Abstract
Youth with severe conduct problems impose a significant cost on society by engaging in high levels of antisocial and aggressive behavior. Within this group, adolescents with high levels of callous- unemotional traits have been found to exhibit more severe and persistent patterns of antisocial behavior than youth with severe conduct problems but normative levels of callous-unemotional traits. Existing neuroimaging studies, along with theoretical accounts of psychopathology, suggest that dysfunction within the paralimbic cortex and limbic system may underlie elevated levels of callous-unemotional traits. The present study examines this hypothesis by investigating gray matter correlates associated with callous-unemotional traits. A sample of incarcerated male adolescents (N = 269), were assessed using voxel-based morphometry. Callous-unemotional traits were assessed using the Inventory of Callous-Unemotional traits (Frick 2004). Total callous-unemotional traits were negatively correlated with anterior temporal lobe gray matter volume (GMV). Callous traits in particular exhibited a reliable negative correlation with gray matter volume in nearly every paralimbic brain region examined. Uncaring traits were positively correlated with GMV in the orbitofrontal and anterior cingulate cortices. These findings demonstrate specific neural features within the paralimbic cortex and limbic system that accompany elevated callous-unemotional traits and serves to expand our understanding of pathophysiological mechanisms that may give rise to severe conduct problems in youth.
Keywords: Callous-unemotional traits, Conduct disorder, Gray matter volume, Voxel-based morphometry, Paralimbic system dysfunction
Abbreviations: ACC, anterior cingulate; aINS, anterior insula; aTL, anterior temporal lobe; CU, callous-unemotional; fMRI, functional magnetic resonance imaging; GMD, gray matter density; GMV, gray matter volume; HCUs, youth with conduct problems who exhibit elevated levels of callous-unemotional traits; ICU, Inventory of Callous-Unemotional Traits; LCUs, youth with conduct problems who exhibit normative levels of Callous-Unemotional traits; mOFC, medial orbitofrontal cortex; PCC, posterior cingulate; PHG, parahippocampal gyrus; pSTS, posterior superior temporal sulcus; sMRI, structural magnetic resonance imaging; vSTR, ventral striatum
Highlights
-
•
Total callous-unemotional traits were negatively correlated with anterior temporal lobe gray matter volume (GMV).
-
•
Callous traits negatively correlated with GMV in nearly every paralimbic regions examined.
-
•
Uncaring traits were positively correlated with GMV in the orbitofrontal and anterior cingulate cortices.
1. Introduction
Youth who exhibit persistent aggressive and antisocial behaviors pose a serious mental health and public policy concern (Erskine et al., 2013). A growing body of research suggests that youth with severe conduct problems can be meaningfully delineated according to the presence of callous-unemotional (CU) traits. CU traits include lack of guilt or remorse, lack of affective empathy, lack of concern for performance in important activities, and deficient or shallow affect (Frick and Moffitt, 2010; Frick et al., 2013). Youth with conduct problems who exhibit elevated levels of CU traits display more severe patterns of antisocial behavior compared to youth with conduct problems but normative levels of CU traits (Byrd et al., 2014; Feilhauer et al., 2012; Longman et al., 2016).
Youth with elevated CU traits also pose a unique treatment challenge, as traditional treatment approaches have shown to be considerably less effective at reducing conduct problems in this population (Falkenbach et al., 2003; Haas et al., 2011; O'Neill et al., 2003; Spain et al., 2004). Nevertheless, some specialized intensive treatment programs have been successful in reducing conduct problems amongst youth with elevated CU traits (Butler et al., 2011; Caldwell et al., 2007; Dadds et al., 2012; Kolko and Pardini, 2010; McDonald et al., 2011; White et al., 2013; for review see Hawes et al., 2014). The success of these programs demonstrates that important functional manifestations of CU traits are malleable and youth high on these traits can be successfully treated in programs tailored to their needs. Moreover, during adolescence individuals may be more amenable to treatment, as reinforcing socially adaptive behaviors and changing their social ecology is likely to have a more significant impact on the adolescent's developing personality and behavior.
Similar to adult psychopaths, neurocognitive abnormalities amongst youth with elevated CU traits include low emotional reactivity (de Wied et al., 2012; Kimonis et al., 2008a), poor emotion recognition (Blair et al., 2005; Sylvers et al., 2011), and deficient reversal learning (Byrd et al., 2014; Finger et al., 2008). Neurobiological accounts of psychopathy have drawn from research conducted on clinical populations with specific brain lesions, functional neuroimaging and electrophysiological studies to identify a series of brain regions in the paralimbic cortex and limbic system which may underpin these neurocognitive abnormalities in adult psychopaths (Kiehl, 2006). These regions are linked cytoarchitecturally as part of the ‘paralimbic system’ – a network of brain regions which includes the primary limbic structures and regions they directly project to (Anderson and Kiehl, 2012; Mesulam, 2000). Regions within the paralimbic system which are thought to play a central role in psychopaths' affective and reinforcement learning deficits include the amygdala, ventral striatum (vSTR), medial orbitofrontal cortex (mOFC), anterior insula (aINS), anterior and posterior cingulate (ACC and PCC), anterior temporal lobe (aTL), posterior superior temporal sulcus (pSTS), and parahippocampal gyrus (PHG) (Anderson and Kiehl, 2012; Blair et al., 2014). The paralimbic system is responsible for generating affective responses to stimuli (i.e., good/bad, approach/avoid) and integrating that information into higher-order emotional processing and decision-making mechanisms (Anderson and Kiehl, 2012; Kiehl, 2006). Moreover, these regions remain relevant in structural studies of youth with elevated psychopathic traits (Cope et al., 2014a; Ermer et al., 2013). Given that youth with severe conduct problems and elevated CU traits exhibit similar affective and reinforcement learning deficits, brain regions within the paralimbic system are promising regions of interest in the study of this population.
Functional magnetic resonance imaging (fMRI) studies on youth with elevated CU traits support the application of the paralimbic dysfunction model to elevated CU traits in youth. Youth with elevated CU traits have consistently exhibited abnormal hemodynamic activity across the paralimbic system during a variety of emotional processing (Lockwood et al., 2013; Lozier et al., 2014; Marsh et al., 2013; Sebastian et al., 2012; Viding et al., 2012) and decision-making tasks (Finger et al., 2008; Finger et al., 2011). A number of structural magnetic resonance imaging (sMRI) studies have focused on youth with severe conduct problems compared to neurotypical controls. They have found that adolescents with severe conduct problems have exhibited reduced gray matter volume (GMV) in the amygdala, aINS, OFC, and aTL (Cope et al., 2014a; Fairchild et al., 2011; Huebner et al., 2008; Sterzer et al., 2007; see Rogers and De Brito, 2016 for a meta-analysis).
A subset of these structural studies have investigated the contribution of elevated CU traits specifically. A number of studies identified positive relationships between CU traits and gray matter structure within the paralimbic brain regions (Fairchild et al., 2011; Wallace et al., 2014). For instance, a recent study found adolescent boys with conduct problems and high CU traits to have greater GMV and gray matter density (GMD) in the posterior mOFC, dorsal ACC, rostral ACC, and aTL compared to typically developing boys (De Brito et al., 2009). However, a more recent group comparison found reduced GMV in the left OFC and right ACC in youth with conduct problems and elevated CU traits compared to youth with conduct problems and normative CU traits and neurotypical controls (Sebastian et al., 2016). Additionally, a few studies have examined the structural correlates of ‘psychopathic traits’ in adolescents with severe conduct problems. These studies have generally found a negative association between psychopathic traits and GMV across multiple paralimbic regions (Ermer et al., 2013; Yang et al., 2015).
The neurological structure that might underlie elevated CU traits has been identified as an under-examined area in the study of CU traits (Frick et al., 2013). The anatomical structure of interconnected brain regions provides the basic architecture underlying a neural network and, through interactions between those regions, facilitates the functions of that network (Bressler and Tognoli, 2006). Thus, while the exact relationship between neural structure and function is not fully understood, identifying the structural correlates of a set of traits provides us with some understanding of the neural functioning from which those traits arise. Similar to how studies examining neurological abnormalities in antisocial adolescents have led to important advances in causal theories (Raine and Yang, 2006), etiological accounts of the development of CU traits are likely to be advanced substantially from a better understanding of the structural brain basis of CU traits (Frick et al., 2013). Moreover, identifying the structural correlates of CU traits may assist future research into the developmental pathways responsible for elevated CU traits, and guide treatment programs by providing a clearer understanding of the neural mechanisms underlying the behavior of youth with severe conduct problems and elevated CU traits.
Additionally, existing structural studies have only examined CU traits as a unified construct, measured by ICU total score without examining the contribution of the individual components of CU traits. A substantial amount of literature in adult psychopathy has demonstrated that the individual factors and facets of psychopathic traits are associated with discriminable neurological effects (Anderson et al., 2018; Baskin-Sommers et al., 2016; Juárez et al., 2013). Moreover, a number of studies of CU traits have identified discriminable behavioral effects amongst the ICU factors (e.g. Byrd et al., 2013; Ezpeleta et al., 2013; Kimonis et al., 2008b). This suggests that CU traits may be a constellation of interrelated yet distinct socio-affective characteristics. This paper advances the study of CU traits by breaking down the factors and showing their individual effects amongst a sample of uniformly antisocial youth.
The purpose of this study is to further explore the structural neurological correlates of CU traits in youth with severe conduct problems, both as a unified construct and divided into factors. To accomplish this, we present results from a voxel-based morphometry analysis on the relationship between brain structure and CU traits in a large sample of maximum-security incarcerated male adolescents. Based on the extant research in the field, as well as theoretical perspectives on the neurobiological basis of psychopathy, we predicted that CU traits would be associated with gray matter volume within the amygdala, aINS, vSTR, mOFC, ACC, PCC, aTL, pSTS, and PHG.
2. Methods
2.1. Participants
The sample (N = 269) included male adolescents recruited from correctional facilities in New Mexico and Wisconsin (mean age = 17.25 years; SD = 1.223; range: 13.75–20.75 years). This research was approved by the relevant institutional review boards and individuals volunteered to participate after providing written informed consent (if ≥ 18 years of age) or after providing written informed assent and parent/guardian written informed consent (if <18 years of age). Participants were paid a flat rate, yoked to the standard institutional hourly pay scale, for participation in the study. Participants were excluded from participation if they had a history of seizures, psychosis, traumatic brain injury, other major medical problems, or failed to show fluency in English at or above a grade four reading level. A portion of this sample was included in previous studies (Cope et al., 2014b; Ermer et al., 2013), but all current data analysis is unique to this study. Demographic information on this sample is provided in Table 1.
Table 1.
Demographic information for incarcerated male adolescents (N = 269).
| Variable | N | Min | Max | Mean | SD | 95% C.I. |
|---|---|---|---|---|---|---|
| Age | 269 | 13.75 | 20.75 | 17.25 | 1.22 | 17.11–17.39 |
| IQ | 269 | 57 | 140 | 90.51 | 13.52 | 88.91–92.19 |
| Callous-unemotional traits | ||||||
| ICU total | 269 | 7 | 65 | 28.51 | 9.59 | 27.41–29.72 |
| ICU callous | 269 | 0 | 24 | 7 | 4.13 | 6.53–7.53 |
| ICU uncaring | 269 | 0 | 22 | 10.34 | 4.96 | 9.75–10.95 |
| ICU unemotional | 269 | 0 | 15 | 8.61 | 3.06 | 8.27–9.00 |
| Substance use history | ||||||
| Cumulative substance usea | 269 | 0 | 215.57 | 52.04 | 35.35 | 47.77–56.31 |
| Variable | n | % | |||
|---|---|---|---|---|---|
| Race | |||||
| American Indian | 30 | 11 | |||
| Black | 58 | 22 | |||
| Pacific Islander | 1 | 1 | |||
| White | 53 | 20 | |||
| Other/decline | 124 | 46 | |||
| Missing | 3 | 1 | |||
| Ethnicity | |||||
| Hispanic | 155 | 58 | |||
| Not Hispanic | 114 | 42 | |||
| Location | |||||
| Wisconsin | 80 | 30 | |||
| New Mexico | 189 | 70 | |||
| Handedness | |||||
| Right | 208 | 77 | |||
| Left | 26 | 10 | |||
| Ambidextrous | 2 | 1 | |||
| Missing | 33 | 12 |
Cumulative Substance Use was calculated by adding years of regular use (three times a week or more) for all substances, dividing by age, multiplied by 100, and a square root transformation was applied to correct for skew.
2.2. Assessments
CU traits were measured using the Inventory of Callous-Unemotional Traits (ICU), a 24-item self-report questionnaire designed to provide a comprehensive assessment of callous and unemotional traits (Frick, 2004). Items are rated on a four-point Likert-type scale (0 = Not at all true to 3 = Definitely true). In addition to a total score, the ICU can be broken down into three factors: callousness, uncaring and unemotional (Essau et al., 2006). The callousness factor encompasses traits such as a lack of empathy, guilt, and remorse for wrongdoing. The uncaring factor focuses on a lack of caring about one's performance on tasks and for the feelings of other people. The final factor (unemotional) captures the absence of emotional expression. Several studies support the construct validity of the ICU in incarcerated youth (e.g., Kimonis et al., 2008b; Pihet et al., 2014). In our sample, the ICU was found to have good internal consistency (Chronbach's alpha for ICU total score, containing all 24 items, was α = 0.84; Callousness, containing 9 items, was α = 0.84; Uncaring, containing 8 items, was α = 0.81; Unemotional, containing 5 items, was α = 0.62).
Full-scale IQ (IQ) was estimated from the Vocabulary and Matrix Reasoning sub-tests of the Wechsler Adult Intelligence Scale (Wechsler, 1997) for participants older than 16 years of age and from the Wechsler Intelligence Scale for Children–Fourth Edition (Wechsler, 2003) for participants younger than 16 years of age.
Trained researchers administered a post-head injury symptoms questionnaire (King et al., 1995) to evaluate history of traumatic brain injury (TBI). Psychiatric disorders were accounted for using two different methods. Diagnoses were coded from comprehensive psychiatric evaluations made by an attending psychiatrist where they were available. For participants that did not receive an admission psychiatric evaluation, psychiatric disorders were evaluated using the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS; Kaufman et al., 1997) completed by trained research assistants (for a breakdown of psychiatric disorders see Table 2). As a result, we had basic diagnostic information (Past, Present, Past & Present) on a variety of psychiatric disorders for our entire sample. Notably, the incidence of conduct disorder was uniformly distributed throughout the sample, as it was present in about 95% of participants (n = 256).
Table 2.
Psychiatric diagnoses for incarcerated male adolescents (N = 269).
| Past disorder | Prevalence (%) | |
|---|---|---|
| Mood disorders | ||
| Major depression | 9 | |
| Melancholic depression | 1 | |
| Dysthymic disorder | 1 | |
| Depressive disorder nos | 3 | |
| Bipolar | 1 | |
| Schizoaffective disorder | 1 | |
| Adjustment disorder w/ depressed mood | 3 | |
| Psychotic disorders | ||
| Schizophreniform | 0 | |
| Psychotic disorder nos | 1 | |
| Anxiety disorders | ||
| Panic | 1 | |
| Phobia | 3 | |
| Obsessive-compulsive | 1 | |
| Post-traumatic stress | 6 | |
| Generalized anxiety | 1 | |
| Anxiety disorder nos | 1 | |
| Behavioral disorders | ||
| Attention deficit hyperactivity disorder | 39 | |
| Oppositional defiant disorder | 47 | |
| Conduct disorder | 95 | |
| Childhood onset | 55 | |
| Adolescent onset | 40 | |
| Group type | 35 | |
| Solitary type | 20 | |
| Undifferentiated | 27 | |
| Substance use disorders | Dependence | Abuse |
| Alcohol | 43 | 57 |
| Sedative-hypnotic-anxiolytic | 7 | 14 |
| Cannabis | 45 | 63 |
| Stimulants | 12 | 14 |
| Opioids | 3 | 6 |
| Cocaine | 20 | 28 |
| Hallucinogens/PCP | 3 | 5 |
A modified version of the Addiction Severity Index (McLellan et al., 1992) was administered across the entire population. Years of regular use were summed for each substance (alcohol and drug) that the participant reported using regularly (three or more times per week for a minimum period of one month). Cumulative substance use was then calculated by adding years of regular use for all substances, dividing by age (to control for opportunity to use), multiplied by 100, and a square root transformation was applied to correct for skew (“Cumulative Substance Use”; Table 1). Diagnoses of substance abuse or dependence are included in the breakdown of psychiatric disorders (see Table 2).
2.3. MRI data acquisition and analysis
High-resolution T1-weighted structural MRI scans were acquired using the Mind Research Network Mobile Siemens 1.5 T Avanto MRI scanner, stationed at the detention facility. A multi-echo MPRAGE pulse sequence (repetition time = 2530 ms, echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms, inversion time = 1100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256) was used, yielding 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm. Data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). T1 images were manually inspected by an operator blind to subject identity and realigned to ensure proper spatial normalization. Images were then analyzed via the Unified Segmentation approach as implemented in SPM12 (Ashburner and Friston, 2005). Unified Segmentation allows for image registration based on Gaussian mixture modelling, tissue classification with warped prior probability maps and bias correction to be combined in the same generative model. During spatial normalization data were resampled to 2 × 2 × 2 mm. Subsequent segmentation partitioned the images into gray matter, white matter, and cerebrospinal fluid. Measures equating to gray matter volume and density were derived from these data. A Jacobian modulation was performed to preserve total volume, i.e., modulated data was interpreted as GMV. A nonlinear transformation without Jacobian determinants was performed on unmodulated images to extract gray matter density (i.e., GMD; Ashburner and Friston, 2000, Ashburner and Friston, 2005). GMD results were included as supplementary material (Table S1). Voxels with a value of <0.15 were excluded in order to remove possible edge effects between gray and white matter. Finally, segmented images were smoothed with a 10-mm full-width at half-maximum Gaussian kernel. Only gray matter segments were used in this analysis.
Multiple regression analyses were performed on a voxel-by-voxel basis over the whole brain using the general linear model to evaluate the relationship between ICU scores and regional GM. In analyses evaluating the relationship between the ICU factors and regional GM, all three factors were included in the model simultaneously to examine relative contribution of each factor, holding the others constant, while also controlling for IQ, total brain volume (TBV), age at scan, and substance use. A separate model was used to examine ICU total scores with the same covariates (IQ, TBV, age, substance use). All ICU scores were used continuously. At the whole brain level, results were considered significant at the voxel level using a statistical threshold of p < .05 after Family-Wise Error (FWE) correction for multiple comparisons. For completeness, the whole brain results were also examined using cluster-level correction and were included in the Supplementary Material (Table S2). Monte Carlo simulation was conducted using 3dClustSim (AFNI: Compile Date = Apr 182,018, Version = AFNI_18.1.05, http://afni.nimh.nih.gov) and accounting for spatial auto correlation function (ACF) in a mixed model to determine significant clusters across the whole brain. For the ICU total score analysis, an initial cluster forming threshold of p < .001 and voxel extent threshold of k = 407 produces an effective family-wise error rate corrected threshold of p < .05 across the whole brain. For models examining ICU factors, an initial threshold of p < .001 and k = 424 effectively controls family wise error at p < .05 across the whole brain.
In addition to the whole-brain analyses, our hypotheses were also tested in a priori regions of interest (ROIs). Bilateral anatomical image masks based on ROIs (amygdala, aINS, vSTR, mOFC, ACC, PCC, aTL, pSTS, PHG) were created using the Wake Forest University (WFU) Pick Atlas Toolbox (Maldjian et al., 2003; Maldjian et al., 2004). For each region, a small volume correction (SVC) was applied to control familywise error rate at p < .05 within the area of each mask; we report the FWE corrected p-values. The WFU Pick Atlas AAL labels used for each anatomical brain region are listed in Table 3. A few a priori ROIs required more than one anatomical mask to encompass the entire region. To correct for multiple comparisons across all ROIs, we employed the Benjamini-Hochberg procedure to perform a false discovery rate (FDR) correction (Benjamini and Hochberg, 1995). The Benjamini-Hochberg critical value was calculated based on applying 14 ROIs to each regression with a false discovery rate of p < .05. Brain regions which emerged as significant during SVC and were significant after FDR correction are marked with a super-scripted psi (ψ) in the imaging results below (Table 5). In a second ROI analysis, included as supplementary material (see Table S2), unilateral masks were used in order to demonstrate which significant effects exhibit left or right hemispheric specificity and, conversely, identify effects which were significant bilaterally.
Table 3.
AAL Labels of WFU Pick Atlas images used to examine each anatomical region of interest.
| Anatomical region | WFU pick atlas AAL labels (Rolls et al., 2015) |
|---|---|
| Amygdala | Amygdala |
| Insula | Insula |
| Ventral striatum | Caudate, Putamen |
| Medial orbitofrontal cortex | Frontal_Med_Orb, Frontal_Sup_Orb, Rectus |
| Anterior cingulate cortex | Cingulum_Ant |
| Posterior cingulate cortex | Cingulum_Post, Cingulum_Mid |
| anterior temporal lobe | Temporal_Pole_Mid, Temporal_Pole_Sup |
| Superior temporal sulcus | Temporal_Mid |
| Parahippocampal gyrus | ParaHippocampal |
Table 5.
Imaging results from sample of incarcerated male adolescents (N = 269).
| Region | BA | x | y | z | t | k | p |
|---|---|---|---|---|---|---|---|
| ICU Total (corrected for age, IQ, TBV, CumSU) | |||||||
| GMV | |||||||
| Positive correlation | |||||||
| None | |||||||
| Negative correlation | |||||||
| R. anterior temporal lobe* | 38 | 32 | 20 | −36 | 3.34 | 853 | 0.052 |
| ICU callousness (corrected for unemotional, uncaring, age, IQ, TBV, CumSU) | |||||||
| GMV | |||||||
| Positive correlation | |||||||
| None | |||||||
| Negative correlation | |||||||
| R. Middle temporal gyrus | 21 | 52 | −22 | −17 | 4.82 | 22 | 0.026 |
| R. Middle temporal gyrus*ψ | 21 | 48 | −6 | −24 | 5.16 | 6936 | 0 |
| R. anterior temporal lobe | 21/38 | 46 | −4 | −26 | 5.21 | 41 | 0.018 |
| R. anterior temporal lobe*ψ | 38 | 38 | 17 | −21 | 3.67 | 1113 | 0.026 |
| R. amygdala*ψ | 53 | 34 | 0 | −29 | 3–56 | 86 | 0.009 |
| L. amygdala*ψ | 53 | −12 | 2 | −20 | 4.01 | 202 | 0.007 |
| R. insula/striatum | 48/49/13 | 24 | 20 | 7 | 4.78 | 27 | 0.023 |
| R. ventral striatum*ψ | 48/49/13 | 26 | 20 | 6 | 4.74 | 1051 | 0 |
| L. anterior insula*ψ | 13 | −26 | 18 | 4 | 3.95 | 2243 | 0.014 |
| R. caudate*ψ | 48 | 22 | 21 | 6 | 3.94 | 30 | 0.008 |
| R. posterior cingulate | 23 | 10 | −22 | 34 | 4.58 | 2 | 0.044 |
| B. dorsal posterior cingulate | 31 | 0 | −37 | 52 | 4.5 | 2 | 0.044 |
| R. posterior cingulate*ψ | 23 | 10 | −22 | 34 | 4.58 | 7647 | 0.001 |
| L. posterior cingulate*ψ | 23 | −9 | −51 | 21 | 3.56 | 1009 | 0.013 |
| L. ventral anterior cingulate*ψ | 32 | −9 | 32 | −3 | 4 | 4884 | 0.008 |
| R. medial orbitofrontal cortex*ψ | 32/11 | 14 | 39 | −2 | 3.61 | 2005 | 0.016 |
| L. medial orbitofrontal cortex*ψ | 11 | −15 | 35 | −15 | 3.75 | 3089 | 0.011 |
| L. medial orbitofrontal cortex*ψ | 11 | −16 | 51 | −11 | 3.93 | 1093 | 0.01 |
| R. dorsomedial prefrontal cortex | 9 | 14 | 42 | 37 | 4.54 | 1 | 0.046 |
| R. ventrolateral prefrontal cortex | 47 | 40 | 38 | −6 | 4.66 | 5 | 0.039 |
| R. precuneus | 7 | 10 | −54 | 60 | 4.76 | 26 | 0.024 |
| R. precuneus | 7 | 3 | −54 | 58 | 4.5 | 3 | 0.042 |
| L. somatosensory cortex | 5 | −16 | −30 | 46 | 4.74 | 3 | 0.042 |
| ICU uncaring (corrected for callousness, unemotional, age, IQ, TBV, CumSU) | |||||||
| GMV | |||||||
| Positive correlation | |||||||
| R. rostral anterior cingulate* | 32 | 12 | 38 | 0 | 3.74 | 2893 | 0.018 |
| R. medial orbitofrontal cortex* | 11 | 12 | 38 | −3 | 3.44 | 898 | 0.026 |
| Negative correlation | |||||||
| None | |||||||
| ICU unemotional (corrected for callousness, uncaring, age, IQ, TBV, CumSU) | |||||||
| GMV | |||||||
| Positive correlation | |||||||
| None | |||||||
| Negative correlation | |||||||
| None |
BA, Brodmann area; t, t score; k, spatial extent of activation. Activations are reported in MNI coordinate space. Regions marked with an asterisk (*) were identified after small volume correction using regions of interest; p-values for these regions represent FWE small-volume-corrected values. Of these regions, those marked with a super-scripted psi (ψ) were found to be significant after FDR correction for multiple comparisons. Regions without an asterisk were identified during whole-brain analysis; p-values for these regions are FWE-corrected p-values.
3. Results
Table 4 provides the correlation matrix for all of the variables included in MRI analysis.
Table 4.
Correlation matrix for variables included in MRI analysis.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 Age | ||||||||
| 2 IQ | 0.17a | |||||||
| 3 CumSUc | 0.15b | 0.12b | ||||||
| 4 TBV | -0.18b | 0.10 | −0.05 | |||||
| 5 ICU total | −0.08 | 0.01 | 0.23a | −0.06 | ||||
| 6 ICU callous | -0.12b | −0.11 | 0.17a | −0.07 | 0.79a | |||
| 7 ICU uncaring | −0.04 | 0.02 | 0.22a | −0.02 | 0.82a | 0.46a | ||
| 8 ICU unemotional | −0.02 | 0.16a | 0.11 | −0.05 | 0.60a | 0.25a | 0.30a |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
CumSU stands for Cumulative Substance Use. This value was calculated by adding years of regular use (three times a week or more) for all substances, dividing by age, multiplied by 100, and a square root transformation was applied to correct for skew.
3.1. Voxel-based morphometry results
In whole-brain analysis, only the Callousness factor of the ICU was significantly correlated with GMV in any regions. The Callousness factor was negatively correlated with GMV in a number of paralimbic regions, including the right aTL, aINS, PCC and precuneus. Additionally, callousness was negatively correlated with GMV in the right middle temporal gyrus, ventrolateral prefrontal cortex and dorsomedial prefrontal cortex. Examining a priori ROIs, ICU total score and all three of its subscales were significantly correlated with gray matter in paralimbic brain regions after small volume correction (FWE). ICU total score was negatively correlated with gray matter volume in both the right aTL (trend level). The Uncaring factor held a positive relationship with GMV in the right mOFC, and rostral ACC. The Unemotional factor was not significantly correlated with GMV in any regions of interest.
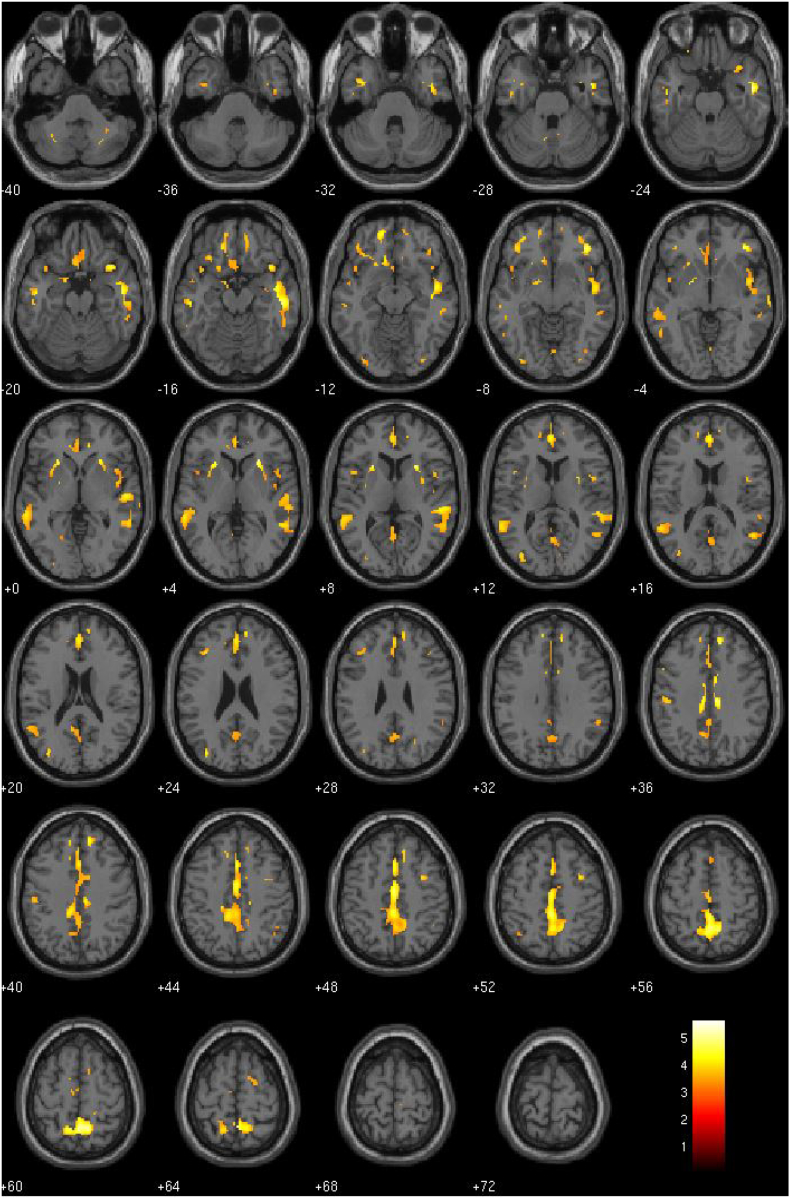
By far the most stable and widespread effects were associated with the Callousness factor of the ICU (see Fig. 1). Callousness scores were negatively correlated with GMV across nearly every paralimbic region examined, including the amygdala, aINS, vSTR, mOFC, aTL, PCC and ACC (See Table 5 for detailed imaging results). There were no regions whose GMV was positively correlated with Callousness.
Fig. 1.
GMV negatively correlated with the Callousness factor of the ICU (p-threshold = 0.001).
4. Discussion
The current study used VBM to examine gray matter volume and density related to varying levels of callous-unemotional traits in a sample of incarcerated male adolescents. Consistent with our hypothesis, ICU total score and two factors of the ICU (Uncaring and Callousness) were associated with gray matter structure in paralimbic brain regions, including the amygdala, aINS, vSTR, mOFC, ACC, PCC and aTL.
Our study is the first to examine CU traits both as a unified construct, through ICU total score, and as a composite of related traits, through the three factors of the ICU. Previous research in this area has looked at overall levels of CU traits only, without examining the contribution of the individual elements of CU traits. As such, only our results relating to the ICU total score are directly comparable to extant findings related to CU traits. In our analysis, ICU total score was inversely related to GMV in the right aTL, and was not significantly related to gray matter structure in any other regions of interest. Existing structural studies of CU traits have largely failed to find associations with the aTL, with the exception of De Brito et al. (2009) who found higher GMV in the right aTL amongst youth with severe conduct problems and elevated callous-unemotional traits when compared to typically developing boys.
Although findings in this area have been limited, there is evidence from extant literature that the aTL might be dysfunctional in adolescents with high callous-unemotional traits. Anatomically, the aTL is highly interconnected with other paralimbic regions such as the amygdala, OFC, aINS and hypothalamus. Based on this anatomical connectivity, it has been suggested that the aTL modulates visceral emotional functions in response to emotionally evocative perceptual stimuli (Frith and Frith, 2010; Kondo et al., 2003, Kondo et al., 2005). Patients with right aTL atrophy often exhibit striking changes in social behavior and emotional processing (Mychack et al., 2001; Thompson et al., 2003). Right aTL atrophy or hypoactivity has been associated with reductions in empathy (Rankin et al., 2006; Sollberger et al., 2011) and impairments on social cognition tasks (Kipps et al., 2009; Zahn et al., 2009).
When the ICU factors were examined individually, the effects were more widespread. Whole-brain analysis of the three ICU factors revealed that the right aTL was strongly negatively associated with Callousness. Given that the relationship between gray matter in the right aTL and ICU total score was relatively weak, it is possible that the Callousness factor drove that correlation. In fact, of the three factors of the ICU, the Callousness factor had the most reliable and widespread results by far. Callousness scores were negatively correlated with gray matter volume across nearly every region examined, including the amygdala, aINS, vSTR, rostral ACC, ventral ACC, mOFC, aTL and PCC. Of our findings, it is these results which are most consistent with previous studies which found CU traits to be negatively associated with GMV across many of these same brain regions (Sebastian et al., 2016; Wallace et al., 2014).
Two prominent neurobiological models of psychopathy have emerged from contemporary neuroimaging research. Both point to components of the limbic system as underlying emotional and behavioral abnormalities in psychopaths. The model put forth by Blair highlights abnormalities in the amygdala and mOFC as contributing to the development of psychopathy (Blair et al., 2014). The paralimbic dysfunction model, proposed by Kiehl, looks at a more widely distributed network of brain regions to account for the neurocognitive abnormalities exhibited by psychopaths. In addition to the amygdala and mOFC, cytoarchitecturally related regions such as the parahippocampal gyrus, anterior temporal lobe, anterior insula and cingulate have been emphasized by Kiehl as particularly relevant to psychopathy (Kiehl, 2006; Kiehl et al., 2001). This model accounts for findings that psychopaths exhibit abnormal neural structure and functioning in regions beyond primary limbic structures (Anderson and Kiehl, 2012; Boccardi et al., 2011; Ermer et al., 2013). Our findings are consistent with both of these neurobiological models. The brain regions in which we found gray matter structure correlated with CU traits, especially for callous traits, are all a part of this paralimbic system. Our pattern of results also supports several previous studies which have found paralimbic gray matter reductions in adolescents to be associated with elevated psychopathic traits (Cope et al., 2014a; Ermer et al., 2013; Yang et al., 2015).
Our findings are also consonant with behavioral and fMRI research regarding several neurocognitive abnormalities which have been exhibited by youth with severe conduct problems and elevated CU traits. Youth with elevated CU traits have consistently exhibited blunted emotional reactivity when presented with negative affective stimuli (Kimonis et al., 2008a; Loney et al., 2003). Specifically, they have shown significantly less autonomic arousal when exposed to emotionally evocative film clips (de Wied et al., 2012), when anticipating aversive stimuli (Fung et al., 2005; Isen et al., 2010), and while viewing others in pain (Cheng et al., 2012; Fanti et al., 2016). Studies using fMRI data to examine these youths' neural responses to viewing others in pain have found associated hypoactivity in several brain areas including the left amygdala, aINS, and ACC (Lockwood et al., 2013; Marsh et al., 2013). Researchers have also consistently found impairments in recognizing facial expressions and body postures of fear and sadness in youth with elevated CU traits (Blair et al., 2005; Dadds et al., 2006; Leist and Dadds, 2009; Muñoz, 2009; Sylvers et al., 2011). Corresponding fMRI studies have shown CU traits to be negatively associated with amygdala activity when presented with fearful facial expressions (Jones et al., 2009; Marsh et al., 2008, Marsh et al., 2013; Sebastian et al., 2012; Viding et al., 2012; White et al., 2012). While the relationship between gray matter structure and function has not been precisely defined, the overlap between significant regional effects in structural and functional investigations lends credence to their interdependence. The consonance between extant fMRI research, related structural gray matter studies and our findings here augment this compelling interpretation.
Characteristic deficits in empathy and emotional processing are widely thought to originate from amygdala dysfunction. The amygdala is a key node in the paralimbic network, and plays a central role in emotional processing—most notably in signaling the affective salience of stimuli (Cunningham and Brosch, 2012; Hamann et al., 2002). When it comes to empathic processes specifically, the amygdala lies at the center of a network of brain areas, including the mOFC, aINS, and ACC, which is believed to be critically involved in generating an aversive response to seeing other people in distress (Decety, 2010). It is thought that, through these aversive responses, individuals are conditioned to regard other people's suffering as something to avoid. Dysfunction within this system, therefore, may prevent someone from developing emotional empathy in this way (Blair, 2013). The negative relationship between Callousness and GMV in all of these paralimbic regions – the amygdala, aINS, mOFC, and ACC – is consistent with the claim that elevated CU traits, specifically elevated callous traits, may be rooted in dysfunction within these structures.
Several studies have found CU traits to be associated with abnormalities in processing punishment cues. Specifically, youth with elevated CU traits tend to disproportionately focus on the potential rewards of a particular behavior, rather than the possibility or magnitude of negative consequences. As a result, they tend to continue responding to previously rewarded cues even when contingencies change and the response results in escalating punishments (Centifanti and Modecki, 2013; Fanti et al., 2016; Finger et al., 2008; Finger et al., 2011; Schwenck et al., 2017). This kind of decision-making deficit is thought to result from dysfunction in the integrated functioning of the amygdala, vSTR, and mOFC (Schoenbaum and Roesch, 2005). The negative relationship between Callousness and GMV in the amygdala, vSTR, and mOFC might be reflective of this decision-making deficit. Moreover, this reward dominant decision-making style might be directly linked to the callous and antisocial behaviors exhibited by youth with elevated CU traits. An individual may be more willing to engage in antisocial behaviors because they are focused on obtaining some personal reward while ignoring the potential harm that action might inflict on themselves or others.
Examining the structural correlates of the ICU construct as a composite of interrelated traits, rather than simply as a singular total score, revealed significant discriminable effects amongst the ICU factors. The factors demonstrated unique relationships with paralimbic gray matter structure in our sample, and this is important for a number of reasons. First of all, in a few areas, the ICU factors had opposing relationships to gray matter structure with each other. For instance, while Callousness was negatively correlated to GMV in the right mOFC, Uncaring was positively correlated to GMV in this same area. This is important for future research into the structural correlates of CU traits, as these factor-level findings may cancel each other out when combined into the ICU total score. Secondly, of the ICU factors, and even compared to ICU total score, it was the Callousness factor which had the most widespread effects and whose findings were most consistent with extant neuroimaging and behavioral research in CU traits and adult psychopathy. There is some evidence to suggest that the Callousness subscale discriminates best at high clinical levels of CU traits (Ray et al., 2016). If significant structural alterations primarily occur at high levels of CU traits, this may partially account for why the Callousness factor had the most reliable structural correlates. Additionally, a recent meta-analysis found that the Callousness factor was more internally consistent and showed stronger associations with measures of psychopathy in detained samples (Cardinale and Marsh, 2017). The Callousness factor was also strongly correlated with behavioral outcomes, along with the ICU total score and Uncaring factor. This raises the possibility that callous traits lie at the core of the affective deficits and behavioral abnormalities commonly found in youth with conduct problems and elevated CU traits.
4.1. Conclusions
Beyond findings related to any specific brain region, the substantial and widespread reduction in paralimbic gray matter associated with elevated callous traits lends strong support to the supposition that the paralimbic system might be involved in the expression of these traits. Future research into CU traits may substantially benefit from expanding their focus and investigating the role of this network as a whole. Moreover, it may be beneficial to examine the role of the ICU factors in addition to the ICU total score with particular focus on the role of callous traits. Finally, treatment focused on youth with elevated CU traits may benefit from understanding the role of paralimbic system dysfunction in this population, and that callous traits specifically appear to play a central role in the behavioral manifestations of this disorder.
4.2. Limitations and future directions
While our findings are highly relevant to the study of CU traits, there are many important questions in this field which our results do not answer on their own.
First, while our results show that elevated CU traits are associated with differences in brain structure amongst youth with conduct problems, they cannot be used to conclude which factors, environmental or genetic, might cause this difference. Additional research, employing much more detailed environmental and genetic information, would be necessary to address these questions.
Second, identifying the neurobiological basis for elevated CU traits does not speak to the treatment prognosis of this population. The treatment challenge posed by youth with elevated CU traits has already been documented and the moderate success of a few select treatment programs suggests that important behavioral manifestations of CU traits can be altered (for review see Hawes et al., 2014). A future study examining what, if any, structural changes occur in youth who are successfully treated by these programs would considerably advance our understanding both of CU traits and the treatment programs which are effective at addressing them.
Third, while our results do suggest that elevated CU traits in antisocial youth are associated with gray matter structure in several paralimbic regions, the cross-sectional design of our study limits what we can conclude about the developmental trajectory of callous-unemotional traits and these brain structures. During childhood and adolescence, gray matter structure changes in heterogeneous ways depending on the brain region examined and the developmental progress of the individual. It is possible, for instance, that youth with elevated CU traits simply experience a delayed development of core paralimbic structures, and that they will catch up to their neurotypical counterparts with or without intervention. In other words, we cannot make conclusions about future brain development or how long any of the observed relationships between neural structure and CU traits will last.
Fourth, our sample was composed of incarcerated youth who predominantly identified as Non-White. As such, our results might be less applicable to predominantly White or community samples. However, considering that ethnic minorities are over-represented in incarcerated settings, these results may be particularly applicable to this population.
Fifth, gray matter volume is a function of both cortical thickness and surface area, which are increasingly viewed as separable endophenotypes (Panizzon et al., 2009; Winkler et al., 2010). Existing structural studies of antisocial populations have commonly focused on gray matter volume overall and our VBM volume measure is commonly used. Future studies, however, might benefit from examining cortical thickness and gyrification as well as volume.
Finally, developmental MRI studies have found that, while early childhood is characterized by gray matter increases across the brain (Gilmore et al., 2012; Knickmeyer et al., 2008), older children and adolescents show cortical gray matter decreases in most regions, increasing white matter volumes and heterogeneous changes in subcortical structures (Brain Development Cooperative Group, 2012; Vijayakumar et al., 2016). Neurotypical brain development seeks to achieve efficient processing in developing networks, and this may require increases or decreases in gray matter volume and density depending on the region. Unlike in adult samples, where decreases in GMV in a given region may be indicative of reduced function (Bassett et al., 2008), increases or decreases in GMV might be indicative of abnormal brain development in our adolescent sample (Paus, 2005). These issues may help to explain why previous studies' findings have diverged. Disentangling these issues to formulate directional hypotheses about the gray matter structure of adolescents will require further study.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was supported by the following grants from the National Institute of Child Health and Human Development and the National Institute of Mental Health: R01HD082257, R01MH071896 (PI: Kiehl).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101703.
Contributor Information
Brendan M. Caldwell, Email: bcaldwell@mrn.org.
Kent A. Kiehl, Email: kkiehl@mrn.org.
Appendix A. Supplementary data
GMD Findings from Primary Analysis
Secondary Imaging Analysis
References
- Anderson N.E., Kiehl K.A. The psychopath magnetized: insights from brain imaging. Trends Cogn. Sci. 2012;16(1):52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N.E., Maurer J.M., Steele V.R., Kiehl K.A. Psychopathic traits associated with abnormal hemodynamic activity in salience and default mode networks during auditory oddball task. Cognit. Affect. Behav. Neurosci. 2018;18(3):564–580. doi: 10.3758/s13415-018-0588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers A.R., Neumann C.S., Cope L.M., Kiehl K.A. Latent-variable modeling of brain gray-matter volume and psychopathy in incarcerated offenders. J. Abnorm. Psychol. 2016;125(6):811. doi: 10.1037/abn0000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28(37):9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(1):289–300. http://www.jstor.org/stable/2346101 [Google Scholar]
- Blair R.J.R. The neurobiology of psychopathic traits in youths. Nat. Rev. Neurosci. 2013;14(11):786–799. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Budhani S., Colledge E., Scott S. Deafness to fear in boys with psychopathic tendencies. J. Child Psychol. Psychiatry. 2005;46(3):327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. Conduct disorder and callous–unemotional traits in youth. N. Engl. J. Med. 2014;371(23):2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M., Frisoni G.B., Hare R.D., Cavedo E., Najt P., Pievani M.…Vaurio O. Cortex and amygdala morphology in psychopathy. Psychiatry Res. Neuroimaging. 2011;193(2):85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Tognoli E. Operational principles of neurocognitive networks. Int. J. Psychophysiol. 2006;60(2):139–148. doi: 10.1016/j.ijpsycho.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Butler S., Baruch G., Hickey N., Fonagy P. A randomized controlled trial of multisystemic therapy and a statutory therapeutic intervention for young offenders. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(12):1220–1235. doi: 10.1016/j.jaac.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Byrd A.L., Kahn R.E., Pardini D.A. A validation of the Inventory of Callous-Unemotional Traits in a community sample of young adult males. J. Psychopathol. Behav. Assess. 2013;35(1):20–34. doi: 10.1007/s10862-012-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A.L., Loeber R., Pardini D.A. Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin. Child. Fam. Psychol. Rev. 2014;17(2):125–156. doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M.F., McCormick D.J., Umstead D., Van Rybroek G.J. Evidence of treatment progress and therapeutic outcomes among adolescents with psychopathic features. Crim. Justice Behav. 2007;34(5):573–587. [Google Scholar]
- Cardinale E.M., Marsh A.A. The reliability and validity of the Inventory of Callous Unemotional Traits: a meta-analytic review. Assessment. 2017 doi: 10.1177/1073191117747392. [DOI] [PubMed] [Google Scholar]
- Centifanti L.C.M., Modecki K. Throwing caution to the wind: Callous-unemotional traits and risk taking in adolescents. J. Clin. Child Adolesc. Psychol. 2013;42(1):106–119. doi: 10.1080/15374416.2012.719460. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Hung A.-Y., Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev. Psychopathol. 2012;24(2):623. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Cope L.M., Ermer E., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. Paralimbic gray matter reductions in incarcerated adolescent females with psychopathic traits. J. Abnorm. Child Psychol. 2014;42(4):659–668. doi: 10.1007/s10802-013-9810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L.M., Ermer E., Gaudet L.M., Steele V.R., Eckhardt A.L., Arbabshirani M.R.…Kiehl K.A. Abnormal brain structure in youth who commit homicide. NeuroImage. 2014;4:800–807. doi: 10.1016/j.nicl.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr. Dir. Psychol. Sci. 2012;21(1):54–59. [Google Scholar]
- Dadds M.R., Perry Y., Hawes D.J., Merz S., Riddell A.C., Haines D.J.…Abeygunawardane A.I. Attention to the eyes and fear-recognition deficits in child psychopathy. Br. J. Psychiatry. 2006;189(3):280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Dadds M.R., Cauchi A.J., Wimalaweera S., Hawes D.J., Brennan J. Outcomes, moderators, and mediators of empathic-emotion recognition training for complex conduct problems in childhood. Psychiatry Res. 2012;199(3):201–207. doi: 10.1016/j.psychres.2012.04.033. [DOI] [PubMed] [Google Scholar]
- De Brito S.A., Mechelli A., Wilke M., Laurens K.R., Jones A.P., Barker G.J.…Viding E. Size matters: increased grey matter in boys with conduct problems and callous–unemotional traits. Brain. 2009;132(4):843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Wied M., van Boxtel A., Matthys W., Meeus W. Verbal, facial and autonomic responses to empathy-eliciting film clips by disruptive male adolescents with high versus low callous-unemotional traits. J. Abnorm. Child Psychol. 2012;40(2):211–223. doi: 10.1007/s10802-011-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Dev. Neurosci. 2010;32(4):257–267. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E., Cope L.M., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(1):94–103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine H.E., Ferrari A.J., Nelson P., Polanczyk G.V., Flaxman A.D., Vos T.…Scott J.G. Research review: epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. J. Child Psychol. Psychiatry. 2013;54(12):1263–1274. doi: 10.1111/jcpp.12144. [DOI] [PubMed] [Google Scholar]
- Essau C.A., Sasagawa S., Frick P.J. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13(4):454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Ezpeleta L., Osa N.D.L., Granero R., Penelo E., Domènech J.M. Inventory of callous-unemotional traits in a community sample of preschoolers. J. Clin. Child Adolesc. Psychol. 2013;42(1):91–105. doi: 10.1080/15374416.2012.734221. [DOI] [PubMed] [Google Scholar]
- Fairchild G., Passamonti L., Hurford G., Hagan C.C., von dem Hagen E.A., van Goozen S.H., Calder …., J A. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am. J. Psychiatr. 2011;168(6):624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Falkenbach D.M., Poythress N.G., Heide K.M. Psychopathic features in a juvenile diversion population: reliability and predictive validity of two self-report measures. Behav. Sci. Law. 2003;21(6):787–805. doi: 10.1002/bsl.562. [DOI] [PubMed] [Google Scholar]
- Fanti K.A., Panayiotou G., Lombardo M.V., Kyranides M.N. Unemotional on all counts: evidence of reduced affective responses in individuals with high callous-unemotional traits across emotion systems and valences. Soc. Neurosci. 2016;11(1):72–87. doi: 10.1080/17470919.2015.1034378. [DOI] [PubMed] [Google Scholar]
- Feilhauer J., Cima M., Arntz A. Assessing callous–unemotional traits across different groups of youths: further cross-cultural validation of the Inventory of Callous–Unemotional Traits. Int. J. Law Psychiatry. 2012;35(4):251–262. doi: 10.1016/j.ijlp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Mitchell D.G., Reid M.E., Sims C., Budhani S.…Pine D.S. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. Gen. Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Blair K.S., Reid M.E., Sims C., Ng P.…Blair R.J.R. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatr. 2011;168(2):152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick P.J. 2004. The Inventory of Callous-unemotional Traits. (Unpublished rating scale) [Google Scholar]
- Frick P.J., Moffitt T.E. American Psychiatric Association; Washington, DC: 2010. A Proposal to the DSM-V Childhood Disorders and the ADHD and Disruptive Behavior Disorders Work Groups to Include a Specifier to the Diagnosis of Conduct Disorder Based on the Presence of Callous-unemotional Traits; pp. 1–36. [Google Scholar]
- Frick P.J., Ray J.V., Thornton L.C., Kahn R.E. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol. Bull. 2013;140(1):1. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C. The social brain: allowing humans to boldly go where no other species has been. Philos. Trans. R. Soc. B. 2010;365(1537):165–176. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M.T., Raine A., Loeber R., Lynam D.R., Steinhauer S.R., Venables P.H., Stouthamer-Loeber M. Reduced electrodermal activity in psychopathy-prone adolescents. J. Abnorm. Psychol. 2005;114(2):187. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Shi F., Woolson S.L., Knickmeyer R.C., Short S.J., Lin W.…Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb. Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S.M., Waschbusch D.A., Pelham W.E., Jr., King S., Andrade B.F., Carrey N.J. Treatment response in CP/ADHD children with callous/unemotional traits. J. Abnorm. Child Psychol. 2011;39(4):541–552. doi: 10.1007/s10802-010-9480-4. [DOI] [PubMed] [Google Scholar]
- Hamann S.B., Ely T.D., Hoffman J.M., Kilts C.D. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol. Sci. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hawes D.J., Price M.J., Dadds M.R. Callous-unemotional traits and the treatment of conduct problems in childhood and adolescence: a comprehensive review. Clin. Child. Fam. Psychol. Rev. 2014;17(3):248–267. doi: 10.1007/s10567-014-0167-1. [DOI] [PubMed] [Google Scholar]
- Huebner T., Vloet T.D., Marx I., Konrad K., Fink G.R., Herpertz S.C., Herpertz-Dahlmann B. Morphometric brain abnormalities in boys with conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(5):540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Isen J., Raine A., Baker L., Dawson M., Bezdjian S., Lozano D.I. Sex-specific association between psychopathic traits and electrodermal reactivity in children. J. Abnorm. Psychol. 2010;119(1):216. doi: 10.1037/a0017777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.P., Laurens K.R., Herba C.M., Barker G.J., Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am. J. Psychiatr. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Juárez M., Kiehl K.A., Calhoun V.D. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Hum. Brain Mapp. 2013;34(8):1921–1930. doi: 10.1002/hbm.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U.M.A., Flynn C., Moreci P.…Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Smith A.M., Hare R.D., Mendrek A., Forster B.B., Brink J., Liddle P.F. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol. Psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis E.R., Frick P.J., Munoz L.C., Aucoin K.J. Callous-unemotional traits and the emotional processing of distress cues in detained boys: Testing the moderating role of aggression, exposure to community violence, and histories of abuse. Dev. Psychopathol. 2008;20(2):569. doi: 10.1017/S095457940800028X. [DOI] [PubMed] [Google Scholar]
- Kimonis E.R., Frick P.J., Skeem J.L., Marsee M.A., Cruise K., Munoz L.C.…Morris A.S. Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional Traits. Int. J. Law Psychiatry. 2008;31(3):241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- King N.S., Crawford S., Wenden F.J., Moss N.E.G., Wade D.T. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Nestor P.J., Acosta-Cabronero J., Arnold R., Hodges J.R. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009;132(3):592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K.…Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolko D., Pardini J. ODD dimensions, ADHD, and Callous-Unemotional traits as predictors of treatment response in children with disruptive behavioral disorders. J. Abnorm. Psychol. 2010;119:713–725. doi: 10.1037/a0020910. [DOI] [PubMed] [Google Scholar]
- Kondo H., Saleem K.S., Price J.L. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2003;465(4):499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kondo H., Saleem K.S., Price J.L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2005;493(4):479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Leist T., Dadds M.R. Adolescents' ability to read different emotional faces relates to their history of maltreatment and type of psychopathology. Clin. Child Psychol. Psychiatry. 2009;14(2):237–250. doi: 10.1177/1359104508100887. [DOI] [PubMed] [Google Scholar]
- Lockwood P.L., Sebastian C.L., McCrory E.J., Hyde Z.H., Gu X., De Brito S.A., Viding E. Association of callous traits with reduced neural response to others' pain in children with conduct problems. Curr. Biol. 2013;23(10):901–905. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney B.R., Frick P.J., Clements C.B., Ellis M.L., Kerlin K. Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. J. Clin. Child Adolesc. Psychol. 2003;32(1):66–80. doi: 10.1207/S15374424JCCP3201_07. [DOI] [PubMed] [Google Scholar]
- Longman T., Hawes D.J., Kohlhoff J. Callous–unemotional traits as markers for conduct problem severity in early childhood: a meta-analysis. Child Psychiatry Hum. Dev. 2016;47(2):326–334. doi: 10.1007/s10578-015-0564-9. [DOI] [PubMed] [Google Scholar]
- Lozier L.M., Cardinale E.M., VanMeter J.W., Marsh A.A. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71(6):627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G., Reid M.E., Sims C., Kosson D.S.…Blair R.J.R. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatr. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Fowler K.A., Adalio C.J., Jurkowitz I.T., Schechter J.C.…Blair R.J.R. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J. Child Psychol. Psychiatry. 2013;54(8):900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R., Dodson M.C., Rosenfield D., Jouriles E.N. Effects of a parenting intervention on features of psychopathy in children. J. Abnorm. Child Psychol. 2011;39(7):1013–1023. doi: 10.1007/s10802-011-9512-8. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Kushner H., Metzger D., Peters R., Smith I., Grissom G.…Argeriou M. The fifth edition of the Addiction Severity Index. J. Subst. Abus. Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. Oxford University Press; 2000. Principles of Behavioral and Cognitive Neurology. [Google Scholar]
- Muñoz L.C. Callous-unemotional traits are related to combined deficits in recognizing afraid faces and body poses. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(5):554–562. doi: 10.1097/CHI.0b013e31819c2419. [DOI] [PubMed] [Google Scholar]
- Mychack P., Kramer J.H., Boone K.B., Miller B.L. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(Suppl. 4):S11–S15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- O'Neill M.L., Lidz V., Heilbrun K. Adolescents with psychopathic characteristics in a substance abusing cohort: Treatment and process outcomes. Law Hum. Behav. 2003;27:299–313. doi: 10.1023/a:1023435924569. [DOI] [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M.…Xian H. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pihet S., Suter M., Meylan N., Schmid M. Factor structure of the youth psychopathic traits inventory: using the total score, three scale scores, and/or 10 subscale scores. Crim. Justice Behav. 2014;41(10):1214–1231. [Google Scholar]
- Raine A., Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc. Cogn. Affect. Neurosci. 2006;1(3):203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J.V., Frick P.J., Thornton L.C., Steinberg L., Cauffman E. Positive and negative item wording and its influence on the assessment of callous-unemotional traits. Psychol. Assess. 2016;28(4):394. doi: 10.1037/pas0000183. [DOI] [PubMed] [Google Scholar]
- Rogers J.C., De Brito S.A. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry. 2016;73(1):64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Joliot M., Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47(5):633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C., Ciaramidaro A., Selivanova M., Tournay J., Freitag C.M., Siniatchkin M. Neural correlates of affective empathy and reinforcement learning in boys with conduct problems: fMRI evidence from a gambling task. Behav. Brain Res. 2017;320:75–84. doi: 10.1016/j.bbr.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., McCrory E.J., Cecil C.A., Lockwood P.L., De Brito S.A., Fontaine N.M., Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch. Gen. Psychiatry. 2012;69(8):814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., De Brito S.A., McCrory E.J., Hyde Z.H., Lockwood P.L., Cecil C.A., Viding E. Grey matter volumes in children with conduct problems and varying levels of callous-unemotional traits. J. Abnorm. Child Psychol. 2016;44(4):639–649. doi: 10.1007/s10802-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M., Neuhaus J., Ketelle R., Stanley C.M., Beckman V., Growdon M.…Rankin K.P. Interpersonal traits change as a function of disease type and severity in degenerative brain diseases. J. Neurol. Neurosurg. Psychiatry. 2011;82(7):732–739. doi: 10.1136/jnnp.2010.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain S.E., Douglas K.S., Poythress N.G., Epstein M. The relationship between psychopathic features, violence and treatment outcome: the comparison of three youth measures of psychopathic features. Behav. Sci. Law. 2004;22(1):85–102. doi: 10.1002/bsl.576. [DOI] [PubMed] [Google Scholar]
- Sterzer P., Stadler C., Poustka F., Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. NeuroImage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Sylvers P.D., Brennan P.A., Lilienfeld S.O. Psychopathic traits and preattentive threat processing in children a novel test of the fearlessness hypothesis. Psychol. Sci. 2011;22(10):1280–1287. doi: 10.1177/0956797611420730. [DOI] [PubMed] [Google Scholar]
- Thompson S.A., Patterson K., Hodges J.R. Left/right asymmetry of atrophy in semantic dementia Behavioral–cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., Lockwood P.L., Cecil C.A., De Brito S.A., McCrory E.J. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am. J. Psychiatr. 2012;169(10):1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yücel M., Simmons J.G., Whittle S. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016;37(6):2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.L., White S.F., Robustelli B., Sinclair S., Hwang S., Martin A., Blair R.J.R. Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(4):456–465. doi: 10.1016/j.jaac.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1997. WAIS-III: Wechsler Adult Intelligence Scale. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) [Google Scholar]
- White S.F., Marsh A.A., Fowler K.A., Schechter J.C., Adalio C., Pope K.…Blair R.J.R. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am. J. Psychiatr. 2012;169(7):750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.F., Frick P.J., Lawing K., Bauer D. Callous–unemotional traits and response to functional family therapy in adolescent offenders. Behavioral Sciences & the Law. 2013;31(2):271–285. doi: 10.1002/bsl.2041. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T.…Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Narr K.L., Baker L.A., Joshi S.H., Jahanshad N., Raine A., Thompson P.M. Frontal and striatal alterations associated with psychopathic traits in adolescents. Psychiatry Res. Neuroimaging. 2015;231(3):333–340. doi: 10.1016/j.pscychresns.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Moll J., Iyengar V., Huey E.D., Tierney M., Krueger F., Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132(3):604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GMD Findings from Primary Analysis
Secondary Imaging Analysis