Abstract
Microtubules are cytoskeletal polymers that dynamically remodel to perform essential cellular functions. Individual microtubules alternate between phases of growth and shrinkage via sudden transitions called catastrophe and rescue, driven by losing and regaining a stabilizing cap at the dynamic microtubule end. New in vitro studies now show that a conserved family of CLASP proteins specifically modulate microtubule catastrophe and rescue transitions. Further, recent cryo-electron microscopy approaches have elucidated new structural features of the stabilizing cap. Together, these new advances provide a clearer view on the complexity of the microtubule end and its regulation.
INTRODUCTION
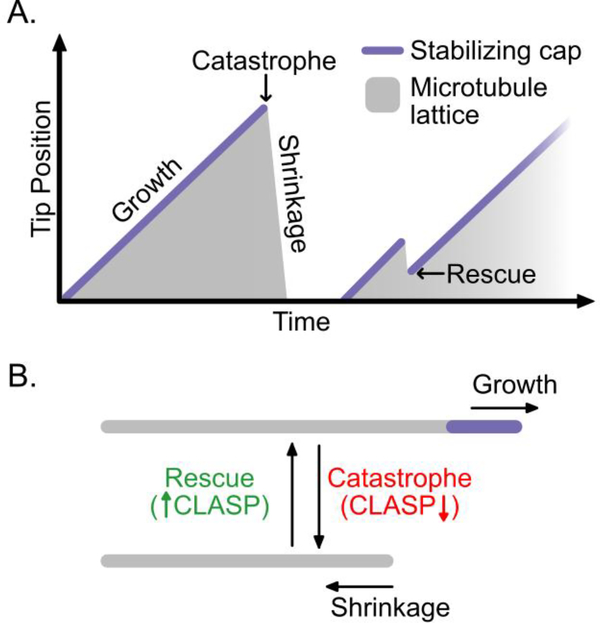
Microtubules constitute an essential component of the cytoskeleton, and are important for establishing cellular architecture, mediating intracellular transport and facilitating dynamic processes including cell division and migration. Microtubules are dynamic polymers composed of αβ-tubulin heterodimers arranged in a head-to-tail fashion. The tubulin subunits in the microtubule lattice associate longitudinally and laterally to form a closed tube with typically 13 protofilaments (although see [1] for examples of structural diversity). A notable feature of microtubules is that they undergo a phenomenon known as dynamic instability [2]: individual microtubules within a population alternate between phases of growth and shrinkage via sudden transitions called catastrophe and rescue (Figure 1A). Catastrophe and rescue are driven by losing and regaining a stabilizing cap at the dynamic microtubule end; however, the mechanistic underpinnings of these transitions remain obscure. In addition, microtubule-associated proteins (MAPs) can regulate one or more aspects of microtubule dynamics and, thus, fine-tune the microtubule network according to the needs of the cell. Several recent studies [3–5] investigate one such family of MAPs, CLASP proteins, known for their ability to both suppress microtubule catastrophe and promote rescue [6–8] (Figure 1B). Here, we explore new insights into the structure and dynamics of the stabilizing cap, and discuss the role of CLASP at the interface of catastrophe and rescue.
Figure 1.
Microtubule dynamic instability. (A) Microtubules alternate between phases of growth and shrinkage via the switch-like transitions called catastrophe and rescue. The schematic shows the phases and transitions of dynamic instability, with the microtubule tip position plotted as a function of time. The grey shaded areas represent the microtubule lattice and the purple line represents the stabilizing cap, which is present only during microtubule growth. The graph depicts a growing microtubule that switches to rapid shrinkage via a catastrophe event. After a short lag, a new microtubule nucleates and grows. However, following catastrophe, shrinkage is interrupted by a rescue event and the microtubule resumes growth. The cap is lost prior to catastrophe and regained when a new microtubule is nucleated or when rescue occurs. (B) CLASP suppresses catastrophe and promotes rescue, but has little effect on the mean rates of microtubule growth and shrinkage.
THE STABILIZING CAP
Dynamic instability is based on the concept of a stabilizing cap of GTP-tubulin at the ends of growing microtubules [2,9]. During growth, GTP-bound tubulin subunits are added to the exposed ends of individual protofilaments. The incorporation of tubulin into the microtubule lattice triggers the hydrolysis of GTP to GDP via an intermediate GDP-Pi state. However, GTP-hydrolysis and phosphate release are not instantaneous [10]. Consequently, the ends of growing microtubules are rich in GTP-tubulin followed by GDP-Pi-tubulin, while the body of the microtubule is composed mainly of GDP-tubulin (Figure 2A). Importantly, microtubules grown with GMPCPP, a slowly hydrolysable GTP analogue, do not undergo dynamic instability [11]. Thus, the GTP-tubulin hydrolysis cycle ultimately underlies microtubule dynamics.
Figure 2.
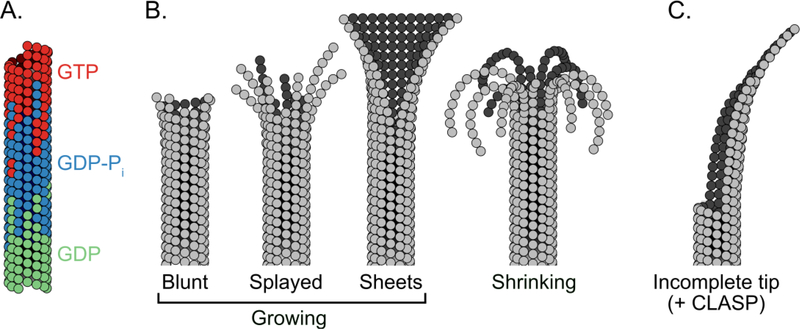
Structure and composition of the microtubule end. (A) Growing microtubule tips are composed of tubulin subunits in different nucleotide states. The very tip of the microtubule is composed of GTP-bound tubulin subunits (red). Subsequent GTP-hydrolysis results in a region proximal to the tip that is rich in GDP-Pi-bound tubulin (blue). Finally, phosphate release results in the body of the microtubule being composed mainly of GDP-bound tubulin (green). (B) Growing microtubule ends can adopt a variety of end structures due to their multi-protofilament nature. Individual microtubules can elongate independently by forming longitudinal interactions with incoming tubulin dimers, which may result in splayed protofilaments at the tip. Protofilaments can also form lateral interactions with adjacent protofilaments resulting in multi-protofilament, sheet-like structures. During shrinkage, lateral interactions between protofilaments are broken, resulting in highly splayed, disconnected and curled protofilaments at the ends of shrinking microtubules. (C) Microtubules grown in the presence of CLASP2α display curved ends, which may arise due to the ability of CLASP to stabilize incomplete end structures.
Previous structural studies have shown that microtubules grown with GMPCPP adopt a slightly extended lattice when compared with GDP microtubule lattice [12]. Specifically, for mammalian microtubules, the longitudinal inter-dimer distance of GTP-like tubulin is approximately 1.7Å larger than GDP-lattices [13]. Until recently, the structure of the lattice in the transient GDP-Pi state has remained elusive. Now, two new high-resolution cryo-EM studies have shed light on the structure of the microtubule lattice in this intermediate hydrolysis state [14,15]. In a pioneering study, Manka and Moores used doublecortin protein to stabilize nascent 13-protofilament microtubules, allowing the structure of microtubules in the GDP-Pi state to be determined for the first time [15]. The authors conclude that hydrolysis and phosphate release sequentially weaken lateral - and strengthen longitudinal - contacts in two distinct steps. A two-step mechanism has also been proposed based on structures of microtubules grown with GTPyS, a GTP-like analogue thought to mimic the GDP-Pi state [14,16]. Interestingly, while microtubules grown with mammalian tubulin undergo compaction upon GTP hydrolysis, recent studies report that microtubules assembled from either purified C. elegans [17] or yeast [18,19] tubulin retain an extended lattice conformation. Even though the structural changes occurring upon nucleotide hydrolysis may be subtle, they are likely crucial for the mechanism of dynamic instability.
The multi-protofilament nature of microtubules adds another layer of structural complexity at microtubule ends. State-of-the-art 3D cryo-electron tomographic reconstructions of microtubules in vitro and in cells now add new insights into the long-standing question regarding the configuration of growing microtubule ends. Some researchers have reported sheet-like ends, in which the protofilaments are laterally connected [20], in agreement with previous reports [21–23]. Others have reported splayed, disconnected protofilaments [24], similar to those observed at shrinking ends [21,23] (Figure 2B). Such variety in microtubule end structure arises from the fact that the protofilaments comprising the microtubule tip can adopt a range of lengths and curvatures, as well as differing degrees of inter-protofilament lateral interactions. These differences are likely accentuated not only by the assay conditions used, but also by the richness of the tubulin code, including species-specific and isotype differences and tubulin post-translational modifications [1,25,26]. Additionally, microtubule end structure has been reported to evolve over time and, thus, microtubule age is likely to contribute to the observed diversity in end structures [27]. Furthermore, in cells, microtubules are regulated by MAPs, which can also influence the microtubule tip configuration.
Several MAPs are known to recognize and modulate specific structural features at the microtubule end. For example, the EB family of microtubule tip tracking proteins bind to microtubules in a nucleotide-state dependent manner [28,29], and have been reported to induce compaction of both mammalian and yeast microtubules [14,18]. Other MAPs, including doublecortin [30] and Tpx2 [31], recognize protofilament curvature. Recently, it was found that a member of the conserved CLASP family of MAPs, human CLASP2γ, preferentially binds to the plus-tips of stabilized microtubules [4]. Furthermore, a comprehensive study found that human CLASP2α not only autonomously tracked growing microtubule tips, but also caused microtubule ends to curve [5]. These curved ends may represent partially closed tubes, as CLASP2α was also found to stabilize growing microtubule ends with lagging protofilaments [5] (Figure 2C). In general, MAPs that exhibit a binding preference for a particular configuration of tubulin can dictate microtubule tip structure: these new studies now reveal that CLASP can be added to this growing list of MAPs.
PREVENTING CATASTROPHE: KEEPING THE CAP
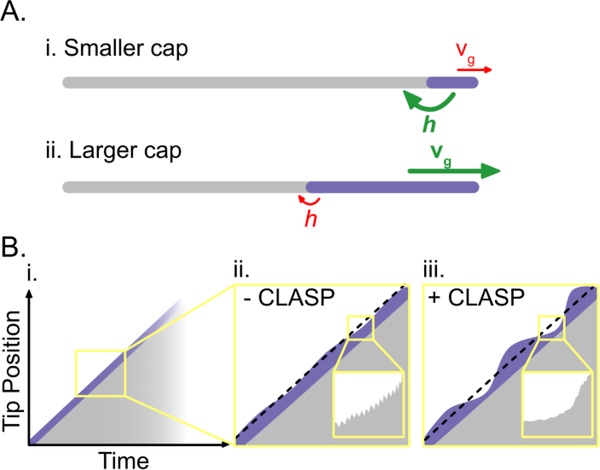
The loss of the stabilizing GTP-cap at the ends of growing microtubules triggers catastrophe, a switch-like transition from growth to rapid shrinkage. However, the extent to which the size of the cap determines microtubule lifetime is unclear. In principle, the size of the stabilizing cap may be changed by modulating the microtubule growth rate (Figure 3A). Indeed, increasing the tubulin concentration in vitro leads to a simultaneous increase in microtubule growth rate and the size of EB comets [32], which mark the stabilizing nucleotide cap [28,29,33]. Early in vitro data showed that increasing the tubulin concentration also results in a lower frequency of catastrophe [34], potentially linking the size of the cap to microtubule stability. A simultaneous increase in growth rate and suppression of catastrophe frequency has been observed in the presence of neuronal protein Tau [35,36], as well as a mammalian microtubule polymerase, Kinesin-5 [37], and the budding yeast polymerases Kip2 and Stu2 [38,39]. By increasing the microtubule growth rate, these MAPs may increase the size of the stabilizing cap, and, thereby, suppress catastrophe.
Figure 3.
Preventing catastrophe: keeping the cap. (A) The size of the stabilizing cap is dependent on the rates of microtubule growth and GTP hydrolysis: (i) slower growth rates and faster hydrolysis rates can decrease the size of the stabilizing cap and promote catastrophe; (ii) faster growth rates and slower hydrolysis rates can increase the size of the stabilizing cap and prevent catastrophe. Growth rates and hydrolysis rates are denoted by vg and h respectively. Fast rates are in green; slow rates are in red; and the stabilizing cap is in purple. (B) (i) On the minute-scale the microtubule growth rate appears linear; however, fluctuations in microtubule growth rate become evident on shorter time-scales. (ii) In the absence of CLASP, fluctuations in microtubule growth rate on the second and sub-second (inset) scales may contribute to catastrophe by eroding the stabilizing cap. (iii) In the presence of CLASP, microtubules tolerate greater fluctuations in growth on the second scale and display smoother growth on the sub-second scale (inset). The black dotted lines represent mean growth rates.
However, several proteins have been shown to decouple the microtubule growth rate and catastrophe frequency. For instance, XMAP215 polymerase increases the microtubule growth rate up to tenfold [40] but does not suppress catastrophe [41]; conversely, doublecortin and Tpx2 suppress catastrophe but do not increase the growth rate [31,42,43]. Recent in vitro studies now show that CLASPs strongly suppress catastrophe without increasing the microtubule growth rate [3–5]. The effects of CLASP were synergistically enhanced by targeting CLASP to microtubule tips with EB [4,5]. In addition to the specific effects of individual or groups of MAPs, species-specific differences in tubulin dimers themselves may encode distinct relationships between microtubule catastrophe and growth. For example, a new study found that microtubules assembled in vitro from purified C. elegans tubulin display both a fast growth rate and a high catastrophe frequency in the absence of any MAPs [17]. Here, the authors suggest that the structure of the C. elegans dimer, displaying ordered lateral contact loops, primes tubulin for fast polymerization in this species. Together, these findings indicate that growth rate and catastrophe frequency can be decoupled, either due to an intrinsic property of tubulin or regulation by MAPs.
The size of the stabilizing cap could also be changed by modulating the rate of GTP-hydrolysis (Figure 3A). Indeed, the finding that EB induces structural changes in the microtubule lattice and may promote hydrolysis [14,16], potentially explains the observation that EB promotes catastrophe in vitro [32,41]. In contrast, pVHL, a MAP important for cilia and spindle maintenance, may prevent microtubule catastrophe by suppressing GTP-hydrolysis [44]. In addition to modulating hydrolysis rate, MAPs could stabilize microtubules by preventing hydrolysis-induced structural changes in the microtubule lattice. This may explain the microtubule stabilizing effects of Tpx2 and Kinesin-1: Tpx2 was recently found to prevent lattice compaction [45], and Kinesin-1 was reported to slightly increase the GDP-lattice length [46], although this observation was not confirmed by cryo-EM [14]. Furthermore, microtubules assembled from yeast T238 mutant tubulin have been reported to undergo GTP hydrolysis but showed increased stability, potentially separating GTP hydrolysis from the structural changes that lead to catastrophe [47]. In this study, the yeast EB homologue, Bim1, strongly bound to the yeast mutant lattice, consistent with EB’s established preference for GTP-like lattice. In the case of CLASPs, both yeast and human CLASPs have now been shown to suppress catastrophe without changing EB comet size [3,4]. Combined, the findings that CLASPs do not increase the microtubule growth rate or alter EB localization at growing microtubule tips imply that their anti-catastrophe mechanism does not involve an increase in the mean cap size.
Although a larger stabilizing cap may be harder to lose, classic studies demonstrated that even a single-layer cap may be sufficient to protect microtubules against catastrophe [48]. Since microtubule lifetimes often last hundreds of seconds, the cap must be maintained over long periods of time, irrespective of its mean size. This may be a particular challenge in the face of rapid fluctuations in microtubule growth, which are a consequence of tubulin on/off kinetics occurring on the sub-second scale [49–53]. Bridging these two temporal scales, several orders of magnitude apart, ultimately holds the answer to the mechanism of microtubule catastrophe. Previous studies proposed that rapid tubulin kinetics lead to the evolution of the microtubule tip structure, resulting in its age-dependent susceptibility to catastrophe [27,54,55]. Dampening sub-second growth fluctuations may protect against catastrophe. Accordingly, the microtubule stabilizing protein Tpx2 has been reported to reduce the variability of microtubule growth [56]. How the fluctuations in microtubule growth rate affect the stabilizing GTP-cap has recently been directly investigated [53]: by correlating the sub-second fluctuations in microtubule growth rate to the fluctuations in the size of fluorescent EB caps, the authors confirmed that rapid tubulin kinetics indeed impact the instantaneous size of the EB cap. However, whereas growth rate fluctuations occur on a sub-second scale, the authors find that the EB-cap size fluctuates on a characteristically longer temporal scale on the order of several seconds, set by the rate of GTP hydrolysis. This finding explains why, even in the absence of MAPs, the microtubule growth rate may slow down for several seconds without triggering catastrophe, and provides another temporal scale for regulation of microtubule stability.
Recently, CLASPs have been shown to modulate growth rate variability on multiple temporal scales [53–56] (Figure 3B). Using mean-squared-displacement analysis of microtubule growth on the sub-second time scale, CLASP was found to promote smoother growth, potentially contributing to its anti-catastrophe activity [5]. Additionally, analysis of growth variability on longer, multi-second scales revealed that, in the presence of CLASP, microtubule ends were capable of withstanding a greater degree of growth variability that would otherwise likely lead to catastrophe [4]. Therefore, it is possible that CLASP stabilizes microtubules even when the GTP-cap is compromised. One way that CLASP could achieve this is by stabilizing individual or subsets of protofilaments, thereby preventing catastrophe of the entire microtubule. Support for such a mechanism comes from the finding that CLASP was found to protect growing microtubules against catastrophe when significant numbers of lagging protofilaments were present [5]. Importantly, CLASPs contain an array of TOG domains, known for their ability to bind soluble tubulin [57–60]. Indeed, an earlier study using fission yeast CLASP, Cls1p, reported that the tubulin-binding activity of Cls1p’s TOG domains is essential for its effects on microtubule dynamics [8]. Surprisingly, recent studies with both budding yeast and human CLASPs have narrowed down the anti-catastrophe activity to a single TOG domain [3,5,61]. The Stu1-TOG2 domain of budding yeast CLASP was found to suppress catastrophe and have moderate binding affinity for both soluble and polymeric tubulin; single point mutations on its tubulin-binding surface were sufficient to disrupt its anti-catastrophe activity [3]. Similarly, the anti-catastrophe activity of the human CLASP2-TOG2 was shown to be dependent on the conserved tubulin-binding residues; however, this domain was not found to bind soluble tubulin [5]. Together these studies indicate that a linked array of TOG domains is not necessary for CLASP’s anti-catastrophe activity. The observations that CLASP prevents catastrophe in the face of incomplete end structures and greater growth fluctuations raises the interesting possibility that CLASP promotes microtubule rejuvenation, acting as an anti-aging factor at growing microtubule ends.
PROMOTING RESCUE: REGAINING THE CAP
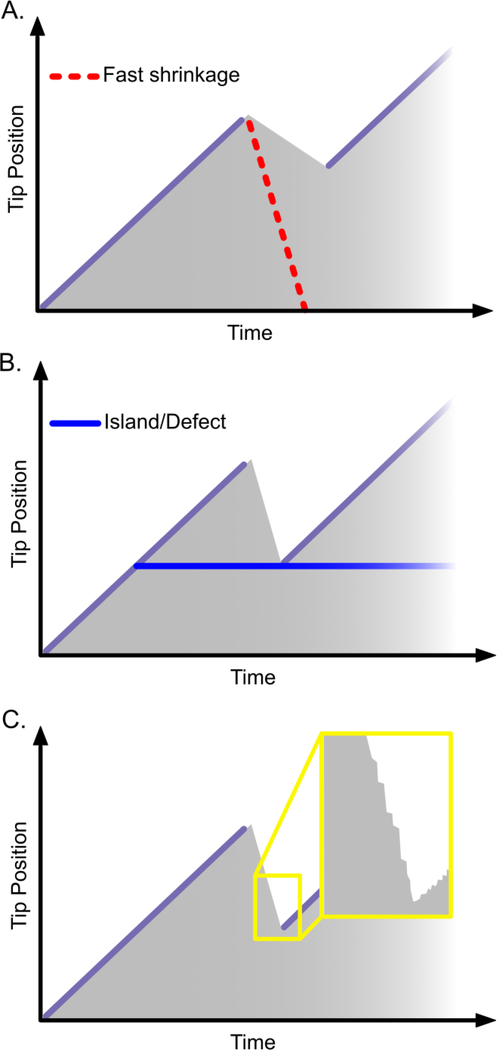
Rescue events are very common in cells, and are critical for processes in which polarized microtubule growth is required, such as cell migration. However, rescues are rarely observed in vitro, and, consequently, remain poorly understood [62,63]. Microtubule rescue occurs when a rapidly shrinking microtubule abruptly switches to growth, presumably by regaining the stabilizing cap. One model states that rescue events occur stochastically with some probability during shrinkage. Therefore, microtubules that spend longer in the shrinkage phase are more likely to undergo rescue; and proteins that slow down shrinkage act as rescue factors (Figure 4A). This has shown to be the case for Tpx2 [43,56] and Tau proteins [35,36], which simultaneously suppress the microtubule shrinkage rate and increase the rescue frequency. Microtubules assembled from yeast T238 tubulin shrink more slowly; whether they also rescue more often has not been determined [47]. Previously, fission yeast CLASP was shown to suppress shrinkage rates, possibly explaining its effect on increasing rescue frequency [8]. However, new studies now show that human CLASPs and Stu1-TOG2 can promote rescue without suppressing the mean microtubule shrinkage rate [3–5]. Furthermore, human CLASPs do not accumulate on shrinking microtubule tips [4,5]. Thus, CLASP-induced rescues do not necessarily involve reduced shrinkage rate.
Figure 4.
Rescue: regaining the cap. Three models of rescue have been proposed for the mechanism of CLASP. (A) CLASP may promote rescue by suppressing microtubule shrinkage. The red dotted line indicates a faster shrinking microtubule tip that does not rescue. (B) CLASP may promote rescue locally on the microtubule lattice: rescue is triggered when the shrinking microtubule tip encounters a defect or island on the microtubule lattice. (C) CLASP may promote rescue by modulating fluctuations during shrinkage (inset).
Another model of microtubule rescue postulates that islands of GTP-tubulin in the microtubule lattice serve as rescue sites [64] (Figure 4B). Supporting this view, rescue has been shown to occur at artificial islands containing a non-hydrolyzable GTP analogue [65]. A recent ultrastructural study found that microtubule lattices contain natural defects in which one or more protofilaments are lost, resulting in gaps, or changes in skew or protofilament number [20]. GTP-islands could then arise by ‘filling-in’ such lattice defects with new GTP-tubulin. Indeed, GTP-tubulin has been shown to incorporate into defects generated mechanically or by photodamage; these sites of self-repair consequently served as sites for microtubule rescue in vitro and in cells [66,67]. A new study using negative stain TEM and TIRF microscopy now finds that two severing enzymes, spastin and katanin, promote GTP-tubulin islands in the microtubule lattice and also increase rescue frequency [68]. By incubating microtubules with severing enzymes directly on EM grids, the authors were able to capture severing intermediates and observed microtubules with nanoscale damage in protofilament structure. Incorporation of GTP-tubulin into microtubule lattices exposed to the severing enzymes was found to both inhibit severing and also correlated with an increased rescue frequency. Together, the findings from these studies imply that tubulin itself can function as a rescue factor. However, whether the incorporated tubulin is able to undergo hydrolysis, and whether the nucleotide state of island-tubulin is critical for rescue is not known.
Lattice defects or GTP-islands could also act to target rescue factors to the microtubule lattice. Previously, it was found that clusters of MAP2 on the microtubule lattice predict sites of rescue [69], though whether these are also sites of lattice defects is not known. Local accumulation of the fission yeast CLASP has been reported to correlate with sites of microtubule rescue [8], and Drosophila CLASP was found to be enriched at sites of microtubule pausing [70]. In contrast, studies with human CLASP found that its rescue-promoting activity does not require local accumulation of CLASP on the microtubule lattice [4,5]. It was found that EB1 localized to the GTP-islands generated by severing enzymes [68], therefore, rescue factors such as CLASPs, which contain EB-recognition motifs could be targeted to defect-sites in this way. In addition to potential defect-recognition, MAPs could generate GTP-islands by suppressing the rate of GTP-hydrolysis. This mechanism is appealing because, in principle, it could explain the action of regulatory proteins that both suppress catastrophe and promote rescue, and has been proposed for pVHL protein [44]. However, in the case of CLASPs, neither human CLASP nor yeast Stu1-TOG2 domain were found to suppress the rate of GTP-hydrolysis, as measured by EB localization [3,4]. Therefore, the exact mechanism by which CLASPs promote microtubule rescue remains elusive.
It is feasible that fluctuations during microtubule shrinkage or transient slow-downs in microtubule shrinkage rate could allow the microtubule to regain the stabilizing cap. Interestingly, brief CLASP binding events have been observed on shrinking microtubule tips immediately prior to rescue [5] and could indicate that CLASPs act locally but transiently to inhibit microtubule shrinkage and promote regrowth; however, the potential modulation of shrinkage-rate fluctuations by CLASPs has not yet been investigated. The global modulation of variability in growth and shrinkage rates could provide a unifying mechanism for anti-catastrophe and rescue activities. Arguing in favour of a unified mechanism, individually mutating the conserved tubulin-binding residues of the Stu1-TOG2 domain consistently abrogated both anti-catastrophe and rescue activities [3]. Interestingly, however, the TOG3 domain of human CLASP mildly promoted rescue without affecting catastrophe [5], thus questioning the classic model that both catastrophe and rescue events transition through a common metastable intermediate state [71].
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, the new studies discussed here highlight the complexity of the microtubule tip structure and its regulation by MAPs including CLASP proteins. While these studies provide novel insights, the relationship between microtubule catastrophe and rescue remains unclear. Another interesting potential relationship is the one linking rescue and nucleation. In the island model, microtubule rescue is conceptually similar to templated microtubule nucleation, an idea that is supported by the finding that many rescue factors, including CLASPs, are also nucleation factors [5,31,43,72]. However, given that microtubules shrink rapidly [34], and that the shrinking microtubule end has highly splayed protofilaments [21], it seems likely that rescuing a shrinking microtubule end would be less favorable than nucleating from a template. Indeed, rescue at extensively-studied microtubule plus-ends is relatively insensitive to tubulin concentration, whereas nucleation is highly sensitive [34]. Perplexingly, and in contrast with plus-ends, rescue occurs frequently at microtubule minus-ends and is sensitive to tubulin concentration, but minus-ends nucleate less readily than plus-ends [34]. The mechanisms underlying microtubule minus-end dynamics remain generally unknown, as they grow substantially slower than plus-ends, but exhibit similar catastrophe frequencies [34]. Interestingly, in spite of the unique dynamic properties of minus-ends, recent studies reported the structures of minus-ends to be quite similar to plus-ends [20,73], indicating that microtubule dynamics are likely defined by a myriad of features at the microtubule end. Although microtubule end complexity can, to some extent, be attributed to the large number of individual protofilaments, it is fascinating that polymers assembled from tubulin-like bacterial proteins, BtubA and BtubB, contain only four protofilaments but display the hallmarks of microtubule dynamic instability [74]. Consolidating the intricate processes occurring on nanometer, sub-second scales with the global transitions on micrometer, minute scales will be essential for a conclusive model of microtubule dynamics. Moreover, whether a mechanism exists that unifies catastrophe, rescue and nucleation, and could be utilized by proteins that regulate all three processes, such as CLASPs, is an enticing concept which remains to be explored.
ACKNOWLEDGEMENTS
We thank J. Alper, G. Brouhard, S. Bechstedt and the members of the Zanic laboratory for discussions and critical feedback on an earlier version of the manuscript. This work was supported by National Institutes of Health grant R35GM119552 to M. Zanic. M. Zanic acknowledges support from the Human Frontier Science Program and the Searle Scholars Program. EJL acknowledges the support of the NIH IBSTO training grant T32CA119925.
Abbreviations:
- MAP
Microtubule-associated protein
- Cryo-EM
Cryo-electron microscopy
- GMPCPP
guanosine-5′-[(α,β)-methyleno]triphosphate
- TIRF
Total Internal Reflection Fluorescence
Footnotes
PAPERS OF OUTSTANDING INTEREST
Chaaban et al., 2018*
Microtubules grown from purified C. elegans tubulin display a high growth rate and catastrophe frequency. Using cryo-EM, the authors find that C. elegans microtubules have ordered lateral contact loops which may ‘prime’ C. elegans tubulin dimers for polymerization. Despite faster growth, EB comets at the tips of C. elegans microtubules are smaller than the corresponding bovine microtubules suggesting a faster maturation time of the stabilizing cap of C. elegans microtubules.
Vemu et al., 2018*
This study combined TIRF microscopy and negative stain TEM to show that two microtubule severing enzymes, katanin and spastin, remove tubulin dimers from microtubule lattices, creating sites of damage that are repaired by the incorporation of new GTP-tubulin. Lattice repair was found to inhibit severing events and also correlated with increased rescue events.
Deng et al., 2017*
This study reports a high-resolution structure of mini-microtubules assembled from bacterial tubulin-like proteins BtubAB, purified from Prosthecobacter. These microtubules share similar structural elements with eukaryotic microtubules, and display dynamic instability, but are composed of only four protofilaments.
Manka et al., 2018**
This study used high resolution cryo-EM to determine the structure of nascent 13-protofilament microtubules, for the first time reporting the structure of the GDP-Pi microtubule lattice, a transient intermediate state between GTP and GDP microtubule lattices. The authors find that microtubules transition through two structural states, sequentially weakening the lateral and strengthening the longitudinal contacts, adding a novel insight into the mechanism of microtubule dynamic instability.
Lawrence et al., 2018*
This study demonstrated that CLASP specifically promotes rescue and suppresses catastrophe without changing the rates of growth and shrinkage, indicating that these parameters of microtubule dynamics are uncoupled in the presence of CLASP. Furthermore, CLASP activity was found to be synergistically enhanced when targeted to microtubule tips with EB1.
Majumdar et al., 2018*
A single TOG domain of CLASP is sufficient to suppress microtubule catastrophe and promote rescue, indicating that the mechanism of CLASP activity does not depend on an array of linked TOGs. Abrogating the conserved tubulin-binding residues inhibited the effects of this TOG domain on microtubule dynamics.
Aher et al., 2018**
In this extensive characterization, the authors found that a single TOG domain of human CLASP tracks the tips of growing microtubules and is sufficient to suppress catastrophes and induce rescue. By inducing catastrophe in multiple ways, the authors show that CLASP employs a general anti-catastrophe mechanism that is not context-specific. Interestingly, this paper also demonstrated that CLASP stabilizes microtubules with damaged tips.
Zhang et al., 2018*
This study uses cryo-EM to provide new insights into the structure of microtubule lattices grown with different nucleotide analogues in the presence and absence of EB protein. This work revealed that microtubules grown with the GTP-analogue, GTPγS, have compacted lattices, and that EB promotes lattice compaction of microtubules grown with all nucleotide analogues.
McIntosh et al., 2018*
This structural analysis using cryo-ET observed splayed and curved protofilaments at growing microtubule ends, both in vitro and in cells, suggesting that microtubules grow by adding GTP-tubulin subunits to the tips of individual protofilaments.
Rickman et al., 2017*
This study investigates the fluctuations in the size of the EB cap during persistent microtubule growth phase. The authors find EB cap size to be directly affected by stochastic fluctuations in growth rate, and that EB cap size fluctuations display a characteristic temporal scale, set by the rate of the GTP-hydrolysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chaaban S, Brouhard GJ: A microtubule bestiary: structural diversity in tubulin polymers. Molecular Biology of the Cell 2017, 28:2924–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchison T, Kirschner M: Dynamic instability of microtubule growth. Nature 1984, 312:237–242. [DOI] [PubMed] [Google Scholar]
- 3.Majumdar S, Kim T, Chen Z, Munyoki S, Tso SC, Brautigam CA, Rice LM: An isolated CLASP TOG domain suppresses microtubule catastrophe and promotes rescue. Mol Biol Cell 2018, 29:1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence EJ, Arpag G, Norris SR, Zanic M: Human CLASP2 specifically regulates microtubule catastrophe and rescue. Molecular Biology of the Cell 2018, 29:1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aher A, Kok M, Sharma A, Rai A, Olieric N, Rodriguez-Garcia R, Katrukha EA, Weinert T, Olieric V,Kapitein LC, et al. : CLASP Suppresses Microtubule Catastrophes through a Single TOG Domain. Dev Cell 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W,Burgering BM, De Zeeuw CI, Grosveld F, et al. : CLASPs are CLIP-115 and −170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 2001, 104:923–935. [DOI] [PubMed] [Google Scholar]
- 7.Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F,Vorobjev I, Tsukita S, et al. : CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. Journal of Cell Biology 2005, 168:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Bassam J, Kim H, Brouhard G, van Oijen A, Harrison SC, Chang F: CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Developmental Cell 2010, 19:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlier MF, Pantaloni D: Kinetic Analysis of Guanosine 5’-Triphosphate Hydrolysis Associated with Tubulin Polymerization. Biochemistry 1981, 20:1918–1924. [DOI] [PubMed] [Google Scholar]
- 10.Carlier MF, Hill TL, Chen Y: Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proceedings of the National Academy of Sciences of the United States of America 1984, 81:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ: Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Molecular biology of the cell 1992, 3:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman AA, Chretien D, Arnal I, Wade RH: Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J Cell Biol 1995, 128:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D and Nogales E, High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis, Cell 157, 2014, 1117–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, LaFrance B, Nogales E: Separating the effects of nucleotide and EB binding on microtubule structure. Proc Natl Acad Sci U S A 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manka SW, Moores CA: The role of tubulin–tubulin lattice contacts in the mechanism of microtubule dynamic instability. Nature Structural & Molecular Biology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Alushin Gregory MM, Brown A, Nogales E: Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins. Cell 2015, 162:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaaban S, Jariwala S, Hsu CT, Redemann S, Kollman JM, Muller-Reichert T, Sept D, Bui KH, Brouhard GJ: The Structure and Dynamics of C. elegans Tubulin Reveals the Mechanistic Basis of Microtubule Growth. Dev Cell 2018. [DOI] [PubMed] [Google Scholar]
- 18.Howes SC, Geyer EA, LaFrance B, Zhang R, Kellogg EH, Westermann S, Rice LM, Nogales E: Structural differences between yeast and mammalian microtubules revealed by cryo-EM. J Cell Biol 2017, 216:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Loeffelholz O, Venables NA, Drummond DR, Katsuki M, Cross R, Moores CA: Nucleotide- and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics. Nat Commun 2017, 8:2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atherton J, Stouffer M, Francis F, Moores CA: Microtubule architecture in vitro and in cells revealed by cryo-electron tomography. Acta Crystallographica Section D Structural Biology 2018, 74:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelkow EM, Mandelkow E, Milligan RA: Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. Journal of Cell Biology 1991, 114:977–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guesdon A, Bazile F, Buey RM, Mohan R, Monier S, García RR, Angevin M, Heichette C, Wieneke R,Tampé R, et al. : EB1 interacts with outwardly curved and straight regions of the microtubule lattice. Nature Cell Biology 2016, 18:1102–1108. [DOI] [PubMed] [Google Scholar]
- 23.Chrétien D, Fuller SD, Karsenti E: Structure of growing microtubule ends: Two-dimensional sheets close into tubes at variable rates. Journal of Cell Biology 1995, 129:1311–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntosh JR, Toole EO, Morgan G, Austin J, Ulyanov E, Ataullakhanov F, Gudimchuk N: Microtubules grow by the addition of bent guanosine triphosphate tubulin to the tips of curved protofilaments. Journal of Cell Biology 2018:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadadhar S, Bodakuntla S, Natarajan K, Janke C: The tubulin code at a glance. Journal of Cell Science 2017, 130:1347. [DOI] [PubMed] [Google Scholar]
- 26.Verhey KJ, Gaertig J: The tubulin code. Cell Cycle 2007, 6:2152–2160. [DOI] [PubMed] [Google Scholar]
- 27.Coombes CE, Yamamoto A, Kenzie MR, Odde DJ, Gardner MK: Evolving tip structures can explain age-dependent microtubule catastrophe. Current Biology 2013, 23:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanic M, Stear JH, Hyman AA, Howard J: EB1 recognizes the nucleotide state of tubulin in the microtubule lattice. PLoS ONE 2009, 4:e7585–e7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer SP, Fourniol FJ, Bohner G, Moores CA, Surrey T: EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell 2012, 149:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bechstedt S, Lu K, Brouhard GJ: Doublecortin recognizes the longitudinal curvature of the microtubule end and lattice. Current Biology 2014, 24:2366–2375. [DOI] [PubMed] [Google Scholar]
- 31.Roostalu J, Cade NI, Surrey T: Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nature Cell Biology 2015, 17:1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bieling P, Laan L, Schek HHT, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T, Dogterom M, Brunner D, et al. : Reconstitution of a microtubule plus-end tracking system in vitro. Nature 2007, 450:1100–1105. [DOI] [PubMed] [Google Scholar]
- 33.Duellberg C, Cade NI, Holmes D, Surrey T: The size of the EB cap determines instantaneous microtubule stability. Elife 2016, 5:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker RA, Brien O, Pryer K, Soboeiro ME, Voter WA, Erickson HP, Salmon ED: Dynamic Instability of Individual Microtubules. The Journal of cell biology 1988, 107:1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW: Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Molecular Biology of the Cell 1992, 3:1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez-Rios S, Denarier E, Prezel E, Vinit A, Stoppin-Mellet V, Devred F, Barbier P, Peyrot V, Sayas CL, Avila J, et al. : Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism. Molecular Biology of the Cell 2016, 27:2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Hancock WO: Kinesin-5 is a microtubule polymerase. Nature Communications 2015, 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podolski M, Mahamdeh M, Howard J: Stu2, the budding yeast XMAP215/Dis1 homolog, promotes assembly of yeast microtubules by increasing growth rate and decreasing catastrophe frequency. Journal of Biological Chemistry 2014, 289:28087–28093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbel A, Bogdanova A, Mahamdeh M, Jannasch A, Storch M, Schaffer E, Liakopoulos D, Howard J: Kinesin Kip2 enhances microtubule growth in vitro through length-dependent feedback on polymerization and catastrophe. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gard DL, Kirschner MW: A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol 1987, 105:2203–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanic M, Widlund PO, Hyman AA, Howard J: Synergy between XMAP215 and EB1 increases microtubule growth rates to physiological levels. Nature Cell Biology 2013, 15:688–693. [DOI] [PubMed] [Google Scholar]
- 42.Moores CA, Perderiset M, Kappeler C, Kain S, Drummond D, Perkins SJ, Chelly J, Cross R, Houdusse A, Francis F: Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO Journal 2006, 25:4448–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wieczorek M, Bechstedt S, Chaaban S, Brouhard GJ: Microtubule-associated proteins control the kinetics of microtubule nucleation. Nature Cell Biology 2015, 17:907–916. [DOI] [PubMed] [Google Scholar]
- 44.Thoma CR, Matov A, Gutbrodt KL, Hoerner CR, Smole Z, Krek W, Danuser G: Quantitative image analysis identifies pVHL as a key regulator of microtubule dynamic instability. The Journal of Cell Biology 2010, 190:991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Roostalu J, Surrey T, Nogales E: Structural insight into TPX2-stimulated microtubule assembly. eLife 2017, 6:e30959–e30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peet DR, Burroughs NJ, Cross RA: Kinesin expands and stabilizes the GDP-microtubule lattice. Nature Nanotechnology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geyer EA, Burns A, Lalonde BA, Ye X, Piedra FA, Huffaker TC, Rice LM: A mutation uncouples the tubulin conformational and GTPase cycles, revealing allosteric control of microtubule dynamics. eLife 2015, 4:e10113–e10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drechsel DN, Kirschner MW: The minimum GTP cap required to stabilize microtubules. Current Biology 1994, 4:1053–1061. [DOI] [PubMed] [Google Scholar]
- 49.Kerssemakers JWJ, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M: Assembly dynamics of microtubules at molecular resolution. Nature 2006, 442:709–712. [DOI] [PubMed] [Google Scholar]
- 50.Howard J, Hyman AA: Growth, fluctuation and switching at microtubule plus ends. Nature reviews. Molecular cell biology 2009, 10:569–574. [DOI] [PubMed] [Google Scholar]
- 51.Schek HT 3rd, Gardner MK, Cheng J, Odde DJ, Hunt AJ: Microtubule assembly dynamics at the nanoscale. Current biology : CB 2007, 17:1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gardner MK, Charlebois BD, Janosi IM, Howard J, Hunt AJ, Odde DJ, Jánosi IM, Howard J, Hunt AJ, Odde DJ: Rapid microtubule self-assembly kinetics. Cell 2011, 146:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rickman J, Duellberg C, Cade NI, Griffin LD, Surrey T: Steady-state EB cap size fluctuations are determined by stochastic microtubule growth and maturation. Proceedings of the National Academy of Sciences 2017, 114:3427–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zakharov P, Gudimchuk N, Voevodin V, Tikhonravov A, Ataullakhanov FI, Grishchuk EL: Molecular and Mechanical Causes of Microtubule Catastrophe and Aging. Biophysical Journal 2015, 109:2574–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner MK, Zanic M, Gell C, Bormuth V, Howard J: Depolymerizing kinesins Kip3 and MCAK shape cellular microtubule architecture by differential control of catastrophe. Cell 2011, 147:1092–1103. [DOI] [PubMed] [Google Scholar]
- 56.Reid TA, Schuster BM, Mann BJ, Balchand SK, Plooster M, McClellan M, Coombes CE, Wadsworth P, Gardner MK: Suppression of microtubule assembly kinetics by the mitotic protein TPX2. Journal of Cell Science 2016, 129:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayaz P, Ye X, Huddleston P, Brautigam CA, Rice LM: A TOG:alphabeta-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 2012, 337:857–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spittle C, Charrasse S, Larroque C, Cassimeris L: The interaction of TOGp with microtubules and tubulin. J Biol Chem 2000, 275:20748–20753. [DOI] [PubMed] [Google Scholar]
- 59.Al-Bassam J, Larsen NA, Hyman AA, Harrison SC: Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 2007, 15:355–362. [DOI] [PubMed] [Google Scholar]
- 60.Al-Bassam J, van Breugel M, Harrison SC, Hyman A: Stu2p binds tubulin and undergoes an open-to-closed conformational change. J Cell Biol 2006, 172:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Funk C, Schmeiser V, Ortiz J, Lechner J: A TOGL domain specifically targets yeast CLASP to kinetochores to stabilize kinetochore microtubules. Journal of Cell Biology 2014, 205:555–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardner MK, Zanic M, Howard J: Microtubule Catastrophe and Rescue. Current Opinion in Cell Biology 2013, 25:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brouhard GJ: Dynamic instability 30 years later: complexities in microtubule growth and catastrophe. Molecular Biology of the Cell 2015, 26:1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimitrov A, Quesnoit M, Moutel S, Cantaloube I, Poüs C, Perez F: Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science 2008, 322:1353–1356. [DOI] [PubMed] [Google Scholar]
- 65.Tropini C, Roth EA, Zanic M, Gardner MK, Howard J: Islands containing slowly hydrolyzable GTP analogs promote microtubule rescues. PLoS ONE 2012, 7:e30103–e30103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aumeier C, Schaedel L, Gaillard JJJ, John K, Blanchoin L, Théry M, Thery M, Théry M: Self-repair promotes microtubule rescue. Nature Cell Biology 2016, 18:1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaedel L, John K, Gaillard J, Nachury MV, Blanchoin L, Théry M: Microtubules self-repair in response to mechanical stress. Nature Materials 2015, 14:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vemu A, Szczesna E, Zehr EA, Spector JO, Grigorieff N, Deaconescu AM, Roll-Mecak A: Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 2018, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itoh TJ, Hotani H: Microtubule-Stabilizing Activity of Microtubule-Associated Proteins (MAPs) Is Due to Increase in Frequency of Rescue in Dynamic Instability: Shortening Length Decreases with Binding of MAPs onto Microtubules. Cell Structure and Function 1994, 19:279–290. [DOI] [PubMed] [Google Scholar]
- 70.Moriwaki T, Goshima G: Five factors can reconstitute all three phases of microtubule polymerization dynamics. Journal of Cell Biology 2016, 215:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tran PT, Walker RA, Salmon ED: A metastable intermediate state of microtubule dynamic instability that differs significantly between plus and minus ends. Journal of Cell Biology 1997, 138:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia ARR, McLeod IX, et al. : Asymmetric CLASP-Dependent Nucleation of Noncentrosomal Microtubules at the trans-Golgi Network. Developmental Cell 2007, 12:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atherton J, Jiang K, Stangier MM, Luo Y, Hua S, Houben K, Van Hooff JJE, Joseph AP, Scarabelli G, Grant BJ, et al. : A structural model for microtubule minus-end recognition and protection by CAMSAP proteins. Nature Structural and Molecular Biology 2017, 24:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng X, Fink G, Bharat TAM, He S, Kureisaite-Ciziene D, Lowe J: Four-stranded mini microtubules formed by Prosthecobacter BtubAB show dynamic instability. Proc Natl Acad Sci U S A 2017, 114:E5950–E5958. [DOI] [PMC free article] [PubMed] [Google Scholar]