Abstract
Seven pairs of enantiomeric isoflavones (1a/1b–7a/7b) were obtained from the ethyl acetate extract of the fruits of Maclura tricuspidata (syn. Cudrania tricuspidata), and successfully separated by chiral high-pressure liquid chromatography (HPLC). The structures and absolute configurations of the enantiomeric isoflavones were established on the basic of comprehensive spectroscopic analyses and quantum chemical calculation methods. Compounds 1, 1a, and 1b exhibited neuroprotective activities against oxygen-glucose deprivation/reoxygenation (ODG/R)-induced SH-SY5Y cells death with EC50 values of 5.5 µM, 4.0 µM, and 10.0 µM, respectively. Furthermore, 1, 1a, and 1b inhibited OGD/R-induced reactive oxygen species generation in SH-5Y5Y cells with IC50 values of 6.9 µM, 4.5 µM, and 9.5 µM, respectively.
Introduction
Maclura tricuspidata (Carr.) Bur. (syn. Cudrania tricuspidata) is a perennial plant, which is mainly distributed in the southern part of Korea. It has been used as folk remedies for gastritis, liver damage, and hypertension in Korean traditional medicine1. Currently, its fruits are consumed fresh and in juices and jams. Further development as a dietary supplement and functional food ingredient has been actively accomplished in many fields2. According to previous reports, various types of flavonoids, including isoflavones3–7, along with xanthones8–12 are considered as the major bioactive constituents of M. tricuspidata, exhibiting antioxidant8, antitherosclerotic, anti-inflammatory13, cytotoxic10, hepatoprotective14, and neuroprotective activities6,7,11,12.
Cerebral ischemia, also known as brain ischemia or ischemic stroke, is one of the most common causes of mortality and morbidity, conducing to major negative social and economic consequences. Accordingly, the prevention of this disease is clearly an important public health priority. It occurs as a result of the cerebral blood flow is disrupted, leading to the starvation of oxygen and glucose to the affected area, causing of irreversible brain damage15,16. Thus far, knowledge about the mechanisms of ischemic brain damage has increased considerably. In general, during ischemia a variety of pathophysiological mechanisms such as calcium influx, glutamate excitotoxicity, inflammation, mitochondrial dysfunction, and oxidative stress were activated, leading to neuronal cell death17–19.
In present study, seven pairs of enantiomeric isoflavones (1a/1b–7a/7b) were obtained from the ethyl acetate extract of the fruits of M. tricuspidata. These enantiomeric isoflavones were further purified by using chiral high-pressure liquid chromatography (HPLC), their structures with absolute configurations were established based on interpretation of their 1D and 2D NMR, and HRESIMS data together with electronic circular dichroism (ECD) calculations. Furthermore, the neuroprotective potentials of the isolated compounds were evaluated.
Results and Discussion
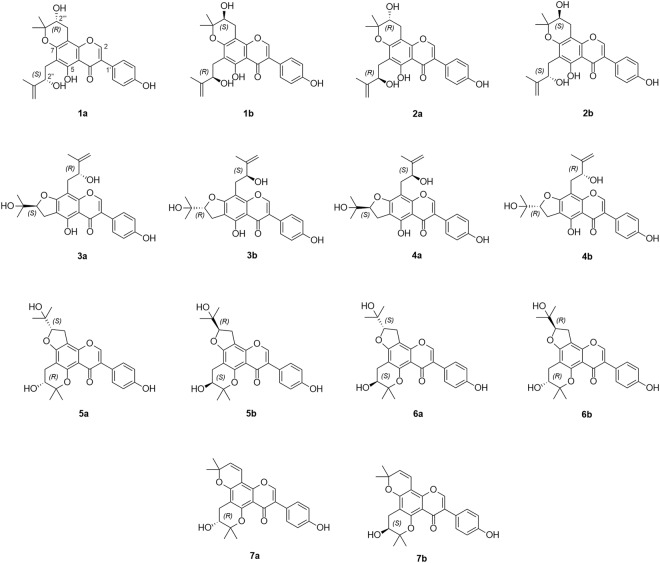
Compound 1 was determined as C25H26O7 by the HRESIMS [M + H]+ ion at m/z 439.1742 (calcd. for C25H25O7, 439.1757). The 1H and 13C NMR spectra resembled those of cudraisoflavone D (Supplementary S.24, Table 1)6, except for the appearance of a 3-hydroxy-2,2-dimethyldihydropyran group [δH 3.07 (1 H, dd, J = 16.5, 5.5 Hz, Ha-1′′′), 2.73 (1 H, dd, J = 16.5, 7.5 Hz, Hb-1′′′), 3.89 (1 H, dd, J = 7.5, 5.0 Hz, H-2′′′), 1.35 (3 H, s, Me-4′′′), and 1.45 (3 H, s, Me-5′′′)] at the C-7 and C-8 positions instead of the furan group, as deduced from the HMBC correlations H-1′′′/C-7 (δC 158.3), C-8 (δC 99.2), and C-9 (δC 154.5). Based on these, compound 1 was established as depicted (Fig. 1) and named cudraisoflavone U.
Table 1.
1H and 13C NMR spectroscopic data of compounds 1–7.
| No. | 1 (Acetone-d6) | 2 (Acetone-d6) | 3 (Acetone-d6) | 4 (Acetone-d6) | 5 (DMSO-d6) | 6 (DMSO-d6) | 7 (DMSO-d6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 2 | 154.0 | 8.24, s | 154.0 | 8.24, s | 154.0 | 8.23, s | 154.0 | 8.22, s | 149.6 | 8.03, s | 149.6 | 8.03, s | 150.0 | 8.08, s |
| 3 | 124.2 | 124.2 | 123.7 | 123.6 | 124.6 | 124.6 | 124.7 | |||||||
| 4 | 181.7 | 181.7 | 182.0 | 182.0 | 173.6 | 173.6 | 173.6 | |||||||
| 5 | 158.5 | 158.6 | 155.8 | 155.9 | 154.0 | 154.0 | 153.7 | |||||||
| 6 | 110.2 | 110.2 | 109.5 | 109.4 | 100.3 | 100.2 | 105.1 | |||||||
| 7 | 158.3 | 158.3 | 166.2 | 166.0 | 162.2 | 162.2 | 154.2 | |||||||
| 8 | 99.2 | 99.3 | 100.5 | 100.5 | 103.5 | 103.4 | 100.7 | |||||||
| 9 | 154.5 | 154.4 | 156.7 | 156.9 | 152.3 | 152.3 | 151.6 | |||||||
| 10 | 106.2 | 106.2 | 106.9 | 106.9 | 107.9 | 108.0 | 108.1 | |||||||
| OH-5 | 13.20, s | 13.20, s | 13.20, s | 13.21, s | ||||||||||
| 1′ | 123.2 | 123.2 | 123.1 | 123.1 | 122.9 | 122.9 | 122.7 | |||||||
| 2′,6′ | 131.2 | 7.46, d (8.5) | 131.2 | 7.46, d (8.5) | 131.1 | 7.47, d (8.5) | 131.1 | 7.47, d (8.5) | 130.3 | 7.27, d (8.5) | 130.3 | 7.27, d (8.5) | 130.2 | 7.29, d (8.5) |
| 3′,5′ | 115.9 | 6.91, d (8.5) | 115.9 | 6.91, d (8.5) | 115.9 | 6.90, d (8.5) | 115.9 | 6.90, d (8.5) | 114.6 | 6.77, d (8.5) | 114.6 | 6.77, d (8.5) | 114.6 | 6.78, d (8.5) |
| 4′ | 158.4 | 158.3 | 158.4 | 158.3 | 156.8 | 156.8 | 156.9 | |||||||
| OH-4′ | 9.44, s | |||||||||||||
| 1′′ | 30.0 | 2.97, dd (6.5, 13.0) 2.86, dd (7.0, 13.0) |
29.9 | 2.97, dd (6.5, 13.0) 2.86, dd (7.0, 13.0) |
27.4 | 3.19, 2H, m | 27.4 | 3.18, 2H, m | 25.7 | 2.82, dd (5.5, 16.5) 2.42, dd (7.5, 16.5) |
25.7 | 2.77, dd (5.5, 17.0) 2.47, dd (7.0, 17.0) |
25.6 | 2.78, dd (5.5, 17.0) 2.42, dd (7.5, 17.0) |
| 2′′ | 75.3 | 4.39, t (7.0Hz) | 75.3 | 4.39, t (7.0Hz) | 92.2 | 4.82, t (8.5) | 92.3 | 4.84, dd (7.5, 9.5) | 66.5 | 3.64, td (5.0, 7.5) | 66.4 | 3.65, q (6.0) | 66.5 | 3.65, td (5.5, 7.5) |
| 3′′ | 149.2 | 149.2 | 71.6 | 71.5 | 77.9 | 77.8 | 78.0 | |||||||
| 4′′ | 110.3 | 4.73, brs 4.64, brs |
110.3 | 4.73, brs 4.63, brs |
25.5 | 1.25, s | 26.0 | 1.27, s | 25.5 | 1.31, s | 25.3 | 1.28, s | 20.5 | 1.19, s |
| 5′′ | 17.7 | 1.83, s | 17.8 | 1.83, s | 25.6 | 1.32, s | 25.2 | 1.29, s | 20.2 | 1.18, s | 20.7 | 1.20, s | 25.3 | 1.30, s |
| OH-2′′ | 5.18, d (5.0) | 5.16, d (4.5) | 5.17, d (5.0) | |||||||||||
| 1′′′ | 26.0 | 3.07, dd (5.5, 16.5) 2.73, dd (7.5, 16.5) |
26.0 | 3.07, dd (5.5, 16.5) 2.73, dd (7.5, 16.5) |
30.4 | 2.95, 2H, m | 30.4 | 2.98, 2H, m | 26.8 | 3.20, 2H, d (8.5) | 26.8 | 3.20, 2H, d (8.0) | 114.5 | 6.68, d (10.0) |
| 2′′′ | 68.6 | 3.89, dd (5.5, 7.0) | 68.7 | 3.89, dd (5.0, 7.5) | 75.2 | 4.38, t (6.5) | 74.9 | 4.45, t (6.5) | 90.9 | 4.76, t (8.5) | 90.9 | 4.75, t (8.5) | 127.4 | 5.73, d (10.0) |
| 3′′′ | 79.8 | 79.9 | 148.9 | 148.9 | 70.1 | 70.0 | 77.7 | |||||||
| 4′′′ | 21.3 | 1.35, s | 20.9 | 1.35, s | 110.6 | 4.69, s 4.79, s |
110.5 | 4.69, s 4.79, s |
24.8 | 1.18, s | 24.7 | 1.16, s | 27.7 | 1.43, s |
| 5′′′ | 25.7 | 1.45, s | 25.9 | 1.45, s | 17.6 | 1.84, s | 17.9 | 1.84, s | 25.6 | 1.15, s | 25.8 | 1.17, s | 27.8 | 1.45, s |
Figure 1.
Structures of enantiomeric isoflavones 1a–7b.
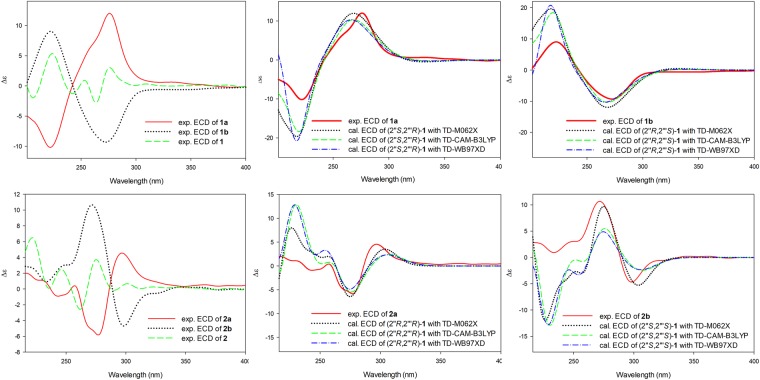
Initially, due to the positive of its specific rotation {[α]24D +4.3 (c 0.01, MeOH)} together with the detection of Cotton effects (CE) in the ECD spectrum (Fig. 2), 1 was supposed to be an optically pure compound. Therefore, a modified Mosher’s experiment was carried out to establish the absolute configurations at the C-2′′ and C-2′′′ positions20. Interestingly, when the (R) and (S)-MTPA esters of 1 were subjected to RP-C18 HPLC, two pairs of diastereomers including (S)-MTPA-1a/(S)-MTPA-1b and (R)-MTPA-1a/(R)-MTPA-1b were observed (Supplementary S.4), suggesting the racemic nature of 1. This suggestion was further confirmed by the detection of two peaks in the chiral HPLC analysis of 1. The enantiomeric separation of 1 by chiral HPLC let to the isolation of the enantiomers 1a (tR 11.14 min, [α]22D +12.7) and 1b (tR 14.49 min, [α]22D -28.7) (Supplementary S.23), which exhibited the mirror image-like ECD curves (Fig. 2).
Figure 2.
Experimental and calculated ECD spectra of 1 and 2 in acetonitrile.
The molecular formula of compound 2 was C25H26O7 (HRESIMS, m/z 439.1741 [M + H]+). The analyzing 1D and 2D NMR data of 2 indicated that 2 was a stereoisomer of 1. Although CE curves were detected in the ECD spectrum of 2 (Fig. 2) along with a measurable optical rotation ([α]24D +2.1), its racemic nature was demonstrated based on chiral HPLC analysis. Further enantiomer separation using chiral HPLC resulted in the isolation of enantiomers 2a (tR 21.48 min, [α]22D −26.2) and 2b (tR 23.52 min, [α]22D +12.0) (Supplementary S.23).
In order to determine the absolute configurations of the enantiomers 1a and 1b, as well as 2a and 2b, quantum chemical ECD calculations were carried out and the results were compared with the experimental data. Four possible stereoisomers based on differences at the C-2′′ and C-2′′′ positions of the gross structure were built and separately subjected to a Merck molecular force field (MMFF) conformation search, followed by geometry optimization in density functional methods. The ECD data of the selected conformers were calculated using the time-dependent DFT (TDDFT) method.
As shown in Fig. 2, the calculated ECD spectra for the (2′′S,2′′′R) and (2′′R,2′′′S)-isomers were well matched with the experimental spectra of 1a and 1b, respectively, and the simulated spectra for the (2′′R,2′′′R) and (2′′S,2′′′S)-isomers were highly consistent with the experimental spectra of 2a and 2b, respectively. Besides, in order to further confirm the results, the additional ECD calculations were carried out using the CAM-B3LYP and WB97XD functionals, which yielded consistent ECD results (Fig. 2). On this basis, the absolute configurations of 1a, 1b, 2a, and 2b were assigned as depicted, which were named as (2′′S,2′′′R)-cudraisoflavone U, (2′′R,2′′′S)-cudraisoflavone U, (2′′R,2′′′R)-cudraisoflavone U, and (2′′S,2′′′S)-cudraisoflavone U, respectively.
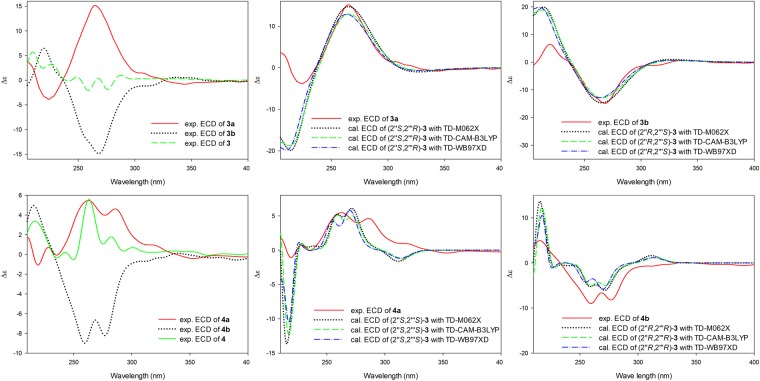
The HRESIMS of compound 3 was indicated the molecular formula of C25H26O7 (m/z 439.1753 [M + H]+). Its 1D NMR spectra were similar to those of cudraisoflavone E6. In opposition to cudraisoflavone E, the HMBC correlations H-1′′′ [δH 2.95 (2H, m)]/C-7 (δC 166.2), C-8 (δC 100.5), and C-9 (δC 156.7) as well as the H-1′′ [δH 3.19 (2H, m)]/C-5 (δC 155.8), C-6 (δC 109.5) and C-7 revealed that the 2-hydroxyl-3-methylbut-3-enyl and 2-(1-hydroxy-1-methylethyl)dihydrofuran groups were located at the C-8 position, as well as the C-6 and C-7 positions, respectively. Thus, compound 3 was elucidated and named cudraisoflavone V.
In additionally, compound 3 was also established to be a racemic mixture due to the lack of CE curves, and further separated into 3a (tR 14.70 min) and 3b (tR 27.68 min) by chiral HPLC (Supplementary S.23). 3a and 3b displayed mirror image-like ECD curves (Fig. 3) and opposite specific rotations (3a: [α]22D +16.2 and 3b: [α]22D −6.2).
Figure 3.
Experimental and calculated ECD spectra of 3 and 4 in acetonitrile.
The HRESIMS spectrum of compound 4 exhibited [M + H]+ signal at m/z 439.1754 (calcd. for C25H27O7, 439.1757), suggesting molecular formula of C25H26O7. The similarity of the NMR data (1D and 2D) of 4 and 3 demonstrated that 4 was a stereoisomer of 3. Considering the racemic nature of 3, 4 was also purified via HPLC using a chiral column to afford a pair of enantiomers 4a (tR 15.13 min, [α]22D +21.5) and 4b (tR 16.36 min, [α]22D −22.5) (Supplementary S.23), which showed antipodal ECD curves (Fig. 3).
Quantum ECD calculations were also applied to measure the absolute configuration of 3a, 3b, 4a, and 4b. The measured spectra of 3a, 3b, 4a, and 4b fit well with the calculated ECD spectra for the (2′′S,2′′′R), (2′′R,2′′′S), (2′′S,2′′′S), and (2′′R,2′′′R)-isomers, respectively (Fig. 3), and the absolute configurations of 3a, 3b, 4a, and 4b were thus assigned as follows: (2′′S,2′′′R)-cudraisoflavone V, (2′′R,2′′′S)-cudraisoflavone V, (2′′S,2′′′S)-cudraisoflavone V, and (2′′R,2′′′R)-cudraisoflavone V, respectively.
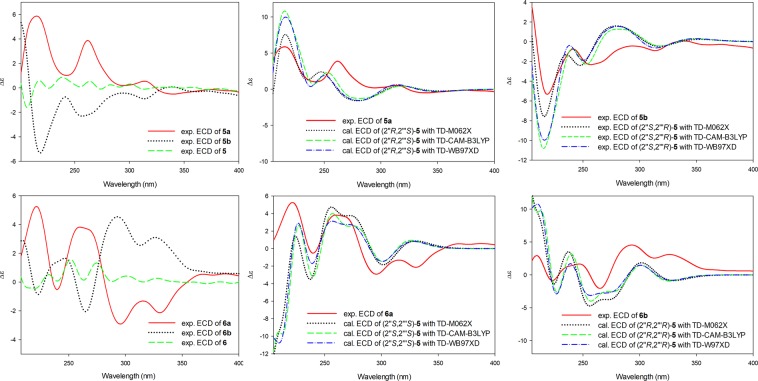
The formula of compound 5 was established as C25H26O7 by the HRESIMS ion [M + H]+ at m/z 439.1740 (calcd. for C25H27O7, 439.1757). The 1D NMR spectra resembled those of cudraisoflavone I (Supplementary S.24)6. However, they differed in the presence of a 2-(1-hydroxy-1-methylethyl)dihydrofuran group [δH 3.20 (2 H, d, J = 8.5 Hz, H-1′′′), 4.76 (1 H, t, J = 8.5 Hz, H-2′′′), 1.18 (3 H, s, Me-4′′′), and 1.15 (3 H, s, Me-5′′′)] at the C-7 and C-8 positions instead of the furan group, confirmed by the HMBC cross-peaks H-1′′′/C-7 (δC 162.2), C-8 (δC 103.5) and C-9 (δC 152.3). Based on these, the structure of compound 5 was determined to be cudraisoflavone W.
The HRESIMS spectra of 6 resulted as the same molecular formula as that of 5. It was a stereoisomer of 5, as elucidated directly from the 1D and 2D NMR spectra. Additionally, no CE curves were detected in the ECD spectra of 5 and 6 (Fig. 4), indicating that these compounds were racemic mixtures, respectively. The racemic nature of 5 and 6 was also confirmed by chiral HPLC analysis. The further purification of 5 and 6 achieved of two pairs of enantiomers 5a (tR 8.06 min, [α]22D +15.7) and 5b (tR 10.16 min, [α]22D −11.2) as well as 6a (tR 12.95 min, [α]22D +12.0) and 6b (tR 20.36 min, [α]22D −10.7) (Supplementary S.23), respectively.
Figure 4.
Experimental and calculated ECD spectra of 5 and 6 in acetonitrile.
Similar to the case for 1–4, the experimental ECD spectra of 5a, 5b, 6a, and 6b were highly consistent with the calculated ECD spectra of the (2′′R,2′′′S), (2′′S,2′′′R), (2′′S, 2′′′S), and (2′′R,2′′′R)-isomers, respectively (Fig. 4). Consequently, the absolute configurations of 5a, 5b, 6a, and 6b were determined as shown [(2′′R,2′′′S)-cudraisoflavone W, (2′′S,2′′′R)-cudraisoflavone W, (2′′S,2′′′S)-cudraisoflavone W, and (2′′R,2′′′R)-cudraisoflavone W, respectively].
The molecular formula of compound 7 was C25H24O6 (HRESIMS). The 1H and 13C NMR signals closely matched those of 6. However, they differed in the replacement of a 2-(1-hydroxy-1-methylethyl)dihydrofuran group by a 2,2-dimethylpyran group [δH 6.68 (1H, d, J = 10.0 Hz, H-1′′′), 5.73 (1H, d, J = 10.0 Hz, H-2′′′), 1.43 (3H, s, Me-4′′′), and 1.45 (3H, s, Me-5′′′)] at the C-7 and C-8 positions, confirmed by the HMBC correlations H-1′′′/C-7 (δC 154.2), C-8 (δC 100.7), and C-9 (δC 151.6). Thus, compound 7 was determined to be cudraisoflavone X.
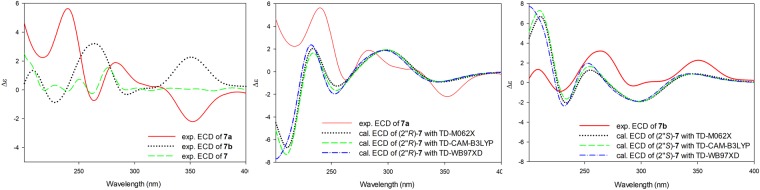
Compound 7 was also found to be a racemic mixture due to the presence of two peaks in the chiral HPLC analysis. Further HPLC separation led to the isolation of two enantiomers 7a (tR 11.40 min, [α]22D +18.0) and 7b (tR 18.58 min, [α]22D −13.2) (Supplementary S.23). 7a and 7b were assigned as (2′′R)-cudraisoflavone X and (2′′S)-cudraisoflavone X, respectively, based on comparison of the experimental ECD spectral data with those of the (2′′R) and (2′′S)-isomers (Fig. 5).
Figure 5.
Experimental and calculated ECD spectra of 7 in acetonitrile.
The racemic compounds 1–7 were evaluated for neuroprotective activity against oxygen-glucose deprivation/reoxygenation (ODG/R)-induced neuronal cell death in SH-SY5Y cells. Of these, 1 exhibited a significant protective effect with an EC50 value of 5.5 µM (carnosine was used as a positive control, EC50 13.4 µM) (Table 2)21. The rest of the compounds were inactive (EC50 > 20 µM). Accordingly, enantiomers 1a and 1b were further separately examined for their neuroprotective potential and both were found to attenuate ODG/R-induced neurotoxicity with EC50 values of 4.0 µM and 10.0 µM, respectively (Table 2).
Table 2.
Neuroprotective and inhibitory of ROS generation activities of isolated compounds.
| Compound | Protective effect against cell death (EC50, μM) | Inhibitory effect against ROS generation (IC50, μM) |
|---|---|---|
| 1 | 5.5 ± 1.4## | 6.9 ± 1.2# |
| 1a | 4.0 ± 1.0### | 4.5 ± 2.5## |
| 1b | 10.0 ± 2.1 | 9.5 ± 3.2 |
| 2 | >20 | —a |
| 3 | >20 | —a |
| 4 | >20 | —a |
| 5 | >20 | —a |
| 6 | >20 | —a |
| 7 | >20 | —a |
| Carnosine | 13.4 ± 1.5 | 14.2 ± 2.3 |
EC50 and IC50 values were determined in a semi-logarithmic graph with 4 different concentrations. aIC50 value not determined. (#p < 0.05, ##p < 0.01, and ###p < 0.001 versus carnosine, a control compound).
Moreover, although the causes of neurodegenerative diseases have not been clearly elucidated, many experimental evidences suggested that oxidative stress resulting in the generation of reactive oxygen species (ROS) plays a pivotal role in neurodegenerative diseases16,17,22. Furthermore, recent biological studies indicate that several isoflavones are beneficial for reducing oxidative stress in neurons and protecting against neurodegenerative diseases22–25. Consequently, the inhibitory effect of 1, 1a, and 1b on the ODG/R-induced intracellular ROS generation in SH-5Y5Y cells was assessed. As shown in Table 2, 1, 1a, and 1b inhibited ROS generation in ODG/R-induced SH-5Y5Y cells with IC50 values of 6.9 µM, 4.5 µM, and 9.5 µM, respectively.
Interestingly, 2 did not inhibit ODG/R-induced neuronal cell death although 2 has the same gross structure as that of 1. On these grounds, it is suggested that the variety of stereochemistry has an apparent effect on the neuroprotective potential of these isoflavones. Recent study demonstrated that isoflavones from M. tricuspidata exerted neuroprotective activity via induction of Nox4-targeting miRNAs and inhibition of the MAPK signal cascade in in vitro and in vivo models of cerebral ischemia26.
Besides, recently studies indicated that ingested flavonoids are mostly metabolized in the small and large intestines, and liver, then enter the bloodstream and can reach the central nervous system (CNS) by transporting across the blood brain barrier (BBB)27–29. However, to date, the knowledge about their capacity of reaching the CNS remain insufficient and inconsistent. The degree to which flavonoids can enter the CNS is still a disagreement, in spite of several studies indicated their presence in brain tissue after oral administration28,29. Therefore, the knowledge regarding flavonoids transport across BBB and how this is regulated is crucial. Recent study reported that flavonoids might pass through the BBB by transmembrane diffusion, which is dependent on the degree of their lipophilicity27,30,31. Furthermore, the evaluations of transmembrane transport of different flavonoids such as genistein, (+)-catechin, hesperidin, and quercetin via blood-brain barrier cells models indicated that after treatment for 3 h, the obtained concentrations of these flavonoids were 3–10 µM, which was sufficient concentration to have beneficial effects30,32–34. In present study, isolated compounds from M. tricuspidata were genistein-based flavonoids, suggesting they may possess the ability to pass through BBB and reach the sufficient concentration.
Consequently, the isolated compounds from M. tricuspidata could be promising candidates for the treatment of cerebral ischemia and more investigations are needed to understand their cellular mechanisms of action in the brain for fully exploring their neuroprotective potential.
Methods
General experimental procedures
IR spectra were recorded on a Varian 640-IR spectrometer. Optical rotation was measured on a JASCO P-2000 polarimeter. UV spectra were recorded on an OPTIZEN POP spectrophotometer. ECD measurements were performed using a JASCO J-1100 spectrometer. 1D and 2D NMR spectra were measured on a Varian VNMRS 500 MHz system. HRESIMS data were obtained on a Waters Q-TOF micromass spectrometer. Column chromatography (CC) was carried out using Kieselgel 60 silica gel (40–60 μm, 70–230 mesh, Merck) and reverse-phase (RP) C18 silica gel (12 μm, YMC, Kyoto, Japan). The HPLC system consisted of a Varian Prostar 210 system, a YMC J′sphere ODS-H80 column (10 × 250 mm, 4 μm, YMC Co., Ltd., Kyoto, Japan), along with Chiralpak IA and IB columns (4.6 × 250 mm, 5 μm, Daicel, Osaka, Japan).
Plant materials
The collection of fruits of Maclura tricuspidata and deposition of voucher specimen (KH1-5-090904) were carried out as previously described7.
Extraction and Isolation
Fresh fruits of M. tricuspidata (10.7 kg) were extracted in 100% MeOH (3 × 10 L) at room temperature over the course of ten days. The extracts were concentrated under vacuum to afford a residue (TH1-1-1, 630.9 g), which was further extracted with n-hexane (48.43 g) and EtOAc (27.8 g).
The EtOAc fraction (TH1-2-2, 27.8 g) was fractionated by silica gel CC using CHCl3–MeOH (1:0 to 1:1) to give six fractions (TH1-4-1–TH1-4-6). Fraction TH1-4-3 (9.68 g) was further separated with a silica gel CC eluted with n-hexane–EtOAc (1:0 to 0:1) to generate seven subfractions (TH1-10-1–TH1-10-7). Fraction TH1-10-4 (4.7 g) was passed over silica gel CC using n-hexane–CHCl3–MeOH (1:0:0 to 0:1:1). Fraction TH1-74-12 (166.3 mg) was further separated into six subfractions (TH3-9-1–TH3-9-6) on RP-C18 silica gel CC using MeOH–H2O (1:1 to 10:0). Fraction TH3-9-1 (71.1 mg) was passed over silica gel CC using n-hexane–EtOAc (1:0 to 0:1), to obtain five fractions (TH3-19-1–TH3-19-5). The racemic mixtures 1 (5.1 mg), 2 (8.1 mg), 3 (4.1 mg), and 4 (6.3 mg) were obtained by preparative HPLC (MeOH–H2O, 60–81%, MeOH in H2O) of fraction TH3-19-3 (40.5 mg). Purification of mixtures 1 (Chiralpak IA; n-hexane–ethanol, 85:15), 2 (Chiralpak IB; n-hexane–ethanol, 90:10), 3 (Chiralpak IA; n-hexane–ethanol, 80:20), and 4 (Chiralpak IB; n-hexane–ethanol, 90:10) by chiral preparative HPLC afforded 1a (1.3 mg, tR 11.14 min), 1b (1.4 mg, tR 14.49 min), 2a (1.6 mg, tR 21.48 min), 2b (1.9 mg, tR 23.52 min), 3a (1.1 mg, tR 14.70 min), 3b (1.4 mg, tR 27.68 min), 4a (1.5 mg, tR 15.13 min), and 4b (1.4 mg, tR 16.36 min), respectively. Purification of fractions TH3-9-2 (24.4 mg) and TH3-9-3 (9.1 mg) via preparative HPLC (MeOH–H2O, 60–85%, MeOH in H2O) yielded the racemic mixture 7 (17.4 mg). The enantiomers 7a (1.6 mg, tR 11.40 min) and 7b (1.6 mg, tR 18.58 min) were obtained by chiral HPLC (Chiralpak IA; n-hexane–ethanol, 85:15). Fraction TH1-74-14 (240.4 mg) was separated into four subfractions TH3-3-1–TH3-3-4 with a RP-C18 silica gel CC using MeOH–H2O (1:1 to 8:2). Fraction TH3-3-2 (96.2 mg) was separated into the racemic mixtures 5 (14.7 mg) and 6 (7.4 mg) with preparative HPLC (MeOH–H2O, 55–75%). Further purification of mixtures 5 (Chiralpak IA; n-hexane–ethanol, 80:20) and 6 (Chiralpak IA; n-hexane–ethanol, 85:15) by chiral preparative HPLC afforded 5a (1.6 mg, tR 8.06 min), 5b (1.6 mg, tR 10.16 min), 6a (1.8 mg, tR 12.95 min), and 6b (1.1 mg, tR 20.36 min), respectively.
Cudraisoflavone U (1): Yellow oil; [α]24D +4.3 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 213 (4.22), 271 (4.31); IR (ATR) νmax cm−1: 3324 (>OH), 1649 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1742 [M + H]+ (calcd. for C25H25O7, 439.1757).
1a: [α]22D +12.7 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −10.18 (222), +12.02 (276).
1b: [α]22D −28.7 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε +9.06 (221), −9.34 (272).
Epi-cudraisoflavone U (2): Yellow oil; [α]24D +2.1 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 214 (4.33), 271 (4.41); IR (ATR) νmax cm−1: 3324 (>OH), 1648 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1741 [M + H]+ (calcd.. for C25H25O7, 439.1757).
2a: [α]22D −26.2 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −0.82 (244), +0.68 (257), −5.97 (275), +4.75 (297).
2b: [α]22D +12.0 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 0.89 (233), +10.64 (272), −4.58 (298).
Cudraisoflavone V (3): Yellow oil; [α]22D −2.8 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 216 (4.37), 270 (4.48); IR (ATR) νmax cm−1: 3286 (>OH), 1660 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1753 [M + H]+ (calcd. for C25H27O7, 439.1757).
3a: [α]22D +16.2 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −4.01 (224), +15.28 (264), −1.01 (341).
3b: [α]22D −6.2 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 6,38 (220), −14.90 (268), +0.62 (338).
Epi-cudraisoflavone V (4): Yellow oil; [α]22D −5.2 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 216 (4.35), 270 (4.48); IR (ATR) νmax cm−1: 3327 (>OH), 1660 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1754 [M + H]+ (calcd. for C25H27O7, 439.1757).
4a: [α]22D +21.5 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −1.13 (219), +0.70 (228), −0.10 (236), +5.47 (262), +4.01 (276), +4.54 (286), −0.53 (352).
4b: [α]22D −22.5 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 4.90 (215), −9.09 (260), −6.79 (268), −8.27 (277), +0.11 (399).
Cudraisoflavone W (5): Yellow oil; [α]24D +3.1 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 213 (4.30), 263 (4.41); IR (ATR) νmax cm−1: 3281 (>OH), 1639 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1740 [M + H]+ (calcd. for C25H27O7, 439.1757).
5a: [α]22D +15.7 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 5.81 (216), +1.02 (243), +3.80 (262), +0.14 (296), +0.43 (315), −0.58 (339).
5b: [α]22D −11.2 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −5.30 (218), −6.71 (241), −2.36 (255), −0.38 (297), −0.91 (314), +0.19 (337).
Epi-cudraisoflavone W (6): Yellow oil; [α]24D −3.2 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 214 (4.30), 263 (4.40); IR (ATR) νmax cm−1: 3365 (>OH), 1640 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 439.1744 [M + H]+ (calcd. for C25H27O7, 439.1757).
6a: [α]22D +12.0 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 5.23 (221), −0.60 (240), +3.91 (260), −2.95 (296), −1.29 (315), −2.26 (332), +0.48 (368).
6b: [α]22D −10.7 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε −0.91 (223), +1.66 (247), −2.05 (265), +4.53 (292), +2.52 (313), +2.97 (328).
Cudraisoflavone X (7): Yellow oil; [α]24D −4.9 (c 0.01, MeOH); UV (MeOH) λmax nm (log ɛ): 210 (4.33), 268 (4.64), 344 (3.63); IR (ATR) νmax cm−1: 3318 (>OH), 1630 (>C=O); 1H and 13C NMR data see Table 1; HRESIMS m/z 419.1476 [M − H]− (calcd. for C25H23O6, 419.1495).
7a: [α]22D +18.0 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 2.14 (218), +5.59 (240), −0.70 (263), +1.83 (283), −2.24 (352).
7b: [α]22D −13.2 (c 0.04, MeOH); CD (c 0.6 mM, ACN) Δε + 1.33 (209), −0.91 (229), +3.17 (264), −0.28 (294), +2.24 (350).
Computational details
The ECD calculations were performed as previously described with some modifications7. The DFT/B3LYP/cc-pTVZ level was employed for optimizing and calculating the relative energies of the initial low-energy conformers. Calculation of the ECD spectra were carried out at the TDDFT/M062X/def2TZVP level. Additional ECD calculations were performed using the CAM-B3LYP and WB97XD functionals in order to further confirm the calculated results.
Measurement of cell viability and intracellular ROS and statistical analysis
The protective effects against ODG/R-induced cell death and intracellular ROS generation in SH-SY5Y cells of test compounds and statistical analysis were carried out as previously described35. All experimental data are expressed as the mean value ± standard deviation from three replicates for each experiment. Statistical significance between multiple groups was determined by one-way ANOVA (PRISM Graph Pad, San Diego, CA, USA). When the ANOVA showed a significant difference, Bonferroni’s multiple comparison post hoc test was conducted. P values less than 0.05 were regarded as statistically significant.
Electronic supplementary material
Supplementary data for: Enantiomeric Isoflavones with neuroprotective activities from the Fruits of Maclura tricuspidata
Acknowledgements
This research was supported by grants from the Korea University, the National Research Foundation of Korea (NRF-2015R1D1A1A01060321 and NRF-2015R1D1A1A01056603), and the BK21 Plus program in 2017 through the NRF, funded by the Ministry of Education of Korea.
Author Contributions
Dongho Lee and Woongchon Mar initiated the project. Nguyen Tuan Hiep, Jaeyoung Kwon, Nahyun Kim, and Sungeun Hong performed the extraction, isolation, structural identification, and biological assays of the compounds. Yuanqiang Guo and Bang Yeon Hwang supported data analysis. Nguyen Tuan Hiep, Dongho Lee, and Woongchon Mar wrote the manuscript. All authors reviewed and confirmed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Woongchon Mar, Email: mars@snu.ac.kr.
Dongho Lee, Email: dongholee@korea.ac.kr.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36095-8.
References
- 1.Kang DG, et al. Effects of Cudrania tricuspidata water extract on blood pressure and renal functions in NO-dependent hypertension. Life Sci. 2002;70:2599–2609. doi: 10.1016/S0024-3205(02)01547-3. [DOI] [PubMed] [Google Scholar]
- 2.Jeong JY, et al. Optimization of pancreatic lipase inhibition by Cudrania tricuspidata fruits using response surface methodology. Bioorg. Med. Chem. Lett. 2014;24:2329–2333. doi: 10.1016/j.bmcl.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto T, Hano Y, Nomura T, Uzawa J. Components of root bark of Cudrania tricuspidata 2. Structures of two new isoprenylated flavones, cudraflavones A and B. Planta Med. 1984;50:161–163. doi: 10.1055/s-2007-969660. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto T, Nomura T. Components of root bark of Cudrania tricuspidata; 31,2 Isolation and structure studies on the flavonoids. Planta Med. 1985;51:190–193. doi: 10.1055/s-2007-969453. [DOI] [Google Scholar]
- 5.Hano Y, et al. Cudraflavone C and cudraflavone C, two new prenylflavones from the root bark of Cudrania tricuspidata (Carr) bur. Heterocycles. 1990;31:1339–1344. doi: 10.3987/COM-90-5416. [DOI] [Google Scholar]
- 6.Hiep NT, et al. Isoflavones with neuroprotective activities from fruits of Cudrania tricuspidata. Phytochemistry. 2015;111:141–148. doi: 10.1016/j.phytochem.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Hiep NT, et al. Neuroprotective constituents from the fruits of Maclura tricuspidata. Tetrahedron. 2017;73:2747–2759. doi: 10.1016/j.tet.2017.03.064. [DOI] [Google Scholar]
- 8.Zou YS, Hou AJ, Zhu GF. Isoprenylated xanthones and flavonoids from Cudrania tricuspidata. Chem. Biodivers. 2005;2:131–138. doi: 10.1002/cbdv.200490164. [DOI] [PubMed] [Google Scholar]
- 9.Lee BW, et al. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005;15:5548–5552. doi: 10.1016/j.bmcl.2005.08.099. [DOI] [PubMed] [Google Scholar]
- 10.Zou YS, et al. Cytotoxic isoprenylated xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. 2004;12:1947–1953. doi: 10.1016/j.bmc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Kwon J, et al. Chemical constituents isolated from the root bark of Cudrania tricuspidata and their potential neuroprotective effects. J. Nat. Prod. 2016;79:1938–1951. doi: 10.1021/acs.jnatprod.6b00204. [DOI] [PubMed] [Google Scholar]
- 12.Kwon J, et al. Neuroprotective xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2014;77:1893–1901. doi: 10.1021/np500364x. [DOI] [PubMed] [Google Scholar]
- 13.Park KH, et al. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2006;16:5580–5583. doi: 10.1016/j.bmcl.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 14.An RB, Sohn DH, Kim YC. Hepatoprotective compounds of the roots of Cudrania tricuspidata on tacrine-induced cytotoxicity in Hep G2 cells. Biol. Pharm. Bull. 2006;29:838–840. doi: 10.1248/bpb.29.838. [DOI] [PubMed] [Google Scholar]
- 15.Hori M, et al. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Model. Mech. 2012;5:270–283. doi: 10.1242/dmm.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, et al. Oxidative stress in ischemic brain damage: Mechanisms of cell ceath and cotential molecular targets for neuroprotection. Antioxid. Redox. Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suresh LM, Raghu V. Mechanisms of Stroke Induced Neuronal Death: Multiple Therapeutic Opportunities. Adv. Anim. Vet. Sci. 2014;2:438–446. doi: 10.14737/journal.aavs/2014/2.8.438.446. [DOI] [Google Scholar]
- 20.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. doi: 10.1021/ja00011a006. [DOI] [Google Scholar]
- 21.Bae ON, Majid A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013;1527:246–254. doi: 10.1016/j.brainres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez-Merino C, et al. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011;18:1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 23.Vauzour D, et al. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inanami O, et al. Oral administration of (−)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Radic. Res. 1998;29:359–365. doi: 10.1080/10715769800300401. [DOI] [PubMed] [Google Scholar]
- 25.Liang HW, et al. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci. Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, et al. The isoflavones and extracts from Maclura tricuspidata fruit protect against neuronal cell death in ischemic injury via induction of Nox4-targeting miRNA-25, miRNA-92a, and miRNA-146a. J. Funct. Foods. 2018;40:785–797. doi: 10.1016/j.jff.2017.12.011. [DOI] [Google Scholar]
- 27.Pilsakova L, Riecansky I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol. Res. 2010;59:651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 28.Rendeiro C, Rhodes JS, Spencer JP. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015;89:126–139. doi: 10.1016/j.neuint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Matias I, Buosi AS, Gomes FC. Functions of flavonoids in the central nervous system: Astrocytes as targets for natural compounds. Neurochem Int. 2016;95:85–91. doi: 10.1016/j.neuint.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, et al. Transport of active flavonoids, based on cytotoxicity and lipophilicity: an evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. In Vitro. 2014;28:388–396. doi: 10.1016/j.tiv.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Barnes S, et al. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011;2:235–244. doi: 10.1039/c1fo10025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faria A, et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2:39–44. doi: 10.1039/C0FO00100G. [DOI] [PubMed] [Google Scholar]
- 33.Tian XJ, Yang XW, Yang X, Wang K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009;367:58–64. doi: 10.1016/j.ijpharm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Faria A, et al. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014;149:190–196. doi: 10.1016/j.foodchem.2013.10.095. [DOI] [PubMed] [Google Scholar]
- 35.Hong S, et al. Mulberrofuran G protects ischemic injury induced cell death via inhibition of Nox4-mediated ROS generation and ER stress. Phytother. Res. 2017;31:321–329. doi: 10.1002/ptr.5754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data for: Enantiomeric Isoflavones with neuroprotective activities from the Fruits of Maclura tricuspidata