Abstract
Interest has been focused on differentiating anatomical, molecular, and physiological characteristics of the types of mammalian adipose tissues. White adipose tissue (WAT) and brown adipose tissue (BAT) are the two main forms of adipose tissue in humans. WAT functions as an endocrine organ and serves as a reservoir of energy in the form of triglycerides. The hormones released by WAT are called adipokines. BAT consists of a group of specialized cells with abundant uncoupling protein 1 (UCP1) in the inner mitochondrial membrane and also fulfills endocrine functions. Following the identification of functional (BAT) in human adults, there has been a great deal of interest in finding out how it is induced, its localization, and the mechanisms by which it regulates thermogenesis. Fibroblast growth factor 21 (FGF21) is a key regulator of the differentiation to brown adipocytes. The main mechanisms occur through enhancing UCP1 expression. In addition, following exposure to cold or exercise, FGF21 induces upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa and thus promotes thermogenesis in adipose tissue and skeletal muscle. FGF21 integrates several pathways allowing the regulation of human energy balance, glucose levels, and lipid metabolism. Such mechanisms and their clinical relevance are summarized in this review.
Keywords: fibroblast growth factor 21, glucose, energy balance, insulin resistance, irisin, exercise, noradrenaline, free fatty acids
Introduction
Fibroblast growth factor 21 (FGF21) has important effects on energy balance, glucose metabolism, and lipid metabolism (Kharitonenkov et al., 2005). Initial reports identified the liver as its main source (Nishimura et al., 2000). On secretion, its most important target is white adipose tissue (WAT), where FGF21 increases expression of GLUT1 and consequently glucose uptake (Kharitonenkov et al., 2005). During fasting or starvation, lipolysis is triggered, with a subsequent increment in circulating free fatty acids (FFAs). FFAs induce the activation of the peroxisome proliferator-activated receptor (PPAR)-alfa in the liver, resulting in the synthesis and release of FGF21. Since carbohydrate ingestion is absent during starvation, FGF21 induces ketone body formation in the liver as an additional energy source (Badman et al., 2007; Inagaki et al., 2008). FGF21 may also be considered an adipokine, since it is also synthesized and released from WAT. It shows complementary actions to adiponectin (Lin et al., 2013), increasing insulin sensitivity, improving the lipid profile, reducing glucose levels without causing hypoglycemia, and ensuring energy availability during starvation (Badman et al., 2007; Inagaki et al., 2008). In human insulin resistance states, FGF21 levels have a positive correlation with the number of metabolic syndrome traits, the severity of oxidative stress, and the presence of type 2 diabetes (Zhang et al., 2008; Cuevas-Ramos et al., 2010; Gómez-Sámano et al., 2017). Other human stress-related conditions that increase circulating FGF21 levels are lactation (Schoenberg et al., 2011), exercise (Cuevas-Ramos et al., 2012b), growth hormone treatment (Chen et al., 2011), and anorexia nervosa (Dostálová, 2008). Interestingly, FGF21 is now considered an important mediator for decreasing oxidative stress, and possibly, preventing microvascular diseases such as diabetic nephropathy (Jian et al., 2012).
The role of FGF21 as a therapeutic option in human metabolic diseases is of increasing importance. Currently, there are multiple recombinant FGF21 analogs in phase 2 and phase 3 clinical trials (Mu et al., 2012; Gaich et al., 2013; Talukdar et al., 2016). Interest has increased even more so after the discovery of the crucial role of FGF21 in inducing the proliferation of brown adipose tissue (BAT) (Fisher et al., 2012). This review describes the mechanisms by which FGF21 induces “browning” of adipose tissue and how it may have a role in the treatment of human metabolic diseases, including obesity and type 2 diabetes.
White, Beige, and Brown Subtypes of Adipose Tissues in Humans
WAT and BAT are the two main subtypes of adipose tissue in humans. WAT has important endocrine functions in addition to its role as a reservoir of energy in the form of triglycerides. The hormones released from WAT, namely adipokines, are a well-recognized group of bioactive factors with endocrine actions that act through specific cell-membrane receptors. Adipokines trigger certain intracellular signaling pathways, which modulate human metabolism (Piya et al., 2013). The most important are leptin and adiponectin, but visfatin, chemerin, omentin, hepcidin, apelin, and vaspin have also been described (Piya et al., 2013).
BAT is also an important endocrine organ. It consists of a group of specialized cells with abundant expression of uncoupling protein 1 (UCP1) in the inner mitochondrial membrane (Aherne and Hull, 1966; Cannon and Nedergaard, 2004). The BAT hormones are named “batokines” (Baboota et al., 2015; Booth et al., 2016). The principle function of BAT is to dissipate stored energy in the form of heat by uncoupling energy oxidation from ATP synthesis (Fedorenko et al., 2012). Initially, BAT was only considered as an energy-producing organ in rodents and human infants (Bartness et al., 2010). However, after the development of 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT), BAT has also been identified in human adults (Nedergaard et al., 2007; Cypess et al., 2009). Nevertheless, the origin of BAT is still under debate, although originally it was thought to be derived from skeletal muscle-like lineage (Myf5+) (Seale et al., 2007). In human adults, a type of adipose tissue showing characteristics between that of white and brown adipocytes has been identified; this kind of adipose tissue is known as beige adipose tissue (brite, brown-in-white) (Jespersen et al., 2013). It appears that beige and white adipocytes arise from both Myf5+ and Myf5- progenitor cells. These findings confirm that the skeletal muscle-like lineage is not the only source of BAT (Wu et al., 2012).
The location of the different types of adipose tissue varies. Beige and WAT have mainly visceral (mesenteric, perigonadal or omental adipose tissue surrounding organs) and subcutaneous (under skin) locations. BAT is located only in axillary, subscapular, interscapular, and periaortic regions in rodents; in cervical, supraclavicular, paravertebral, mediastinal regions in humans; and in perirenal regions in both (Park et al., 2014; Sanchez-Gurmaches and Guertin, 2014). Brite or beige adipocytes have basal metabolic actions similar to those seen in white adipocytes, and with the enough stimulus, they are able to transform into thermogenic adipocytes with higher UCP1 expression similar to BAT (Wu et al., 2012). This process is referred as “browning” and it describes the capacity of white adipocytes to acquire a phenotype similar to that of BAT, leading to increased thermogenesis. It is achieved when white adipocytes are exposed to cold or to beta 3-adrenoreceptor agonists (Young et al., 1984, Harms and Seale, 2013). Browning occurs mainly in subcutaneous white adipose fat depots. The underlying molecular mechanisms for this trans-differentiation are currently under intensive research (Luo and Liu, 2016). In addition, there are important structural differences among WAT, brite, and BAT. WAT is a large lipid droplet, with a peripheral nucleus and a small amount of cytoplasm, whereas BAT has a central nucleus with more cytoplasm but smaller lipid droplets. In between these is the brite or beige tissue; this has the mixed structural characteristics of both. Sometimes the different structures are found together; for example, the ectopic expression of UCP1 and the presence of the PR domain containing 16 (PRDM16) suggests that brite adipocytes are mixed with white adipocyte depots (Wu et al., 2013). The balance between WAT and BAT, and their endocrine regulation, are key elements to better understand the development of weight gain and human metabolic diseases.
Molecular Pathways and Clinical Relevance of Browning Induced by FGF21
Since the discovery of FGF21, it has been appreciated that its synthesis is strongly related to cold exposure (Badman et al., 2007; Inagaki et al., 2008). In mice, during hypothermia, FGF21 induces torpor, a short-term hibernation state in which animals can save energy by reducing body temperature and physical activity (Badman et al., 2007). More recently, studies have shown a higher expression of FGF21 in inguinal WAT after cold exposure. The role of FGF21 produced in WAT includes both paracrine and autocrine actions; this results in the local upregulation of peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa and thus an increase in thermogenesis (Hondares et al., 2010; Fisher et al., 2012; Adams et al., 2013; Emanuelli et al., 2014). PGC1-alfa is a protein involved in modulating several effects in post-exercise skeletal muscle, including the improvement of energy and glucose metabolism (Summermatter et al., 2013). Interestingly, PGC1-alfa is also induced after irisin or insulin exposure, both hormones showing a clear interaction with FGF21 post-exercise (Cuevas-Ramos et al., 2010, 2012b; Bostrom et al., 2012; Fisher et al., 2012; Hu and Christian, 2017). Irisin-induced phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and extracellular signal-related kinase (ERK) show a positive correlation with shivering intensity (Bostrom et al., 2012; Zhang et al., 2014). FGF21 also shows a direct relationship with exercise intensity (Cuevas-Ramos et al., 2010, 2012b). The consequence of these PGC1-alfa inducers is to promote adaptive thermogenesis with “browning” of WAT (Fisher et al., 2012). The main mechanism following FGF21 action is PPAR-gamma activation in WAT, together with the irisin effect inducing MAPK and ERK pathways. This results in differentiation of pre-adipocytes to mature white adipocytes, which are then available for “browning” (Hondares et al., 2011; Zhang Y. et al., 2016).
Some animal models have reported findings consistent with these actions. For example, FGF21 deficiency in mice results in increased body weight with excessive adiposity, higher serum cholesterol, insulin resistance, and hyperglycemia (Kharitonenkov et al., 2005). The finding of a 30–40% lower nuclear content of PGC1-alfa at the hepatic mitochondrial level in Fgf21 KO mice compared with WT mice, is a potential explanation for these results (Fletcher et al., 2016). FGF21 induces palmitate oxidation and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity. In the Fgf21 KO model, these enzymatic activities are decreased, indicating lower lipid oxidation, a reduction in glucose metabolism, and a lower degree of energy waste (Fletcher et al., 2016). In contrast, overexpression of FGF21 effectively decreases weight, adiposity, levels of FFAs, triglycerides, glucose, and insulin, all due to the normalization of mitochondrial oxidation (Fletcher et al., 2016) and the improvement in insulin sensitivity (Kharitonenkov et al., 2005). Interestingly, exercise, irisin, and noradrenaline were necessary to restore PGC1-alfa content in the liver despite overexpression of FGF21, emphasizing the key interaction of such inducers with FGF21 (Fletcher et al., 2016). Insulin, the most important regulator of energy and glucose metabolism, enhanced differentiation of WAT to brite and brown adipocytes through pro-opiomelanocortin (POMC) neurons (Table 1 and Figure 1); (Dodd et al., 2015). Therefore, multiple mechanisms may be inter-connected to improve metabolism in humans, with FGF21 functioning as an important link between them.
Table 1.
Most important hormones, drugs, and nutritional inducers of browning.
| Effector | Effect and mechanism on WAT |
|---|---|
| AMPK activators | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis. Enhance PGC1-alfa and UCP1. Ej: with AICAR (Gaidhu et al., 2009) |
| BMPs | BMP7 and BMP 8 induce higher thermogenesis, increase energy expenditure and mitochondrial biogenesis. Enhance PGC1-alfa and UCP1. Increases lipid oxidation (Schulz et al., 2011; Whittle et al., 2012). |
| Beta-3-adrenergic stimulation | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis, enhance UCP1, and activation of c-AMP, PKA, p38 MAPK, PGC1-alfa, and PPAR-alfa (Jimenez et al., 2003; Li et al., 2005). |
| Fenofibrate | Effects are through PPAR-alfa agonism (Magliano et al., 2013; Rachid et al., 2015) |
| FGF21 recombinant analogs | After cold exposure or adrenergic stimulation induced higher thermogenesis, increase energy expenditure and mitochondrial biogenesis. Enhance PGC1-alfa and UCP1. After exercising, possible interaction with irisin reducing fat depots (Dostálová, 2008; Cuevas-Ramos et al., 2012b; Lee et al., 2014). |
| FGFR1/KLB antibodies | Higher thermogenesis but through UCP1-independent mechanism (Chen et al., 2017). |
| Insulin and leptin (adipoinsular axis) | Acts in hypothalamic POMC neurons to induce browning (Dodd et al., 2015). |
| Irisin | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis, enhance UCP1, through PPAR-alfa agonism. Irisin also stimulated browning after exercising (Bostrom et al., 2012; Wu et al., 2012; Zhang Y. et al., 2016). |
| Thyroid hormones | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis (Lee et al., 2012). |
| Natriuretic peptides (ANP) | Synergism with beta-3-adrenergic receptor stimulation after exercising inducing higher thermogenesis, enhancing UCP1 expression, and lipolysis through PKA, c-GMP and PKG (Lafontan et al., 2008; Bordicchia et al., 2012). |
| Thiazolidinediones | Higher thermogenesis, enhancing UCP1 expression and inducing insulin sensitivity. Synergism after adrenergic stimulus (Petrovic et al., 2010). |
| Capsaicin | Adrenergic stimulation causing higher thermogenesis, through TRPV1 protein activating neurons (Teodoro et al., 2014; Baskaran et al., 2016). |
| Bile acids | Higher thermogenesis, enhancing UCP1 expression through TGR5 (Watanabe et al., 2006; Teodoro et al., 2014). |
| Citrulline | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis, enhance UCP1, and PPAR-alfa agonism (Joffin et al., 2015). |
| Fucoxanthin | Higher thermogenesis, enhancing UCP1 expression (Maeda et al., 2005). |
| Luteolin | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis, enhance UCP1 (Zhang X. et al., 2016). |
| Methionine restriction | Higher thermogenesis, enhancing UCP1 expression (Hasek et al., 2010). |
| n-3 PUFAs | Higher thermogenesis, enhancing UCP1 expression (Zhao and Chen, 2014; Bargut et al., 2016). |
| Resveratrol | Higher thermogenesis, increase energy expenditure and mitochondrial biogenesis, enhancing PGC1-alfa and UCP1. Also increase PRDM16 expression, and increases lipid oxidation activating AMPK (Wang et al., 2015). |
| Retinoic acid | Higher thermogenesis, enhancing UCP1 expression. Also, PPAR-beta/delta expression (Murholm et al., 2013). |
| Beta-hydroxybutyrate | Higher thermogenesis, enhancing UCP1 expression (Carriere et al., 2014). |
AICAR, Activator 5-aminoimidazole-4-carboxamide ribonucleoside; AMPK, adenosin monophosphate activated protein kinase; ANP, atrial natriuretic peptide; c-GMP, cyclic-guanosine monophosphate protein; FFA, free fatty acids; FGF21, fibroblast growth factor 21; MAPK, Mitogen-activated protein kinase; PGC1-alfa, peroxisome proliferator-activated receptor gamma coactivator (PGC) 1-alfa; PKA, protein kinase A; PKG, protein kinase G; PPAR, peroxisome proliferator-activated receptor; PRDM16, PR domain containing 16; PUFA, polyunsaturated fatty acids; TRG5, G protein-coupled receptor 5; TRPV1, transient receptor potential V1; UCP1, uncoupled protein 1; WAT, white adipose tissue.
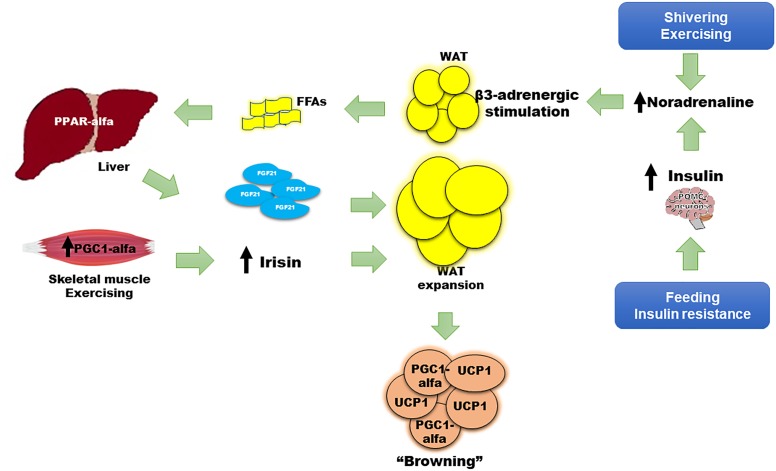
FIGURE 1.
Role of FGF21 in the “browning” of adipose tissue. Adaptive thermogenesis following cold exposure, shivering, or exercise, and physiologic (i.e., feeding) or pathologic (i.e., insulin resistance) states, begins a compensatory process to induce “browning” of WAT, thus enhancing thermogenesis, energy waste, and improving cell metabolism. The principle mechanisms to induce “browning” involve insulin, irisin, and FGF21. Insulin increases adrenergic stimulation and noradrenaline secretion after acting on POMC neurons in the central nervous system. FGF21 also has a WAT-independent mechanism acting directly to the CNS, increasing noradrenaline release. Noradrenaline induces beta 3-adrenergic receptor stimulation and greater lipolysis that produces FFAs as main substrate for PPAR-alfa agonism and FGF21 synthesis and release from liver. Irisin is mainly released from skeletal muscle after shivering or exercise. Noradrenaline, irisin, and FGF21 promote uncoupling protein 1 (UCP1) expression, a protein that increases thermogenesis at the mitochondrial inner membrane, and upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa on white adipocytes, turning them to brite or brown adipose tissue. WAT expansion is also induced by FGF21, increasing insulin sensitivity.
The exogenous administration of an FGF21 analog in animal models has been associated with a thermogenic effect, an improvement in glucose homeostasis, lipid profile, and a reduction in body weight (Wu et al., 2011; Veniant et al., 2012). However, in humans, recombinant FGF21 analogs have not shown these effects. FGF21 is paradoxically increased in insulin-resistant states such as obesity or type 2 diabetes; this suggests either a resistance to FGF21 effects or a compensatory response to these metabolic disarrangements (Zhang et al., 2008; Cuevas-Ramos et al., 2010). Nevertheless, the key metabolic role of FGF21 is clear. Firstly, WAT increases FGF21 expression in response to feeding, which has an autocrine action on PPAR-gamma activity; PPAR-gamma is an important inducer of insulin sensitivity pathways, adipocyte maturation, and function (Dutchak et al., 2012). Secondly, FGF21 is significantly induced after moderate to intensive physical activity (Cuevas-Ramos et al., 2010, 2012b), and interestingly, its release has been correlated with sympathetic nervous system activation and lipolysis markers, such as noradrenaline and serum free fatty acids, respectively (Cuevas-Ramos et al., 2012b). Thirdly, FGF21 regulates metabolism through increasing insulin sensitivity at WAT and BAT, but this may happen in an acute and a chronic manner. Acute and chronic exogenous administration of FGF21 induce insulin sensitivity independently of adiponectin action (BonDurant et al., 2017). However, the chronic effect of FGF21 is not necessarily via a direct effect on WAT since it also induces signaling in the central nervous system via POMC neurons, and on brown adipocytes, enhancing thermogenesis and insulin-sensitivity in mice (Figure 1). These effects require functional insulin signaling in adipose tissue, otherwise the FGF21-benefit is lost. In addition, the action of FGF21 on the central nervous system is important to induce energy expenditure, mainly by increasing noradrenaline release (Figure 1) (Owen et al., 2014). Noradrenaline plays an important role in the regulation of “browning.” It is the most studied activator of thermogenesis, increasing UCP1 transcription, enhancing lipolysis and mitochondrial oxidation (Figure 1; (Puigserver et al., 1996). After cold-induced shivering or physical activity stress, noradrenergic pathways increase thermogenic gene expression through c-AMP-mediated mechanisms (Hondares et al., 2011). Lipolysis and FFAs increase following noradrenaline release. With acute intensive exercise, the increase in beta 3-receptor activity also causes an increment in circulating FGF21 (Cuevas-Ramos et al., 2012b). Increased circulating FFAs induce PPAR-alfa expression in the liver, increasing FGF21 synthesis and release into circulation (Badman et al., 2007; Inagaki et al., 2008). FGF21 can also increase insulin sensitivity by promoting the expansion of subcutaneous WAT. Fgf21 knockout mice show less subcutaneous WAT and a greater degree of insulin resistance. After treatment with recombinant FGF21, subcutaneous adipose tissue was restored with a subsequent improvement in insulin sensitivity (Li et al., 2018). The expression of co-factor beta klotho is necessary to accomplish the FGF21-related expansion of subcutaneous fat (Li et al., 2018). Finally, chronic pharmacologic administration of FGF21 in obese mice has been shown to suppress growth hormone (GH) and the insulin growth factor-1 (IGF1) signaling axis in the liver, increasing lifespan through an improvement in insulin sensitization, normalization of glycemia, and a reduction in body weight (Zhang Y. et al., 2012).
Under normal conditions, FGF21 is synthetized and released from the liver. However, in certain circumstances, such as adaptive thermogenesis induced by cold exposure or exercise, BAT expresses and releasees FGF21 (Chartoumpekis et al., 2011; Hondares et al., 2011; Giralt et al., 2015; Lee et al., 2015). The production of FGF21 by BAT is not negligible; it significantly contributes to systemic FGF21 levels (Hondares et al., 2011). Following exposure to cold, the production of FGF21 by BAT is greater than that of the liver, enhancing thermogenesis, confirming the key roles of BAT in regulating FGF21 levels (Hondares et al., 2011). Moreover, a dramatic rise in Fgf21 expression in BAT has also been reported in Ucp1-null mice or after genetic inactivation of UCP1 protein. In these situations, there is an increase in serum FGF21 levels without changes in FGF21 gene expression in the liver (Keipert et al., 2015; Samms et al., 2015). This suggests thermogenic regulation of FGF21 through both UCP1-dependent as well as UCP1-independent mechanisms (Keipert et al., 2015; Samms et al., 2015).
Taken together, adipose tissue, liver, and skeletal muscle respond to multiple stimuli in order to increase adaptive thermogenesis and induce the browning of WAT (Figure 1). Expression and release of FGF21 by the liver, BAT, and skeletal muscle is induced by shivering (Badman et al., 2007; Inagaki et al., 2008; Hondares et al., 2011), physical activity (Cuevas-Ramos et al., 2010, 2012b; Kim et al., 2013), protein synthesis after growth hormone treatment (Chen et al., 2011), and as a consequence of experimental or clinical mitochondrial dysfunction following DNA mutations (Suomalainen et al., 2011; Keipert et al., 2014). FGF21, together with irisin, insulin, and noradrenaline, provokes metabolically healthy effects that are concomitantly associated with the browning of WAT (Mossenbock et al., 2014; Lee et al., 2015; Villarroya et al., 2017). The higher BAT activity and increased heat production may benefit human health, reducing weight, preventing hyperglycemia, and hyperlipidemia, and protecting against obesity through enhancement of energy waste (Cuevas-Ramos et al., 2012a). These effects explain the association of FGF21 and explain its key role in the “browning” of WAT. This was probably aimed to allow the adaptation of human metabolism to obesity, diabetes, dyslipidemia, metabolic syndrome, and other insulin resistance states (Figure 1).
FGF21 as Potential Medical Treatment to Induce Browning of WAT
The beneficial metabolic consequences of “browning” may be useful to treat metabolic diseases in humans (Barquissau et al., 2016). There are multiple medical drugs or nutritional inducers that may help to stimulate browning (Table 1). Recently, the role of FGF21 in the browning of WAT has been evaluated with the development of recombinant FGF21-analogs. Initially, clinical trials with analogs LY2405319 and PF05231023 were focused on treating human metabolic diseases including obesity, metabolic syndrome and type 2 diabetes (Gaich et al., 2013; Talukdar et al., 2016). However, resistance to their actions, resulting in an inadequate clinical effect, has been a problem; in humans, only a slight glucose, weight or triglyceride reduction was achieved. The subcutaneous depots of WAT are small cells with greater potential to differentiate (Gustafson and Smith, 2015). Therefore, it is feasible to hypothesize a positive effect if “browning” can be induced, following a sufficient stimulus using FGF21-analogs. In addition, the FGF receptor type 1 together with beta klotho cofactor, called the FGFR1/KLB complex, is the functional target for FGF21 (Kolumam et al., 2015). The use of FGF21 agonist antibodies that specifically activate this complex largely mimic the action of recombinant FGF21 in mice (Kolumam et al., 2015). These include the agonist antibodies BFKB8488 and NGM313, which are currently under clinical research (ClinicalTrials.gov, NCT02593331, NCT02708576, and NCT03060538). Both FGF21 recombinant analogs (Gaich et al., 2013; Talukdar et al., 2016) and the FGFR1/KLB complex agonist antibodies induce higher thermogenesis and browning of BAT through UCP1-dependent pathways. Although UCP1 has traditionally been thought of as indispensable for browning and thermogenesis (Golozoubova et al., 2001; Feldmann et al., 2009), recent research suggests a role of a UCP1-independent pathway (Chen et al., 2017). In Ucp1 KO mice, higher thermogenesis with weight loss and beneficial changes in cardiometabolic markers have been reported (Veniant et al., 2015; Chen et al., 2017). The origin of this UCP1-independent thermogenesis is still controversial, with different reports suggesting the opposite (Keipert et al., 2015). Further investigation is warranted to clarify if higher thermogenesis can be obtained without overexpression of UCP1.
In addition to FGF21-recombinant analogs or the FGFR1-KLB complex agonist antibodies, other drugs have been used with similar aims; however, most have shown little clinical utility. For example, PPAR-alfa agonists (fibrates), adrenergic beta-3-receptor stimulators, thyroid hormones, and more recently irisin have been tested (Table 1). There are also certain nutritional inducers of “browning” of WAT that may be considered as therapeutic options. The most important hormones, drugs and nutritional inducers of browning are summarized on Table 1. It is important to mention that although nicotine has been associated with body weight reduction, mainly due to the associated decreased appetite, greater lipolysis, and increased energy waste (Zoli and Picciotto, 2012), it has never been confirmed that smoking cigarettes can induce browning (Chen et al., 2005).
Conclusion
FGF21 is a key regulator of the differentiation of WAT to brown adipocytes, resulting in enhanced thermogenesis and energy waste. The main action seems to be through UCP1-dependent and -independent mechanisms. In addition, after cold exposure or exercising, FGF21-induced upregulation of local peroxisome proliferator-activated receptor gamma co-activator (PGC)-1-alfa increases thermogenesis in adipose tissue and skeletal muscle. Potential mechanisms involve higher noradrenaline levels that act on the WAT-beta-3 adrenergic receptor, inducing lipolysis, and higher serum free fatty acids, which in turn increase PPAR-alfa agonism at liver, and higher FGF21 synthesis and then release into circulation. This effect contributes to other FGF21-related mechanisms that integrate metabolic pathways to regulate human energy balance, glucose and lipid levels. Therefore, the development of better recombinant FGF21 analogs as a potential treatment for metabolic diseases in humans is necessary.
Author Contributions
All authors made substantial contributions to the conception and/or design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; and approved the final version of the article to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This review was supported by Department of Endocrinology and Metabolism at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
References
- Adams A. C., Coskun T., Cheng C. C., Ls O. F., Dubois S. L., Kharitonenkov A. (2013). Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol. Metab. 2 205–214. 10.1016/j.molmet.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne W., Hull D. (1966). Brown adipose tissue and heat production in the newborn infant. J. Pathol. Bacteriol. 91 223–234. 10.1002/path.1700910126 [DOI] [PubMed] [Google Scholar]
- Baboota R. K., Sarma S. M., Boparai R. K., Kondepudi K. K., Mantri S., Bishnoi M. (2015). Microarray based gene expression analysis of murine brown and subcutaneous adipose tissue: significance with human. PLoS One 10:e0127701. 10.1371/journal.pone.0127701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. (2007). Hepatic fibroblast growth factor 21 is regulated by PPAR-alfa and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5 426–437. 10.1016/j.cmet.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Bargut T. C., Souza-Mello V., Mandarim-de-Lacerda C. A., Aguila M. B. (2016). Fish oil diet modulates epididymal and inguinal adipocyte metabolism in mice. Food Funct. 7 1468–1476. 10.1039/c5fo00909j [DOI] [PubMed] [Google Scholar]
- Barquissau V., Beuzelin D., Pisani D. F., Beranger G. E., Mairal A., Montagner A., et al. (2016). White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 5 352–365. 10.1016/j.molmet.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T. J., Vaughan C. H., Song C. K. (2010). Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34(Suppl. 1), S36–S42. 10.1038/ijo.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P., Krishnan V., Ren J., Thyagarajan B. (2016). Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 173 2369–2389. 10.1111/bph.13514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant L. D., Ameka M., Naber M. C., Markan K. R., Idiga S. O., Acevedo M. R., et al. (2017). FGF21 regulates metabolism through adipose-dependent and independent-mechanisms. Cell Metab. 25 935–944. 10.1016/j.cmet.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A., Magnuson A., Fouts J., Foster M. T. (2016). Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 26 25–42. 10.1515/hmbci-2015-0073 [DOI] [PubMed] [Google Scholar]
- Bordicchia M., Liu D., Amri E. Z., Ailhaud G., Dessi-Fulgheri P., Zhang C., et al. (2012). Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122 1022–1036. 10.1172/JCI59701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., et al. (2012). A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481 463–468. 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84 277–359. 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Carriere A., Jeanson Y., Berger-Muller S., Andre M., Chenouard V., Arnaud E., et al. (2014). Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63 3253–3265. 10.2337/db13-1885 [DOI] [PubMed] [Google Scholar]
- Chartoumpekis D. V., Habeos I. G., Ziros P. G., Psyrogiannis A. I., Kyriazopoulou V. E., Papavassiliou A. G., et al. (2011). Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol. Med. 17 736–740. 10.2119/molmed.2011.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Vlahos R., Bozinovski S., Jones J., Anderson G. P., Morris M. J. (2005). Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology 30 713–719. 10.1038/sj.npp.1300597 [DOI] [PubMed] [Google Scholar]
- Chen M. Z., Chang J. C., Zavala-Solorio J., Kates L., Thai M., Ogasawara A., et al. (2017). FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/Beta Klotho complex in non-adipocytes. Mol. Metab. 6 1454–1467. 10.1016/j.molmet.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Hoo R. L., Konishi M., Itoh N., Lee P. C., Ye H. Y., et al. (2011). Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 286 34559–34566. 10.1074/jbc.M111.285965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos D., Aguilar-Salinas C. A., Gomez-Perez F. J. (2012a). Metabolic actions of fibroblast growth factor 21. Curr. Opin. Pediatr. 24 523–529. 10.1097/MOP.0b013e3283557d22 [DOI] [PubMed] [Google Scholar]
- Cuevas-Ramos D., Almeda-Valdés P., Meza-Arana C. E., Brito-Córdova G., Gómez-Pérez F. J., Mehta R., et al. (2012b). Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 7:e38022. 10.1371/journal.pone.0038022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos D., Almeda-Valdes P., Gomez-Perez F. J., Meza-Arana C. E., Cruz-Bautista I., Arellano-Campos O., et al. (2010). Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur. J. Endocrinol. 163 469–477. 10.1530/EJE-10-0454 [DOI] [PubMed] [Google Scholar]
- Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., et al. (2009). Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360 1509–1517. 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd G. T., Decherf S., Loh K., Simonds S. E., Wiede F., Balland E., et al. (2015). Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160 88–104. 10.1016/j.cell.2014.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostálová I., Kaválková P., Haluzíková D., Lacinová Z., Mráz M., Papezová H., et al. (2008). Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 93 3627–3632. 10.1210/jc.2008-0746 [DOI] [PubMed] [Google Scholar]
- Dutchak P. A., Katafuchi T., Bookout A. L., Choi J. H., Yu R. T., Mangelsdorf D. J., et al. (2012). Fibroblast growth factor-21 regulates PPARg activity and the antidiabetic actions of thiazolidinediones. Cell 148 556–567. 10.1016/j.cell.2011.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli B., Vienberg S. G., Smyth G., Cheng C., Stanford K. I., Arumugam M., et al. (2014). Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Investig. 124 515–527. 10.1172/JCI67353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A., Lishko P. V., Kirichok Y. (2012). Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151 400–413. 10.1016/j.cell.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H. M., Golozoubova V., Cannon B., Nedergaard J. (2009). UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9 203–209. 10.1016/j.cmet.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., et al. (2012). FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26 271–281. 10.1101/gad.177857.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. A., Linden M. A., Sheldon R. D., Meers G. M., Morris E. M., Butterfield A., et al. (2016). Fibroblast growth factor 21 and exercise-induced hepatic mitochondrial adaptations. Am. J. Physiol. Gastrointest. Liver Physiol. 310 G832–G843. 10.1152/ajpgi.00355.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G., Chien J. Y., Fu H., Glass L. C., Deeg M. A., Holland W. L., et al. (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18 333–340. 10.1016/j.cmet.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Gaidhu M. P., Fediuc S., Anthony N. M., So M., Mirpourian M., Perry R. L., et al. (2009). Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 50 704–715. 10.1194/jlr.M800480-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M., Gavalda-Navarro A., Villarroya F. (2015). Fibroblast growth factor-21, energy balance and obesity. Mol. Cell. Endocrinol. 418 66–73. 10.1016/j.mce.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Golozoubova V., Hohtola E., Matthias A., Jacobsson A., Cannon B., Nedergaard J. (2001). Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15 2048–2050. 10.1096/fj.00-0536fje [DOI] [PubMed] [Google Scholar]
- Gómez-Sámano M. Á., Grajales-Gómez M., Zuarth-Vázquez J. M., Navarro-Flores M. F., Martínez-Saavedra M., Juárez-León Ó. A., et al. (2017). Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 11 335–341. 10.1016/j.redox.2016.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B., Smith U. (2015). Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 241 27–35. 10.1016/j.atherosclerosis.2015.04.812 [DOI] [PubMed] [Google Scholar]
- Harms M., Seale P. (2013). Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19 1252–1263. 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- Hasek B. E., Stewart L. K., Henagan T. M., Boudreau A., Lenard N. R., Black C., et al. (2010). Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299 R728–R739. 10.1152/ajpregu.00837.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E., Iglesias R., Giralt A., Gonzalez F. J., Giralt M., Mampel T., et al. (2011). Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286 12983–12990. 10.1074/jbc.M110.215889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E., Rosell M., Gonzalez F. J., Giralt M., Iglesias R., Villarroya F. (2010). Hepatic FGF21 expression is induced at birth via PPAR alpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 11 206–212. 10.1016/j.cmet.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Christian M. (2017). Hormonal factors in the control of the browning of white adipose tissue. Horm. Mol. Biol. Clin. Invest. 31 1868–1891. 10.1515/hmbci-2017-0017 [DOI] [PubMed] [Google Scholar]
- Inagaki T., Lin V. Y., Goetz R., Mohammadi M., Mangelsdorf D. J., Kliewer S. A. (2008). Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8 77–83. 10.1016/j.cmet.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen N. Z., Larsen T. J., Peijs L., Daugaard S., Homoe P., Loft A., et al. (2013). A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17 798–805. 10.1016/j.cmet.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Jian W.-X., Peng W.-H., Jin J., Chen X.-R., Fang W.-J., Wang W.-X., et al. (2012). Association between serum fibroblast growth factor 21 and diabetic nephropathy. Metabolism 61 853–859. 10.1016/j.metabol.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Jimenez M., Barbatelli G., Allevi R., Cinti S., Seydoux J., Giacobino J. P., et al. (2003). Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur. J. Biochem. 270 699–705. 10.1046/j.1432-1033.2003.03422.x [DOI] [PubMed] [Google Scholar]
- Joffin N., Jaubert A. M., Bamba J., Barouki R., Noirez P., Forest C. (2015). Acute induction of uncoupling protein 1 by citrulline in cultured explants of white adipose tissue from lean and high-fat-diet-fed rats. Adipocyte 4 129–134. 10.4161/21623945.2014.989748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keipert S., Kutschke M., Lamp D., Brachthäuser L., Neff F., Meyer C. W., et al. (2015). Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol. Metab. 4 537–542. 10.1016/j.molmet.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keipert S., Ost M., Johann K., Imber F., Jastroch M., van Schothorst E. M., et al. (2014). Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 306 E469–E482. 10.1152/ajpendo.00330.2013 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., et al. (2005). FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115 1627–1635. 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Kim S. H., Yang H. M., Lee J. B., Lee M. S. (2013). Acute exercise induces FGF21 expression in mice and healthy humans. PLoS One 8:e63517. 10.1371/journal.pone.0063517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam G., Chen M. Z., Tong R., Zavala-Solorio J., Kates L., van Bruggen N., et al. (2015). Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/betaKlotho complex. EBioMedicine 2 730–743. 10.1016/j.ebiom.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M., Moro C., Berlan M., Crampes F., Sengenes C., Galitzky J. (2008). Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 19 130–137. 10.1016/j.tem.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Lee J. O., Kim N., Kim J. K., Kim H. I., Lee Y. W., et al. (2015). Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol. Endocrinol. 29 873–881. 10.1210/me.2014-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Takahashi N., Yasubuchi M., Kim Y. I., Hashizaki H., Kim M. J., et al. (2012). Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol. Cell Physiol. 302 C463–C472. 10.1152/ajpcell.00010.2011 [DOI] [PubMed] [Google Scholar]
- Lee P., Linderman J. D., Smith S., Brychta R. J., Wang J., Idelson C., et al. (2014). Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19 302–309. 10.1016/j.cmet.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu G., Fang Q., Zhang M., Hui X., Sheng B., et al. (2018). Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2:272. 10.1038/s41467-017-02677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Zhu Z., Lu Y., Granneman J. G. (2005). Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am. J. Physiol. Endocrinol. Metab. 289 E617–E626. 10.1152/ajpendo.00010.2005 [DOI] [PubMed] [Google Scholar]
- Lin Z., Tian H., Lam K. S., Lin S., Hoo R. C., Konishi M., et al. (2013). Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17 779–789. 10.1016/j.cmet.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Luo L., Liu M. (2016). Adipose tissue in control of metabolism. J. Endocrinol. 231 R77–R99. 10.1530/JOE-16-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. (2005). Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 332 392–397. 10.1016/j.bbrc.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Magliano D. C., Bargut T. C., de Carvalho S. N., Aguila M. B., Mandarim-de-Lacerda C. A., Souza-Mello V. (2013). Peroxisome proliferator-activated receptors-alpha and gamma are targets to treat offspring from maternal diet-induced obesity in mice. PLoS One 8:e64258. 10.1371/journal.pone.0064258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossenbock K., Vegiopoulos A., Rose A. J., Sijmonsma T. P., Herzig S., Schafmeier T. (2014). Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One 9:e110428. 10.1371/journal.pone.0110428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J., Pinkstaff J., Li Z., Skidmore L., Li N., Myler H., et al. (2012). FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes 61 505–512. 10.2337/db11-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murholm M., Isidor M. S., Basse A. L., Winther S., Sorensen C., Skovgaard-Petersen J., et al. (2013). Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol. 14:41. 10.1186/1471-2121-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Bengtsson T., Cannon B. (2007). Unexpected evidence for active brown adipose tissue in adult humans. Ame. J. Physiol. Endocrinol. Metabol. 293 E444–E452. 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Nakatake Y., Konishi M., Itoh N. (2000). Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochem. Biophys. Acta 1492 203–206. 10.1016/S0167-4781(00)00067-1 [DOI] [PubMed] [Google Scholar]
- Owen B. M., Ding X., Morgan D. A., Coate K. C., Bookout A. L., Rahmouni K., et al. (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20 670–677. 10.1016/j.cmet.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A., Kim W. K., Bae K. H. (2014). Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 6 33–42. 10.4252/wjsc.v6.i1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2010). Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285 7153–7164. 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya M. K., McTernan P. G., Kumar S. (2013). Adipokine inflammation and insulin resistance: the role of glucose, lipids, and endotoxin. J. Endocrinol. 216 T1–T15. 10.1530/JOE-12-0498 [DOI] [PubMed] [Google Scholar]
- Puigserver P., Pico C., Stock M. J., Palou A. (1996). Effect of selective beta-adrenoceptor stimulation on UCP synthesis in primary cultures of brown adipocytes. Mol. Cell. Endocrinol. 117 7–16. 10.1016/0303-7207(95)03727-6 [DOI] [PubMed] [Google Scholar]
- Rachid T. L., Penna-de-Carvalho A., Bringhenti I., Aguila M. B., Mandarim-de-Lacerda C. A., Souza-Mello V. (2015). Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 402 86–94. 10.1016/j.mce.2014.12.027 [DOI] [PubMed] [Google Scholar]
- Samms R. J., Smith D. P., Cheng C. C., Antonellis P. P., Perfield J. W., II, Kharitonenkov A., et al. (2015). Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 11 991–999. 10.1016/j.celrep.2015.04.046 [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Guertin D. A. (2014). Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5:4099. 10.1038/ncomms5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg K. M., Giesy S. L., Harvatine K. J., Waldron M. R., Cheng C., Kharitonenkov A., et al. (2011). Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 152 4652–4661. 10.1210/en.2011-1425 [DOI] [PubMed] [Google Scholar]
- Schulz T. J., Huang T. L., Tran T. T., Zhang H., Townsend K. L., Shadrach J. L., et al. (2011). Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. U.S.A. 108 143–148. 10.1073/pnas.1010929108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., et al. (2007). Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6 38–54. 10.1016/j.cmet.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summermatter S., Shui G., Maag D., Santos G., Wenk M. R., Handschin C. H. (2013). PGC-1 alfa improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62 85–95. 10.2337/db12-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A., Elo J. M., Pietiläinen K. H., Hakonen A. H., Sevastianova K., Korpela M., et al. (2011). FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10 806–818. 10.1016/S1474-4422(11)70155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., et al. (2016). A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23 427–440. 10.1016/j.cmet.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Teodoro J. S., Zouhar P., Flachs P., Bardova K., Janovska P., Gomes A. P., et al. (2014). Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int. J. Obes. 38 1027–1034. 10.1038/ijo.2013.230 [DOI] [PubMed] [Google Scholar]
- Veniant M. M., Hale C., Helmering J., Chen M. M., Stanislaus S., Busby J., et al. (2012). FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One 7:e40164. 10.1371/journal.pone.0040164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant M. M., Sivits G., Helmering J., Komorowski R., Lee J., Fan W., et al. (2015). Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21 731–738. 10.1016/j.cmet.2015.04.019 [DOI] [PubMed] [Google Scholar]
- Villarroya F., Cereijo R., Villarroya J., Giralt M. (2017). Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13 26–35. 10.1038/nrendo.2016.136 [DOI] [PubMed] [Google Scholar]
- Wang S., Liang X., Yang Q., Fu X., Rogers C. J., Zhu M., et al. (2015). Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP activated protein kinase (AMPK) alpha1. Int. J. Obes. 39 967–976. 10.1038/ijo.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., et al. (2006). Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439 484–489. 10.1038/nature04330 [DOI] [PubMed] [Google Scholar]
- Whittle A. J., Carobbio S., Martins L., Slawik M., Hondares E., Vazquez M. J., et al. (2012). BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149 871–885. 10.1016/j.cell.2012.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. L., Kolumam G., Stawicki S., Chen Y., Li J., Zavala-Solorio J., et al. (2011). Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 3:113ra126. 10.1126/scitranslmed.3002669 [DOI] [PubMed] [Google Scholar]
- Wu J., Bostrom P., Sparks L. M., Ye L., Choi J. H., Giang A. H., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150 366–376. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Cohen P., Spiegelman B. M. (2013). Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27 234–250. 10.1101/gad.211649.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Arch J. R., Ashwell M. (1984). Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167 10–14. 10.1016/0014-5793(84)80822-4 [DOI] [PubMed] [Google Scholar]
- Zhang X., Yeung D. C., Karpisek M., Stejskal D., Zhou Z. G., Liu F., et al. (2008). Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57 1246–1253. 10.2337/db07-1476 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Q. X., Wang X., Zhang L., Qu W., Bao B., et al. (2016). Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1alpha pathway-mediated mechanism. Int. J. Obes. 40 1841–1849. 10.1038/ijo.2016.108 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li R., Meng Y., Li S., Donelan W., Zhao Y., et al. (2014). Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63 514–525. 10.2337/db13-1106 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xie C., Wang H., Foss R. M., Clare M., George E. V., et al. (2016). Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 311 E530–E541. 10.1152/ajpendo.00094.2016 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xie Y., Berglund E. D., et al. (2012). The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1:e00065. 10.7554/eLife.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Chen X. (2014). Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 450 1446–1451. 10.1016/j.bbrc.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Zoli M., Picciotto M. R. (2012). Nicotinic regulation of energy homeostasis. Nicotine Tob. Res. 14 1270–1290. 10.1093/ntr/nts159 [DOI] [PMC free article] [PubMed] [Google Scholar]