Abstract
Placebo effects benefit a wide range of clinical practice, which can be profoundly influenced by expectancy level and personal characteristics. However, research on the issue of whether these factors independently or interdependently affect the placebo effects is still in its infancy. Here, we adopted a 3-day between-subject placebo analgesia paradigm (2-day conditioning and 1-day test) to investigate the influence of expectancy levels (i.e., No, Low, and High) and personal characteristics (i.e., gender, dispositional optimism, and anxiety state) on placebo effects in 120 healthy participants (60 females). Our results showed that the reduction of pain intensity in the test phase was influenced by the interaction between expectancy and gender, as mainly reflected by greater reductions of pain intensity in females at Low expectancy level than females at No/High expectancy levels, and greater reductions of pain intensity in males than in females at High expectancy level. Additionally, the reduction of pain unpleasantness was not only modulated by the interaction between expectancy and gender, but also by the interaction between expectancy and dispositional optimism, as well as the interaction between expectancy and anxiety state. Specifically, participants who were more optimistic in Low expectancy group, or those who were less anxious in High expectancy group showed greater reductions of pain unpleasantness. To sum up, we emphasized on regulating the expectancy level individually based on the assessment of personal characteristics to maximize placebo effects in clinical conditions.
Keywords: expectancy, placebo analgesia, gender, dispositional optimism, anxiety state
Introduction
Placebo commonly refers to an inert substance or a medicinally inactive treatment that can generate clinically-useful effects. A person who receives a placebo treatment usually experiences actual improvements in his/her physical condition, which is well-known as the placebo effect. The placebo effect can be beneficial in a wide range of clinical situations, such as modulating the therapeutic effects of deep brain stimulation on Parkinson's disease, generating antidepressant responses in depression, and reducing unpleasantness in patients with anxiety (1, 2). It can also enhance the effectiveness of physical interventions (3–5) and provide an alternative approach to avoid side effects of drug treatments (6, 7).
Although placebo effect is a complex phenomenon that can be affected by multiple factors (e.g., memory, motivation, anxiety, learning, patient-provider interaction, and previous treatment experience) (1, 8–10), response expectancy has been recognized as one of the main psychological mechanisms underlying this effect (11). Response expectancy has been defined as the expectancy to the occurrence of non-volitional responses (i.e., responses experienced as occurring automatically without volitional efforts, including fear, sadness, sexual arousal, pain, etc.) to situational cues (12). According to Response Expectancy Theory, such response expectancy could affect the probability that an individual would engage in a particular behavior (e.g., increased/decreased pain responses), as non-volitional responses have positive and negative reinforcement values (12). Consistent with this theory, accumulating evidence has shown that the placebo effect (e.g., placebo analgesia) could be altered by changing individual expectancy (3, 7, 13–19).
In general, response expectancy is composed of two distinct aspects: (1) the expected magnitude of a change (i.e., expectancy level), and (2) the subjective probability that the change will occur (i.e., individual belief) (12). With regards to the first aspect of response expectancy, a greater placebo effect is usually associated with a higher level of positive expectancy (4, 5, 20–23). However, these observations are not guaranteed under some circumstances. For example, in laboratory settings, even individual expectation of placebo effects has been successfully acquired during the classical conditioning phase, unrealistically high expectancy that does not match with one's present experience would weaken individual belief in the placebo treatment during the test phase (24).
In terms of the second aspect of response expectancy, previous studies have demonstrated that individual belief in the current experience has a critical influence on response expectancy through learning mechanisms (21, 24). Such a belief is easily affected by personal characteristics, thus contributing to the differentiation of individual expectancy (25, 26), and subsequently leading to individual variability in response to placebos (27, 28). For instance, gender has been verified as a factor contributing to the variability of placebo effects—some studies suggested that males reported greater pain reductions after placebo treatments compared to females (29–31), whereas other studies described a better respondence to placebos in females than in males (29, 32–34). Additionally, the influence of other personal characteristics on placebo responses has been frequently reported in the literature. For example, dispositional optimism, referred to a generalized positive outcome expectancy for the future (35), is inextricably linked to proneness of increased placebo effects (36, 37). Comparatively, individuals with low anxiety level are more likely to respond to a placebo treatment (36).
However, research on the issue of whether expectancy level and personal characteristics independently or interdependently affect the placebo effects is still in its infancy. Here, we adopted a between-subject placebo analgesia paradigm to test the influence of expectancy levels (i.e., No, Low, and High) and personal characteristics (i.e., gender, dispositional optimism, and anxiety state) on placebo effects.
Materials and Methods
Participants
A total of 120 healthy, right-handed participants (60 females) were recruited from the local community. None of them reported a history of illness or concurrent medication. Participants were informed that they were attending a study aimed to test the effect of lidocaine (a local anesthetic that could be topically applied on the skin) on alleviating pain, and they were asked not to consume products containing caffeine, alcohol, or nicotine at least 12 h before the experiment. All the participants gave their written informed consents and were told their rights to discontinue participation at any time during the study. Each participant was randomly assigned to one of the three experimental groups divided by the manipulated expectancy levels (i.e., No, Low, and High) during the Conditioning phase (as described below), with 40 participants (20 females) in each group. After the whole experiment, all participants were fully debriefed.
Experimental Materials
Pain Stimuli
The electrical pain stimuli were delivered using a constant-current stimulator (model DS7A; Digitimer, UK) with three stainless steel concentric bipolar needle electrodes (38, 39). Pain stimuli were intraepidermal electrical pulses delivered to the inner side of the left forearm through the electrodes (located according to an equilateral triangle shape), which have been proved to preferentially activate the Aδ nociceptive fibers in the superficial skin layers (40, 41). Each electrode consisted of a needle cathode (length = 0.1 mm, diameter = 0.2 mm) surrounded by a cylindrical anode (diameter = 1.4 mm). Each stimulus consisted of 100 rapidly succeeding constant-current, square-wave pulses at 50 Hz (0.5-ms duration for each pulse).
Dispositional Optimism
The Chinese version of the Life Orientation Test-Revised (LOT-R) was adopted to assess participants' dispositional optimism, as its reliability has been well-established (Cronbach alpha of positive subscale = 0.73, N = 479; Cronbach alpha of negative subscale = 0.82, N = 479) (42). In the current sample, the reliability of the scale was satisfactory (Cronbach alpha = 0.66, N = 120).
Anxiety State
The state subscale of Chinese version of State-Trait Anxiety Inventory (STAI-S) was adopted to assess participants' anxiety state. The reliability of the Chinese version of STAI-S (Cronbach alpha = 0.90, N = 2,150) (43) has been well-established. Notably, the reliability of the subscale in the current sample was satisfactory (STAI-S: Cronbach alpha = 0.89, N = 120).
Experimental Procedure
A randomized, single-blinded between-subject experimental paradigm of placebo analgesia was adopted in the present study (7). Participants were firstly familiarized with the electrical stimulation prior to the formal experiment. The stimulus intensities were adjusted individually using the method of limits, to identify the thresholds for each participant that would elicit a low sensation (~2 rating), moderate sensation (~4 rating), and high sensation (~6 rating) on an 11-point self-report Numeric Rating Scale (NRS, 0 = no sensation, 10 = unbearable pain). Specifically, the stimuli at ~2 rating elicited a non-painful sensation, whereas the stimuli at ~4 and ~6 ratings elicited a painful pinprick sensation. Once these stimulus intensities were determined, a randomized sequence of pain stimuli with different intensities was delivered to participants until they were able to reliably distinguish the intensities of these stimuli. Notably, these determined stimuli with varied intensities were used during the conditioning procedure (see Conditioning Phase section) to ensure a successful manipulation of expectancy level during the experiment.
The experiment consisted of two phases in three consecutive days: Conditioning phase (Day 1 and Day 2) and Test phase (Day 3). On each day, participants underwent three sessions: (1) a pre-treatment session, (2) a treatment session, and (3) a post-treatment session (see Figure 1). To rule out possible confounding effects related to the gender of experimenter (44, 45), half of participants in each group with an equal number of males (n = 20) and females (n = 20) were instructed by a female experimenter, while the rest were guided by a male experimenter. Both female and male experimenter wore white coats and had received systematic training of procedure prior to the formal experiment.
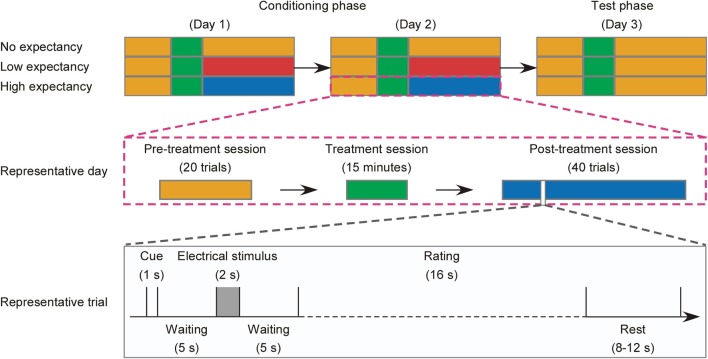
Figure 1.
Flowchart of the experimental design. (Top) For each experimental group (No, Low, and High expectancy), the experiment consisted of two phases: Conditioning phase (Day 1 and Day 2) and Test phase (Day 3), and each day consisted of three sessions: a pre-treatment session, a treatment session, and a post-treatment session. Sessions, in which the electrical stimuli elicit a low sensation at ~2 rating, moderate sensation at ~4 rating, and high sensation at ~6 rating on the 11-point Numerical Rating Scale, are marked in blue, red, and orange, respectively. Treatment sessions are marked in green. (Middle) The experimental procedure in a representative day contained a 20-trial pre-treatment session, a 15 min treatment session, and a 40-trial post-treatment session, which are marked in orange, green, and blue, respectively. (Bottom) A representative trial of the pre-treatment session or the post-treatment session was starting with a 1-s cue, followed by a 5 s waiting, a 2 s electrical stimulus, another 5 s waiting, and a 16 s rating of the perception of pain intensity, unpleasantness, anxiety level, and satisfaction of drug efficacy (post-treatment session in Low and High expectancy groups). The trial was ended with a rest of 8–12 s.
Conditioning Phase
The Conditioning phase started with a pre-treatment session consisting of 20 trials. Each trial started with a 1 s white fixation centered on the screen with a black background. After a 5 s waiting, an electrical stimulus at ~6 ratings (0.80 ± 0.29 mA) lasting for 2 s was delivered to the left forearm of the participant. Being waiting for another 5 s, participants were required to verbally rate the perceived intensity (0 = no sensation, 10 = unbearable pain) and unpleasantness (0 = not unpleasant, 10 = extremely unpleasant) of pain evoked by the electrical stimulus (with 8 s per each rating, 16 s in total). The inter-trial interval varied between 8 and 12 s. The stimulus intensity in the pre-treatment session was identical across groups and the whole session lasted for ~16 min.
In the treatment session, a non-active skin cream was applied on the palmar side of the participant's left forearm. Being waiting for 5 min, participants were instructed to remove the cream and have a 10 min rest. Meanwhile, in order to strength expectancy level, participants were given one of the following verbal interventions, depending on treatment assignment:
(1) participants in No expectancy group were told that “the skin cream is ineffective to relieve or eliminate pain”;
(2) participants in Low expectancy group were told that “the skin cream can reduce but not eliminate pain”;
(3) participants in High expectancy group were told that “the skin cream can completely eliminate pain.”
The treatment session lasted for ~15 min.
The Conditioning phase ended with a post-treatment session consisting of 40 trials. The procedure was identical to the pre-treatment session, except that different intensities of electrical stimuli were set for different groups: inducing a painful sensation at ~6 rating (0.69 ± 0.16 mA) for No expectancy group, at ~4 rating (0.47 ± 0.17 mA) for Low expectancy group, and a non-painful sensation at ~2 rating (0.28 ± 0.08 mA) for High expectancy group. Such changes of stimulus intensity for different groups were intended to strengthen the power of verbal intervention to response expectancy, which has been frequently applied in previous placebo-related studies (46–48). The post-treatment session lasted for ~37 min.
Test Phase
The Test phase also consisted of a pre-treatment session, a treatment session, and a post-treatment session. The procedure of this phase was identical to the Conditioning phase, except that the intensity of electrical stimuli applied in the post-treatment session was identical for all participants across groups, i.e., inducing a painful pinprick sensation at ~6 rating (0.78 ± 0.26 mA). Participants were first required to complete the psychological questionnaires upon arriving to the laboratory on the Test day. To make sure that the expectancy manipulation was successful, participants in the Low and High Expectancy groups were required to verbally rate the strength of expectancy to drug efficacy on an 11-point NRS (0 = without any expectancy, 10 = full expectancy) at the end of the test. The average ratings of expectancy to drug efficacy were significantly different between groups (Low: 6.90 ± 1.31, High: 8.39 ± 0.80, t = −6.11, P < 0.001), indicating a successful expectancy manipulation. For participants in No expectancy group, the expectancy to drug efficacy was not assessed to avoid extra response bias to the expectancy manipulation.
Statistical Analysis
To assess the magnitude of placebo effects, we calculated the changes of subjective pain intensity and unpleasantness by subtracting the ratings in the post-treatment session from those in the pre-treatment session in Test phase (49). To demonstrate the influence of personal characteristics on the modulation of expectancy level on placebo effects, we performed a statistical analysis using a “split into three subgroups” strategy (13-14-13 split). Specifically, we sorted the LOT-R scores in ascending order and split the data into low (13 participants), middle (14 participants), and high LOT-R subgroups (13 participants) for each experimental condition. Following, we performed three-way analyses of variance (ANOVAs) on the indicators of placebo effects, with “expectancy level” (No, Low, and High), “dispositional optimism (LOT-R)” (low and high), and “gender” (female and male) as between-subject factors. Likewise, scores of anxiety state (STAI-S) were sorted and analyzed using the same statistical strategy. The statistical P-values were adjusted with Greenhouse-Geisser correction to avoid violation of the sphericity assumption, when necessary. Post hoc pairwise comparisons were performed with Bonferroni adjustments, when the main effects or interactions reach statistical significance. The effect size and statistical power in the present sample were estimated by partial eta-squared and 1-β, respectively. For partial eta-squared (), an effect size of 0.0099 is deemed as a “small” effect, around 0.0588 as a “medium” effect, and 0.1379 to infinity as a “large” effect (50). For 1- β, 0.8 is the commonly acceptable statistical power. To detect the effectiveness of sample size used in the current study, we performed a prior computation on the required sample size using G*power (an online free software for power analysis, available at http://www.gpower.hhu.de/en.html) by setting statistical power at 0.8 with large effect size ( = 0.1379). The result showed a minimal sample size of 64 in total to detect the main effects and interactions between independent variables, which indicated that our sample size (N = 120) was enough to detect these effects. All statistical analyses were carried out in SPSS 22.0 statistical analysis package (SPSS Inc., New York, USA). Statistical threshold was set at 0.05.
Results
Participant Characteristics
Participant characteristics for each experimental group are summarized in Table 1. The age was not significantly associated with “expectancy level” (No, Low, and High) and “gender” (female and male) [F(2, 114) = 2.84, P = 0.06, = 0.05]. This result, together with the counterbalanced experimental design for gender, indicated that all the participants across groups were age- and gender-matched, thus avoiding possible bias when assessing placebo effects.
Table 1.
Characteristics of participants in each experimental group (data are expressed as mean ± standard deviation, M ± SD).
| Group | Gender | N | Age |
|---|---|---|---|
| No expectancy | F | 20 | 20.20 ± 1.15 |
| M | 20 | 21.30 ± 1.38 | |
| Low expectancy | F | 20 | 20.95 ± 1.28 |
| M | 20 | 21.55 ± 1.73 | |
| High expectancy | F | 20 | 21.40 ± 1.39 |
| M | 20 | 21.00 ± 1.59 |
F, Female; M, Male.
Influence of Expectancy Level, Dispositional Optimism, and Gender on Placebo Effects
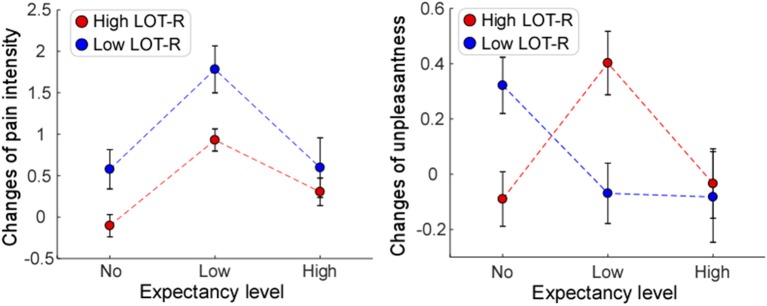
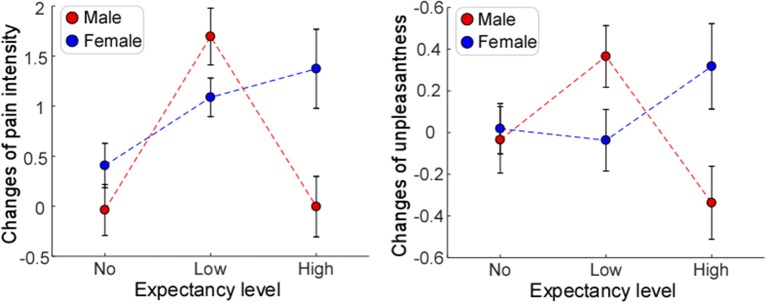
Significant main effects of “expectancy level” [F(2, 72) = 7.06, P = 0.002, ηp2 = 0.172] and “dispositional optimism (LOT-R)” [F(1, 72) = 5.18, P = 0.026, ηp2 = 0.071] were observed, while no significant main effect of “gender” [F(1, 114) = 0.04, P = 0.848, ηp2 = 0.001] was showed in the reduction of pain intensity (see Table 2). Post hoc comparisons on “expectancy level” showed that participants in Low expectancy group elicited a greater reduction of pain intensity than both No (P < 0.001) and High (P = 0.055) expectancy groups, while the latter two had no significant difference (P = 0.29). Neither the interaction between “expectancy level” and “dispositional optimism (LOT-R)” [F(1, 72) = 0.79, P = 0.460, ηp2 = 0.023] (Figure 3, left panel), nor the interaction between “dispositional optimism (LOT-R)” and “gender” [F(2, 72) = 0.20, P = 0.655, ηp2 = 0.003] (see Figure 2, left panel) was significant. However, the interaction between “expectancy level” and “gender” [F(2, 114) = 4.29, P = 0.018, ηp2 = 0.112] was significant. Post-hoc comparison on this interaction revealed that (1) female participants in the Low expectancy group reported a greater reduction of pain intensity due to placebo treatment than females in No (P < 0.001) and High (P = 0.001) expectancy groups; (2) for participants in High expectancy group, males reported a greater reduction of pain intensity due to placebo treatment than females (P = 0.01).
Table 2.
The changes of pain intensity from pre-treatment to post-treatment sessions in all experimental groups (data are expressed as M ± SD).
| Personal characteristics | Response expectancy | |||
|---|---|---|---|---|
| No | Low | High | ||
| Gender | F | −0.04 ± 1.14 | 1.70 ± 1.27 | −0.004 ± 1.35 |
| M | 0.41 ± 0.99 | 1.09 ± 0.87 | 1.37 ± 1.77 | |
| LOT-R | Low | 0.58 ± 1.21 | 1.78 ± 1.44 | 0.59 ± 1.83 |
| High | −0.10 ± 0.68 | 0.93 ± 0.68 | 0.31 ± 0.85 | |
| STAI-S | Low | −0.22 ± 1.16 | 0.94 ± 0.69 | 1.21 ± 1.83 |
| High | 0.39 ± 1.31 | 1.54 ± 1.38 | 0.75 ± 1.89 | |
F, Female; M, Male; LOT-R, Life Orientation Test-Revised; STAI-S, State subscale of State-Trait Anxiety Inventory. Changes of pain intensity were obtained by subtracting the ratings in post-treatment sessions from those in pre-treatment sessions.
Figure 3.
The influences of expectancy level and dispositional optimism (LOT-R) on placebo effects. Significant main effect of expectancy level was only observed for changes of pain intensity (Left). Significant interaction of expectancy level and dispositional optimism (LOT-R) was observed for changes of unpleasantness (Right), but not for changes of pain intensity (Left). Error bars indicate standard error, and data from participants with low and high scores of Life Orientation Test-Revised (Low LOT-R and High LOT-R) are marked in blue and red, respectively.
Figure 2.
The influences of expectancy level and gender on placebo effects. Significant main effect of expectancy level was only observed for changes of pain intensity (Left). Significant interactions of expectancy level and gender were observed for changes of pain intensity (Left) and unpleasantness (Right). Error bars indicate standard error, and data from female and male participants are marked in blue and red, respectively.
In contrast, main effects of “expectancy level” [F(2, 72) = 1.00, P = 0.374, ηp2 = 0.029], “dispositional optimism (LOT-R)” [F(1, 72) = 0.05, P = 0.832, ηp2 = 0.001], and “gender” [F(1, 72) = 0.03, P = 0.874, ηp2 < 0.001] were not significant on the reduction of pain unpleasantness (see Table 3). Except of non-significant interaction between “dispositional optimism (LOT-R)” and “gender” [F(2, 72) = 0.78, P = 0.379, ηp2 = 0.011], both the interaction between “expectancy level” and “dispositional optimism (LOT-R)” [F(2, 72) = 3.26, P = 0.044, ηp2 = 0.084] (Figure 3, right panel), and the interaction between “expectancy level” and “gender” [F(2, 72) = 3.38, P = 0.040, ηp2 = 0.091] (Figure 2, right panel) were statistically significant. Post-hoc comparison on the interaction between “expectancy level” and “dispositional optimism (LOT-R)” showed that (1) for participants with high LOT-R scores, those in Low expectancy group experienced a greater reduction of unpleasantness due to placebo treatment than those in No expectancy group (P = 0.046); (2) for Low expectancy group, participants with high LOT-R scores had a tendency to report a greater reduction of unpleasantness than those with low LOT-R scores (P = 0.056; see Table 3). Similar to the results of pain intensity, post-hoc pairwise comparisons showed that (1) female participants in the Low expectancy group reported a greater reduction of unpleasantness due to placebo treatment than those in High expectancy group (P = 0.039); (2) for participants in High expectancy group, males tended to report a greater reduction of unpleasantness due to placebo treatment than females (P = 0.073).
Table 3.
The changes of pain unpleasantness from pre-treatment to post-treatment sessions in all experimental groups (data are expressed as M ± SD).
| Personal characteristics | Response expectancy | |||
|---|---|---|---|---|
| No | Low | High | ||
| Gender | F | −0.03 ± 0.71 | 0.36 ± 0.66 | −0.34 ± 0.78 |
| M | 0.02 ± 0.54 | −0.04 ± 0.66 | 0.32 ± 0.92 | |
| LOT-R | Low | 0.32 ± 0.52 | −0.07 ± 0.56 | −0.08 ± 0.84 |
| High | −0.09 ± 0.50 | 0.40 ± 0.58 | −0.03 ± 0.64 | |
| STAI-S | Low | −0.25 ± 0.56 | 0.07 ± 0.72 | 0.47 ± 1.11 |
| High | 0.35 ± 0.71 | 0.21 ± 0.81 | −0.13 ± 0.86 | |
F, Female; M, Male; LOT-R, Life Orientation Test-Revised; STAI-S, State subscale of State-Trait Anxiety Inventory. Changes of pain unpleasantness were obtained by subtracting the ratings in post-treatment sessions from those in pre-treatment sessions.
Influence of Expectancy Level, Anxiety State, and Gender on Placebo Effects
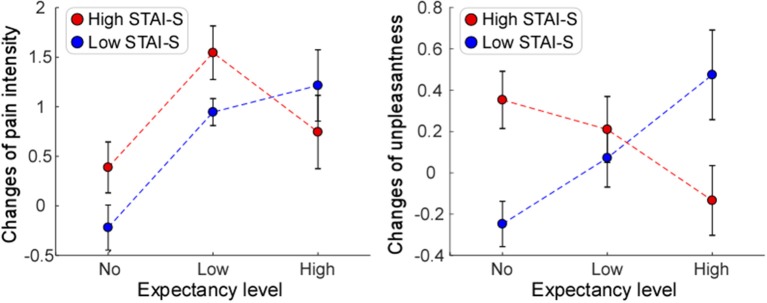
For the reduction of pain intensity, there was a significant main effect of “expectancy level” [F(2, 72) = 4.14, P = 0.02, ηp2 = 0.026]. Post-hoc comparisons showed that participants in Low expectancy group reported a greater reduction of pain intensity than those in No expectancy group (P = 0.007). No significant main effect of “gender” [F(1, 72) = 3.07, P = 0.082, ηp2 = 0.09] or “anxiety state (STAI-S)” [F(1, 72) = 1.21, P = 0.26, ηp2 = 0.02] was found. No significant interaction between “expectancy level” and “anxiety state (STAI-S)” [F(2, 72) = 1.68, P = 0.19, ηp2 = 0.05] (see Figure 4, left panel), or between “anxiety state” and “gender” [F(2, 72) = 1.27, P = 0.26, ηp2 = 0.02] was observed. However, the interaction between “expectancy level” and “gender” was significant [F(2, 72) = 4.04, P = 0.02, ηp2 = 0.11]. Post-hoc comparison on this interaction revealed the same pattern as the results reported in the previous section (“Influence of expectancy level, dispositional optimism, and gender on placebo effects”).
Figure 4.
The influences of expectancy level and anxiety state (STAI-S) on placebo effects. Significant main effect of expectancy level was only observed for changes of pain intensity (Left). Significant interaction of expectancy level and anxiety state (STAI-S) was observed for changes of unpleasantness (Right), but not for changes of pain intensity (Left). Error bars indicate standard error, and data from participants with low and high scores of State subscale of State-Trait Anxiety Inventory (Low STAI-S and High STAI-S) are marked in blue and red, respectively.
With regard to the reduction of unpleasantness, no main effect of “expectancy level” [F(2, 72) = 0.12, P = 0.891, ηp2 = 0.003], “anxiety state (STAI-S)” [F(1, 72) = 0.07, P = 0.796, ηp2 = 0.001], or “gender” [F(1, 72) = 1.28, P = 0.263, ηp2 = 0.018] was observed. The interaction between “expectancy level” and “anxiety state (STAI-S)” [F(2, 72) = 4.05, P = 0.022, ηp2 = 0.106] (Figure 4, right panel), and the interaction between “expectancy level” and “gender” [F(2, 72) = 4.49, P = 0.015, ηp2 = 0.117] (Figure 2, right panel) were significant. Post-hoc pairwise comparisons on the interaction between “expectancy level” and “anxiety state (STAI-S)” showed that for participants with low STAI-S scores, those in High expectancy group felt less pain unpleasantness due to the placebo treatment than those in No expectancy group (P = 0.027). Post-hoc comparison on interaction between “expectancy level” and “gender” revealed the same pattern as the results reported in the previous section (“Influence of expectancy level, dispositional optimism, and gender on placebo effects”). No significant interaction was observed between “anxiety state (STAI-S)” and “gender” [F(2, 72) = 2.39, P = 0.127, ηp2 = 0.034].
Discussion
In the present study, we demonstrated that placebo effects were not only influenced by expectancy level or personal characteristics alone, but also depended on their interactions. Specifically, we observed that the reductions of pain intensity and pain unpleasantness in the Test phase were influenced by the interaction between expectancy level and gender, as mainly reflected by greater reductions of pain intensity and pain unpleasantness in females at Low expectancy level than females at No/High expectancy levels, and greater reductions of pain intensity and pain unpleasantness in males than in females at High expectancy level. Additionally, the reduction of pain unpleasantness was modulated by the interaction between expectancy level and dispositional optimism, as well as the interaction between expectancy level and anxiety state. Participants who were more optimistic in Low expectancy group, or those who were less anxious in High expectancy group showed greater reductions of pain unpleasantness.
Firstly, placebo effects, defined as the reduction of pain intensity or unpleasantness, depended on the interaction between expectancy level and gender. We found female participants elicited maximal placebo effects in Low rather than No and High expectancy groups, which is in line with the previous study showing that placebo effects, as quantified by systolic blood pressure, alertness, and tension, were stronger at the moderate expectancy level than at the extremely low and high expectancy levels (24). In other words, a realistically reasonable expectancy, rather than the unrealistic expectancy level, is more likely to enhance individual belief of the treatment, which is essential to maximize placebo effects (21, 24, 51). However, when compared with female participants, males exhibited a different pattern: in High expectancy group, they reported a greater reduction of pain intensity/unpleasantness due to placebo treatment. These findings suggested that a placebo response to an expectancy manipulation can vary tremendously by gender. However, we failed to observe the main effect of gender in placebo responses, which is inconsistent with a few previous studies (33, 34, 52). Admittedly, the issue of gender discrepancy in placebo responses is still controversial, and further investigation on this issue is highly needed. Please note that gender-specific placebo effects would have tremendous implications for medical research and clinical conditions, such as pain and neurological disorders, in which placebo responses are commonly considered relevant (53, 54).
Secondly, we provided evidence showing that placebo effects, defined as the reduction of pain unpleasantness, were influenced by the interaction between expectancy level and other personal characteristics, such as dispositional optimism and anxiety state. Previous evidence proved that individuals with high scores of dispositional optimism or low scores of anxiety state were more likely to respond to placebo treatment (36, 37). This is in line with our results demonstrating that optimists (those with high LOT-R scores) or participants with low STAI-S scores showed greater placebo responses after treatments. Obviously, being more optimistic or less anxious has a positive influence on the experience to a placebo treatment, as these individuals have a tendency to hold positive expectation (55). In particular, the present results might help explain the consistent correlation between dispositional optimism and positive medical outcomes (56–58). It is suggested that the different respondence between optimists and pessimists (those with low LOT-R scores) to placebo-related expectations may contribute to placebo response discrepancy. Noted that such an effect of dispositional optimism on placebo effects was confirmed in our study, but only observed in Low expectancy group, suggesting that a realistically reasonable, but not an overly-positive expectancy could optimize the influence of dispositional optimism on the placebo response. In other words, since optimists cannot be frequently driven by negative expectancy as forcefully as pessimists can, they might experience fewer negative events. Further, may it be the optimists, not the pessimists, who could be most likely to respond to a placebo-related expectancy for positive outcomes. The above speculation is consistent with a study on persuasion, in which optimists were more likely than pessimists to be persuaded by positively structural arguments (59). Therefore, an individual with high dispositional optimism might not only be less susceptible to negative expectancy, but also be more possible than those with lower dispositional optimism to benefit from positive expectancy, particularly at realistically optimized expectancy level. This is also prompted that patients with high dispositional optimism should be informed more frequently about a certain treatment with realistically positive expectancy to strengthen their responses in medical care. Future studies are needed to explore this issue under clinical conditions.
Importantly, in line with previous studies demonstrating that people's belief can be influenced by personal characteristics, such as optimism, neuroticism, and extraversion (25, 26, 60), our observation provides further evidence suggesting that the placebo effect can be jointly affected by the expectancy level and personal characteristics, which is fitted well with the Response Expectancy Theory (24, 51). Notably, there are tremendous differences in personal characteristics between healthy population and patients. For example, depressive, persistent social phobic, neurotic, fearful, and obsessive-compulsive personality characteristics are very common in pain sufferers compared to healthy population, whereas patients undergoing injectable aesthetic treatments scored significantly higher on extraversion, agreeableness, openness to experience, and neuroticism (61–63). Thus, the next important step is to replicate the main findings of the present study in clinical conditions. To note, a growing body of neurobiological researches on placebo effects indicated the influence of cognitive progressing on the modulation of pain perception (64, 65), which implied that an integrated model combining cognitive factors with psychological factors is warranted to comprehensively explore the placebo mechanisms.
Limitations
There are two limitations in the present study. First, although we assigned both male and female experimenters randomly to the participants, we did not control the potential influence of experimenters' gender well-enough, which still could have increased error variance. Selecting either a male or a female experimenter might be more suitable for further investigations. Second, we examined the effect of the interaction between expectancy level and personal characteristics on placebo effects within a non-clinical population, and it calls for clinical studies to replicate the main findings of the present study.
Conclusion
Considering that placebo effects have been recognized as effective psychobiological events attributing to the improvement of the overall therapeutic outcomes, we believe that our findings not only advance our understanding of the psychological underpinnings of placebo effects, but also suggest a constructive way (regulating the expectation level individually based on the assessment of personal characteristics) to maximize placebo effects in various clinical applications.
Ethics Statement
This experiment was approved by the Ethics Committee of Southwest University, China, and registered with ChiCTR1800014737 through Chinese Clinical Trial Register Centre. All procedure was carried out in accordance with the relevant approved lines.
Author Contributions
HW, LW, XJL, and LH conceived and designed the experiments. HW and HZ performed the experiments. LZ, XJL, and LH analyzed the data. LZ, HW, XYL, CB, LW, XJL, and LH wrote the paper. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (No. 31671141, 31701000, 31822025), the Informatization Special Project of Chinese Academy of Sciences (No. XXH13506-306), and the Scientific Foundation project of Institute of Psychology, Chinese Academy of Sciences (No. Y6CX281007, Y6CX021008, KLMH2018ZG02). The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. (2008) 48:33–60. 10.1146/annurev.pharmtox.48.113006.094711 [DOI] [PubMed] [Google Scholar]
- 2.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet (2010) 375:686–95. 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. (2011) 3:70ra14. 10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- 4.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. (2004) 3:679–84. 10.1016/s1474-4422(04)00908-1 [DOI] [PubMed] [Google Scholar]
- 5.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain (2001) 93:77–84. 10.1016/S0304-3959(01)00296-2 [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. (2006) 26:12014–22. 10.1523/jneurosci.2947-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. (2003) 23:4315–23. 10.1523/JNEUROSCI.23-10-04315.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. (2008) 59:565–90. 10.1146/annurev.psych.59.113006.095941 [DOI] [PubMed] [Google Scholar]
- 9.Kessner S, Forkmann K, Ritter C, Wiech K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: psychological and neurobiological underpinnings. PLoS ONE (2014) 9:e109014. 10.1371/journal.pone.0109014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern Med. (2013) 173:1468–9. 10.1001/jamainternmed.2013.6705 [DOI] [PubMed] [Google Scholar]
- 11.Milling LS, Reardon JM, Carosella G.M. Mediation and moderation of psychological pain treatments: response expectancies and hypnotic suggestibility. J Consult Clin Psychol. (2006) 74:253–62. 10.1037/0022-006x.74.2.253 [DOI] [PubMed] [Google Scholar]
- 12.Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. (1985) 40:1189 10.1037/0003-066X.40.11.1189 [DOI] [Google Scholar]
- 13.Kirsch I. Response expectancy and the response to antidepressant medication. EBioMedicine (2017) 25:13. 10.1016/j.ebiom.2017.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. (2005) 102:12950–5. 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch IE. How Expectancies Shape Experience. Washington, DC: American Psychological Association; (1999). [Google Scholar]
- 16.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. (2006) 26:381–8. 10.1523/jneurosci.3556-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain (1997) 72:107–13. [DOI] [PubMed] [Google Scholar]
- 18.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (2004) 303:1162–7. 10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- 19.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. (2005) 25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain (2002) 96:393–402. 10.1016/S0304-3959(01)00485-7 [DOI] [PubMed] [Google Scholar]
- 21.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. (2013) 12:191–204. 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- 22.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain (1999) 83:147–56. 10.1016/S0304-3959(99)00081-0 [DOI] [PubMed] [Google Scholar]
- 23.Wei H, Zhou L, Zhang H, Chen J, Lu X, Hu L. The influence of expectation on nondeceptive placebo and nocebo effects. Pain Res Manag. (2018) 2018:8459429. 10.1155/2018/8459429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirsch I, Weixel LJ. Double-blind versus deceptive administration of a placebo. Behav Neurosci. (1988) 102:319–23. [DOI] [PubMed] [Google Scholar]
- 25.Evans AM, Revelle W. Survey and behavioral measurements of interpersonal trust. J Res Pers. (2008) 42:1585–93. [Google Scholar]
- 26.Warren ME. Democracy and Trust. Cambridge: Cambridge University Press; (1999). [Google Scholar]
- 27.Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L. The placebo effect: from concepts to genes. Neuroscience (2015) 307:171–90. 10.1016/j.neuroscience.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colloca L, Grillon C. Understanding placebo and nocebo responses for pain management. Curr Pain Headache Rep. (2014) 18:419. 10.1007/s11916-014-0419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxon L, Hiltunen AJ, Hjemdahl P, Borg S. Gender-related differences in response to placebo in benzodiazepine withdrawal: a single-blind pilot study. Psychopharmacology (2001) 153:231–7. 10.1007/s002130000574 [DOI] [PubMed] [Google Scholar]
- 30.Aslaksen PM, Bystad M, Vambheim SM, Flaten MA. Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosom Med. (2011) 73:193–9. 10.1097/PSY.0b013e3182080d73 [DOI] [PubMed] [Google Scholar]
- 31.Butcher BE, Carmody JJ. Sex differences in analgesic response to ibuprofen are influenced by expectancy: a randomized, crossover, balanced placebo-designed study. Eur J Pain (2012) 16:1005–13. 10.1002/j.1532-2149.2011.00104.x [DOI] [PubMed] [Google Scholar]
- 32.Pud D, Yarnitsky D, Sprecher E, Rogowski Z, Adler R, Eisenberg E. Can personality traits and gender predict the response to morphine? An experimental cold pain study. Eur J Pain (2006) 10:103. 10.1016/j.ejpain.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 33.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender-and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain (1999) 83:339–45. 10.1016/S0304-3959(99)00119-0 [DOI] [PubMed] [Google Scholar]
- 34.Olofsen E, Romberg R, Bijl H, Mooren R, Engbers F, Kest B, et al. Alfentanil and placebo analgesiano sex differences detected in models of experimental pain. Anesthesiol J Am Soc Anesthesiol. (2005) 103:130–9. 10.1097/00000542-200507000-00020 [DOI] [PubMed] [Google Scholar]
- 35.Brissette I, Scheier MF, Carver CS. The role of optimism in social network development, coping, and psychological adjustment during a life transition. J Personal Soc Psychol. (2002) 82:102. 10.1037//0022-3514.82.1.102 [DOI] [PubMed] [Google Scholar]
- 36.Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain (2009) 146:194–8. 10.1016/j.pain.2009.07.026 [DOI] [PubMed] [Google Scholar]
- 37.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. J Pain (2010) 11:1165–71. 10.1016/j.jpain.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu L, Zhao C, Li H, Valentini E. Mismatch responses evoked by nociceptive stimuli. Psychophysiology (2013) 50:158–73. 10.1111/psyp.12000 [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Valentini E, Hu L. Functional features of crossmodal mismatch responses. Exp Brain Res. (2015) 233:617–29. 10.1007/s00221-014-4141-4 [DOI] [PubMed] [Google Scholar]
- 40.Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Adelta fibers by intra-epidermal needle electrode in humans. Pain (2002) 96:247–52. 10.1016/S0304-3959(01)00453-5 [DOI] [PubMed] [Google Scholar]
- 41.Kakigi R, Inui K, Tamura Y. Electrophysiological studies on human pain perception. Clin Neurophysiol. (2005) 116:743–63. 10.1016/j.clinph.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 42.Li CH. Validation of the Chinese version of the life orientation test with a robust weighted least squares approach. Psychol Assess. (2012) 24:770–6. 10.1037/a0026612 [DOI] [PubMed] [Google Scholar]
- 43.Shek DT. Reliability and factorial structure of the Chinese version of the State-Trait Anxiety Inventory. J Psychopathol Behav Assess. (1988) 10:303–17. [Google Scholar]
- 44.Aslaksen PM, Myrbakk IN, Høifødt RS, Flaten MA. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain (2007) 129:260–8. 10.1016/j.pain.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 45.Kállai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain (2004) 112:142–7. 10.1016/j.pain.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 46.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain (2006) 124:126–33. 10.1016/j.pain.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 47.Jensen K, Kirsch I, Odmalm S, Kaptchuk TJ, Ingvar M. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci USA. (2015) 112:7863–7. 10.1073/pnas.1504567112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, et al. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci USA. (2012) 109:15959–64. 10.1073/pnas.1202056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. (2008) 28:13354–62. 10.1523/jneurosci.2944-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J. Statistical Power Analysis for the Social Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; (1988). [Google Scholar]
- 51.Kirsch I. Changing Expectations: A Key to Effective Psychotherapy. Pacific Grove, CA: Thomson Brooks/Cole Publishing Co; (1990). [Google Scholar]
- 52.Averbuch M, Katzper M. Gender and the placebo analgesic effect in acute pain. Clin Pharmacol Ther. (2001) 70:287–91. 10.1067/mcp.2001.118366 [DOI] [PubMed] [Google Scholar]
- 53.Castelnuovo G, Giusti EM, Manzoni GM, Saviola D, Gabrielli S, Lacerenza M, et al. What is the role of the placebo effect for pain relief in neurorehabilitation? Clinical implications from the italian consensus conference on pain in neurorehabilitation. Front Neurol. (2018) 9:310. 10.3389/fneur.2018.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skyt I, Moslemi K, Baastrup C, Grosen K, Svensson P, Jensen TS, et al. Does conditioned pain modulation predict the magnitude of placebo effects in patients with neuropathic pain? Eur J Pain (2018) 22:784–92. 10.1002/ejp.1164 [DOI] [PubMed] [Google Scholar]
- 55.Hodo DW. Personality characteristics of patients with pain. Am J Psychiatry (2002) 159:336. [Google Scholar]
- 56.Williams NL. Optimism and pessimism: implications for theory, research, and practice. J Cogn Psychother. (2003) 17:200 10.1891/088983903780906174 [DOI] [Google Scholar]
- 57.Geers AL, Helfer SG, Kosbab K, Weiland PE, Landry SJ. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. J Psychosom Res. (2005) 58:121–7. 10.1016/j.jpsychores.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 58.Scheier MF, Carver CS. Effects of optimism on psychological and physical well-being: theoretical overview and empirical update. Cogn Ther Res. (1992) 16:201–28. [Google Scholar]
- 59.Geers AL, Handley IM, McLarney AR. Discerning the role of optimism in persuasion: the valence-enhancement hypothesis. J Personal Soc Psychol. (2003) 85:554. 10.1037/0022-3514.85.3.554 [DOI] [PubMed] [Google Scholar]
- 60.Hiraishi K, Yamagata S, Shikishima C, Ando J. Maintenance of genetic variation in personality through control of mental mechanisms: a test of trust, extraversion, and agreeableness. Evol Hum Behav. (2008) 29:79–85. 10.1016/j.evolhumbehav.2007.07.004 [DOI] [Google Scholar]
- 61.Naylor B, Boag S, Gustin SM. New evidence for a pain personality? A critical review of the last 120 years of pain and personality. Scand J Pain (2017) 17:58–67. 10.1016/j.sjpain.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 62.Scharschmidt D, Mirastschijski U, Preiss S, Brähler E, Fischer T, Borkenhagen A. Body image, personality traits, and quality of life in Botulinum Toxin A, and dermal filler patients. Aesthetic Plast Surg. (2018) 42:1119–25. 10.1007/s00266-018-1165-3 [DOI] [PubMed] [Google Scholar]
- 63.Taiminen T, Kuusalo L, Lehtinen L, Forssell H, Hagelberg N, Tenovuo O, et al. Psychiatric (axis I) and personality (axis II) disorders in patients with burning mouth syndrome or atypical facial pain. Scand J Pain (2011) 2:155–60. 10.1016/j.sjpain.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 64.Wiech K. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science (2016) 354:584–7. 10.1126/science.aaf8934 [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Zhou L, Wei H, Lu X, Hu L. The sustained influence of prior experience induced by social observation on placebo and nocebo responses. J Pain Res. (2017) 10:2769. 10.2147/JPR.S147970 [DOI] [PMC free article] [PubMed] [Google Scholar]