Abstract
There is a need for a rapid, robust, and sensitive biosensor to identify low concentrations of pathogens in their native sample matrix without enrichment or purification. Nucleic acid-based detection methods are widely accepted as the gold standard in diagnostics, but robust detection of low concentrations of pathogens remains challenging. Amplified nucleic acids produce more viscous solutions, which can be measured by combining these products with fluorescent particles and measuring the change in the particle diffusion coefficient using a technique known as particle diffusometry. Here, we utilize Vibrio cholerae (V. cholerae) as a proof-of-concept for our detection system due to its inherently low concentration in environmental water samples. We demonstrate that particle diffusometry can be used to detect down to 1 V. cholerae cell in molecular-grade water in 20 minutes and 10 V. cholerae cells in pond water in just 35 minutes in 25 µL reaction volumes. The detection limit in pond water is environmentally relevant and does not require any enrichment or sample preparation steps. Particle diffusometry is 10-fold more sensitive than current gold standard fluorescence detection of nucleic acid amplification. Therefore, this novel measurement technique is a promising approach to detect low levels of pathogens in their native environments.
Introduction
Environmental pathogen detection presents unique challenges in the development of novel biosensors due to the exceedingly low concentrations of pathogens in their native environments. For example, despite surviving at only 100 cells/mL in environmental water sources, the Vibrio cholerae (V. cholerae) bacteria that causes the devastating diarrheal disease cholera, leads to over 150,000 deaths worldwide each year1,2. Further, the current gold standard for the detection of V. cholerae in water sources is an 8-hour process involving bacteria enrichment and culture followed by polymerase chain reaction (PCR)3. Despite being one of the most sensitive laboratory detection methods, PCR is still not sensitive or robust enough to directly detect V. cholerae from the environment1. Hence, there is a need for a biosensor that can rapidly detect pathogens, such as V. cholerae, in their native environments.
Next generation mechanical, electrical, or optical signal transducers for biosensing have the potential to detect pathogens and biomolecular species with high sensitivity. For example, mechanical micro and nano-cantilever systems have been used extensively to detect E. coli in the range of 1–100 cells/mL4–8 and B. anthracis at less than 300 cells/mL4,9–11. Additionally, electrical and electrochemical transducers, such as impedance-based sensing of carbon nanotubes where the signal change is caused by E. coli binding to the surface, have been shown to have a limit of detection (LOD) of 50 colony forming units (cfu)/mL12. Further, optical and spectroscopic-based biosensing techniques have been used to achieve highly sensitive detection of pathogens in complex samples such as mixed cultures or food matrices. As few as 10 cfu/mL of E. coli, S. typhimurium, and S. aureus have been detected using surface enhanced Raman spectroscopy (SERS)13,14. Dark-field microscopy techniques that detect light scattered from nanoparticles also show promise in pathogen detection applications. For instance, gold nanoparticles were functionalized with antibodies against E. coli surface antigens and imaged. A color and shape analysis algorithm was applied to the E. coli dark-field images to detect as little as 104 cfu/mL of bacteria in only 30 minutes15. As promising as these technologies are, no single technique overcomes all of the challenges incurred in pathogen identification. In particular, these methods require extensive pre-processing techniques to purify or label samples prior to detection. Indeed, designing an integrated biosensor that rapidly detects pathogens at a low limit of detection in the presence of complex sample matrices continues to be a primary goal in biosensor development16–18.
Due to their exquisite sensitivity, nucleic acid amplification methods, such as PCR and loop-mediated isothermal amplification (LAMP), provide excellent target DNA enrichment for biosensor detection. LAMP is a particularly attractive DNA amplification method because it operates at a single temperature and provides rapid and robust amplification even in the presence of complex sample matrices19. Amplicon detection has been integrated into LAMP assays using fluorescent, visual, and electrochemical methods20–22. Okada et al. showed that visual detection of LAMP assays are robust enough to identify V. cholerae found in clinical rectal swabs23. The promising results from Okada et al. indicate that LAMP can be used for the detection of the low concentrations of V. cholerae in complex sample matrices. Indeed, we wish to employ LAMP for the development of an environmental-based biosensor for ultrasensitive detection of V. cholerae in water samples. Taking advantage of the primer design from Okada et al., we have developed a LAMP protocol for V. cholerae that is efficient (under 30-minute amplification), specific (targeting 6 different regions of the cholera toxin gene), and robust (usable in non-pretreated environmental water sources).
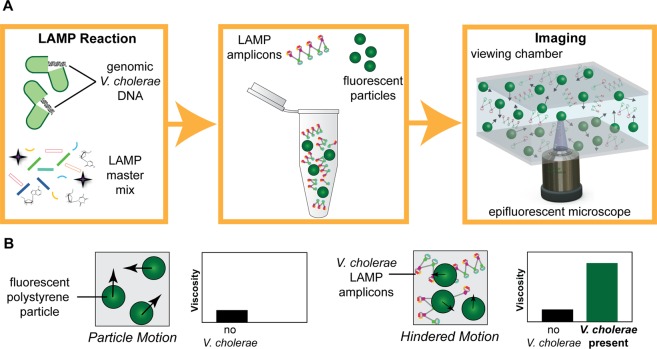
In this work, we develop a highly accurate and sensitive biosensor for the rapid detection of V. cholerae in environmental water sources by pairing LAMP with particle diffusometry (PD) (Fig. 1A). PD involves rapid optical measurements of particle Brownian motion24–26 following amplification. When V. cholerae DNA is present in the solution, the LAMP assay polymerizes DNA targets into a variety of base pair lengths up to 25 kilobases27. This polymerization causes the particle Brownian motion to decrease25. We can calculate this change in the particle Brownian motion with PD using correlation-based algorithms of the particle images24 (Fig. 1B). In this work, we show the applicability of PD-LAMP to detect the presence of V. cholerae. We can use PD-LAMP to successfully detect V. cholerae presence down to 1 cell/reaction, which is 100-fold more sensitive than fluorescence-based measurements and similar to the detection sensitivity of next-generation signal transducer-based biosensors. We further show robust and rapid V. cholerae detection in complex sample matrices down to 10 cells/reaction using PD-LAMP. With these results, we envision that PD-LAMP will enable rapid and sensitive detection of other pathogens at low concentrations in their native environment.
Figure 1.
Illustration of PD-LAMP set-up. (A) The LAMP assay is performed in the presence of V. cholerae DNA (left). LAMP amplicons combined with polystyrene fluorescent particles (middle) are imaged under fluorescence microscopy (right). (B) Relationship of particle motion and viscosity. Particles undergo Brownian motion in a solution (left). In the presence of LAMP amplicons, the viscosity of the solution increases and particles experience hindered motion, indicating the presence of V. cholerae DNA in the sample (right).
Results
PD-LAMP Blinded Study
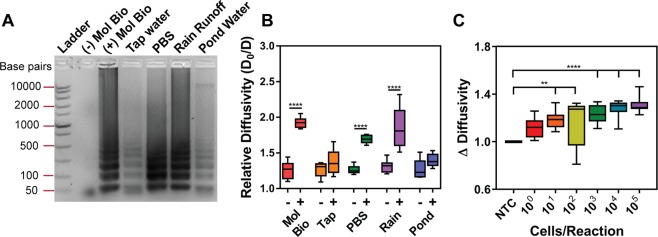
To validate that PD measurements of relative solution viscosity could be used to detect successful LAMP amplification, a series of blinded studies were performed. The sample containing the amplified genomic V. cholerae DNA was correctly identified with statistical significance (p-value < 0.0001) in every circumstance (n = 3). Data from one representative blinded study is presented in Fig. 2. The sample containing amplified genomic V. cholerae DNA had the greatest relative viscosity (, calculated from equation (3)) as compared to the control samples, meaning that the presence of polymerized LAMP amplicons in the solution increased the fluid viscosity (Fig. 2B). Successfully identifying V. cholerae LAMP amplicons in blinded studies demonstrated that viscosity measurements are a feasible approach in determining pathogen presence.
Figure 2.
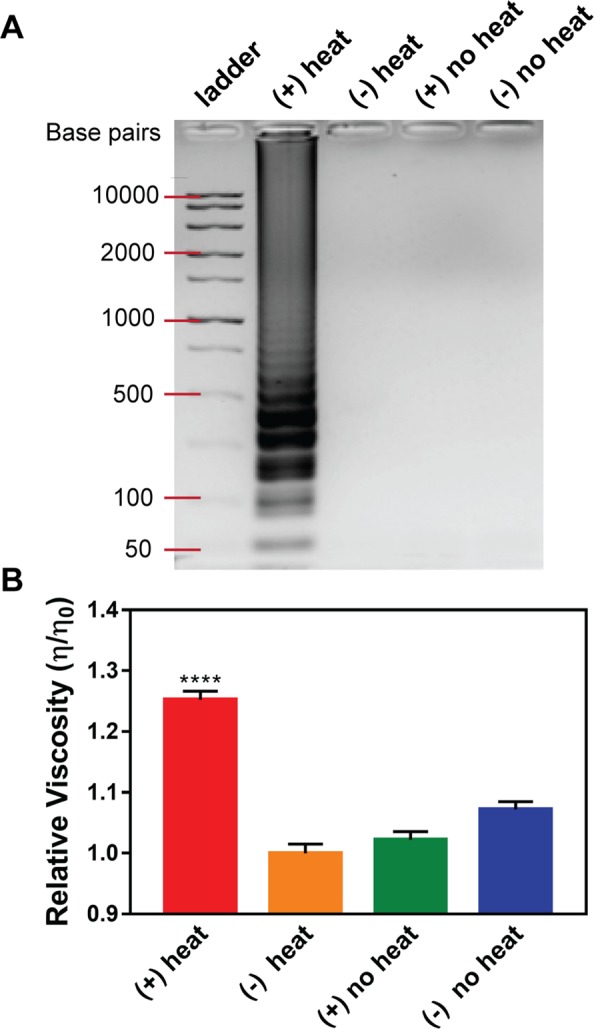
Relative viscosity blinded study. Here, genomic V. cholerae DNA that underwent the 65 °C heating of LAMP is represented as (+) heat, no genomic V. cholerae DNA that underwent the 65 °C heating of LAMP is (−) heat, genomic V. cholerae DNA that did not undergo heating is (+) no heat, and no genomic V. cholerae DNA that did not undergo heating is (−) no heat. (A) A 2% agarose gel of a representative blinded test to show DNA amplification in the positive sample. (B) Relative PD results show the experimental sample (+) heat is statistically significantly more viscous than control samples (****p-value < 0.0001). Nine PD measurements were made for each sample to allow for statistical comparison.
PD versus Fluorescence Measurements
LAMP was performed with genomic V. cholerae DNA at concentrations ranging from 100–105 DNA copies/reaction (n = 3, data of repeats in Fig. S1). As expected, real-time visualization of the change in fluorescence showed that LAMP samples with higher initial concentrations of genomic V. cholerae DNA amplify more rapidly (Fig. 3A). Samples with lower concentrations showed slower, if any, amplification and resulted in a lower fluorescence signal at the end of the 20-minute period (corresponding CT values are presented in Fig. 3B). Gel electrophoresis was used to confirm amplification of the DNA (Fig. 3C). The change in EvaGreen/Rox (), from the signal measured at 0 minutes and 20 minutes, was calculated by equation (4) (n = 3, Fig. 3D). The data indicated that a higher initial concentration of DNA corresponds with a greater signal at 20 minutes. We performed a one-way ANOVA with Dunnett’s post-hoc against the NTC sample and saw statistically significant differences for samples 102, 103, 104, and 105 DNA copies/reaction (p-value < 0.001 for 102 and p-value < 0.0001 for 103, 104, and 105 DNA copies/reaction).
Figure 3.
Detection of V. cholerae amplification using purified DNA. NTC represents no added V. cholerae DNA. (A) Real-time fluorescence was monitored over a 20-minute LAMP reaction for initial DNA concentrations between 100–105 DNA copies/reaction and (B) the corresponding CT values were recorded for each reaction and are not available (NA) for samples that did not amplify. (C) A 2% agarose gel confirms amplification and presents the DNA banding pattern of LAMP amplicons at the different dilutions. (D) Box plots of the average change in fluorescence () shows a trend of a greater change in fluorescence signal at higher initial V. cholerae DNA concentrations with statistical differences for samples 102 (***p-value < 0.001), 103, 104, and 105 (****p-value < 0.0001) DNA copies/reaction when compared to NTC. (E) Particle diffusometry measurements of the viscosity change of LAMP reactions show statistically significant measurements for 102 (*p-value < 0.05), 104, and 105 (****p-value < 0.0001) DNA copies/reaction when compared to NTC. (D,E) Statistical analysis was a one-way ANOVA with Dunnett’s post-hoc relative to NTC (n = 3). (F) A positive correlation between change in fluorescence and PD yields a Pearson’s correlation coefficient of 0.81.
PD was used to measure the change in the viscosity of V. cholerae DNA samples after the 20-minute LAMP reaction (data for individual repeats is presented in Fig. S1). Similar to the change in fluorescence, we found that as the initial concentration of V. cholerae DNA increased, there was a greater change in viscosity (, Fig. 3E, calculated with equation (4)). Like the fluorescence measurements, statistically significant differences were seen between the of NTC compared to 102, 104, and 105 DNA copies/reaction (p-value < 0.05 and p-value < 0.0001, respectively) (Fig. 3E).
Correlation between the (PD) and measurements confirmed agreement between the two methods. The correlation plot demonstrated in Fig. 3F with a calculated Pearson correlation coefficient (R) of 0.81 indicating that the two methods are strongly positively correlated with one another28. A slight discrepancy between measurements is expected since the polymerized DNA chains produced in each LAMP reaction vary in length29. Chain length has a major effect on solution viscosity30, and in turn the PD measurement25. However, the strong positive correlation between the two measurements demonstrated the feasibility of PD as a measurement technique for detection of V. cholerae.
Measuring the Combined Effect of Changes in Particle Size and Viscosity with PD-LAMP
Despite successful detection of as few as 104 DNA copies/reaction, we sought to improve the sensitivity of the of the PD measurements by combining detection of the change in viscosity with change in the hydrodynamic radius of the particles. Particle diffusivity (equation (2)) is a function of both viscosity of the solution (), and the size of the measured particles (). Similar to Tian et al.31, we used streptavidin conjugated polystyrene particles and biotinylated LAMP primer (LF) to bind the polymerized biotin-tagged DNA to the streptavidin polystyrene particles, creating multi-particle aggregates (Fig. 4A). First, we sought to experimentally validate that biotin-streptavidin induced aggregation occurs as a response only to V. cholerae DNA amplification in our system (Fig. 4B,C). In the negative sample (NTC), the streptavidin conjugated particles were uniformly distributed in the image (Fig. 4B, top). However, when particles were added to a positive sample after amplification, a cluster of particles was seen by the increase in fluorescence in regions of the image (Fig. 4B, bottom). Quantitative analysis showed that there was a narrower distribution of particle size around 10 pix2 in the negative samples and greater variability in the positive samples (Fig. 4C).
Figure 4.
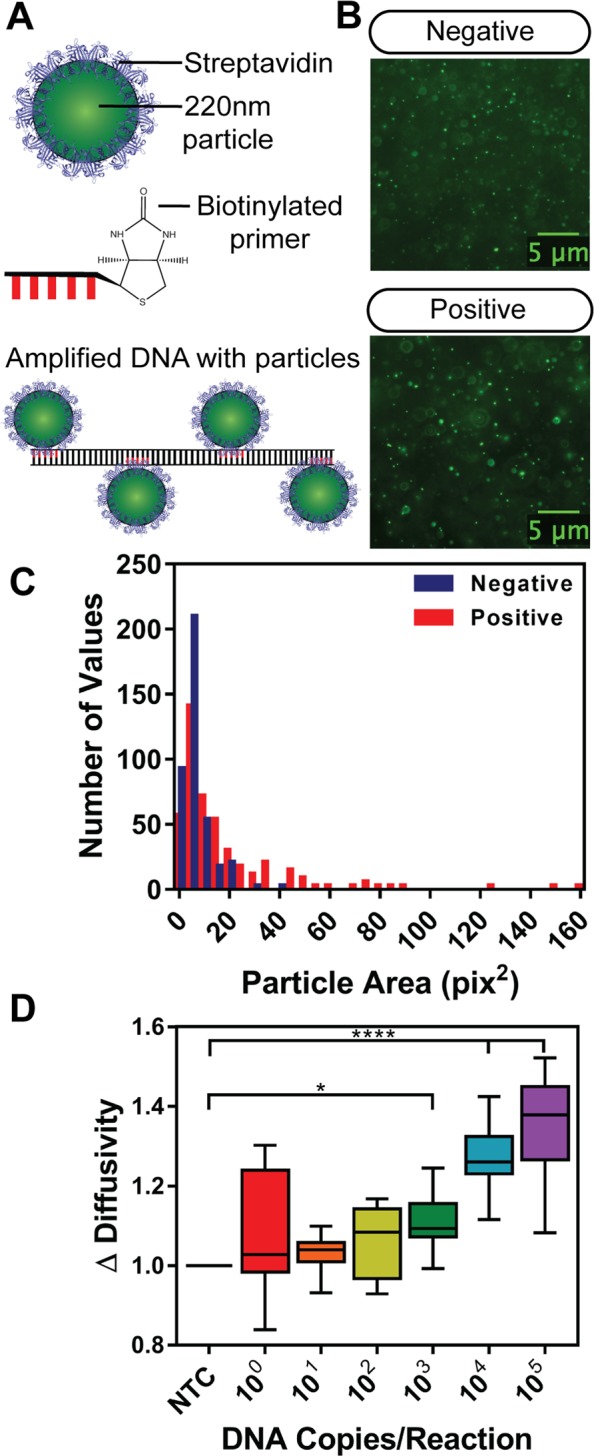
Biotinylated LAMP products measured using streptavidin conjugated particles. (A) Schematic of fluorescent streptavidin-coated polystyrene particles combined with DNA tagged with a biotinylated LAMP primer. (B) Representative image of fluorescent particles in a negative LAMP sample (top, no V. cholerae DNA). (C) Images in (B) are processed to quantify particle area (in pix2) for negative and positive LAMP reactions. (D) Measurement of diffusivity after amplification of various initial concentrations of V. cholerae DNA is statistically significant for 103 (*p-value < 0.05), 104, and 105 (****p-value < 0.0001) DNA copies/reaction compared to NTC. Statistical analysis was a one-way ANOVA with Dunnett’s post-hoc relative to NTC (n = 4).
To quantify the relative change in particle motion upon changes in both viscosity and particle size, we measured the change in diffusivity, , instead of the change in viscosity, (equation (4)). We measured the in samples containing 100–105 DNA copies/reaction (n = 4, data of repeats in Fig. S2). The data showed a similar trend as observed in the uncoated beads; a higher initial DNA concentration leads to a higher measurement (Fig. 4D). Further, there was an increase in the baseline relative diffusivity when streptavidin conjugated particles were used compared to the uncoated 200 nm polystyrene beads (comparing the y-axis in Fig. 3E to Fig. 4D in which is directly proportional to ). This baseline increase occurred as both the particle size and solution viscosity were considered (equation (2)). There was a statistically significant difference for the 103, 104 and 105 DNA copies/reaction samples (p-value < 0.05 and p-value < 0.0001, one-way ANOVA with a post-hoc Dunnett’s test against NTC).
We wanted to determine if changes in diffusivity could be measured after amplification of DNA from whole cells. The cell lysate contained extra proteins that may potentially alter solution viscosity or cause changes in particle stability. It is important to note that V. cholerae cells, like other gram-negative bacteria, lysed due to thermal effects alone at 65 °C32, such that an additional cell lysis step beyond the LAMP assay was not necessary (validated in Fig. S3). LAMP assays with biotinylated primers and varying concentrations of whole V. cholerae cells (100–105 cells/reaction) were performed and the of streptavidin particles was measured with PD. As the initial concentration of V. cholerae cells increased, the and of the solutions also increased (Fig. 5). Real-time fluorescence curves and for each repeat are presented in Fig. S4. There was a statistically significant difference in the down to 100 cells/reaction when compared to NTC (p-value < 0.01). In contrast, the measurements showed statistically significant differences down to 102 cells/reaction when compared to NTC (p-value < 0.01). Interestingly, the PD-LAMP assay detected V. cholerae an order of magnitude lower using whole cells versus purified DNA (compare Figs 5B with 4D). These results are in agreement with Linnes et al. showing a 10-fold lower limit of detection when using isothermal amplification on whole cell Chlamydia trachomatis samples as compared to purified corresponding DNA33. Relative to fluorescence measurements, the PD measurements were 100-fold more sensitive (comparing Fig. 5A,B). This is promising in the implementation of PD as a pathogen detection technique considering that environmental samples collected for testing would contain very low concentrations of V. cholerae cells.
Figure 5.
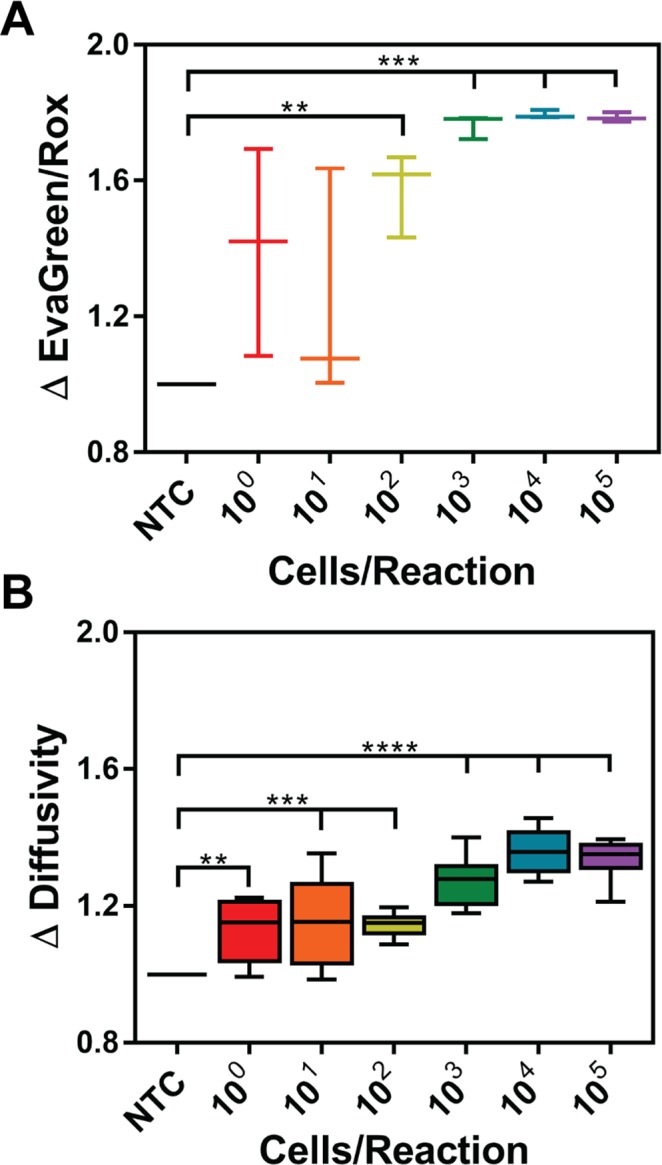
Measuring LAMP amplification from V. cholerae whole cells. Cells were spiked into LAMP reactions at concentrations ranging from 100–105 cells/reaction. The change in (A) EvaGreen/Rox is significant at 102 (**p < 0.01), 103, 104, and 105 (***p < 0.001) cells/reaction. (B) The change in diffusivity measurements show an increasing trend as a function of starting cell concentration with significance at 100 (**p < 0.01), 101, 102 (***p < 0.001), 103, 104, and 105 (****p < 0.0001) cells/reaction. Statistical analysis was a one-way ANOVA with Dunnett’s post-hoc relative to NTC (n = 3).
Environmental Water Sources
V. cholerae is a pathogen found in environmental water sources34,35, thus it is essential to perform PD-LAMP on cells in complex matrices other than molecular biology water. PD-LAMP was performed in 1X PBS, tap water, rain runoff, and pond water (and molecular biology water as a control) with 105V. cholerae cells spiked into each 25 µL reaction (positive samples) or with no cells (negative control) for each water source. These water sources presented three potential challenges for accurate PD detection of DNA amplification: (1) inhibition of the LAMP assay, (2) adverse effects to the particles during the measurement including degradation or aggregation, and (3) unaccounted changes that may occur due to excess particulates that may non-specifically bind to the particles by increasing apparent size or create viscosity changes.
Gel electrophoresis (Fig. 6A) and quantitative fluorescence measurements (Fig. S5) showed little DNA amplification in tap water. We measured the relative diffusivity using PD to investigate this difference (, equation (3), repeat data presented in Fig. S5). There was no statistically significant difference in relative diffusivity of positive samples in tap water compared to the negative controls (Fig. 6B, p-value > 0.05, student t-test). This was to be expected because tap water contains chlorine, which likely inhibited the activity of the Bst 2.0 enzyme required for the LAMP assay36.
Figure 6.
LAMP amplification from whole V. cholerae cells in environmental water sources. Different water sources were used in the LAMP reactions for (A) and (B). Molecular biology water (Mol Bio) was used as a control ((−) no V. cholerae cells, (+) V. cholerae cells) for each water source in (A) and (B). (A) Gel electrophoresis shows less DNA amplification in pond and tap water groups compared to other water sources. (B) PD measurement of relative diffusivity shows a statistically significant difference between negative and positive samples for molecular biology water, PBS, and rain runoff groups (****p-values < 0.0001). (C) V. cholerae cells were spiked into pond water at concentrations ranging from 100–105 cells/reaction. The change in diffusivity measurements show an increasing trend as a function of starting cell concentration with significance at 101, 102 (**p < 0.01), 103, 104, and 105 (****p < 0.0001) cells/reaction. Statistical analysis was a one-way ANOVA with Dunnett’s post-hoc relative to NTC (n = 3).
Since V. cholerae can be harbored in sea water, we performed LAMP in 1X PBS to determine whether salt affects the LAMP assay or PD measurements. LAMP assays performed in 1X PBS did not show any inhibition when analyzing both fluorescence measurements and gel electrophoresis (Figs 6A and S5). Similarly, there was a statistically significant difference in PD measurements (p-value < 0.0001, student t-test) between the negative and positive PBS samples. This confirmed that salt content did not inhibit the LAMP assay or PD measurements (Fig. 6B).
Using PD, we analyzed LAMP assays performed in rain runoff and pond water, both which contained sediment and therefore added to sample matrix complexity. PD measurements showed a statistically significant difference in the relative diffusivity of rain runoff samples with V. cholerae cells compared to its negative control (Fig. 6B, p-value < 0.0001, student t-test). This is extremely promising given that the water source was collected outside of the laboratory space and potentially contained particulates that inhibit the LAMP reaction. In contrast, LAMP assays in pond water demonstrated a decrease in amplification signal (Fig. S5). This was supported with the faded banding pattern in the agarose gel (Fig. 6A). Due to the minimal amplification, PD reflected little-to-no change in the diffusivity between the negative and positive V. cholerae samples in pond water (Fig. 6B, p-value > 0.05, student t-test).
Further characterization of PD-LAMP in pond water was performed, as this source is the best surrogate of the native environment for V. cholerae37,38. LAMP assays with biotinylated primers and varying concentrations of whole V. cholerae cells (100–105 cells/reaction) were performed in pond water (where 50% of the total LAMP reaction volume was pond water). The of streptavidin particles in the presence of the LAMP reaction products was measured with PD. Because the debris and ions in pond water slightly inhibit nucleic acid amplification reactions39, the LAMP assay was run for 35 minutes. As the initial concentration of V. cholerae cells increased, the and of the solutions also increased (Fig. 6C). There was a statistically significant difference in the down to 10 cells/reaction when compared to NTC (p-value < 0.01). Real-time fluorescence curves and for each repeat are presented in Fig. S6. In contrast, the measurements showed statistically significant differences down to 102 cells/reaction when compared to NTC (p-value < 0.05) (Fig. S7). Relative to fluorescence measurements, the PD measurements were 10-fold more sensitive (comparing Figs 6C to S7).
Discussion
In this work, we demonstrate that PD-LAMP can be used as a rapid, sensitive, and robust method for the detection of V. cholerae in environmental water samples. In blinded studies PD-LAMP could detect the presence of V. cholerae DNA with 100% accuracy (Fig. 2B). Additionally, there is a strong, positive correlation (R = 0.81) between (quantitative fluorescence) and (PD measurements). Our studies indicate that PD-LAMP can detect as few as 1 V. cholerae cell/reaction in molecular-grade water (Fig. 5B) which is 100-fold more sensitive than gold standard fluorescence measurements1,40. Furthermore, we demonstrate that PD-LAMP is robust enough to detect down to 10 cells/reaction of V. cholerae DNA from cells lysed in situ during a 35-minute reaction in pond water without the need for additional sample preparation (Fig. 6C). This detection method is 10-fold more sensitive than current gold standard fluorescence detection of nucleic acid amplification. This is the first study directly comparing fluorescence detection and the novel PD-LAMP method. We have demonstrated that PD-LAMP offers at least a 10-fold increase in sensitivity over fluorescence detection. These results establish the utility of combining both changes in size and viscosity for improved signal-to-noise measurements with PD-LAMP for the rapid detection of V. cholerae. PD is an alternate method to fluorescence detection for nucleic acid amplification products that has a significant improvement in sensitivity and is robust enough to detect the amplified products in their native sample matrix.
PD-LAMP as a biosensor is pathogen agnostic; it is not limited to V. cholerae identification, but it can serve as a platform for the detection of a wide variety of pathogens. Due to the success in detecting V. cholerae, we envision PD-LAMP as an effective detection method to identify pathogenic DNA for other infectious diseases including E. coli and salmonella. PD-LAMP is particularly attractive due to the passive nature of the detection method (i.e. optically detecting Brownian motion) compared to current DNA detection techniques that require chemical reactions involving fluorophore intercalation, colorimetric, or turbidimetric readouts derived from magnesium products, or electrochemical techniques. Future development of PD-LAMP would involve designing the biosensor as a handheld device. This is plausible considering that PD-LAMP involves only a microscope, camera, and computer. Miniaturization and integration of these components would allow for the translation of a field deployable biosensor for pathogen detection.
Methods
Bacteria Culture
The V. cholerae strain N16961, a toxigenic O1 serogroup, was provided by Dr. Afsar Ali, from the Department of Environmental and Global Health at the University of Florida. All cultures were grown aerobically in Lysogeny Broth (LB) overnight at 37 °C using a miniature incubating shaker at 300 rpm (Thermo Fisher, Waltham, MA). Cultures were diluted in LB media to an OD600 of 1, (Ultrospec 10, Biochrom, Cambourne, UK) representing 6 × 108 cells/mL of V. cholerae as determined by counting colony forming units of serially diluted samples.
Loop-Mediated Isothermal Amplification (LAMP)
Purified genomic DNA from V. cholerae N16961 (American Type Culture Collection 39315D-5, Manassas, VA) was maintained in 2.2 ng/µL aliquots for use in preliminary testing and at specified concentrations in experiments thereafter. LAMP primers were devised to target the cholera toxin A (ctxA) gene within the toxigenic V. cholerae strain23. There is one ctxA gene copy per genome. The primer sequences are provided in Table S1 Supplemental. For all amplification experiments, 25 µL reactions consisted of 23 µL of LAMP master mix (components shown in Table S2 Supplemental). 2 µL of sample (purified V. cholerae DNA or whole cells) or negative control (molecular biology water (Invitrogen, Carlsbad, CA)) was added just prior to heating. LAMP was performed at 65 °C for 20, 25, or 35 minutes using an Applied Biosystems 7500 Real-Time PCR System (Foster City, CA), and then the samples were stored at 4 °C until analyzed with PD.
LAMP was performed with V. cholerae purified genomic DNA or whole cells. 10-fold serial dilutions of template (both DNA and cells) were prepared for experimentation (100 to 105 copies of DNA or cells per reaction) in molecular biology water. Real-time fluorescent data was collected for each experiment to visually track the amplification progress. LAMP amplicons were also characterized via gel electrophoresis using a 2% agarose gel at 100 V for 60 minutes, stained with ethidium bromide, and imaged using an ultraviolet light gel imaging system (c400, Azure Biosystems, Dublin, CA). All gel images were collected using the Azure cSeries software and settings of UV302 with an exposure time of 15 seconds. The gel electrophoresis images, exported as JPG files, were not cropped nor edited.
Particle Preparation
For viscosity measurements, red fluorescent 200 nm polystyrene particles (Fluoro-max red dyed aqueous spheres, Thermo Scientific, Erie, NY, USA) were combined with the LAMP products. Polystyrene particles were chosen due because they are similar in density to water, making them relatively neutrally buoyant, allowing the effects of gravity to remain negligible for particle diffusometry measurements. Further, the 200 nm particle size was chosen to achieve more sensitive measurements as smaller particles exhibit greater changes in diffusivity These particles were washed three times in MilliQ water by centrifugation at 13,000 x g for 15 minutes. Following, the particles were added to the LAMP products at a final concentration of 6 × 109 particles/mL and stored at 4 °C until imaging.
For combined size and viscosity measurements (i.e. diffusivity), streptavidin coated 220 nm green polystyrene fluorescent particles were used (Bangs Labs, Fishers, IN, USA) to maintain a particle diameter as close as possible to the 200 nm unmodified polystyrene particles used for viscosity measurements. Due to supplier constraints, 220 nm was the nearest particle diameter with streptavidin-modified surface chemistry. Particles were washed three times in MilliQ water by centrifugation at 13,000 x g for 15 minutes. Washed particles were added to the LAMP products at a final concentration of 1.49 × 109 particles/mL (note that the final concentrations of the 200 and 220 nm particle samples differ due to their size to eliminate hindered diffusion). Particles and LAMP amplicons were incubated at 4 °C by gentle rotation for two hours to allow binding of the biotinylated LF primer to the streptavidin particles and then imaged.
Particle Diffusometry Theory
PD involves recording a series of images of fluorescent particles undergoing Brownian motion in a quiescent volume and calculating the particle diffusion coefficient using correlation analysis24. Each individual image is partitioned into smaller interrogation areas where the size of each interrogation area is defined such that 8–10 particles, on average, are present41. Within these interrogation areas, autocorrelations and cross-correlations of the images are computed for the entire image stack. Cross-correlation involves correlating two sequential images, for example taken at time and at time (where is a function of the frame rate). Greater particle displacement, during the elapsed time , creates broader cross-correlation peaks42. The cross-correlation peak width, (pixels) at a height of , is used to calculate the diffusion coefficient43. Further, autocorrelation is performed by correlating an image captured at time with itself. The autocorrelation peak width, at a height of , is taller and narrower when compared to the cross-correlation peak42. With autocorrelation and cross-correlation, the diffusion coefficient can be calculated by the equation derived by Olsen and Adrian44:
| 1 |
where M is the magnification of the microscope objective. Because the peak width is in units of pixels, using equation (1), we see that the squared difference in the peak widths, , corresponds to the change in the cross-sectional area of the correlation peak at . By experimentally determining the diffusion coefficient, D, from the series of particle images, the Stokes-Einstein relationship can be algebraically rearranged (equation (2)) to calculate the viscosity, η, of a solution45,46.
| 2 |
Here, is the Boltzmann constant, is the absolute temperature, and is the hydrodynamic radius of fluorescent spheres that are imaged. It is important to note that smaller diameter particles will provide a greater signal-to-noise ratio in solutions where there are only modest changes in viscosity.
We are specifically interested in characterizing how the presence of LAMP amplicons affects diffusivity of particles for pathogen detection. The change in diffusivity in this context are due to changes in solution viscosity and/or particle size. Therefore, we compute the relative solution viscosity or the combined relative size and solution viscosity, forgoing the magnitude in either case. Algebraic manipulation of equation (2), where is the viscosity of the buffer solution without the LAMP amplicons (but still including LAMP primers and fluorescent particles), produces the relative viscosity ().
| 3 |
Further, the equation (2) can be manipulated to include relative size. In equation (3), is the size of nanoparticles where no DNA is amplified and is the size of the particles after amplification. This approach is optimal in binary situations, where an investigator is interested in the presence or absence of pathogens in a solution.
Sample-to-sample variation often occurs in the quantitative measurements of LAMP assays due to the polymerization process29. Therefore, when measuring the change in the diffusion coefficient as a function of the concentration of LAMP amplicons, we calculate the change () in the signal. This approach is used when comparing real-time LAMP fluorescence measurements with PD viscosity measurements. In equation (4) the signal change (, either fluorescence or viscosity) is a function of the signal after amplification () and before amplification ().
| 4 |
Experimental Particle Diffusometry Measurements
A fluid well for the LAMP-particle solution was made by punching a 6 mm diameter hole (120 μm thickness) through double-sided adhesive (Therm-O-Web, Wheeling, IL, USA) which was then adhered to a cover glass slide (Thickness No. 1, Thermo Scientific, Erie, NY, USA). The 3 μL LAMP-particle solution was added to the fluid well and sealed with a second cover glass slide to limit convective evaporation during imaging.
The LAMP-particle solutions were imaged at room temperature using an inverted fluorescence microscope (Nikon TE-2000U, Nikon, Japan) equipped with an X-cite lamp and 40X magnification objective using PCO Camware software (PCO, Kelheim, Germany). Images were recorded using a PCO 1600 CCD camera (PCO, Kelheim, Germany) using a 802 × 802 pixel2 imaging window with 2 × 2 binning at 13.3 fps. Individual pixels were 7.4 × 7.4 μm2. For imaging of the 200 nm red polystyrene spheres, a Q-Dot 585 filter cube was used (Chroma, Bellows Falls, VT) and for the 220 nm green polystyrene spheres, a B3-A filter cube was used (Nikon, Japan).
Particles were imaged at the mid-plane of the chip to ensure the effects of hindered diffusion caused by the proximity of particles to any wall was avoided. We analyzed particles which were located in the depth of correlation, 4.2 μm, by using an expression derived by Meinhart et al.47,48. Therefore, particles located within the depth of correlation form the correlation function and the remainder of particles contribute to the background signal.
Particle images were processed and auto- and cross-correlation analysis was performed using an in-house MATLAB code. 64 × 64 pixel2 interrogation areas contain, on average, 8–10 particles per 100 image frame stacks (~8 seconds of data). This allowed for a high signal-to-noise ratio while maintaining a statistically relevant number of data points. Nine measurements, of which 100 images constituted one measurement, were performed for every sample. A two-dimensional Gaussian curve fit was used to calculate the auto- and cross-correlation peak widths for both the XZ- and YZ-planes. The width of the correlation peak is defined by and the width of the XZ- and YZ-Gaussian curves are averaged as one peak width value43.
Blinded Study
Control samples contained (1) no V. cholerae DNA that underwent the 65 °C heating for 20 minutes ((−) heat), (2) genomic V. cholerae DNA that did not undergo heating ((+) no heat), and (3) no V. cholerae DNA that did not undergo heating ((−) no heat). The experimental sample contained genomic V. cholerae DNA and was amplified at 65 °C for 20 minutes ((+) heat). The four samples were unknown to the researcher performing the PD testing in order to obtain unbiased measurements. The four samples contained 200 nm red fluorescent unmodified polystyrene spheres that were added to the samples after amplification and were imaged under fluorescence microscopy (data in Table S3 Supplemental). Data was represented in terms of relative viscosity (, equation (3)).
Water Testing
All LAMP reactions in various water sources were prepared using the standard master mix reagents with the biotinylated LF primer and were performed for 25 minutes. The different water sources were 50% by volume of the LAMP reaction. Water sources included laboratory tap water, autoclaved 1X phosphate buffered-saline (PBS) (Sigma-Aldrich, St. Louis, MO) pH 7.4, rain runoff, pond water, and molecular biology water. Rain runoff and pond water collected from the bank of a small stagnant pond were stored at 4 °C until LAMP was performed. In the experiments that investigated the limit of detection of whole V. cholerae cells in pond water, the LAMP assay was run for a total of 35 minutes due to the slight delay caused by the inhibitors in pond water39.
Statistical Analysis
The blinded test groups were statistically analyzed with a one-way ANOVA with multiple comparisons using a 95% confidence interval, comparing data represented in terms of relative viscosity (, equation (3)). In measuring the 10-fold dilutions and determining the LOD, data was represented in terms of or (equation (4)). When comparing a series of 10-fold dilutions, a one-way ANOVA post-hoc Dunnett’s test was performed with multiple comparisons against a negative control with no template (no template control, NTC) with a 95% confidence interval. To compare and , the Pearson’s correlation coefficient was calculated. A Student’s paired t-test with a 95% confidence interval was used when comparing the negative control and positive samples in different water types, with data again represented in terms of relative viscosity (, equation (3)). Box-and-whisker plots were made for the PD and fluorescent data for the 10-fold dilutions where the upper and lower bounds represent the 75th and 25th percentile about the median, respectively, and the minimum and maximum values represented by the upper and lower whiskers.
Supplementary information
Acknowledgements
This work was supported by the Vodafone Americas Foundation Wireless Innovation Project Award and the Purdue Global Engineering Programs Innovations in International Development Lab (I2D) (TKU and JCL) as well as the Whitaker Foundation (TJM). Additionally, the authors would like to thank Dr. Afsar Ali from the Department of Environmental and Global Health at the University of Florida for providing the Vibrio cholerae cells.
Author Contributions
K.N.C. designed and performed PD experiments, contributed to the design of LAMP experiments, and analyzed the data. T.J.M. designed and performed LAMP experiments, performed cell culturing, and analyzed data. D.L. performed PD experiments. K.N.C. and T.J.M. wrote the manuscript with contributions from S.T.W., J.C.L., and T.K.U. Further, S.T.W., J.C.L. and T.K.U. provided guidance on modeling of the experiments.
Competing Interests
Drs Clayton, Kinzer-Ursem, Linnes, and Wereley are co-founders of OmniVis, LLC and own stock in the company. Dr. Clayton currently is employed by OmniVis, LLC. Taylor Moehling and Dong Hoon Lee declare no potential conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Katherine N. Clayton and Taylor J. Moehling contributed equally.
Contributor Information
Jacqueline C. Linnes, Email: jlinnes@purdue.edu
Tamara L. Kinzer-Ursem, Email: tursem@purdue.edu
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38056-7.
References
- 1.Alam, M. T. et al. Increased Isolation Frequency of Toxigenic Vibrio cholerae O1 from Environmental Monitoring Sites in Haiti. PLoS One10 (2015). [DOI] [PMC free article] [PubMed]
- 2.WHO|Number of cholera cases. World Health Organization (2016).
- 3.Wang D, et al. Detection of Vibrio cholerae O1 and O139 in environmental water samples by an immunofluorescent-aggregation assay. Appl. Environ. Microbiol. 2010;76:5520–5525. doi: 10.1128/AEM.02559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell GA, deLesdernier D, Mutharasan R. Detection of airborne Bacillus anthracis spores by an integrated system of an air sampler and a cantilever immunosensor. Sensors Actuators B Chem. 2007;127:376–382. doi: 10.1016/j.snb.2007.04.038. [DOI] [Google Scholar]
- 5.Campbell GA, Mutharasan R. Detection of pathogen Escherichia coli O157:H7 using self-excited PZT-glass microcantilevers. Biosens. Bioelectron. 2005;21:462–473. doi: 10.1016/j.bios.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Ilic B, Craighead HG. Topographical Patterning of Chemically Sensitive Biological Materials Using a Polymer-Based Dry Lift Off. Biomed. Microdevices. 2000;2:317–322. doi: 10.1023/A:1009911407093. [DOI] [Google Scholar]
- 7.Maraldo D, Mutharasan R. 10-minute assay for detecting Escherichia coli O157:H7 in ground beef samples using piezoelectric-excited millimeter-size cantilever sensors. J Food Prot. 2007;70:1670–1677. doi: 10.4315/0362-028X-70.7.1670. [DOI] [PubMed] [Google Scholar]
- 8.Maraldo D, Mutharasan R. Preparation-free method for detecting Escherichia coli O157:H7 in the presence of spinach, spring lettuce mix, and ground beef particulates. J Food Prot. 2007;70:2651–2655. doi: 10.4315/0362-028X-70.11.2651. [DOI] [PubMed] [Google Scholar]
- 9.Campbell GA, Mutharasan R. Method of measuring Bacillus anthracis spores in the presence of copious amounts of Bacillus thuringiensis and Bacillus cereus. Anal. Chem. 2007;79:1145–1152. doi: 10.1021/ac060982b. [DOI] [PubMed] [Google Scholar]
- 10.Davila AP, et al. Microresonator mass sensors for detection of Bacillus anthracis Sterne spores in air and water. Biosens. Bioelectron. 2007;22:3028–3035. doi: 10.1016/j.bios.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Fu L, Barbaree JM, Cheng Z-Y. Resonance behavior of magnetostrictive micro/milli-cantilever and its application as a biosensor. Sensors Actuators B Chem. 2009;137:692–699. doi: 10.1016/j.snb.2009.01.038. [DOI] [Google Scholar]
- 12.Zhou, Y. & Ramasamy, R. P. Phage-based Electrochemical Biosensors for Detection of Pathogenic Bacteria (2015).
- 13.Craig AP, Franca AS, Irudayaraj J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013;4:369–380. doi: 10.1146/annurev-food-022811-101227. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lee K, Irudayaraj J. Silver nanosphere SERS probes for sensitive identification of pathogens. J. Phys. Chem. C. 2010;114:16122–16128. doi: 10.1021/jp1015406. [DOI] [Google Scholar]
- 15.Xu, X. et al. Counting Bacteria Using Functionalized Gold Nanoparticles as the Light-Scattering Reporter (2012). [DOI] [PubMed]
- 16.Fujioka R, Sian-Denton C, Borja M, Castro J, Morphew K. Soil: the environmental source of Escherichia coli and Enterococci in Guam’s streams. J. Appl. Microbiol. 1998;85:83–89. doi: 10.1111/j.1365-2672.1998.tb05286.x. [DOI] [PubMed] [Google Scholar]
- 17.Waswa J, Irudayaraj J, DebRoy C. Direct detection of E. Coli O157:H7 in selected food systems by a surface plasmon resonance biosensor. LWT - Food Sci. Technol. 2007;40:187–192. doi: 10.1016/j.lwt.2005.11.001. [DOI] [Google Scholar]
- 18.Ahmed A, Rushworth JV, Hirst NA, Millner PA. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014;27:631–646. doi: 10.1128/CMR.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009;15:62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safavieh M, Ahmed MU, Ng A, Zourob M. High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosens. Bioelectron. 2014;58:101–106. doi: 10.1016/j.bios.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto T, Sonobe T, Hayashi K. Loop-Mediated Isothermal Amplification for Direct Detection of Mycobacterium tuberculosis Complex intracellulare in Sputum Samples. J. Clin. Microbiol. 2003;41:2616–2622. doi: 10.1128/JCM.41.6.2616-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connelly JT, Rolland JP, Whitesides GM. ‘Paper Machine’ for Molecular Diagnostics. Anal. Chem. 2015;87:7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, et al. A rapid, simple, and sensitive loop-mediated isothermal amplification method to detect toxigenic Vibrio cholerae in rectal swab samples. Diagn. Microbiol. Infect. Dis. 2010;66:135–139. doi: 10.1016/j.diagmicrobio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics. 2016;10:1–15. doi: 10.1063/1.4962992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton KN, Berglund GD, Linnes JC, Kinzer-Ursem TL, Wereley ST. DNA Microviscosity Characterization with Particle Diffusometry for Downstream DNA Detection Applications. Anal. Chem. 2017;89:13334–13341. doi: 10.1021/acs.analchem.7b03513. [DOI] [PubMed] [Google Scholar]
- 26.Clayton KN, Lee DH, Wereley ST. & Kinzer-Ursem, T. L. Measuring Biotherapeutic Viscosity and Degradation On-Chip with Particle Diffusometry. Lab on a chip. 2017;17:4148–4159. doi: 10.1039/C7LC00507E. [DOI] [PubMed] [Google Scholar]
- 27.Notomi T, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:63–63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leydesdorff L. Similarity measures, author cocitation analysis, and information theory. J. Am. Soc. Inf. Sci. Technol. 2005;56:769–772. doi: 10.1002/asi.20130. [DOI] [Google Scholar]
- 29.Karanis P, et al. Development and Preliminary Evaluation of a Loop-Mediated Isothermal Amplification Procedure for Sensitive Detection of Cryptosporidium Oocysts in Fecal and Water Samples. Appl. Environ. Microbiol. 2007;73:5660–5662. doi: 10.1128/AEM.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman A, Tseng Y, Wirtz D. Effect of length, topology, and concentration on the microviscosity and microheterogeneity of DNA solutions. J. Mol. Biol. 2002;323:199–215. doi: 10.1016/S0022-2836(02)00893-8. [DOI] [PubMed] [Google Scholar]
- 31.Tian B, et al. Attomolar Zika virus oligonucleotide detection based on loop-mediated isothermal amplification and AC susceptometry. Biosens. Bioelectron. 2016;86:420–425. doi: 10.1016/j.bios.2016.06.085. [DOI] [PubMed] [Google Scholar]
- 32.Packard M, Wheeler E, Alocilja E, Shusteff M. Performance Evaluation of Fast Microfluidic Thermal Lysis of Bacteria for Diagnostic Sample Preparation. Diagnostics. 2013;3:105–116. doi: 10.3390/diagnostics3010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linnes JC, et al. Paper-based molecular diagnostic for Chlamydia trachomatis. RSC Adv. 2014;4:42245–42251. doi: 10.1039/C4RA07911F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam MT, et al. Monitoring water sources for environmental reservoirs of toxigenic Vibrio cholerae O1, Haiti. Emerg. Infect. Dis. 2014;20:356–363. doi: 10.3201/eid2003.131293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 2002;26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 36.Miyagi K, et al. An improved method for detecting faecal Vibrio cholerae by PCR of the toxin A gene. J. Med. Microbiol. 1999;48:883–889. doi: 10.1099/00222615-48-10-883. [DOI] [PubMed] [Google Scholar]
- 37.Huq A, et al. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabral JPS. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health. 2010;7:3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors - occurrence, properties and removal. J. Appl. Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee P, et al. Evaluation of a rapid immunochromatographic dipstick kit for diagnosis of cholera emphasizes its outbreak utility. Jpn. J. Infect. Dis. 2010;63:234–238. [PubMed] [Google Scholar]
- 41.Lu, L. & Sick, V. High-speed particle image velocimetry near surfaces. J. Vis. Exp. (2013). [DOI] [PMC free article] [PubMed]
- 42.Chamarthy P, Garimella SV, Wereley ST. Non-intrusive temperature measurement using microscale visualization techniques. Exp. Fluids. 2009;47:159–170. doi: 10.1007/s00348-009-0646-1. [DOI] [Google Scholar]
- 43.Adrian RJ, Yao C-S. Pulsed laser technique application to liquid and gaseous flows and the scattering power of seed materials. Appl. Opt. 1985;24:44. doi: 10.1364/AO.24.000044. [DOI] [PubMed] [Google Scholar]
- 44.Olsen MG, Adrian RJ. Out-of-focus effects on particle image visibility and correlation in microscopic particle image velocimetry. Exp. Fluids. 2000;29:166–174. doi: 10.1007/s003480070018. [DOI] [Google Scholar]
- 45.Einstein A. Investigations on the Theory of the Brownian Movement. Ann. Phys. 1905;17:549. doi: 10.1002/andp.19053220806. [DOI] [Google Scholar]
- 46.Chuang H-S, Sie Y-S. A micro-volume viscosity measurement technique based on microPIV diffusometry. Microfluid. Nanofluidics. 2014;16:65–72. doi: 10.1007/s10404-014-1364-4. [DOI] [Google Scholar]
- 47.Raffel, M., Willert, C. E., Wereley, S. T. & Kompenhans, J. Particle Image Velocimetry: A Practical Guide. Particle Image Velocimetry 2nd (2007).
- 48.Meinhart CD, Wereley ST, Gray MHB. Volume illumination for two-dimensional particle image velocimetry. Meas. Sci. Technol. 2000;11:809–814. doi: 10.1088/0957-0233/11/6/326. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.