Abstract
Vitamin D is a secosteroid hormone regulating calcium and phosphate metabolism, immune response and brain development. Low blood 25(OH)D levels have been reported in patients affected by infectious diseases caused by parasites, including malaria. Despite the high effectiveness of antimalarials, malaria is burdened with high morbidity and mortality, and the search for additional therapies is rapidly growing. Furthermore, available preventive measures have proved to be barely effective so far. Finding new prevention and therapy tools is a matter of urgency. Studies on animal models and humans have hypothesized some mechanisms by which the hormone can influence malaria pathogenesis, and the role of Vitamin D supplementation in preventing and treating this disease has been suggested. Few studies on the association between Vitamin D and malaria are available and disagreeing results have been reported. Studies in humans reporting an association between low 25(OH)D circulating levels and Malaria have a small sample size and observational study-set. Randomized controlled trials are needed in order to understand if Vitamin D administration might play a role in preventing and treating malaria.
Keywords: Infectious disease, Biochemistry, Immunology
1. Introduction
Vitamin D is a secosteroid hormone regulating the expression of almost 900 genes [1] and it is involved in the regulation of calcium and phosphate metabolism, immune response and brain development [2, 3]. Low blood Vitamin D levels have been reported in patients affected by infectious diseases, including those caused by parasites [4]. Among these, the pathogen causing malaria in humans, Plasmodium falciparum, was responsible for the death of almost 365.000 African children under the age of five in 2016 [5, 6]. Even though existing antimalarials are highly-effective, malaria is burdened with high morbidity and mortality [7], which means that the search for additional therapies to administer along with antimalarial treatments is rapidly growing. Furthermore, available preventive measures, including vector control programs, chemoprevention and vaccinations, have proved to be barely effective so far, mainly due to insecticides and drugs resistance in vectors and plasmodium [8]. Finding new prevention and therapy tools is a matter of urgency. Studies on animal models and humans have hypothesised some mechanisms by which the hormone can influence malaria pathogenesis, and the role of Vitamin D analogues supplementation in preventing and treating this disease has been suggested [9].
The purpose of this paper is to highlight the mechanisms by which Vitamin D can influence the pathogenesis of malaria and provide a critical review of the studies evaluating the association of Vitamin D and malaria, in order to determine whether and how supplementation can be useful in preventing and treating patients affected by this disease.
2. Main text
2.1. Vitamin D: a brief overview
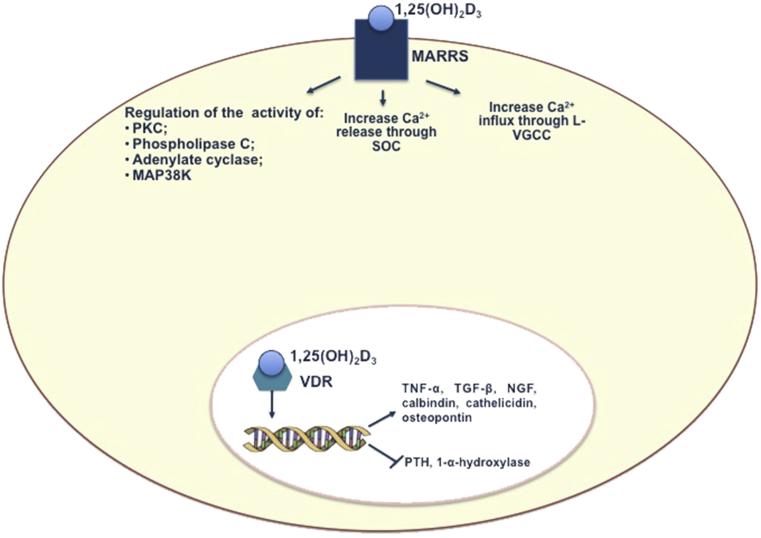
Ultraviolet B-rays action in the skin produces cholecalciferol, which requires two different hydroxylations to be converted into active Vitamin D [1,25(OH)2D]. The enzyme 25-α-hydroxylase catalyses in the liver the first hydroxylation and produces 25(OH)D, which is the main circulating form of Vitamin D. The second hydroxylation produces 1,25(OH)2D in the kidney and is performed by the enzyme 1-α-hydroxylase. In the past two decades, new insights in the Vitamin D metabolism led to uncovering that many tissues and cytotypes, including brain and immune cells, can produce 1,25(OH)2D, having 1-α-hydroxylase [10]. Within these tissues and cell-types, the active hormone acts in an autocrine/paracrine fashion, regulating cell growth and proliferation [11]. Vitamin D signalling pathways include a nuclear receptor (Vitamin D Receptor-VDR) pathway and a surface receptor (protein disulphide isomers family A members 3- PDIA3, also known as membrane-associated rapid response steroid binding- MARRS or ERp57) pathway [12]. Both nuclear and surface receptors signalling carry out genomic and non-genomic actions (Fig. 1) [13]. While VDR- mediated activities seem to be involved in modulation of the immune response [14], the PDIA3 signalling pathway is deemed to be implicated in the regulation of brain development and function [15].
Fig. 1.
Genomic and non-genomic actions of Vitamin D. Active Vitamin D include a nuclear receptor, VDR, and a surface receptor, MARRS, with whom 1,25(OH)2D interacts to carry out genomic and non-genomic actions. After binding the ligand, VDR forms a heterodimer with RXR receptor. VD/VDR/RXR complex interacts with VDREs, located within chromatin, resulting in genes transcription activation and genes suppression. Non-genomic actions carried out by MARRS receptors include the regulation of the activity of some proteins, like adenylyl cyclase, phospholipase C, protein kinase C, p38 MAP kinase, and the increase of Ca2+ influx through L-type voltage-gated calcium channels (L-VGCC) and Ca2+ release from intracellular stores through store-operated channels (SOC). Vitamin D Receptor: VDR; MARRS: membrane-associated rapid response steroid binding; RXR: retinoic acid receptor; VDREs: VD responsive elements; TNF-α: Tumor Necrosis Factor-α; TGF- β: Transforming Growth Factor- β; NGF: Nerve Growth Factor; L-VGCC = L-type voltage-gated calcium channels; SOC = store-operated channels; p38MAPK: 38-mitogen-activated protein kinase; PKC: Protein Kinase C.
Low 25(OH)D circulating levels, as well as genetic variants of VDR, have been reported in patients affected by infectious, autoimmune and neurological diseases [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. Notably, multiple lines of evidence show that Vitamin D may play a role in the pathogenesis of infectious diseases [27, 28]. In autoimmune diseases, a growing body of data documented a role for Vitamin D either as a pathogenic factor or a serum biomarker [24]. Also, Vitamin D has been proposed as a serum biomarker in neurodegenerative disorders, along with inflammatory and cardiovascular markers [29, 30, 31, 32, 33, 34].
Low blood 25(OH)D levels have been widely reported in the whole healthy population, regardless of demographic variables such as age, race or geographical collocation [35]. Although some debate exists about Vitamin D reference values [36, 37], Vitamin D sufficiency is widely defined as a serum 25(OH)D level greater or equal to 30 ng/mL; Vitamin D insufficiency as a serum 25(OH)D level from 20 to 30 ng/mL; Vitamin D deficiency as a serum 25(OH)D level lower than 20 ng/mL [38].
2.2. Immunomodulation by vitamin D
The immune system is designed to protect the body against pathogens and cell damage acting in conjunction with the Central Nervous System (CNS) [39].
The immune response consists of two types: an innate response and an adaptive response. Innate immunity is the first line of defence using monocytes, macrophages, neutrophils, NK cells and dendritic cells; macrophages and dendritic cells work as antigen presenting cells (APCs) to B and T lymphocytes, which proliferate and mature after antigen recognition. T lymphocytes are effector cells performing the antigen-specific immune response and are divided in T-helper (CD4+), which include the subsets Th1, Th2 and Th17, T cytotoxic (CD8+) and T regulatory (Treg). Immune response against pathogens starts when pathogen-associated molecular patterns (PAMPs) are detected by pattern recognition receptors (PRRs), that include Toll-like receptors (TLRs) and NOD-like receptors (NLR). PAMPS/TRL binding results in the activation of transcription factors, such as NF-kB, leading to chemokines, proinflammatory cytokines and growth factors synthesis. These are responsible for the amplification of the response and immune cells migration to the site of inflammation. An inflammation resolution phase follows the proinflammatory elicitation phase, that is mediated by cells and molecules which are recruited to avoid damages for an excessive immune response, such as autoimmune diseases and allergies [39, 40, 41].
Active Vitamin D influences the immune system in different ways. Macrophages, dendritic cells and activated B and T lymphocytes express 1α-hydroxylase and VDR. 1,25(OH)2D upregulates the expression of CD14, the TLR4 co-receptor, and the expression of 1- increases after TLR2 stimulation in macrophages and keratinocytes. Furthermore, 1,25(OH)2D inhibits NF-kB activity in lymphocytes [42, 43, 44, 45]. While the role of active Vitamin D in the differentiation of dendritic cells is discussed, there is no doubt that it decreases their antigen-presenting capacity and survival [46, 47, 48, 49]. 1,25(OH)2D plays a key role in Th1, Th2 and Th17 lymphocytes balance, by inhibiting the production of Th1 and Th17 cells and their cytokines (IFN-γ, TNF-α, IL-1, IL-2, IL12, IL-23 and IL-17, IL-21) and fostering the differentiation of Th2 and their products (IL-4 and IL-10); further, 1,25(OH)2D enhances the development of Treg cells and the production of their cytokines, including FoxP3 [50]. Influence of active Vitamin D to T cytotoxic CD8+ cells has also been suggested [51]. Finally, the hormone determines the expression of cathelicidin and defensins, antimicrobial peptides involved in the defence against pathogens [52, 53]. Vitamin D comprehensive action results in the increase of antimicrobial activity of the innate response and the decreasing of the adaptive response proinflammatory effects. It is also known that 1,25(OH)2D acts on the endothelium, e.g. increasing the expression of vascular cell adhesion molecule 1 (VCAM-1) [54].
2.3. The role of vitamin D in protection against pathogens
Active Vitamin D exerts an antibacterial and antiviral action mediated by defensins and cathelicidin. An antiparasitic action through the production of lactoferrin, cathelicidin-related antimicrobial peptide, reactive oxygen species and the phospholipids biosynthesis inhibition has been documented in Plasmodium falciparum and Plasmodium yoelii [55, 56, 57, 58]. Besides, 1,25(OH)2D influences the defence against pathogens by modulating T-helper lymphocytes subsets balance. When considering the role of the active hormone in T cells differentiation, it has to be borne in mind that both Th balance and Treg function have an impact on immune response efficacy and safety against pathogens. Indeed, Th1 cells provide an effective defence against pathogens, but, on the other hand, a Th1 uncontrolled response can result in self-reactive and pathological phenomena. Th2 cells exert an anti-inflammatory action along with the defence against helminth, but, on the other hand, a Th2 excessive response can undermine pathogens clearance and infections containment. Finally, Treg cells play a role in regulating/suppressing effector T cells and they also suppress pro-inflammatory cytokines action [59]. Active Vitamin D can exert a protective role against pathogens by modulating Th cells balance and enhancing the development of Treg.
1,25(OH)2D immunomodulatory activity has been associated with some parasitic infections, such as malaria (Fig. 2). Th1 excessive response, Th2 response mitigation and Treg cells dysfunction represent mechanisms involved in the onset and development of malaria [8, 9], and these effects can be limited by the action of 1,25(OH)2D on the immune response. Further, the hormone inhibits the synthesis of some pro-inflammatory cytokines such as IFN-γ and TNF-α, which are involved in the development of cerebral malaria (CM), an often fatal multifactoral pathogenesis syndrome [60].
Fig. 2.
Vitamin D influence on the pathogenesis of malaria. The activity of 1,25(OH)2D has been related to the pathogenesis of malaria, due to its action on Th cells and Treg cells. The onset and progression of malaria partly depend on Th1 overwhelming response, Th2 response mitigation and Treg cells dysfunction. Active Vitamin D might influence the pathogenesis of malaria by inhibiting Th1 cells production, fostering Th2 cells differentiation and enhancing the development of Treg cells. Further, 1,25(OH)2D inhibits the syntesis of IFN-γ, TNF-α, which are involved in the development of malaria and its severe complication, CM. IFN- γ: Interferon- γ; TNF- α: Tumor Necrosis Factor α; Th: T-helper; Treg: T regulatory; CM: cerebral malaria.
2.4. Vitamin D in the bacteria, virus, and fungal diseases: a brief summary
25(OH)D circulating levels, along with Vitamin D analogues therapeutic supplementation, have been studied in patients affected by respiratory tract infections (RTI), tuberculosis, virus infections (Human Immunodeficiency Virus-HIV, Epstein Barr Virus), parasitic and fungal infections and sepsis [61, 62, 63, 64, 65]. Vitamin D in such diseases has been studied i) in relation to pathogenesis; ii) as a risk factor for the onset of the infection and for the development of sepsis (when <30 ng/ml); iii) as a biomarker of disease severity, along with well-established biomarkers [55, 65, 66, 67].
Many studies carried out on large samples have shown an association between 25(OH)D circulating levels and RTI onset, both in children and adults, but, a more recent small sample size study has shown opposite results [68, 69, 70]. Some of the randomized controlled trials (RCTs) evaluating Vitamin D analogues supplementation effects in patients affected by RTI supposedly show encouraging results, also in terms of safety (no adverse reactions reported in most RCTs) [70]. However, other RTCs contradicted these results [71, 72]. It should be noted that Vitamin D trials generally enrol subjects who are not 25(OH)D deficient, thus, failure in finding a beneficial effect of supplementation could depend on this issue [73].
The association between Vitamin D deficiency and tuberculosis has been widely documented. Vitamin D deficiency has been considered as an independent risk factor for tuberculosis onset [74, 75, 76, 77, 78]. However, it has to be noted that RCTs on Vitamin D analogues supplementation in patients affected by tuberculosis present some limitations, e.g. large discrepancies as far as doses and primary outcomes are concerned [79, 80, 81, 82].
Vitamin D has been widely studied in HIV and Epstein Barr virus infections. As regards patients with HIV infection or affected by acquired immunodeficiency syndrome (AIDS), an association between 25(OH)D blood levels and CD4+ lymphocytes count, all-cause mortality and bone fractures risk has been reported [83, 84, 85]. However, interventional studies in this setting are not available. The relationship between Vitamin D and Epstein Barr Virus infection has been mainly evaluated in patients affected by multiple sclerosis (MS), suggesting the role of Vitamin D as an environmental factor involved in the pathogenesis of the disease [86].
Few studies on animal models have shown that low dose supplementation of Vitamin D analogues in Candida-infected mice can reduce fungal burden and improve their survival when compared with Vitamin D-untreated mice [62]. Furthermore, some in vitro studies have shown that Vitamin D deficiency represents a risk factor for the nephrotoxicity induced by Amphotericin B, an antifungal agent used to treat systemic mycoses [87].
Finally, few studies have investigated the role of Vitamin D in sepsis and, although two systematic reviews have shown an association between sepsis risk and mortality in these patients, results should be taken with a grain of salt, because most of the studies included were retrospective and because Vitamin D deficiency and insufficiency definition differed considerably according to the studies [64]. Few RCTs have been carried out on septic patients, reporting disheartening results [88]. However, a recent review suggested that high-dose Vitamin D analogues supplementation could be beneficial in intensive care units patients (ICU) [89]. Further, the Authors suggested that ICU patients might display Vitamin D deficiency due to many reasons, including hepatic and kidney dysfunction.
2.5. The role of vitamin D in malaria
The study on the role of Vitamin D in malaria is rooted in the past. At the end of the '50s, and then in the '80s, some studies have shown apparently promising results suggesting an antiplasmodial activity of active Vitamin D [90, 91, 92]. On the heels of a sort of enthusiasm, many researchers have deepened this topic to the present day. The scientific rationale supporting these studies seemed to be reasonable and intriguing: indeed, it is known that, when parasitic diseases occur, the modulation of many physiological pathways takes place, including Vitamin D metabolism pathway [93]; moreover, as mentioned above, Vitamin D influences the differentiation of Th1, Th2 and Treg cells, that play a pivotal role in the pathogenesis of the disease. Broadly speaking, studies carried out in vitro and on animal models have shown disagreeing results; those carried out on humans, having observational study set, have achieved unclear evidence. Since there are no RCTs evaluating the benefits of Vitamin D supplementation in patients with malaria, set out below studies have to be carefully considered [94].
2.5.1. Studies on animal models
A study aiming to evaluate melanin protective effect on malaria infection by Weisberg [95] shows that Vitamin D does not affect the survival of Plasmodium berghei ANKA (PbA)-infected B6 mice. The Authors used intraperitoneal Vitamin D injections (0.5 μg/Kg every other day, starting three days before the development of the infection); it could be noted that other Authors employed different administration doses and ways. For example, He [48] carried out a careful experiment in order to evaluate Vitamin D effects on mortality caused by CM and on the integrity of the blood-brain barrier (BBB), which shows characteristic alterations during CM, due to parasitised red blood cells (pRBC) cytoadherence to the endothelium of brain microvessels. He et al. orally administered Vitamin D in PbA-infected C57BL/6 mice (the most used experimental CM murine model), before (50 μg/Kg/day, for four days before infection) and after infection (50 μg/Kg/day, for four days after infection) and finally evaluated, also, T lymphocytes trafficking to the brain, the growth of Th1 and Treg cells in the spleen and DC differentiation and function. The Authors highlighted that Vitamin D treatment, especially when administered after the infection, increased the survival of treated mice (15 days more than Vitamin D-untreated mice) and they explained this result by Vitamin D effects on Th1 response inhibition, evaluated through IFNγ and TNF synthesis inhibition. Even if the Authors interpreted these results resoundingly speaking about a Vitamin D direct protective effect against ECM, it would be necessary to recognise that all the mice died after three weeks, regardless of the treatment with Vitamin D. Maybe it would be more appropriate to discuss about lengthening of the survival rather than a protection. The same team carried out another experiment this year, orally administering Vitamin D before infecting C57BL/6 mice with PbA (50 μg/Kg/day before infection for five consecutive days) to evaluate beneficial effects of Vitamin D pretreatment on CM progression [9]. Achieving a similar result (treated mice survived from 4 to 9 days more than untreated mice), the Authors present it as a protection by Vitamin D against CM, but it should not be overlooked that, regardless of the Vitamin D pretreatment, all the mice developed CM symptoms within 5 days from the infection and then died. It is also worth mentioning the experiment conducted by Yamamoto [5]: he tested the efficacy of Vitamin D and 22-oxacalcitriol (22-OCT); the latter was used because it causes less hypercalcaemia and weight loss than Vitamin D. Using VDR-knockout mice, the Authors suggested that both Vitamin D and 22-OCT perform their alleged antiplasmodial action through direct and indirect mechanisms, both VDR-independent and VDR-mediated. However, the Authors suggested that further studies are needed to explain the physiological mechanisms underlying this antiplasmodial activity. Moreover, the Authors infected BALB/c mice and B6 mice with a PcAS subspecies that is lethal in the first rodent type and that leads to partial mortality in the second type. Administering Vitamin D and 22-OCT, they obtain opposite effects on the two types of animals: 50% of BALB/c mice died before the control, because of their high susceptibility to Vitamin D toxic effects, while 100% of treated-B6 mice survived. It would be interesting to investigate the reasons why Vitamin D and 22-OCT exerted marked toxicity in a case and a beneficial effect on the other.
Dwivedi [7] has recently tested the efficacy against CM of combined treatment Vitamin D+α/β arteether. Arteether is an antimalarial agent belonging to the family of antimalarials derived from artemisinin. Dwivedi found that Vitamin D has no efficacy per se. After the onset of symptoms, mice were divided into four groups: one did not receive any treatment, one received only Vitamin D, another only α/β arteether and the last one Vitamin D+α/β arteether. Vitamin D-group (50 μg/kg−1 for 3 days via intramuscular injection) and arteether-group (25 mg/kg−1 α/β arteether on day 6 followed by 12.5 mg/kg−1 arteether on day 7 and 8 via intramuscular injection) died before the end of the treatment. In the Vitamin D+α/β arteether-group (25 mg/kg−1 α/β arteether on day 6 followed by 12.5 mg/kg−1 arteether on day 7 and 8 via intramuscular injection along with 50 μg/kg−1 for 3 days via intramuscular injection) a significant survival rate compared to the other groups was observed. It should be noted that an additional α/β arteether high dose-group (50 mg/kg−1 α/β arteether on day 6 followed by 25 mg/kg−1 arteether on day 7 and 8 via intramuscular injection) survived more than Vitamin D+α/β arteether-group (77% vs 73% respectively). Moreover, the evaluation of blood-brain barrier integrity, affected by injection of Evan Blue and calculating its concentration in brain parenchyma, showed that there were no differences between α/β arteether-treated group and Vitamin D+α/β arteether-treated one.
2.5.2. Studies on humans
There are few studies on humans, and they could be challenging to interpret, because they have often been carried out on small samples and, in any case, they have produced mixed results [63, 96] (Table 1). Sudfeld made considerable efforts to study the association between Vitamin D circulating levels and the onset of malaria in children and adults, testing large patient cohorts [4]. In 2013, the Authors studied 1105 adults affected by HIV from Tanzania and measured Vitamin D in order to understand if an association between the hormone and the occurrence of some infectious diseases, including malaria, exists [97]. People receiving the diagnosis at baseline and within the first month after the baseline visit were excluded from the study, in order to minimise reverse causation risk. Furthermore, he analysed 25(OH)D levels continuously, to get around the issue of consensus lacking on ideal hormone levels. 52% of patients showed 25(OH)D < 30 ng/ml levels and there were 356 incident malaria cases during a median follow-up of 20.6 months. No association between Vitamin D status and malaria incidence has been found using univariate and multivariate analyses.
Table 1.
Studies on the association between VD and malaria in humans.
| Authors | Study design | Patients | No of patients | Cut-off for Vitamin D deficiency | Association of hypovitaminosis D and Malaria |
|---|---|---|---|---|---|
| Sudfeld et al., 2017 | Prospective cohort study | Tanzanian infants born to human immunodeficiency virus-uninfected mothers | 581 | <20 ng/mL | No |
| Sudfeld et al., 2015 | Prospective cohort | HIV-infected and -exposed children (<2ys) | 253 and 948, respectively | 20–29.9 ng/mL | Yes |
| Cusick etl., 2014 | Prospective cohort study | Children aged 18 months-12 years with severe malaria | 40 | <30 ng/mL | Yes |
| Sudfeld et al., 2013 | Prospective observational cohort study | HIV-infected adults | 1103 | 20–30 ng/mL | No |
| Newens et al., 2006 | Prospective cohort study | Healthy, well-nourished adults presenting with clinical Plasmodium falciparum malaria | 14 | No | |
| Toko et al., 2016 | Longitudinal study | Pregnant women | 63 | <50 nmol/L | No |
In 2015, the same Authors reported surprising results when they investigated the association between 25(OH)D levels and mortality in 1200 HIV-infected and non-infected children [98]. Indeed, they indicated an association between higher 25(OH)D (>30 ng/ml) levels and malaria diagnosis in HIV-infected children (Incident Rate Ratio-IRR: 1.71; 95%: 1.15, 2.54; p < 0,01 and IRR:1.37; CI: 1.09 1.73; p < 0,01). The clinical diagnosis was made according to WHO's Integrated Management of Childhood Illness guidelines [99] during a 24 months follow-up, and, in some cases, was confirmed by parasitemia in a Giemsa-stained blood smear. In order to provide a mechanistic explanation of the result, the Authors suggested that the Th2 excessive response promoted by Vitamin D could play a role in reducing plasmodium clearance. However, they pointed out that the observational nature of the study leaves room for unmeasured and residual confounders that might have influenced the association between malaria and Vitamin D. For instance, children more exposed to sunlight, who synthesise more Vitamin D, are also more exposed to mosquitos, malaria and other pathogens causing malaria-like symptoms. Sudfeld has recently obtained different results carrying out a study on 581 children born to HIV-uninfected and exposed mothers, in whom found no association between serum 25(OH)D and malaria incidence [4]. Instead, Cusik [100] reported opposite results carrying out a study on 40 children from Uganda in 2014, who suffer from severe forms of malaria including CM and Severe Malarial Anemia (SMA) (20 CM+20 SMA vs 20 controls). 95% of children with severe malaria presented plasma 25(OH)D < 30 ng/ml and logistic regression analysis showed that the odds having severe malaria decreased by 9% for each ng/ml of Vitamin D (OR: 0.9; CI 95% 0.84. 1.0). However, interpreting Cusik results, it should be taken into account that he tested a small sample and that 80% of controls presented Vitamin D insufficiency. It is well known that dark-skinned populations are more exposed to lower 25(OH)D levels, because of some factors including low-Vitamin D diet [96, 101]. More recently, Toko et al. have assessed the association between plasma 25(OH)D concentrations and pregnancy outcomes in 63 women from western Kenya [63]. 39% of women included in this study presented malaria infection and the binomial regression analysis showed that no association between malaria infection and Vitamin D status.
Studies have shown that malaria is more often diagnosed in the rainy season, also because rainfalls and floods can contribute to developing an ecosystem that favours mosquito breeding [102]. Since Vitamin D concentration in this season is lower, supplementation might help to reduce the risk of malaria. Further, supplementing in the rainy season would not increase the risk of some bacterial infections which have been positively correlated with Vitamin D and UVB [103].
VDR up-regulation has been related to the intestinal damage occurring during Plasmodium chabaudi infection, suggesting that it could depend on VDR-mediated NF-kB activation [104]. Finally, a very recent study has assayed 109 patients affected by malaria for VDR single nucleotide polymorphism (SNP rs 731236), finding no association between SNP rs 731236 and both infection susceptibility and disease severity [105].
3. Conclusions
There are few studies on the association between Vitamin D and malaria producing disagreeing results. Although the pathogenic mechanisms by which immunomodulation by Vitamin D can influence the onset and progression of malaria have been suggested, it can not be stated that low 25(OH)D circulating levels contribute to the pathogenesis of the disease. Generally speaking, there is not clear evidence on the preventive and/or therapeutic efficacy of Vitamin D against malaria. RCTs are needed in order to understand whether Vitamin D administration might play a role in preventing or in treating malaria.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Wang T.T., Tavera-Mendoza L.E., Laparriere D. Large-scale in silico and microarray-based identification of direct 1, 25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 2.Sharif K., Sharif Y., Watad A. Vitamin D, autoimmunity and recurrent pregnancy loss: more than an association. Am. J. Reprod. Immunol. 2018;80(3) doi: 10.1111/aji.12991. [DOI] [PubMed] [Google Scholar]
- 3.Ali A., Cui X., Eyles D. Developmental vitamin D deficiency and autism: putative pathogenic mechanisms. J. Steroid Biochem. Mol. Biol. 2018;175:108–118. doi: 10.1016/j.jsbmb.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Sudfeld C.R., Manji K.P., Smith E.R. Vitamin D deficiency is not associated with growth or the incidence of common morbidities among Tanzanian infants. J. Pediatr. Gastroenterol. Nutr. 2017;65(4):467–474. doi: 10.1097/MPG.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto K., Iwagami M., Seki T. Dual antiplasmodial activity of vitamin D3 and its analog, 22-oxacalcitriol, by direct and indirect mechanisms. Parasitrol. Int. 2017;66(2):89–99. doi: 10.1016/j.parint.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization, World Malaria Report 2017, http://www.who.int/malaria/media/world-malaria-report-2017/en/. (date when the reference was last accessed: 30/11/2018).
- 7.Dwivedi H., Singh S.K., Chauhan B.S. Potential cerebral malaria therapy: intramuscular arteether and vitamin D co-administration. Parasitology. 2016;143(12):1557–1568. doi: 10.1017/S0031182016001207. [DOI] [PubMed] [Google Scholar]
- 8.Blayneh kw, Mojammed-Awel J. Insecticide-resistant mosquitoes and malaria control. Math. Biosci. 2014;252:14–26. doi: 10.1016/j.mbs.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Wu B., Du Y., Feng Y. Oral administration of vitamin D and importance in prevention of cerebral malaria. Int. Immunopharmacol. 2018;64:356–363. doi: 10.1016/j.intimp.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Hewison M., Adams J.S. Extrarenal 1α-hydroxylase. In: Feldman D., Pike J.W., Adams J.S., editors. Vitamin D. third ed. Academic Press; San Diego, CA: 2011. pp. 777–804. [Google Scholar]
- 11.Gil A., Plaza-Diaz J., Mesa A.D. Vitamin D: classic and novel action. Ann. Nutr. Metab. 2018;72:87–95. doi: 10.1159/000486536. [DOI] [PubMed] [Google Scholar]
- 12.Gezen-Ak D., Atasoy I.L., Candaş E. Vitamin D receptor regulates amyloid beta 1-42 production with protein disulfide isomerase A3. ACS Chem. Neurosci. 2017;8(10):2335–2346. doi: 10.1021/acschemneuro.7b00245. [DOI] [PubMed] [Google Scholar]
- 13.Cui X., Gooch H., Petty A. Vitamin D and the brain: genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017:131–143. doi: 10.1016/j.mce.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Bivona G., Agnello L., Ciaccio M. The immunological implication of the new vitamin D metabolism. Centr. Eur. J. Immunol. 2018;43(3):331–334. doi: 10.5114/ceji.2018.80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landel V., Stephan D., Cui X. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J. Steroid Biochem. Mol. Biol. 2018;177:129–134. doi: 10.1016/j.jsbmb.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z., Li W. The role of vitamin D and its analogs in inflammatory diseases. Curr. Top. Med. Chem. 2016;16(11):1242–1261. doi: 10.2174/1568026615666150915111557. [DOI] [PubMed] [Google Scholar]
- 17.Trinko J.R., Land B.B., Solecki B.W. Vitamin D3: a role in dopamine circuit regulation, diet-induced obesity, and drug consumption. eNeuro. 2016;3(2) doi: 10.1523/ENEURO.0122-15.2016. pii: ENEURO.0122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trochoutsou A.I., Kloukina V., Samitas K. Vitamin D in the immune system: genomic and non-genomic actions. Mini Rev. Med. Chem. 2015;15(11):953–963. doi: 10.2174/1389557515666150519110830. [DOI] [PubMed] [Google Scholar]
- 19.Autier P., Mullie P., Macacu A. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet. 2017;5(12):986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- 20.Miller J.W., Harvey D.J., Beckett L.A. Vitamin D status and rates of cognitive decline in a multi-ethnic cohort of older adults. JAMA Neurol. 2015;72(11):1295–1303. doi: 10.1001/jamaneurol.2015.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti R., Morelli M.E., Caruso P. Vitamin D in neurological diseases: a rational for a pathogenic impact. Int. J. Mol. Sci. 2018;19:2245. doi: 10.3390/ijms19082245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnello L., Scazzone C., Lo Sasso B. VDBP, 1α-Hydroxylase, and 25-hydroxyvitamin D gene polymorphism analyses in a group of Sicilian multiple sclerosis patients. Biochem. Genet. 2017;55(2):183–192. doi: 10.1007/s10528-016-9783-4. [DOI] [PubMed] [Google Scholar]
- 23.Mak A. The impact of vitamin D on the immunopathophysiology, disease activity, and extra-muskuloskeletal manifestations of systemic erithematosus lupus. Int. J. Mol. Sci. 2018;19(8) doi: 10.3390/ijms19082355. Pii:E2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bivona G., Agnello L., Pivetti A. Association between hypovitaminosis D and systemic sclerosis: true or fake? Clin. Chim. Acta. 2016;458:115–119. doi: 10.1016/j.cca.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Scazzone C., Agnello L., Ragonese P. Association of CYP2R1 rs10766197 with MS risk and disease progression. J. Neurosci. Res. 2018;96(2):297–304. doi: 10.1002/jnr.24133. [DOI] [PubMed] [Google Scholar]
- 26.Kim D. The role of vitamin D in thyroid diseases. Int. J. Mol. Sci. 2017;18(9) doi: 10.3390/ijms18091949. Pii:E1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martineau A.R., Jolliffe D.A., Hooper R.L. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urashima M., Segawa T., Okazaki M. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Zhang S., Lin F. Elevated galectin-3 levels in the serum of patients with Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 2015;30(8):729–732. doi: 10.1177/1533317513495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q., Fan Y., Mu L.Y., Ma L. S100B and ADMA in cerebral small vessel disease and cognitive dysfunction. J. Neurol. Sci. 2015;354(1-2):27–32. doi: 10.1016/j.jns.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Guo L.H., Alexopoulos P., Perneczky R. Heart-type fatty acid binding protein and vascular endothelial growth factor: cerebrospinal fluid biomarker candidates for Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(7):553–560. doi: 10.1007/s00406-013-0405-4. [DOI] [PubMed] [Google Scholar]
- 32.Agnello L., Bivona G., Lo Sasso B. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017;50(13-14):797–803. doi: 10.1016/j.clinbiochem.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Zinellu A., Sotgia S., Porcu P. Carotid restenosis is associated with plasma ADMA concentrations in carotid endarterectomy patients. Clin. Chem. Lab. Med. 2011;49(5):897–901. doi: 10.1515/CCLM.2011.121. [DOI] [PubMed] [Google Scholar]
- 34.Agnello L., Bivona G., Novo G. Heart-type fatty acid binding protein is a sensitive biomarker for early AMI detection in troponin negative patients: a pilot study. Scand. J. Clin. Lab. Invest. 2017;77(6):428–432. doi: 10.1080/00365513.2017.1335880. [DOI] [PubMed] [Google Scholar]
- 35.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 36.Bivona G., Agnello L., Ciaccio M. Vitamin D and immunomodulation: is it time to change the reference value? Ann. Clin. Lab. Sci. 2017;47(4):508–510. [PubMed] [Google Scholar]
- 37.Rahman A., Al-Tiar A., Shaban L. Plasma 25-hydroxy vitamin D is not associated with either cognitive function or academic performance in adolescents. Nutrients. 2018;10(9) doi: 10.3390/nu10091197. pii: E1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoel D.G., Berwick M., de Gruijl F.R. The risk and benefit of sun exposure. Derm. Endocrinol. 2016;8:e1248325. doi: 10.1080/19381980.2016.1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 2018;98(1):477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlov V.A., Tracey K.J. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 2017;20(2):156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 41.Kalinec G.M., Lomberck G., Urrutia R.A. Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise- and age-related hearing loss. Front. Cell. Neurosci. 2017;11:192. doi: 10.3389/fncel.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansdottir S., Monick M.M., Hinde S.L. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P.T., Stenger S., Li H. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 44.Schauber J., Dorschner R.A., Coda A.B. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toniato E., Spinas E., Saggini A. Immunomodulatory effects of vitamin D on skin inflammation. J. Biol. Regul. Homeost. 2015;29(3):563–567. [PubMed] [Google Scholar]
- 46.Enioutina E.Y., Bareyan D., Daynes R.A. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J. Immunol. 2009;182(7):4296–4305. doi: 10.4049/jimmunol.0804344. [DOI] [PubMed] [Google Scholar]
- 47.Voisine C., Matselic B., Sponaas A.M. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int. J. Parasitol. 2010;40(6):711–719. doi: 10.1016/j.ijpara.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 48.He X., Yan J., Zhu X. Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. J. Immunol. 2014 1;193(3):1314–1323. doi: 10.4049/jimmunol.1400089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sassi F., Tamone C., D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11) doi: 10.3390/nu10111656. pii: E1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoenfeld Y., Giacomelli R., Azrielant S. Vitamin D and systemic lupus erythematosus – the hype and the hope. Autoimmun. Rev. 2018;17(1):19–23. doi: 10.1016/j.autrev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar S., Hewison M., Studzinski G.P. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit. Rev. Clin. Lab. Sci. 2016;53(2):132–145. doi: 10.3109/10408363.2015.1094443. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes de Abreu D.A., Eyles D., Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34(Suppl. 1):S265–S277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 53.Hewison M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011;7(6):337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 54.Stach K., Kälsch A.I., Nguyen X.D. 1α,25-dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology. 2011;118(2):107–115. doi: 10.1159/000327547. [DOI] [PubMed] [Google Scholar]
- 55.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller S., Kapper B. Vitamin and cofactor biosynthesis pathways in plasmodium and other apicomplexan parasites. Trends Parasitol. 2007;23(3):112–121. doi: 10.1016/j.pt.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luong K.V., Nguyen L.T. The role of vitamin D in malaria. J. Infect. Dev. Ctries. 2015;9(1):8–19. doi: 10.3855/jidc.3687. [DOI] [PubMed] [Google Scholar]
- 58.Parra M., Liu X., Derrick S.C. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0066115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spellberg B., Edwards J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001;32(1):76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 60.Schofield L. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol. Cell Biol. 2007;85(2):130–137. doi: 10.1038/sj.icb.7100040. [DOI] [PubMed] [Google Scholar]
- 61.Kim J.H., Park J.S., Cho Y.J. Low serum 25-hydroxyvitamin D level: an independent risk factor for tuberculosis? Clin. Nutr. 2014;33(6):1081–1086. doi: 10.1016/j.clnu.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Lim J.H., Ravikumar S., Wang Y.M. Bimodal influence of vitamin D in host response to systemic Candida infection-vitamin D dose matters. J. Infect. Dis. 2015;212(4):635–644. doi: 10.1093/infdis/jiv033. [DOI] [PubMed] [Google Scholar]
- 63.Toko E.N., Sumba O.P., Daud Maternal vitamin D status and adverse birth outcomes in children from rural western Kenya. Nutrients. 2016;8(12) doi: 10.3390/nu8120794. pii: E794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Haan K., Groeneveld A.B.J., de Geus H.R.H. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit. Care. 2014;18(6):660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brance M.L., Miljevic J.N., Tizziani R. Serum 25-hydroxyvitamin D levels in hospitalized adults with community-acquired pneumonia. Clin. Respir. J. 2018;12(7):2220–2227. doi: 10.1111/crj.12792. [DOI] [PubMed] [Google Scholar]
- 66.Giulia B., Luisa A., Concetta S. Procalcitonin and community-acquired pneumonia (CAP) in children. Clin. Chim. Acta. 2015;451(Pt B):215–218. doi: 10.1016/j.cca.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 67.Berry D.J., Hesketh K., Power C. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011;106(9):1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 68.Ginde A.A., Mansbach J.M., Camargo C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination survey. Arch. Intern. Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sismanlar T., Aslan A.T., Gulbahar O. The effect of vitamin D on lower respiratory tract infections in children. Turk. Pediatr. Ars. 2016;51(2):94–99. doi: 10.5152/TurkPediatriArs.2016.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergman P., Lindh A.U., Björkhem-Bergman L. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das R.R., Singh M., Naik S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst. Rev. 2018;7:CD011597. doi: 10.1002/14651858.CD011597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aglipay M., Birken C., Parkin P.C. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grant W.B., Boucher B.J., Bhattoa H.P. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018;177:266–269. doi: 10.1016/j.jsbmb.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Ho-Pham L.T., Nguyen N.D., Nguyen T.T. Association between vitamin D insufficiency and tuberculosis in a Vietnamese population. BMC Infect. Dis. 2010;10:306. doi: 10.1186/1471-2334-10-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nnoaham K.E., Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int. J. Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 76.Hong J.Y., Kim S.Y., Chung K.S. Association between vitamin D deficiency and tuberculosis in a Korean population. Int. J. Tuberc. Lung Dis. 2014;18(1):73–78. doi: 10.5588/ijtld.13.0536. [DOI] [PubMed] [Google Scholar]
- 77.Davies P.D., Martineau A.R. Vitamin D and tuberculosis: more effective in prevention than treatment? Int. J. Tuberc. Lung Dis. 2004;8(6):737–742. doi: 10.5588/ijtld.15.0506. [DOI] [PubMed] [Google Scholar]
- 78.Zeng J., Wu G., Yang W. A serum vitamin D level <25 nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One. 2015;10(5):e0126014. doi: 10.1371/journal.pone.0126014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daley P., Jagannathan V., John K.R. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015;15(5):528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 80.Ralph A.P., Waramori G., Pontororing G.J. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martineau A.R., Timms P.M., Bothamley G.H. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wejse C., Gomes V.F., Rabna P. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 83.Coelho L., Cardoso Sw, Luz P.M. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutr. J. 2015;14:81. doi: 10.1186/s12937-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haug C., Muller F., Aukrust P. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J. Infect. Dis. 1994;169(4):889–893. doi: 10.1093/infdis/169.4.889. [DOI] [PubMed] [Google Scholar]
- 85.Hileman C.O., Overton E.T., McComsey G.A. Vitamin D and bone loss in HIV. Curr. Opin. HIV AIDS. 2016;11(3):277–284. doi: 10.1097/COH.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosio E., Lossius A., Abdelmagid N. Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein-Barr virus in relapsing-remitting multiple sclerosis. Mult. Scler. 2017;23(3):395–402. doi: 10.1177/1352458516654310. [DOI] [PubMed] [Google Scholar]
- 87.Ferreira D., Canale D., Volpini R.A. Proceedings of the ASN 2016-American Society of Nephrology Kidney Week, Chicago, IL, USA. 15–20 November 2016. Vitamin D deficiency induces acute kidney injury un rats treated with lipid formulation of amphotericin B; p. 670. [Google Scholar]
- 88.Upala S., Sanguankeo A., Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15:84. doi: 10.1186/s12871-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amrein K., Papinutti A., Mathew E. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr. Connect. 2018;7(12):R304–R315. doi: 10.1530/EC-18-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sautet J., Vuillet J., Arnaud G. Effects of the immediate adjunction of cod liver oil or vitamin D and calcium biphosphate to antimalarial drugs used in the treatment of Plasmodium berghei infections. II. Bull. Soc. Pathol. Exot. Filiales. 1957;50(1):44–49. [PubMed] [Google Scholar]
- 91.IuIu Sergacheva, Sokhanenkova T.L., Soprunov F.F. Effect of vitamins D and E on the development of Plasmodium berghei infection in mice. Med. Parazitol. (Mosk) 1986;4:15–18. [PubMed] [Google Scholar]
- 92.Vial H.J., Thuet M.J., Philippot J.R. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D-3 derivatives. Mol. Biochem. Parasitol. 1982;5(3):189–198. doi: 10.1016/0166-6851(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 93.Ray S., Kamath K., Srivastava R. Serum proteome analysis of vivax malaria: an insight into the disease pathogenesis and host immune response. J. Proteomics. 2012;75(10):3063–3080. doi: 10.1016/j.jprot.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 94.Yakoob M.Y., Salam R.A., Khan F.R. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst. Rev. 2016;9:11. doi: 10.1002/14651858.CD008824.pub2. CD008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weisberg M., Vickers B.K., Yager S.B. Testing in mice the hypothesis that melanin is protective in malaria infections. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newens K., Filteau S., Tomkins A. Plasma 25-hydroxyvitamin D does not vary over the course of a malarial infection. Trans. R. Soc. Trop. Med. Hyg. 2006;100(1):41–44. doi: 10.1016/j.trstmh.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 97.Sudfeld C.R., Giovannucci E.L., Isanaka S. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J. Infect. Dis. 2013;207(3):378–385. doi: 10.1093/infdis/jis693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sudfeld C.R., Duggan C., Aboud S. Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV-infected and HIV-exposed Tanzanian infants. J. Nutr. 2015;145(1):121–127. doi: 10.3945/jn.114.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization . World Health Organization; Geneva: 2004. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Tratment Guidelines for a Public Health Approach. [Google Scholar]
- 100.Cusik S.E., Opoka R.O., Lund T.C. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PLoS One. 2014;9(12):e113185. doi: 10.1371/journal.pone.0113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacKeown J.M., Cleaton-Jones P.E., Edwards A.W. Energy, macro- and micronutrient intake of 5-year-old urban black South African children in 1984 and 1995. Paediatr. Perinat. Epidemiol. 1998;12(3):297–312. doi: 10.1046/j.1365-3016.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 102.Ould Ahmedou Salem M.S., Basco L.K., Ouldabdallahi M. Malaria-associated morbidity during the rainy season in Saharan and Sahelian zones in Mauritania. Acta Trop. 2015;152:1–7. doi: 10.1016/j.actatropica.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Bojang A., Jafali J., Egere U.E. Seasonality of pneumococcal nasopharyngeal carriage in rural gambia determined within the context of a cluster randomized pneumococcal vaccine trial. PLoS One. 2015;10(7):e0129649. doi: 10.1371/journal.pone.0129649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mubaraki M.A., Dkhil M.A., Hafiz T.A. Vitamin D receptor regulates intestinal inflammatory response in mice infected with blood stage malaria. Microb. Pathog. 2018;117:299–303. doi: 10.1016/j.micpath.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 105.Ojurongbe O., Funwei R.I., Snyder T.J. Genetic variants of tumor necrosis factor-α -308G/A (rs1800629) but not Toll-interacting proteins or vitamin D receptor genes enhances susceptibility and severity of malaria infection. Immunogenetics. 2018;70(2):135–140. doi: 10.1007/s00251-017-1032-4. [DOI] [PubMed] [Google Scholar]