Abstract
Hematopoietic stem cell (HSC) gene therapy is curative for various hereditary diseases; however, high-efficiency transduction in HSCs remains crucial to improve the prospects for hemoglobinopathies. We previously optimized lentiviral transduction in human CD34+ cells with serum-free medium containing minimal cytokines, allowing efficient transduction (∼50%) and robust xenograft engraftment. In this study, we further improved lentiviral transduction in human CD34+ cells. High-density culture conditions (4e6/mL) resulted in ∼5-fold more efficient transduction in CD34+ cells (p < 0.01) compared with standard cell density (1e5/mL). After co-culturing vector-exposed CD34+ cells with non-transduced CD34+ cells, high-density culture conditions enhanced lentiviral gene marking in the non-transduced population (p < 0.01) compared with low-density conditions, suggesting that increasing cell-to-cell contact allows more efficient transduction. Two adjuvants, poloxamer 407 (100 μg/mL) and prostaglandin E2 (10 μM), were added to high-density CD34+ cells, resulting in ∼4-fold more efficient transduction (p < 0.01) without significant toxicity compared with no adjuvant control. In summary, we developed a highly efficient lentiviral transduction method in high-density CD34+ cell culture with poloxamer 407 and prostaglandin E2, allowing overall ∼10-fold improvement in transduction efficiency and consistently achieving more than 90% transduction and an average vector copy number of ∼10. Our optimized transduction method should improve gene therapy approaches using lentiviral vectors targeting HSCs.
Keywords: lentiviral vector, hematopoietic stem cells, transduction efficiency
Introduction
Hematopoietic stem cell (HSC)-targeted gene therapy has been reported to cure various hereditary immunodeficiencies1, 2, 3, 4, 5 and is under development for hemoglobin disorders, including β-thalassemia and sickle cell disease (SCD).6, 7 In HSC-targeted gene therapy, the defective protein is compensated by delivery of a normal or therapeutic gene to patient HSCs using HIV type 1 (HIV-1)-based lentiviral vectors. Following transplantation of lentivirally transduced HSCs, the normal or therapeutic gene can be expressed in targeted cells, allowing long-term phenotypic correction in gene therapy patients. We have previously performed extensive optimization for lentiviral transduction; however, high-efficiency transduction of CD34+ cells remains crucial for successful gene therapy, especially for SCD, because pancellular expression of the transgene at high levels is important for phenotypic correction. In our current gene therapy trial for SCD, preliminary data suggest that high-level transduction in CD34+ cells is important for improving phenotypic correction.8
Our lentiviral transduction method for human CD34+ cells employs serum-free X-VIVO10 medium containing cytokines (stem cell factor [SCF], fms-like tyrosine kinase 3 ligand [FL], and thrombopoietin [TPO]) on fibronectin-coated plates after overnight pre-stimulation.9 Using these optimized transduction conditions, high-level gene marking with lentiviral vectors can be obtained in hematopoietic repopulating cells capable of long-term engraftment in humanized xenograft mouse models and rhesus gene therapy models.9, 10, 11 Increasing proliferation with cytokine pre-stimulation improves transduction efficiency but decreases engraftment in xenograft mice, suggesting that lentiviral transduction methods in CD34+ cells must balance transduction efficiency and engraftability. Our current optimized methods are capable of transducing around 50% of human CD34+ cells with lentiviral vectors.
In this study, we sought to further optimize lentiviral transduction in human CD34+ cells without concurrent increases in proliferation and toxicity. We hypothesized that culturing CD34+ cells at high density would save time and money in large-scale transduction settings and improve transduction efficiency because cell-to-cell contacts in high-density cell culture may mimic bone marrow conditions more than normal low-density cell culture. In addition, we hypothesized that adjuvant supplementation would improve lentiviral transduction in high-density CD34+ cell culture because cell-to-cell as well as vector-to-cell contacts may be enhanced by some adjuvants. To investigate these hypotheses, we evaluated lentiviral transduction in human CD34+ cells using high-density CD34+ cell culture and adjuvant supplementation in addition to our previously optimized transduction conditions.
Results
High-Density Culture of Human CD34+ Cells Enhances Transduction Efficiency with Lentiviral Vectors
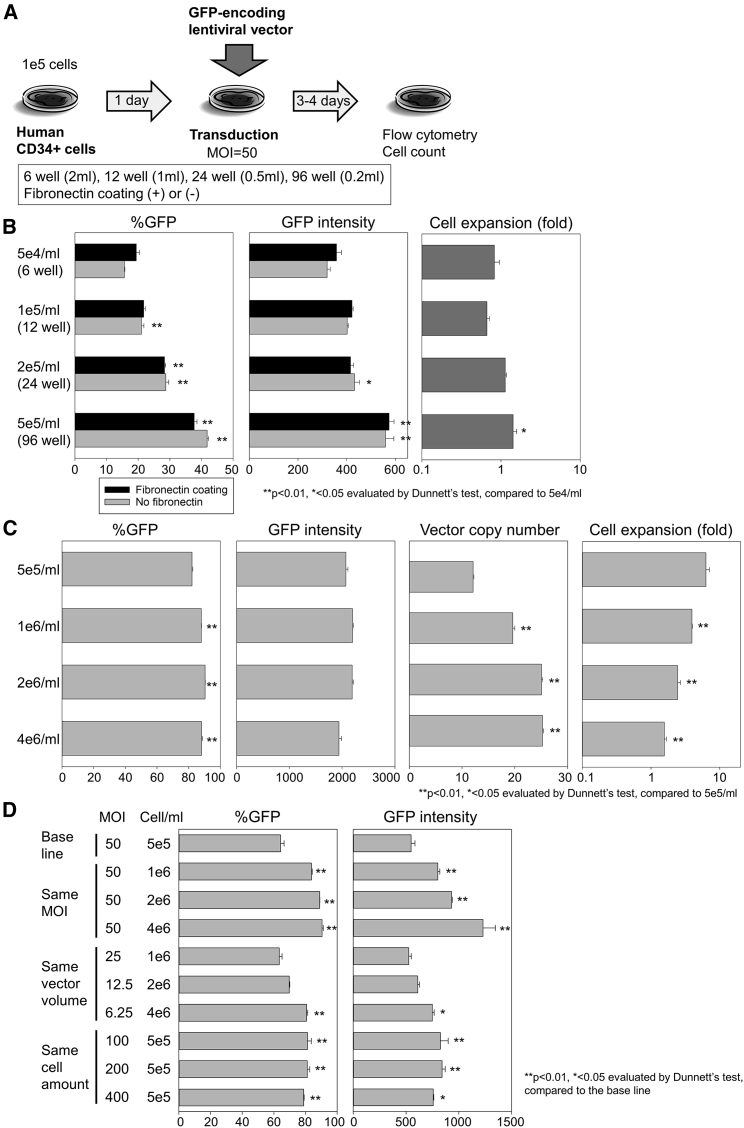
To investigate whether cell culture density affects transduction efficiency with lentiviral vectors, we transduced a fixed amount (1e5) of human CD34+ cells with an EGFP-encoding lentiviral vector at MOI 50 after overnight pre-stimulation in 6-well (5e4/mL), 12-well (1e5/mL), 24-well (2e5/mL), and 96-well (5e5/mL) plates with or without fibronectin coating (Figure 1A). Three days later, transduction efficiency was evaluated by GFP-positive percentage (%GFP) and GFP intensity in GFP-positive fractions, and cell proliferation was evaluated by cell counts. We observed up to 2.7-fold higher %GFP (up to 42%) (1–5e5/mL, p < 0.01) and up to 1.8-fold higher GFP intensity (2–5e5/mL, p < 0.05) in high-density CD34+ cell culture compared with low-density culture (5e4/mL) (Figure 1B, left and center). Higher cell proliferation was observed in 5e5/mL CD34+ cell culture compared with low-density culture (5e4/mL) (Figure 1B, right). Transduction efficiency was slightly increased by fibronectin coating in low-density CD34+ cell culture; however, it was more strongly affected by cell density than fibronectin coating.
Figure 1.
High-Density Culture of Human CD34+ Cells Enhances Transduction Efficiency with Lentiviral Vectors
(A) We transduced human CD34+ cells (1e5) with an EGFP-encoding lentiviral vector at MOI 50 after overnight pre-stimulation (100 ng/mL each of stem cell factor, fms-related tyrosine kinase 3 ligand, and thrombopoietin) among 6-well (5e4/mL), 12-well (1e5/mL), 24-well (2e5/mL), and 96-well (5e5/mL) plates with or without fibronectin coating. (B) We evaluated transduction efficiency (%GFP), GFP intensity in GFP-positive fractions, and cell counts 3 days later. (C) We then compared lentiviral transduction at various concentrations of CD34+ cells (5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL) in a 96-well plate (200 μL/well) at the same MOI 50 without fibronectin coating. We evaluated %GFP, GFP intensity, and cell counts 4 days after transduction. In addition, average vector copy number (VCN) per cell was analyzed 6 days after transduction. (D) We evaluated higher cell densities of CD34+ cell transduction (1e6/mL, 2e6/mL, and 4e6/mL) in the same vector volume as well as higher vector volumes of CD34+ cell transduction in the same cell amount (5e5/mL). Values: mean ± SE. All experiments were performed in triplicate.
To investigate CD34+ cell densities greater than 5e5/mL, we transduced escalating doses of CD34+ cells (5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL) in a fixed amount of culture medium (200 μL/well in a 96-well plate) at MOI 50 without fibronectin coating (Figure 1C). We observed higher %GFP in high-density CD34+ cell culture (1–4e6/mL, p < 0.01) compared with 5e5/mL culture (Figure 1C, left); however, it was difficult to evaluate differences in GFP expression because ∼90% of GFP positivity was achieved uniformly under these conditions. Therefore, we evaluated average vector copy number (VCN) per cell in transduced CD34+ cells 6 days after transduction. Up to 2.1-fold higher VCNs (up to 25) were obtained in high-density CD34+ cell culture (1–4e6/mL, p < 0.01) compared with 5e5/mL culture (Figure 1C, center right). Cell proliferation was reduced in high-density CD34+ cell culture (1–4e6/mL, p < 0.01) compared with 5e5/mL culture (Figure 1C, right).
Because greater vector volumes must be added to maintain the same MOI 50 (vector amounts per cell) for higher cell densities of CD34+ cells in a fixed amount of medium (200 μL in a 96-well plate), we separately evaluated the factors of (1) higher cell densities of CD34+ cell transduction (1e6/mL, 2e6/mL, and 4e6/mL) in the same vector volume (MOI 25, 12.5, and 6.25, respectively) and (2) greater vector volumes (MOI 100, 200, and 400) of CD34+ cell transduction with the same cell amount (5e5/mL) for 200 μL culture medium in a 96-well plate (Figure 1C). Higher cell densities of CD34+ cells gradually increased %GFP and GFP intensity until a maximal cell density of 4e6/mL (p < 0.05), whereas %GFP and GFP intensity arrived at plateau levels at MOI 100 (p < 0.01). The combination of high-density culture (4e6/mL) with high MOI (50) resulted in the highest %GFP and GFP intensity in transduced CD34+ cells. These data demonstrate that high-density CD34+ cell culture significantly improves lentiviral transduction.
Cell-to-Cell Contact Is Important to Improve Transduction Efficiency in High-Density Human CD34+ Cell Culture
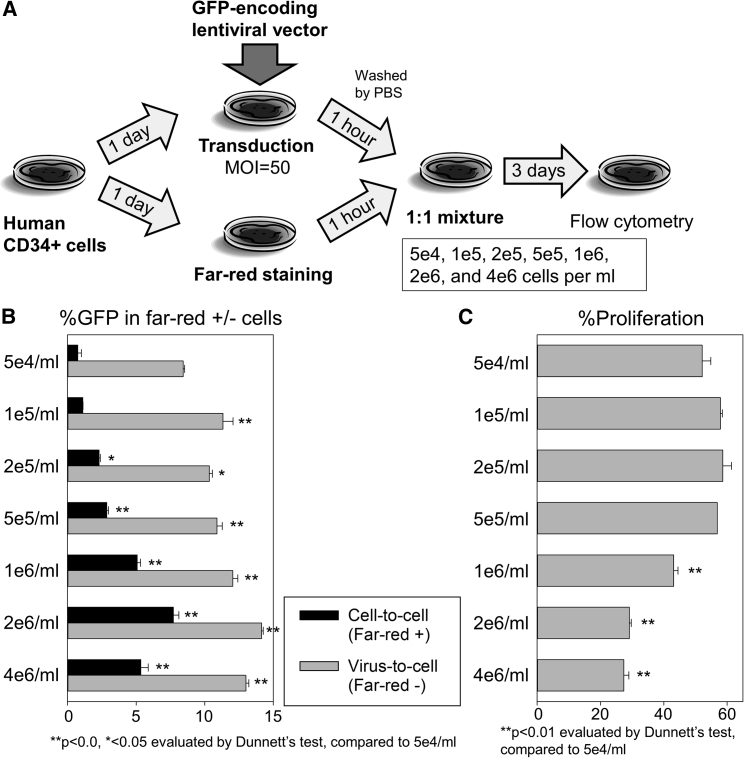
Because transduction efficiency reached plateau levels in high MOI transduction, but high-density cell culture increased transduction efficiency even at high MOIs, we hypothesized that high-density cell culture might increase vector receptor amounts on CD34+ cells. Recently, low-density lipoprotein receptor (LDL-R) has been reported to mediate human CD34+ cell transduction with vesicular stomatitis virus G protein (VSV-G)-pseudotyped lentiviral vectors.12 Therefore, we evaluated LDL-R expression in high-density CD34+ cell culture (Figure S1); however, similar LDL-R expression was observed among various cell densities (5e4/mL, 1e5/mL, 2e5/mL, 5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL). Because faster cell growth can increase transduction efficiency with lentiviral vectors, we also evaluated cell proliferation in high-density CD34+ cell culture using a more reliable method (cell proliferation dye) without lentiviral transduction (Figure 2C); however, we again observed lower cell proliferation in high-density culture on day 7 (1–4e6/mL, p < 0.01) compared with low-density culture (5e4/mL).
Figure 2.
Cell-to-Cell Contacts Are Important to Improve Lentiviral Transduction in High-Density Human CD34+ Cell Culture
(A) We transduced half of the CD34+ cells with a GFP-expressing lentiviral vector at MOI 50, whereas the other half of CD34+ cells were stained with a far-red fluorescent dye. One hour later, transduced CD34+ cells were washed with PBS medium and co-cultured (1:1 ratio) with far-red stained CD34+ cells (non-transduced) among various cell densities (5e4/mL, 1e5/mL, 2e5/mL, 5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL). (B) We evaluated %GFP in far-red positive and -negative cells 3 days after transduction. (C) In addition, we evaluated cell proliferation of human CD34+ cells cultured for 7 days at various cell densities using cell proliferation dye. Values: mean ± SE. Experiments were performed in duplicate (B) or triplicate (C).
We then hypothesized that direct cell-to-cell contact is important to improve lentiviral transduction in human CD34+ cells because high-density cell culture conditions produce cell layers in culture plates. To investigate this, we transduced half of the CD34+ cells with a GFP-expressing lentiviral vector at MOI 50 after overnight pre-stimulation, whereas the other half was stained with a far-red fluorescent dye (Figure 2A). One hour later (to permit internalization of the lentiviral genome), transduced CD34+ cells were washed with PBS medium and co-cultured (1:1 ratio) with far-red-stained CD34+ cells (non-transduced) at various cell densities (5e4/mL, 1e5/mL, 2e5/mL, 5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL). Three days after transduction, %GFP in far-red-positive cells was strongly increased (up to 10-fold) in high-density CD34+ cell culture (2e5–4e6, p < 0.05) compared with the lowest-density condition (5e4/mL), whereas slightly higher %GFP (1.2- to 1.7-fold) was observed in far-red-negative cells (1e5–4e6, p < 0.05) (Figure 2B). These data demonstrate that cell-to-cell contact is important to increase transduction efficiency in human CD34+ cells.
Addition of Poloxamer and Prostaglandin E2 to High-Density CD34+ Cell Culture Increases Lentiviral Vector Transduction Efficiency
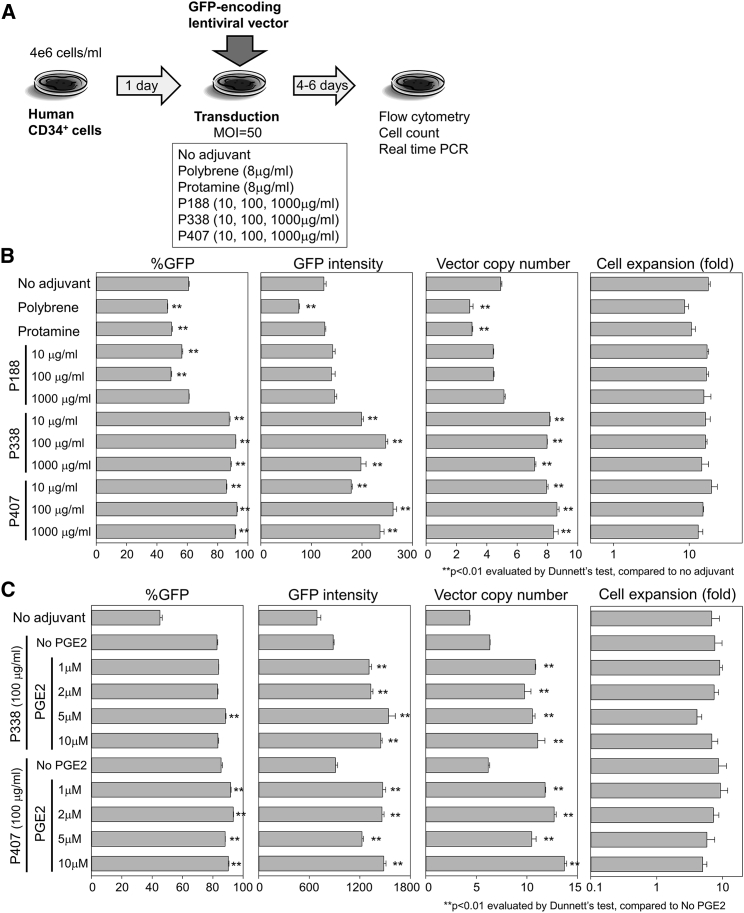
We hypothesized that lentiviral vectors might remain on the surface of CD34+ cells for a period before internalization and that these surface-bound vectors could be transferred to adjacent cells by cell-to-cell contact. Based on this hypothesis, we decided to add poloxamers, which are used for drug delivery to various cells, because their surfactant properties may improve vector-to-cell binding. Thus, we tested 3 sizes of poloxamers, poloxamer 188 (P188), poloxamer 338 (P338), and poloxamer 407 (P407), in a K562 erythroleukemia cell line and compared their performance with the traditional polycation-based adjuvants polybrene and protamine. After transduction of K562 cells (1e5/mL) with a GFP-expressing lentiviral vector at MOI 0.5 supplemented with one type of adjuvant, we observed higher %GFP and GFP intensity with polybrene (8 μg/mL), protamine (8 μg/mL), P338 (100 and 1,000 μg/mL), and P407 (100 and 1,000 μg/ml) compared with a no-adjuvant control (p < 0.01) (Figure S2). We then transduced high-density CD34+ cells (4e6/mL) after 1-day pre-stimulation with a GFP-expressing lentiviral vector at MOI 50 supplemented with each reagent individually, including polybrene (8 μg/mL), protamine (8 μg/mL), P188 (10, 100, and 1,000 μg/mL), P338 (10, 100, and 1,000 μg/mL), and P407 (10, 100, and 1,000 μg/mL) (Figure 3A). We observed up to 1.5-fold higher %GFP, up to 2.1-fold higher GFP intensity, and up to 1.8-fold higher VCNs in P338 and P407 among all concentrations (p < 0.01), whereas %GFP, GFP intensity, and VCNs decreased in polybrene and protamine (p < 0.01 except for GFP intensity in protamine) (Figure 3B, left, center left, and center right). Cell counts (Figure 3B, right) and CD34 expression (Figure S3A) were similar among all conditions (except slightly lower CD34 expression in P188). We further analyzed the adjuvant effects of P338 and P407 at a smaller range of dose escalation (100, 200, 500, and 1,000 μg/mL) in high-density CD34+ cells (4e6/mL) (Figure S2B), and both P338 and P407 similarly increased %GFP, GFP intensity, and VCNs in all concentrations (p < 0.01 except GFP intensity in 1,000 μg/mL P407 and VCN in 500 μg/mL P338). Therefore, we selected 100 μg/mL of P338 and P407 for further experiments.
Figure 3.
Addition of Poloxamer and Prostaglandin E2 (PGE2) to High-Density CD34+ Cell Culture Results in More Efficient Transduction with a Lentiviral Vector
(A) After 1-day pre-stimulation, we transduced high-density CD34+ cells (4e6/mL) with a GFP-expressing lentiviral vector at MOI 50 supplemented with various single reagents, including polybrene (8 μg/mL), protamine (8 μg/mL), poloxamer 188 (P188; 10, 100, and 1,000 μg/mL), P338 (10, 100, and 1,000 μg/mL), and P407 (10, 100, and 1,000 μg/mL) for 1 day. (B) %GFP, GFP intensity, and cell counts were evaluated 4 days after transduction, and VCNs were evaluated 6 days after transduction. (C) For high-density CD34+ cell transduction, we added P338 or P407 as well as PGE2 (1, 2, 5, and 10 μM) for 1 day and evaluated %GFP (day 4), GFP intensity (day 4), cell counts (day 4), and VCNs (day 6). Values: mean ± SE. All experiments were performed in triplicate.
For further improvement of lentiviral transduction, we used another adjuvant, prostaglandin E2 (PGE2), which may improve vector replication (reverse transcription) by stimulating CD34+ cells because PGE2 exposure improves engraftment of cultured CD34+ cells in xenografted mice and is safe in large animals.9, 13 We added PGE2 (1, 2, 5, and 10 μM) to high-density CD34+ cell transduction with P338 (100 μg/mL) or P407 (100 μg/mL) (Figure 3C). The combination of PGE2 and P407 resulted in up to 1.1-fold higher %GFP, up to 1.6-fold higher GFP intensity, and up to 2.2-fold higher VCNs (up to 14) at all concentrations (p < 0.01) compared with no PGE2 control with P407 (Figure 3C, left, center left, and center right), whereas similar %GFP, up to 1.7-fold higher GFP intensity, and up to 1.7-fold higher VCNs were observed with PGE2 addition to P338 at all concentrations (p < 0.01 except %GFP in 1, 2, and 10 μM PGE2 with P338). Cell counts were similar under all conditions (Figure 3C, right). These data demonstrate that the combination of P407 (or P338) and PGE2 results in an additional ∼4-fold improvement of transduction efficiency in high-density CD34+ cell culture.
P407 More Strongly Improves Lentiviral Transduction in High-Density CD34+ Cell Culture Compared with PGE2
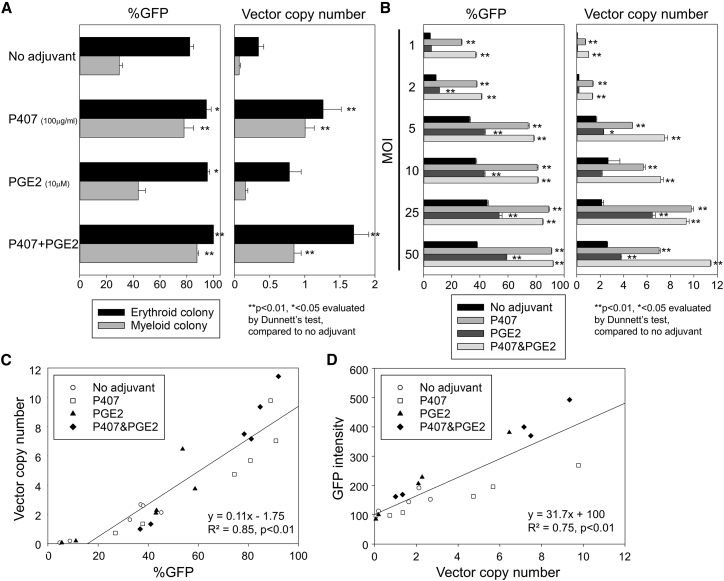
Based on overall data, we selected P407 (100 μg/mL) and PGE2 (10 μM) for optimal lentiviral transduction in high-density CD34+ cell culture (4e6/mL). To compare the adjuvant effects between P407 and PGE2, we transduced high-density CD34+ cells (4e6/mL) with a GFP-expressing lentiviral vector at various MOIs (1, 2, 5, 10, 25, and 50) supplemented with P407 (100 μg/mL) and/or PGE2 (100 μM) (Figure 4B). In addition, transduced cells at MOI 50 were cultured in semi-solid medium to analyze colony-forming units (CFUs) (Figure 4A; Figure S4). In the CFU assay, higher %GFP and higher VCNs in vector-positive colonies were observed in both P407 and PGE2 in erythroid colonies (p < 0.05 except VCN in PGE2) compared with no adjuvant, whereas P407 alone and P407&PGE2 combination increased both %GFP and VCNs in myeloid colonies (p < 0.01).
Figure 4.
P407 More Strongly Improves Lentiviral Transduction in High-Density CD34+ Cell Culture compared with PGE2
(A) After 1-day pre-stimulation, we transduced high-density CD34+ cells with a GFP-expressing lentiviral vector at MOI 50 with P407 (100 μg/mL), PGE2 (100 μM), and a combination of P407 and PGE2. One day later, transduced cells were cultured in semi-solid medium for 9 days, and colony-forming units (CFUs), %GFP, and VCNs were evaluated by UV microscopy as well as qPCR. (B) We transduced high-density CD34+ cells with a GFP-expressing lentiviral vector at various MOIs (1, 2, 5, 10, 25, and 50) supplemented with P407 (100 μg/ml) and/or PGE2 (100 μM) and evaluated %GFP (days 3–4) and VCNs (day 6). (C and D) We analyzed correlation between %GFP and VCNs (C) and between VCNs and GFP intensity (D). Values: mean ± SE. All experiments were performed in triplicate.
In MOI escalation of lentiviral transduction in high-density CD34+ cell culture, higher %GFP and higher VCNs were observed in P407 alone and P407&PGE2 combination at all MOIs (p < 0.01), whereas high-MOI transduction only allowed for an increase in %GFP (MOI 2, 5, 10, 25, and 50; p < 0.05) and VCNs (MOI 5, 25, and 50; p < 0.05) in PGE2 alone. These data demonstrate that P407 more strongly improves transduction efficiency in high-density CD34+ cells compared with PGE2 and that the P407&PGE2 combination is optimal for efficient transduction in high-density CD34+ cell culture. In addition, we queried the correlation between %GFP and VCNs (Figure 4C) as well as between VCNs and GFP intensity (Figure 4D) and found positive correlations for both %GFP-VCN (R2 = 0.85, p < 0.01) and VCN-GFP intensity (R2 = 0.75, p < 0.01). When %GFP-VCN and VCN-GFP intensity data for no adjuvant, P407, PGE2, and P457&PGE2 were analyzed separately, similar regression lines were obtained under all conditions.
When we evaluated lentiviral transduction among our standard cell densities (1e5/mL) without adjuvant as well as high-density culture (4e6/mL) with P407 (100 μg/mL) and/or PGE2 (100 μM), 3-fold higher %GFP (p < 0.01) and 9.2-fold higher VCNs (p < 0.01) were observed in high-density culture with P407 and PGE2 compared with our standard cell density culture (Figure S5). Overall, these data demonstrate that high-density culture (4e6/mL) with P407 and PGE2 results in ∼10-fold improvement in transduction efficiency in human CD34+ cells compared with our standard cell density culture (1e5/mL).
High-Density Culture with or without P407 and PGE2 Improves Lentiviral Transduction in Engrafting Human CD34+ Cells in Xenograft Mice
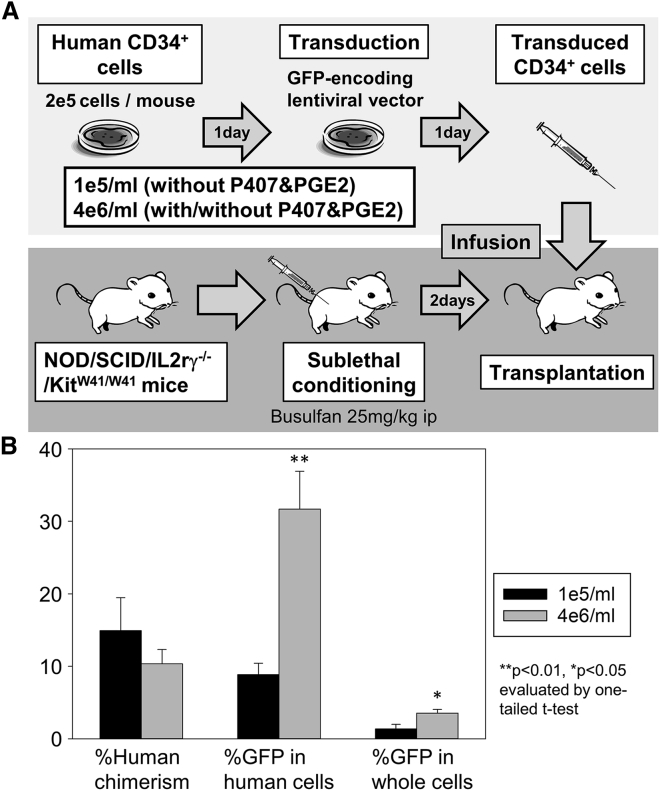
To evaluate the engrafting ability of transduced CD34+ cells with high-density culture, we transduced human CD34+ cells (2e5 cells/mouse) with a GFP-expressing lentiviral vector at MOI 50 in our standard cell density culture (1e5/mL) without adjuvant and high-density culture (4e6/mL) with or without P407 (100 μg/mL) and PGE2 (100 μM) after 1-day pre-stimulation (Figure 5A). One day later, transduced cells were infused into immunodeficient mice (NOD.Cg-KitW-41J Tyr+ Prkdcscid Il2rgtm1Wjl/ThomJ) 2 days after sublethal busulfan conditioning (25 mg/kg). Efficient in vitro transduction was obtained in infusion products (transduced CD34+ cells) among all groups (%GFP and VCNs: 19% ± 0% and 0.7 – 0.0 in 1e5/mL, 28% ± 0% and 1.3 ± 0.0 in 4e6/mL without adjuvant, and 86% ± 0% and 12.7 ± 0.0 in 4e6/mL with P407 and PGE2, respectively). Twelve weeks after transplantation, similar human CD45-positive percentages (human cell engraftment), higher %GFP in human cells (transduction efficiency) (p < 0.01), and higher %GFP in whole blood cells (engraftment of transduced cells) (p < 0.05) were observed in high-density culture (4e6/mL, n = 3) compared with our standard cell density (1e5/mL, n = 3) (Figure 5B). We also observed higher %GFP in engrafting human cells in high-density culture (4e6/mL) with P407 and PGE2 (p < 0.01, n = 2) compared with our standard cell density culture (1e5/mL) without adjuvant (Figure S6). These data demonstrate that high-density culture with or without P407 and PGE2 improves lentiviral transduction in engrafting human CD34+ cells evaluated in xenograft mice.
Figure 5.
High-Density Culture with or without P407 and PGE2 Improves Lentiviral Transduction in Engrafting Human CD34+ Cells in Xenograft Mice
(A) After 1-day pre-stimulation, human CD34+ cells (2e5 cells/mouse) were transduced with a GFP-expressing lentiviral vector at MOI 50 in our standard cell density culture (1e5/mL) without adjuvant and high-density culture (4e6/mL) with or without P407 (100 μg/mL) and PGE2 (100 μM). One day later, transduced cells were transplanted into immunodeficient mice (NOD.Cg-KitW-41J Tyr+ Prkdcscid Il2rgtm1Wjl/ThomJ) 2 days after sublethal busulfan conditioning of 25 mg/kg by intraperitoneal (i.p.) injection. (B) Twelve weeks after transplantation, we evaluated peripheral blood cells for human cell engraftment (human CD45-positive percentages), %GFP in human cells, and %GFP in whole cells (including both human and mouse cells). Values: mean ± SE. All experiments were performed in triplicate. 1e5/mL, n = 3; 4e6/mL, n = 3.
Robust βT87Q-globin Production in Erythroid Cells Differentiated from SCD CD34+ Cells Results from Lentiviral Transduction with High-Density Culture with P407 and PGE2 Supplementation
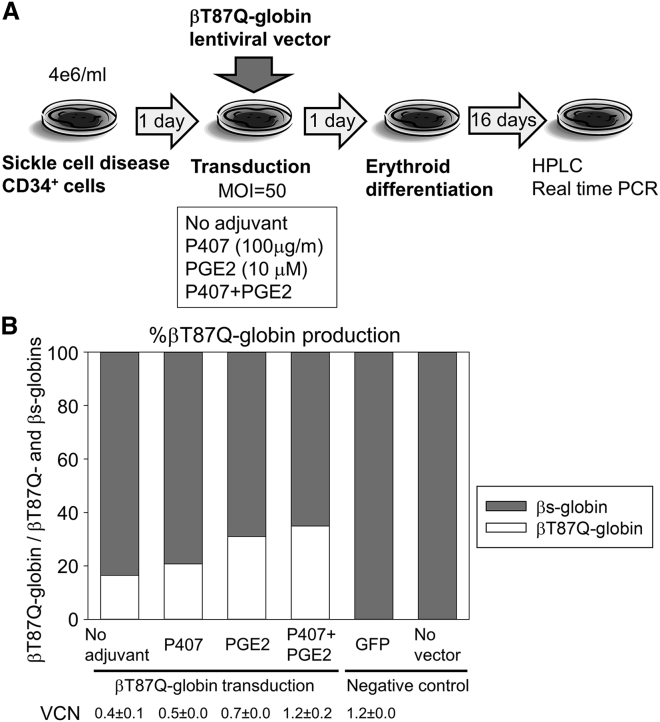
To investigate these improvements in an SCD gene therapy setting, plerixafor-mobilized CD34+ cells from an SCD patient were pre-stimulated for 1 day and transduced in high-density culture (4e6/mL) with a lentiviral vector encoding βT87Q-globin (including an anti-sickling mutation) at MOI 50 with P407 (100 μg/mL), PGE2 (100 μM), and a combination of P407 and PGE2 (Figure 6A). Following 16-day erythroid differentiation, we evaluated globin production at the protein level by reverse-phase high-performance liquid chromatography (HPLC) and VCNs at the DNA level. Lentiviral transduction for CD34+ cell-derived erythroid cells was less efficient with the large-sized βT87Q-globin vector (7.5 kb) (0.4 ± 0.1, p < 0.01) compared with a GFP vector (3.6 kb) (1.2 ± 0.0), but P407 and PGE2 supplementation increased VCNs with the βT87Q-globin vector (1.2 ± 0.2, p < 0.01) to levels similar to those in GFP transduction without adjuvant (Figure 6B). After erythroid differentiation, mostly βs-globin production was observed in both the untransduced control and GFP transduction control, and 2-fold higher βT87Q-globin production (35%) was detected at the protein level with βT87Q-globin transduction in high-density culture with P407 and PGE2 compared with the no-adjuvant control (17%) (Figure 6B). These data demonstrate that high-density culture with P407 and PGE2 allows more efficient lentiviral transduction in the SCD gene therapy setting.
Figure 6.
Robust βT87Q-globin Production in Erythroid Cells Differentiated from SCD CD34+ Cells Results from Lentiviral Transduction with High-Density Culture with P407 and PGE2 Supplementation
(A) After 1-day pre-stimulation, plerixafor-mobilized CD34+ cells from an SCD patient were transduced in high-density culture with a lentiviral vector expressing βT87Q-globin (including an anti-sickling mutation) at MOI 50 with P407 (100 μg/mL), PGE2 (100 μM), and a combination of P407 and PGE2. One day later, transduced cells were differentiated into erythroid cells for 16 days. (B) Globin production was evaluated by reverse-phase high-performance liquid chromatography (HPLC), and VCNs were evaluated by qPCR. Values: mean ± SE. All experiments were performed in triplicate, except for HPLC (single run).
Discussion
We improved lentiviral transduction using high-density CD34+ cell culture (4e6/mL) with P407 and PGE2 adjuvants, resulting in ∼10-fold higher transduction efficiency in human CD34+ cells compared with our current standard method (1e5/mL) that was optimized previously,9 consistently achieving more than 90% transduction and VCNs of ∼10 in human CD34+ cells. The combination of high-density CD34+ cell culture with P407 and PGE2 improves transduction efficiency and saves time and cost for large-scale transduction (40-fold less culture media and cytokines). This practical, high-efficiency transduction method in human CD34+ cells should be desirable for various gene therapy trials because achieving high-level transduction in CD34+ cells is important for improving phenotypic correction.7
We demonstrated that high-density CD34+ cell culture (4e6/mL) alone significantly improves transduction efficiency ∼5-fold compared with our current standard method (1e5/mL) (Figure 1). Usually, improvement in transduction efficiency results from higher cell proliferation because of increased reverse transcription, but these efforts ultimately reduce the engraftment ability of transduced cells.14 Therefore, we have previously optimized lentiviral transduction in low-density cell culture of CD34+ cells (1e5/mL) with minimal cytokine stimulation (100 ng/mL SCD, FL, and TPO) to balance efficient transduction with high-level engraftment.9, 10, 11 However, high-density CD34+ cell culture resulted in more efficient lentiviral transduction while decreasing cell proliferation (Figures 1 and 2), suggesting that it has a unique mechanism to improve lentiviral transduction and possibly does not affect engraftment abilities. We observed that improvements in transduction efficiency plateaued at MOI 100, whereas transduction efficiency gradually increased at higher densities of CD34+ cell culture until 4e6/mL. Therefore, we hypothesized that a receptor for lentiviral vectors is saturated in high MOI transduction and that expression of this receptor might be enhanced by high-density culture. Recently, VSV-G-pseudotyped lentiviral vectors have been reported to enter target cells through LDL-R. We evaluated LDL-R in high-density CD34+ cell culture; however, LDL-R did not increase in high-density culture (Figure S1). We then hypothesized that cell-to-cell contacts effect more efficient lentiviral transduction in high-density cell culture at high MOI. When we co-cultured vector-exposed CD34+ cells with non-vector-exposed CD34+ cells at various cell-densities, higher levels of secondary transduction in non-vector-exposed cells were observed in high-density culture (Figure 2), suggesting that cell-to-cell contacts allow secondary transduction with the lentiviral vector on vector-exposed cells. Previously, surface-bound lentiviral particles have been reported to affect subsequent transduction in secondary target cells.15 The enhancement in lentiviral transduction observed with high-density CD34+ cell culture may be due to secondary transduction with residual vector particles on the surface of transduced CD34+ cells.
We then added adjuvants to further improve lentiviral transduction in high-density CD34+ cell culture. Traditionally, polycations, including polybrene and protamine, are used as an adjuvant to improve lentiviral transduction for cell lines,16, 17 but both adjuvants are less effective in CD34+ cells because of their toxicity (Figure 3). We hypothesized that lentiviral transduction in high-density CD34+ cell culture could be enhanced by poloxamers, which are useful for drug delivery to various cells, because poloxamers might allow vector particles to stay on the surface of CD34+ cells due to their surfactant properties.18 The adjuvant effects of poloxamers has been reported previously in cell lines and peripheral blood cells using P338,19 and, in this study, we observed the strong adjuvant effects of not only P338 but also P407 in human CD34+ cells with lentiviral transduction without significant toxicity. Slightly stronger adjuvant effects were observed in P407 compared with P338 in high-density CD34+ cell transduction (Figure 3; Figure S3). These data demonstrate that P407 and P338 enhance lentiviral transduction in high-density CD34+ cell culture, probably because of improved vector-to-cell contact as a result of their surfactant function.
We added another adjuvant, PGE2, in high-density CD34+ cell culture with lentiviral transduction with P407 because PGE2 might improve vector replication (reverse transcription) by stimulating CD34+ cells. PGE2 exposure has been reported to improve xenograft engraftment and lentiviral transduction of CD34+ cells without increasing lentiviral entry.13, 20 We added both P407 and/or PGE2 in high-density CD34+ cell transduction, and PGE2 slightly increased transduction efficiency in CD34+ cells (Figure 4). The P407&PGE2 combination improved lentiviral transduction more than simply increasing the dosage of P407 or PGE2 alone, suggesting that these adjuvants may work by different mechanisms (Figures 3 and 4). Additionally, we compared P407 and PGE2 at MOI escalation in high-density CD34+ cell transduction (Figure 4). P407 and/or PGE2 increased both %GFP and VCNs in CD34+ cells in a similar manner, demonstrating that P407 and/or PGE2 improve the percentage of transduced cells as well as vector amounts per transduced cell.
We performed xenograft mouse transplantation of transduced human CD34+ cells in high-density culture with or without P407 and PGE2 (Figure 5; Figure S6) and demonstrated higher transduction efficiency and similar human cell engraftment in high-density culture (4e6/mL) compared with our standard cell density (1e5/mL). We demonstrated previously that CD34+ cell culture conditions favoring expansion resulted in higher transduction efficiency but lower engrafting abilities in xenograft mouse transplantation.14 However, high-density CD34+ cell culture enhanced transduction efficiency and reduced cell expansion (Figure 2), suggesting that this low-expansion state might allow CD34+ cells to maintain engrafting ability with efficient transduction. In addition, P407 and PGE2 supplementation further improved transduction of engrafting human cells in xenograft mice (Figure S6). Overall, these data suggest that high-density culture with P407 and PGE2 allows more efficient transduction in engrafting CD34+ cells in xenograft mice. Human cell engraftment was slightly lower (1.4-fold, not significant) in high-density culture (4e6/mL) with P407 and PGE2 compared with our standard cell density (1e5/mL) (Figure S6). Further analysis of long-term engraftment is preferable in a large animal model or secondary xenograft transplantation to characterize how it is affected by high-density culture and P407 and PGE2 supplementation.
To investigate in an SCD gene therapy setting, SCD CD34+ cells were transduced with a βT87Q-globin (a therapeutic globin)-encoding vector in high-density culture with P407 and PGE2 (Figure 6), and, following erythroid differentiation, more efficient transduction at the DNA level and robust βT87Q-globin production at the protein level were observed in high-density culture with P407 and PGE2 supplementation. Preliminary data in a gene therapy trial for SCD suggest that efficient transduction in patient CD34+ cells in vitro is important to enhance therapeutic effects in gene therapy patients.8 Our data suggest that high-density CD34+ cell culture with P407 and PGE2 can improve gene therapy for SCD.
In summary, we developed an optimized method for lentiviral transduction in high-density human CD34+ cell culture with P407 and PGE2 adjuvants, resulting in ∼10-fold more efficient transduction without significant toxicity and consistently achieving more than 90% transduction and ∼10 VCNs in CD34+ cells. For high-density CD34+ culture, 40-fold smaller amounts of culture media and cytokines are required compared with a standard cell density, making this method both time- and cost-effective for large-scale transduction. Our optimized transduction method should strongly improve HSC-targeted gene therapy.
Materials and Methods
High-Density Human CD34+ Cell Culture with Lentiviral Transduction
Granulocyte colony-stimulating factor-mobilized CD34+ cells and peripheral blood mononuclear cells (PBMCs) from healthy donors and plerixafor-mobilized CD34+ cells from patients with SCD were collected under studies 08-H-0156, 03-H-0015, and 17-H-0124, which were approved by the institutional review board of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). All individuals gave written informed consent for sample donation, and consent documents are maintained in the donors’ medical records. Consent documents were approved by the institutional review board prior to study initiation and are reviewed and updated yearly.
Human CD34+ cells were cultured in serum-free X-VIVO10 medium (Lonza, Basel, Switzerland) containing 100 ng/mL each of SCF, FL, and TPO (R&D Systems, Minneapolis, MN, USA) among various cell densities, including 1e5/2 mL in a 6-well plate (5e4/mL), 1e5/1 mL in a 12-well plate (1e5/mL), 1e5/0.5 mL in a 24-well plate (2e5/mL), 1e5/0.2 mL in a 96-well plate (5e5/mL), 2e5/0.2 mL in a 96-well plate (1e6/mL), 4e5/0.2 mL in a 96-well plate (2e6/mL), and 8e5/0.2 mL in a 96-well plate (4e6/mL).9, 11 Culture plates were coated with fibronectin CH-296 (RetroNectin; Takara, Shiga, Japan) for the initial experiment (Figure 1B). After overnight pre-stimulation, the culture medium was changed to fresh medium containing identical cytokines and a self-inactivating HIV-1-based lentiviral vector encoding GFP under the control of a murine stem cell virus promoter with a VSV-G envelope at MOI 50.21, 22 After 1-day transduction, culture and transduction media were changed to fresh media containing the same cytokines. GFP expression was analyzed by flow cytometry (FACSCalibur, Becton Dickinson, East Rutherford, NJ, USA) 3–4 days after transduction, whereas VCNs were measured by qPCR (QuantStudio 6 Flex real-time PCR system, Thermo Fisher Scientific, Waltham, MA, USA) 6–7 days after transduction. In addition, we counted cell amounts (Countess II automated cell counter, Thermo Fisher Scientific) 3–4 days after transduction.
To evaluate the effects of cell-to-cell contacts in high-density CD34+ cell culture on lentiviral transduction, after overnight pre-stimulation, half of the CD34+ cells were transduced with the GFP-expressing lentiviral vector at MOI 50, and the other half was labeled with cell proliferation dye (CellTrace FarRed, Thermo Fisher Scientific) without transduction (Figure 2A). One hour later, CD34+ cells were washed with PBS (Mediatech, Manassas, VA, USA), and GFP-transduced CD34+ cells and far-red-labeled CD34+ cells were mixed in a 1:1 ratio among various cell densities (5e4/mL, 1e5/mL, 2e5/mL, 5e5/mL, 1e6/mL, 2e6/mL, and 4e6/mL). Three days after transduction, %GFP in far-red-positive and -negative cells was evaluated by flow cytometry.
After overnight pre-stimulation of high-density CD34+ cells (4e6/mL, 4e5 cells/0.1 mL in a 96-well plate), the culture medium was changed to fresh medium containing the same cytokines, the GFP-expressing lentiviral vector (MOI 50), and various single adjuvants: polybrene (8 μg/mL, Sigma-Aldrich, St. Louis, MO, USA), protamine (8 μg/mL, Sigma-Aldrich), P188 (10, 100, and 1,000 μg/mL, Sigma-Aldrich), P338 (10, 100, and 1,000 μg/mL, Sigma-Aldrich), P407 (10, 100, and 1,000 μg/mL, Sigma-Aldrich), and PGE2 (1, 2, 5, and 10 μM, R&D Systems).16, 17, 19, 20 After 1-day transduction, the cells were split into 3 wells in a 12-well plate containing 1 mL fresh medium with the same cytokines. GFP expression was evaluated by flow cytometry 3–6 days after transduction, and VCNs were evaluated by qPCR 6–7 days after transduction.
Flow Cytometry
LDL-R expression in high-density CD34+ cell culture was evaluated by flow cytometry (FACSCalibur, Becton Dickinson) with LDL-R antibody (Clone C7, Becton Dickinson) 1 day after pre-stimulation (at the time of transduction).12 CD34 expression in high-density CD34+ cell culture was evaluated with CD34 antibody (Clone 563, Becton Dickinson) 4 days after transduction.
High-density CD34+ cells were labeled with cell proliferation dye (CellTrace FarRed, Thermo Fisher Scientific) without lentiviral transduction, and 7 days later, cell proliferation was evaluated by reduction in FarRed fluorescent color (Figure 2C).
qPCR
Genomic DNA was extracted from CD34+ cells in high-density culture 6–7 days after lentiviral transduction. VCNs were measured by qPCR (QuantStudio 6 Flex real-time PCR system, Thermo Fisher Scientific) with an integrating vector-specific self-inactivating long terminal repeat (SIN-LTR) probe and primers and ribosomal RNA probe and primers (TaqMan ribosomal RNA control reagents, Applied Biosystems, Foster City, CA, USA).23
CFU Assay
After overnight pre-stimulation of high-density CD34+ cells (4e6/mL), CD34+ cells were transduced with a GFP-expressing lentiviral vector at MOI 50 supplemented with 100 μg/mL P407, 10 μM PGE2, or a combination of P407 (100 μg/mL) and PGE2 (10 μM). The following day, the cells were added to semi-solid medium (1e3/mL) in triplicate as described previously.11 Nine days later, GFP expression in erythroid and myeloid colonies was evaluated by UV microscopy, and VCNs in each colony were evaluated by qPCR.
Erythroid Differentiation from Transduced CD34+ Cells
After overnight pre-stimulation of high-density SCD CD34+ cells (4e6/mL), cells were transduced at MOI 50 with a lentiviral vector encoding a βT87Q-globin gene under the control of an erythroid-specific β-globin promoter with locus control regions, as described previously.24, 25 The following day, transduced cells were differentiated into erythroid cells using serum-free differentiation medium (SCF, interleukin-3, erythropoietin, dexamethasone, estradiol, and knockout serum replacement) for 5 days and maturation medium (erythropoietin, insulin, transferrin, BSA, and knockout serum replacement) for 11 days.25 Following erythroid differentiation, globin production was evaluated by specific elution time of peaks in reverse-phase HPLC as described previously.25, 26
Humanized Xenograft Mouse Transplantation of Transduced CD34+ Cells
After 1-day pre-stimulation, human CD34+ cells (2e5 cells/mouse) were transduced with a GFP-expressing lentiviral vector at MOI 50 in our standard cell density culture (1e5/mL) without adjuvant as well as high-density culture (4e6/mL) without adjuvant or with a combination of P407 (100 μg/mL) and PGE2 (100 μM). One day later, transduced cells were transplanted into immunodeficient mice (NOD.Cg-KitW-41J Tyr+ Prkdcscid Il2rgtm1Wjl/ThomJ) 2 days after sublethal busulfan conditioning of 25 mg/kg by intraperitoneal injection. Twelve weeks after transplantation, we evaluated peripheral blood cells for human cell engraftment (human CD45-positive percentages), %GFP in human CD45-positive cells, and %GFP in whole cells (including both human and mouse cells).
Statistical Analysis
Statistical analyses were performed using JMP 13 software (SAS Institute, Cary, NC, USA). The averages under various conditions were evaluated by Dunnett’s test (one-way ANOVA for a control). Correlation was evaluated by t test for coefficient of correlation and R2 in regression analysis. p < 0.01 or 0.05 was deemed significant. SEM is shown as error bars in all figures. All experiments, except those shown in Figure 2B (duplicate) and Figure 6B (single run), were performed in triplicate.
Author Contributions
N.U. designed the research, performed experiments, analyzed results, made the figures, and wrote the paper. T.N. C.M.D., J.G., M.Y., and M.M.H. performed experiments. S.D. and J.J.H.-M. designed the research. J.F.T. designed the research and wrote the paper.
Conflicts of Interest
No competing financial interests exist.
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the NIH. We thank Dr. Duck-Yeon Lee from the NHLBI Biochemistry Core for help with reverse-phase HPLC analysis.
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.omtm.2019.01.005.
Supplemental Information
References
- 1.Aiuti A., Cattaneo F., Galimberti S., Benninghoff U., Cassani B., Callegaro L., Scaramuzza S., Andolfi G., Mirolo M., Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A., Slavin S., Aker M., Ficara F., Deola S., Mortellaro A., Morecki S., Andolfi G., Tabucchi A., Carlucci F. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 3.Boztug K., Schmidt M., Schwarzer A., Banerjee P.P., Díez I.A., Dewey R.A., Böhm M., Nowrouzi A., Ball C.R., Glimm H. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W. Gene Therapy in a Patient with Sickle Cell Disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 8.Kanter J., Walters M.C., Hsieh M., Krishnamurti L., Kwiatkowski J.L., Kamble R., von Kalle C., Kuypers F.A., Cavazzana M., Leboulch P. Interim Results from a Phase 1/2 Clinical Study of Lentiglobin Gene Therapy for Severe Sickle Cell Disease. Blood. 2017;128:1176. [Google Scholar]
- 9.Uchida N., Hsieh M.M., Hayakawa J., Madison C., Washington K.N., Tisdale J.F. Optimal conditions for lentiviral transduction of engrafting human CD34+ cells. Gene Ther. 2011;18:1078–1086. doi: 10.1038/gt.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida N., Hargrove P.W., Lap C.J., Evans M.E., Phang O., Bonifacino A.C., Krouse A.E., Metzger M.E., Nguyen A.D., Hsieh M.M. High-efficiency transduction of rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol. Ther. 2012;20:1882–1892. doi: 10.1038/mt.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida N., Washington K.N., Hayakawa J., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Donahue R.E., Tisdale J.F. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J. Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelshtein D., Werman A., Novick D., Barak S., Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goessling W., Allen R.S., Guan X., Jin P., Uchida N., Dovey M., Harris J.M., Metzger M.E., Bonifacino A.C., Stroncek D. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida N., Green R., Ballantine J., Skala L.P., Hsieh M.M., Tisdale J.F. Kinetics of lentiviral vector transduction in human CD34(+) cells. Exp. Hematol. 2016;44:106–115. doi: 10.1016/j.exphem.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y.W., Scarlett J.M., Luoh T.T., Kurre P. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J. Virol. 2007;81:639–649. doi: 10.1128/JVI.01089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornetta K., Anderson W.F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J. Virol. Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- 17.Manning J.S., Hackett A.J., Darby N.B., Jr. Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl. Microbiol. 1971;22:1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabanov A.V., Lemieux P., Vinogradov S., Alakhov V. Pluronic block copolymers: novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002;54:223–233. doi: 10.1016/s0169-409x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 19.Höfig I., Atkinson M.J., Mall S., Krackhardt A.M., Thirion C., Anastasov N. Poloxamer synperonic F108 improves cellular transduction with lentiviral vectors. J. Gene Med. 2012;14:549–560. doi: 10.1002/jgm.2653. [DOI] [PubMed] [Google Scholar]
- 20.Heffner G.C., Bonner M., Christiansen L., Pierciey F.J., Campbell D., Smurnyy Y., Zhang W., Hamel A., Shaw S., Lewis G. Prostaglandin E2 Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells. Mol. Ther. 2018;26:320–328. doi: 10.1016/j.ymthe.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanawa H., Kelly P.F., Nathwani A.C., Persons D.A., Vandergriff J.A., Hargrove P., Vanin E.F., Nienhuis A.W. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N., Hanawa H., Dan K., Inokuchi K., Shimada T. Leukemogenesis of b2a2-type p210 BCR/ABL in a bone marrow transplantation mouse model using a lentiviral vector. J. Nippon Med. Sch. 2009;76:134–147. doi: 10.1272/jnms.76.134. [DOI] [PubMed] [Google Scholar]
- 23.Uchida N., Evans M.E., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Sellers S.E., Dunbar C.E., Donahue R.E., Tisdale J.F. Integration-specific In Vitro Evaluation of Lentivirally Transduced Rhesus CD34(+) Cells Correlates With In Vivo Vector Copy Number. Mol. Ther. Nucleic Acids. 2013;2:e122. doi: 10.1038/mtna.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida N., Washington K.N., Lap C.J., Hsieh M.M., Tisdale J.F. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol. Ther. 2011;19:133–139. doi: 10.1038/mt.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida N., Demirci S., Haro-Mora J.J., Fujita A., Raines L.N., Hsieh M.M., Tisdale J.F. Serum-free Erythroid Differentiation for Efficient Genetic Modification and High-Level Adult Hemoglobin Production. Mol. Ther. Methods Clin. Dev. 2018;9:247–256. doi: 10.1016/j.omtm.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida N., Haro-Mora J.J., Fujita A., Lee D.Y., Winkler T., Hsieh M.M., Tisdale J.F. Efficient Generation of β-Globin-Expressing Erythroid Cells Using Stromal Cell-Derived Induced Pluripotent Stem Cells from Patients with Sickle Cell Disease. Stem Cells. 2017;35:586–596. doi: 10.1002/stem.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.