Abstract
Graves’ disease is characterized by two sonographic features, hypoechogenicity and increased blood flow. The aim of this study was to review retrospectively ultrasound features and biochemical data of a cohort of untreated Graves’ disease patients. We reviewed charts of 42 such patients, who were referred to our Endocrinology Unit from January 2013 to May 2018. One operator performed all the thyroid sonographic scans. Serum TSH, FT3, FT4 and TSH-receptor antibodies (TRAb) levels at the time of ultrasound examination were evaluated. Over a mean follow-up of 30.9 months, about one in three patients (38%) experienced at least one recurrence of hyperthyroidism (1.4 ± 0.6 recurrence per patient), either on or off antithyroid drugs. We found that thyroid vascularization correlated directly with thyroid volume and that larger thyroids tended to be more vascularized. We also found that greater vascularization was associated with marked hypoechogenicity, and greater FT4 and TRAb levels. Patients who experienced recurrence(s) had 1.7-fold higher levels of TRAb at onset. In conclusion, thyroid hypervascularization at onset of Graves’ disease is an important sonographic feature.
Abbreviations: FT3, triiodothyronine; FT4, thyroxine; TRAb, thyrotropin-receptor antibodies; TSH, thyrotropin
Keywords: Graves disease, Hyperthyroidism, Ultrasonography, Vascularization, Doppler, Ultrasonographic pattern, Hypoechoic, Echopattern
Introduction
Graves’ disease is a common autoimmune thyroid disease and the most frequent cause of thyrotoxicosis, in that it has a prevalence of 5–10 cases per 1000 inhabitants in the United States [1]. Like other autoimmune diseases, it develops in genetically predisposed individuals [2] upon exposure to environmental factors, such as stress [2], [3]. TSH-receptor antibodies (TRAb) are found in almost all patients with untreated Graves’ disease, and they are responsible for thyroid hyperstimulation and ensuing both goiter and hyperthyroidism [1].
Although thyroid ultrasound examination is not obligatory for the diagnosis of Graves’ disease in case of TRAb-positive hyperthyroidism, it may help differentiate Graves’ disease from other causes of thyrotoxicosis when TRAb assay is not available or in the few TRAb-negative cases [4], [5]. Ultrasonography may also help in predicting relapse of hyperthyroidism in Graves’ disease patients off antithyroid drugs [6], [7], [8]. In these patients, hypoechogenicity [7], [8] and increased thyroid blood flow [6], both detected prior to discontinuing the medical treatment, predict a recurrence shortly after. These two sonographic features can be found also in untreated Graves’ disease patients [5], [8]. Increased thyroid blood flow consists of increased thyroid artery diameter (3–4 mm compared to the normal 1–2 mm), increased flow rate (>100 cm/sec), and reduced peripheral resistance [5], [10]. Clinically, the hypervascularized thyroid can be accompanied by two clinical signs, namely thyroid bruit and thrill [11].
Correlation between thyroid sonographic pattern and thyroid function tests is controversial [12], [13].
The aim of this study was to review retrospectively ultrasound features and biochemical data of a cohort of untreated Graves’ disease patients at onset, and to correlate such parameters with recurrences of hyperthyroidism.
Materials and methods
Patients
From the total 56 patients referred to our Endocrinology Unit from January 2013 to May 2018, and who were experiencing Graves’s disease associated thyrotoxicosis, we selected those who: (i) had not suffered prior episodes of thyrotoxicosis; (ii) had not started antithyroid drugs prior to the ultrasound examination; (iii) had their ultrasound examination performed by the same operator (R.V.) by means of the same ultrasound system (GE Loqiq 3, General Electric, Milwaukee, USA), with a linear-array transducer (7–15 MHz). The diagnosis of Graves’ disease was based on goiter, clinical signs of thyrotoxicosis (such as tremors, high pulse rate, diaphoresis, diarrhea), ophthalmopathy (when present) and typical thyroid function tests (suppressed TSH levels, elevated FT3 and FT4 levels, and positivity of TRAb).
We excluded patients (i) with thyrotoxicosis from causes other than Graves’ disease; (ii) with Graves’ disease not at onset and who had been already treated with antithyroid drugs, radioiodine or thyroidectomy.
Forty-two patients with Graves’ disease at onset and who had not started treatment with antithyroid drugs prior to our visit were finally selected for this retrospective study. Thyroid ultrasound report included evaluation of thyroid lobes diameters (anterior-posterior, transverse and longitudinal diameters), thyroid volume (calculated by the ellipsoid volume formula, namely the three diameters expressed in centimeters multiplied by π/6), isthmus thickness, echostructure, homogeneity, vascularization, and nodularity (yes/no; for the purpose of this study we limited our analysis only to the presence/absence of nodules, disregarding their ultrasonographic features). In particular, echostructure was subjectively scored by the operator (R.V.) from 0 to 3 (0 = normal, i.e. higher echogenicity compared to the neck strap muscles, and similar echogenicity compared to salivary glands, 1 = modestly hypoechoic, 2 = moderately hypoechoic, 3 = markedly hypoechoic, i.e. similar to the neck strap muscles), as well as homogeneity (0 = homogeneous, 1 = finely/diffusely inhomogeneous, 2 = grossly inhomogeneous without pseudonodules, 3 = grossly inhomogeneous with pseudonodules, i.e. discrete nodular areas with different echogenicity), and vascularization (0 = absent/minimal spots with subcapsular vessels, 1 = modest, patchy hypervascularization, 2 = moderate hypervascularization, 3 = intense hypervascularization, i.e. “thyroid inferno”). Furthermore, thyroid blood flow was evaluated by color Doppler mode.
We also reviewed patients’ charts, taking note of their serum TSH, FT3, FT4 and TRAb levels at onset of hyperthyroidism. Assay of these analytes preceded the time of ultrasound examination by one to eight weeks. Serum FT3, FT4 and TRAb were expressed as fold increase over the upper normal limit. This is because patients come from different parts of our province, north-eastern Sicily and southern Calabria, so that analytes were measured in different laboratories with different kits. No repetition of these assays is required if results are consistent with the diagnosis of Graves’ disease.
Statistical analysis
Continuous variables are expressed as mean ± SD, whereas categorical variables are expressed as proportions. Differences between continuous variables were managed with the Mann-Withney test, while difference between proportions with the Fisher’s exact test or the ??2-test, as appropriate. Bivariate analysis was used to assess correlation between two variables. Finally, linear multiple regression, binary logistic regression or ordinal regression were used to assess influence between variables for continuous, dichotomous or polytomous dependent variables, respectively.
Results
Of the 42 selected patients, 37 were women (female to male ratio = 7.4:1) (Table 1). On the average, patients presented with goiter and a moderately hypoechoic, pseudonodular and moderately hypervascularized parenchyma. As expected [14], FT3 levels were relatively higher compared to FT4 levels (Table 1).
Table 1.
Demographics, thyroid ultrasonographic and biochemical data of the 42 selected patients with Graves’ disease.
| Index | N (%) or m ± SD | |
|---|---|---|
| F:M (ratio) | 37:5 or 7.4:1 | |
| Age (years) | 42.2 ± 15.9 | |
| Thyroid volume (ml) | 23.9 ± 13.9 | |
| Isthmus thickness (mm) | 7.4 ± 7.0 | |
| Echogenicity score (zero to 3) | 0 = normal | 0 |
| 1 = modestly hypoechoic | 8 (19.0%) | |
| 2 = moderately hypoechoic | 22 (52.4%) | |
| 3 = markedly hypoechoic | 12 (28.6%) | |
| Omogeneity score (zero to 3) | 0 = homogeneous | 0 |
| 1 = finely/diffusely inhomogeneous | 5 (11.9%) | |
| 2 = grossly inhomogenenous without pseudonodules | 5 (11.9%) | |
| 3 = grossly inhomogeneous with pseudonodules | 32 (76.2%) | |
| Vascularization score (zero to 3) | 0 = normal | 0 |
| 1 = modest hypervascularization | 15 (35.7%) | |
| 2 = moderate hypervascularization | 17 (40.5%) | |
| 3 = intense hypervascularization (“thyroid inferno”) | 10 (23.8%) | |
| Presence of thyroid nodules [no. of nodules; no. of patients (%)] | 23; 17 (40.5%) | |
| Serum TSH (mU/L) | 0.01 ± 0.02 | |
| Serum FT3, fold increase over the upper normal limit | 3.2 ± 1.3 | |
| Serum FT4, fold increase over the upper normal limit | 2.2 ± 1.0 | |
| Serum TRAb, fold increase over the upper normal limit | 9.4 ± 7.0 | |
Compared to females, males had larger thyroid (37.1 ± 15.7 ml vs. 22.1 ± 12.9 ml, P = 0.01) and less frequently grossly echotexture (3/5 [60%] vs. 34/37 [91.9%], P = 0.04). Indeed, a thyroid volume ≥20 ml was observed in all 5 males but in 17/37 females (45.9%, P = 0.049). Larger thyroids (≥20 ml) were trendwisely more vascularized than smaller thyroids (score ≥2, 77.3% vs. 50%, P = 0.06).
Younger patients (≤40 years) had a more vascularized thyroid, as the proportion of patients with a moderately or intensely vascularized thyroid was greater compared to those with a modestly vascularized thyroid (17/21 [80.9%] vs. 10/21 [47.6%], P = 0.05). Alternatively, patients with moderately or intensely vascularized thyroid were 13 years younger compared to those with a modestly vascularized gland (37.7 ± 12.5 vs. 50.4 ± 18.7, P = 0.048).
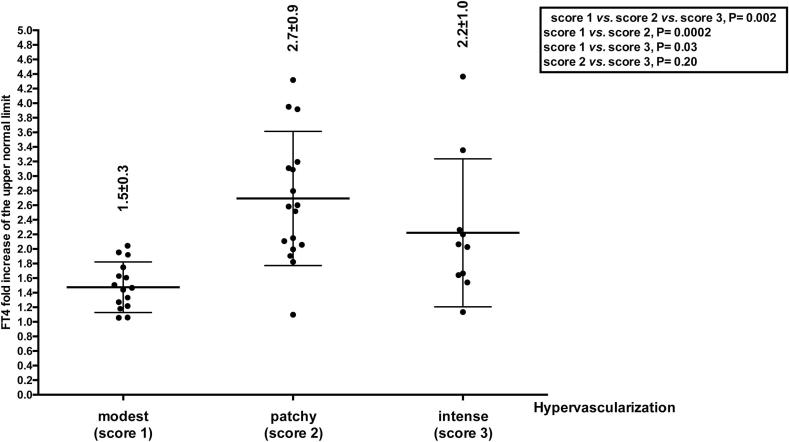
Patients with moderately or markedly hypoechoic thyroid echotexture showed greater vascularity compared to those with only modest hypoechogenicity (16/22 [72.2%] vs. 9/12 [75%] vs. 2/8 [25%], P = 0.036; 75% vs. 25%, P = 0.06; 72.2% vs. 25%, P = 0.03). Furthermore, patients with greater vascularization of their thyroid had greater FT4 levels (Fig. 1). Even if this difference was noted also for FT3 levels, it was not statistically significant (score 1 = 2.9 ± 1.5, score 2 = 3.5 ± 1.5, score 3 = 3.0 ± 1.0).
Fig. 1.
FT4 fold increase over the upper normal limit in the 42 Graves’ disease patients stratified based on the degree of thyroid vascularization. No patient had normal vascularization (score = 0).
Marked hypoechogenicity was found in patients who tended to have higher TRAb levels compared to those with modest to moderate hypoechogenicity (score 3 = 18.4 ± 11.4 vs. score 1 or 2 = 11.7 ± 9.5 IU/L, P = 0.07). Thyroid parenchyma with pseudonodular echostructure was more likely to harbor nodules, as compared to non-pseudonodular parenchyma, even though this difference was not significant (15/32 [46.9%] vs. 2/10 [20%], P = 0.32).
Patients with FT3 or FT4 increased by at least 3-fold over the upper normal limit were 10 years younger compared to the remaining patients (36.7 ± 15.6 vs. 46.1 ± 14.2 years, P = 0.06 or 32.7 ± 13.6 vs. 44.5 ± 15.0 years, P = 0.06). Also, those with FT4 elevated by ≥3 times had a more vascularized thyroid compared to the remaining patients (score ≥2, 100% vs. 61.5%, P = 0.07). Concerning TRAb levels, patients with levels increased by at least 5-fold over the upper normal limit showed more frequently intense vascularization of thyroid compared with none of those with TRAb levels below this cut-off (score 3, 40% vs. 0%, P = 0.03).
During the follow-up, which ranged from 6 to 69 months (30.9 ± 21.1), about one in three patients (16/42, 38%) experienced at least one exacerbation (i.e., recurrence of hyperthyroidism while on antithyroid drugs) or relapse (i.e., recurrence of hyperthyroidism after withdrawal of antithyroid drugs) (1.4 ± 0.6 recurrence per patient, range 1–3). Such patients did not differ at onset from those who did not either relapse or exacerbate subsequently for none of the clinical, sonographic and biochemical parameters, except for TRAb levels, which were 1.7-fold higher in the former patients (12.6 ± 7.0 vs. 7.5 ± 6.5, P = 0.02).
Correlation analysis
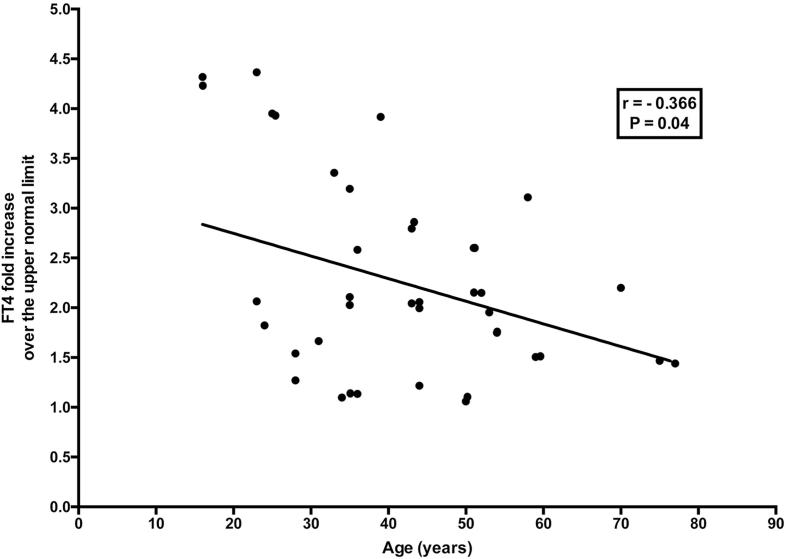
At bivariate analysis, FT4 fold increase over the upper normal limit correlated negatively with age at onset of hyperthyroidism (r = −0.366, P = 0.04) (Fig. 2) and, even if not significantly, positively with the homogeneity score (r = 0.317, P = 0.07). In turn, age at onset correlated negatively with the vascularization score (r = −0.350, P = 0.03).
Fig. 2.
Correlation between age at onset and FT4 fold increase over the upper normal limit.
The number of recurrences of hyperthyroidism correlated positively with thyroid volume and TRAb levels (r = 0.388, P = 0.02 and r = 0.399, P = 0.03). Moreover, the younger the patients, the shorter the interval between onset and the first recurrence of hyperthyroidism (r = 0.618, P = 0.03).
At stepwise linear regression analysis, the negative correlation between age at onset and vascularization score was confirmed (β = −0.412, P = 0.01). Age at onset correlated positively with nodularity (β = 0.372, P = 0.02), as follow-up duration did (β = 0.402, P = 0.02). In the logistic ordinal model, vascularization was influenced positively by thyroid volume (Wald = 3.776; P = 0.05) and the homogeneity score (Wald = 6.638; P = 0.01).
Discussion
Autoimmune thyroid diseases are chronic inflammatory conditions that share similar ultrasonographic features, hypoechogenicity being the hallmark of thyroid autoimmunity. Microscopically, hypoechogenicity results from lymphocytic infiltration and disruption of tissue architecture. Whereas in Hashimoto’s thyroiditis reduced echogenicity is the consequence of lymphocytic infiltration together with different degree of fibrosis, in Graves’ disease it stems from lymphocytic infiltration, hypercellularity, scanty colloid and hypervascularization [9]. Hypoechogenicity is also a valuable sign for detecting autoimmune thyroid disease in apparently healthy subjects [9], and for predicting thyroid failure in thyroid antibodies positive patients [15]. Finally, it can predict relapse of hyperthyroidism in patients with Graves’ disease after antithyroid drug withdrawal [7], [8].
The Doppler measurement of blood flow is based on the Doppler effect, namely the change in wave frequency under reflection by a moving structure. This change depends on the blood flow velocity, the probe emission frequency and the Doppler angle, that is the angle between the ultrasound beam and the blood flow [5].
Thyroid color Doppler can help distinguish untreated Graves’ disease from Hashimoto’s thyroiditis, as blood flow is increased much more in the first condition [16]. Indeed, the combination of thyroid hypoechogenicity and color Doppler r evaluation identified all but one patient with Graves’ disease [16]. In 1988, Ralls et al demonstrated for the first time the increased thyroid vascularization of 15 patients with Graves’ disease, and named it the “thyroid inferno”, this pattern consisting of “multiple small areas of intrathyroidal flow seen diffusely throughout the gland in both systole and diastole” [17]. Eight years later, Castagnone et al confirmed the usefulness of color Doppler sonography in Graves’ disease patients with active hyperthyroidism, who had enlarged thyroid and greater intrathyroid vascularization compared to controls and patients off antithyroid drugs [6]. Interestingly, different color Doppler appearance results in different histological features in terms of aspect of vessels, fibrosis and cellular hyperplasia [18].
Thyroid color Doppler has been successfully used to differentiate the autoimmune type 1 amiodarone-induced thyrotoxicosis from the nonautoimmune type 2 amiodarone-induced thyrotoxicosis [19], and Graves’ disease from destructive thyroiditis [20], [21], painless thyroiditis [12] and thyrotoxicosis factitia [22]. In subacute thyroiditis and in the early phases of Hashimoto’s thyroiditis, any degree of vascularization is possible, rendering the differential diagnosis a challenging one [13], [23], [24], [25]. Furthermore, Cappelli et al proved the high sensitivity and specificity (both at 95%) of color flow Doppler ultrasonography, which are very close to those of the more expensive and time-consuming thyroid scintigraphy [26].
Thyroid hypervascularization results from hemodynamic changes during hyperthyroidism, and include increased heart rate and contractility, increased output, and decreased peripheral resistance [11]. Particularly, vascular endothelial growth factor and interferon-γ inducible chemokines have been reported to be upregulated in Graves’ disease [13], [27]. The increased blood flow has been also measured quantitatively [12], [13], [16], [20], [21], [28], [29], [30], [31], [10], [32]. However, quantitative Doppler techniques need careful adjustments of the ultrasound system in order to prevent artifacts, can be challenging in certain patients, and yet, they have not been standardized (for instance, it is debated which artery should be sampled). Therefore, quantitative techniques have been not included in national and international guidelines for the diagnosis and management of hyperthyroidism [1], [9], [33].
In the present study we found that larger thyroids were trendwisely more vascularized, and in turn, vascularization was influenced positively by thyroid volume at logistic analysis. These findings are in keeping with those of Huang et al, who found that blood flow rate measured at superior thyroid artery correlated with thyroid gland weight [29]. In contrast with Hodgson [34], we found no significant correlation between vascularity and FT3 levels, but we did so for FT4 and TRAb levels, in accordance with Hiraiwa et al [12]. In this regard, a role of TSH-receptor stimulation by TRAb in determining the increased thyroid blood flow in Graves’ disease has been proposed [11]. Interestingly, patients who experienced at least one recurrence of hyperthyroidism, either on or off antithyroid drugs, had about 2-fold higher levels of TRAb at onset. In these patients, the number of recurrences correlated positively with both TRAb levels and thyroid volume at onset. Thus, it could be inferred that these two parameters may have prognostic relevance. Overall, young patients with a large, markedly hypoechoic, hypervascularized thyroid, and elevated TRAb levels at onset underpinning the increased hormonal output, are more likely to recur in the short term.
The main strength of this study is the limited variability in reporting ultrasonographic examinations, since only one operator performed them. For this reason, and for the other restrictive inclusion criteria, the major limitation is the limited number of patients selected. In light of these restraints, some comparisons and correlations yielded only trendwisely significant P values. Also, the operator was not blinded to the severity of hyperthyroidism in most cases.
In conclusion, hypervascularization appears to be an important sonographic feature in patients with Graves’ disease at onset.
Declaration of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L. American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid: Off J Am Thyroid Assoc. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 2.Vita R., Lapa D., Trimarchi F., Benvenga S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves' disease. Endocrine. 2015;48(1):254–263. doi: 10.1007/s12020-014-0289-8. [DOI] [PubMed] [Google Scholar]
- 3.Vita R., Lapa D., Trimarchi F., Vita G., Fallahi P., Antonelli A. Certain HLA alleles are associated with stress-triggered Graves' disease and influence its course. Endocrine. 2017;55(1):93–100. doi: 10.1007/s12020-016-0909-6. [DOI] [PubMed] [Google Scholar]
- 4.Leger J., Oliver I., Rodrigue D., Lambert A.S., Coutant R. Graves' disease in children. Ann Endocrinol. 2018 doi: 10.1016/j.ando.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Goichot B., Leenhardt L., Massart C., Raverot V., Tramalloni J., Iraqi H. Diagnostic procedure in suspected Graves' disease. Ann Endocrinol. 2018 doi: 10.1016/j.ando.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Castagnone D., Rivolta R., Rescalli S., Baldini M.I., Tozzi R., Cantalamessa L. Color Doppler sonography in Graves' disease: value in assessing activity of disease and predicting outcome. AJR Am J Roentgenol. 1996;166(1):203–207. doi: 10.2214/ajr.166.1.8571877. [DOI] [PubMed] [Google Scholar]
- 7.Vitti P., Rago T., Mancusi F., Pallini S., Tonacchera M., Santini F. Thyroid hypoechogenic pattern at ultrasonography as a tool for predicting recurrence of hyperthyroidism after medical treatment in patients with Graves' disease. Acta Endocrinol. 1992;126(2):128–131. doi: 10.1530/acta.0.1260128. [DOI] [PubMed] [Google Scholar]
- 8.Zingrillo M., D'Aloiso L., Ghiggi M.R., Di Cerbo A., Chiodini I., Torlontano M. Thyroid hypoechogenicity after methimazole withdrawal in Graves' disease: a useful index for predicting recurrence? Clin Endocrinol. 1996;45(2):201–206. doi: 10.1046/j.1365-2265.1996.d01-1563.x. [DOI] [PubMed] [Google Scholar]
- 9.Rago T., Cantisani V., Ianni F., Chiovato L., Garberoglio R., Durante C. Thyroid ultrasonography reporting: consensus of Italian Thyroid Association (AIT), Italian Society of Endocrinology (SIE), Italian Society of Ultrasonography in Medicine and Biology (SIUMB) and Ultrasound Chapter of Italian Society of Medical Radiology (SIRM) J Endocrinol Invest. 2018;41(12):1435–1443. doi: 10.1007/s40618-018-0935-8. [DOI] [PubMed] [Google Scholar]
- 10.Caruso G., Attard M., Caronia A., Lagalla R. Color Doppler measurement of blood flow in the inferior thyroid artery in patients with autoimmune thyroid diseases. Eur J Radiol. 2000;36(1):5–10. doi: 10.1016/s0720-048x(00)00147-9. [DOI] [PubMed] [Google Scholar]
- 11.Bogazzi F., Bartalena L., Brogioni S., Burelli A., Manetti L., Tanda M.L. Thyroid vascularity and blood flow are not dependent on serum thyroid hormone levels: studies in vivo by color flow doppler sonography. Eur J Endocrinol. 1999;140(5):452–456. doi: 10.1530/eje.0.1400452. [DOI] [PubMed] [Google Scholar]
- 12.Hiraiwa T., Tsujimoto N., Tanimoto K., Terasaki J., Amino N., Hanafusa T. Use of color Doppler ultrasonography to measure thyroid blood flow and differentiate graves' disease from painless thyroiditis. Eur Thyroid J. 2013;2(2):120–126. doi: 10.1159/000350560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corona G., Biagini C., Rotondi M., Bonamano A., Cremonini N., Petrone L. Correlation between, clinical, biochemical, color Doppler ultrasound thyroid parameters, and CXCL-10 in autoimmune thyroid diseases. Endocr J. 2008;55(2):345–350. doi: 10.1507/endocrj.k07e-052. [DOI] [PubMed] [Google Scholar]
- 14.Sriphrapradang C., Bhasipol A. Differentiating Graves' disease from subacute thyroiditis using ratio of serum free triiodothyronine to free thyroxine. Ann Med Surg. 2016;10:69–72. doi: 10.1016/j.amsu.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcocci C., Vitti P., Cetani F., Catalano F., Concetti R., Pinchera A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab. 1991;72(1):209–213. doi: 10.1210/jcem-72-1-209. [DOI] [PubMed] [Google Scholar]
- 16.Vitti P., Rago T., Mazzeo S., Brogioni S., Lampis M., De Liperi A. Thyroid blood flow evaluation by color-flow Doppler sonography distinguishes Graves' disease from Hashimoto's thyroiditis. J Endocrinol Invest. 1995;18(11):857–861. doi: 10.1007/BF03349833. [DOI] [PubMed] [Google Scholar]
- 17.Ralls P.W., Mayekawa D.S., Lee K.P., Colletti P.M., Radin D.R., Boswell W.D. Color-flow Doppler sonography in Graves disease: “thyroid inferno”. AJR Am J Roentgenol. 1988;150(4):781–784. doi: 10.2214/ajr.150.4.781. [DOI] [PubMed] [Google Scholar]
- 18.Morosini P.P., Simonella G., Mancini V., Argalia G., Lucarelli F., Montironi R. Color Doppler sonography patterns related to histological findings in Graves' disease. Thyroid: Off J Am Thyroid Assoc. 1998;8(7):577–582. doi: 10.1089/thy.1998.8.577. [DOI] [PubMed] [Google Scholar]
- 19.Bogazzi F., Bartalena L., Brogioni S., Mazzeo S., Vitti P., Burelli A. Color flow Doppler sonography rapidly differentiates type I and type II amiodarone-induced thyrotoxicosis. Thyroid: Off J Am Thyroid Assoc. 1997;7(4):541–545. doi: 10.1089/thy.1997.7.541. [DOI] [PubMed] [Google Scholar]
- 20.Donkol R.H., Nada A.M., Boughattas S. Role of color Doppler in differentiation of Graves' disease and thyroiditis in thyrotoxicosis. World J Radiol. 2013;5(4):178–183. doi: 10.4329/wjr.v5.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thalavai Sundarram K.S., Sadacharan D., Ravikumar K., Kalpana S., Suresh R.V. Role of color doppler ultrasonography in differentiation of graves’ disease from thyroiditis: a prospective study. World J Endocr Surgery. 2017;9(2):5. [Google Scholar]
- 22.Bogazzi F., Bartalena L., Vitti P., Rago T., Brogioni S., Martino E. Color flow Doppler sonography in thyrotoxicosis factitia. J Endocrinol Invest. 1996;19(9):603–606. doi: 10.1007/BF03349025. [DOI] [PubMed] [Google Scholar]
- 23.Akhter A.A., Fariduddin M., Jahan S., Sultana N., Hasan M., Rahman A.Q., Hasanat M.A. Can color doppler ultrasonography differentiate thyrotoxicoisis in graves’ disease from subacute thyroiditis? J Endocrinol Thyroid Res. 2017;2(5):5. [Google Scholar]
- 24.Erdogan M.F., Anil C., Cesur M., Baskal N., Erdogan G. Color flow Doppler sonography for the etiologic diagnosis of hyperthyroidism. Thyroid: Off J Am Thyroid Assoc. 2007;17(3):223–228. doi: 10.1089/thy.2006.0104. [DOI] [PubMed] [Google Scholar]
- 25.Levine R.A. Doppler ultrasound. In: Baskin H.J., Duick D.S., Levine R.A., editors. Thyroid ultrasound and ultrasound-guided FNA. second ed. Springer; New York: 2008. pp. 27–43. [Google Scholar]
- 26.Cappelli C., Pirola I., De Martino E., Agosti B., Delbarba A., Castellano M. The role of imaging in Graves' disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65(1):99–103. doi: 10.1016/j.ejrad.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Antonelli A., Ferrari S.M., Corrado A., Di Domenicantonio A., Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Ueda M., Inaba M., Kumeda Y., Nagasaki T., Hiura Y., Tahara H. The significance of thyroid blood flow at the inferior thyroid artery as a predictor for early Graves' disease relapse. Clin Endocrinol. 2005;63(6):657–662. doi: 10.1111/j.1365-2265.2005.02397.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang S.M., Chow N.H., Lee H.L., Wu T.J. The value of color flow Doppler ultrasonography of the superior thyroid artery in the surgical management of Graves disease. Arch Surg. 2003;138(2):146–151. doi: 10.1001/archsurg.138.2.146. discussion 51. [DOI] [PubMed] [Google Scholar]
- 30.Wu C. Duplex doppler ultrasonography for the functional evaluation of diffuse thyroid diseases. J Med Ultrasound. 2009;17(4):4. [Google Scholar]
- 31.Hari Kumar K.V., Pasupuleti V., Jayaraman M., Abhyuday V., Rayudu B.R., Modi K.D. Role of thyroid Doppler in differential diagnosis of thyrotoxicosis. Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2009;15(1):6–9. doi: 10.4158/EP.15.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Kumar K.V., Vamsikrishna P., Verma A., Muthukrishnan J., Rayudu B.R., Modi K.D. Utility of colour Doppler sonography in patients with Graves' disease. West Indian Med J. 2009;58(6):566–570. [PubMed] [Google Scholar]
- 33.Biondi B., Bartalena L., Cooper D.S., Hegedus L., Laurberg P., Kahaly G.J. The 2015 European thyroid association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J. 2015;4(3):149–163. doi: 10.1159/000438750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodgson K.J., Lazarus J.H., Wheeler M.H., Woodcock J.P., Owen G.M., McGregor A.M. Duplex scan-derived thyroid blood flow in euthyroid and hyperthyroid patients. World J Surg. 1988;12(4):470–475. doi: 10.1007/BF01655423. [DOI] [PubMed] [Google Scholar]