds the promise of further reducing the final infarct size and hence limiting heart failure in survivors1. The initial phase of reperfusion is characterized by ionic imbalance and distortion of the Na+/Ca2+ and Na+/H+ exchangers, leading to mitochondrial damage and hypercontraction of the myocardium2, 3. Microembolization, production of reactive oxygen species, and instability of vessel wall integrity further threaten myocardial recovery4. Finally, excessive leukocyte recruitment into the affected myocardium occurs in the early hours after reperfusion (neutrophils are the predominant invaders during the first 24 hours)5. The effect of neutrophil infiltration on outcome appears to follow a U-shaped curve with an optimum of modest neutrophil recruitment benefiting post-infarction healing, while excessive infiltration is detrimental, because neutrophils aggravate reperfusion injury6.
Mitigating the excess of neutrophil infiltration may restrict reperfusion injury while maintaining the beneficial healing process.
For transmigration through the vessel wall into inflamed tissues, neutrophils interact with activated endothelial cells directly7 or under the influence of platelet-mediated cell-cell communication8. Endothelial selectins mediate neutrophil tethering and rolling, followed by integrin-mediated firm cell adhesion that allows for subsequent transmigration9. Interfering with selectin and integrin function reduced experimental myocardial reperfusion injury, illustrating the relevance of inflammatory cell recruitment and the potential of pharmacological intervention10.
We recently reported that platelet-derived serotonin (5-hydroxytryptamine, 5-HT) enhanced the recruitment of neutrophils to sites of acute inflammation and tissue damage11. However, the underlying mechanism remained elusive. In the context of MI and coronary intervention, observational studies proposed that the depletion of platelet serotonin stores by long-term administration of serotonin reuptake inhibitors (a side-effect of many antidepressants) may improve outcome after MI and vasomotion post percutaneous coronary intervention (PCI)12.
We hypothesized that platelet serotonin aggravates myocardial reperfusion injury by promoting excessive neutrophil recruitment.
Indeed, serotonin induced exocytosis of neutrophil granules, thereby increasing the surface expression of the β2-integrin CD11b, mediating cell adhesion to platelets and the endothelium, and releasing myeloperoxidase (MPO) and hydrogen peroxide (H2O2), which altogether impaired healing after myocardial infarction13.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. All data, methods, materials are available upon personal request at the Department of Cardiology and Angiology I, Heart Center, University of Freiburg(contact: daniel.duerschmied@universitaets-herzzentrum.de).
A detailed section of all used methods is available in the online version of this manuscript.
Animals
Ten to twelve week-old tryptophan hydroxylase-1-deficient (Tph1−/−) mice were kindly provided by Michael Bader from the Max-Delbrück-Center for Molecular Medicine (MDC) (Berlin, Germany). Wild type (WT) littermates of heterozygous breeding were on a C57BL/6N background. All mice were housed in the local animal facility. All experiments were conducted strictly according to the German animal protection law and in accordance with good animal practice as defined by the Federation of Laboratory Animal Science Associations (www.felasa.eu) and the national animal welfare body GV-SOLAS (www.gv-solas.de). The examinations undertaken in this study were approved by the federal authorities in Freiburg and the Institutional Review Board.
Murine model of myocardial ischemia reperfusion
All surgeries were performed between 10a.m. and 2p.m. to avoid circadian rhythm-associated irregularities according to recently published guidelines for experimental models of myocardial ischemia and infarction14. Briefly, WT and Tph1−/− mice were anesthetized by intraperitonially (i.p.) injection of 80mg/kg Ketamine (Ketavet, Pfizer Pharmacia, Berlin, Germany) and 4,8mg/kg Xylazine (Rompun, Bayer Vital, Leverkusen, Germany). Analgesia was provided by subcutaneous (s.c.) injection of 125µg/kg buprenorphine. To avoid dehydration 500µL warm 0.9% NaCl (9mg/mL) containing 5% glucose (G5, B.Braun Melsungen, Melsungen, Germany) was injected i.p. Mice were placed on a heating pad to maintain body temperature at 37°C. The operation table was tilted 20% to allow visualization of the vocal cords and mice were intubated under sight. Ventilation was set to a maximal end-inspiratory pressure of 10 cm H2O, at a respiratory rate of 110 breaths per minute, and an inspiration/expiration ratio of 1/1.5 with a small-animal respirator (TSE Systems, Bad Homburg, Germany). Oxygen saturation, heart rate, and respiratory rate were monitored throughout the procedure by a MouseOX system (Starr Life Sciences, Oakmont, PA). Anesthesia was maintained by addition of 1.2% isoflurane (Abbott, Wiesbaden, Germany) during surgery. After right lateral positioning of the animal, left lateral thoracotomy was performed and the left anterior descending (LAD) coronary artery was identified. To induce ischemia an 8.0 nylon suture (Prolene, Ethicon, Norderstedt, Germany) was placed around the vessel and a loose loop was formed. A fine bore 0.61mm polythene tube was placed on the LAD and the loop was tied. Ligation was visually confirmed by paling of the left ventricular anterior wall. The tube was removed after 30 minutes to allow reperfusion of myocardium indicated by a bright red color within the LAD. After evacuation of the pneumothorax chest and skin wounds were closed using a 6–0 prolene suture (Prolene, Ethicon, Norderstedt, Germany). Mice were monitored until spontaneous breathing occurred and placed under a heating lamp. A second dose of buprenorphine was administered 10 hours after surgery.
Determination of infarct size
Hearts were excised 24 hours after surgery, placed in a petri dish containing 0.9% NaCl (B.Braun Melsungen, Melsungen, Germany) solution, the aorta was cannulated using a 22G safety iV catheter with injection port (Vasofix Safety, B.Braun Melsungen, Melsungen, Germany) and fixated using a 6–0 prolene suture. Ligation was reestablished by placing a polythene tube under the existing knot. Hearts were perfused with warm NaCl to remove any blood residues for 5 minutes by gravity flow. 200µL 5% MONOLITE Blue (Heubach, Langelsheim, Germany) diluted in 0.9% NaCl containing household detergent was injected into the system via a three-way cock to visualize affected myocardium. Hearts were frozen at −20°C for 10 minutes afterwards and cut in five to seven 1mm sections using a cold razor blade. Slices were put in phosphate buffer (0.2M Na2HPO4 / 0.2M NaH2PO4, adjusted to pH 7.4) containing 1% triphenyltetrazolium chloride (TTC, Sigma-Aldrich CHEMIE, Steinheim, Germany) and incubated in a water bath set to 37°C for 15 minutes. Necrotic tissue appeared white and healthy myocardium was stained red. Heart sections were placed on a cover slip, embedded with 5% agarose and covered with a second slip. Slices were photographed from both sides using an EOS 1000D camera (Canon, Deutschland, Krefeld, Germany) under a Stemi 2000-C binocular (Carl Zeiss, Oberkochen, Germany). Infarct size was calculated as the percentage of necrotic tissue in relation to non-monolite blue area with ImageJ software.
Echocardiography
Mice were anesthetized by 2% isoflurane i.n. and placed on a heating pad. Chest hair was removed using an electric shaver and animals were fixated on the back. Echocardiography loops were recorded in B and M modes in longitudinal and short axis views on a Vevo 3100 equipped with a MX550D transducer ( both FUJIFILM VisualSonics, Inc., Toronto, ON, Canada). Mice were fixed on a heated table and heart rate was monitored during the procedure. Systole and diastole were defined based on concomitant electrocardiography (ECG) recordings. The end-systolic time point for LV diameter measurement was defined as the maximum of ventricle contraction just before complete closure of the aortic valve. End-diastole was defined as the maximum of LV dilation and filling just before mitral valve closing (when visible) and aortic valve opening. Left ventricular ejection fraction (LVEF) was determined by LV tracing relating end systolic LV area as the minimal LV cross-sectional area to end-diastolic LV area as the maximum LV cross-sectional area in long axis views. Fractional shortening (FS) was assessed from short axis M-mode using VevoLab software (FUJIFILM VisualSonics).
Quantitative real-time polymerase chain reaction
Quantitative real-time PCR was performed using using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Braunschweig, Germany) or IQ SYBR Green Supermix (Bio-Rad Laboratories, München, Germany) on a C1000 Touch Thermal Cycler (Bio-Rad Laboratories). Samples were amplified using An overview of used Taqman probes (Supplemental table 1) or oligonucleotides (Supplemental table 2). Data were statistically analyzed using the 2−▵▵Ct method.
Flow cytometry
Murine blood samples
Blood from WT ant Tph1−/− mice was obtained by cardiac puncture using a 30 gauge needle coated with unfractionated heparin (B. Braun Melsungen, Melsungen, Germany). 10µL were incubated with 190µL lysis buffer (BD Bioscience, Heidelberg, Germany) for 5 minutes and leukocytes were counted in a Neubauer chamber. Blood was immediately transferred to an Eppendorf tube containing 1.5µg/µL enoxaparine (Sanofi Aventis, Frankfurt a. M., Germany) in 50 µL PBS containing 0.9mmol/L calcium, 0.5mmol/L magnesium, and 0.1% BSA (PBS+/+, BSA). For in vitro stimulation blood was incubated with either vehicle, or 100µmol/L 5-hydroxytryptamine (5-HT, serotonin, Sigma-Aldrich CHEMIE, Steinheim, Germany) for 15 minutes, as previously described15–18. Platelet rich plasma (PRP) was prepared as described above. Samples were diluted 1:6 in 37°C warm PBS. 90µL diluted blood or PRP was mixed with 10µL antibody mix and incubated for 15 minutes in the dark at room temperature. Stained whole blood samples were mixed gently with 400µL 37°C warm 1x Phosflow Lyse/Fix buffer (BD Bioscience, Heidelberg, Germany) and incubated for 30 minutes at room temperature. Intracellular analysis was performed using Fixation/Permeabilization Solution Kit (BD Bioscience, Heidelberg, Germany) according to suppliers protocol. Data was acquired on a BD FACSCanto II and analyzed with FlowJo v10 software (Tree Star, Ashland, OR, USA).
To evaluate leukocyte subsets, platelet neutrophil complexes, integrin, and selectin expression after 24 hours of reperfusion the following antibodies were used in 1:16 dilution in PBS+/+, BSA: anti-CD41-PECy7 (clone MWReg30), anti-CD42d-PerCPCy5.5 (clone 1C2), anti-CD45.2-AmCyan (clone 104), anti-CD45.2-PacificBlue (clone 104), anti-CD115-APC (clone CSF-1R), anti-F4/80-PE (clone BM8), anti-CD182-FITC (clone SA045E1), anti-CD3-FITC (clone 145–2C11), anti-CD19-PECy7 (clone 1D3), anti-Ly6C-PerCP/Cy5.5 (clone HK 1.4), anti-CD11a/CD18-PerCPCy5.5, (clone H155–78; all BioLegend, Fell, Germany), anti-CD62P-PacificBlue (clone RB40.34), anti-CD11b-APCCy7 (clone M1/70), anti-CD162-PacificBlue (clone 2PH1), anti-Ly6G-PE (clone 1A8; all Becton Dickinson, Heidelberg, Germany), anti-GPIIb/IIIa (clone JON/A) anti-GPVI-FITC (clone JAQ1, all EMFRET Analytics, Eibelstadt, Germany), anti-Ly6G(Gr1)-PECy7 (clone RB6–8C5) and anti-CD62L-APC (clone MEL-14; both eBiosceince, Frankfurt, Germany).
Human blood samples
Whole blood from healthy volunteers, depressed patients, and patients suffering from acute coronary syndrome (ACS) was obtained by peripheral venous puncture. Blood was drawn from ACS patients on the day after percutaneous intervention. ACS patients and patients with depression gave written informed consent as approved by the Institutional Review Board of the University Hospital of Freiburg: 287/12, and 18/11_160618, respectively. In a separate experiment, neutrophils of healthy volunteers were isolated from whole blood using Polymorphprep (AXIS-SHIELD, Rodelokka, Norway) according to manufacturers´ protocol. For in vitro stimulation blood was incubated with either vehicle, or 100µmol/L 5-hydroxytryptamine for 15 minutes at room temperature. For receptor blocking experiments blood was incubated with 100µmol/L of a 5-HT receptor antagonist (5HTR4: SB207266; 5HTR7 : SB269970; both Sigma-Aldrich CHEMIE, Steinheim, Germany) prior to stimulation. Blood was diluted 1:6 in warm FACS buffer and 90µL were stained with 10µL antibody mix and incubated for 15 minutes in the dark at room temperature. Stained whole blood samples were mixed gently with 400µL 37°C warm 1x Phosflow Lyse/Fix buffer (BD Bioscience, Heidelberg, Germany) and incubated for 30 minutes at room temperature. Data were acquired on a BD FACSCanto II and analyzed with FlowJo v10 software. Antibody mix to analyze PNC content, integrin, and selectin expression contained 1:16 dilution of the following monoclonal antibodies antibodies in PBS+/+, BSA: anti-CD41-PECy7 (clone HIP8), anti-CD45-APCCy7 (clone HI30), anti-CD66b-PerCP/Cy5.5 (clone G10F5), anti CD182-APC (clone 5E8/CXCR2), anti CD162-PE (clone KPL-1), anti-CD11a/CD18-AlexaFluor 488 (clone m24), anti-CD62L-BrilliantViolet 421 (clone DREG-56), anti-CD11a-PECy7 (clone HI111), and anti-CD11b-AlexaFluor 488 (clone ICRF44; all BioLegend, Fell, Germany).
Statistical analysis
Experimental results are presented as mean values ± SEM. One-way ANOVA with post hoc Tukey multiple comparisons test was performed when comparing >2 groups. Two-way Anova with Bonferroni’s multiple comparisons testing was used when more than two groups and variables were compared. Repeated measures ANOVA was used when the observations were not independent. Paired t-test was used when samples were split and analyzed under various conditions. Pearson correlation analysis, Fisher´s exact test, Mann-Whitney U test or Student´s t-test were used as indicated. P-values from multiple t-tests were adjusted using the Holm-Sidak method. Adjusted p-values of ≤0.05 denote significant changes. Statistics were calculated using GraphPad Software.
Results
Platelet serotonin aggravated myocardial ischemia and reperfusion injury
Myocardial infarction was induced by temporary coronary artery ligation in mice. At baseline serotonin plasma levels were low (73.5±8.7 ng/mL), but surged within 24 hours following 30min of myocardial ischemia with subsequent reperfusion in wild type (WT) mice (153.3±31.3 ng/mL*)(Figure 1a). In blood, serotonin is stored in platelets at millimolar concentrations and is quickly released upon their activation, which raises serotonin levels from nanomolar (in resting plasma) to micromolar concentrations (locally in vivo during thrombosis or inflammation or in vitro in serum preparations)19, 20. Tryptophan hydroxylase 1 deficient mice (Tph1−/−) are devoid of serotonin in blood, and platelets in particular, while serotonin levels in the central nervous system remain unaffected21 (Figure 1a). When Tph1−/− mice were subjected to myocardial ischemia with reperfusion, the infarct size was significantly reduced by about 35% compared to WT controls (36.4±2.4 vs. 55.3±3.2 % area at risk (AAR)*)(Figure 1b). In line, echocardiography documented less compromised left ventricular (LV) function early on and up to 3 weeks post MI in Tph1−/− mice (∼ 40% relative preservation of ejection fraction (EF) on d2 and 25% improved ejection fraction (EF) on d7*) (Figure 1c, Supplemental table 3). Moreover, inflammatory gene expression of myeloperoxidase (MPO) (−65.3±19 %), Tumor necrosis factor alpha (TNFα) (−59.8±16%), and chemokine KC (−48.5±16%) were reduced in the infarcts of Tph1−/− mice. The changes were considered significant between groups for MPO and TNFα following multiple comparisons testing. (Supplemental table 1, Figure 1d).
Figure 1.
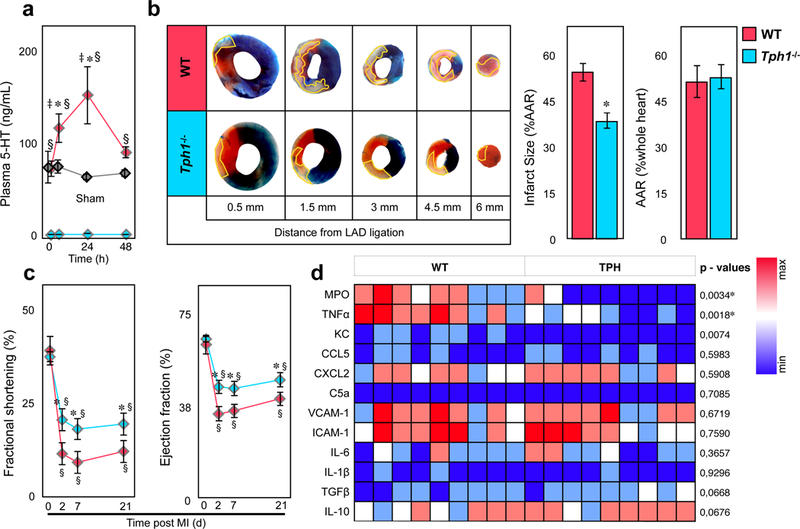
Myocardial reperfusion injury is dampened in Tph1−/− mice. (a) Plasma serotonin levels in WT (red) and Tph1−/− (blue) before, 6, 24, and 48 hours after myocardial infarction with reperfusion. Values in sham (thoracotomy was performed without ligation of the LAD) operated WT mice are shown in grey. Results are presented as mean ± SEM, ✽p<0.05 denote significant changes compared to time point 0 hours within groups, § p<0.05 denote significant changes between WT and Tph1−/− mice, and ‡ p<0.05 between WT and Sham, two-way ANOVA with Bonferroni’s multiple comparisons testing, n≥6 independent animals per group and time point. (b) Representative images of heart sections of WT and Tph1−/− mice after 30 minutes of LAD ligation with 24 hours of reperfusion at increasing distance from the site of ligation. The infarct area (white tissue) is circumscribed in yellow and quantified as percentage of area at risk (AAR; non-blue tissue). The AAR is presented as percentage of the entire heart section. Viable tissue within the AAR was stained in red. Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n≥10 per group, WT (red) and Tph1−/− (blue). (c) Time course of fractional shortening (FS) and left ventricular ejection fraction of WT and Tph1−/− mice recorded under baseline (d0) conditions and repetitively over 3 weeks (at day 2, 7, and 21) following myocardial infarction with reperfusion. Results are presented as mean ± SEM, ✽,§p<0.05, ✽ denotes significant changes between different genotypes; § denotes significance compared to d0 within groups; Two-way repeated measures ANOVA with Bonferroni’s multiple comparison testing, n=8 per group, WT (red) and Tph1−/− (blue). (d) Expression of pro- and anti-inflammatory target genes in homogenized heart tissue after I/R injury. Results are presented in a heat map depicting 2−▵Ct values, n=9 per group, each column represents one WT or Tph1−/− mouse. P values were derived from comparison of respective 2−▵▵Ct values by unpaired t-tests, and asterisks denote significant differences according to correction for multiple comparisons using the Holm-Sidak method.
Platelet serotonin promoted myocardial neutrophil infiltration
Neutrophils have previously been recognized as decisive promoters of myocardial reperfusion injury1, 22. We confirmed that neutrophils were the most abundant immune cell population within the left ventricle at 24 hours post infarction (44.8±5.4 % of leukocytes in WT controls*)(Figure 2a). Coinciding with reduced infarct size, we found fewer neutrophils within the infarcted tissue of Tph1−/− mice as compared to WT controls (13.9±1.5 vs. 27.1±2.7 per mm2 infarct area*)(Figure 2a-b) while their numbers in the blood, spleen and bone marrow did not differ significantly (Figure 2c). Monocyte counts in the infarct, blood, spleen and bone marrow were similar between the groups, pointing towards a mechanism that influenced neutrophil infiltration, in particular (Figure 2a, Supplemental Figure 1). Indeed, neutrophil depletion protected WT mice from myocardial reperfusion injury (39.2±3 vs. 53.2±4.2 % AAR* IgG treatment) to a similar extent as serotonin depletion in Tph1−/− mice, and did not alter the effects seen in Tph1−/− mice (Figure 2d, e, Supplemental Figure 1f). When hearts from WT and Tph1−/− mice were perfused ex vivo in the absence of circulating leukocytes and subjected to myocardial ischemia with reperfusion, both loss in cardiac function and cardiomyocyte viability were comparable in both groups (Supplemental Figure 2). We concluded that the deleterious effects of platelet serotonin on myocardial reperfusion injury mainly depended on neutrophil influx.
Figure 2.
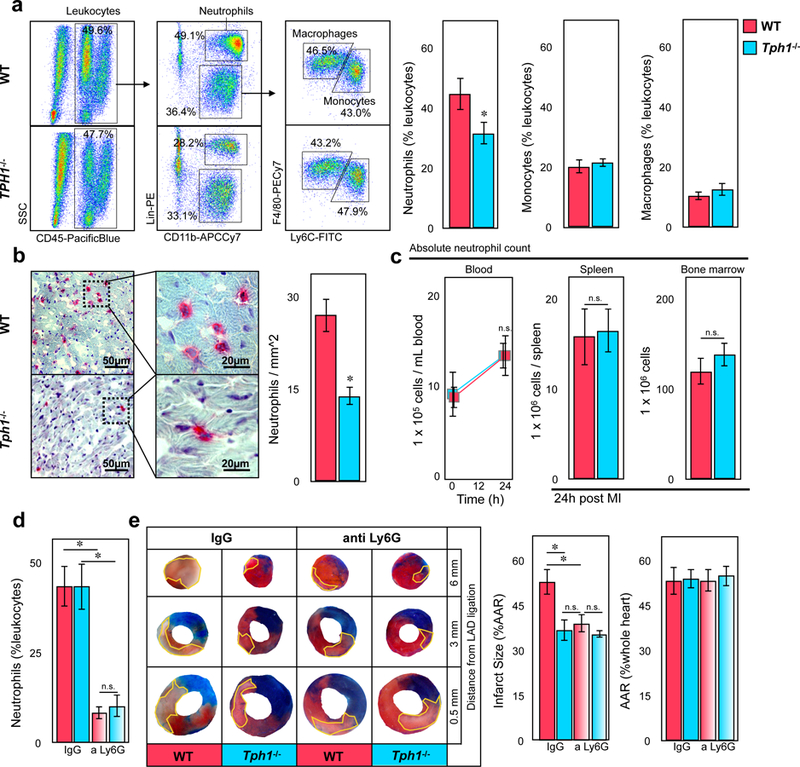
Reduced neutrophil accumulation in hearts of Tph1−/− mice following myocardial I/R injury. (a) Representative flow cytometric dot plots of digested cardiac tissues of WT and Tph1−/− mice 24 hours after myocardial ischemia and subsequent reperfusion (left panels). Quantification of neutrophils, monocytes, and macrophages in cardiac tissue of WT (red) and Tph1−/− (blue) mice 24 hours after myocardial I/R injury (right panels). Results are presented as mean percent of CD45.2+ leukocytes ± SEM, ✽p<0.05, Student´s t-test, n≥7 per group. (b) Representative immunohistology of myocardial infarct tissue. Ly6G+ neutrophils are stained in red (left panels) and quantified per mm2 tissue section of WT (red) and Tph1−/− (blue) mice. Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n≥9 per group. (c) Neutrophil counts of WT (red) and Tph1−/− (blue) mice in blood before, and in blood, spleen and bone marrow after myocardial I/R injury. Results are presented as mean ± SEM, n.s., two-way ANOVA with Bonferroni’s multiple comparison testing was used for blood samples, Student´s t-test was used for spleen and bone marrow, n=6 independent animals per group and time point. (d) Quantification of blood neutrophils following depletion with anti-Ly6G versus IgG control. Results are presented as mean ± SEM, ✽p<0.05, two-way ANOVA with Bonferroni’s multiple comparison testing, n=6 per group, WT (red) and Tph1−/− (blue). (e) Representative images of heart sections of WT and Tph1−/− mice after 30 minutes of LAD ligation followed by 24 hours of reperfusion at increasing distance from the site of ligation. The infarct area (white tissue) is circumscribed in yellow and quantified as percentage of area at risk (AAR; non-blue tissue). The AAR is presented as percentage of the entire heart section. Viable tissue within the AAR was stained in red. Results are presented as mean ± SEM, ✽p<0.05, two-way ANOVA with Bonferroni’s multiple comparison testing, n=6 per group, WT (red) and Tph1−/− (blue)
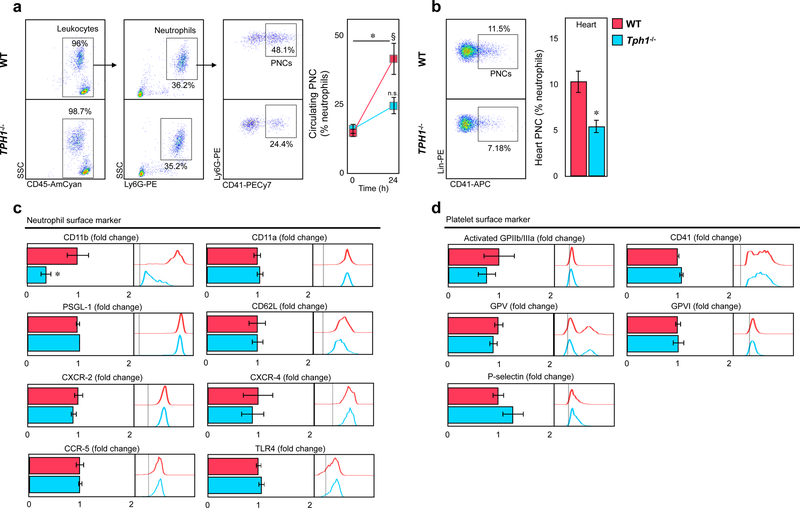
Serotonin increased integrin alpha M surface expression on neutrophils
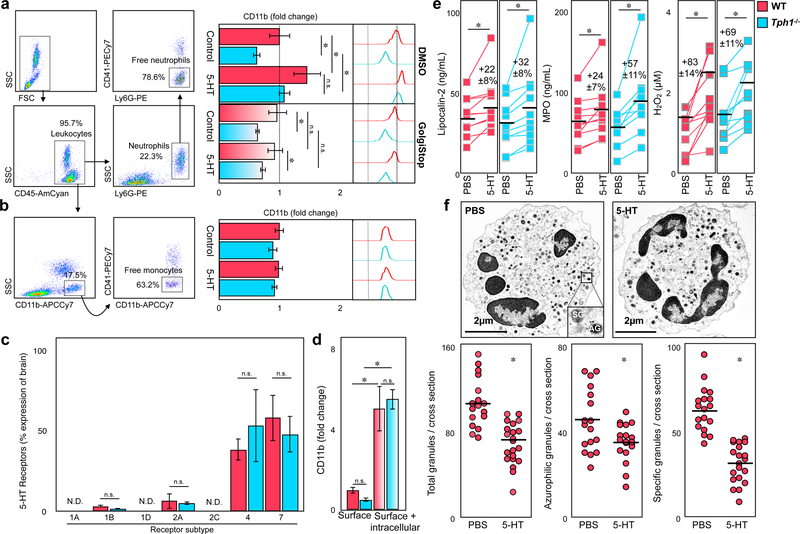
Platelets can directly interact with neutrophils to form complexes23, 24. Platelet neutrophil complex (PNC) formation in blood and heart doubled in WT mice 24 hours after myocardial reperfusion injury but remained low in Tph1−/− mice (41.3±5.8 vs. 24.2±3.1 % blood neutrophils*; 10.3±1.2 vs. 5.5±0.7 %MI tissue neutrophils*)(Figure 3a, b). Platelet monocyte complex formation however was similar in both groups (Supplemental Figure 1e). Interestingly, surface expression of CD11b on neutrophils was significantly increased in WT mice compared to Tph1−/− mice, with CD11b (integrin alpha M, alpha-subunit of Mac-1) known to mediate leukocyte adhesion to endothelial cells and platelets 19, 25 (Figure 3c, Supplemental Figure 3a). Other neutrophil surface components such as CD11a (part of LFA-1), L-selectin (CD62L), P-selectin glycoprotein ligand 1 (PSGL-1), and chemokine receptors remained largely unaffected (Figure 3c, Supplemental Figure 3a). Indeed, intravital microscopy of TNFα stimulated mesenteric venules revealed reduced numbers of adherent leukocytes in Tph1−/− mice (Supplemental Figure 4a) an effect limited to 5-HT mediated effects on neutrophils (Supplemental Figure 4b) while their migratory capacity appeared normal in vitro (Supplemental Figure 4c). Platelet surface marker expression in PNCs was similar in both groups (Figure 3d). Circulating platelets in Tph1−/− mice expressed lower levels of activated GPIIb/IIIa and GPV after myocardial infarction (Supplemental Figure 3b), while in vitro stimulation of platelets with serotonin in the absence of co-stimulators had no significant effects on platelet activation marker expression and integrity (Supplemental Figure 5, c), supporting serotonin´s role as a platelet co-activator that does not induce classical platelet activation without additional stimuli26. In contrast, incubating blood neutrophils with serotonin selectively raised CD11b surface expression by approximately 40% (Figure 4a; Supplemental Figure 5a).
Figure 3.
Platelet neutrophil complex formation is reduced in Tph1−/− mice after reperfusion injury. (a) Representative dot plots displaying platelet neutrophil complexes (PNCs) in blood of WT and Tph1−/− mice (left panel). PNCs were quantified before and after 24h of reperfusion (right panel). Results are presented as mean ± SEM, ✽p<0.05 comparing time points, § p<0,05 comparing groups, two-way ANOVA with Bonferroni’s multiple comparison testing, n≥10 independent animals per group and time point, WT (red) and Tph1−/− (blue).(b) Representative dot plots and quantification of PNCs within the AAR of WT (red) and Tph1−/− (blue) mice at 24 hours after myocardial ischemia with reperfusion. Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n≥7 per group. (c) Quantification of CD11b, CD11a, CXCR-2, CD62L and PSGL-1 surface expression in PNC of WT (red) and Tph1−/− (blue) mice 24 hours after myocardial I/R injury. Results are presented as normalized mean ± SEM, ✽p<0.05, Student´s t-test, n=10 per group. (d) Activated GPIIb/IIIa, GPV, GPVI, and P-selectin platelet marker expression in PNC of WT and Tph1−/− mice 24 hours after myocardial I/R injury. Results are presented as normalized mean ± SEM, n.s., Student´s t-test, n=10 per group. Bar graphs are accompanied by representative histograms of mean fluorescence intensities (MFI) of respective markers.
Figure 4.
Serotonin mediates degranulation of neutrophils in vitro. (a) Representative dot plots displaying uncomplexed (free) neutrophils (SSChigh, Ly6G+, CD41−) in blood (left panel). Quantification of neutrophil CD11b expression in WT (red) and Tph1−/− (blue) mice upon stimulation with PBS (control) or 100µM serotonin co-incubated with DMSO or GolgiStop/Plug reagent (right panel). Results are presented as mean ± SEM, ✽p<0.05, two-way ANOVA with Bonferroni test, n≥12 per group (b) Dot plots of uncomplexed (free) monocytes (SSClow, CD11b+, CD41−) in blood (left panel) and quantification of CD11b expression following serotonin or PBS treatment (right panel). Results are presented as mean ± SEM, ✽p<0.05, two-way ANOVA with Bonferroni test, n≥12 per group, WT (red) and Tph1−/− (blue). (c) Expression of different serotonin receptors in isolated neutrophils in WT (red) and Tph1−/− (blue) mice. Results are presented as normalized mean ± SEM, n.s., Student´s t-test, n=5 per group.
(d) Intracellular expression of CD11b in neutrophils compared to matched surface expression in blood of WT (red) and Tph1−/− (blue) mice. Results are presented as mean ± SEM normalized to WT surface staining, ✽p<0.05 denotes significant changes between surface and intracellular staining, two-way ANOVA for repeated measures with Bonferroni’s multiple comparison testing, n=5 per group. (e) Plasma concentration of lipocalin-2,MPO, and H2O2 in WT (red) and Tph1−/− (blue) blood after stimulation with either serotonin or PBS. Results are presented as normalized mean ± SEM, ✽p<0.05, Paired Student´s t-test, n=9 per group. (f) Electron microscopic images of neutrophils after treatment with serotonin or PBS (top panel). Quantification of azurophilic granules (AG) and specific granules (SG) per cross section after stimulation with serotonin (lower panels). Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n≥15 per treatment.
Serotonin induced degranulation of neutrophils
Neutrophil stimulation with serotonin increased CD11b surface expression both in wild type and Tph1−/− mice, thereby normalizing CD11b levels in the knockout animals (Figure 4a). In contrast, CD11b surface expression in monocytes was similar in both groups before and after incubation with serotonin (Figure 4b). These observations pointed to a neutrophil-specific and inducible serotonin effect. Identification of several serotonin receptors, most notably subtype 4 and 7, on isolated neutrophils suggested that neutrophils were able to respond to serotonin, directly (Figure 4c, Supplemental table 2). Unlike monocytes, neutrophils carry intracellular granules that are shuttled to the surface upon stimulation. CD11b, but not CD11a, is stored in secretory granules alongside other neutrophil mediators such as lipocalin-2 and MPO27. Flow cytometric staining following membrane permeabilization documented that most of the neutrophil’s CD11b was stored intracellularly. Tph1−/− mice showed similar total CD11b protein levels to those in wild type mice while CD11b surface expression was relatively reduced by trend (Figure 4d). These data suggested that serotonin engaged in granule release in neutrophils. Consequently, stimulation of cultured blood neutrophils with serotonin did not only increase CD11b surface expression but also lipocalin-2 and MPO levels in the supernatants (Figure 4e). We also detected a release of hydrogen peroxide (H2O2), a highly reactive oxidative burst product, upon stimulation (Figure 4e). Conversely, inhibition of granule release by treatment with protein transport inhibitors brefeldin A and monensin abolished the rise of CD11b in wild type and Tph1−/− mice (Figure 4a). Comparing electron microscopic images of neutrophils before and after stimulation with serotonin, we were able to visualize and quantify the disposal of azurophilic (45.9±3.9 vs. 35.6±2.3*) and specific granules (64.1±3.5 vs. 35.2±4.8*)(Fig4f). Taken together, our data indicate a critical role for platelet serotonin in the regulation of neutrophil degranulation.
Platelet serotonin depletion by fluoxetine protected from myocardial ischemia reperfusion injury
We wondered wether we could phenocopy our observations made in Tph1−/− mice by treating WT mice with the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Flx) for 3 weeks. The longterm SSRI treatment (mimicking SSRI treatment of patients with depression) resulted in a near complete depletion of serotonin stores in platelets (Figure 5a). Consequently, plasma serotonin remained at baseline in Flx treated mice even after myocardial ischemia with reperfusion (Figure 5b). Serotonin depletion resulted in a decreased PNC frequency with lower surface expression of CD11b on neutrophils, and reduced plasma MPO levels post MI (Figure 5d, e). Surface expression of CD11a and other adhesion molecules did not change and circulating platelets showed a decrease of activation markers with SSRI treatment similar to that observed in Tph1−/− mice (Supplemental Figure 6a, b). Lastly, infarct size (39.9±1.9 vs. 56.4±2.8 % AAR*) and heart function (20.2±0.7 vs. 12.6±0,5 % FS*) improved with SSRI treatment (Figure 5f, g). P2Y12 inhibitors are routinely used in the acute treatment of MI and have been described to limit myocardial ischemia and reperfusion injury28, 29. We tested whether this protection was at least partly mediated by preventing the release of serotonin from platelets during myocardial infarction (Supplemental Figure 7a). While Flx treatment alone mildly reduced adenosine diphosphate (ADP) stimulated platelet aggregation, ticagrelor administration blocked ADP effects completely and blunted platelet P-selectin externalization both in Flx and vehicle treated mice (Supplemental Figure 7b, e). Serotonin release, however, remained unaffected by ticagrelor (Supplemental Figure 7c). In accord, ADP and many other platelet agonists except for thrombin and LPS failed to stimulated 5-HT release from platelets in vitro (Supplemental Figure 7d). In neutrophils, Flx or ticagrelor alone reduced CD11b surface expression to a similar extent. The combination of both drugs, however, reduced neutrophil CD11b expression and PNC formation by an additional 30–50%* (Supplemental Figure 7f, g). Consequently, the complementary blockade of ADP-dependent platelet activation and serotonin release proved to reduce the infarct size even more effectively than either drug alone (Supplemental Figure 7h).
Figure 5.
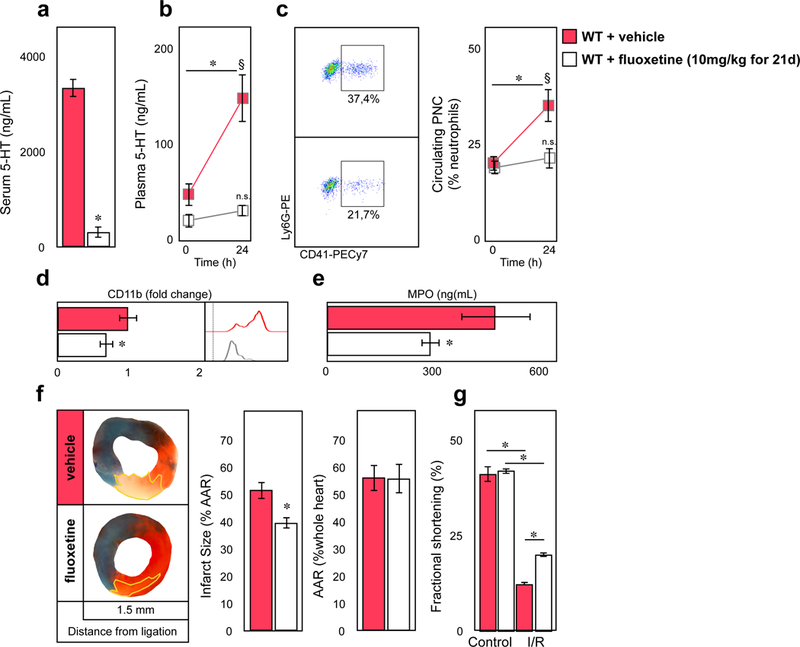
Pharmacologic platelet serotonin depletion protects from myocardial ischemia reperfusion injury. (a) Three week administration of fluoxetine (Flx) depletes platelet dense granule serotonin storage (indirectly assessed by freshly provoked serum). Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n=7 per vehicle (red) or Flx (white) treatment. (b) Plasma serotonin levels in vehicle (red) and Flx (white) before, and 24 hours after myocardial infarction with reperfusion. Results are presented as mean ± SEM, ✽p<0.05 comparing time points within groups, § p<0.05 comparing groups, two-way ANOVA with Bonferroni’s multiple comparison testing, n=7 independent animals per treatment and time point. (c) Representative dot plots of circulating PNCs in vehicle (red) and Flx (white) treated mice (left panel) and quantification before and after 24 hours following myocardial I/R injury. Results are presented as mean ± SEM, p<0.05 comparing time points within groups, § p<0.05 comparing groups, two-way ANOVA with Bonferroni’s multiple comparison testing, n=7 independent animals per treatment and time point. (d) Quantification of CD11b surface expression in PNC and (e) plasma MPO levels of vehicle (red) and Flx (white) treated mice 24 hours after myocardial ischemia and reperfusion. Results are presented as normalized mean ± SEM, ✽p<0.05, Student´s t-test, n=7 per group. (f) Representative images of heart sections of vehicle and fluoxetine treated mice after 30 minutes of LAD ligation with 24 hours of reperfusion. Infarcted areal (white tissue) is circumscribed in yellow and quantified as percentage of area at risk (AAR; non-blue tissue). The AAR is presented as percentage of the entire heart section. Viable tissue within the AAR was tained in red. Results are presented as mean ± SEM, ✽p<0.05, Student´s t-test, n=7 per vehicle (red) and Flx (white) treatment. (g) LV fractional shortening in vehicle (red) and Flx (white) treated mice following I/R injury. Results are presented as mean ± SEM, ✽p<0.05, two-way ANOVA with Bonferroni test, n=7 per group.
Plasma serotonin correlated with the neutrophil release of CD11b and MPO in ACS patients
In accord with our findings in mice, human neutrophils showed strong upregulation ofCD11b surface expression and elevated plasma MPO levels upon stimulation with serotonin in vitro. These effects were reversed by blocking the 5HT receptor subtype 7, specifically (Figure 6a), and by vesicle transport inhibition (Supplemental Figure 8a). CD11b expression on neutrophils gradually increased within minutes of incubation with serotonin (Supplemental Figure 8b). In contrast, CD11b expression on human monocytes as well as CD11a expression on either cell type did not change in response to serotonin (Supplemental Figure 7c). In patients with acute coronary syndrome (see Supplemental Table 4 for demographics), CD11b surface expression and plasmatic MPO concentration strongly correlated with serotonin levels in plasma (Pearson r=0.846*, r=0.6606*, and r=0.516* respectively), while no significant correlations were observed for CD11a, CD62L, PSGL-1 and CXCR2 (Figure 6b c, d, Supplemental Figure 8d). Of note, all patients studied had been treated with aspirin plus a P2Y12 inhibitor for at least 24 hours prior to blood sampling. Conversely, in patients with depression, longterm SRI use suppressed plasma serotonin levels, and reduced neutrophil CD11b expression significantly and MPO levels by trend (Figure 6e) compared to controls (Supplemental Table 5). We concluded that activated platelets released serotonin during myocardial ischemia and reperfusion injury leading to the release of secretory granules containing CD11b and MPO in neutrophils. Elevated CD11b surface expression supported platelet neutrophil complex formation and cell adhesion to the endothelium while MPO aggravated inflammation (Figure 7).
Figure 6.
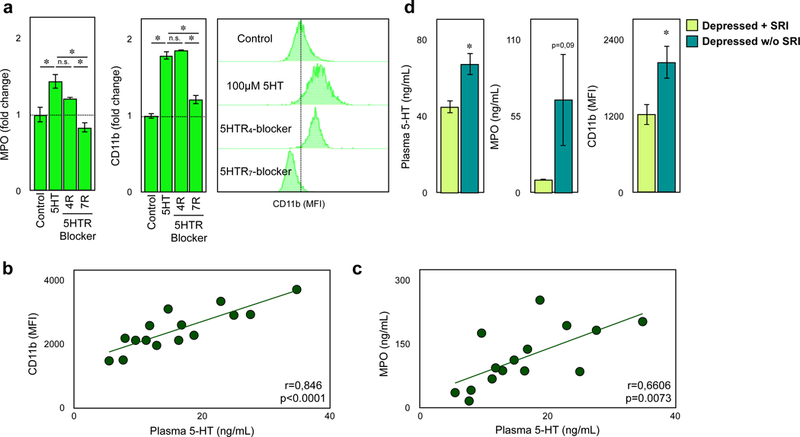
Human neutrophil degranulation is regulated by serotonin. (a) Supernatant MPO concentration and surface CD11b expression on human neutrophils after stimulation with 100µM serotonin or PBS with and without addition of specific serotonin receptor antagonist (5HTR4 and 5HTR7). Representative histograms of changes in CD11b expression are shown on the right. Results are presented as mean ± SEM, ✽p<0.05, One-way ANOVA with Tukey t-test, n=4 per treatment. (b) Correlation of plasma serotonin concentration and CD11b (p<0.0001) on blood neutrophils and (c) plasma MPO concentration of patients with acute coronary syndrome. Each point represents an individual patient, r: Pearson correlation coefficient. (d) Quantification of plasma serotonin and MPO levels and CD11b expression in blood of depressed patients treated with (lime green) or without (blue) antidepressants. ✽p<0.05, Student´s t-test, n=10 per group
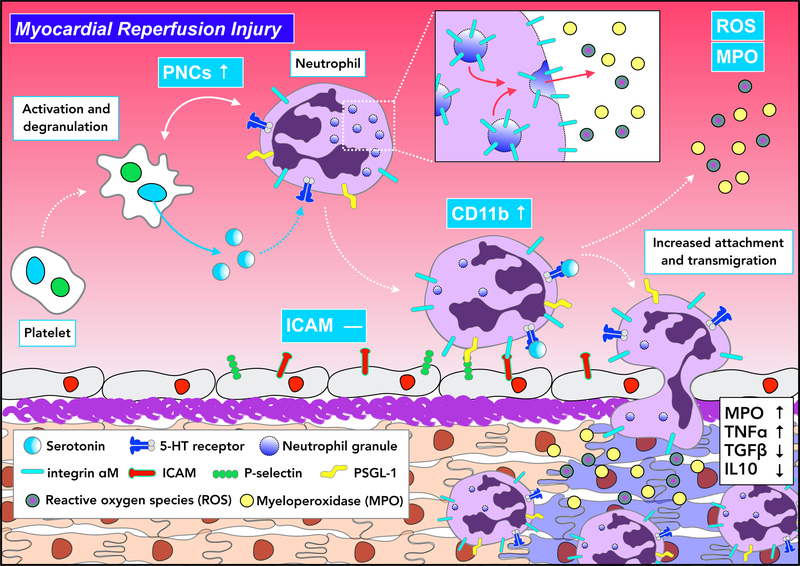
Figure 7.
Proposed model of platelet serotonin-neutrophil interactions during myocardial reperfusion injury. Serotonin released by platelets triggers the degranulation of neutrophils, subsequently leading to increased CD11b surface expression. This enhances circulating PNC levels and supports the accumulation of pro-inflammatory cytokine-releasing neutrophils within the injured myocardium.
Taken together, longterm fluoxetine treatment, an inducible model of platelet serotonin depletion in adult WT mice, phenocopied Tph1−/− mice that underwent temporary LAD ligation with respect to CD11b and MPO release and protection from experimental myocardial ischemia and reperfusion injury. Our clinical data suggest that similar mechanisms operate in humans.
Discussion
Serotonin is well known as a neurotransmitter and modulator of vasomotion30–32. Platelets are the dominant store of serotonin outside the nervous system and discharge serotonin upon activation. Coronary artery disease and myocardial infarction provide a platelet-stimulating micro-environment that leads to the release of serotonin from platelets33. We identified thrombin and TLR4 ligands, but not ADP, as stimulators of serotonin release. In particular younger patients with coronary artery disease and high serotonin levels are at increased risk for future cardiovascular events34. Mechanistically, serotonin was thought to promote platelet aggregation and vasoconstriction of diseased coronary arteries and influence cardiomyocyte function and survival35, 36. Experimental studies in animals investigating the role of serotonin in myocardial ischemia and reperfusion injury yielded conflicting results, which has to date prevented a successful translation into clinical studies33, 37. Discrepancies may have originated from different experimental setups, different animal models, and most notably, different serotonin receptor antagonists being investigated.
In an early study, the 5-HT2 receptor antagonist LY53857 did not influence infarct size after 90 min of ligation of the left circumflex coronary artery followed by 5 h of reperfusion in living dogs38. In contrast, the combined Ca2+ and 5-HT2 receptor antagonist nexopamil reduced infarct size by 55% after 1 h of LAD ligation and 3 h of reperfusion in living minipigs39. While LY53857 rather increased ex vivo reperfusion injury of isolated rat hearts perfused with platelets in an experimental setup with 15 min global ischemia followed by 10 min of reperfusion40, several 5-HT2 receptor antagonists reduced ex vivo reperfusion injury of rat hearts after 25 min of ischemia and 30 min of reperfusion41. Circulating serotonin levels were significantly increased after 30 min of myocardial ischemia in living rabbits as well as in isolated rabbit hearts and treatment with the 5-HT2A receptor antagonist sarpogrelate reduced infarct size by 32% (after 48h of reperfusion) in a more recent study33. We report now the first study on myocardial reperfusion injury investigating platelet serotonin depletion in mice.
Transient ischemia of ex vivo perfused hearts of Tph1−/− mice devoid of peripheral serotonin did not affect the loss in cardiac function in the absence of circulating cells, while neutrophil depletion in vivo completely abolished the platelet serotonin-mediated aggravation of reperfusion injury.
Intrigued by these neutrophil-dependent effects, we identified platelet-derived serotonin as a potent stimulator of neutrophils in vitro and in vivo. We provided several lines of evidence that serotonin induced degranulation in neutrophils increasing the surface expression of granule-stored CD11b27, 42 and the release of MPO known to enhance tissue damage in concert with reactive oxygen species13, 43. First, we found that serotonin elicits CD11b but not CD11a surface expression in neutrophils but not monocytes, as CD11a is not stored in intracellular granules44 and monocytes are almost devoid of those. Second, vesicle transport inhibition blocked the increase of CD11b surface expression. Third, electron microscopy of neutrophils directly visualized the depletion of intracellular granules upon serotonin stimulation. The prompt and gradual increase of CD11b surface expression in neutrophils resembled degranulation kinetics reported for other granule-derived mediators such as MPO, CD66b, lactoferrin, lysozyme, gelatinase, and neutrophil gelatinase-associated lipocalin (NGAL)45. Interestingly, the P2Y12 antagonist ticagrelor inhibited platelet activation and lowered CD11b surface expression on neutrophils as well. However, these inhibitory effects were independent from serotonin. Serotonin depletion resulted in an additional reduction in CD11b expression. Our data indicate that neutrophil degranulation is non-redundantly controlled by several stimuli, of which serotonin is being described in this work for the first time.
We discovered several serotonin receptor subtypes on neutrophils, most prominently 5-HT4 and 5-HT7. Neutrophil degranulation depends on intracellular calcium increase that is typically mediated by chemokine/cytokine-induced G protein-coupled receptor signaling46. Direct serotonin effects on neutrophil degranulation have not been described before. However, serotonin has been shown to stimulate Ca2+ influx and intracellular Ca2+ mobilization in vascular smooth muscle cells both via G protein coupled/IP3-dependent (e.g 5-HT2 receptor subset) and cAMP/PKA-dependent (e.g. 5-HT4–7 receptor subsets) signaling pathways18, 47. We demonstrated that serotonin stimulated neutrophil degranulation via the receptor subtype 7 in humans.
Increased CD11b surface expression renders neutrophils more effective in adhering to the activated endothelium, the extracellular matrix7, 25, and to platelets, forming complexes19. Consequently, lack of platelet serotonin resulted in reduced numbers of PNC and neutrophil deposition in the injured heart. Our findings are in line with previous reports suggesting that limiting neutrophil influx protected from myocardial reperfusion injury48. Serotonin has further been shown to aggravate other neutrophil-dependent inflammatory diseases like arthritis, endotoxic shock, asthma, or viral hepatitis11, 49, 50, but the cellular mechanisms have not been completely evaluated.
Finally, we verified that longterm SSRI treatment emptied platelet serotonin stores, providing us with an inducible model of peripheral serotonin deficiency in adult mice. Longterm SSRI treatment protected from myocardial reperfusion injury similar to TPH1 deficiency, resulting in a comparable reduction in CD11b surface expression in neutrophils. Intriguingly, SSRI treatment protected from myocardial ischemia and reperfusion injury even on top of P2Y12 inhibition, which is an essential part of standard of care for ACS patients, clinically, and was reported to limit myocardial I/R injury, experimentally in rats and rabbits 28, 29. Platelets, our data suggest, govern neutrophil responses in serotonin-dependent and -independent pathways. Wholesome platelet depletion, however, may offset the protection conferred by selective serotonin depletion and P2Y12 inhibition in exchange for bleeding complications and is not a viable option in practice29. We therefore advocate for targeting undesirable platelet effects in MI, such as those mediated by serotonin, while preserving beneficial ones.
This view is supported by clinical data showing that chronic SSRI use was associated with fewer cardiovascular events in patients with depression12. Thus, we extended our experimental studies to humans. We confirmed the stimulatory effects of serotonin on CD11b surface expression and MPO release in human neutrophils mediated via the 5HT7 receptor. Elevated plasma serotonin levels correlated with neutrophil CD11b surface expression and MPO plasma levels in patients with acute coronary syndrome. Platelet serotonin depletion in non-cardiac patients treated with SSRI for their depression, we showed, suppressed CD11b expression and MPO levels.
Thus, we identified a novel mode of platelet-neutrophil crosstalk during myocardial ischemia and reperfusion injury that is relevant to humans: platelets secrete serotonin to promote degranulation of neutrophils with CD11b externalization, and MPO and H2O2 secretion (Figure 7). These findings provide a pathomechanistic rationale for targeting the formerly unrecognized platelet serotonin-neutrophil interactome to ameliorate the inflammatory response in myocardial ischemia/reperfusion injury.
Supplementary Material
Clinical Perspective.
What is new?
Markers of neutrophil degranulation correlate with plasmatic serotonin levels in ACS patients
Neutrophils express serotonin receptors 5HT4 and 5HT7
Platelet-derived serotonin triggers neutrophil degranulation
Genetic or pharmacologic depletion of serotonin in platelets protects from myocardial ischemia and reperfusion injury
What are the clinical implications?
Platelet-derived serotonin stimulating neutrophil infiltration, degranulation and oxidative burst represents a druggable target to mitigate the inflammatory burden in myocardial ischemia and reperfusion injury.
Inhibiting serotonin uptake into platelets of patients treated for depression reduced neutrophil degranulation even under otherwise healthy conditions, indicating that the platelet serotonin–neutrophil axis is of relevance to humans and modifiable.
Acknowledgment:
The authors thank Prof. Karlheinz Peter, Baker Heart and Diabetes Institute, Melbourne, Australia, Prof. Michael Reth, Max Planck Institute for Immunobiology and Epigenetics, Freiburg, Germany, and Prof. Marco Idzko, Department of Pneumology, Medical Center Freiburg, Germany for their intellectual support and discussion of the data. We thank Dr. Klaus Kaier and Moritz Hess for their assistance with statistical analysis. Prof. Denisa D. Wagner, Harvard Medical School, Boston, USA, has been a long-standing mentor, supporter, and source of ideas for N.H. and D.D.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG) DU 1190/3–1 (D.D.) and IH 1573/2 (I.H.), the National Institutes of Health NIH R01 HL115232 (K.L.), the Ernst und Berta Grimmke Stiftung 3/16, and the German Heart Foundation K/02/16.
Footnotes
Disclosures: The authors declare that there are no conflicts of interest to disclose.
References
- 1.Yellon DM and Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ and Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM and Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heusch G and Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017;38:774–784. [DOI] [PubMed] [Google Scholar]
- 5.Vinten-Johansen J Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovascular research 2004;61:481–497. [DOI] [PubMed] [Google Scholar]
- 6.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O and Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
- 7.Ley K, Laudanna C, Cybulsky MI and Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 8.Diacovo TG, Roth SJ, Buccola JM, Bainton DF and Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood 1996;88:146–157. [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K, McEver RP and Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011;118:6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D and Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 2011;17:581–588. [DOI] [PubMed] [Google Scholar]
- 11.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C and Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013;121:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer WH, Berlin JA and Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003;108:32–36. [DOI] [PubMed] [Google Scholar]
- 13.Ali M, Pulli B, Courties G, Tricot B, Sebas M, Iwamoto Y, Hilgendorf I, Schob S, Dong A, Zheng W, Skoura A, Kalgukar A, Cortes C, Ruggeri R, Swirski FK, Nahrendorf M, Buckbinder L and Chen JW. Myeloperoxidase Inhibition Improves Ventricular Function and Remodeling After Experimental Myocardial Infarction. JACC: Basic to Translational Science 2016;1:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey ML, Bolli R, Canty JM Jr., Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE and Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 2018;314:H812–H838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durk T, Panther E, Muller T, Sorichter S, Ferrari D, Pizzirani C, Di Virgilio F, Myrtek D, Norgauer J and Idzko M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol 2005;17:599–606. [DOI] [PubMed] [Google Scholar]
- 16.Pracharova L, Okenkova K, Lojek A and Ciz M. Serotonin and its 5-HT(2) receptor agonist DOI hydrochloride inhibit the oxidative burst in total leukocytes but not in isolated neutrophils. Life Sci 2010;86:518–523. [DOI] [PubMed] [Google Scholar]
- 17.Patocka N, Sharma N, Rashid M and Ribeiro P. Serotonin signaling in Schistosoma mansoni: a serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog 2014;10:e1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okubo M, Satoh Y, Hirakawa M, Sasaki K, Masu K, G JM, Ikeda-Kurosawa C, Kurosaka D and Saino T. Different effect of serotonin on intracellular calcium ion dynamics in the smooth muscle cells between rat posterior ciliary artery and vorticose vein. Biomed Res 2016;37:101–115. [DOI] [PubMed] [Google Scholar]
- 19.Zarbock A, Polanowska-Grabowska RK and Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 2007;21:99–111. [DOI] [PubMed] [Google Scholar]
- 20.Gros A, Ollivier V and Ho-Tin-Noe B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H and Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003;299:76. [DOI] [PubMed] [Google Scholar]
- 22.Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL and Lucchesi BR. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J 1986;112:682–690. [DOI] [PubMed] [Google Scholar]
- 23.Page C and Pitchford S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. International immunopharmacology 2013;17:1176–1184. [DOI] [PubMed] [Google Scholar]
- 24.Mauler M, Seyfert J, Haenel D, Seeba H, Guenther J, Stallmann D, Schoenichen C, Hilgendorf I, Bode C, Ahrens I and Duerschmied D. Platelet-neutrophil complex formation-a detailed in vitro analysis of murine and human blood samples. J Leukoc Biol 2016;99:781–789. [DOI] [PubMed] [Google Scholar]
- 25.Kolaczkowska E and Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology 2013;13:159–175. [DOI] [PubMed] [Google Scholar]
- 26.Vanags DM, Rodgers SE, Duncan EM, Lloyd JV and Bochner F. Potentiation of ADP-induced aggregation in human platelet-rich plasma by 5-hydroxytryptamine and adrenaline. Br J Pharmacol 1992;106:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borregaard N and Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997;89:3503–3521. [PubMed] [Google Scholar]
- 28.Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S and Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 2015;35:1805–1814. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MV, Yang XM, White J, Yellon DM, Bell RM and Downey JM. Cangrelor-Mediated Cardioprotection Requires Platelets and Sphingosine Phosphorylation. Cardiovasc Drugs Ther 2016;30:229–232. [DOI] [PubMed] [Google Scholar]
- 30.Berger M, Gray JA and Roth BL. The expanded biology of serotonin. Annu Rev Med 2009;60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duerschmied D and Bode C. [The role of serotonin in haemostasis]. Hamostaseologie 2009;29:356–359. [PubMed] [Google Scholar]
- 32.Watts SW. Oh, the places you’ll go! My many colored serotonin (apologies to Dr. Seuss). Am J Physiol Heart Circ Physiol 2016;311:H1225–H1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu Y, Minatoguchi S, Hashimoto K, Uno Y, Arai M, Wang N, Chen X, Lu C, Takemura G, Shimomura M, Fujiwara T and Fujiwara H. The role of serotonin in ischemic cellular damage and the infarct size-reducing effect of sarpogrelate, a 5-hydroxytryptamine-2 receptor blocker, in rabbit hearts. J Am Coll Cardiol 2002;40:1347–1355. [DOI] [PubMed] [Google Scholar]
- 34.Vikenes K, Farstad M and Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events. Circulation 1999;100:483–489. [DOI] [PubMed] [Google Scholar]
- 35.Doggrell SA. The role of 5-HT on the cardiovascular and renal systems and the clinical potential of 5-HT modulation. Expert Opin Investig Drugs 2003;12:805–823. [DOI] [PubMed] [Google Scholar]
- 36.Nebigil CG, Etienne N, Messaddeq N and Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2003;17:1373–1375. [DOI] [PubMed] [Google Scholar]
- 37.Muto T, Usuda H, Yamamura A, Yoshida K, Ohashi A, Mitsui-Saitoh K, Sakai J, Sugimoto Y, Mizutani H, Nonogaki T and Hotta Y. Protective effects of fluvoxamine against ischemia/reperfusion injury in isolated, perfused guinea-pig hearts. Biological & pharmaceutical bulletin 2014;37:731–739. [DOI] [PubMed] [Google Scholar]
- 38.Simpson PJ, Schelm JA, Jakubowski JA and Smallwood JK. The role of serotonin (5HT2) receptor blockade in myocardial reperfusion injury: effects of LY53857 in a canine model of myocardial infarction. The Journal of pharmacology and experimental therapeutics 1991;258:979–985. [PubMed] [Google Scholar]
- 39.Hohlfeld T, Braun M, Strobach H and Schror K. Protection of reperfused ischemic pig myocardium by nexopamil, a new combined Ca2+ and serotonin antagonist. J Cardiovasc Pharmacol 1994;23:922–931. [DOI] [PubMed] [Google Scholar]
- 40.Yang BC, Virmani R, Nichols WW and Mehta JL. Platelets protect against myocardial dysfunction and injury induced by ischemia and reperfusion in isolated rat hearts. Circ Res 1993;72:1181–1190. [DOI] [PubMed] [Google Scholar]
- 41.Grover GJ, Sargent CA, Dzwonczyk S, Normandin DE and Antonaccio MJ. Protective effect of serotonin (5-HT2) receptor antagonists in ischemic rat hearts. J Cardiovasc Pharmacol 1993;22:664–672. [DOI] [PubMed] [Google Scholar]
- 42.Cowland JB and Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev 2016;273:11–28. [DOI] [PubMed] [Google Scholar]
- 43.Dale DC, Boxer L and Liles WC. The phagocytes: neutrophils and monocytes. Blood 2008;112:935–945. [DOI] [PubMed] [Google Scholar]
- 44.Faurschou M and Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes and infection / Institut Pasteur 2003;5:1317–1327. [DOI] [PubMed] [Google Scholar]
- 45.Naegelen I, Beaume N, Plancon S, Schenten V, Tschirhart EJ and Brechard S. Regulation of Neutrophil Degranulation and Cytokine Secretion: A Novel Model Approach Based on Linear Fitting. J Immunol Res 2015;2015:817038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacy P Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2006;2:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong DT, Perry KW and Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 2005;4:764–774. [DOI] [PubMed] [Google Scholar]
- 48.Arai M, Lefer DJ, So T, DiPaula A, Aversano T and Becker LC. An anti-CD18 antibody limits infarct size and preserves left ventricular function in dogs with ischemia and 48-hour reperfusion. J Am Coll Cardiol 1996;27:1278–1285. [DOI] [PubMed] [Google Scholar]
- 49.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Lohning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM and Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 2008;14:756–761. [DOI] [PubMed] [Google Scholar]
- 50.Cloutier N, Pare A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S and Boilard E. Platelets can enhance vascular permeability. Blood 2012;120:1334–1343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.