Abstract
Pleuroparenchymal fibroelastosis (PPFE) is a newly described entity of interstitial lung disease, which has been recently recognized as a rare complication of bone marrow transplantation. We report a case of 30-year-old man who developed a unique combination of pleuroparenchymal fibroelastosis with cellular and fibrotic non-specific interstitial pneumonia (NSIP) and bronchiolitis obliterans (BO) sixteen years after hematopoietic stem cell transplantation. Histological examination revealed almost exclusive infiltration of CD3-positive T lymphocytes associated with lymphoepithelial lesions and multi-focal denudation of covering epithelial cells in all components. This case suggests PPFE, NSIP, and BO might be conditions of the same spectrum, pathogenetically related to chronic graft-versus-host disease. Immunostaining for CD3 and CD20 in transbronchial lung biopsies may be helpful for identifying graft-versus-host-driven interstitial lung disease.
Keywords: Graft-versus-host disease, HRCT, Interstitial lung disease, Pathology, Pulmonary fibroelastosis
1. Introduction
Hematopoietic stem cell transplantation (HSCT) is increasingly used in the management of various hematologic malignancies. In Japan, more than 5000 procedures are performed annually [1]. Pulmonary complications of HSCT are the major cause of mortality and morbidity in the post-transplant period [2]. With the better management of infectious complications, the prevalence of non-infectious pulmonary sequelae shows a growing trend [3]. Among them, late-onset non-infectious respiratory complications such as bronchiolitis obliterans (BO), organizing pneumonia (OP), and interstitial pneumonia were reported among life-threatening post-HSCT conditions with the incidence of 1.7–25.6% [2]. Very recently, pleuroparenchymal fibroelastosis (PPFE) has been added to this spectrum [4]; however, the underlying mechanism of PPFE in HSCT is not fully understood.
Herein, we present a case with the unique constellation of PPFE, non-specific interstitial pneumonia (NSIP) and BO developed after HSCT. Furthermore, we elaborate on the possible pathophysiology based on immunohistochemical findings.
2. Case report
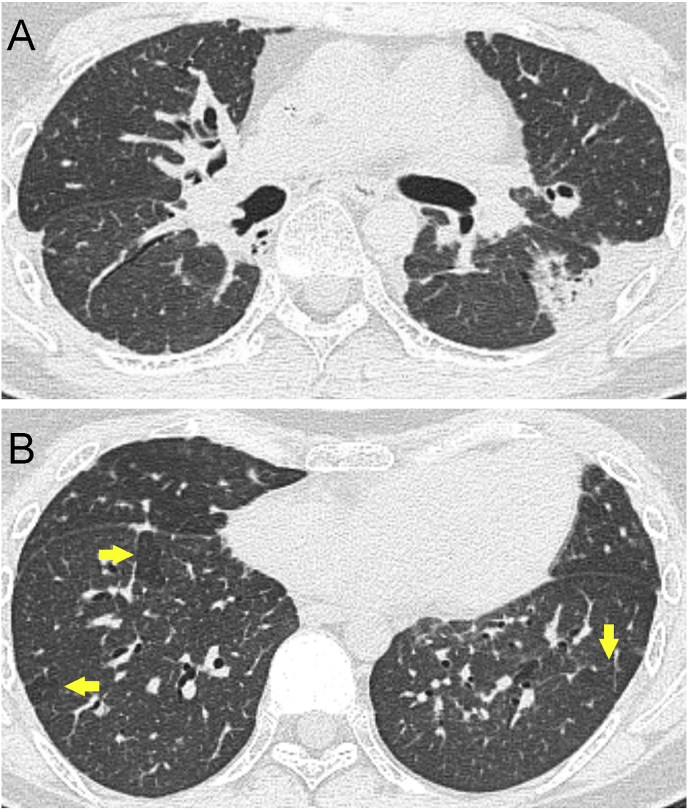
A 30-year-old male patient who suffered from acute lymphoblastic leukemia was treated with allogenic HSCT in 2000. Sixteen years later, he suffered with dry cough and progressive dyspnea on exertion. Later on, he developed pneumothorax thrice. Blood tests revealed a slight elevation of C-reactive protein (0.91 mg/dl) but no elevation of KL-6 and surfactant protein D. Pulmonary function tests were not performed because of pneumothorax. Chest high-resolution computed tomography (HRCT) showed pleural and subpleural thickening with severe fibrotic changes in the marginal parenchyma, flat chest, mosaic perfusion, and reticular shadows in both lungs, added by pneumomediastinum (Fig. 1).
Fig. 1.
HRCT of the patient. Subpleural consolidation associated with pleural thickening (A) and mosaic attenuation (arrows) (B).
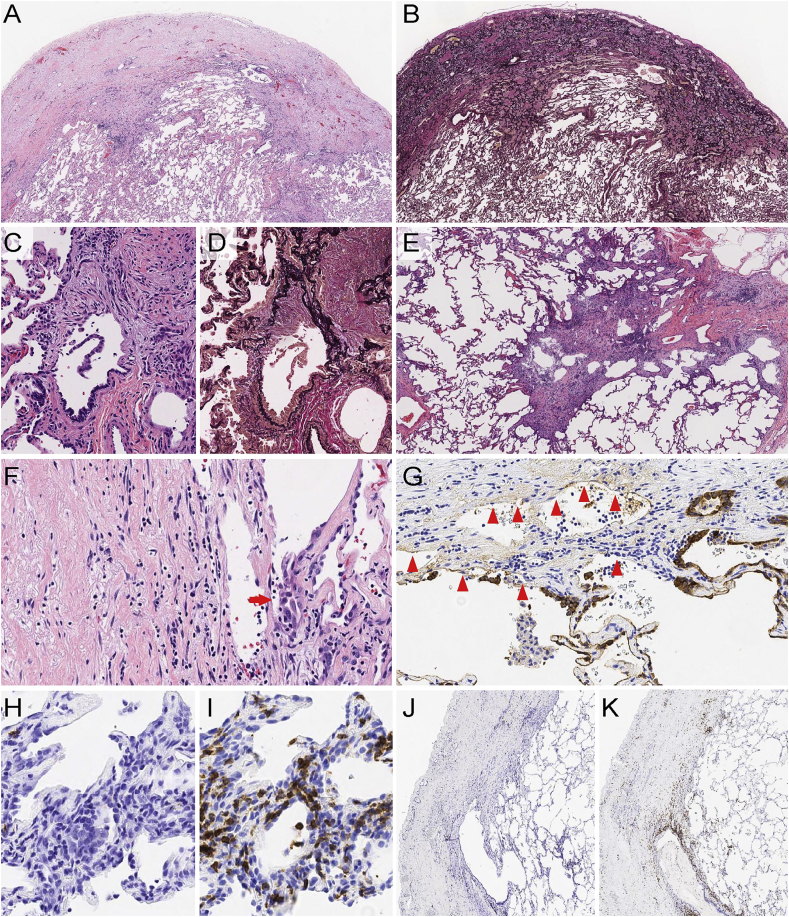
Video-assisted thoracoscopic surgery was performed and wedge biopsies from segments 5 and 10 of the right lung were taken. Microscopically, subpleural and peribronchial fibroelastosis (Fig. 2A and B), constrictive bronchiolitis (Fig. 2C and D) and cellular and fibrotic non-specific interstitial pneumonia (NSIP pattern) (Fig. 2E) were found. Immunohistochemistry revealed that cellular infiltrates were almost exclusively made of CD3-positive cells with just a few occasional CD20-positive cells (Fig. 2H–K). These inflammatory cells permeated into both alveolar and bronchial epithelial lining cells creating lymphoepithelial lesions (LELs) and erosions (Fig. 2F). AE1/AE3 immunostaining confirmed multiple foci of alveolar cell denudation (Fig. 2G). Such LELs and erosions were consistently observed in all the areas with morphology of PPFE, NSIP, and BO. The patient is currently on immunosuppressive therapy and the condition is stable.
Fig. 2.
Histological and immunohistochemical findings (A) Area of PPFE showing markedly thickened pleura and subpleural fibroelastosis. (B) Elastica van Gieson staining of the same field shows the increase of elastic fibers. (C–D) Area of BO shows constrictive change by collagenous fibrosis. Note denudation of covering epithelia. (E) Diffuse cellular infiltrates showing NSIP pattern. (F) The transitional area between PPFE and adjacent lung shows denudation and inflammatory cells infiltration into epithelial lining cells creating lymphoepithelial lesions (arrow). (G) AE1/AE3 immunostaining highlights denuded lining epithelia (arrowheads). (H–K). Immunostaining for CD20 (H, J) and CD3 (I, K) reveals almost exclusive CD3 positive cells in NSIP (H, I) and PPFE (J, K). Hematoxylin and eosin (A, C, E, F), elastica van Gieson (B, D), immunohistochemistry (G–K); × 50 (A, B), × 400 (C, D, F, G–I), × 100 (E, J, K).
3. Discussion
We herein report a case of cellular and fibrotic NSIP in association with PPFE and BO after HSCT. PPFE is a rare complication of bone marrow transplantation accounting for 0.3% of all HSCT cases [4]. However, it is becoming increasingly recognized in the last decade, especially in the Asian population. We found eight case reports and case series, which described 36 patients who developed PPFE after HSCT (Table 1) [[4], [5], [6], [7], [8], [9], [10], [11]]. Mean age was 31.6 (range – 8–61 years) and male to female ratio was 21:15. Twenty-eight cases were from Japan, two from Korea and six were from European countries. Mean interval between bone marrow transplantation and respiratory symptoms onset was 3.5 years, ranged from just 3 months to as long as 16 years. Thirty patients were already expired at a time of the report, two patients had progressive deterioration, two had stable to slowly progressive pattern and another two were not reported with regard to disease outcome. A combination of PPFE and BO was found in 21 patients and variable degrees of inflammatory cell infiltrations were found in nine patients whereas the exact pattern was not mentioned apart from cases reported by Takeuchi et al. [6] The commonly considered potential causes included chronic graft-versus-host disease (cGVHD), total body irradiation, and chemotherapy, e.g. cyclophosphamide conditioning treatment. However, the exact mechanism of development of PPFE after bone marrow transplantation has not been addressed.
Table 1.
Summary of reported PPFE cases after bone marrow transplantation.
| Source | Patients | Age | Sex | Underlying disease | Manifestation after BMT (years) | Histological findings |
|---|---|---|---|---|---|---|
| Present case | 1 | 30 | M | ALL | 16 | PPFE, BO, cellular and fibrotic NSIP |
| Thusen, 2011 [5] | 4 | 13 | F | AA | 2.8 | subpleural fibrosis, BO, mild patchy inflammation |
| 23 | M | AML | 12 | subpleural and paraseptal fibroelastosis, BO | ||
| 32 | M | ALL | 16 | subpleural fibroelastosis, BO, pneumothorax | ||
| 55 | F | AML | 2 | PPFE, BO, widespread pulmonary fibrosis | ||
| Fujikura, 2014 [7] | 1 | 31 | F | ALL | 9 | PPFE, BO |
| Takeuchi, 2015 [6] | 20 | 27.5 (8–57) | 11 M 9 F |
AML (12) MDS (2) ALL (1) Primary macroglobulinemia (1) SCID (1) Aplastic anemia (1) Chediak-Higashi syndrome (1) Neuroblastoma (1) |
1.2 (0.4–3.8) | BO (20) PPFE (15) NSIP fibrotic (9) NSIP cellular (6) |
| Mariani, 2016 [4] | 2 | 33 | M | AML | 13 | PPFE was diagnosed by HRCT |
| 61 | F | AML | 5 | |||
| Matsui, 2016 [11] | 1 | 40 | M | HL | 2.7 | PPFE, diffuse interstitial fibrosis |
| Ishii, 2016 [8] | 5 | 44 | F | MDS (RA) | 3.3 | PPFE, fibrotic lesions, no significant inflammatory cell infiltration |
| 27 | M | MDS (RA) | 1.1 | |||
| 38 | M | CML | 9.1 | |||
| 28 | M | ALL | 6.4 | |||
| 50 | M | AA | 8.8 | |||
| Cha, 2017 [9] | 2 | 54 | M | AML | 2 | PPFE, diffuse lymphocytic infiltration |
| 16 | M | AML | n/a | PPFE, BO, patchy lymphocytic infiltration | ||
| Okimoto, 2018 [10] | 1 | 45 | F | MDS | 5 | PPFE |
AA, aplastic anemia.
ALL, acute lymphoblastic leukemia.
AML, acute myeloid leukemia.
BO, bronchiolitis obliterans
BMT, bone marrow transplantation.
CML, chronic myeloid leukemia.
HL, Hodgkin lymphoma.
HRCT, high resolution computed tomography.
MDS, myelodysplastic syndrome.
NSIP, non-specific interstitial pneumonia.
RA, refractory anemia.
SCID, severe combined immunodeficiency.
BO is considered diagnostic for pulmonary cGVHD [12] and the present case showed that in addition to BO, areas of PPFE in NSIP and BO showed LELs dominated by lymphocytes along with multiple foci of alveolar erosion. Immunostaining with CD3 and CD20 showed almost exclusive infiltration of T lymphocytes. It is widely accepted that activated donor T cells are the key pathogenic players in cGVHD [13]. The finding of exclusive infiltration by CD3+ cells in the present case allowed us to speculate that NSIP and PPFE may also be in the same spectrum of cGVHD similar to BO.
Another intriguing finding was the presence of frequent LELs and erosions confirmed by AE1/AE3. The denudation of alveolar and bronchiolar lining epithelia was scattered at the transition zone between the affected area and adjacent normal parenchyma. This raised a hypothesis that the graft T cells might attack epithelial cells and create erosions followed by healing scar as fibrosis to develop lesions of PPFE, NSIP, and BO. PPFE may follow after the collapse due to the insufficiency of surfactant triggered by the denudation of epithelial cells. Takeuchi et al. reported 20 cases of interstitial lung disease after HSCT and found that PPFE, NSIP, and BO were seen frequently together with six of out twenty cases having cellular NSIP similarly to the present case [6]. They also speculated that all lesions could be a part of the cGVHD although the possible mechanism was not addressed.
Furthermore, our patient developed pneumothorax thrice and pneumomediastinum once. Usually, air-leak syndrome (ALS) such as pneumothorax, pneumomediastinum, and subcutaneous emphysema is considered as a complication of BO due to collapsed fibrosis [8]. Interestingly, there is also a possible link between ALS and PPFE. Ishii et al. reported five cases of PPFE without BO, which suggests that PPFE itself may also lead to ALS [8].
Additionally, a case reported by Okimoto et al. developed PPFE without BO after HSCT [10]. As BO is a diagnostic feature for pulmonary cGVHD, Okimoto et al. speculated that the development of PPFE was not associated with GVHD. However, the patient reported by Okimoto et al. did have cGVHD in other organs such as liver and skin so it may not be justifiable to deny cGVHD as the possible contributor just because of the lack of BO and unresponsiveness to immunosuppressive therapy. It is obvious that the development of PPFE after HSCT may have heterogeneous mechanisms other than cGVHD; nonetheless, this does not rule out the possibility of cGVHD as one of the mechanisms for PPFE. Further studies focusing on the nature and interactions of inflammatory cells and LELs in the cases of PPFE with cGVHD are highly anticipated.
In conclusion, we report a case of PPFE after allogenic HSCT in which cellular and fibrotic NSIP was found in addition to BO. Although it is a rare combination, our case, in addition to the previous cases reported by Takeuchi et al. highlights the necessity to evaluate the composition of cellular infiltrates in post-HSCT lung disease. This can be a key in understanding the pathophysiology of PPFE in cases with chronic GVHD after HSCT. Moreover, in cases with contraindications for video-assisted thoracoscopic surgery biopsy, performing immunostaining for CD3 and CD20 in transbronchial lung biopsies may be helpful to identify GVHD-driven interstitial lung disease.
Conflicts of interest
None.
Informed consent
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Financial support
No grants or funding have been received for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.02.001.
Contributor Information
Zun Pwint Oo, Email: dr.zunpwintoo@gmail.com.
Andrey Bychkov, Email: bychkov.andrey@kameda.jp.
Yoshiaki Zaizen, Email: zaizen_yoshiaki@nagasaki-u.ac.jp.
Mari Yamasue, Email: sai-mari@oita-u.ac.jp.
Jun-ichi Kadota, Email: kadota@oita-u.ac.jp.
Junya Fukuoka, Email: fukuokaj@nagasaki-u.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Japanese Data Centre for Hematopoietic Cell Transplantation, Activities and Outcomes of Hemopoietic Stem Cell Transplantation in Japan. 2016. http://www.jdchct.or.jp/en/data/slide/2016/ [Google Scholar]
- 2.Yoshihara S., Yanik G., Cooke K.R., Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Maria D., Jihane Z., Ayman O.S. Major pulmonary complications after hematopoietic stem cell transplant. Exp. Clin. 2016;14:259–270. doi: 10.6002/ect.2015.0275. [DOI] [PubMed] [Google Scholar]
- 4.Mariani F., Gatti B., Rocca A., Bonifazi F., Cavazza A., Fanti S., Tomassetti S., Piciucchi S., Poletti V., Zompatori M. Pleuroparenchymal fibroelastosis: the prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 2016;22:400–406. doi: 10.5152/dir.2016.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Von Der Thüsen J.H., Hansell D.M., Tominaga M., Veys P.A., Ashworth M.T., Owens C.M., Nicholson A.G. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod. Pathol. 2011;24:1633–1639. doi: 10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi Y., Miyagawa-Hayashino A., Chen F., Kubo T., Handa T., Date H., Haga H. Pleuroparenchymal fibroelastosis and non-specific interstitial pneumonia: frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology. 2015;66:536–544. doi: 10.1111/his.12553. [DOI] [PubMed] [Google Scholar]
- 7.Fujikura Y., Kanoh S., Kouzaki Y., Hara Y., Matsubara O., Kawana A. Pleuroparenchymal fibroelastosis as a series of airway complications associated with chronic graft-versus-host disease following allogeneic bone marrow transplantation. Intern. Med. 2014;53:43–46. doi: 10.2169/internalmedicine.53.1124. [DOI] [PubMed] [Google Scholar]
- 8.Ishii T., Bandoh S., Kanaji N., Tadokoro A., Watanabe N., Imataki O., Dobashi H., Kushida Y., Haba R., Yokomise H. Air-leak syndrome by pleuroparenchymal fibroelastosis after bone marrow transplantation. Intern. Med. 2016;55:105–111. doi: 10.2169/internalmedicine.55.4539. [DOI] [PubMed] [Google Scholar]
- 9.Cha Y.J., Han J., Chung M.P., Kim T.J., Shin S. Pleuroparenchymal fibroelastosis in heterogeneous clinical conditions: clinicopathologic analysis of 7 cases. Clin. Res. J. 2018;12:1495–1502. doi: 10.1111/crj.12696. [DOI] [PubMed] [Google Scholar]
- 10.Okimoto T., Tsubata Y., Hamaguchi M., Sutani A., Hamaguchi S., Isobe T. Pleuroparenchymal fibroelastosis after haematopoietic stem cell transplantation without graft-versus-host disease findings. Respirol. Case Rep. 2018;6 doi: 10.1002/rcr2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui T., Maeda T., Kida T., Fujita J., Tsuji H., Morii E., Kanakura Y. Pleuroparenchymal fibroelastosis after allogenic hematopoietic stem cell transplantation: important histological component of late-onset noninfectious pulmonary complication accompanied with recurrent pneumothorax. Int. J. Hematol. 2016;104:525–530. doi: 10.1007/s12185-016-2038-7. [DOI] [PubMed] [Google Scholar]
- 12.Filipovich A.H., Weisdorf D., Pavletic S., Socie G., Wingard J.R., Lee S.J., Martin P., Chien J., Przepiorka D., Couriel D., Cowen E.W., Dinndorf P., Farrell A., Hartzman R., Henslee-Downey J., Jacobsohn D., McDonald G., Mittleman B., Rizzo J.D., Robinson M., Schubert M., Schultz K., Shulman H., Turner M., Vogelsang G., Flowers M.E.D. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Presland R.B. Biology of chronic graft- vs -host disease: immune mechanisms and progress in biomarker discovery. World J. Transplant. 2016;6:608. doi: 10.5500/wjt.v6.i4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.