Abstract
Background
Helicobacter pylori (H pylori) is one of the most common pathogens to establish and cause infection in human beings, affecting about 50% of the world's population. Prevalence may be as high as 83% in Latin American countries and as low as 17% in North America. Approximately 20% of infected people will manifest disease; people at high risk include those who live in low‐ and middle‐income countries with poor sanitary conditions, since the mechanism of transmission seems to be oral‐oral or faecal‐oral (mostly during infancy). There are several antibiotic regimens to treat the infection, but antibiotic resistance is growing around the world. New adjuvant drugs — such as probiotics, statins, curcumin, and N‐acetylcysteine (NAC) — are being tested to enhance eradication rates.
N‐acetylcysteine can destabilise the biofilm structure; it also has synergic action with antibiotics, and bactericidal effects. In addition, NAC has antioxidant properties, and has a primary mucolytic effect by reducing the thickness of the gastric mucus layer, both of which may exert beneficial adjuvant effects on H pylori eradication.
Objectives
To assess the efficacy and safety of N‐acetylcysteine as an adjuvant therapy to antibiotics for Helicobacter pylori eradication.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966 to April 2018), Embase (1988 to April 2018), CINAHL (1982 to April 2018), LILACS (1982 to April 2018), grey literature databases and trials registries. We handsearched the reference lists of relevant studies. We screened 726 articles and assessed 18 for eligibility.
Selection criteria
We included randomised controlled trials (RCTs) of any antibiotic regimen plus NAC, in adults infected with H pylori. To be included, trials had to use a control consisting of the same antibiotic regimen with or without placebo. Outcomes of interest were eradication rates, and gastrointestinal, toxic, and allergic adverse events. Reporting of the primary outcomes listed here was not an inclusion criterion for the review.
Data collection and analysis
Two review authors independently reviewed and extracted data and completed the 'Risk of bias' assessments. A third review author independently confirmed the 'Risk of bias' assessments. We used Review Manager 5 software for data analysis. We contacted study authors if there was missing information.
Main results
We included eight RCTs (with a total of 559 participants) in this review. The studies recruited outpatients aged between 17 and 76 years who were referred to endoscopy centres in several different countries. The certainty of evidence was reduced for most outcomes due to the poor methodological quality of included studies; issues mainly related to the generation of allocation sequence, allocation concealment, and blinding (this last domain related specifically to adverse outcomes).
We are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates, compared with the addition of placebo or no NAC (38.8% versus 49.1%, risk ratio (RR) 0.74, 95% confidence interval (CI) 0.51 to 1.08; participants = 559; studies = eight; very low‐certainty evidence). A post‐hoc sensitivity analysis, in which we removed studies that tested antibiotic regimens no longer recommended in clinical practice, showed that the addition of NAC may improve eradication rates compared to control (27.2% versus 37.6%, RR 0.71, 95% CI 0.53 to 0.94; participants = 397; published studies = five).
We are uncertain whether NAC is associated with a higher risk of gastrointestinal adverse events compared to control (23.9% versus 18.9%, RR 1.25, 95% CI 0.85 to 1.85; participants = 336; studies = five; very low‐certaintyevidence), or allergic adverse events (2% versus 0%, RR 2.98, 95% CI 0.32 to 27.74; participants = 336; studies = five; very low‐certainty evidence). There were no reports of toxic adverse events amongst included studies.
Authors' conclusions
We are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates compared with the addition of placebo or no NAC. Due to the clinical, statistical and methodological heterogeneity found in included studies, and the uncertainty observed when analysing therapy subgroups, any possible beneficial effect of NAC should be regarded cautiously.
We are uncertain whether NAC is associated with a higher risk of gastrointestinal or allergic adverse events compared with placebo or no NAC. There were no reports of toxic adverse events amongst the included studies.
Further large, well‐designed, randomised clinical studies should be conducted, with good reporting standards and appropriate collection of efficacy and safety outcomes, especially for current recommended antibiotic regimens.
Plain language summary
N‐acetylcysteine taken with antibiotics for treatment of Helicobacter pylori infection
Review question
Is the addition of N‐acetylcysteine to antibiotics safe and does it improve cure rates for Helicobacter pylori infection?
Background
Helicobacter pylori (H pylori) is a bacteria that lives in the stomach and might cause several diseases such as gastric cancer, ulcer disease, and others. Colonisation occurs in about one‐half of the world's population and is more common in countries with poor sanitary conditions. People become infected by consuming contaminated water.
The infection is treated using antibiotics and a drug which reduces acid production in the stomach. However, rates of antibiotic resistance are rising around the world, which is reducing the cure rates even with treatment. New medications are being tested to improve cure rates. One of these medications is N‐acetylcysteine (NAC). NAC is a drug that helps to dissolve mucus in respiratory diseases. It can be taken by mouth (orally) or injected into a vein (intravenous). NAC can destroy some mechanisms of survival of H pylori and could improve cure rates.
Study characteristics
We included eight studies (specifically, randomised controlled trials (RCTs)) with a total of 559 people aged between 17 and 76 years old. The evidence is current to April 2018. All studies recruited outpatients from endoscopy centres (centres that specialise in an examination done with a flexible tube with a camera that is inserted into stomach) in several countries. The antibiotic combinations tested were very different in the included studies, as were the doses of NAC (600 mg to 1800 mg per day). NAC was compared with placebo (dummy pill) or nothing.
Key results
We are uncertain whether the addition of NAC to antibiotics improves H pylori cure rates compared with the addition of placebo or no NAC. Any possible beneficial effect of NAC should be regarded cautiously because the included studies were very different and of low certainty, with some flaws that could have compromised their results and consequently, the results of this review.
We are uncertain whether NAC is associated with a higher risk of gastrointestinal or allergic adverse events compared with placebo or no NAC. There were no reports of toxic adverse events amongst the included studies.
Further large, well‐designed randomised clinical studies, with good reporting standards and appropriate collection of effectiveness and safety outcomes should be done, especially for current recommended antibiotic combinations.
Quality of the evidence
The overall certainty of the evidence for eradication rates ranged from very low to low. Five studies provided information on adverse events (side effects), and the certainy of evidence was very low. The included studies were poorly conducted and this reduced our confidence in the results.
Summary of findings
Summary of findings for the main comparison. Any antibiotic regimen plus N‐acetylcysteine compared to the same regimen without N‐acetylcysteine (with or without placebo) for Helicobacter pylori infection.
| Any antibiotic regimen plus NAC compared to the same regimen without NAC (with or without placebo) for H pylori infection | ||||||
| Patient or population: people withH pylori infection Setting: outpatients Intervention: any antibiotic regimen plus NAC Comparison: the same antibiotic regimen (with or without placebo) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with any antibiotic regimen without NAC | Risk with any antibiotic regimen plus NAC | |||||
|
Eradication rate Follow‐up: 38 to 175 days |
Study population | RR 0.74 (0.51 to 1.08) | 559 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW1 2 3 | ||
| 491 per 1000 | 363 per 1000 (250 to 530) | |||||
| Triple therapies | ||||||
| 483 per 1000 | 300 per 1000 (203 to 440) |
RR 0.62 (0.42 to 0.91) |
177 (3 RCTs) |
|||
| Dual therapies | ||||||
| 702 per 1000 | 435 per 1000 (295 to 632) |
RR 0.62 (0.42 to 0.90) |
92 (2 RCTs) |
|||
| Sequential therapy | ||||||
| 420 per 1000 | 328 per 1000 (193 to 546) |
RR 0.78 (0.46 to 1.30) |
99 (1 RCT) |
|||
| Bismuth quadruple therapy | ||||||
| 183 per 1000 | 180 per 1000 (84 to 383) |
RR 0.98 (0.46 to 2.09) |
121 (1 RCT) |
|||
| No PPI, antibiotic monotherapy | ||||||
| 400 per 1000 | 456 per 1000 (264 to 788) |
RR 1.14 (0.66 to 1.97) |
70 (1 RCT) |
|||
|
Gastrointestinal adverse events Follow‐up: 38 to 175 days |
Study population | RR 1.25 (0.85 to 1.85) | 336 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW3 4 | ||
| 189 per 1000 | 237 per 1000 (161 to 350) | |||||
| Triple therapies | ||||||
| 300 per 1000 | 399 per 1000 (171 to 942) |
RR 1.33 (0.57 to 3.14) |
40 (1RCT) |
|||
| Dual therapies | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 92 (2 RCTs) |
|||
| Sequential therapy | ||||||
| 262 per 1000 | 267 per 1000 (131 to 550) |
RR 1.02 (0.50 to 2.10) |
83 (1 RCT) |
|||
| Bismuth quadruple therapy | ||||||
| 250 per 1000 | 345 per 1000 (198 to 603) |
RR 1.38 (0.79 to 2.41) |
121 (1 RCT) |
|||
| No PPI, antibiotic monotherapy | ||||||
| Not measured | ||||||
|
Allergic adverse events Follow‐up: 38 to 175 days |
Study population | RR 2.98 (0.32 to 27.74) | 336 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW3 4 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Triple therapies | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 40 (1 RCT) |
|||
| Dual therapies | ||||||
| 0 per 1000 | 0 per 1000 | RR 3.00 (0.13 to 68.84) |
92 (2 RCTs) |
|||
| Sequential therapy | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 83 (1 RCT) |
No events in either group | ||
| Bismuth quadruple therapy | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) |
RR 2.95 (0.12 to 71.05) |
121 (1RCT) |
|||
| No PPI, antibiotic monotherapy | ||||||
| Not measured | ||||||
|
Toxic adverse events Follow‐up: 38 to 175 days |
Study population | not estimable | 336 (5 RCTs) | ⊕⊕⊝⊝ LOW3 | No events in either group | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Triple therapies | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 40 (1 RCT) |
|||
| Dual Therapies | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 92 (2 RCTs) |
|||
| Sequential therapy | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 83 (1 RCT) |
|||
| Bismuth quadruple therapy | ||||||
| 0 per 1000 | 0 per 1000 | not estimable | 121 (1 RCT) |
|||
| No PPI, antibiotic monotherapy | ||||||
| Not measured | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; PPI: proton pump inhibitor; NAC: N‐acetylcysteine; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded by one level due to risk of bias (lack of information on sequence generation and allocation concealment).
2 We downgraded by one level due to imprecision (the confidence interval includes both null effect and appreciable benefit (RR 0.75)).
3 We downgraded by two levels due to inconsistency (large variation of effect, confidence intervals do not overlap, I2 > 50% and P < 0.05).
4 We downgraded by two levels due to risk of bias (lack of blinding of participants, personnel, and outcome assessors).
5 We downgraded by two levels due to imprecision (wide confidence interval that includes appreciable harm (RR 1.25)).
Background
Description of the condition
Since 1875, several scientists have observed that gastric tissue specimens and gastric aspirates contained bacteria (Blaser 2005). However, it was only in 1982 that two Australian physicians isolated a gram‐negative microaerophilic spiral bacterium from gastric specimens which appeared initially to be of the Campylobacter genus (Marshall 1984), and that were supposed to be associated with gastritis. Some years later, observations of the bacterial structure demonstrated that they did not fulfil some characteristics of the Campylobacter genus. A new species was created, entitled Helicobacter, and later the micro‐organism was named H pylori.
H pylori is one of the most successful pathogens to establish and cause infection in human beings, after only Streptococcus mutans (S mutans) (Cammarota 2012). Colonisation occurs in 50% of the adult population (Hunt 2011), with a wide variation in prevalence according to the region studied. Prevalence may be as high as 83% in Latin American countries and as low as 17% in North America (Calvet 2013). Approximately 20% of infected people will manifest disease (Venerito 2013). Those at high risk include people who live in low‐ and middle‐income countries with poor sanitary conditions, since the mechanism of transmission seems to be oral‐oral or faecal‐oral, mostly during infancy (Dunn 1997). Strains of bacteria are present in faeces, saliva, gastric mucosa, and dental plaque, reinforcing the hypothesis of these routes of transmission (Momtaz 2012). A positive result in the urea breath test, serology, stool antigen test, endoscopic biopsies with rapid urease reaction, histology or culture, confirms the diagnosis of H pylori infection.
The micro‐organism acts by disrupting the mucosal layer of the stomach, adhering to epithelial cells and leading to chronic inflammation of gastric mucosa. Persistent inflammation leads to chronic gastritis, atrophy, intestinal metaplasia, dysplasia, and neoplasm (Correa 1992). Important virulence factors of H pylori contribute to successful colonisation and infection. These include:
urease synthesis (to inactivate toxicity by gastric urea and to resist in acidic milieu);
flagella, lipases, and proteases (to penetrate the intimacy of mucus);
adhesins (to attach to epithelial cells and allow interaction);
effector toxins (including cytotoxin‐associated gene A (CagA), vacuolating cytotoxin gene A (VacA), and others, which induce changes in epithelium cytoskeleton and secretion of interleukin 8 (IL‐8)) (Kao 2016).
Current data show that H pylori infection is associated with a range of gastric diseases (most as a result of chronic inflammation), including atrophic gastritis, mucosal‐associated lymphoid tissue (MALT) lymphoma, peptic ulcer disease, gastric cancer, and functional dyspepsia (Kuipers 1997; Malfertheiner 2009; Zhao 2014). Some non‐gastric conditions are also associated with H pylori infection, such as idiopathic thrombocytopenic purpura (Stasi 2009), and idiopathic iron deficiency anaemia (Chaabane 2011).
See Appendix 1 for a glossary of terms.
Description of the intervention
Several regimens of antibiotic treatment are available for H pylori eradication. Most of them are associated with a proton pump inhibitor (PPI) to enhance bioavailability and chemical properties of antibiotics and raise cure rates. Since the 1990s PPI‐clarithromycin triple therapy has become the first‐line treatment of H pylori infection. This regimen consists of a PPI plus clarithromycin plus amoxicillin or metronidazole. Only one decade later, the efficacy of such therapy declined, with clarithromycin resistance emerging as the most important cause. Other explanations for the decrease in eradication rates of PPI‐clarithromycin triple therapy are compliance, type of strains, high gastric acidity and high bacterial load. Eradication rates dropped from about 80% in the early 1990s to less than 70% a decade later (Graham 2010).
In 2012, the European Helicobacter Study Group (EHSG) published the Maastricht IV/Florence Consensus Report (EHSG 2012), proposing an approach based on local clarithromycin resistance patterns, assuming a threshold of 15% to 20% to separate regions with high and low clarithromycin resistance. Other antibiotic regimens using combinations of metronidazole, fluoroquinolones, tetracycline, and bismuth exhibit the same concerns about increasing resistance rates. If eradication was unsuccessful after an initial therapy (so‐called 'first‐line therapy'), people would need a second treatment with a different regimen. If this second‐line treatment failed, the third regimen would need to be guided by culture and antibiogram (Malfertheiner 2012).
New approaches are being tested to enhance H pylori eradication rates, such as probiotics, statins, curcumin, and N‐acetylcysteine (NAC).
NAC is a component of the amino acid L‐cysteine and is available in intravenous or oral preparations. After oral ingestion, it is almost entirely absorbed and metabolised by the small intestine and liver. Only a small concentration of intact NAC reaches the plasma and tissues (De Caro 1989). Peak plasma levels are observed after less than one hour; half‐life is about two hours and it is not detectable in plasma after 10 to 12 hours (Borgstrom 1986). NAC may be administered orally or intravenously, and it seems to have a good safety profile at dosages of 1200 mg twice daily or lower. Severe adverse effects are rare and include gastrointestinal, cutaneous, and allergic effects (Kelly 1998; Millea 2009).
N‐acetylcysteine metabolites stimulate glutathione synthesis, promoting detoxification and acting mainly as free oxygen radical scavengers. NAC promotes the cleavage of disulfide bonds of mucus glycoproteins, reducing viscosity and thickness of mucus, including the gastric mucus layer. Due to these properties, NAC has been used in several clinical situations, such as chronic obstructive pulmonary disease, influenza, idiopathic pulmonary fibrosis, polycystic ovary syndrome, prevention of contrast‐induced nephropathy, acetaminophen overdose, cancer, heart disease, and heavy metal toxicity (Kelly 1998; Millea 2009; Sherwood 2002).
N‐acetylcysteine was first proposed as an adjuvant therapy for H pylori in a study by Zala and colleagues (Zala 1994); it demonstrated improved eradication rates in people randomised to receive antibiotics and NAC, compared with a control group that received only antibiotics. Following on from this, other studies demonstrated conflicting results. Some reported better eradication rates when NAC was associated with antibiotics (Cammarota 2010; Gurbuz 2005; Hamidian 2015), and others reported no beneficial effect on eradication rates (Emami 2014; Hansen 1994; Karbasi 2013; Yoon 2015).
How the intervention might work
Biofilms are complex biological systems produced by various species of bacteria. H pylori produce an extracellular polymeric matrix (polysaccharides, DNA, proteins and lipids) with water channels as a strategy to overcome environmental stress and protect itself. Colonies of H pylori can live embedded in biofilms in two primary forms: spiral or coccoid. Spiral forms are cultivable and virulent, while coccoid forms are viable but non‐cultivable, latent, and more resistant to adverse environmental conditions and antibiotics (Cammarota 2012).
The biofilm complex allows micro‐organisms to adhere to surfaces and proliferate under adverse conditions, and also to cause refractory clinical infections (Hall‐Stoodley 2009). Current data show that biofilms are responsible for about 80% of chronic infections. Bacteria in biofilms are 1000 times more resistant to antibiotics and human defences than free‐living ones. Biofilms can worsen resistance rates to antimicrobials by retarding antibiotic diffusion, allowing expression of gene resistance, having chemical properties that impair the effect of some antibiotics, producing beta‐lactamases, decreasing the bacterial growth ratio (target of some antibiotics), and producing reactive oxygen species (Cammarota 2012; Garcia 2014).
N‐acetylcysteine can destabilise the biofilm structure, act synergistically with antibiotics, and has bactericidal effects (Aslam 2007; Aslam 2011). Also, NAC has a primary mucolytic effect by reducing the thickness of the gastric mucus layer and has antioxidant properties, both of which may exert beneficial adjuvant effects on H pylori eradication.
Helicobacter is capable of living as free micro‐organisms in gastric mucus, but more frequently they colonise and form biofilm ecosystems. This was demonstrated in vivo (Carron 2006; Coticchia 2006), and in vitro (Yonezawa 2010).
A study by Gurbuz and colleagues showed positive results for antibiotics plus NAC, compared with placebo plus antibiotics (Gurbuz 2005). An open‐label randomised study of 40 people infected with H pylori showed that pretreatment with NAC plus antibiotics was effective when compared with antibiotics only (Cammarota 2010). In Hamidian 2015, another randomised, placebo‐controlled study, it was reported that infection was eradicated in 72.9% of people in the experimental group (NAC plus antibiotic) and in 60.9% in the control group (antibiotics alone). However, there are some studies which reported an additive effect on eradication rates with the use of NAC, although no statistical significance was detected (Karbasi 2013; Yoon 2015). In Hansen 1994, a double‐blind study, no difference was observed in eradication rates using NAC plus antibiotics. One open‐label study showed similar eradication rates comparing NAC plus quadruple therapy (bismuth, amoxicillin, clarithromycin, and omeprazole) with the same quadruple therapy alone (Emami 2014).
We hypothesise that treatment failure may occur because of biofilm properties and resistant strains. NAC may have a role in first‐line and rescue therapies because of its properties in biofilm formation. This Cochrane Review intends to examine if NAC, used as an adjuvant to antibiotics, has a benefit in H pylori eradication rates. If so, this could change current practice.
Why it is important to do this review
H pylori infection is a major problem for public health, and is one of the most common infectious diseases worldwide. This infection is associated with high morbidity and mortality, poor quality of life, and high costs to healthcare systems. Although several regimens to eradicate H pylori are available, resistance to antibiotics is rising around the world, leading to very low eradication rates (less than 80%) for an infectious disease.
Biofilm formation can be a barrier to H pylori eradication (Cammarota 2012). NAC is used as an adjuvant therapy in an attempt to enhance success in eradication rates (Ermis 2015).
The current literature shows some contradictory results for the efficacy of NAC as an adjuvant to antibiotics to eradicate H pylori. While some authors have found positive effects (Cammarota 2010; Gurbuz 2005), others have found no effect, or worsening results, when NAC is combined with antibiotics compared with antibiotics only (Hansen 1994; Karbasi 2013). We conducted this Cochrane Review to explore the uncertainty arising from conflicting results from studies in this area.
Objectives
To assess the efficacy and safety of N‐acetylcysteine (NAC) as an adjuvant therapy to antibiotics for Helicobacter pylori (H pylori) eradication.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full text, those published as abstract only, and unpublished data. We did not include cluster‐randomised, cross‐over, or quasi‐randomised controlled trials.
Types of participants
We included adults (equal or over 16 years of age) with a diagnosis of Helicobacter pylori (H pylori) infection, confirmed by urea breath test, stool antigen test, validated immunoglobulin G (IgG), serology, endoscopic biopsies with rapid urease reaction, histology, or culture. We included people in first‐, second‐ or third‐line eradication therapy.
Types of interventions
We included studies comparing any antibiotic regimen plus NAC (intervention group) with the same antibiotic regimen without NAC (control group). In the control group, we included studies with a placebo replacing the NAC, as well as studies of antibiotic regimens alone (without placebo).
We included studies in which NAC was offered by any route of administration (orally or intravenously) and with any dose of NAC, provided the same route and the same dose were used in both experimental and control groups.
We included studies with any drug, dose, or duration of antibiotic regimen, provided they were equal in both groups.
We included studies with any drug, dose, or duration of proton pump inhibitor (PPI) when they were part of the eradication therapy tested, provided they were equal in both groups.
Types of outcome measures
Primary outcomes
The primary outcomes were:
successful H pylori eradication; and
gastrointestinal adverse events (e.g. diarrhoea, nausea, vomiting, or any other reported adverse event).
H pylori eradication was defined as a negative test, at least four weeks after treatment, confirmed by urea breath test, stool antigen test, endoscopic biopsies with rapid urease reaction, histology, or culture. We excluded studies that used serology to confirm eradication and/or studies where eradication was confirmed by a test performed within four weeks of treatment, as this could lead to misleading test results (Malfertheiner 2012).
In case any study reported outcomes at more than one time point, we considered the last available follow‐up.
We planned to contact study authors if a study did not report eradication rates.
Reporting of the primary outcomes listed here was not an inclusion criterion for the review.
Secondary outcomes
Secondary outcomes were:
allergic adverse events (cutaneous rash, pruritus, or any other reported adverse event); and
toxic adverse events (hypotension, headache, anaphylactoid reactions, fever, or any other reported adverse event).
We collected reports of adverse events, regarding the number and type of events (allergic, toxic, etc.), proportions of participants, and interference in compliance. We analysed each type of adverse event separately.
Reporting of the secondary outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
We placed no restrictions on the date, language or status of publication when searching the electronic databases or other resources.
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies with no limits to the year of publication. We translated the non‐English language papers and fully assessed them for potential inclusion in the review as necessary.
We searched the following electronic databases for identifying potential studies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4) (Appendix 2).
MEDLINE (1966 to 12 April 2018) (Appendix 3).
Embase (1988 to 12 April 2018) (Appendix 4).
CINAHL (1982 to 12 April 2018) (Appendix 5).
LILACS (1982 to 12 April 2018) (Appendix 6).
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified studies and asked them to identify other published and unpublished studies. We also contacted manufacturers and experts in the field.
We searched for errata or retractions from eligible studies on PubMed and reported the date this was done within the review. We searched the grey literature databases and clinical trials registers below.
Grey literature databases
Health Management Information Consortium (HMIC) database (ovid.com/site/catalog/DataBase/99.jsp)
National Technical Information Service (NTIS) database (ntis.gov/products/ntisdb.aspx)
OpenGrey (opengrey.eu)
Clinical trials registers/trial result registers
AstraZeneca Clnical Trials
Bristol‐Myers Squibb Clinical Trial Registry
ClinicalTrials.gov
-
Current Controlled Trials metaRegister of Controlled Trials (mRCT)
active registers (controlled‐trials.com/mRCT)
archived registers (controlled‐trials.com/mrct/archived)
-
Eli Lilly and Company Clinical Trial Registry
lillytrials.com
lillytrials.com/initiated
EU Clinical Trials Register
GlaxoSmithKline Clinical Study Register
International Clinical Trials Registry Platform Search Portal
International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Clinical Trials Portal
Roche Clinical Trials Results Database
Data collection and analysis
Selection of studies
Two review authors (LESF, CSB) independently screened titles and abstracts of all the potential studies we identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and two review authors (LESF, CSB) independently screened the full text and identified studies for inclusion and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (RR). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
We used a Cochrane standard data collection form (ERC 2014) for study characteristics and outcome data, which had been piloted on at least one study in the review. Two review authors (LESF, CGZ) extracted the following study characteristics from the included studies.
General information of the study: report title, year of publication, author contacts, and publication type (abstract or full report).
Methods: aim of study, study design, unit of allocation, start date, end date, duration of participation, and ethical approval.
Participants: population description, setting, inclusion criteria, exclusion criteria, age, method of recruitment, informed consent obtained, total number randomised, baseline imbalances, withdrawals and exclusions, gender, race/ethnicity, severity of condition, comorbidities, diagnostic criteria, subgroups measured, subgroups reported, and other relevant sociodemographics.
Interventions: number randomised in each group, dose, duration of treatment period, timing, delivery, providers, cointerventions, economic information, resource requirements, integrity of delivery, and compliance.
Outcomes: primary and secondary outcomes specified and collected, time points measured and reported, outcome definition, person measuring/reporting, unit of measurement, scales, imputation of missing data, assumed risk estimates, and power.
Notes: funding for study, and notable conflicts of interest of study authors.
Two review authors (LESF, CGZ) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were reported in an unusable way. We resolved disagreements by consensus or by involving a third review author (RR). One review author (LESF) copied across the data from the data collection form into the Review Manager 5 file (RevMan 2014). We double‐checked that the data were entered correctly by comparing the study reports with how the data were presented in the review. A second review author spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (LESF, ALCM) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third review author (RR). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
For each study, we graded each potential source of bias as high, low, or unclear, and provided a quote form the study report together with a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a researcher, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the review
We conducted the review according to this published protocol and reported any deviations from it in Differences between protocol and review.
Measures of treatment effect
We analysed dichotomous data as risk ratios and continuous data as a mean differences or standardised mean differences, providing 95% confidence intervals for the results. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction of effect to the reader, and reported where the directions were reversed (if this was necessary).
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
A common way that researchers indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed and considered the implication of this.
Where multiple study arms were reported in a single study, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were entered into the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
The unit of analysis was the individual, and a single measurement of each outcome for each participant was collected and analysed.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). If we could not obtain the numerical outcome data, we planned to carry out an intention‐to‐treat analysis, assuming that missing participants have failed the treatment.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis (Higgins 2003). If we identified substantial heterogeneity, we explored it by prespecified subgroup analysis. We investigated statistical diversity by estimates of treatment effect through forest plots produced using Review Manager 5 software (RevMan 2014). We considered an I2 value greater than 50% as substantial heterogeneity (Higgins 2011). In this case, as well as in the presence of clinical or methodological heterogeneity (or both), we used a random‐effects model, rather than a fixed‐effect model.
Assessment of reporting biases
We attempted to contact study authors, in order to ask them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We could not pool more than 10 studies, so we did not create a funnel plot to explore possible publication biases.
Data synthesis
We combined the results across studies, and used a random‐effects model meta‐analysis for dichotomous and continuous outcomes if participants, interventions, comparisons, and outcomes were sufficiently similar to make clinical sense.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
Line of treatment (participants in first‐ versus second‐ versus third‐line therapy): we expected that those receiving the interventions of interest as a first‐line therapy have better outcomes.
Smoking status (smokers versus non‐smokers: we expected that smoking participants have poorer eradication rates (Itskoviz 2017).
Type of test used to assess eradication (urea breath test versus stool antigen test versus endoscopic methods): these tests are different in terms of accuracy.
We used the outcome of successful eradication rate in subgroup analysis. We used the I² statistic to measure heterogeneity among the subgroups in each analysis. If we identified substantial heterogeneity (more than 50%), we explored it using the subgroup analyses prespecified above. Additionally, in case of substantial heterogeneity (clinical , statistical or methodological), we used a random‐effects model rather than a fixed‐effect model.
Sensitivity analysis
We performed a sensitivity analysis, defined a priori, to assess the robustness of our conclusions. This involved excluding studies with a high risk of bias (those classified as having high risk of bias in at least one of the following criteria: randomisation, allocation concealment, and blinding). We also performed a post‐hoc sensitivity analysis, in which we excluded studies that tested antibiotic regimens no longer recommended in current clinical practice due to low efficacy.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice if the results of the review were not statistically significant, and our implications for research gave the reader a clear sense of where the focus of any future research in the area should be, and what the remaining uncertainties are.
'Summary of findings' table
We created a 'Summary of findings' table for the comparison: any antibiotic regimen plus NAC versus the same antibiotic regimen alone.
Into this comparison, we have created subgroups with each correspondent analysis for:
triple therapies plus NAC versus triple therapies alone;
dual therapies plus NAC versus dual therapies alone;
sequential therapy plus NAC versus sequential therapy alone;
bismuth quadruple therapy plus NAC versus bismuth quadruple therapy alone;
antibiotic monotherapy plus NAC, without PPI, versus antibiotic monotherapy alone, without PPI.
We had planned to create a 'Summary of Findings' table for the comparisons below, but we did not find studies for them.
Concomitant therapy plus NAC versus concomitant therapy alone.
Hybrid therapy plus NAC versus hybrid therapy alone.
Quinolone‐based therapy plus NAC versus quinolone‐based therapy alone (We found a study that used a quinolone plus bismuth instead of amoxicillin. We have considered this combination as a triple therapy.)
Definitions for these regimens are as follows.
Triple therapies = PPI + two antibiotics.
Dual therapies = PPI + one antibiotic.
Sequential therapy = PPI + amoxicillin (five days), followed by PPI + clarithromycin + metronidazole (five days).
Bismuth quadruple therapy = PPI + bismuth + tetracycline + metronidazole (10 to 14 days).
Concomitant therapy = PPI + clarithromycin + metronidazole + amoxicillin (7 to 10 days).
Hybrid therapy = PPI + amoxicillin (10 days), followed by PPI + clarithromycin + metronidazole + amoxicillin (10 days).
Quinolone‐based therapy = PPI + quinolone + amoxicillin (10 days).
We assessed the body of the evidence for all pre‐defined outcomes: successful H pylori eradication, adverse events (gastrointestinal, cutaneous, toxic, and other serious adverse events). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2006). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b; Schünemann 2011), and used GRADEpro GDT to generate the tables (GRADEpro GDT 2015). We used footnotes to record and justify all decisions to downgrade or upgrade our assessments of the quality of evidence, and made comments to aid the reader's understanding of the review where necessary. We considered whether there was any additional outcome information that was not able to be incorporated into meta‐analyses, noted this in the comments, and stated if it supported or contradicted the information from the meta‐analyses.
Results
Description of studies
Results of the search
Initially, 765 records were retrieved through database searching. After resolving duplicates, we selected 226 records for the screening process. After discarding 208 reports that were clearly not relevant, we identified 18 records as potentially eligible studies. After the full‐text assessment, we included eight studies from 18 references in the review. The process of selection of the studies is described in Figure 1.
1.
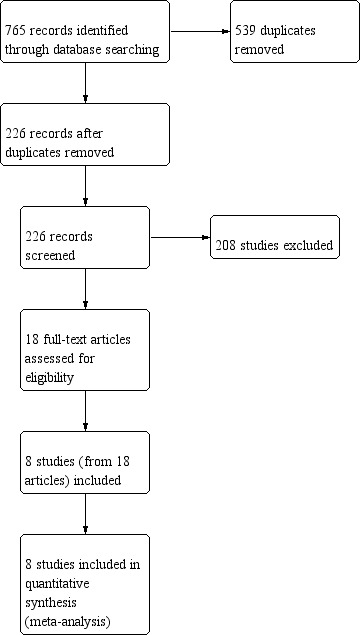
Study flow diagram.
Included studies
Full details on the individual eight studies are available in Characteristics of included studies. We contacted the authors of all studies in order to obtain missing information on methods used in their studies, but we received no feedback from them.
Design
All studies were randomised controlled trials (RCTs), with a parallel design with two groups of comparison.
Sample Size
There were 559 participants enrolled in this review and the sample size of studies ranged from 34 (Zala 1994), to 121 (Emami 2014).
Setting
All studies recruited outpatients who were referred to endoscopy centres with different indications for endoscopy examination. Studies were performed in several different countries: Italy (Cammarota 2009), Switzerland (Zala 1994), Iran (Emami 2014; Hamidian 2015; Karbasi 2013), Denmark (Hansen 1994),Turkey (Gurbuz 2005), and South Korea (Yoon 2014). The first of the included studies published was the Zala and colleagues in 1994 (Zala 1994), and the most recent was published in 2015 (Hamidian 2015).
Participants
All participants were adults between 17 and 76 years with a gender distribution of 287 males and 285 females. The method of diagnosis of Helicobacter pylori (H pylori) infection varied between histology, rapid urease test, stool antigen test, urea breath test or culture, according to each study protocol.
The conditions affecting participants with H pylori infection were dyspepsia, peptic ulcer disease, gastric cancer or dysplasia, family history of gastric cancer, gastritis, oesophagitis, and duodenitis.
Only one study reported the ethnicity of participants (Zala 1994), although the studies were performed in a wide range of countries as described above.
Interventions and comparisons
The studies tested several different antibiotic regimens, using the same antibiotic drugs (dose, frequency, duration) for both comparison arms. The intervention groups received N‐acetylcysteine (NAC) and the controls did not. NAC dose ranged from 600 mg/day to 1800 mg/day. The duration of treatment with antibiotics ranged from 7 to 28 days according to each study protocol. Three studies tested one antibiotic plus NAC (Gurbuz 2005; Hansen 1994; Zala 1994), three studies tested two antibiotics plus NAC (Cammarota 2009; Hamidian 2015; Karbasi 2013), and two studies tested three antibiotics plus NAC (Emami 2014; Yoon 2014). All studies included a proton pump inhibitor (PPI) in both treatment arms, except the study by Hansen and colleagues (Hansen 1994). Compliance was reported in four studies (Cammarota 2009; Emami 2014; Hansen 1994; Yoon 2014). All drugs were delivered in capsules or pills and administered orally. NAC was tested in first‐line therapy in two studies (Emami 2014; Yoon 2014), and in rescue therapy in one study (Cammarota 2009). The other studies included did not mention clearly if they were testing first‐ or second‐line eradication therapy. The comparisons tested are described in Table 2.
1. Comparisons in included studies.
| Intervention | Control (no NAC) | Reference |
| NAC 600 mg orally twice daily plus amoxicillin 1000 mg twice daily, omeprazole 20 mg twice daily and clarithromycin 500 mg twice daily | Same regimen, placebo controlled | Hamidian 2015 |
| NAC 600 mg orally twice daily plus amoxicillin 500 mg four times daily, bismuth citrate 120 mg four times daily, omeprazole 20 mg twice daily, and clarithromycin 500 mg twice daily | Same regimen, without placebo | Emami 2014 |
| NAC 400 mg orally twice daily plus sequential therapy with rabeprazole 20 mg and amoxicillin 1g (both twice daily) for the first five days, followed by rabeprazole 20 mg, clarithromycin 500 mg and metronidazole 500 mg (all drugs twice daily) for the remaining five days | Same regimen, without placebo | Yoon 2014 |
| NAC 600 mg orally twice daily plus ciprofloxacin 500 mg twice daily, pantoprazole 40 mg twice daily and bismuth subcitrate 120 mg two tablets twice daily | Same regimen, placebo controlled | Karbasi 2013 |
| NAC 600 mg orally once a day for one week before a culture‐guided regimen, including a proton pump inhibitor (PPI) plus two antibiotics | Same regimen, without placebo | Cammarota 2009 |
| NAC 400 mg orally three times a day plus clarithromycin 500 mg twice daily and lansoprazole 30 mg twice daily | Same regimen, without placebo | Gurbuz 2005 |
| NAC 600 mg orally twice daily plus omeprazole 40 mg twice daily and amoxicillin 750 mg twice daily | Same regimen, without placebo | Zala 1994 |
| NAC 600 mg orally three times a day plus amoxicillin 375 mg three times a day | Same regimen, placebo controlled | Hansen 1994 |
NAC: N‐acetylcysteine
Outcomes
The following outcomes of interest were assessed by the included studies.
Eradication rate: all included studies.
Adverse events: five studies (Cammarota 2009; Emami 2014; Gurbuz 2005; Yoon 2014; Zala 1994).
Eradication rates were tested and described in all included studies. Criteria for considering eradication were different among studies.Three studies defined eradication as a negative result in urea breath test alone (Cammarota 2009; Hamidian 2015; Karbasi 2013). One study considered eradication as a negative result in either urea breath test, histology, or rapid urease test (Yoon 2014). One study considered eradication as a negative result in stool antigen test (Emami 2014), while another defined eradication as a negative result in rapid urease test and histology (Gurbuz 2005). Finally, two studies considered eradication as a negative result in all of the following: histology, rapid urease test, and culture (Hansen 1994; Zala 1994).
The study by Hansen and colleagues used six months after treatment as the time point for eradication assessment (Hansen 1994), in contrast with all others which used four weeks after the end of treatment.
The most observed class of adverse event were gastrointestinal symptoms. The adverse events cited in the studies were: abdominal pain or discomfort, stomatitis, diarrhoea, vomiting, nausea, dyspepsia, epigastric soreness, regurgitation, abdominal distention, metallic or altered taste. Other adverse events observed included skin rash, headache, dizziness, and thirst.
Excluded studies
We did not exclude any studies following full‐text assessment.
Ongoing studies
There are two ongoing studies. One study intends to assess second‐line eradication rate of H pylori by adding NAC or metronidazole to the conventional triple therapy (NCT01572597). The other is testing the efficacy of acetylcysteine‐containing triple therapy in the first‐line treatment of H pylori infection (NCT02249546). These studies are listed in Characteristics of ongoing studies.
Risk of bias in included studies
The 'Risk of bias' assessments for all included studies are depicted in Figure 2 and Figure 3.
2.
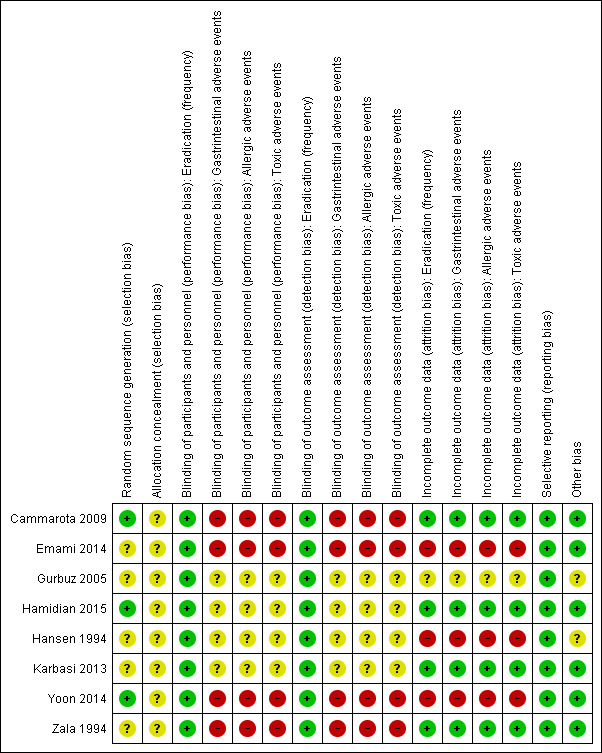
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
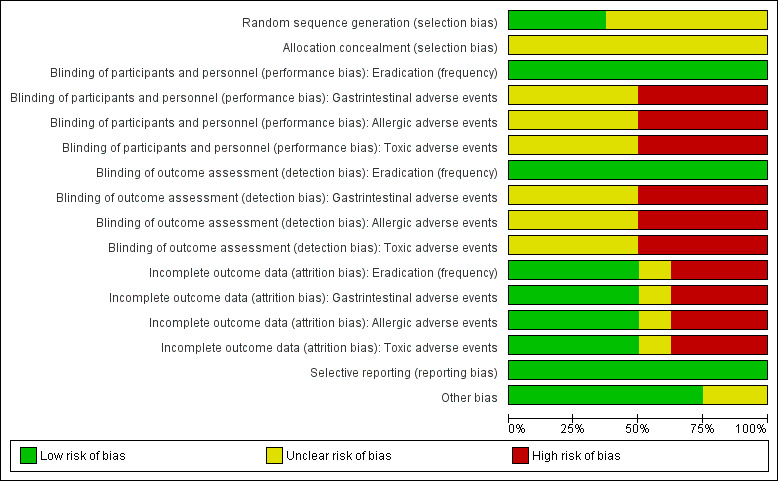
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Random sequence allocation method was adequate in three studies (Cammarota 2009; Hamidian 2015; Yoon 2014), which we classified as having a low risk of bias for this domain. The remaining studies did not present the method used and we judged them as having an unclear risk of bias.
None of the studies provided information about allocation concealment methods (even after email contact), and we classified them as having an unclear risk of bias.
Blinding
We assessed performance and detection bias for each outcome of interest.
Blinding of participants and personnel
Eradication rate
Although the method of assessment of eradication varied, this outcome was always assessed through an objective laboratory test. Therefore, we judged that performance bias was unlikely to influence results and we classified all studies as having a low risk of bias.
Gastrointestinal, allergic and toxic adverse events
Three studies did not assess these outcomes (Hamidian 2015; Hansen 1994; Karbasi 2013). Another study did not provide sufficient information (Gurbuz 2005), so we classified it as having an unclear risk of bias. We considered all other studies to have a high risk of bias provided they were performed in an open‐label design.
Blinding of outcome assessment
Eradication rate
The method of assessment of eradication was always a laboratory objective test, meaning detection bias was very unlikely. We judged all studies as having a low risk of bias.
Gastrointestinal, allergic and toxic adverse events
Three studies did not assess these outcomes (Hamidian 2015; Hansen 1994; Karbasi 2013). Four studies were open‐label, with no blinding for the outcome assessor, and so we considered them to be at high risk of bias (Cammarota 2009; Emami 2014; Yoon 2014; Zala 1994). The final study did not provide sufficient information and so we judged it as having an unclear risk of bias (Gurbuz 2005).
Incomplete outcome data
We judged one study to have an unclear risk of bias because there was a loss of 5% of participants with an unbalance between groups and no reason provided (Gurbuz 2005).
We deemed three studies to have a high risk of bias. In Emami 2014, there was a loss of 32.7% of the participants and no reason was provided for this. In Hansen 1994, there was a loss of 24.7% of the participants. Reasons were provided, but no information about the balance between groups or the methods used for data imputation were given. Finally, in Yoon 2014, there was a loss of 18% of the participants (with a balance between groups). An intention‐to‐treat (ITT) analysis was used, but the methods used for data imputation were also not provided.
We classified four studies as having a low risk of bias for this domain. There were no losses in three studies (Cammarota 2009; Hamidian 2015; Zala 1994). In Karbasi 2013, there was a loss of 6% of the participants in the control group, which we judged as being unlikely to influence the results.
Selective reporting
We judged all studies to be free from selective outcome reporting provided they described all outcomes planned.
Other potential sources of bias
We judged two studies as having an unclear risk of potential sources of bias. One study declared that Astra Group provided NAC and placebo but provided no information about Astra Group's influence in design, analysis or reporting (Hansen 1994). In the other study, the author did not report funding sources or conflict of interest (Gurbuz 2005). There was insufficient information to allow judgement about this.
Effects of interventions
See: Table 1
Any antibiotic regimen plus N‐acetylcysteine (NAC) versus the same antibiotic regimen without NAC
Eradication rate
All eight included studies provided data for this outcome (Cammarota 2009; Emami 2014; Gurbuz 2005; Hamidian 2015; Hansen 1994; Karbasi 2013; Yoon 2014; Zala 1994). In Hansen 1994, eradication rates were assessed immediately after treatment (this was termed "clearance"), and at six months after treatment (this was termed "eradication"). For this study, we considered eradication data available at six months, following our criteria for studies that have assessed outcomes at more than one time point, as mentioned in this review's protocol (Fontes 2016). All studies apart from one included a proton pump inhibitor (PPI) in both treatment arms.
Overall, we are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates compared with the addition of placebo or no NAC (38.8% versus 49.1%, risk ratio (RR) 0.74, 95% confidence interval (CI) 0.51 to 1.08; participants = 559; studies = eight; very low certainty of evidence) (Analysis 1.1).
1.1. Analysis.
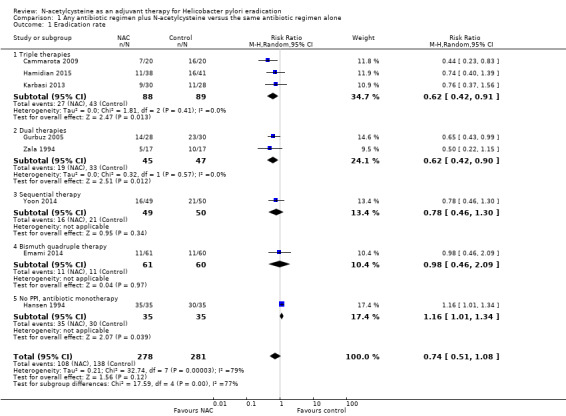
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 1 Eradication rate.
Subgroup analysis: triple therapies (PPI + two antibiotics) plus NAC versus triple therapies without NAC
Three studies provided data for this outcome (Cammarota 2009; Hamidian 2015; Karbasi 2013). We are uncertain whether the addition of NAC to triple therapy improves eradication rates compared with the addition of placebo or no NAC (51.6% versus 69.3%, RR 0.62, 95% CI 0.42 to 0.91; participants = 177; studies = three) (Analysis 1.1).
Subgroup analysis: dual therapies (PPI + one antibiotic) plus NAC versus dual therapies without NAC
Two studies provided data for this outcome (Gurbuz 2005; Zala 1994). We are uncertain whether NAC improves eradication rates compared with the addition of placebo or no NAC (29.8% versus 57.8%, RR 0.62, 95% CI 0.42 to 0.90; participants = 92; studies = two) (Analysis 1.1).
Subgroup analysis: sequential therapy (PPI + amoxicillin (five days), followed by PPI + clarithromycin + metronidazole (five days)) plus NAC versus sequential therapy without NAC
One study provided data for this outcome (Yoon 2014). We are uncertain whether NAC improves eradication rates compared with the addition of placebo or no NAC (58% versus 67.3%, RR 0.78, 95% CI 0.46 to 1.30; participants = 99; studies = one) (Analysis 1.1).
Subgroup analysis: bismuth quadruple therapy (PPI + bismuth + tetracycline + metronidazole) plus NAC versus bismuth quadruple therapy without NAC
One study provided data for this outcome (Emami 2014). We are uncertain whether NAC improves eradication rates compared with the addition of placebo or no NAC (81.7% versus 82%, RR 0.98, 95% CI 0.46 to 2.09; participants = 121; studies = one) (Analysis 1.1).
Subgroup analysis: no PPI, antibiotic monotherapy plus NAC versus antibiotic monotherapy without NAC
One study provided data for this outcome (Hansen 1994). We are uncertain whether NAC improves eradication rates compared with the addition of placebo or no NAC (54.2% versus 60%, RR 1.14, 95% CI 0.66 to 1.97; participants = 70; studies = one) (Analysis 1.1).
Subgroup analysis: smoking status
The results were based on only one study (Hamidian 2015). We are uncertain whether NAC improves eradication rates in smokers (50% versus 57.1%, RR 0.86, 95% CI 0.27 to 2.77; participants = 13; studies = one) or in non‐smokers (62.8% versus 74.1%, RR 0.69, 95% CI 0.33 to 1.45; participants = 66; studies = 1) (Analysis 1.5).
1.5. Analysis.
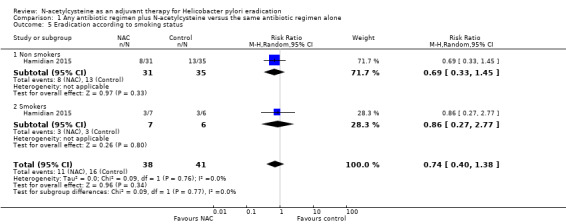
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 5 Eradication according to smoking status.
The test for subgroup differences showed no interaction related to smoking status (Chi² = 0.09, df = 1 (P = 0.77), I² = 0%).
Subgroup analysis: line of treatment
We did not find sufficient information in included studies to organise subgroups as first‐, second‐, third‐line as we planned. Only one study stated that they were testing NAC in first‐line therapy (Yoon 2014), and one study stated that they tested NAC in association with culture‐guided antibiotic therapy, after at least four failures (Cammarota 2009).
Subgroup analysis: type of test used to assess eradication
Four studies tested eradication using the urea breath test (Cammarota 2009; Hamidian 2015; Karbasi 2013; Yoon 2014). NAC may improve eradication rates measured this way, compared with the addition of placebo or no NAC (31.3% versus 46%, RR 0.67, 95% CI 0.49 to 0.91; participants = 276; studies = four) (Analysis 1.6).
1.6. Analysis.
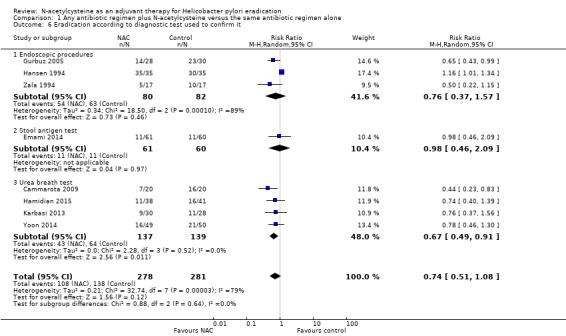
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 6 Eradication according to diagnostic test used to confirm it.
Three studies used endoscopic methods (Gurbuz 2005; Hansen 1994; Zala 1994). We are uncertain whether NAC improves eradication rates measured this way, compared with the addition of placebo or no NAC (43.7% versus 57.3%, RR 0.75, 95% CI 0.48 to 1.17; participants = 162; published studies = three) (Analysis 1.6).
Finally, one study used a stool antigen test (Emami 2014). We are uncertain whether NAC improves eradication rates measured this way, compared with the addition of placebo or no NAC (18% versus 18.3%, RR 0.98, 95% CI 0.46 to 2.49; participants = 121 ; published studies = one) (Analysis 1.6)
Sensitivity analysis: currently used regimens
Five studies tested eradication with currently used antibiotic regimens (Cammarota 2009; Emami 2014; Hamidian 2015; Karbasi 2013; Yoon 2014). Adding NAC to these regimens may improve eradication rates, compared with adding placebo or no NAC (27.2 % versus 37.6 %, RR 0.71, 95% CI 0.53 to 0.94; participants = 397 ; published studies = five) (Analysis 1.7).
1.7. Analysis.
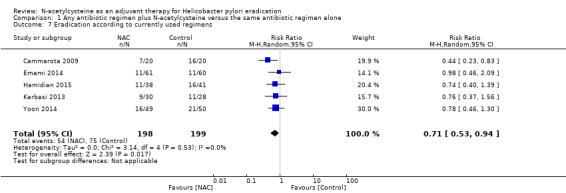
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 7 Eradication according to currently used regimens.
Gastrointestinal adverse events
Five included studies provided data for this outcome (Cammarota 2009; Emami 2014; Gurbuz 2005; Yoon 2014; Zala 1994).
We are uncertain whether NAC leads to a higher rate of gastrointestinal adverse events compared with the addition of placebo or no NAC (23.9% versus 18.9%, RR 1.25, 95% CI 0.85 to 1.85; participants = 336; studies = five; very low certainty of evidence) (Analysis 1.2).
1.2. Analysis.
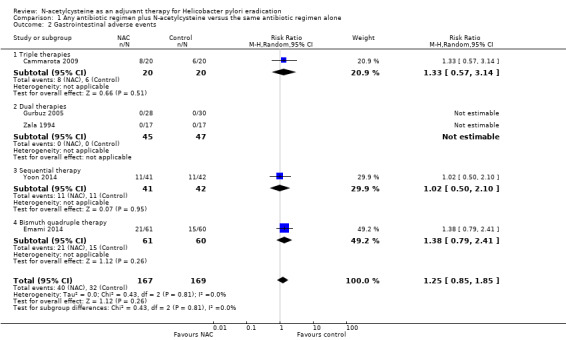
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 2 Gastrointestinal adverse events.
Triple therapies plus NAC versus triple therapies without NAC
One study provided data for this outcome (Cammarota 2009). We are uncertain whether NAC leads to a higher rate of gastrointestinal adverse events compared with the addition of placebo or no NAC (40% versus 30%, RR 1.33 , 95% CI 0.57 to 3.14; participants = 40; studies = one) (Analysis 1.2).
Dual therapies plus NAC versus dual therapies without NAC
Two studies provided data for this outcome (Gurbuz 2005; Zala 1994). No events were observed in both arms of the included studies (RR not estimable; participants = 92; studies = two) (Analysis 1.2).
Sequential therapy plus NAC versus sequential therapy without NAC
One study provided data for this outcome (Yoon 2014). We are uncertain whether NAC leads to a higher rate of gastrointestinal adverse events compared with the addition of placebo or no NAC (26.8% versus 26.1%, RR 1.02, 95% CI 0.50 to 2.10; participants = 83; studies = one) (Analysis 1.2).
Bismuth quadruple therapy plus NAC versus bismuth quadruple therapy without NAC
One study provided data for this outcome (Emami 2014). We are uncertain whether NAC leads to a higher rate of gastrointestinal adverse events compared with the addition of placebo or no NAC (34.4% versus 25%, RR 1.38, 95% CI 0.79 to 2.41; participants = 121; studies = one) (Analysis 1.2).
No PPI, antibiotic monotherapy plus NAC versus antibiotic monotherapy without NAC
None of the studies assessed this outcome.
Allergic adverse events
Five included studies provided data for this outcome (Cammarota 2009; Emami 2014; Gurbuz 2005; Yoon 2014; Zala 1994).
We are uncertain whether NAC leads to a higher rate of allergic adverse events compared with the addition of placebo or no NAC (1.2% versus 0%, RR 2.98, 95% CI 0.32 to 27.74; participants = 336 ; published studies = five; very low certainty of evidence) (Analysis 1.3).
1.3. Analysis.
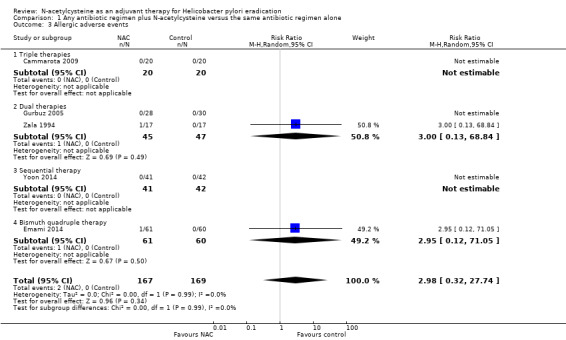
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 3 Allergic adverse events.
Triple therapies plus NAC versus triple therapies without NAC
One study provided data for this outcome (Cammarota 2009). No events were observed in both arms (RR not estimable; participants = 40; studies = one) (Analysis 1.3).
Dual therapies plus NAC versus dual therapies without NAC
Two studies provided data for this outcome (Gurbuz 2005; Zala 1994). We are uncertain whether NAC leads to a higher rate of allergic adverse events compared with the addition of placebo or no NAC (2.2% versus 0%, RR 3.00, 95% CI 0.13 to 68.84; participants = 92; studies = two) (Analysis 1.3).
Sequential therapy plus NAC versus sequential therapy without NAC
One study provided data for this outcome (Yoon 2014). No events were observed in both arms (RR not estimable; participants = 83; studies = one) (Analysis 1.3).
Bismuth quadruple therapy plus NAC versus bismuth quadruple therapy without NAC
One study provided data for this outcome (Emami 2014). We are uncertain whether NAC leads to a higher rate of allergic adverse events compared with the addition of placebo or no NAC (1.2% versus 0%, RR 2.95, 95% CI 0.12 to 71.05; participants = 121; studies = one) (Analysis 1.3).
No PPI, antibiotic monotherapy plus NAC versus antibiotic monotherapy without NAC
None of the studies assessed this outcome.
Toxic adverse events
There were no reports of toxic adverse events amongst the five studies that reported this outcome (Cammarota 2009; Emami 2014; Gurbuz 2005; Yoon 2014; Zala 1994).
Discussion
Summary of main results
This systematic review assessed the efficacy and safety of N‐acetylcysteine (NAC) as an adjuvant therapy to antibiotics for Helicobacter pylori (H pylori) eradication. We found eight randomised controlled trials (RCTs), recruiting 559 participants. The results are depicted in Table 1.
The studies were different with respect to the participants (i.e. whether they had been previously treated or not), type of antibiotics, dose of NAC, duration of treatment, and measurement of the outcomes.
Eradication rates: we are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates compared with the addition of placebo or no NAC. Due to the clinical, statistical and methodological heterogeneity found in included studies, and the uncertainty observed when we analysed therapy subgroups, any possible beneficial effect of NAC should be regarded cautiously. Furthermore, we are uncertain if there are differences in eradication rates between smokers and non‐smokers who use NAC in addition to antibiotics.
Adverse events: we are uncertain whether the addition of NAC to antibiotics is associated with a higher risk of gastrointestinal or allergic adverse events, compared with the addition of placebo or no NAC. There were no reports of toxic adverse events amongst the included studies.
Overall completeness and applicability of evidence
The regimens compared in the included studies were very different. Overall, the studies had small sample sizes and measured the outcomes in the short term, using different tests. Importantly, three studies tested regimens that are no longer recommended due to low efficacy (Gurbuz 2005; Hansen 1994; Zala 1994), and their external validity must be regarded cautiously. We performed a post‐hoc sensitivity analysis to see whether the results are still applicable when these studies are excluded. In contrast with the main analysis, the results were in favour of adding NAC to antibiotics.
The following treatment schemes, with versus without NAC, were assessed.
NAC plus amoxicillin, a proton pump inhibitor (PPI), and clarithromycin (Hamidian 2015).
NAC plus amoxicillin, a PPI, bismuth citrate, and clarithromycin (Emami 2014).
NAC plus sequential therapy with amoxicillin and a PPI for the first five days, followed by a PPI, clarithromycin, and metronidazole for the remaining five days (Yoon 2014).
NAC plus ciprofloxacin, a PPI, and bismuth subcitrate (Karbasi 2013).
NAC for one week before a culture‐guided regimen including a PPI plus two antibiotics (Cammarota 2009).
NAC plus clarithromycin, and a PPI (Gurbuz 2005).
NAC plus amoxicillin and a PPI (Zala 1994).
NAC plus amoxicillin (Hansen 1994).
Therefore, the available evidence is limited to these specific interventions, and a restricted follow‐up period of four weeks. We did not find two or more studies testing the same drug regimen.
Doses of NAC ranged from 600 mg to 1800 mg among studies, which led to uncertainty concerning optimal dose and safety issues.
There is a lack of evidence for the following outcomes:
eradication rates of currently recommended regimens, such as concomitant therapy, hybrid therapy, and conventional bismuth triple therapy (there were no studies for these comparisons of interest);
adverse events: the outcomes were not originally planned at the protocol stage of the included RCTs, or they were not properly reported.
We considered the studies identified insufficient to address all of the objectives of the review.
Quality of the evidence
We have presented our assessments of the certainty of the body of evidence obtained for each outcome and comparison in Table 1.
For eradication rate, we judged the certainty of the pooled evidence as very low (eight studies, 559 participants). We downgraded our assessment of the certainty of the evidence because of serious or very serious concerns related to the 'Risk of bias' assessment, inconsistency and imprecision amongst included studies.
For adverse events, we judge the certainty of the pooled evidence as low to very low (five studies, 336 participants). We downgraded our assessment of the certainty of the evidence because of serious or very serious concerns related to the 'Risk of bias' assessment and imprecision amongst included studies.
The overall certainty of the evidence was reduced at study or outcome levels due to the poor methodological quality (mainly related to generation of allocation sequence, allocation concealment, and blinding of participants, personnel or outcome assessors, the last domain for adverse event outcomes), or due to imprecision and inconsistency (mainly related to wide confidence intervals and small samples). The small sample size that each study contributed to each comparison can be associated with an increased risk of type 2 error.
Potential biases in the review process
To avoid the introduction of bias in this review, we followed all of the recommendations on searching, study selection, data collection, and analysis in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The strengths of this review include an extensive literature search and the use of intention‐to‐treat analyses for dichotomous data. The eradication rate was measured in all included studies by an objective test, reducing the risk of bias related to the blinding of personnel, participants and outcome assessors.
We decided to perform a post‐hoc sensitivity analysis, in which we removed studies that tested antibiotic regimens which are no longer in use because of low efficacy. Importantly, two of the three studies removed for this reason contributed considerable weight to the primary analysis: 24.7% (Gurbuz 2005), and 14.8% (Hansen 1994).
In the next update of this review, we intend to perform an overall analysis including only studies that have tested recommended regimens on date, and a sensitivity analysis including all studies available, even if the regimens tested are outdated.
We also decided to add a subgroup analysis for smoking status, as tobacco use could interfere in H pylori eradication rates.
This review has some limitations, as follows.
We found a lack of outcome data in the included RCTs, even after trying contact the correspondent authors via email. In some studies, authors removed participants from an analysis because of non‐compliance (even because of adverse effects), without stating which group they were allocated to.
There weren't any comparisons with two or more studies testing the same antibiotic regimen, leading to consequent clinical heterogeneity.
Agreements and disagreements with other studies or reviews
At the end of reporting this review, there was no other review in progress or already published with a similar clinical question.
Two ongoing studies are testing NAC for H pylori eradication:
comparing the efficacy of standard triple therapy with or without NAC in the first line of H pylori infection (NCT02249546); and
comparing efficacy and safety of 10‐day triple therapy (rabeprazole, clarithromycin, and amoxicillin) plus NAC versus 10‐day concomitant therapy (rabeprazole, clarithromycin, amoxicillin, and metronidazole) for re‐eradication H pylori infection (NCT01572597).
Authors' conclusions
Implications for practice.
The rate of development of new antibiotic drugs has been declining in the last few years (Fair 2014). Clinicians and those infected are exposed to rising rates of resistance to antibiotics. Therefore, new regimens and associations of drugs are currently being tested for H pylori infection. The recently published Maastricht V/Florence Consensus Report on the management of H pylori has changed the recommendation about who should be treated; the guideline advises that all people with confirmed infection should receive treatment, regardless of having gastric abnormalities (Malfertheiner 2017). More people will have to be treated, and as a result, antibiotic resistance should increase with this practice.
We are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates compared with the addition of placebo or no NAC. Due to the clinical, statistical and methodological heterogeneity found in the studies included in this review, and the uncertainty observed when analysing therapy subgroups, any possible beneficial effect of NAC should be regarded cautiously. Furthermore, we are uncertain if there are differences in eradication rates between smokers and non‐smokers who take NAC added to the antibiotic regimen.
We are uncertain whether the addition of NAC is associated with higher risk of adverse events (gastrointestinal or allergic) compared with the addition of placebo or no NAC. There were no reports of toxic adverse events amongst included studies.
Implications for research.
Due to the lack of available data and low quality of the current evidence, as well as the uncertainty observed in subgroup analysis, future research still needs to answer the following questions.
Is NAC addition effective and safe forH pylori eradication in currently recommended antibiotic regimens?
What is the optimal dose of NAC?
Is NAC effective for both newly and previously treated people?
Further large, well‐designed randomised clinical studies, with good reporting standards and appropriate collection of efficacy and safety outcomes, should be done. A particular focus for investigation should be currently recommended antibiotic regimens, such as standard clarithromycin triple therapies, bismuth‐based therapies, and quinolone‐based therapies.
Acknowledgements
We acknowledge the help and support of the Cochrane Upper Gastrointestinal Diseases (UGPD) group. The authors would also like to thank the following editors and peer referees who provided comments to improve the protocol: Sarah Rhodes and Huan Song (editors), Eduardo Villatoro (clinical reviewer), Xavier Calvet (clinical reviewer), Marilyn Walsh (consumer reviewer), two anonymous clinical reviewers, and to Karin Dearness for copy‐editing the protocol and Jessica Sharp for copy‐editing the review. Finally, we would like to thank Ruy Alberto Kux from Petrópolis Medical School for translating an article from German into Portuguese (Zala 1994), and Therese Dalbo for using Cochrane's Task Exchange to help us obtain the full text of Hansen 1994.
The methods section of this protocol is based on a standard template used by the UGPD group.
Appendices
Appendix 1. Glossary of terms
Acetylated: product of a chemical reaction
Adjuvant therapy: therapy that is given in addition to the primary therapy
Antagonist: a substance that acts against and blocks an action
Antioxidant: a substance that blocks or neutralises oxidation
Atrophic gastritis: a pre‐cancerous type of inflammation of the lining of the stomach, characterised by the wasting away of stomach glands
Atrophy: the wasting away, or decrease in size of something
Bactericidal: capable of killing bacteria
Beta‐lactamases: enzymes which give resistance to a group of antibiotics, so‐called beta‐lactams
Bioavailability: the degree to which a substance is absorbed
Biopsies: the removal and examination of small tissue, cell, or fluid samples
Bismuth: a type of chemical substance with antimicrobial properties
Campylobacter: a genus of bacteria
Chronic: long‐term
Cleavage: the breaking down into smaller components
Culture: a method to cultivate bacteria
Cutaneous: skin‐related
Compliance: a person’s willingness to follow prescribed treatment
Cultivable: capable of growth
Detoxification: the removal of a poisonous substance from the body
Diffusion: distribution
Dyspepsia: indigestion
Dyspeptic: related to indigestion
Endoscopy: the insertion of a thin illuminated tube into a hollow internal organ to allow visualisation and the passage of small surgical instruments
Epithelial cells: a protective lining of membranous tissue
Extracellular polymeric matrix: a type of biological substance produced by bacteria that can survive under difficult conditions
Eradication: destruction
Faecal‐oral transmission: transfer characterised by the intake of food or water into the mouth that has been contaminated with bodily waste
Free oxygen radical scavengers: substance that blocks toxins released by oxygen metabolism
Gastric: related to the stomach
Gastric aspirates: contents suctioned from the stomach
Gastritis: inflammation of the lining of the stomach
Genus: origin
Glutathione: a protein with important biochemical properties to protect all body cells
Gram‐negative microaerophilic spiral bacterium: a type of bacterium with a spiral shape, not reacting to a dye test to detect bacteria, and with the capacity to survive in low oxygen environments.
Histology: examination under a microscope
Idiopathic: occurring suddenly
IgG serology: a test in blood serum using immunoglobulin G
In vivo: in the body
In vitro: in an artificial environment outside the body
Iron deficiency anaemia: a condition of low red blood cells
Latent: capable of living in the body without showing visible symptoms
Lesions: abnormal changes in the structure of an organ
Mucosal‐asssociated lymphoid tissue (MALT) lymphoma): a type of stomach cancer
Metabolites: product of body's metabolism
Morbidity: degree of illness or disease
Mortality: death rate
Mucolytic: tending to break down the thickness of human and animal secretions and tissues such as saliva and the lining of the stomach
NAC: N‐acetylcysteine
Oral‐oral transmission: transfer by the exchange of secretions (e.g. saliva, vomiting) of one person’s mouth to another person’s mouth
Oxidative: product of chemical reaction, so‐called oxidation
Pathogen: a micro‐organism capable of causing disease
Peptic: related to the digestive tract
Plasma: the fluid part of blood
Proliferate: grow
Pruritus: itching
Proton pump inhibitor (PPI): a medication that lowers stomach acid production
Rapid urease reaction: a test used to detect urea in gastric specimens
Reactive oxygen species: toxic substances which are products of normal metabolism and could cause cellular damage
Refractory: resistant to treatment or cure
Stool antigen test: a laboratory test that detects parts of bacteria and serves to demonstrate its presence
Streptococcus mutans: one species of bacteria which colonises humans
Synergic: working together
Synthesis: creating something whole by combining other smaller parts
Thrombocytopenia purpura: a condition where purplish discolorations occur due to excessive bleeding into the skin and mucous membranes from the inability of the blood to clot
Toxicity: poisoning
Urea breath test: a laboratory test to detect the presence of urea in expired breath and to confirm the presence of Helicobacter pylori (H pylori)
Viable: capable of living
Virulent: capable of being extremely dangerous or poisonous
Viscosity: inability to flow
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Helicobacter] explode all trees 1989
#2 MeSH descriptor: [Helicobacter Infections] explode all trees 1949
#3 helicobacter or pylori or pyloridis or "HP" or campylobacter:ti,ab,kw (Word variations have been searched) 5064
#4 #1 or #2 or #3 5064
#5 MeSH descriptor: [Acetylcysteine] explode all trees 612
#6 acetylcystein* or (acetyl near/2 cistein*) or (acetyl near/2 cystein*):ti,ab,kw (Word variations have been searched) 1261
#7 NAC or Mucomyst or Acetadote or cilol or flumucil or Gluton or Mucare or mucinac:ti,ab,kw (Word variations have been searched) 701
#8 mucohelp or mucomelt or mucomix or mucosys or mucotyle or mucyst or nacel or nacfil:ti,ab,kw (Word variations have been searched) 0
#9 Genac or Solmucol or Acetabs or Acetyst or Airbron or Alveolex or Azubronchin or Bromuc:ti,ab,kw (Word variations have been searched) 0
#10 Broncho Fips or BronchoFips or Broncholysin or Broncoclar or Codotussyl or Cystamucil:ti,ab,kw (Word variations have been searched) 0
#11 Dampo Mucopect or durabronchal or Eurespiran or Exomuc or Fabrol or Fluprowit or Muco Sanigen:ti,ab,kw (Word variations have been searched) 4
#12 Frekatuss or Hoestil or Ilube or Jenacystein or Jenapharm or Lantamed or Lindocetyl or M‐Pectil or M Pectil or MPectil:ti,ab,kw (Word variations have been searched) 3
#13 Muciteran or Acetylin or Mucosil or Mucosol or Mucosolvin or Siccoral or Siran or acebraus:ti,ab,kw (Word variations have been searched) 4
#14 acerac or acetain or acypront or acys‐5 or brunac or cetilan or drenaflen or ecomucyl or encore or flemex‐ac:ti,ab,kw (Word variations have been searched) 42
#15 fluimicil or fluimucil or fluimukan or flutafin or hidonac or inspire or lappe or libramucil or menaxol or mercapturic acid:ti,ab,kw (Word variations have been searched) 1497
#16 mucocil or mucofillin or mucolator or mucomiste or mucoserin or mucosof or mucosten or mucoza or mukolit:ti,ab,kw (Word variations have been searched) 0
#17 muteran or parvolex or reolin or respaire or sigamucil or siran 200 or spatam or sputoprompt or stecin or tixair or zifluvis:ti,ab,kw (Word variations have been searched) 0
#18 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 2916
#19 #4 and #18 14
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to 12 April 2018>
1 exp Helicobacter/ or exp Helicobacter infection/ (33279)
2 (helicobacter or pylori or pyloridis or "HP" or campylobacter).ti,ab,kw. (64294)
3 1 or 2 (66915)
4 exp Acetylcysteine/ (11078)
5 (acetylcystein* or (acetyl adj2 cistein*) or (acetyl adj2 cystein*)).tw,kw. (15547)
6 (NAC or Mucomyst or Acetadote or cilol or flumucil or Gluton or Mucare or mucinac).tw,kw. (12595)
7 (mucohelp or mucomelt or mucomix or mucosys or mucotyle or mucyst or nacel or nacfil).tw,kw. (0)
8 (Genac or Solmucol or Acetabs or Acetyst or Airbron or Alveolex or Azubronchin or Bromuc).tw,kw. (4)
9 (Broncho Fips or BronchoFips or Broncholysin or Broncoclar or Codotussyl or Cystamucil).tw,kw. (2)
10 (Dampo Mucopect or durabronchal or Eurespiran or Exomuc or Fabrol or Fluprowit or Muco Sanigen).tw,kw. (6)
11 (Frekatuss or Hoestil or Ilube or Jenacystein or Jenapharm or Lantamed or Lindocetyl or M‐Pectil or M Pectil or MPectil).tw,kw. (36)
12 (Muciteran or Acetylin or Mucosil or Mucosol or Mucosolvin or Siccoral or Siran or acebraus).tw,kw. (28)
13 (acerac or acetain or acypront or acys‐5 or brunac or cetilan or drenaflen or ecomucyl or encore or flemex‐ac).tw,kw. (157)
14 (fluimicil or fluimucil or fluimukan or flutafin or hidonac or inspire or lappe or libramucil or menaxol or mercapturic acid).tw,kw. (2902)
15 (mucocil or mucofillin or mucolator or mucomiste or mucoserin or mucosof or mucosten or mucoza or mukolit).tw,kw. (0)
16 (muteran or parvolex or reolin or respaire or sigamucil or siran 200 or spatam or sputoprompt or stecin or tixair or zifluvis).tw,kw. (6)
17 or/4‐16 (27714)
18 3 and 17 (58)
19 remove duplicates from 18 (56)
Appendix 4. Embase search strategy
Database: Embase <1974 to 12 April 2018>
1 exp Helicobacter/ or exp Helicobacter infection/ (55182)
2 (helicobacter or pylori or pyloridis or "HP" or campylobacter).ti,ab,kw. (82108)
3 1 or 2 (91470)
4 exp acetylcysteine/ (27713)
5 (acetylcystein* or (acetyl adj2 cistein*) or (acetyl adj2 cystein*)).tw,kw. (19474)
6 (NAC or Mucomyst or Acetadote or cilol or flumucil or Gluton or Mucare or mucinac).tw,kw. (17114)
7 (mucohelp or mucomelt or mucomix or mucosys or mucotyle or mucyst or nacel or nacfil).tw,kw. (1)
8 (Genac or Solmucol or Acetabs or Acetyst or Airbron or Alveolex or Azubronchin or Bromuc).tw,kw. (45)
9 (Broncho Fips or BronchoFips or Broncholysin or Broncoclar or Codotussyl or Cystamucil).tw,kw. (23)
10 (Dampo Mucopect or durabronchal or Eurespiran or Exomuc or Fabrol or Fluprowit or Muco Sanigen).tw,kw. (31)
11 (Frekatuss or Hoestil or Ilube or Jenacystein or Jenapharm or Lantamed or Lindocetyl or M‐Pectil or M Pectil or MPectil).tw,kw. (185)
12 (Muciteran or Acetylin or Mucosil or Mucosol or Mucosolvin or Siccoral or Siran or acebraus).tw,kw. (128)
13 (acerac or acetain or acypront or acys‐5 or brunac or cetilan or drenaflen or ecomucyl or encore or flemex‐ac).tw,kw. (476)
14 (fluimicil or fluimucil or fluimukan or flutafin or hidonac or inspire or lappe or libramucil or menaxol or mercapturic acid).tw,kw. (3987)
15 (mucocil or mucofillin or mucolator or mucomiste or mucoserin or mucosof or mucosten or mucoza or mukolit).tw,kw. (16)
16 (muteran or parvolex or reolin or respaire or sigamucil or siran 200 or spatam or sputoprompt or stecin or tixair or zifluvis).tw,kw. (83)
17 or/4‐16 (42667)
18 3 and 17 (137)
19 remove duplicates from 18 (136)
Appendix 5. CINAHL search strategy
| # | Query | Results |
| S18 | S3 AND S17 | 21 |
| S17 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 3,368 |
| S16 | TX muteran or parvolex or reolin or respaire or sigamucil or siran 200 or spatam or sputoprompt or stecin or tixair or zifluvis | 0 |
| S15 | TX mucocil or mucofillin or mucolator or mucomiste or mucoserin or mucosof or mucosten or mucoza or mukolit | 0 |
| S14 | TX fluimicil or fluimucil or fluimukan or flutafin or hidonac or inspire or lappe or libramucil or menaxol or mercapturic acid | 1,905 |
| S13 | TX acerac or acetain or acypront or acys‐5 or brunac or cetilan or drenaflen or ecomucyl or encore or flemex‐ac | 124 |
| S12 | TX Muciteran or Acetylin or Mucosil or Mucosol or Mucosolvin or Siccoral or Siran or acebraus | 17 |
| S11 | TX Frekatuss or Hoestil or Ilube or Jenacystein or Jenapharm or Lantamed or Lindocetyl or M‐Pectil or M Pectil or MPectil | 1 |
| S10 | TX Dampo Mucopect or durabronchal or Eurespiran or Exomuc or Fabrol or Fluprowit or Muco Sanigen | 0 |
| S9 | TX Broncho Fips or BronchoFips or Broncholysin or Broncoclar or Codotussyl or Cystamucil | 0 |
| S8 | TX Genac or Solmucol or Acetabs or Acetyst or Airbron or Alveolex or Azubronchin or Bromuc | 0 |
| S7 | TX mucohelp or mucomelt or mucomix or mucosys or mucotyle or mucyst or nacel or nacfil | 0 |
| S6 | TX NAC or Mucomyst or Acetadote or cilol or flumucil or Gluton or Mucare or mucinac | 505 |
| S5 | TX acetylcystein* or (acetyl and cistein*) or (acetyl and cystein*) | 1,050 |
| S4 | (MH "Acetylcysteine") | 703 |
| S3 | S1 OR S2 | 8,709 |
| S2 | TX helicobacter or pylori or pyloridis or "HP" or campylobacter | 8,709 |
| S1 | (MH "Helicobacter+") OR (MH "Helicobacter Infections") | 2,280 |
Appendix 6. LILACS search strategy
((tw:(helicobacter OR pylori OR "H.pylori" OR "HP" OR campylobacter)) OR (mh:(helicobacter))) AND ((tw:(acetylcystein* OR (acetyl AND cistein*) OR (acetyl AND cystein*) OR nac)) OR (mh:(acetylcysteine)))
Data and analyses
Comparison 1. Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication rate | 8 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 1.1 Triple therapies | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.91] |
| 1.2 Dual therapies | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.90] |
| 1.3 Sequential therapy | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.46, 1.30] |
| 1.4 Bismuth quadruple therapy | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.46, 2.09] |
| 1.5 No PPI, antibiotic monotherapy | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.01, 1.34] |
| 2 Gastrointestinal adverse events | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.85, 1.85] |
| 2.1 Triple therapies | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.14] |
| 2.2 Dual therapies | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Sequential therapy | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.50, 2.10] |
| 2.4 Bismuth quadruple therapy | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.79, 2.41] |
| 3 Allergic adverse events | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [0.32, 27.74] |
| 3.1 Triple therapies | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Dual therapies | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.84] |
| 3.3 Sequential therapy | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Bismuth quadruple therapy | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 71.05] |
| 4 Toxic adverse events | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Triple therapies | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Dual therapies | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Sequential therapy | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Bismuth quadruple therapy | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Eradication according to smoking status | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.38] |
| 5.1 Non smokers | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.33, 1.45] |
| 5.2 Smokers | 1 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.27, 2.77] |
| 6 Eradication according to diagnostic test used to confirm it | 8 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 6.1 Endoscopic procedures | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.37, 1.57] |
| 6.2 Stool antigen test | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.46, 2.09] |
| 6.3 Urea breath test | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.49, 0.91] |
| 7 Eradication according to currently used regimens | 5 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.94] |
1.4. Analysis.
Comparison 1 Any antibiotic regimen plus N‐acetylcysteine versus the same antibiotic regimen alone, Outcome 4 Toxic adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cammarota 2009.
| Methods |
Aim of study: to test the hypothesis that a mucolytic pretreatment was able to demolish the biofilm architecture, rendering H pylori–resistant strains more vulnerable to antibiotics. Study design: randomised controlled study, open‐label. Study grouping: parallel group. Unit of allocation: by individuals. Country: Italy. Start date: not available in report. End date: not available in report. Duration of participation: 9 weeks. Ethical approval: approved by the ethics committee of Catholic University of Medicine and Surgery. |
|
| Participants |
Total number randomised: 40. Method of recruitment: consecutive participants who had a history of at least fourH pylori eradication failures, and were therefore referred to a tertiary endoscopy centre for endoscopic examination and H pylori culture. Informed consent obtained: participants provided written informed consent to participate in the study. Baseline imbalances: groups were similar according to age and gender. Withdrawals and exclusions: none. Subgroups measured: none. Subgroups reported: none. Mean age: group A (49 years), group B (47 years). Gender (M/F): group A (8/12), group B (10/10). Race/ethnicity: not available in report. Severity of condition: not available in report. Comorbidities: not available in report. Diagnostic criteria: two gastric biopsy specimens were used for biofilm demonstration by scanning electron microscopy. A biofilm architecture was defined as a dense accumulation of bacteria within an amorphous matrix. Two additional specimens were used for H pylori culture. Setting: tertiary endoscopy centre, single‐centre, outpatients. Inclusion criteria: participants who had a history of at least 4 H pylori eradication failures. Exclusion criteria: participants affected by serious concomitant illnesses and those with recent or continuing use of antibiotics. |
|
| Interventions |
Number randomised in each group: group A (n = 20), group B (n = 20). Dose
Duration of treatment period: 7 days. Timing: once a day. Delivery: oral. Providers: not reported. Cointerventions: none. Economic information: not available. Resource requirements: not reported. Integrity of delivery: all participants received intervention. Compliance: all participants received intervention. |
|
| Outcomes |
Primary outcome: eradication rate. Secondary outcomes
Time points measured and reported
Outcome definition
Person measuring/reporting: not reported. Unit of measurement: % of cure for H pylori eradication. Scales: variable according to outcome. Imputation of missing data: there were no losses. Assumed risk estimates: there were no losses. Power: 80%. |
|
| Notes |
Publication status: full text. Funding source: the study was funded by the Italian Ministry for University, Scientific, and Technological Research. Conflict of interest: authors declared no conflicts. Contact with authors: we contacted the authors on 26 January 2017 but received no answer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly, using a computer‐assisted allocation method". Comment: the authors used an appropriate method for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "In an open‐label, randomized controlled trial..."; "...patients underwent urea breath testing to determine their H pylori status". Comment: it is an open label study, but eradication rate was assessed by urea breath test (objective test). |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Quote: "In an open‐label, randomized controlled trial...patients underwent urea breath testing to determine their H pylori status" Comment: it is an open label study, but eradication rate was assessed by urea breath test (objective test). |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | Low risk | No losses |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | Low risk | No losses |
| Incomplete outcome data (attrition bias) Allergic adverse events | Low risk | No losses |
| Incomplete outcome data (attrition bias) Toxic adverse events | Low risk | No losses |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Low risk | This study seems to be free of other sources of bias. |
Emami 2014.
| Methods |
Aim of study: this study evaluated the impact of adding NAC to quadruple regimens ofH pylori eradication. Study design: randomised controlled study, open‐label. Study grouping: parallel group. Unit of allocation: by individuals. Country: Iran. Start date: September 2010. End date: July 2012. Duration of participation: 8 weeks. Ethical approval: this study was approved by the local ethics committee. |
|
| Participants |
Total number randomised: 180. Method of recruitment: consecutive participants between 17 and 80 years of age complaining of dyspeptic symptoms who referred to the Gastroenterology Clinics of Al‐Zahra hospital, Noor, Poursina Hakim Research Center and Ardakan hospital for endoscopy by a gastroenterologist between September 2010 and July 2012 recruited with full consent if H pylori tests were positive (confirmed by histology, rapid urease test, or stool antigen test). Informed consent obtained: full consent. Baseline imbalances: no difference observed between groups. Withdrawals and exclusions: 59 participants were lost for follow‐up and only one drop out in the ABCO group with significant side effects, leading to therapy cessation before completion of treatment. Subgroups measured: not reported. Subgroups reported: not reported. Mean age: group A (42.3 ± 13.9 years) and group B (47.1 ± 13.9 years). Gender (M/F): group A (27/33) and group B (30/31). Race/ethnicity: not available in report. Severity of condition: non‐ulcer disease (44%), duodenal ulcer (15%), gastric ulcer (1%). Comorbidities: not available in report. Diagnostic criteria:H pylori tests positive (confirmed by histology, rapid urease test, or stool antigen test). Setting: Gastroenterology Clinics of Al‐Zahra hospital, Noor, Poursina Hakim Research Center and Ardakan hospital. Inclusion criteria: all participants between 17 and 80 years of age complaining of dyspeptic symptoms with H pylori tests positive (confirmed by histology, rapid urease test, or stool antigen test). Exclusion criteria: participants having previously received eradication of H pylori treatment, presence of underlying disease such as cirrhosis, renal failure, severe cardiac disease, malignancy outside the gastrointestinal (GI) tract, the need for simultaneous use of non steroid anti inflammatory drugs (NSAIDs), the need for concomitant use of steroids or other immunosuppressive drugs, recent gastrointestinal bleeding, pregnancy, or lactating mothers. |
|
| Interventions |
Number randomised in each group: group A (n = 60), group B (n = 61). Dose
Duration of treatment period: 14 days. Timing: as described above. Delivery: as described above. Providers: not reported. Cointerventions: none. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: a total of 107 (87.2%) participants took > 85% of the prescribed treatment, which was considered good compliance. |
|
| Outcomes |
Primary outcomes: eradication rates, defined as the number of participants with negative H pylori stool antigen test (generic assay‐Dahlewitz‐Germany) after treatment. Secondary outcomes: side effects, defined as complaints determined by a specific questionnaire completed by the participants during the treatment period. Time points measured and reported: four to six weeks after treatment. Person measuring/reporting: not reported. Unit of measurement: individual. Scales: none. Imputation of missing data: the eradication rates, their 95% confidence intervals (CIs) at ITT analysis (all included participants), and per protocol (PP) analysis (all participants who took > 85% of prescribed treatment) were calculated. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: Isfahan University of Medical Sciences. Conflict of interest: authors have no competing interests. Contact with authors: we contacted the authors on 26 January 2017 but received no answer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned in one of two H pylori eradication regimens..." Comment: insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "In an open‐label, randomized controlled trial...H pylori stool antigen test (generic assay‐Dahlewitz‐Germany) was performed to evaluate the effectiveness of the eradication" Comment: it was an open label study, but eradication rate was assessed byH pylori stool antigen test (generic assay‐Dahlewitz‐Germany) (objective test). |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Quote: "In an open‐label, randomized controlled trial...H pylori stool antigen test (generic assay‐Dahlewitz‐Germany) was performed to evaluate the effectiveness of the eradication" Comment: it was an open label study, but eradication rate was assessed by H pylori stool antigen test (generic assay‐Dahlewitz‐Germany) (objective test). |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | High risk | Quote: "In an open‐label, randomized controlled trial..." Comment: this outcome could be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | High risk | Comment: there was 32.7% losses. No reasons were provided, nor the methods used for data imputation. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | High risk | Comment: there was 32.7% losses. No reasons were provided, nor the methods used for data imputation. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Allergic adverse events | High risk | Comment: there was 32.7% losses. No reasons were provided, nor the methods used for data imputation. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Toxic adverse events | High risk | Comment: there was 32.7% of losses. No reasons were provided, nor the methods used for data imputation. This fact could substantially influence the results. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Low risk | This study seems to be free of other sources of bias. |
Gurbuz 2005.
| Methods |
Aim of study: to evaluate wether the addition of NAC to the eradication regimen would affect eradication rates. Study design: randomised controlled study. Study grouping: parallel group. Unit of allocation: by individuals. Country: Turkey. Start date: January 2004. End date: January 2005. Duration of participation: 40 days. Ethical approval: not reported. |
|
| Participants |
Total number randomised: 70. Method of recruitment: consecutive participants scheduled to upper endoscopy. Informed consent obtained: not reported. Baseline imbalances: no difference observed between groups. Withdrawals and exclusions: seven losses in intervention group (four losses of follow‐up and three indeterminate result in post‐treatment testing) and five losses in control group (one loss of follow‐up and four indeterminate result in post‐treatment testing). Subgroups measured: not reported. Subgroups reported: not reported. Mean age: group A (46 ± 13.9) and group B (51.2 ± 1.4). Gender (M/F): group A (18/17) and group B (19/16). Race/ethnicity: not available in report. Severity of condition: dyspeptic participants. Comorbidities: not available in report. Diagnostic criteria: positive result in both histology and rapid urease test. Inclusion criteria: all participants scheduled for upper GI endoscopy during the study period. Exclusion criteria: recent use of antibiotics, bismuth salts or proton pump inhibitors, chronic use of nonsteroidal anti‐inflammatory drugs or corticosteroids, severe comorbid diseases, pregnancy or lactation, prior gastric surgery, history of peptic ulcers, and participant or family history of gastric malignancy. |
|
| Interventions |
Number randomised in each group: group A (n = 28), group B (n = 30). Dose:
Duration of treatment period: 10 days. Timing: as described above. Delivery: oral. Providers: not reported. Cointerventions: none. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: not reported. |
|
| Outcomes |
Primary outcome: eradication rate, defined as the number of participants with negative results in both histology and rapid urease test after treatment. Secondary outcome: side effects, defined as complaints by the participants during the treatment period. Time points measured and reported: one month after treatment. Person measuring/reporting: not reported. Unit of measurement: ‐ Scales: ‐ Imputation of missing data: not reported. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: not reported. Conflict of interest: not reported. Contact with authors: we contacted the authors on 26 January 2017 but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with positive results were randomly assigned into two groups..." Comment: insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Comment: insufficient information. However, since an objective test was used for assessing this outcome, we judge it unlikely that the lack of blinding could influence the results. |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | Unclear risk | Comment: insufficient information. This outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | Unclear risk | Comment: insufficient information. This outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | Unclear risk | Comment: insufficient information. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Comment: insufficient information. However, since an objective test was used for assessing this outcome, we judge it unlikely that the lack of blinding could influence the results. |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | Unclear risk | Comment: insufficient information. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | Unclear risk | Comment: Insufficent information. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | Unclear risk | Comment: insufficient information. This outcome could be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | Unclear risk | Comment: there were 5% losses. There was a unbalance between groups and no reason was provided. We are not sure about the extent to which these factors could influence the results. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | Unclear risk | Comment: there were 5% losses. There was a unbalance between groups and no reason was provided. We are not sure about the extent to which these factors could influence the results. |
| Incomplete outcome data (attrition bias) Allergic adverse events | Unclear risk | Comment: there were 5% losses. There was a unbalance between groups and no reason was provided. We are not sure about the extent to which these factors could influence the results. |
| Incomplete outcome data (attrition bias) Toxic adverse events | Unclear risk | Comment: there were 5% losses. There was a unbalance between groups and no reason was provided. We are not sure about the extent to which these factors could influence the results. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Unclear risk | The author did not report funding sources or conflict of interest. There is insufficient information to allow judgement. |
Hamidian 2015.
| Methods |
Aim of study: to evaluate whether the addition of N‐acetyl cysteine to the treatment regimen of H pylori infection would affect eradication rates of the disease. Study design: randomised controlled study, double‐blind. Study grouping: parallel group. Unit of allocation: by individuals. Country: Iran. Start date: June 2012. End date: June 2012 and July 2013. Duration of participation: 10 to 12 weeks. Ethical approval: the study was approved by the Ethical Committee of Tehran University of Medical Sciences. |
|
| Participants |
Total number randomised: 79. Method of recruitment: all participants with peptic‐like epigastric pain and dyspeptic symptoms, referred to the endoscopy unit and scheduled for upper GI endoscopy, were recruited for this prospective study. Informed consent obtained: informed consent was obtained from all the participants prior to the treatments. Baseline imbalances: no difference observed between groups. Withdrawals and exclusions: none. Subgroups measured: gender, age, drinking habit, smoking habit, type of disease. Subgroups reported: gender, age, drinking habit, smoking habit, type of disease. Mean age: group A (41.61 ± 10.45 years) and group B ( 42.41 ± 14.11 years). Gender (M/F): group A (17/21) and group B (13/28). Race/ethnicity: not available in report. Severity of condition: dyspepsia and peptic‐like epigastric pain. Non‐ulcer dyspepsia, gastric ulcer, and duodenal ulcer. Comorbidities: not available in report. Diagnostic criteria: the diagnosis of H pylori infection was made based on positive rapid urease test. Setting: endoscopy ward of Imam Khomeini Hospital of Tehran, Iran. Inclusion criteria: participants with positive rapid urease test. Exclusion criteria: recent use of antibiotics, bismuth salts, or proton pump inhibitors (PPIs), chronic use of nonsteroidal anti‐inflammatory drugs or corticosteroids, gastric outlet obstruction, pregnancy, renal failure, gastric malignancy, and prior gastric surgery. |
|
| Interventions |
Number randomised in each group: group A (n = 38), group B (n = 41). Dose
Duration of treatment period: 14 days. Timing: as described above. Delivery: oral. Providers: not reported. Cointerventions: none. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: not reported. |
|
| Outcomes |
Primary outcome: eradication rates, defined as the number of participants with negative urea breath test after treatment. Secondary outcome: none. Time points measured and reported: four weeks after treatment. Unit of measurement: ‐ Scales: ‐ Imputation of missing data: not reported. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: supported by a grant from Tehran University of Medical Sciences. Conflict of interest: the authors had no potential conflict of interests to declare. Contact with authors: we contacted the authors on 26 January 2017 but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized using a computerized random‐number generator to one of two therapeutic groups". Comment: the authors used an appropriate method for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "This study had a double‐blind,randomized placebo‐controlled design". Comment: the authors stated that placebo was used in control group. |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Comment: the authors stated that an objective test was used to evaluate eradication. |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Allergic adverse events | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Toxic adverse events | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Low risk | This study seems to be free from other sources of bias. |
Hansen 1994.
| Methods |
Aim of study: to evaluate the efficacy of NAC with amoxacillin in people with H pylori associated gastritis. Study design: randomised, double‐blind. Study grouping: parallel group. Unit of allocation: by individuals. Country: Denmark. Start date: October 1989. End date: December 1990. Duration of participation: 25 weeks. Ethical approval: approved by regional ethics committee. |
|
| Participants |
Total number randomised: 93. Method of recruitment: consecutive participants. Informed consent obtained: participants consented participation. Baseline imbalances: not reported. Withdrawals and exclusions: eleven excluded by non‐compliance, five because of side effects, three decided not to participate, four were withdrawn for other reasons. Subgroups measured: not reported. Subgroups reported: not reported. Mean age: 58 years. Gender (M/F): (42/28). Race/ethnicity: not reported. Severity of condition: not available in report. Comorbidities: not reported. Diagnostic criteria: positive test for H pylori either by histology, urease test or culture. Setting: not reported. Inclusion criteria: participants referred to gastroscopy because of dyspepsia with positive test for H pylori either by histology, urease test or culture. Exclusion criteria: participants with peptic ulcers, decreased renal function, treatment with colloidal bismuth subcitrate or antibiotics ‐ ongoing or within the last month, ongoing treatment with potential ulcer provoking or mucosa irritating medication such as steroids, NSAIDs and salicylates. |
|
| Interventions |
Number randomised in each group: group A (n = 35) group B (n = 35). Dose:
Duration of treatment period: 28 days. Timing: as described above. Delivery: not reported. Providers: not reported. Cointerventions: not reported. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: measured by pill count. Eleven participants failed to take treatment as planned and were removed from analysis. |
|
| Outcomes |
Primary outcomes: eradication rates, defined as the number of participants with negative histology, culture and rapid urease test six months after treatment. Secondary outcomes: none. Time points measured and reported: immediately after treatment and six months after treatment. Person measuring/reporting: not reported. Unit of measurement: not reported. Scales: not reported. Imputation of missing data: not reported. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: Astra Group A/S provided NAC and placebo. Conflict of interest: not reported. Contact with authors: we contacted the authors on 26 January 2017 and got no answer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "This study was designed as a prospective, randomised, double‐blind, placebo‐controlled trial" Comment: the authors stated that placebo was used in control group. |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Comment: blinding unlikely to introduce bias provided the outcome was assessed by objective test. |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | High risk | Comment: there were 24.7% of participants lost to follow up. Reasons were provided but no information about balance between groups or the methods used for data imputation were given. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | High risk | Comment: there were 24.7% of participants lost to follow up. Reasons were provided but no information about balance between groups or the methods used for data imputation were given. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Allergic adverse events | High risk | Comment: there were 24.7% of participants lost to follow up. Reasons were provided but no information about balance between groups or the methods used for data imputation were given. This fact could substantially influence the results. |
| Incomplete outcome data (attrition bias) Toxic adverse events | High risk | Comment: there were 24.7% of participants lost to follow up. Reasons were provided but no information about balance between groups or the methods used for data imputation were given. This fact could substantially influence the results. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods were measured and analysed. |
| Other bias | Unclear risk | Astra Group provided NAC and placebo. |
Karbasi 2013.
| Methods |
Aim of study: to determine the efficacy of NAC on eradication of H pylori infections in participants suffering from dyspepsia. Study design: randomised controlled study. Study grouping: parallel group. Unit of allocation: by individuals. Country: Iran. Start date: not reported. End date: not reported. Duration of participation: 6 weeks. Ethical approval: approved by the ethical committee of the Tehran Islamic Azad Medical University. |
|
| Participants |
Total number randomised: 60. Method of recruitment: not reported. Informed consent obtained: informed written consent, approved by the ethical committee of the Tehran Islamic Azad Medical University, was obtained from all participants before their participation in the study. Baseline imbalances: the participants were matched for age and sex. Information about other types of imbalances was not available. Withdrawals and exclusions: two participants dropped out from the placebo group for unknown reasons. Subgroups measured: endoscopic findings, age, gender. Subgroups reported: endoscopic findings, age, gender. Mean age: 41.5 ± 13.53 years, range 17 to 76 years overall. Not reported in details about group A or group B. Gender (M/F): group A (14/16), group B ( 14/14). Race/ethnicity: not reported. Severity of condition: dyspepsia. Normal oesophagus, non‐ulcerative oesophagitis, erosive oesophagitis, normal stomach, antral gastritis, pangastritis, erosive gastritis, normal duodenum, erosive duodenitis, duodenitis and duodenal ulcer were observed. Comorbidities: not reported. Diagnostic criteria: diagnosis of positive infection with H pylori was performed on the basis of endoscopic examination and H pylori rapid urease test. Setting: not reported. Inclusion criteria: history of chronic dyspepsia at least for three months, diagnosis of H pylori infection, no participation in clinical studies or not being under treatment with other drugs. Exclusion criteria: withdrawal, severe and debilitating underlying disease, recent consumption of antibiotics, bismuth, NSAID drugs, corticosteroids during the past one month, pregnancy or lactation, and a positive history of surgery or gastric cancer. |
|
| Interventions |
Number randomised in each group: group A (n = 30) , group B (n = 28). Dose
Duration of treatment period: 14 days. Timing: as described above. Delivery: oral. Providers: not reported. Cointerventions: none. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: not reported. |
|
| Outcomes |
Primary outcomes: eradication rates, defined as the number of participants with negative urea breath test after treatment. Secondary outcomes: none. Time points measured and reported: four weeks after treatment. Person measuring/reporting: a gastroenterologist. Unit of measurement: ‐ Scales: ‐ Imputation of missing data: not reported. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: not declared. Conflict of interest: authors declared no conflicts. Contact with authors: we contacted the authors on 26 January 2017 but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this randomized double‐blinded placebo‐control study 60 patients were enrolled." Comment: insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Comment: the method used for assessment of eradication was an objective test, unlikely to be influenced by blinding. |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Comment: the method used for assessment of eradication was an objective test, unlikely to be influenced by blinding of assessor. |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | Low risk | Comment: there was a loss of 6% (2/30) in the control group. Participants dropped out because of unknown reasons. It is unlikely that this fact could influence the results. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | Low risk | Comment: there was a loss of 6% (2/30) in the control group. Participants dropped out because of unknown reasons. It is unlikely that this fact could influence the results. |
| Incomplete outcome data (attrition bias) Allergic adverse events | Low risk | Comment: there was a loss of 6% (2/30) in the control group. Participants dropped out because of unknown reasons. It is unlikely that this fact could influence the results. |
| Incomplete outcome data (attrition bias) Toxic adverse events | Low risk | Comment: there was a loss of 6% (2/30) in the control group. Participants dropped out because of unknown reasons. It is unlikely that this fact could influence the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes that are of interest in this review were presented. |
| Other bias | Low risk | This study seems to be free of other sources of bias. |
Yoon 2014.
| Methods |
Aim of study: to evaluate the adjuvant effects of N‐acetylcysteine (NAC) on first‐line sequential therapy (SQT) for H pylori infection. Study design: randomised controlled study, open‐label. Study grouping: parallel group. Unit of allocation: by individuals. Country: South Korea. Start date: July 2013. End date: January 2014. Duration of participation: 38 days. Ethical approval: approved by the Institutional Review Board of Seoul National University Bundang Hospital. |
|
| Participants |
Total number randomised: 100. Method of recruitment: not reported. Informed consent obtained: all participants were provided informed consent. Baseline imbalances: there was no significant difference in baseline demographics between the two groups. Withdrawals and exclusions: group A: there were 8 losses before follow‐up and one participant who withdrew consent. Group B: there were 8 losses before follow‐up and 2 exclusions for noncompliance. Subgroups measured: not reported. Subgroups reported: not reported. Mean age: group A (57.2 ± 11.1 years), group B (58.8 ± 12.7 years). Gender (M/F): group A (25/25), group B (23/27). Race/ethnicity: not reported. Severity of condition: dyspepsia, peptic ulcer disease, gastric cancer or dysplasia, family history of gastric cancer, chronic atrophic gastritis. Comorbidities: not reported. Diagnostic criteria:H pylori infection was defined based on the results of at least one of the following three tests: 1) a positive C13‐urea breath test (UBT) results; 2) histological evidence of H pylori in the stomach by modified Giemsa staining; and 3) a positive rapid urease test (CLO test; Delta West, Bentley, Australia) result by gastric mucosal biopsy. Setting: not reported. Inclusion criteria: people with positive test defined above. Exclusion criteria: people with active peptic ulcer disease, a history of the use of PPIs, histamine‐2 receptor antagonists, or antibiotics within the previous two months. |
|
| Interventions |
Number randomised in each group: group A (n = 49), group B (n = 50). Dose
Duration of treatment period: 10 days. Timing: described above. Delivery: not reported. Providers: not reported. Cointerventions: not reported. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: compliance was very good in both groups (group A: 100%; group B: 95.2%). |
|
| Outcomes |
Primary outcomes: eradication rates, defined as the number of participants with negative urea breath test, histology or rapid urease test after treatment. Secondary outcomes: adverse events, recorded by an interview after treatment was finished. Time points measured and reported: 4 weeks after treatment. Person measuring/reporting: not reported. Unit of measurement: ‐ Scales: ‐ Imputation of missing data: eradication rates of H pylori were determined on intention‐to‐treat (ITT) and per‐protocol (PP) bases. All enrolled participants were included in the ITT analysis. Participants who were lost to follow‐up, were noncompliant, or dropped out due to adverse events were excluded from the PP analysis. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: not reported. Conflict of interest: no potential conflict of interest relevant to this article was reported. Contact with authors: we contacted the authors on 26 January 2017 and got no answer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to the SQT‐only or SQT+NAC group using a computer‐generated table in blocks of four." Comment: the authors used an appropriate method for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "...each patient was administrated 100 mg of 13C‐urea powder (UBiTkitTM; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) dissolved in 100 mL of water..." Comment: it was an open label study, but eradication rate was assessed by C‐urea breath test (objective test). |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Quote: "...each patient was administrated 100 mg of 13C‐urea powder (UBiTkitTM; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) dissolved in 100 mL of water..." Comment: it was an open label study, but eradication rate was assessed by C‐urea breath test (objective test). |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) Toxic adverse events | High risk | Quote: "...randomized open‐labeled pilot study at ..." Comment: this outcome could be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | High risk | Comment: there was a loss of 18% (with a balance between groups), but no reason was provided. An ITT analyses was used, but the methods used for data imputation were also not provided. We are not sure if these factors could influence the results. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | High risk | Comment: There was a loss of 18% (with a balance between groups), but no reason was provided. An ITT analyses was used, but the methods used for data imputation were also not provided. We are not sure if these factors could influence the results. |
| Incomplete outcome data (attrition bias) Allergic adverse events | High risk | Comment: There was a loss of 18% (with a balance between groups), but no reason was provided. An ITT analyses was used, but the methods used for data imputation were also not provided. We are not sure if these factors could influence the results. |
| Incomplete outcome data (attrition bias) Toxic adverse events | High risk | Comment: There was a loss of 18% (with a balance between groups), but no reason was provided. An ITT analyses was used, but the methods used for data imputation were also not provided. We are not sure if these factors could influence the results. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Low risk | This study seems to be free of other sources of bias. |
Zala 1994.
| Methods |
Aim of study: to test the hypothesis whether better eradication results could be achieved by addition of NAC to omeprazole/amoxicillin. Study design: randomised controlled study, open‐label. Study grouping: parallel group. Unit of allocation: by individuals. Country: Switzerland. Start date: not available in report. End date: not available in report. Duration of participation: 8 weeks. Ethical approval: not reported. |
|
| Participants |
Total number randomised: 34. Method of recruitment: not reported. Informed consent obtained: not reported. Baseline imbalances: not reported. Withdrawals and exclusions: none. Subgroups measured: smokers/non‐smokers. Subgroups reported: smokers/ non‐smokers. Mean age: group A (46 years), group B (39 years). Gender (M/F): group A (12/5), group B (15/2). Race/ethnicity: group A (1 Swiss, 10 from Eastern Europe/Mediterranean, 1 Iranian), group B (3 Swiss, 11 from Eastern Europe/Mediterranean, 3 from other countries). Severity of condition: duodenal ulcer. Comorbidities: not reported. Diagnostic criteria:H pylori infection confirmed by histology (3 biopsy specimens from gastric antrum and 2 from gastric body) and at least positive urease test or culture. Setting: outpatients. Inclusion criteria: outpatients with endoscopically documented recurrent duodenal ulcer. Exclusion criteria: alcoholism, previous gastric surgery, or intake of antibiotics, omeprazole, bismuth salts, corticosteroids or NSAIDs 4 weeks before study entry. |
|
| Interventions |
Number randomised in each group: group A (n = 17), group B (N = 17). Dose:
Duration of treatment period: 10 days. Timing: as described above. Delivery: not reported. Providers: not reported. Co‐interventions: not reported. Economic information: not reported. Resource requirements: not reported. Integrity of delivery: not reported. Compliance: not reported. |
|
| Outcomes |
Primary outcome: eradication rates, defined as the number of participants with rapid urease test negative, culture negative and absence of bacteria in histologic examination. Secondary outcome: adverse events (undefined). Time points measured and reported: 4 weeks after treatment. Person measuring/reporting: not reported. Unit of measurement: ‐ Scales: ‐ Imputation of missing data: not reported. Assumed risk estimates: not reported. Power: not reported. |
|
| Notes |
Publication status: full text. Funding source: not reported. Conflict of interest: authors declared no conflicts. Contact with authors: we contacted the authors on 26 January 2017 but received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Ineinerprospektiven,randomisierten offenen studie wurden duodenalulkuspatient ennachdem H.‐pylori‐Nachweis einer von zwei Therapie gruppenzugeteilt" (translation: "In a prospective, randomized open study, duodenal ulcer patients were randomized to H pylori detection of one of two therapy groups.") Comment: insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) Eradication (frequency) | Low risk | Quote: "Ineinerprospektiven,randomisierten offenen studie wurden duodenalulkuspatient ennachdem H.‐pylori‐Nachweis einer von zwei Therapie gruppenzugeteilt" (translation: "In a prospective, randomized open study, duodenal ulcer patients were randomized to H pylori detection of one of two therapy groups.") Comment: it was an open label study, but eradication rate was assessed by rapid urease test (objective test). |
| Blinding of participants and personnel (performance bias) Gastrintestinal adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Allergic adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Blinding of participants and personnel (performance bias) Toxic adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Eradication (frequency) | Low risk | Quote: "Ineinerprospektiven,randomisierten offenen studie wurden duodenalulkuspatient ennachdem H.‐pylori‐Nachweis einer von zwei Therapie gruppenzugeteilt" (translation: "In a prospective, randomized open study, duodenal ulcer patients were randomized to H pylori detection of one of two therapy groups.") Comment: it was an open label study, but eradication rate was assessed by rapid urease test (objective test). |
| Blinding of outcome assessment (detection bias) Gastrintestinal adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Allergic adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Toxic adverse events | High risk | Quote: "In a prospective, randomized open study.." Comment: this was an open label study. This outcome could be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Eradication (frequency) | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Gastrintestinal adverse events | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Allergic adverse events | Low risk | There were no losses. |
| Incomplete outcome data (attrition bias) Toxic adverse events | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were properly presented. |
| Other bias | Low risk | This study seems to be free of other sources of bias. |
GI: gastrointestinal H pylori: Helicobacter pylori ITT: intention‐to‐treat mg: milligram n: number NAC: N‐acetylcysteine NSAIDs: non‐steroidal anti‐inflammatory drugs
Characteristics of ongoing studies [ordered by study ID]
NCT01572597.
| Trial name or title | Increased second‐line eradication rate of helicobacter pylori by adding N‐acetylcystein or metronidazole to the conventional triple therapy |
| Methods | Allocation: randomised. Intervention model: parallel assignment. Masking: open label. Primary purpose: treatment. |
| Participants | Participant (20 to 80 years old) after treatment for H pylori eradication Still clinically with evidence of gastric H pylori infection |
| Interventions |
Experimental: acetylcysteine 10‐day triple therapy plus N‐acetylcysteine to remove the biofilm. Active comparator: metronidazole 10‐day triple therapy plus metronidazole (concomitant therapy) as the active comparator. |
| Outcomes |
Primary outcome measures: re‐eradication rate (time frame: 4 weeks after complete use of drug for treatment). A negative post‐treatment C13‐urea breath test result at more than 4 weeks after complete use of drug for treatment. Secondary outcome measures: influence of participant's CYP2C19 genotype on re‐eradication rate (time frame: 4 weeks after complete use of drug for treatment). Influence of participant's CYP2C19 genotype (EM, IM or PM) on re‐eradication rate of H pylori |
| Starting date | June 2011 |
| Contact information | Ming‐Cheh CHEN, M.D. MingCheh_chen@tzuchi.com.tw |
| Notes |
NCT02249546.
| Trial name or title | Efficacy of acetylcysteine‐containing triple therapy in the first line of H pylori infection |
| Methods | Allocation: randomised. Intervention model: parallel assignment. Masking: open label. Primary purpose: treatment. |
| Participants | H pylori infected participants (20 years old or more) who have willingness to receive eradication therapy. |
| Interventions |
Experimental: acetylcysteine + PPI‐amoxicillin‐clarithromycin N‐acetylcysteine 600 mg twice a day dexlansoprazole 60 mg once a day amoxicillin 1000 mg twice a day clarithromycin 500 mg twice a day (14 days). Active comparator: PPI‐amoxicillin‐clarithromycin dexlansoprazole 60 mg once a day amoxicillin 1000 mg twice a day clarithromycin 500 mg twice a day (14 days). |
| Outcomes |
Primary outcome measures:H pylori eradication rate (time frame: 6 weeks). Secondary outcome measures
|
| Starting date | September 2014 |
| Contact information | Chieh‐Chang Chen, MD, MSc chiehchang.chen@gmail.com |
| Notes |
H pylori: Helicobacter pylori mg: milligram PPI: proton pump inhibitor
Differences between protocol and review
We decided to perform a subgroup analysis taking into account smoking habit, because there is evidence that smoking could reduce eradication rates (Itskoviz 2017), and two studies assessed this subgroup among participants (Hamidian 2015; Zala 1994).
At the protocol phase we planned to use random‐effects model only if we detected statistical heterogeneity (I2 more than 50%). However, during the review phase, we agreed that the random‐effects model should be used in the presence of any kind of heterogeneity (clinical, methodological or statistical, or both). This decision was based on the fact that even in the absence of statistical heterogeneity, an important clinical or methodological heterogeneity could be found.
After peer review, and statistical and methodological advice, we decided to change the planned 'Summary of findings' table, showing one pooled comparison with subgroups into it: "NAC plus any antibiotic regimen versus the same antibiotic regimen alone".
Standard bismuth quadruple therapy described in protocol was not tested in addition with NAC. However, a bismuth quadruple therapy was tested in Emami 2014, replacing metronidazole and tetracycline by amoxicillin and clarithromycin. We decided to include this comparison.
One author (CSB) decided to no longer contribute to this review after the protocol was published.
We performed a post‐hoc sensitivity analysis to see whether the results are still applicable when the studies that used antibiotic regimens that are no longer recommended are excluded. This could improve the external validity of the review.
Contributions of authors
Conceiving the protocol: LESF.
Designing the protocol: LESF, RR.
Co‐ordinating the protocol: LESF.
Designing search strategies: Information Specialist of the UGPD group.
Writing the protocol: LESF, RR.
Providing general advice on the protocol: RR.
Securing funding for the protocol: LESF.
Performing previous work that was the foundation of the current study: LESF.
Sources of support
Internal sources
-
PETRÓPOLIS MEDICAL SCHOOL, Brazil.
Luis Eduardo Fontes is a professor at Petrópolis Medical School and receives a salary.
External sources
No sources of support supplied
Declarations of interest
None of the review authors are sponsored, employed, or involved in clinical studies of antimicrobial H pylori therapy, or the manufacture or use of N‐acetylcysteine (NAC).
LEF: none known.
CB: none known.
ALM: none known.
CGZ: none known.
RR: none known.
New
References
References to studies included in this review
Cammarota 2009 {published data only}
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clinical Gastroenterology and Hepatology 2010;8(9):817‐20.e3. [DOI] [PubMed] [Google Scholar]
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Histopathologic and morphologic evaluations of resistant strains of H. pylori, and consequent new therapeutic approaches: usefulness of N‐acetil‐cysteine and culture‐guided antibiotics. Helicobacter 2009;14(4):334. [Google Scholar]
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Pre‐treatment with N‐acetil‐cysteine and culture‐guided rescue therapy for eradication of Helicobacter pylori infection. Gastroenterology 2009;136(5 Suppl 1):A345W. [Google Scholar]
Emami 2014 {published data only}
- Emami MH, Zobeiri M, Rahimi H, Arjomandi F, Daghagzadeh H, Adibi P, et al. N‐acetyl cysteine as an adjunct to standard anti‐Helicobacter pylori eradication regimen in patients with dyspepsia: A prospective randomized, open‐label trial. Advanced Biomedical Research 2014;3:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurbuz 2005 {published data only}
- Gurbuz AK, Ozel AM, Ozturk R, Yildirim S, Yazgan Y, Demirturk L. Effect of N‐acetyl cysteine onHelicobacter pylori. Southern Medical Journal 2005;98(11):1095‐7. [DOI] [PubMed] [Google Scholar]
Hamidian 2015 {published data only}
- Hamidian SMT, Aletaha NS, Taslimi R, Montazeri M. An additive effect of oral N‐acetyl cysteine on eradication of Helicobacter pylori. Journal of Pathogens 2015;2015:540271. [DOI: 10.1155/2015/540271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 1994 {published data only}
- Hansen IM, Larsen JF, Qvist N, Lundborg CJ, Brandslund I, Axelsson CK. N‐acetylcysteine does not enhance eradication of Helicobacter pylori by amoxycillin. European Journal of Clinical Research 1994;5:185‐91. [Google Scholar]
Karbasi 2013 {published data only}
- Karbasi A, Hossein HS, Shohrati M, Amini M, Najafian B. Effect of oral N‐acetyl cysteine on eradication of Helicobacter pylori in patients with dyspepsia. Minerva Gastroenterologica e Dietologica 2013;59(1):107‐12. [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon H, Lee DH, Jang ES, Kim J, Shin CM, Park YS, et al. Effects of N‐acetylcysteine on first‐line sequential therapy for Helicobacter pylori Infection: A randomized controlled pilot trial. Gut and Liver 2016;10(4):520‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Lee DH, Jang ES, Kim J, Shin CM, Park YS, et al. The effects of N‐acetylcysteine on first‐line 10‐day sequential therapy for Helicobacter pylori infection: A randomized controlled trial. United European Gastroenterology Journal 2014;2(1 Suppl 1):A4301. [Google Scholar]
Zala 1994 {published data only}
- Zala G, Flury R, Wust J, Meyenberger C, Ammann R, Wirth HP. N‐acetylcysteine improves eradication of Helicobacter pylori by omeprazole/amoxicillin in cigarette smokers. Schweizerische Medizinische Wochenschrift 1994;124(31‐32):1391‐7. [PubMed] [Google Scholar]
References to ongoing studies
NCT01572597 {published data only}
- NCT01572597. Increased re‐eradication rate of Helicobacter pylori by adding N‐acetylcysteine or metronidazole to the triple therapy [Increased second‐line eradication rate of Helicobacter pylori by adding N‐acetylcysteine or metronidazole to the conventional triple therapy]. clinicaltrials.gov/ct2/show/NCT01572597 (first received 6 April 2012).
NCT02249546 {published data only}
- NCT02249546. Efficacy of acetylcysteine‐containing triple therapy in the first line of Helicobacter pylori infection [Comparison of the efficacy of triple therapy with or without acetylcysteine in the first line of Helicobacter pylori infection ‐ a multicenter randomized comparative trial]. clinicaltrials.gov/ct2/show/NCT02249546 (first received 25 September 2014).
Additional references
Aslam 2007
- Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N‐acetylcysteine reduces biofilm‐embedded bacteria on vascular catheters. Antimicrobial Agents and Chemotherapy 2007;51(4):1556‐8. [PUBMED: 17220399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aslam 2011
- Aslam S, Darouiche RO. Role of antibiofilm‐antimicrobial agents in controlling device‐related infections. International Journal of Artificial Organs 2011;34(9):752‐8. [PUBMED: 22094553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blaser 2005
- Blaser MJ. An endangered species in the stomach. Scientific American 2005;292(2):38‐45. [PUBMED: 15715390] [DOI] [PubMed] [Google Scholar]
Borgstrom 1986
- Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N‐acetylcysteine in man. European Journal of Clinical Pharmacology 1986;31(2):217‐22. [PUBMED: 3803419] [DOI] [PubMed] [Google Scholar]
Calvet 2013
- Calvet X, Ramirez Lazaro MJ, Lehours P, Megraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013;18 Suppl 1:5‐11. [PUBMED: 24011238] [DOI] [PubMed] [Google Scholar]
Cammarota 2010
- Cammarota G, Branca G, Ardito F, Sanguinetti M, Ianiro G, Cianci R, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clinical Gastroenterology and Hepatology 2010;8(9):817‐20.e3. [PUBMED: 20478402] [DOI] [PubMed] [Google Scholar]
Cammarota 2012
- Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Alimentary pharmacology & therapeutics 2012;36(3):222‐30. [PUBMED: 22650647] [DOI] [PubMed] [Google Scholar]
Carron 2006
- Carron MA, Tran VR, Sugawa C, Coticchia JM. Identification of Helicobacter pylori biofilms in human gastric mucosa. Journal of Gastrointestinal Surgery 2006;10(5):712‐7. [PUBMED: 16713544] [DOI] [PubMed] [Google Scholar]
Chaabane 2011
- Chaabane NB, Mansour IB, Hellara O, Loghmeri H, Bdioui F, Safer L, et al. Role of Helicobacter pylori infection in iron deficiency anemia [Role de l'infection par l'Helicobacter pylori dans l'anemie ferriprive]. Presse Medicale 2011;40(3):239‐47. [PUBMED: 21196096] [DOI] [PubMed] [Google Scholar]
Correa 1992
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process ‐ First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Research 1992;52(24):6735‐40. [PUBMED: 1458460] [PubMed] [Google Scholar]
Coticchia 2006
- Coticchia JM, Sugawa C, Tran VR, Gurrola J, Kowalski E, Carron MA. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. Journal of Gastrointestinal Surgery 2006;10(6):883‐9. [PUBMED: 16769546] [DOI] [PubMed] [Google Scholar]
De Caro 1989
- Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittel‐Forschung 1989;39(3):382‐6. [PUBMED: 2757663] [PubMed] [Google Scholar]
Dunn 1997
- Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clinical Microbiology Reviews 1997;10(4):720‐41. [PUBMED: 9336670] [DOI] [PMC free article] [PubMed] [Google Scholar]
EHSG 2012
- European Helicobacter Study Group (EHSG). Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. GUT 2012;61(5):302‐84. [DOI] [PubMed] [Google Scholar]
ERC 2014
- Cochrane Editorial Resources Committee (ERC). ERC data collection form for intervention review for RCTs only. www.community‐archive.cochrane.org/sites/default/files/uploads/forums/u389/ERC%20data%20collection%20form%20for%20intervention%20reviews%20for%20RCTs%20only.doc (accessed 12 September 2016).
Ermis 2015
- Ermis F, Senocak Tasci E. Current Helicobacter pylori treatment in 2014. World Journal of Methodology 2015;5(2):101‐7. [PUBMED: 26140276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fair 2014
- Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspectives in medicinal chemistry 2014;6:25‐64. [PUBMED: 25232278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontes 2016
- Fontes LES, Batista CS, Martimbianco ALC, Zanin CG, Riera R. N‐acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2014
- Garcia A, Salas‐Jara MJ, Herrera C, Gonzalez C. Biofilm and Helicobacter pylori: from environment to human host. World Journal of Gastroenterology 2014;20(19):5632‐8. [PUBMED: 24914322] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 9 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Graham 2010
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59(8):1143‐53. [PUBMED: 20525969] [DOI] [PubMed] [Google Scholar]
Guyatt 2006
- Guyatt G, Gutterman D, Baumann MH, Addrizzo‐Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest 2006;129(1):174‐81. [PUBMED: 16424429] [DOI] [PubMed] [Google Scholar]
Hall‐Stoodley 2009
- Hall‐Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cellular Microbiology 2009;11(7):1034‐43. [PUBMED: 19374653] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, Cochrane Statistical Methods Group, Cochrane Bias Methods Group. Chapter 8.5: The Cochrane Collaboration's tool for assessing risk of bias. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunt 2011
- Hunt RH, Xiao SD, Megraud F, Leon‐Barua R, Bazzoli F, Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. Journal of Gastrointestinal and Liver Diseases 2011;20(3):299‐304. [PUBMED: 21961099] [PubMed] [Google Scholar]
Itskoviz 2017
- Itskoviz D, Boltin D, Leibovitzh H, Tsadok Perets T, Comaneshter D, Cohen A, et al. Smoking increases the likelihood of Helicobacter pylori treatment failure. Digestive and Liver Disease 2017;49(7):764‐8. [PUBMED: 28427781] [DOI] [PubMed] [Google Scholar]
Kao 2016
- Kao CY, Sheu BS, Wu JJ. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomedical Journal 2016;39(1):14‐23. [PUBMED: 27105595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1998
- Kelly GS. Clinical applications of N‐acetylcysteine. Alternative Medicine Review 1998;3(2):114‐27. [PUBMED: 9577247] [PubMed] [Google Scholar]
Kuipers 1997
- Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Alimentary Pharmacology & Therapeutics 1997;11 Suppl 1:71‐88. [PUBMED: 9146793] [DOI] [PubMed] [Google Scholar]
Malfertheiner 2009
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374(9699):1449‐61. [PUBMED: 19683340] [DOI] [PubMed] [Google Scholar]
Malfertheiner 2012
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection — the Maastricht IV/ Florence Consensus Report. Gut 2012;61(5):646‐64. [PUBMED: 22491499] [DOI] [PubMed] [Google Scholar]
Malfertheiner 2017
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence Consensus Report. Gut 2017;66(1):6‐30. [PUBMED: 27707777] [DOI] [PubMed] [Google Scholar]
Marshall 1984
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1(8390):1311‐5. [PUBMED: 6145023] [DOI] [PubMed] [Google Scholar]
Millea 2009
- Millea PJ. N‐acetylcysteine: multiple clinical applications. American Family Physician 2009;80(3):265‐9. [PUBMED: 19621836] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Momtaz 2012
- Momtaz H, Souod N, Dabiri H, Sarshar M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World Journal of Gastroenterology 2012;18(17):2105‐11. [PUBMED: 22563199] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sherwood 2002
- Sherwood PV, Wibawa JI, Atherton JC, Jordan N, Jenkins D, Barrett DA, et al. Impact of acid secretion, gastritis, and mucus thickness on gastric transfer of antibiotics in rats. Gut 2002;51(4):490‐5. [PUBMED: 12235069] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stasi 2009
- Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113(6):1231‐40. [PUBMED: 18945961] [DOI] [PubMed] [Google Scholar]
Venerito 2013
- Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta‐analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 2013;88(1):33‐45. [PUBMED: 23880479] [DOI] [PubMed] [Google Scholar]
Yonezawa 2010
- Yonezawa H, Osaki T, Kurata S, Zaman C, Hanawa T, Kamiya S. Assessment of in vitro biofilm formation by Helicobacter pylori. Journal of Gastroenterology and Hepatology 2010;25 Suppl 1:S90‐4. [PUBMED: 20586874] [DOI] [PubMed] [Google Scholar]
Yoon 2015
- Yoon H, Lee DH, Jang ES, Kim J, Shin CM, Park YS, et al. Effects of N‐acetylcysteine on first‐Line sequential therapy for Helicobacter pylori infection: a randomized controlled pilot trial. Gut and Liver 2015;9:1‐6. [PUBMED: 26347514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2014
- Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta‐analysis of randomized controlled studies with 12‐month follow‐up. Journal of Clinical Gastroenterology 2014;48(3):241‐7. [PUBMED: 24002127] [DOI] [PubMed] [Google Scholar]


