Abstract
Pain is the root cause for the overwhelming majority of emergency department (ED) visits worldwide. However, pain is often undertreated due to inappropriate analgesic dosing and ineffective utilization of available analgesics. It is essential for emergency providers to understand the analgesic armamentarium at their disposal and how it can be used safely and effectively to treat pain of every proportion within the emergency setting. A ‘balanced analgesia’ regimen may be used to treat pain while reducing the overall pharmacologic side effect profile of the combined analgesics. Channels-Enzymes-Receptors Targeted Analgesia (CERTA) is a multimodal analgesic strategy incorporating balanced analgesia by shifting from a system-based to a mechanistic-based approach to pain management that targets the physiologic pathways involved in pain signaling transmission. Targeting individual pain pathways allows for a variety of reduced-dose pharmacologic options – both opioid and non-opioid – to be used in a stepwise progression of analgesic strength as pain advances up the severity scale. By developing a familiarity with the various analgesic options at their disposal, emergency providers may formulate safe, effective, balanced analgesic combinations unique to each emergency pain presentation.
Keywords: Pain management, Balanced analgesia, CERTA, Opioids, Non-opioids, Emergency medicine
1. Introduction
Pain is the root cause for the overwhelming majority of emergency department (ED) visits, encompassing up to 75–80% of all presenting chief complaints.1,2 In the United States alone, pain is the chief complaint of over 100 million patients presenting to the ED each year.3 Despite this, pain management in the ED is frequently delayed due to overcrowded emergency rooms4,5 or pain is undertreated (oligoanalgesia) due to inappropriate analgesic dosing and ineffective utilization of available analgesics.6, 7, 8 While overcrowding remains a continued logistical challenge, the latter can be prevented with sufficient knowledge of available analgesic options.
The primary goal of emergency pain management is not zero pain, but a reduction in pain to an acceptable level that will allow for a bridge to inpatient care or a safe discharge with return to the patients' daily activities.9 In the midst of the United States opioid epidemic, a discerning eye has been placed on how providers are achieving this goal and the analgesic options being used to do so. In selecting a particular analgesic regimen, emergency providers must familiarize themselves with the myriad of pharmacologic choices that will allow them to balance the intensity of the pain with the safety and efficacy of various treatment options.
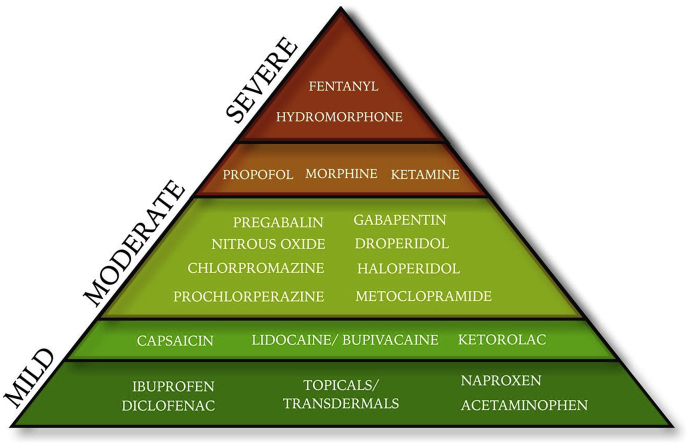
The ultimate analgesic approach provides a quick onset, limited side effects, and sustained relief tailored to each pain presentation. Analgesic optimization requires a patient-centered, pain-syndrome specific regimen. A combined analgesic regimen – collectively referred to as ‘balanced analgesia’ – may be used to treat pain while reducing the overall side effect profile.10 Channels-Enzymes-Receptors Targeted Analgesia (CERTA) is a multimodal analgesic strategy that incorporates balanced analgesia11 by promoting a shift from a system-based to a mechanistic-based approach to pain management that targets the physiologic pathways involved in pain signaling transmission (see Table 1).12 By targeting individual pain signaling pathways, a variety of reduced-dose analgesics may be used to optimize the safety and efficacy of the analgesic regimen. CERTA emphasizes a stepwise approach of opioid and non-opioid analgesics with progression in analgesic strength as pain progresses up the severity scale (see Fig. 1).
Table 1.
Channels-Enzymes-Receptors Targeted Analgesia (CERTA) approach to analgesia. By targeting individual pain signaling pathways, a variety of analgesics may be used at reduced doses in order to optimize the safety and reduce the side effect profile.
| CERTA (Analgesic) Target | Target Analgesics |
|---|---|
| COX-1, COX-2 Enzyme Inhibitors | Ibuprofen, Diclofenac, Naproxen, Ketorolac, Ketoprofen |
| TRPV1 Receptor Agonists | Capsaicin, Acetaminophen, Paracetamol |
| Sodium Channel Blockers | Lidocaine, Mepivacaine, Bupivacaine, Chloroprocaine, Procaine |
| Dopamine Receptor (D1-R, D2-R) Antagonists | Metoclopramide, Prochlorperazine, Chlorpromazine, Haloperidol, Droperidol |
| Glutamate/NMDA Receptor Antagonists | Ketamine, Nitrous Oxide, Magnesium, Propofol |
| GABA Receptor Agonists | Propofol |
| Serotonin (5HT-1) Receptor Agonists | Sumatriptan |
| Calcium Channel Blockers | Gabapentin, Pregabalin |
| Mu-opioid Receptor Agonists | Morphine, Oxycodone/Hydrocodone, Fentanyl, Hydromorphone, Tramadol, Buprenorphine (partial), Nitrous Oxide (partial) |
| Central Alpha-2 Receptor Agonists | Dexmedetomidine, Clonidine |
Fig. 1.
Analgesic Pyramid. The analgesic pyramid emphasizes a stepwise approach to analgesics - opioid and non-opioid - with progression in analgesic strength as pain progresses up the severity scale.
The purpose of this review is to provide an overview of the essential pharmacologic options available to the emergency pain management armamentarium and the evidence to support their use. This review focuses on utilizing a variety of analgesics to cover the entire spectrum of pain - mild to severe - specific to the patient and pain presentation, and consistent with the CERTA concept of balanced analgesia. Examples of multimodal CERTA pain management will be discussed throughout this review, with a listing of suggested CERTA combination therapies listed in Table 6.
Table 6.
CERTA recommendations by pain presentation. By choosing one of the suggested analgesics per CERTA class for each of the pain presentations , providers can optimize the safety and efficacy of an analgesic regimen. Note - these are only recommendations; a full consideration of the patients's presentation and comorbidites should be given prior to initiating an analgesic regimen.
2. COX-1, COX-2 inhibitors: ibuprofen, diclofenac, naproxen, ketorolac
Cyclooxygenase (COX-1 and COX-2) inhibitors (NSAIDs) reduce pain by inhibiting prostaglandin synthesis involved in both acute and chronic pain conditions.13 Though classically used for mild to moderate pain, NSAIDs are a complement to the entire spectrum of pain severity.
Ibuprofen may be administered via PO, IV, IM, PR routes, and topically. 400 mg PO ibuprofen every 8 h (1200 mg/per day) is the dosing regimen consistent with analgesic ceiling– the dose of drug above which no further analgesic efficacy is achieved.12,14,15 Alternatively, 50 mg PO diclofenac every 8 h or 250–500 mg PO naproxen every 8–12 h can be used with similar analgesic effect.12
NSAIDs are commonly used for headaches,16 renal colic,17 musculoskeletal pain,18 dysmenorrhea,19 and non-radicular back pain.20,21 The synergistic combination of 400 mg ibuprofen with 1000 mg paracetamol (acetaminophen) is a classic example of balanced analgesia and has long been considered a first line analgesic regimen for mild to moderate acute pain presentations.14,22 Pain reduction from this combination has analgesic efficacy comparable to oral opioid combinations (oxycodone/hydrocodone-acetaminophen) in treating acute musculoskeletal pain in the emergency setting.18
Ketorolac is the most commonly used parenteral NSAID in the emergency setting in the United States (diclofenac is the most commonly used parenteral NSAID worldwide).23 Due to an unpredictable absorption rate, delayed onset of analgesia, and injection pain associated with intramuscular (IM) administration, IV ketorolac is preferred. Research has shown 10 mg IV ketorolac to be the analgesic ceiling for acute pain in the emergency setting.24 Administration of 10–15 mg IV ketorolac every 6 h (based on the analgesic ceiling dose) is the recommended dosing regimen for moderate to severe pain presentations.12
Similar to other NSAIDs, ketorolac is effectively used for management of pain associated with headaches,16 renal colic,10 biliary colic, and acute musculoskeletal pain.24 The combination of 15 mg IV ketorolac-5mg IV morphine (administered twice at 0 and 20 min) has shown to more effectively reduce acute renal colic pain than either ketorolac or morphine alone and required less rescue analgesia.10
Topical NSAIDs – diclofenac (gel, patch), ketoprofen, ibuprofen (5%) - offer the advantage of preferential localization to cartilage, meniscus, and tendons at concentrations up to 100-fold parenteral administration.25 Each agent may be used to effectively treat acute musculoskeletal injuries,26 neuropathies, soft-tissue strains and sprains, contusions, burns, skin ulcers, acute herpetic zoster, low back pain, osteoarthritis, cancer-related pain, and visceral pain.25,26 Topical agents should be administered with gloves to avoid mucous membrane irritation and used without heating pads to prevent excessive absorption.
Adverse effects of NSAIDs include gastric ulcers, platelet function inhibition, helicobacter pylori infection, and nephrotoxicity.12 Patients with gastrointestinal ulcers, active hemorrhage, hepatic or renal disease, heart failure, and elderly patients with multiple comorbidities should consider alternative analgesics.27,28 Although data suggests the short-term use of NSAIDs for mild/moderate acute pain may be used without a profound reduction in renal perfusion, the recommendation remains to avoid NSAIDs in patients with renal disease.29,30 Further, NSAIDs should be avoided in cirrhotic patients where renal perfusion is reliant on prostaglandin synthesis to prevent hepatorenal syndrome.29 NSAIDS during the second and third trimester of pregnancy should be avoided due to adverse effects on fetal development and risk of peripartum hemorrhage.31,32
3. TRPV1 receptor agonist: capsaicin, acetaminophen
Transient Receptor Potential (TRP) channels play a dual role in pain signaling.33 Whereas immediate activation of TRP channels stimulates pain signaling, repeated stimulation desensitizes the pain-conducting neurons, silencing the pain signal.34 Capsaicin and acetaminophen both function as TRPV1 receptor agonists.34,35
Capsaicin comes in both patch (8%) and cream (0.025–0.075%) formulations.26 Capsaicin is used to treat osteoarthritis,36 postherpetic neuralgias,36 diabetic neuropathy,36,37 and cannabinoid hyperemesis syndrome (CHS) when applied to the abdomen during acute pain presentations.38
Acetaminophen (APAP, Paracetamol) administered PO, PR, and IV may be used as a first-line agent for acute pain presentations of mild intensity.15,18 325 to 1000 mg PO acetaminophen every 4–6 h (maximum 4 g per day) is a recommended dosing regimen.12 1000 mg PO acetaminophen is considered the analgesic ceiling dose for acute pain.39
The combination of 1000 mg acetaminophen-400 mg ibuprofen has long been considered the base standard for mild to moderate acute pain management.22 As mentioned, this combination has been shown to be as effective as oral opioid combinations (oxycodone/hydrocodone-acetaminophen) and may be used as the baseline regimen for mild to moderate musculoskeletal injuries.18
Intravenous acetaminophen is an effective analgesic in patients who cannot tolerate oral or rectal delivery and may reduce overall opioid consumption when used as an analgesic adjunct.40 In the emergency setting, however, the use of IV paracetamol as an adjunct for pain management offered no superiority over oral paracetamol.41
Acetaminophen is considered safe for a developing fetus and is the first-line analgesic agent in pregnant and lactating women with mild to moderate pain.32
4. Sodium channel blockers: lidocaine, mepivacaine, bupivacaine, chloroprocaine, procaine
Sodium channel blockers such as lidocaine, mepivacaine, bupivacaine, chloroprocaine, and procaine function as analgesics through the noncompetitive inhibition of nerve signal propagation.42,43
Nerve blocks (regional anesthesia) refer to the infiltration of peripheral nerves with sodium channel-blocking agents creating localized analgesic relief (see Table 2 for a list of commonly used anesthetics in the emergency setting).42,44 Nerve blocks prevent systemic loading of analgesic medications, improve door-to-analgesia timing,45,46 and are optimally used when combined as part of a multimodal analgesic regimen. Epinephrine may be added to anesthetic injection to provide local vasoconstriction that prolongs analgesic duration by delaying anesthetic clearance.42,44
Table 2.
Commonly used regional anesthetics in the emergency setting. Recommended dosing and duration of commonly used anesthetics used in the ED. Example use calculation: 1% lidocaine = 1 g/100 ml = 10 mg/ml; 7 mg/kg x 70 kg patient = 490 mg; 490 mg @ 10 mg/ml = 49 ml 1% lidocaine Table adapted with permission courtesy of painandpsa.org.a
| Anesthetic | Class | Onset | Common Concentration | Dose | Max Dose | Duration |
|---|---|---|---|---|---|---|
| Chloroprocaine (w/epi) | Ester | Rapid | 2%(20 mg/ml) | 14 mg/kg | 1000 mg | ∼0.5–1 h |
| Chloroprocaine (w/o epi) | Ester | Rapid | 2%(20 mg/ml) | 11 mg/kg | 800 mg | 0.5–1 h |
| Lidocaine (w/epi) | Amide | Rapid | 1% (10 mg/ml) | 7 mg/kg | 500 mg | 1.5–3 h |
| Lidocaine (w/o epi) | Amide | Rapid | 1% (10 mg/ml) | 4.5 mg/kg | 300 mg | 1–2 h |
| Bupivacaine (w/epi) | Amide | Slow | 0.5% (5 mg/ml) | 3 mg/kg | 225 mg | 5–8 h |
| Bupivacaine (w/o epi) | Amide | Slow | 0.5% (5 mg/ml) | 2.5 mg/kg | 175 mg | 3–6 h |
| Ropivacaine (w/epi) | Amide | Slow | 0.5% (5 mg/ml) | 3 mg/kg | 225 mg | 3–6 h |
| Ropivacaine (w/o epi) | Amide | Slow | 0.5% (5 mg/ml) | 3 mg/kg | 225 mg | 3–6 h |
Odashima K, Strasberg S, Dickman E. Ultrasound-Guided Regional Nerve Blocks in Emergency Medicine Brooklyn, NY2017 [June 27, 2018]. Available from: http://painandpsa.org/rnb/.
Pain syndromes that benefit from either nerve blocks or local anesthetic infiltration include laceration repair,46 headaches and migraines,47 extremity and hip fractures,48,49 abscess drainage,50 shoulder reductions,51 chest tube placements,52 and paraphimosis reductions.53
Intravenous lidocaine can also be used to treat chronic regional pain syndrome,54 post-herpetic neuralgia, and post-spinal cord injury radiculopathy.55 Several clinical trials have also shown 1.5 mg/kg IV lidocaine administered over 10 min was successful in reducing renal colic refractory to traditional NSAID and opioid regimen56,57 with efficacy comparable to 0.1 mg/kg IV morphine.58
Local anesthetic systemic toxicity (LAST) may occur when excessive quantities accumulate intravascularly. Adverse effects include confusion, anxiety, headaches, drowsiness, lightheadedness, tremors, seizures, cardiac dysrhythmias, and hemodynamic instability.46,57 Patients receiving systemic anesthetic should be placed on cardiac monitoring to assess for systemic toxicity.46 Ultrasound guidance with nerve blocks may prevent direct injection of anesthetic into vasculature causing similar toxicity.46
5. Dopamine Receptor (D1-R, D2-R) Antagonists: metoclopramide, prochlorperazine, chlorpromazine, haloperidol, droperidol
Beyond its role in motivational behavior, dopamine plays an active role in the pain signaling pathway where inhibition may prevent signal propagation.59 Common dopamine antagonists used in the emergency setting include metoclopramide, prochlorperazine, chlorpromazine, haloperidol, and droperidol.
Metoclopramide, prochlorperazine, and chlorpromazine are commonly used to treat migraine headaches.16 10 mg IV metoclopramide may be used as a first-line agent in the treatment of acute migraine attacks.60 10 mg IV prochlorperazine has also demonstrated significant headache relief61 and superiority over opioids in the treatment of migraines.62 10 mg IV chlorpromazine has also been shown to be as effective as metoclopramide for headache relief.63
Droperidol and haloperidol - classically known for their function as high-potency, first generation antipsychotics64 - have also shown efficacy in treating headaches in the emergency setting.60,65,66 2.5 mg IV (or IM) droperidol has been shown to be the minimum effective dose for migraine relief66 with efficacy comparable to 10 mg IV prochlorperazine.65 Alternatively, 5 mg IV haloperidol has been shown to be as efficacious as 10 mg IV metoclopramide.60,64 Further off-label utilization of haloperidol has also been studied for its antiemetic and analgesic efficacy in treating gastroparesis, cannabinoid-induced hyperemesis, and cycling vomiting syndrome.67,68
Side effects of dopamine-receptor antagonists include QT prolongation, extrapyramidal side effects (akathisia, dystonia), anti-muscarinic effects (drowsiness, dry mouth, constipation, hypotension), and neuroleptic malignancy syndrome (hyperpyrexia, muscle rigidity, rhabdomyolysis).12,69 Metoclopramide, prochlorperazine, and chlorpromazine can be administered over a 15–30 min infusion to reduce extrapyramidal effects.63,70 25 mg IV or PO diphenhydramine may also be used with prochlorperazine to offset the associated akathisia.69,70
6. Glutamate/NMDA receptor antagonists: ketamine, nitrous oxide
Glutamate serves as an excitatory neurotransmitter in the central nervous system.71 Pharmacologic agents focused on reducing central pain sensitization and hyperalgesia by blocking the N-methyl-d-aspartate (NMDA) glutamate receptor are key analgesic targets.71 Examples used in the emergency setting include ketamine and nitrous oxide.
Ketamine is used for analgesia in subdissociative doses (SDK) - 0.1–0.3 mg/kg IV or subcutaneous (SQ) - that provide analgesic relief while preserving respiratory and cardiopulmonary stability in the emergency setting.72,73 SDK has shown to be effective in treating acute abdominal pain,73,74 renal colic,73, 74, 75 back pain,73,74 headaches,76,77 and extremity pain.75,78 0.3 mg/kg IV ketamine has been shown to be as efficacious as 0.1 mg/kg IV morphine for acute pain78 while doses as low as 0.15 mg/kg IV ketamine have been shown to reduce overall morphine consumption up to 26% when used as an adjunct analgesic.79
The side effects of ketamine include a feeling of unreality, gastric irritation, sedation, dizziness, and nystagmus (seen shortly after onset).73 These effects are increasingly prominent among geriatric patients where SDK should be used cautiously.80 Administering ketamine as a slow infusion over 15 min (versus IV push) can reduce these adverse side effects.74 Co-administration of prophylactic ondansetron and midazolam can be used to treat post-ketamine nausea and emergence reaction respectively.12
Nitrous oxide (N2O) is an odorless gas with a fast-onset and a short half-life.81,82 N2O effects range from analgesia at low doses to neurologic depression and medullary paralysis at high doses.83 Low-dose N2O administration allows for conscious, rational thought with a decreased pain perception.83 Analgesic N2O is administered as a combined equimolar mixture of 50% oxygen – 50% nitrous oxide.84 Average onset is 3–5 min with recovery occurring within 5 min of discontinuation.82
N2O research in the pediatric population has demonstrated efficacy for bone and joint procedures,85laceration repairs, abscess drainage, foreign body removal, and urinary catheterization placement.81,86 N2O has increased utility when IV access is undesired or difficult to attain.
Side effects of N2O include gastrointestinal disturbance, dizziness, headaches, increased intracranial pressure, oral distaste (delivery system tubing), and respiratory depression.81,82 N2O should be administered under continuous pulse oximetry and cardiac monitoring in a setting where airway management is available.81,86
7. GABA receptor agonists: propofol
The gamma-aminobutyric acid (GABA) receptor agonist propofol has traditionally been used for anesthesia induction, procedural sedation, and in the treatment of status epilepticus.87 However, propofol (administered in sub-anesthetic doses) has been shown to decrease pain perception88 and its use as an adjunct analgesic has been promoted to support balanced analgesia.89
Propofol may be used to treat acute headaches in the emergency setting. 30–40 mg IV propofol (sub-anesthetic dosing) with repeat boluses of 10 mg every 3–5 min (maximum 120 mg) has shown efficacy in the treatment of migraines refractory to a traditional migraine regimen90 and as effective as 6 mg subcutaneous sumatriptan with less gastrointestinal disturbance.91
Adverse effects of propofol include respiratory depression, hypotension, sedation, pain at the injection pain, and propofol infusion syndrome (PRIS).87 Concomitant use of opioids may potentiate the risk of respiratory failure and should be avoided.
Propofol should be administered under continuous pulse oximetry and cardiac monitoring in a setting of airway management accessibility.
8. Serotonin (5HT-1) receptor agonists: sumatriptan
Serotonin receptor agonists such as sumatriptan (triptans) are hypothesized to prevent pain signal propagation by inhibiting calcitonin gene-related peptide (CGRP) release.92
Triptans are effective in treating migraines and cluster headaches.93,94 Administration of 6 mg subcutaneous sumatriptan may be used to treat acute migraines in the emergency setting93 with repeat delivery of 6 mg after 1 h if symptoms persist (max 12 mg per day).12 Additionally, 100 mg PO sumatriptan has been shown to reduce 24-h migraine recurrence.93 Sumatriptan is most effective in treating migraines when used as part of a balanced analgesic regimen including acetaminophen, NSAIDs, metoclopramide (or prochlorperazine), and IV fluids.16
Adverse effects of triptans include paresthesias, flushing, palpitations, fatigue/drowsiness, transient neck tightness, chest pressure, and dysrhythmias.94,95 The American Headache Society concluded that the incidence of serious cardiovascular events was extremely low and that the chest pain generally not serious or explained by ischemia, thus favoring triptan use in the absence of contraindications.95
9. Calcium channel blockers: gabapentin, pregabalin
The calcium channel blocker anticonvulsants pregabalin and gabapentin reduce central pain sensitization by preventing voltage-gated postsynaptic neurotransmitter release.96 Pregabalin functions similar to gabapentin but with increased potency and binding affinity.97
Both pregabalin and gabapentin are effective in treating neuropathic pain, where other analgesics offer little relief. Pregabalin was the first FDA-approved drug for the management of post-herpetic and diabetic neuropathic pain.96,97 Administration of 150 mg daily PO pregabalin divided into 3 daily doses with titration up to 300–600 mg daily as tolerated is the recommended approach.12,97 Research has shown that 600 mg pregabalin daily was effective for postherpetic neuralgia, diabetic neuropathy, and fibromyalgia though medication compliance was hindered by an unfavorable side effect profile.97
Gabapentin is also recommended for postherpetic neuralgia and diabetic neuropathy.98 A gabapentin titration of 300 mg on day 1, 300 mg twice a day on day 2, 300 mg three times a day on day 3, and further titration up to 1800 to 3600 mg per day divided into 3 doses as tolerated is the recommended approach.12 Similar to pregabalin, multiple studies have shown its use hindered by a high percentage of intolerable side effects.98
Adverse effects of both gabapentin and pregabalin include dizziness, fatigue, ataxia, peripheral edema, nystagmus, tremor, rhabdomyolysis, angioedema, and weight gain.96 Pregabalin/gabapentin should be used cautiously among elderly populations to avoid precipitating cognitive impairments.96 Both gabapentin and pregabalin are excreted un-metabolized in the urine and are contraindicated in patients with impaired renal function.99 Neither gabapentin nor pregabalin should be administered with opioids as both may potentiate the euphoric effects of opioids when taken concomitantly, increasing susceptibility to abuse100 and a worsening respiratory and CNS depression.
A detailed summary of commonly used non-opioid analgesic options in the emergency setting can be found in Table 3.
Table 3.
Non-opioid analgesic options used in the emergency setting. The medications, doses, and durations listed are estimates based on average responses in the general population; a full consideration of the individual patient's presentation and comorbidities should be given prior to initiating an analgesic regimen.
| Medication | Average Dose | Duration | Max Dose | Side effects |
|---|---|---|---|---|
| Ibuprofen | 400 mg PO | 8 h | 1200 mg per day | GI irritation, bleeding, renal dysfunction, bronchospasm, delayed wound healing |
| Diclofenac | 50 mg PO | 8 h | 150 mg per day | GI irritation, bleeding, renal dysfunction, bronchospasm, delayed wound healing |
| Naproxen | 250 mg PO (every 8 h) 500 mg PO (every 12 h) |
8–12 h | 1000 mg per day | GI irritation, bleeding, renal dysfunction, bronchospasm, delayed wound healing |
| Ketorolac | 10–15 mg IV | 6 h | 60 mg per day | GI irritation, bleeding, renal dysfunction, bronchospasm, delayed wound healing |
| Capsaicin | thin film to effected area | 6–8 h | Varies | Localized pain, erythema (rare - transient hypertension, pruritus, swelling, papules) |
| Acetaminophen | 325-1000 mg PO | 4–6 h | 4 g per day | Nausea, vomiting, liver toxicity |
| Lidocaine | 1.5 mg/kg (admin over 10 min) | Varies | 200 mg per administration | Confusion, anxiety, sense of impending doom, headache, drowsiness, cardiac dysrhythmias |
| Metoclopramide | 10 mg IV | 1–2 h | 40 mg per day | Akathisia, dystonia, drowsiness, QT prolongation, torsade de pointes |
| Prochlorperazine | 10 mg IV, PO | 3–4 h | 40 mg per day | Akathisia, dystonia, drowsiness, QT prolongation, torsade de pointes |
| Chlorpromazine | 10 mg IV, PO | 4–6 h | 25 mg per day | Akathisia, dystonia, drowsiness, QT prolongation, torsade de pointes |
| Haloperidol | 2–10 mg PO, IV, IM | 2–4 h | 20 mg (based on side effect profile, ECG findings) | Akathisia, dystonia, drowsiness, QT prolongation, torsade de pointes |
| Droperidol | 2.5 mg IV | 2–4 h | Unknown (based on side effect profile, ECG findings) | Akathisia, dystonia, drowsiness, QT prolongation, torsade de pointes |
| Ketamine | 0.15–0.3 mg/kg IV | Varies | Dependent on infusion | Dizziness, agitation, emergence reaction, nystagmus, sensation of unreality, nausea, vomiting |
| Nitrous Oxide | 50/50 (%NO/%Oxygen mixture) | 3–5 min | Varies | Nausea, vomiting, headache, euphoria, dizziness, tingling, oral distaste mild increase in intracranial pressure |
| Propofol | 30–40 mg IV | Varies (repeat 10 mg every 3–5 min as needed) | 120 mg per day | Respiratory depression, hypotension, sedation, hypertriglyceridemia, pain at injection Propofol Infusion Syndrome |
| Sumatriptan | 6 mg SQ 25–100 mg PO |
Varies | 12 mg SQ per day 200 mg PO per day |
Tingling sensation, dizziness, hot flashes, palpitations, drowsiness, dysrhythmia |
| Gabapentin | 300 mg PO (titrated up to 1200 mg three times per day) | 24 h (requires titration to effect) | 3600 mg per day | Fatigue, dizziness, weight gain, ataxia, nystagmus, leukopenia, rhabdomyolysis |
| Pregabalin | 50–75 mg PO (titrate up to 150–300 mg) | 12 h | 600 mg per day (following slow titration) | Fatigue, dizziness, weight gain, ataxia, nystagmus, thrombocytopenia, angioedema |
| Dexmedetomidine | 0.5–1 μg/kg IV | Varies | Dependent on infusion | Hypotension, bradycardia |
10. Opioid Receptor Agonists: Morphine, Oxycodone/Hydrocodone, Fentanyl, Hydromorphone, Tramadol
Opioid receptor agonists produce analgesic and euphoric effect by modulating three main opioid receptors - mu, kappa, and delta.101 The most commonly used opioids in the emergency setting are morphine, oxycodone/hydrocodone, fentanyl, hydromorphone, and tramadol. There is no evidence that one opioid is more effective than the others at equianalgesic doses and it is prudent to titrate one drug to desired effect prior to using multiple agents.102 However, not all opioids are metabolized the same and individuals with allelic variances in the metabolizing enzymes can have varying degrees of analgesic response.103 If an opioid is not achieving an anticipated analgesic effect, consider an alternative opioid.
Morphine is considered the “gold standard” for moderate to severe pain and the baseline by which other opioids are measured.104 Administration of 5–10 mg IV (0.1 mg/kg IV) in titratable fashion or 10–15 mg PO morphine every 3–6 h is the recommended standard dose.12,105,106 A ‘start low, go slow’ approach is often advised to prevent overdosing. However, substandard dosing may fail to achieve pain reduction as even standard dosing (0.1 mg/kg IV) has been demonstrated to leave up to two-thirds patients with insufficient pain reduction 30 min post-administration.105,106 A comparison of the standard-dose (0.10 mg/kg) versus higher-dose (0.15 mg/kg) IV morphine showed a statistically significant improvement in pain reduction with the higher dose, though the clinically significant minimum of 1.3 NRS unit difference between the two doses was not seen.8,106 Ultimately, close monitoring is advised and re-dosing every 30–60 min as needed to balance analgesia with adverse effect while avoiding extended periods of sub-therapeutic analgesia.
A variety of morphine delivery routes are available as safe alternatives when IV access cannot be obtained. Research comparing nebulized versus intravenous morphine has shown 10–20 mg nebulized morphine (with repeat dosing every 10 min for a maximum of 3 boluses) had similar efficacy and an improved safety profile compared to IV morphine in patients with severe posttraumatic pain.107 Additionally, 10 mg intramuscular (IM), 10 mg subcutaneous (SQ), or 10–20 mg rectal (PR) morphine may be administered every 4 h as needed for moderate to severe pain presentations.12
Oxycodone (OC) and hydrocodone (HC) were oral opioids marketed with the advantage of reduced first pass metabolism over oral morphine.108 Oxycodone can be administered as a stand-alone or in combination with acetaminophen (Roxicet, Percocet - 5/7.5/10 mg OC + 325 mg acetaminophen), or as an extended release formulation (OxyContin).12 Hydrocodone is typically administered in combination with acetaminophen (Lortab, Lorcet – 5/7.5/10 mg + 325 mg acetaminophen) or as an oral solution (Hycet - 7.5 mg OC + 325 mg acetaminophen).12,109 Both OC and HC are initially dosed at 5–10 mg with re-dosing every 3–6 h as needed.109
Despite the analgesic efficacy of OC/HC, several studies have demonstrated non-opioid alternatives to be non-inferior in reducing pain. In the treatment of acute extremity pain in the emergency setting, neither 5 mg oxycodone-325 acetaminophen nor 5 mg hydrocodone-300 mg acetaminophen were superior to 400 mg ibuprofen-1000 mg acetaminophen for pain reduction.18 Similarly, the addition of acetaminophen-oxycodone to 500 mg PO naproxen (twice daily) for acute, non-radicular low back pain was no more efficacious than naproxen alone.20 Further, the OC/HC-acetaminophen combination pills increase the risk of under-dosing acetaminophen when attempting to minimize opioid consumption or over-dosing acetaminophen when attempting to reach sufficient opioid equianalgesic effect. When available, alternative analgesic options (or acetaminophen alone) are recommended for acute pain presentations to maximize safety and efficacy.
Due to convenient oral formulations, OC/HC are commonly prescribed analgesics from the emergency room. However, the use of OC comes with an increased abuse potential. Data extrapolated from experimentation on healthy volunteers has shown a higher likeability (surrogate marker for abuse potential) and lower incidence of negative subjective effects with OC compared to either HC or morphine.110 Alternatively, morphine sulfate immediate release (MSIR) has a decreased likability (i.e., abuse potential), increased dysphoria,110,111 and a decreased risk of acetaminophen under-/over-dosing making it a preferred choice when a short outpatient analgesic course is required (e.g., 3-day supply of MSIR 15 mg every 6 h with plan for reevaluation if pain persists beyond three days).102,112
Fentanyl is 100-times more potent that morphine allowing for a quick onset and short duration when needed for rapid titration during severe pain unremitting to traditional analgesics.113 0.5 mcg/kg IV fentanyl (25–50 mcg) every 30 min as needed is the recommended dosing for severe pain presentations in the emergency setting.12
A variety of fentanyl delivery routes are available as safe alternatives when IV access cannot be obtained. Research has shown 1.5 to 3.0 mcg/kg nebulized fentanyl as effective as IV fentanyl for acute abdominal and musculoskeletal pain in the emergency setting.114 Research comparing 2 mcg/kg nebulized fentanyl to 0.1 mg/kg IV morphine showed more rapid pain relief, higher patient and physician satisfaction, and similar rates of adverse effects when using fentanyl for acute abdominal pain.115 Though research is limited to the pediatric population, data has shown 1 to 2 mcg/kg intranasal (IN) fentanyl can be used for both safe and effective analgesia in the emergency setting116,117 and may also improve the door-to-analgesia time.118
Compared to morphine and hydromorphone, which are metabolized by the liver to active metabolites requiring renal clearance, fentanyl is metabolized by the liver to inactive metabolites that are relatively safer in the setting of renal failure.119 In contrast to other opioids, fentanyl has less gastrointestinal disturbance and histamine-induced hypotension making it a better choice in hemodynamically borderline patients.120
Hydromorphone (HM) is a highly lipophilic opioid, approximately 7-times more potent than morphine.121,122 A traditional regimen utilizing a “1 + 1” dosing protocol - 1 mg HM IV bolus given at baseline followed by a repeat 1 mg HM IV bolus at 15 min as needed - has shown clinically significant pain reduction and limited adverse effects in non-elderly patients presenting with moderate to severe acute pain.123 The “1 + 1” protocol is further supported by a data comparing the protocol to a baseline 2 mg IV bolus where both arms resulted in similar analgesic efficacy, though an opioid-sparing advantage in the “1 + 1” group where only one-third of the patients requested the second 1 mg dose.124 In opioid-naïve patients, however, initial doses as low as 0.25–0.5 mg IV HM should be administered to avoid nausea, pruritus, and rapid (over) sedation with early reassessment and titration as needed.12,122 Similar to morphine, the analgesic effect of HM lasts approximately 3–6 h.12,125
Hydromorphone has been cited as a recurrent source of preventable iatrogenic over-dosing,126 and caution should be taken when calculating the desired equianalgesic dose (see Fig. 2). Research has shown 2 mg IV HM is capable of causing desaturations (<95% O2 saturation) in up to one-third of patients, though successfully reversed with oxygen via nasal cannula.127 Age is directly correlated with risk of desaturation and increased caution is advised with HM use among the elderly.127
Fig. 2.
Comparison of common opioid analgesic potency. Hydromorphone is approximately 7-fold more potent than morphine; fentanyl is approximately 100-fold more potent than morphine.
Tramadol is a partial mu-opioid receptor (MOR) agonist with dual functionality as a serotonin and norepinephrine reuptake inhibitor (SNRI).128 The mu-opioid receptor affinity of tramadol and its more active metabolite O-desmethyltramadol (ODT, M1) are significantly less than that of morphine.129 The analgesic efficacy of tramadol has shown to be inferior to the combination regimen 500 mg acetaminophen-5 mg hydrocodone for acute musculoskeletal pain,130 non-superior to 1 mg/kg IV diclofenac for extremity injury,131 and non-superior to 5–10 mg IV morphine for limb pain.132 Tramadol toxicity results in nausea, agitation, tachycardia, confusion, hypertension, hypoglycemia, hyponatremia,133,134 and a lowered seizure threshold.125,135 Additionally, tramadol has shown to be a major contributor to abuse-related ED visits.136 Due to the increased risk of adverse effects, abuse potential, and non-superiority compared to analgesic alternatives, tramadol use is not recommend in the emergency setting.
Side effects common to all opioids include respiratory depression, miosis, cardiovascular depression (hypotension, bradycardia), pruritus (through central mu-receptor agonism and histamine release; seen in a minority of cases), urinary retention, and constipation/gastrointestinal motility depression.122,137 Respiratory depression is the leading preventable adverse side effect seen in the emergency setting secondary to over-dosing.126 Muscle (chest wall) rigidity is an adverse effect unique to fentanyl toxicity113 directly correlated to the overall dose and rate of administration.
A detailed summary of commonly used opioid analgesic options in the emergency setting can be found in Table 4.
Table 4.
Common opioids used in the emergency setting. A variety of opioids are available to address moderate to severe pain. There is no evidence that one opioid is more effective than the others at equianalgesic doses and it is prudent to titrate one drug to desired effect prior to using multiple agents.
| Opioid | Dose (oral) | Dose (IV) | Onset IV (oral) | Duration |
|---|---|---|---|---|
| Morphine (MSIR) | 10–15 mg | 0.1 mg/kg (5–10 mg) | 5–10 min (15–30 min) | 3–6 h (IV, oral) |
| Hydromorphone (Dilaudid) | 2 mg | 0.25–0.5 mg | 5–10 min (15–30 min) | 3–6 h (IV, oral) |
| Oxycodone (Percocet) | 5–10 mg | – | (15–20 min) | 3–6 h (PO) |
| Hydrocodone (Lorcet, Norco) | 5–10 mg | – | (15–20 min) | 3–6 h (PO) |
| Fentanyl | – | 0.5 mcg/kg (25–50 mcg) | 1–2 min | 0.5–1 h (IV) |
[Note: Codeine was intentionally excluded from this analgesic review. Codeine is a pro-drug with analgesic effects dependent on the metabolic conversion from the pro-drug form to codeine-6-glucuronide and morphine by the liver (similar to tramadol). This enzymatic reaction has significant allelic (genetic) variability.103 Both poor and ultra-rapid metabolizers exist108 resulting in uncontrolled variability in analgesic response. Based on this increased analgesic variability, codeine should not be used in the emergency setting.]
11. Future emergency analgesic options
Dexmedetomidine (DXMT): The alpha-2-adrenergic receptor agonist DXMT produces analgesia by dampening the centrally-activated sympathetic response.138 DXMT utilization has expanded from it classic role as a sedative among mechanically ventilated patients in intensive care settings, to a promising analgesic adjunct.138 DXMT can be delivered IV or IN with recommended dosing of 0.5–1.0 μg/kg IV (1–2 μg/kg intranasal).138 Concomitant DXMT use has been shown to reduce opioid (oxycodone) consumption, decrease opioid side effect profile, and improve patient satisfaction in the postoperative setting.139 When combined with regional nerve blocks (ropivacaine, bupivacaine) 1 μg/kg dexmedetomidine has resulted in a prolonged duration of sensory blockade140 and a shorter time to anesthesia onset.141 At this time, however, a high drug cost has limited research and utilization of DXMT in the emergency setting and its use remains an emerging concept in need of further research.
Buprenorphine: Buprenorphine is a partial opioid receptor agonist approximately 25–100 times more potent than morphine.142 A higher binding affinity and slower dissociation rate of buprenorphine result in a longer duration of action compared to other opioids.143 Predominantly used to treat opioid withdrawal and opioid use disorder,144,145 research has shown buprenorphine can successfully treat neuropathic pain,142 cancer pain,142,146 and post-operative pain.147 One randomized trial found 2 mg sublingual buprenorphine to be as effective as 30 mg IV ketorolac for acute renal colic relief but with increased nausea, vomiting, and dizziness in the buprenorphine group.148 A separate randomized trial found 2 mg sublingual buprenorphine to be as effective as 0.1 mg/kg IV morphine for acute renal colic relief, again with increased dizziness.149 Furthermore, 0.4 mg sublingual buprenorphine has been shown to be as effective and safe as 5 mg IV morphine for acute bone fractures in the emergency setting.150 Additional research is needed to understand the full utility of analgesic buprenorphine in the emergency setting.
Intranasal Analgesics: Intranasal (IN) analgesic delivery is a safe, simple, and effective method of analgesic administration that bypasses oral absorption and first-pass metabolism with onset comparable to IV administration.151 Analgesics commonly used intranasally include fentanyl, ketamine, dexmedetomidine, and hydromorphone (see Table 5 for a descriptive listing of commonly used intranasal analgesics).117,152 IN administration allows for quick delivery of low-volume, high-concentrate analgesics without the need for IV access or site sterilization.151,153 Though topical administration is also feasible, mucosal atomizer devices (MAD) are preferred to optimize distribution of approximately 0.5–1.0 cc analgesic per nostril.152 IN analgesic research has predominantly focused on the pain reduction among the pediatric population where IV access is often limited.116 Future studies are required to determine the efficacy and feasibility of IN analgesics among the adult population in acute emergency settings.
Table 5.
Common intranasal analgesics used in the emergency setting. For optimal delivery divide the total dose equally among each nostril to maximize absorption surface area. Total volume per nostril should not exceed 1–2 cc to avoid excess run-off.
| Drug | Indication | Dose | IN amount (cc) | Peak Onset (min) | Duration (min) | Adverse effects |
|---|---|---|---|---|---|---|
| Dexmedetomidine (100 mcg/ml) | Analgesia, procedural sedation | 1-2 mcg/kg | 0.7–1.5 | 20 | 90–105 | Bradycardia, hypotension |
| Ketamine (100 mg/ml) | Analgesia | 0.7–1.0 mg/kg | 0.5–1 | 5–10 | 70–75 | Distaste, hypersalivation, emergence reaction |
| Fentanyl (50 mcg/ml) | Analgesia | 1-2 mcg/kg | 1.5–3 | 5–15 | 30–60 | Nasal irritation, rhinitis, respiratory depression, nausea/vomiting |
| Hydromorphone (2 mg/ml) | Analgesia | 2–5 mg | 1–3 | 20–25 | 120–240 | Distaste, nasopharyngeal irritation, somnolence, dizziness |
12. Conclusion
The presentation of pain within the emergency department is an inevitable part of every shift. Despite under or improper utilization of pain medication, a wide variety of analgesic options exist for the effective management of pain in every proportion. Utilizing the multimodal Channels-Enzymes-Receptors Targeted Analgesia (CERTA) approach to pain allows providers to take advantage of a variety of analgesic options at reduced doses, thereby optimizing the safety and efficacy of the analgesic regimen. By developing a familiarity with the various analgesic options at their disposal emergency providers may formulate a safe, effective, balanced analgesic combination unique to each patient presentation.
Disclaimer
Dr. Cisewski - No financial disclaimers.
Dr. Motov - No financial disclaimers.
Author contribution statement
I, David Cisewski, do verify and confirm that everyone who contributed to this manuscript is either listed as an author or acknowledged as a contributor in the acknowledgement section, and that the title page details any professional writing assistance or others paid to provide manuscript support.
Both authors provide equal weight in writing and reviewing the manuscript and creating the images and tables.
Conflict of interest statement
Neither providers have a conflict of interest.
Funding
No funding was received for this review.
Acknowledgments
We would like to thank the editors at the Turkish Journal of Medicine for sharing an interest and appreciation for effective pain management in the emergency setting.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
David H. Cisewski, Email: dhc0626@gmail.com.
Sergey M. Motov, Email: smotov@gmail.com.
References
- 1.Todd K.H., Ducharme J., Choiniere M. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007;8(6):460–466. doi: 10.1016/j.jpain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Cordell W.H., Keene K.K., Giles B.K. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165–169. doi: 10.1053/ajem.2002.32643. [DOI] [PubMed] [Google Scholar]
- 3.CDC . U.S. Department of Health and Human Services; 2015. National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables.www.cdc.gov [Google Scholar]
- 4.Pines J.M., Hollander J.E. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008;51(1):1–5. doi: 10.1016/j.annemergmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Mills A.M., Shofer F.S., Chen E.H. The association between emergency department crowding and analgesia administration in acute abdominal pain patients. Acad Emerg Med. 2009;16(7):603–608. doi: 10.1111/j.1553-2712.2009.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher R.M. Physician variability in pain management: are the JCAHO standards enough? Pain Med. 2003;4(1):1–3. doi: 10.1046/j.1526-4637.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor A.B., Zwemer F.L., Hays D.P. Outcomes after intravenous opioids in emergency patients: a prospective cohort analysis. Acad Emerg Med. 2009;16(6):477–487. doi: 10.1111/j.1553-2712.2009.00405.x. [DOI] [PubMed] [Google Scholar]
- 8.Bijur P.E., Latimer C.T., Gallagher E.J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee T.H. Zero pain is not the goal. J Am Med Assoc. 2016;315(15):1575–1577. doi: 10.1001/jama.2016.1912. [DOI] [PubMed] [Google Scholar]
- 10.Hosseininejad S.M., Amini Ahidashti H., Bozorgi F. Efficacy and safety of combination therapy with ketorolac and morphine in patient with acute renal colic; a triple-blind randomized controlled clinical trial. Bull Emerg Trauma. 2017;5(3):165–170. [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen V., Motov S., Rockoff B. Development of an opioid reduction protocol in an emergency department. Am J Health Syst Pharm. 2015;72(23):2080–2086. doi: 10.2146/ajhp140903. [DOI] [PubMed] [Google Scholar]
- 12.Motov S., Hossain R. Jones and Bartlett Learning LLC; Burlington, MA: 2018. Tarascon Pain Pocketbook. [Google Scholar]
- 13.Cashman J.N. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(Suppl 5):13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 14.Seymour R.A., Ward-Booth P., Kelly P.J. Evaluation of different doses of soluble ibuprofen and ibuprofen tablets in postoperative dental pain. Br J Oral Maxillofac Surg. 1996;34(1):110–114. doi: 10.1016/s0266-4356(96)90147-3. [DOI] [PubMed] [Google Scholar]
- 15.Optimizing the Treatment of Acute Pain in the Emergency Department. Ann Emerg Med. 2017;70(3):446–448. doi: 10.1016/j.annemergmed.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Friedman B.W. Managing migraine. Ann Emerg Med. 2017;69(2):202–207. doi: 10.1016/j.annemergmed.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Golzari S.E., Soleimanpour H., Rahmani F. Therapeutic approaches for renal colic in the emergency department: a review article. Anesthesiol Pain Med. 2014;4(1) doi: 10.5812/aapm.16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang A.K., Bijur P.E., Esses D. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. J Am Med Assoc. 2017;318(17):1661–1667. doi: 10.1001/jama.2017.16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lethaby A., Duckitt K., Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400. doi: 10.1002/14651858.CD000400.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Friedman B.W., Dym A.A., Davitt M. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. J Am Med Assoc. 2015;314(15):1572–1580. doi: 10.1001/jama.2015.13043. [DOI] [PubMed] [Google Scholar]
- 21.Friedman B.W., Irizarry E., Solorzano C. Diazepam is No better than placebo when added to naproxen for acute low back pain. Ann Emerg Med. 2017;70(2):169–176 e1. doi: 10.1016/j.annemergmed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey E., Worthington H., Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216(8):451–455. doi: 10.1038/sj.bdj.2014.330. [DOI] [PubMed] [Google Scholar]
- 23.Motov S., Nelson L. In: Anesthesiology Clinics Review - Pain Management: Advanced Concepts and Controversies in Emergency Department Pain Management. Fine P.G., Ashburn L.A., editors. Elsevier; New York: 2016. [DOI] [PubMed] [Google Scholar]
- 24.Motov S., Yasavolian M., Likourezos A. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177–184. doi: 10.1016/j.annemergmed.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 25.McCarberg B., D'Arcy Y. Options in topical therapies in the management of patients with acute pain. Postgrad Med. 2013;125(4 Suppl 1):19–24. doi: 10.1080/00325481.2013.1110567011. [DOI] [PubMed] [Google Scholar]
- 26.Derry S., Wiffen P.J., Kalso E.A. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;5:CD008609. doi: 10.1002/14651858.CD008609.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Rheumatology Ad Hoc Group on Use of S, Nonselective Nonsteroidal Antiinflammatory D Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59(8):1058–1073. doi: 10.1002/art.23929. [DOI] [PubMed] [Google Scholar]
- 28.Danelich I.M., Wright S.S., Lose J.M. Safety of nonsteroidal antiinflammatory drugs in patients with cardiovascular disease. Pharmacotherapy. 2015;35(5):520–535. doi: 10.1002/phar.1584. [DOI] [PubMed] [Google Scholar]
- 29.Chandok N., Watt K.D. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham P.C., Toscano E., Pham P.M. Pain management in patients with chronic kidney disease. NDT Plus. 2009;2(2):111–118. doi: 10.1093/ndtplus/sfp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel S., Koren G., Lunenfeld E. Fetal exposure to nonsteroidal anti-inflammatory drugs and spontaneous abortions. CMAJ (Can Med Assoc J) 2014;186(5):E177–E182. doi: 10.1503/cmaj.130605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black R.A., Hill D.A. Over-the-counter medications in pregnancy. Am Fam Physician. 2003;67(12):2517–2524. [PubMed] [Google Scholar]
- 33.Jara-Oseguera A., Simon S.A., Rosenbaum T. TRPV1: On the road to pain relief. Curr Mol Pharmacol. 2008;1(3):255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallet C., Barriere D.A., Ermund A. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghanem C.I., Perez M.J., Manautou J.E. Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol Res. 2016;109:119–131. doi: 10.1016/j.phrs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anitescu M., Benzon H.T., Argoff C.E. Advances in topical analgesics. Curr Opin Anaesthesiol. 2013;26(5):555–561. doi: 10.1097/01.aco.0000432514.00446.22. [DOI] [PubMed] [Google Scholar]
- 37.Derry S., Rice A.S., Cole P. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1:CD007393. doi: 10.1002/14651858.CD007393.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dezieck L., Hafez Z., Conicella A. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: a case series. Clin Toxicol. 2017;55(8):908–913. doi: 10.1080/15563650.2017.1324166. [DOI] [PubMed] [Google Scholar]
- 39.Barden J., Edwards J., Moore A. Single dose oral paracetamol (acetaminophen) for postoperative pain. Cochrane Database Syst Rev. 2004;(1):CD004602. doi: 10.1002/14651858.CD004602. [DOI] [PubMed] [Google Scholar]
- 40.Yeh Y.C., Reddy P. Clinical and economic evidence for intravenous acetaminophen. Pharmacotherapy. 2012;32(6):559–579. doi: 10.1002/j.1875-9114.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 41.Furyk J., Levas D., Close B. Intravenous versus oral paracetamol for acute pain in adults in the emergency department setting: a prospective, double-blind, double-dummy, randomised controlled trial. Emerg Med J. 2018;35(3):179–184. doi: 10.1136/emermed-2017-206787. [DOI] [PubMed] [Google Scholar]
- 42.Becker D.E., Reed K.L. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90–101. doi: 10.2344/0003-3006-59.2.90. quiz 2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golzari S.E., Soleimanpour H., Mahmoodpoor A. Lidocaine and pain management in the emergency department: a review article. Anesthesiol Pain Med. 2014;4(1) doi: 10.5812/aapm.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinnott C.J., Cogswell I.L., Johnson A. On the mechanism by which epinephrine potentiates lidocaine's peripheral nerve block. Anesthesiology. 2003;98(1):181–188. doi: 10.1097/00000542-200301000-00028. [DOI] [PubMed] [Google Scholar]
- 45.Johnson B., Herring A., Shah S. Door-to-block time: prioritizing acute pain management for femoral fractures in the ED. Am J Emerg Med. 2014;32(7):801–803. doi: 10.1016/j.ajem.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Wilson C. Feeling blocked? Another pain management tool in the emergency department. Ann Emerg Med. 2018;72(2):120–126. doi: 10.1016/j.annemergmed.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y., Kang J., Zhang Y. Influence of greater occipital nerve block on pain severity in migraine patients: a systematic review and meta-analysis. Am J Emerg Med. 2017;35(11):1750–1754. doi: 10.1016/j.ajem.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 48.Beaudoin F.L., Haran J.P., Liebmann O. A comparison of ultrasound-guided three-in-one femoral nerve block versus parenteral opioids alone for analgesia in emergency department patients with hip fractures: a randomized controlled trial. Acad Emerg Med. 2013;20(6):584–591. doi: 10.1111/acem.12154. [DOI] [PubMed] [Google Scholar]
- 49.Haines L., Dickman E., Ayvazyan S. Ultrasound-guided fascia iliaca compartment block for hip fractures in the emergency department. J Emerg Med. 2012;43(4):692–697. doi: 10.1016/j.jemermed.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 50.Flores S., Herring A.A. Ultrasound-guided greater auricular nerve block for emergency department ear laceration and ear abscess drainage. J Emerg Med. 2016;50(4):651–655. doi: 10.1016/j.jemermed.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Blaivas M., Adhikari S., Lander L. A prospective comparison of procedural sedation and ultrasound-guided interscalene nerve block for shoulder reduction in the emergency department. Acad Emerg Med. 2011;18(9):922–927. doi: 10.1111/j.1553-2712.2011.01140.x. [DOI] [PubMed] [Google Scholar]
- 52.Stone M.B., Carnell J., Fischer J.W. Ultrasound-guided intercostal nerve block for traumatic pneumothorax requiring tube thoracostomy. Am J Emerg Med. 2011;29(6):697. doi: 10.1016/j.ajem.2010.06.014. e1-2. [DOI] [PubMed] [Google Scholar]
- 53.Flores S., Herring A.A. Ultrasound-guided dorsal penile nerve block for ED paraphimosis reduction. Am J Emerg Med. 2015;33(6):863 e3–5. doi: 10.1016/j.ajem.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Wallace M.S., Ridgeway B.M., Leung A.Y. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000;92(1):75–83. doi: 10.1097/00000542-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Tremont-Lukats I.W., Hutson P.R., Backonja M.M. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22(3):266–271. doi: 10.1097/01.ajp.0000169673.57062.40. [DOI] [PubMed] [Google Scholar]
- 56.Firouzian A., Alipour A., Rashidian Dezfouli H. Does lidocaine as an adjuvant to morphine improve pain relief in patients presenting to the ED with acute renal colic? A double-blind, randomized controlled trial. Am J Emerg Med. 2016;34(3):443–448. doi: 10.1016/j.ajem.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 57.Sin B., Cao J., Yang D. Intravenous lidocaine for intractable renal colic unresponsive to standard therapy. Am J Therapeut. 2018 doi: 10.1097/MJT.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 58.Soleimanpour H., Hassanzadeh K., Vaezi H. Effectiveness of intravenous lidocaine versus intravenous morphine for patients with renal colic in the emergency department. BMC Urol. 2012;12:13. doi: 10.1186/1471-2490-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood P.B. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 60.Gaffigan M.E., Bruner D.I., Wason C. A randomized controlled trial of intravenous haloperidol vs. Intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326–334. doi: 10.1016/j.jemermed.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Friedman B.W., Esses D., Solorzano C. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Ann Emerg Med. 2008;52(4):399–406. doi: 10.1016/j.annemergmed.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 62.Friedman B.W., Irizarry E., Solorzano C. Randomized study of IV prochlorperazine plus diphenhydramine vs IV hydromorphone for migraine. Neurology. 2017;89(20):2075–2082. doi: 10.1212/WNL.0000000000004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bigal M.E., Bordini C.A., Speciali J.G. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141–148. doi: 10.1016/s0736-4679(02)00502-4. [DOI] [PubMed] [Google Scholar]
- 64.Honkaniemi J., Liimatainen S., Rainesalo S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781–787. doi: 10.1111/j.1526-4610.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 65.Weaver C.S., Jones J.B., Chisholm C.D. Droperidol vs prochlorperazine for the treatment of acute headache. J Emerg Med. 2004;26(2):145–150. doi: 10.1016/j.jemermed.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Thomas M.C., Musselman M.E., Shewmaker J. Droperidol for the treatment of acute migraine headaches. Ann Pharmacother. 2015;49(2):233–240. doi: 10.1177/1060028014554445. [DOI] [PubMed] [Google Scholar]
- 67.Roldan C.J., Chambers K.A., Paniagua L. Randomized controlled double-blind trial comparing haloperidol combined with conventional therapy to conventional therapy alone in patients with symptomatic gastroparesis. Acad Emerg Med. 2017;24(11):1307–1314. doi: 10.1111/acem.13245. [DOI] [PubMed] [Google Scholar]
- 68.Ramirez R., Stalcup P., Croft B. Haloperidol undermining gastroparesis symptoms (HUGS) in the emergency department. Am J Emerg Med. 2017;35(8):1118–1120. doi: 10.1016/j.ajem.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Vinson D.R., Drotts D.L. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann Emerg Med. 2001;37(2):125–131. doi: 10.1067/mem.2001.113032. [DOI] [PubMed] [Google Scholar]
- 70.D'Souza R.S., Mercogliano C., Ojukwu E. Effects of prophylactic anticholinergic medications to decrease extrapyramidal side effects in patients taking acute antiemetic drugs: a systematic review and meta-analysis. Emerg Med J. 2018;35(5):325–331. doi: 10.1136/emermed-2017-206944. [DOI] [PubMed] [Google Scholar]
- 71.Salter M.W. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Curr Top Med Chem. 2005;5(6):557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- 72.Yeaman F., Meek R., Egerton-Warburton D. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australasia (EMA) 2014;26(3):237–242. doi: 10.1111/1742-6723.12173. [DOI] [PubMed] [Google Scholar]
- 73.Motov S., Drapkin J., Likourezos A. Continuous intravenous sub-dissociative dose ketamine infusion for managing pain in the emergency department. West J Emerg Med. 2018;19(3):559–566. doi: 10.5811/westjem.2017.12.36174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motov S., Mai M., Pushkar I. A prospective randomized, double-dummy trial comparing IV push low dose ketamine to short infusion of low dose ketamine for treatment of pain in the ED. Am J Emerg Med. 2017;35(8):1095–1100. doi: 10.1016/j.ajem.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Miller J.P., Schauer S.G., Ganem V.J. Low-dose ketamine vs morphine for acute pain in the ED: a randomized controlled trial. Am J Emerg Med. 2015;33(3):402–408. doi: 10.1016/j.ajem.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 76.Goltser A., Soleyman-Zomalan E., Kresch F. Short (low-dose) ketamine infusion for managing acute pain in the ED: case-report series. Am J Emerg Med. 2015;33(4):601 e5–7. doi: 10.1016/j.ajem.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 77.Zitek T., Gates M., Pitotti C. A comparison of headache treatment in the emergency department: prochlorperazine versus ketamine. Ann Emerg Med. 2018;71(3):369–377 e1. doi: 10.1016/j.annemergmed.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 78.Motov S., Rockoff B., Cohen V. Intravenous subdissociative-dose ketamine versus morphine for analgesia in the emergency department: a randomized controlled trial. Ann Emerg Med. 2015;66(3):222–229 e1. doi: 10.1016/j.annemergmed.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Galinski M., Dolveck F., Combes X. Management of severe acute pain in emergency settings: ketamine reduces morphine consumption. Am J Emerg Med. 2007;25(4):385–390. doi: 10.1016/j.ajem.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Motov S., Mann S., Drapkin J. Intravenous subdissociative-dose ketamine versus morphine for acute geriatric pain in the Emergency Department: a randomized control trial. AJEM (Am J Emerg Med) 2018 doi: 10.1016/j.ajem.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 81.Huang C., Johnson N. Nitrous oxide, from the operating room to the emergency department. Curr Emerg Hosp Med Rep. 2016;4:11–18. doi: 10.1007/s40138-016-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasaron R., Burnweit C., Zerpa J. Nitrous oxide procedural sedation in non-fasting pediatric patients undergoing minor surgery: a 12-year experience with 1,058 patients. Pediatr Surg Int. 2015;31(2):173–180. doi: 10.1007/s00383-014-3608-5. [DOI] [PubMed] [Google Scholar]
- 83.Frolich M.A., Zhang K., Ness T.J. Effect of sedation on pain perception. Anesthesiology. 2013;118(3):611–621. doi: 10.1097/ALN.0b013e318281592d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.American Society of Anesthesiologists Task Force on S, Analgesia by N-A Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96(4):1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 85.Reinoso-Barbero F., Pascual-Pascual S.I., de Lucas R. Equimolar nitrous oxide/oxygen versus placebo for procedural pain in children: a randomized trial. Pediatrics. 2011;127(6):e1464–e1470. doi: 10.1542/peds.2010-1142. [DOI] [PubMed] [Google Scholar]
- 86.Tobias J.D. Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care. 2013;29(2):245–265. doi: 10.1097/PEC.0b013e318280d824. [DOI] [PubMed] [Google Scholar]
- 87.Marik P.E. Propofol: therapeutic indications and side-effects. Curr Pharmaceut Des. 2004;10(29):3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 88.Bandschapp O., Filitz J., Ihmsen H. Analgesic and antihyperalgesic properties of propofol in a human pain model. Anesthesiology. 2010;113(2):421–428. doi: 10.1097/ALN.0b013e3181e33ac8. [DOI] [PubMed] [Google Scholar]
- 89.Godwin S.A., Burton J.H., Gerardo C.J. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2014;63(2):247–258. doi: 10.1016/j.annemergmed.2013.10.015. e18. [DOI] [PubMed] [Google Scholar]
- 90.Soleimanpour H., Taheraghdam A., Ghafouri R.R. Improvement of refractory migraine headache by propofol: case series. Int J Emerg Med. 2012;5(1):19. doi: 10.1186/1865-1380-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moshtaghion H., Heiranizadeh N., Rahimdel A. The efficacy of propofol vs. Subcutaneous sumatriptan for treatment of acute migraine headaches in the emergency department: a double-blinded clinical trial. Pain Pract. 2015;15(8):701–705. doi: 10.1111/papr.12230. [DOI] [PubMed] [Google Scholar]
- 92.Juhasz G., Zsombok T., Jakab B. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 93.Akpunonu B.E., Mutgi A.B., Federman D.J. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Ann Emerg Med. 1995;25(4):464–469. doi: 10.1016/s0196-0644(95)70259-8. [DOI] [PubMed] [Google Scholar]
- 94.Loder E. Triptan therapy in migraine. N Engl J Med. 2010;363(1):63–70. doi: 10.1056/NEJMct0910887. [DOI] [PubMed] [Google Scholar]
- 95.Dodick D., Lipton R.B., Martin V. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414–425. doi: 10.1111/j.1526-4610.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 96.Kremer M., Salvat E., Muller A. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience. 2016;338:183–206. doi: 10.1016/j.neuroscience.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 97.Moore R.A., Straube S., Wiffen P.J. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiffen P.J., Derry S., Bell R.F. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. doi: 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLean M.J. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44(6 Suppl 5) S17-22;discussion S31-S32. [PubMed] [Google Scholar]
- 100.Smith R.V., Havens J.R., Walsh S.L. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–1174. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waldhoer M., Bartlett S.E., Whistler J.L. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 102.Motov S., Hayes B., Reiter M. Vol. 7. 2017. https://www.aaem.org/UserFiles/file/WhitePaperAcutePainManaginED102417.pdf (AAEM White Paper on Acute Pain Management in the Emergency Department). Available from: [Google Scholar]
- 103.Smith H.S. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruiz-Garcia V., Lopez-Briz E. Morphine remains gold standard in breakthrough cancer pain. BMJ. 2008;337:a3104. doi: 10.1136/bmj.a3104. [DOI] [PubMed] [Google Scholar]
- 105.Bijur P.E., Kenny M.K., Gallagher E.J. Intravenous morphine at 0.1 mg/kg is not effective for controlling severe acute pain in the majority of patients. Ann Emerg Med. 2005;46(4):362–367. doi: 10.1016/j.annemergmed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Birnbaum A., Esses D., Bijur P.E. Randomized double-blind placebo-controlled trial of two intravenous morphine dosages (0.10 mg/kg and 0.15 mg/kg) in emergency department patients with moderate to severe acute pain. Ann Emerg Med. 2007;49(4):445–453. doi: 10.1016/j.annemergmed.2006.06.030. 53 e1-2. [DOI] [PubMed] [Google Scholar]
- 107.Grissa M.H., Boubaker H., Zorgati A. Efficacy and safety of nebulized morphine given at 2 different doses compared to IV titrated morphine in trauma pain. Am J Emerg Med. 2015;33(11):1557–1561. doi: 10.1016/j.ajem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 108.Eichelbaum M., Evert B. Influence of pharmacogenetics on drug disposition and response. Clin Exp Pharmacol Physiol. 1996;23(10-11):983–985. doi: 10.1111/j.1440-1681.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 109.Pain P.A.M.I. University of Florida College of Medicine; 2016. Management and Dosing Guide American Pain Society.http://pami.emergency.med.jax.ufl.edu/ Available from: [Google Scholar]
- 110.Wightman R., Perrone J., Portelli I. Likeability and abuse liability of commonly prescribed opioids. J Med Toxicol. 2012;8(4):335–340. doi: 10.1007/s13181-012-0263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zacny J.P., Lichtor S.A. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berlin) 2008;196(1):105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kattan J.A., Tuazon E., Paone D. Public health detailing-A successful strategy to promote judicious opioid analgesic prescribing. Am J Public Health. 2016;106(8):1430–1438. doi: 10.2105/AJPH.2016.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burns S.M., Cunningham C.W., Mercer S.L. DARK classics in chemical neuroscience: fentanyl. ACS Chem Neurosci. 2018 doi: 10.1021/acschemneuro.8b00174. [DOI] [PubMed] [Google Scholar]
- 114.Thompson J.P., Thompson D.F. Nebulized fentanyl in acute pain: a systematic review. Ann Pharmacother. 2016;50(10):882–891. doi: 10.1177/1060028016659077. [DOI] [PubMed] [Google Scholar]
- 115.Deaton T., Auten J.D., Darracq M.A. Nebulized fentanyl vs intravenous morphine for ED patients with acute abdominal pain: a randomized double-blinded, placebo-controlled clinical trial. Am J Emerg Med. 2015;33(6):791–795. doi: 10.1016/j.ajem.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 116.Reynolds S.L., Bryant K.K., Studnek J.R. Randomized controlled feasibility trial of intranasal ketamine compared to intranasal fentanyl for analgesia in children with suspected extremity fractures. Acad Emerg Med. 2017;24(12):1430–1440. doi: 10.1111/acem.13313. [DOI] [PubMed] [Google Scholar]
- 117.Rech M.A., Barbas B., Chaney W. When to pick the nose: out-of-hospital and emergency department intranasal administration of medications. Ann Emerg Med. 2017;70(2):203–211. doi: 10.1016/j.annemergmed.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 118.Schaefer J.A., Mlekoday T.J. Time to opioid administration after implementation of an intranasal fentanyl protocol. Am J Emerg Med. 2015;33(12):1805–1807. doi: 10.1016/j.ajem.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 119.Poklis A. Fentanyl: a review for clinical and analytical toxicologists. J Toxicol Clin Toxicol. 1995;33(5):439–447. doi: 10.3109/15563659509013752. [DOI] [PubMed] [Google Scholar]
- 120.Monk J.P., Beresford R., Ward A. Sufentanil. A review of its pharmacological properties and therapeutic use. Drugs. 1988;36(3):286–313. doi: 10.2165/00003495-198836030-00003. [DOI] [PubMed] [Google Scholar]
- 121.Sarhill N., Walsh D., Nelson K.A. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Canc. 2001;9(2):84–96. doi: 10.1007/s005200000183. [DOI] [PubMed] [Google Scholar]
- 122.Mazer-Amirshahi M., Motov S., Nelson L.S. Hydromorphone use for acute pain: misconceptions, controversies, and risks. J Opioid Manag. 2018;14(1):61–71. doi: 10.5055/jom.2018.0430. [DOI] [PubMed] [Google Scholar]
- 123.Chang A.K., Bijur P.E., Campbell C.M. Safety and efficacy of rapid titration using 1mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: the “1+1” protocol. Ann Emerg Med. 2009;54(2):221–225. doi: 10.1016/j.annemergmed.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 124.Chang A.K., Bijur P.E., Lupow J.B. Randomized clinical trial of the 2 mg hydromorphone bolus protocol versus the “1+1” hydromorphone titration protocol in treatment of acute, severe pain in the first hour of emergency department presentation. Ann Emerg Med. 2013;62(4):304–310. doi: 10.1016/j.annemergmed.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 125.Trescot A.M., Datta S., Lee M. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–S153. [PubMed] [Google Scholar]
- 126.Beaudoin F.L., Merchant R.C., Janicki A. Preventing iatrogenic overdose: a review of in-emergency department opioid-related adverse drug events and medication errors. Ann Emerg Med. 2015;65(4):423–431. doi: 10.1016/j.annemergmed.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 127.Chang A.K., Bijur P.E., Napolitano A. Two milligrams i.v. hydromorphone is efficacious for treating pain but is associated with oxygen desaturation. J Opioid Manag. 2009;5(2):75–80. doi: 10.5055/jom.2009.0008. [DOI] [PubMed] [Google Scholar]
- 128.Minami K., Sudo Y., Miyano K. micro-Opioid receptor activation by tramadol and O-desmethyltramadol (M1) J Anesth. 2015;29(3):475–479. doi: 10.1007/s00540-014-1946-z. [DOI] [PubMed] [Google Scholar]
- 129.Grond S., Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 130.Turturro M.A., Paris P.M., Larkin G.L. Tramadol versus hydrocodone-acetaminophen in acute musculoskeletal pain: a randomized, double-blind clinical trial. Ann Emerg Med. 1998;32(2):139–143. doi: 10.1016/s0196-0644(98)70127-1. [DOI] [PubMed] [Google Scholar]
- 131.Hoogewijs J., Diltoer M.W., Hubloue I. A prospective, open, single blind, randomized study comparing four analgesics in the treatment of peripheral injury in the emergency department. Eur J Emerg Med. 2000;7(2):119–123. doi: 10.1097/00063110-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 132.Vergnion M., Degesves S., Garcet L. Tramadol, an alternative to morphine for treating posttraumatic pain in the prehospital situation. Anesth Analg. 2001;92(6):1543–1546. doi: 10.1097/00000539-200106000-00039. [DOI] [PubMed] [Google Scholar]
- 133.Fournier J.P., Azoulay L., Yin H. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186–193. doi: 10.1001/jamainternmed.2014.6512. [DOI] [PubMed] [Google Scholar]
- 134.Fournier J.P., Yin H., Nessim S.J. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418–425 e5. doi: 10.1016/j.amjmed.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 135.Ryan N.M., Isbister G.K. Tramadol overdose causes seizures and respiratory depression but serotonin toxicity appears unlikely. Clin Toxicol. 2015;53(6):545–550. doi: 10.3109/15563650.2015.1036279. [DOI] [PubMed] [Google Scholar]
- 136.DAWN Emergency department visits for drug misuse or abuse involving the pain medication tramadol 2015. June 16, 2018. https://www.samhsa.gov/data/sites/default/files/report_1966/ShortReport-1966.html Available from: [PubMed]
- 137.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155(1-3):11–17. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mahmoud M., Mason K.P. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 139.Tang C., Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–1904. doi: 10.2147/JPR.S139387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rancourt M.P., Albert N.T., Cote M. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115(4):958–962. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 141.Abdulatif M., Fawzy M., Nassar H. The effects of perineural dexmedetomidine on the pharmacodynamic profile of femoral nerve block: a dose-finding randomised, controlled, double-blind study. Anaesthesia. 2016;71(10):1177–1185. doi: 10.1111/anae.13603. [DOI] [PubMed] [Google Scholar]
- 142.Davis M.P. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209–219. doi: 10.1016/j.suponc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 143.Tzschentke T.M. Behavioral pharmacology of buprenorphine, with a focus on preclinical models of reward and addiction. Psychopharmacology (Berlin) 2002;161(1):1–16. doi: 10.1007/s00213-002-1003-8. [DOI] [PubMed] [Google Scholar]
- 144.D'Onofrio G., O'Connor P.G., Pantalon M.V. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. J Am Med Assoc. 2015;313(16):1636–1644. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cisewski D.H., Santos C., Koyfman A. Approach to buprenorphine use for opioid withdrawal treatment in the emergency setting. Am J Emerg Med. 2018 doi: 10.1016/j.ajem.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 146.Davis M.P. Buprenorphine in cancer pain. Support Care Canc. 2005;13(11):878–887. doi: 10.1007/s00520-005-0849-9. [DOI] [PubMed] [Google Scholar]
- 147.Raffa R.B., Haidery M., Huang H.M. The clinical analgesic efficacy of buprenorphine. J Clin Pharm Therapeut. 2014;39(6):577–583. doi: 10.1111/jcpt.12196. [DOI] [PubMed] [Google Scholar]
- 148.Mozafari J., Masoumi K., Forouzan A. Sublingual buprenorphine efficacy in renal colic pain relief: a randomized placebo-controlled clinical trial. Pain Ther. 2017;6(2):227–234. doi: 10.1007/s40122-017-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Payandemehr P., Jalili M., Mostafazadeh Davani B. Sublingual buprenorphine for acute renal colic pain management: a double-blind, randomized controlled trial. Int J Emerg Med. 2014;7(1):1. doi: 10.1186/1865-1380-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jalili M., Fathi M., Moradi-Lakeh M. Sublingual buprenorphine in acute pain management: a double-blind randomized clinical trial. Ann Emerg Med. 2012;59(4):276–280. doi: 10.1016/j.annemergmed.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 151.Kalviainen R. Intranasal therapies for acute seizures. Epilepsy Behav. 2015;49:303–306. doi: 10.1016/j.yebeh.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 152.Bailey A.M., Baum R.A., Horn K. Review of intranasally administered medications for use in the emergency department. J Emerg Med. 2017;53(1):38–48. doi: 10.1016/j.jemermed.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 153.Tsze D.S., Ieni M., Fenster D.B. Optimal volume of administration of intranasal midazolam in children: a randomized clinical trial. Ann Emerg Med. 2017;69(5):600–609. doi: 10.1016/j.annemergmed.2016.08.450. [DOI] [PMC free article] [PubMed] [Google Scholar]