Abstract
Dynamic stretching (DS) is often performed during warm-up to help avoid hamstring muscle injuries, increase joint flexibility, and optimize performance. We examined the effects of DS of the hamstring muscles on passive knee extension range of motion (ROM), passive torque (PT) at the onset of pain (as a measure of stretch tolerance), and passive stiffness of the muscle-tendon unit over an extended period after stretching. Twenty-four healthy subjects participated, with 12 each in the experimental and control groups. Stretching was performed, and measurements were recorded using an isokinetic dynamometer pre-intervention, and at 0, 15, 30, 45, 60, 75, and 90 min post-intervention. DS consisted of ten 30-s sets of 15 repetitions of extension and relaxation of the hamstrings. ROM increased significantly (range, 7%–10%) immediately after DS, and the increase was sustained over 90 min. PT at the onset of pain also increased immediately by 10% but returned to baseline by 30 min. Passive stiffness decreased significantly (range, 7.9%–16.7%) immediately after DS, and the decrease was sustained over 90 min. Post-DS values were normalized to pre-DS values for the respective outcomes in both groups. ROM was significantly higher (range, 7.4%–10%) and passive stiffness was significantly lower (range, 5.4%–14.9%) in the experimental group relative to the control group at all time points. Normalized PT values at the onset of pain were significantly higher in the experimental group at 0–15 min than in the controls, but the differences were smaller at 30–45 min and not significant thereafter. We conclude that DS increases ROM and decreases passive stiffness in a sustained manner, and increases PT at the onset of pain for a shorter period. Overall, our results indicate that when performed prior to exercise, DS is beneficial for the hamstring muscles in terms of increasing flexibility and reducing stiffness.
Key points.
Effect of dynamic stretching (DS) on hamstring muscle flexibility was examined.
DS increased range of motion and stretch tolerance, and decreased passive stiffness.
Stretch tolerance returned more rapidly after DS than other measurement indexes.
DS performed prior to exercise is useful for improving hamstring muscle flexibility.
Key words: Flexibility, muscle stretching, retention time, muscle-tendon unit, stretch tolerance, exercise
Introduction
Hamstring strain injuries are one of the most common types of noncontact injuries experienced during sports activities and exercise (Opar et al., 2012), accounting for 12%–16% of injuries in athletes, with a reported reinjury rate as high as 22%–34% (Schmitt et al., 2012). These injuries typically occur during sports activities that involve rapid acceleration/deceleration and fast running (Opar et al., 2012). The eccentric contraction of the hamstrings that occurs during the late swing phase of running to decelerate knee extension has been reported to be associated with such injuries (Opar et al., 2012). Lack of flexibility in the hamstrings may also result in major muscle imbalances, which predispose to muscle injuries, patellar tendinopathy, patellofemoral pain, and the development of low back pain (Heiderscheit et al., 2010; Lankhorst et al., 2013; Sadler et al., 2017; van der Worp et al., 2011). In an attempt to increase joint flexibility and avoid injury, the hamstrings are frequently stretched prior to exercise (Behm et al., 2016; Chen et al., 2018b).
Stretching is often performed during the warm-up before exercise to increase joint flexibility (defined as the ability to move a joint through its complete range of motion [ROM]) (Medicine ACoS, 2017), reduce the stiffness of muscle-tendon units, and optimize performance (Behm et al., 2016). Various stretching techniques, such as static stretching (SS), dynamic stretching (DS), ballistic stretching (BS), and proprioceptive neuromuscular facilitation, are often practiced. Of these, SS, in which the target muscle is held in a lengthened position for a defined period of time (Kisner and Colby, 2017), is the most commonly performed because of its beneficial effects on reducing muscle-tendon unit stiffness and increasing stretch tolerance (Kataura et al., 2017; Magnusson et al., 1996; Matsuo et al., 2015; Mizuno et al., 2013; Morse et al., 2008; Nordez et al. 2006; Ryan et al., 2008). However, concerns have been raised that SS, BS, and proprioceptive neuromuscular facilitation may reduce muscle strength, muscle power, maximum number of repetitions, and vertical jump performance (Barroso et al., 2012; Bradley et al., 2007; Lima et al., 2016; Marek et al., 2005). In particular, if SS is prolonged and performed in isolation, it can lead to impairments (Behm et al., 2011; 2016; Kay et al., 2012), though if performed for an appropriate length of time and within a full warm-up regimen, impairments are inconsequential (Blazevich et al., 2018; Reid et al., 2018). In comparison, DS, in which limbs are moved through their active ROM by contracting the muscle group antagonist to the target muscle group without bouncing (Samukawa et al., 2011; Yamaguchi and Ishii, 2005), has been reported to improve muscle strength, muscle power, sprint time, vertical jump performance, and golf swing performance (Hough et al., 2009; Little and Williams, 2006; Moran et al., 2009; Sekir et al., 2010; Yamaguchi and Ishii, 2005). As a result, DS has been recently promoted for warm-up prior to exercise (Behm et al., 2016).
While DS has been reported to improve joint ROM (Chaouachi et al., 2017; Chen et al., 2018a; Herda et al., 2013; Mizuno, 2017; Mizuno and Umemura, 2016; Samukawa et al., 2011), there have been conflicting reports about its effect on muscle-tendon unit stiffness (Chen et al., 2018a; Herda et al., 2013; Mizuno, 2017; Mizuno and Umemura, 2016; Samukawa et al., 2011). Furthermore, it has been suggested that the improvement in joint ROM seen after DS is due to increased stretch tolerance rather than reduced stiffness of the muscle-tendon unit (Mizuno, 2017; Mizuno and Umemura, 2016). As stretching routines are typically performed 15–60 min prior to competition or exercise (Woods et al., 2007), it is important to not only clarify the effect of DS on the stiffness of the muscle-tendon unit and stretch tolerance, but also to investigate the temporal profile of these effects. The purpose of this study was to examine the effects of DS of the hamstring muscles on the ROM of passive knee extension, passive torque (PT) at the onset of pain (as a measure of stretch tolerance) (Kataura et al., 2017; Matsuo et al., 2015; Mizuno et al., 2013), and passive stiffness of the muscle-tendon unit over an extended period after stretching. We hypothesized that DS performed before exercise would improve performance of the hamstring muscles by increasing ROM and PT at the onset of pain and reducing the passive stiffness of the muscle-tendon unit, and that the effects on passive stiffness would not be maintained as long as the changes in ROM or PT at the onset of pain.
Methods
Participants
Twenty-four healthy individuals between the ages of 20 and 23 years were recruited from a university population and sequentially assigned to either an experimental or control group (n = 12/group). All of the participants were selected on the basis of a screening test to confirm that they were not able to fully extend their knee from the measuring start position (Figure 1A). Potential subjects who had a history of hip or lower extremity joint surgery, had lower extremity neurological complications, were receiving hormones and/or medications that could affect their muscles, or who were involved in competitive sports or regular resistance, aerobic, or flexibility training were excluded from participation. There were no significant differences in age (21.8 ± 0.8 years vs. 21.0 ± 0.9 years), sex (6 males and 6 females in both groups), height (1.68 ± 0.08 m vs. 1.64 ± 0.08 m), body mass (61.9 ± 9.8 kg vs. 57.5 ± 7.4 kg), or body mass index (22.0 ± 2.2 kg/m2 vs. 21.5 ± 2.3 kg/m2) between the experimental and control groups.
Figure 1.
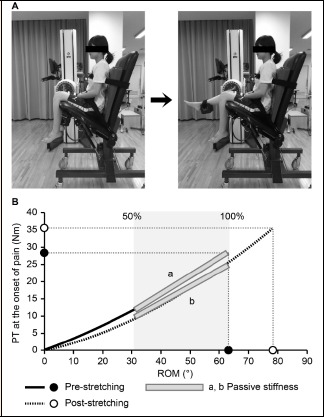
(A) Position for passive knee extension test using an isokinetic dynamometer. Left - Start position with the knee and hip both in approximately 110° of flexion. Right - The knee is extended passively until the subject experiences the onset of pain (maximum knee extension). (B) Typical torque-angle curves showing pre-stretching and 15-min post-stretching data. Stiffness was determined using a regression line between 50% and 100% of the pre-stretching range of motion (ROM)-passive torque (PT) relationship. PT at the onset of pain and ROM were determined using the PT and knee extension angle at the onset of pain.
Study design
The study protocol was reviewed and approved by the Ethics Committee for Research on Human Subjects at Nihon Fukushi University (approval number 14-23). The study abided by the principles of the Declaration of Helsinki, and written informed consent was obtained from each participant prior to participation in the study.
The participants were instructed to maintain their normal dietary habits and to refrain from vigorous physical activity for two days before the experiment. At least one day before the testing day, the participants reconfirmed their health history and familiarized themselves with the equipment and experimental procedure. The experiment was performed in the university’s temperature-controlled laboratory at 26lC. The ROM of passive knee extension, PT at the onset of pain, and passive stiffness of the hamstring muscles were measured using an isokinetic dynamometer (Primus RS, BTE Technologies, Hanover, MD, USA) at various time points before and after DS, as shown in Figure 1. Specifically, measurements were taken before DS (PRE) and at 0, 15, 30, 45, 60, 75, and 90 min after DS (POST0, POST15, POST30, POST45, POST60, POST75, and POST90, respectively) in the experimental group. Measurements were obtained at the same time points but without any DS in the control group.
Dynamic stretching protocol
Each subject performed DS of their right leg only to keep experimental conditions consistent between subjects. The right leg was dominant in all 24 subjects; this was determined by asking the subjects which leg they use to kick a soccer ball. No warm-up was performed in any subjects to eliminate any potential interaction between warm-up and stretching. The subjects stood upright with their feet parallel and facing forward while holding onto parallel bars with both hands (original standing position; Figure 2A). The subjects were then instructed to contract their hip flexors intentionally once every 2 s so that their hip joints flexed while keeping their knees extended so that their right leg swung up to the anterior aspect of their body and their right hamstring muscles were stretched (Figure 2B). Each subject performed 5 repetitions of the stretching exercise slowly while learning to accurately perform the motion, and then 10 repetitions as quickly and powerfully as possible without bouncing and in synchrony with the rhythm of a digital metronome set to 30 beats/min (0.5 Hertz) (Yamaguchi and Ishii, 2005). DS was performed for 5 min, comprising ten 30-s long sets (2-s stretch × 15 repetitions) with a 20-s rest period between sets during which the subject was allowed to rest in the original standing position. A 5-min period of DS was selected because our previous studies showed that 5 min of static stretching at a tolerable intensity and without pain significantly increased ROM and PT, and decreased passive stiffness after stretching (Matsuo et al., 2013; 2015).
Figure 2.
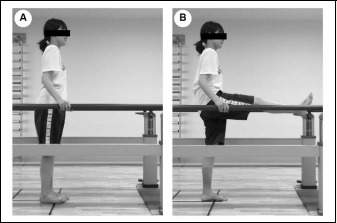
The procedure for dynamic stretching of the hamstring muscles. (A) The subjects started in a standing position with their feet parallel and facing forward and their hands holding the parallel bars (original standing position). (B) The subjects contracted their hip flexors intentionally with the knee extended and the hip joint flexed so that the leg swung up anteriorly (the subjects’ hamstrings were stretched).
Measurement procedure and outcomes
Measurements were made with the participant seated on a chair (Figure 1A) with an attached isokinetic dynamometer. The seat of the chair was tilted maximally to 35° from the horizontal position and a wedge-shaped cushion was inserted between the trunk and the backrest; this set the angle between the seat and the back at approximately 60°. The participant’s chest, pelvis, and right thigh were stabilized with Velcro® straps. The knee joint was aligned with the axis of rotation of the isokinetic dynamometer, and the lever arm attachment was placed just proximal to the medial malleolus and stabilized with Velcro® straps. Next, the knee was extended passively at 5°/s to the point of maximum knee extension just before the subjective onset of pain (Figure 1A). We confirmed with electromyography that the muscles did not contract during this passive knee extension. With the isokinetic dynamometer programmed in passive mode, the torque and angle signals were continuously measured and recorded. Gravity correction was not performed when the torque-angle curve was measured, per our previous protocols (Kataura et al., 2017; Matsuo et al., 2013; 2015). All output signals underwent analog-to-digital conversion and were recorded using LabChart 8.0 (PowerLab 4/20T, ADInstruments, NSW, Australia) for torque-angle relationship analyses (Figure 1B) (Kataura et al., 2017; Matsuo et al., 2013; 2015). In the initial position (0°), there were no significant differences between groups with regard to the average hip and knee flexion angles, as described below. ROM (in degrees) was defined as the maximum knee extension angle from the initial position (0°) until the onset of pain, and PT at the onset of pain (in Nm) was defined as the torque at the onset of pain (Kataura et al., 2017; Matsuo et al., 2015; Mizuno et al., 2013). Passive stiffness (in Nm/°) was calculated as the slope of the regression line (using the least-squares method (Kataura et al., 2017; Matsuo et al., 2013; 2015)) of the torque-angle relationship between 50% and 100% of the maximum knee extension angle (Figure 1B), as we and others have described (Kataura et al., 2017; Kubo et al., 2007; 2017; Matsuo et al., 2013; 2015). Passive stiffness was calculated from the same knee extension angle range before and after stretching, and the pre-stretching value was compared with that after stretching. The lower pre-stretching and post-stretching maximum knee extension angles were used for passive stiffness calculation.
Test/retest reliability was determined for all measurement outcomes in a separate cohort of four men and three women (age, 21.9 ± 0.7 years; height, 1.67 ± 0.10 m; body mass, 61.3 ± 7.3 kg) using two tests performed 1–7 days apart at the same time of day (±1 h). Intraclass correlation coefficients and their 95% confidence intervals were highly reliable for all the measures (ROM: 0.94 [0.74–0.99], PT at the onset of pain: 0.95 [0.76–0.99], and passive stiffness: 0.90 [0.59–0.98]) (Shrout and Fleiss, 1979). The coefficient of variation for the measures also showed acceptable reliability (ROM: 6.5%, PT at the onset of pain: 3.9%, and passive stiffness: 7.5%) (Dow, 1976).
Statistical analysis
G*Power software (version 3.1.9.2; University of Kiel, Kiel, Germany) was used to estimate the sample size required for this repeated-measures study (Faul et al., 2007); a minimum sample size of n = 10/group was required to reach a statistical power level (1 − β) of 0.95 based on an α level of 0.05 and a predicted effect size of 0.40. To allow for possible dropout, 12 participants were recruited for each group.
Sigma Plot 13 software (Systat Software Inc., San Jose, CA, USA) was used for all statistical analyses. Nonparametric tests were performed on the passive stiffness data, which was shown to be non-normally distributed using the Shapiro-Wilk normality test (Shapiro and Wilk, 1965). The data for ROM and PT at the onset of pain were normally distributed. The Friedman repeated-measures analysis of variance on ranks was used to calculate temporal changes in outcome measures (ROM, PT at the onset of pain, and passive stiffness) within each group. When a significant time effect was found, post-hoc analysis with Wilcoxon signed-rank tests was conducted with a Bonferroni correction applied to detect significant differences from pre-DS values. Between-group differences in pre-DS values for all outcome measures were assessed using the Mann-Whitney U test. Post-DS values (POST0, POST15, POST30, POST45, POST60, POST75, and POST90) were normalized to the pre-DS values for the respective outcome in both groups. Between-group differences in post-DS value for all outcome measures were then assessed for each time point using the Mann-Whitney U test. For transparency, both significant differences (p < 0.05) and differences approaching significance (0.05 ≤ p < 0.1) are reported where appropriate. Owing to the non-normal data distribution, all data are presented as medians and interquartile ranges unless otherwise noted.
Results
Baseline measures
There were no significant between-group differences in the average angles of hip flexion (111.3° ± 1.7° vs. 111.4° ± 1.6°) and knee flexion (110.7° ± 1.4° vs. 109.8° ± 2.3°) at the beginning of the test. There were also no significant between-group differences in the pre-DS values of any of the outcome measures (Table 1).
Table 1.
Changes in range of motion, passive torque at the onset of pain, and passive stiffness before and after dynamic stretching. Values are expressed as median (interquartile range).
| Condition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome measure | PRE | POST0 | POST15 | POST30 | POST45 | POST60 | POST75 | POST90 |
| Experimental group (n = 12) | ||||||||
| ROM (°) | 84.9 (77.5–90.0) |
92.5* (86.0–97.4) |
94.0* (86.0–98.9) |
91.9* (85.7–99.6) |
91.5* (85.5–98.9) |
91.0* (83.7–96.9) |
90.5* (84.7–95.2) |
89.4* (85.4–95.4) |
| PT at the onset of pain (Nm) | 27.9 (24.5–35.7) |
33.6* (26.5–41.5) |
32.5* (26.4–41.3) |
30.7 (25.8–36.0) |
30.7 (25.6–38.6) |
30.6 (25.4–36.4) |
30.0 (23.7–38.1) |
29.8 (25.0–36.8) |
| Passive stiffness (Nm/°) | .388 (.336–.491) |
.372* (.296–.447) |
.344* (.290–.435) |
.337* (.290–.377) |
.335* (.284–.403) |
.324* (.306–.426) |
.329* (.276–.423) |
.336* (.287–.387) |
| Control group (n = 12) | ||||||||
| ROM (°) | 82.8 (81.0–89.6) |
84.9 (79.7–92.0) |
82.9 (80.7–91.8) |
82.5 (81.0–91.1) |
83.5 (81.7–91.9) |
83.9 (81.7–91.8) |
84.4 (82.5–90.4) |
83.5 (81.7–91.1) |
| PT at the onset of pain (Nm) | 24.8 (22.1–32.4) |
23.9 (22.9–33.1) |
25.1 (22.1–30.8) |
23.9 (22.3–30.7) |
23.9 (21.9–32.8) |
23.7 (22.5–31.8) |
24.4 (21.8–31.8) |
23.9 (22.2–31.2) |
| Passive stiffness (Nm/°) | .366 (.290–.410) |
.351 (.299–.422) |
.358 (.274–.410) |
.342 (.285–.404) |
.340 (.279–.410) |
.332 (.284–.411) |
.330 (.280–.401) |
.320 (.279–.403) |
ROM, range of motion; PT, passive torque; PRE, before stretching; POST, after stretching. The asterisk indicates a significant (p < 0.05) difference value.
Intragroup analysis of changes in outcome measures after DS
No significant temporal changes in any of the outcome measures in the control group were found, but significant changes in all outcome measures were observed over time in the experimental group (Table 1, Figure 3). In the experimental group, knee ROM increased significantly by a median of 8.1° (range, 6.8°–11.5°; 10% increase over pre-DS values) immediately after DS. The significant increase in knee ROM was sustained over 90 min (15 min: 9.5° [8.0°–11.5°], 30 min: 9.0° [6.0°–11.9°], 45 min: 8.0° [5.5°–10.3°], 60 min: 7.0° [4.9°–8.0°], 75 min: 8.0° [3.8°–9.2°], and 90 min: 8.0° [3.6°–9.0°]) and was 9.4% greater than the pre-DS value at 90 min after DS.
Figure 3.
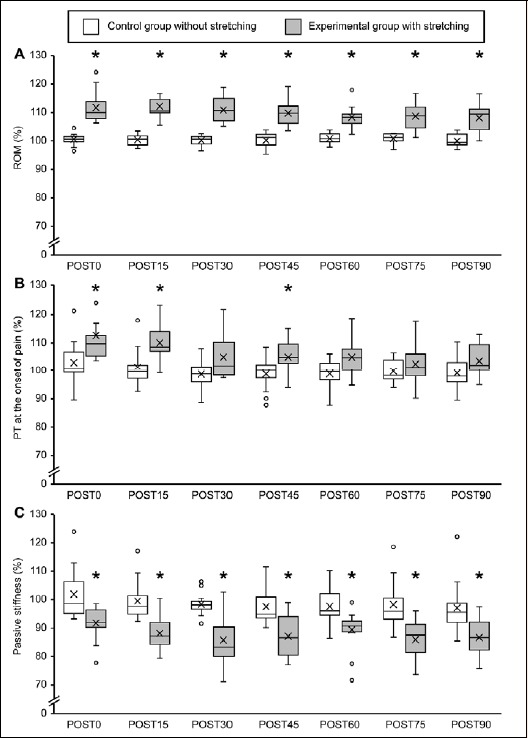
Boxplot figures showing percent changes in (A) range of motion, (B) passive torque at the onset of pain, (C) passive stiffness before and after dynamic stretching. The line within the box marks the median, crosses represent the mean, the boundary of the box indicates the 25th and 75th percentiles, the error bars indicate the 10th and 90th percentiles, and the dots above and below the error bars represent outliers. The asterisk indicates a significant (p < 0.05) difference from the time-matched control group. ROM, range of motion; PT, passive torque; PRE, before stretching; POST, after stretching
Similarly, PT at the onset of pain increased significantly by 9.7% immediately after DS; however, post-DS PT values were not significantly different from pre-DS values 30 min after DS. Passive stiffness decreased significantly by 7.9% immediately after DS, continued to be lower than the control group over 90 min, and was 13.4% lower than the pre-DS value at 90 min after DS.
Intergroup analysis of changes in normalized outcome measures after DS
Normalized ROM values were significantly higher in the experimental group at all time points than in the control group (higher by 9.4% at 0 min, 9.8% at 15 min, 10.0% at 30 min, 8.5% at 45 min, 7.4% at 60 min, 7.5% at 75 min, and 9.8% at 90 min; Figure 3A). Similarly, normalized PT values at the onset of pain were significantly higher (by 8.8%) in the experimental group at 0 and 15 min, approached significance at 30 min (3.2% higher, P = 0.069), and were slightly but significantly higher by 4.7% at 45 min after DS (Figure 3B). Normalized passive stiffness values were significantly lower in the experimental group at all time points after DS (lower by 6.5% at 0 min, 10.5% at 15 min, 14.9% at 30 min, 8.4% at 45 min, 5.4% at 60 min, 8.3% at 75 min, and 9.0% at 90 min; Figure 3C).
Discussion
We investigated whether DS would influence the following parameters of hamstring flexibility: ROM, PT at the onset of pain, and passive stiffness of the hamstring muscles. In addition, we studied the long-term effects of DS by evaluating the temporal changes in each flexibility parameter after DS. We found that DS produced an immediate increase in passive knee extension ROM, a decrease in passive stiffness of the hamstrings, and an increase in PT at the onset of pain. Knee ROM increased significantly by 10% (median, 8.1°) relative to baseline immediately after DS. Although the extent of the effect of DS on ROM differed between individuals, the significant increase in ROM was sustained over 90 min (median, 8.0°).
The reduction in passive stiffness was also maintained for ≥ 90 minutes following DS. In contrast, reductions in passive stiffness have been reported to be less prolonged after SS. For example, SS reduced the passive stiffness of the triceps surae for only 15–20 min (Mizuno et al., 2013; Ryan et al., 2008) and the passive stiffness of the hamstrings for less than 60 min in a previous report (Magnusson et al., 1996). Furthermore, in our study the increase in PT at the onset of pain lasted for a shorter duration (15–30 min) than the passive stiffness reduction following DS of the hamstrings. In contrast, the effects of SS of the triceps surae on PT at the onset of pain and on ROM lasted longer than its effects on passive stiffness (Mizuno et al., 2013).
Our results also differed from those of previous studies that investigated the effects of DS on the triceps surae (Mizuno, 2017; Mizuno and Umemura, 2016). DS of the triceps surae did not influence its stiffness (Mizuno, 2017; Mizuno and Umemura, 2016) but did increase ROM; however, the increase in ROM lasted for only 10 min (Mizuno and Umemura, 2016). We speculate that these differences in the effects of DS could be due to differences in the target muscles (hamstrings vs. triceps surae) and in the DS conditions of the studies. The DS of the triceps surae was performed by adding a light resistance to the antagonistic muscles of the triceps and using a lower number of sets (1–7) than we used in the current study (Mizuno, 2017). The prolonged reduction in passive stiffness following our DS protocol might have resulted in the more sustained increase (90 min) in ROM in our study.
Passive stiffness, which is calculated from the torque–angle curve, is thought to reflect the viscoelasticity of the muscle-tendon unit (Magnusson et al., 1996). In contrast, stretch tolerance is thought to be the tolerance to the tensile strength produced in muscles that are subjected to passive extension. The index used to reflect this in previous studies has been PT at the onset of pain (Kataura et al., 2017; Matsuo et al., 2015; Mizuno et al., 2013). Thus, the immediate increase in knee ROM that occurs following DS likely results from both a decrease in passive stiffness (i.e., changes in the viscoelasticity of the muscle-tendon unit) and an increase in PT at the onset of pain (i.e., an increased stretch tolerance due to altered sensation). Since the passive stiffness changed greatly following DS, it can be inferred that a physiological response that causes elasticity change in the muscle-tendon complex occurred. Specifically, there is a possibility that some elastic elements in the muscle-tendon complex were changed, such as fascia, elastic proteins such as titin (also known as connectin), and tendons. However, it is important to note that the changes in passive stiffness of the hamstring muscles after DS persisted for a longer period than did the changes in PT at the onset of pain. These results suggest that the sustained increase in knee ROM following DS is primarily attributable to the reduced passive stiffness of the hamstring muscle–tendon unit (Opplert and Babault, 2018) and not to stretch tolerance.
Our finding that ten 30-s sets of 15 repetitions of DS increased knee extension ROM is consistent with the results of previous studies (Chen et al., 2018a; Herda et al., 2013). Both the duration and intensity of stretching have been shown to influence passive stiffness of the muscle-tendon unit (Opplert and Babault, 2018). For example, while relatively short periods of SS did not affect passive stiffness of the hamstrings (Magnusson et al., 1998; 2000), longer SS techniques decreased passive stiffness (Magnusson et al., 1996; Matsuo et al., 2013; Nordez et al., 2006) in a more sustained manner (Ryan et al., 2008). In addition, the intensity of SS has been reported to decrease passive stiffness in a dose-dependent manner (Kataura et al., 2017). Similarly, a higher number of repetitions and addition of resistance to the antagonistic muscles have both been shown to increase ROM following DS of the triceps surae (Mizuno, 2017). Taken together, these data suggest that the relatively high intensity of DS in our study resulted in a greater dose-dependent change in hamstring flexibility, which in turn resulted in a more prolonged effect on passive stiffness. Thus, the reduction in passive stiffness was sustained for a longer time than the effect on PT at the onset of pain.
This study had several limitations. First, we used a nonrandomized, sequential study design where the control group was selected to match the baseline characteristics of the experimental group. The experimental group was unavailable for a follow-up visit; this prevented us from investigating outcomes with and without DS in the same subjects. Such paired analysis could presumably have allowed more robust investigations of the effect of DS. Second, we investigated only the effect of DS, but not other stretching techniques such as SS and BS. Finally, we did not measure hamstring tension or extension during DS. As DS relies on a subject consciously and actively contracting antagonist muscles to stretch the targeted muscle (Samukawa et al., 2011; Yamaguchi and Ishii, 2005), quantifying the loaded tension in real time is challenging. Newer techniques using videotaping and electrogoniometry to measure angular velocity and ROM could potentially allow real-time quantification of the loaded tension in the hamstrings.
Conclusion
We investigated the acute and sustained effects of DS on flexibility parameters in healthy volunteers who performed 10 sets of DS (15 repetitions per set) of the hamstring muscles. We found that DS caused a sustained reduction in passive stiffness of the hamstrings and increase in knee ROM, as well as a less lasting increase in PT at the onset of pain. As increased passive stiffness of the hamstrings and decreased knee ROM are both risk factors for hamstring injury during sports (Goldman and Jones, 2011; Woods et al., 2004), our results suggest that DS of the hamstrings before exercise may help to prevent injuries.
Acknowledgements
We thank Wakako Tsuchida, PhD, from Nihon Fukushi University for providing encouragement and support during this study. We also thank Brielle Rosa, DVM, PhD, and Peter Mittwede, MD, PhD from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported in part by JSPS KAKENHI Grant Nos. 26750288 (SM) and 16K01503 (SS) from the Japan Society for the Promotion of Science (JSPS) and in part by a grant from the Public Advertisement Research Project of Nihon Fukushi University. The funding sources had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The experiments comply with the current laws of the country in which they were performed. The authors have no conflicts of interests to declare.
Biographies

Masahiro IWATA
Employment
Associate professor, Department of Rehabilitation, Faculty of Health Sciences, Nihon Fukushi University, Handa, Japan
Degree
Ph.D. in Physical Therapy
Research interests
Exercise physiology, skeletal muscle biology, and mechanobiology
E-mail: iwata-m@n-fukushi.ac.jp

Ayano YAMAMOTO
Employment
Kamiiida Daiichi General Hospital, Nagoya, Japan
Degree
B.H.S. in Physical Therapy
Research interests
Renal rehabilitation in patients with chronic kidney disease
E-mail: hr110529@n-fukushi.ac.jp

Shingo MATSUO
Employment
Assistant professor, Department of Rehabilitation, Faculty of Health Sciences, Nihon Fukushi University, Handa, Japan
Degree
Ph.D. in Rehabilitation Science
Research interests
Stretching exercise and muscle flexibility.
E-mail: matsuo@n-fukushi.ac.jp

Genki HATANO
Employment
Lead Researcher, Institute of Sport Science, ASICS Corporation, Kobe, Japan
Degree
M.S. in Physical Therapy
Research interests
Exercise physiology, biomechanics, and sports engineering
E-mail: genki.hatano@asics.com

Manabu MIYAZAKI
Employment
Assistant professor, Department of Physical Therapy, Faculty of Medical Science for Health, Teikyo Heisei University, Toshima-ku, Japan
Degree
M.S. in Physical Therapy
Research interests
Exercise physiology and human medical engineering
E-mail: m.miyazaki@thu.ac.jp

Taizan FUKAYA
Employment
Kyoto Kujo Hospital, Kyoto, Japan
Degree
M.S. in Physical Therapy
Research interests
Exercise physiology and evaluation for physical performance
E-mail: fukaya.taizan@gmail.com

Mitsuhiro FUJIWARA
Employment
Kamiiida Rehabilitation Hospital, Nagoya, Japan
Degree
Ph.D. in Physical Therapy
Research interests
Muscle weakness and pain with work-related musculoskeletal disorders
E-mail: 32fujiwara@gmail.com

Yuji ASAI
Employment
Professor, Department of Rehabilitation, Faculty of Health Sciences, Nihon Fukushi University, Handa, Japan
Degree
Ph.D. in Medicine
Research interests
Postural control and vestibular rehabilitation
E-mail: asai@n-fukushi.ac.jp

Shigeyuki SUZUKI
Employment
Professor, Department of Health and Sports Sciences, School of Health Sciences, Asahi University, Mizuho, Japan
Degree
Ph.D. in Medicine
Research interests
Evidence based stretching for exercise and rehabilitation
E-mail: suzuki@met.nagoya-u.ac.jp
References
- Barroso R., Tricoli V., Santos Gil S.D., Ugrinowitsch C., Roschel H. (2012) Maximal strength, number of repetitions, and total volume are differently affected by static-, ballistic-, and proprioceptive neuromuscular facilitation stretching. Journal of Strength and Conditioning Research 26, 2432-2437. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Chaouachi A. (2011) A review of the effects of static and dynamic stretching on performance. European Journal of Applied Physiology 111, 2633-2651. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Blazevich A.J., Kay A.D., McHugh M. (2016) Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Applied Physiology, Nutrition, and Metabolism 41, 1-11. [DOI] [PubMed] [Google Scholar]
- Blazevich A.J., Gill N.D., Kvorning T., Kay A.D., Goh A.G., Hilton B., Drinkwater E.J., Behm D.G. (2018) No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Medicine and Science in Sports and Exercise 50, 1258-1266. [DOI] [PubMed] [Google Scholar]
- Bradley P.S., Olsen P.D., Portas M.D. (2007) The effect of static, ballistic, and proprioceptive neuromuscular facilitation stretching on vertical jump performance. Journal of Strength and Conditioning Research 21, 223-226. [DOI] [PubMed] [Google Scholar]
- Chaouachi A., Padulo J., Kasmi S., Othmen A.B., Chatra M., Behm D.G. (2017. Unilateral static and dynamic hamstrings stretching increases contralateral hip flexion range of motion. Clinical Physiology and Functional Imaging 37, 23-29. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Xin Y., Lee K.W., Lin M.J., Lin J.J. (2018a) Acute effects of different dynamic exercises on hamstring strain risk factors. PLoS One 13, e0191801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Ye X., Wang W.T., Chen Y.S., Tseng W.C. (2018b) Differential effects of different warm-up protocols on repeated sprint-induced muscle damage. Journal of Strength and Conditioning Research 32, 3276-3284. [DOI] [PubMed] [Google Scholar]
- Dow D.D. (1976) The use and misuse of the coefficient of variation in analysing geographical variation in birds. Emu 76, 25-29. [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175-191. [DOI] [PubMed] [Google Scholar]
- Goldman E.F., Jones D.E. (2011) Interventions for preventing hamstring injuries: a systematic review. Physiotherapy 97, 91-99. [DOI] [PubMed] [Google Scholar]
- Heiderscheit B.C., Sherry M.A., Silder A., Chumanov E.S., Thelen D.G. (2010) Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. The Journal of Orthopaedic and Sports Physical Therapy 40, 67-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herda T.J., Herda N.D., Costa P.B., Walter-Herda A.A., Valdez A.M., Cramer J.T. (2013) The effects of dynamic stretching on the passive properties of the muscle-tendon unit. Journal of Sports Sciences 31, 479-487. [DOI] [PubMed] [Google Scholar]
- Hough P.A., Ross E.Z., Howatson G. (2009) Effects of dynamic and static stretching on vertical jump performance and electromyographic activity. Journal of Strength and Conditioning Research 23, 507-512. [DOI] [PubMed] [Google Scholar]
- Kataura S., Suzuki S., Matsuo S., Hatano G., Iwata M., Yokoi K., Asai Y. (2017) Acute effects of the different intensity of static stretching on flexibility and isometric muscle force. Journal of Strength and Conditioning Research 31, 3403-3410. [DOI] [PubMed] [Google Scholar]
- Kay A.D., Blazevich A.J. (2012) Effect of acute static stretch on maximal muscle performance: a systematic review. Medicine and Science in Sports and Exercise 44, 154-164. [DOI] [PubMed] [Google Scholar]
- Kisner C., Colby L.A. (2017) Therapeutic exercise: foundations and techniques. 7th edition Philadelphia, PA: F. A. Davis. [Google Scholar]
- Kubo K., Morimoto M., Komuro T., Tsunoda N., Kanehisa H., Fukunaga T. (2007) Influences of tendon stiffness, joint stiffness, and electromyographic activity on jump performances using single joint. European Journal of Applied Physiology 99, 235-243. [DOI] [PubMed] [Google Scholar]
- Kubo K., Ishigaki T., Ikebukuro T. (2017) Effects of plyometric and isometric training on muscle and tendon stiffness in vivo. Physiological Reports 5, e13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankhorst N.E., Bierma-Zeinstra S.M., van Middelkoop M. (2013) Factors associated with patellofemoral pain syndrome: a systematic review. British Journal of Sports Medicine 47, 193-206. [DOI] [PubMed] [Google Scholar]
- Lima C.D., Brown L.E., Wong M.A., Leyva W.D., Pinto R.S., Cadore E.L., Ruas C.V. (2016) Acute effects of static vs. ballistic stretching on strength and muscular fatigue between ballet dancers and resistance-trained women. Journal of Strength and Conditioning Research 30, 3220-3227. [DOI] [PubMed] [Google Scholar]
- Little T., Williams A.G. (2006) Effects of differential stretching protocols during warm-ups on high-speed motor capacities in professional soccer players. Journal of Strength and Conditioning Research 20, 203-207. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Aagaard P., Nielson J.J. (2000) Passive energy return after repeated stretches of the hamstring muscle-tendon unit. Medicine and Science in Sports and Exercise 32, 1160-1164. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Aagard P., Simonsen E., Bojsen-Møller F. (1998) A biomechanical evaluation of cyclic and static stretch in human skeletal muscle. International Journal of Sports Medicine 19, 310-316. [DOI] [PubMed] [Google Scholar]
- Magnusson S.P., Simonsen E.B., Aagaard P., Kjaer M. (1996) Biomechanical responses to repeated stretches in human hamstring muscle in vivo. The American Journal of Sports Medicine 24, 622-628. [DOI] [PubMed] [Google Scholar]
- Marek S.M., Cramer J.T., Fincher A.L., Massey L.L., Dangelmaier S.M., Purkayastha S., Culbertson J.Y. (2005) Acute effects of static and proprioceptive neuromuscular facilitation stretching on muscle strength and power output. Journal of Athletic Training 40, 94-103. [PMC free article] [PubMed] [Google Scholar]
- Matsuo S., Suzuki S., Iwata M., Hatano G., Nosaka K. (2015) Changes in force and stiffness after static stretching of eccentrically-damaged hamstrings. European Journal of Applied Physiology 115, 981-991. [DOI] [PubMed] [Google Scholar]
- Matsuo S., Suzuki S., Iwata M., Banno Y., Asai Y., Tsuchida W., Inoue T. (2013) Acute effects of different stretching durations on passive torque, mobility, and isometric muscle force. Journal of Strength and Conditioning Research 27, 3367-3376. [DOI] [PubMed] [Google Scholar]
- Medicine ACoS. (2017) ACSM’s guidelines for exercise testing and prescription. 10th edition Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Mizuno T. (2017) Changes in joint range of motion and muscle-tendon unit stiffness after varying amounts of dynamic stretching. Journal of Sports Sciences 35, 2157-2163. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Matsumoto M., Umemura Y. (2013) Viscoelasticity of the muscle-tendon unit is returned more rapidly than range of motion after stretching. Scandinavian Journal of Medicine & Science in Sports 23, 23-30. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Umemura Y. (2016) Dynamic stretching does not change the stiffness of the muscle-tendon unit. International Journal of Sports Medicine 37, 1044-1050. [DOI] [PubMed] [Google Scholar]
- Moran K.A., McGrath T., Marshall B.M., Wallace E.S. (2009) Dynamic stretching and golf swing performance. International Journal of Sports Medicine 30, 113-118. [DOI] [PubMed] [Google Scholar]
- Morse C.I., Degens H., Seynnes O.R., Maganaris C.N., Jones D.A. (2008). The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. The Journal of Physiology 586, 97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordez A., Cornu C., McNair P. (2006) Acute effects of static stretching on passive stiffness of the hamstring muscles calculated using different mathematical models. Clinical Biomechanics (Bristol, Avon) 21, 755-760. [DOI] [PubMed] [Google Scholar]
- Opar D.A., Williams M.D., Shield A.J. (2012). Hamstring strain injuries: factors that lead to injury and re-injury. Sports Medicine 42, 209-226. [DOI] [PubMed] [Google Scholar]
- Opplert J., Babault N. (2018). Acute effects of dynamic stretching on muscle flexibility and performance: an analysis of the current literature. Sports Medicine 48, 1-27. [DOI] [PubMed] [Google Scholar]
- Reid J.C., Greene R., Young J.D., Hodgson D.D., Blazevich A.J., Behm D.G. (2018) The effects of different durations of static stretching within a comprehensive warm-up on voluntary and evoked contractile properties. European Journal Applied Physiology 118, 1427-1445. [DOI] [PubMed] [Google Scholar]
- Ryan E.D., Beck T. W., Herda T.J., Hull H.R., Hartman M.J., Costa P.B., Cramer J. T. (2008. The time course of musculotendinous stiffness responses following different durations of passive stretching. The Journal of Orthopaedic and Sports Physical Therapy 38, 632-639. [DOI] [PubMed] [Google Scholar]
- Sadler S.G., Spink M.J., Ho A., De Jonge X.J., Chuter V.H. (2017) Restriction in lateral bending range of motion, lumbar lordosis, and hamstring flexibility predicts the development of low back pain: a systematic review of prospective cohort studies. BMC Musculoskeletal Disorders 18, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samukawa M., Hattori M., Sugama N., Takeda N. (2011) The effects of dynamic stretching on plantar flexor muscle-tendon tissue properties. Manual Therapy 16, 618-622. [DOI] [PubMed] [Google Scholar]
- Schmitt B., Tim T., McHugh M. (2012) Hamstring injury rehabilitation and prevention of reinjury using lengthened state eccentric training: a new concept. International Journal of Sports Physical Therapy 7, 333-341. [PMC free article] [PubMed] [Google Scholar]
- Sekir U., Arabaci R., Akova B., Kadagan S.M. (2010) Acute effects of static and dynamic stretching on leg flexor and extensor isokinetic strength in elite women athletes. Scandinavian Journal of Medicine & Science in Sports 20, 268-281. [DOI] [PubMed] [Google Scholar]
- Shapiro S.S., Wilk M.B. (1965) An analysis of variance test for normality (complete samples). Biometrika 52, 591-611. [Google Scholar]
- Shrout P.E., Fleiss J.L. (1979) Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin 86, 420-428. [DOI] [PubMed] [Google Scholar]
- van der Worp H., van Ark M., Roerink S., Pepping G.J., van den Akker-Scheek I., Zwerver J. (2011) Risk factors for patellar tendinopathy: a systematic review of the literature. British Journal of Sports Medicine 45, 446-452. [DOI] [PubMed] [Google Scholar]
- Woods C., Hawkins R.D., Maltby S., Hulse M., Thomas A., Hodson A., Football Association Medical Research P. (2004) The Football Association Medical Research Programme: an audit of injuries in professional football--analysis of hamstring injuries. British Journal of Sports Medicine 38, 36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K., Bishop P., Jones E. (2007) Warm up and stretching in the prevention of muscular injury. Sports Medicine 37, 1089-1099. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Ishii K. (2005) Effects of static stretching for 30 seconds and dynamic stretching on leg extension power. Journal of Strength and Conditioning Research 19, 677-683. [DOI] [PubMed] [Google Scholar]


