Abstract
This study aimed to verify the effect of beetroot juice on post-exercise ambulatory blood pressure (BP) in obese individuals. Fourteen non-hypertensive obese males were randomly assigned to three experimental sessions: 1) Beetroot juice with exercise (BJE, 200ml with ≈ 800mg nitrate and 40 minutes of moderate-intensity aerobic exercise at an intensity of 50% of the heart rate reserve), 2) fruit soda with exercise (FSE, 200ml of a low-nitrate drink and the same exercise session) and 3) control (CON, 200ml of water, an insignificant nitrate drink without exercise). The concentration of total nitrites and nitrates in plasma (NOx) after the drinks and the 24-hour ambulatory BP were evaluated. A two-way (condition vs. time) ANOVA for repeated measures, with a Bonferroni post hoc was used to analyze variables. The plasma NOx concentration increased significantly after ingestion of beetroot juice (from 9.9 ± 8.4 μM to 47.0 ± 16.9 μM, p < 0.001) and remained elevated until 1 hour post-intervention (54.7 ± 10.1 μM, p < 0.001), while it did not change in FSE and CON groups. The BJE session decreased ambulatory systolic BP in 5.3 mmHg (IC95%, -10.1 to -0.6, p = 0.025) in the period of 1-6 h after the BJE session compared to the CON session and reduction of 3.8 mmHg (IC95%, -7.5 to -0.007, p = 0.05) compared to the FSE session. No significant changes were observed for ambulatory diastolic BP (p > 0.05). BJE enhanced the reduction of systolic ambulatory BP up to 6 hours following a moderate-intensity aerobic exercise in obese individuals with an elevated cardiovascular risk profile.
Key points.
Inorganic nitrate may have important therapeutic applications to decrease the blood pressure response to exercise when individuals have cardiovascular risk.
Beetroot may be a co-adjuvant to increase post-exercise blood pressure response.
Supplementation of beetroot juice significantly increased the plasma NOx concentration.
Key words: Aerobic exercise, nitric oxide, post-exercise hypotension, obesity, cardiovascular disease
Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality worldwide (Joseph et al., 2017). In 2013 there were > 54 million deaths (95% uncertainty interval, 53.6–56.3 million) globally, and 32% were occasioned to cardiovascular disease (GBD, 2015). Hypertension and obesity are a major risk factor for cardiovascular disease (Roth el al., 2017). In 2015, 603.7 million adults were obese around the world, and the overall prevalence of obesity was 12% (GBD, 2017). Interestingly, the hallmark and shared feature of CVD and metabolic syndrome is the reduced bioavailability of nitric oxide (NO) signaling (Newsholme et al., 2009).
Non-pharmacological intervention (lifestyle modification) offers an attractive alternative for preventing and treating hypertension, and nutritional strategies and exercise are widely recommended (Samadian et al., 2016). Exercise has been considered an important approach for reducing cardiovascular risk, promoting clinically acute and chronic effects on blood pressure (BP). According to the American College of Cardiology, regular physical exercise reduces systolic BP (SBP) and diastolic BP (DBP) by 4-5 mmHg and 2-4 mmHg, respectively (Whelton et al., 2018). In fact, even a single bout of exercise can induce short-term reductions on BP, a phenomenon called post-exercise hypotension (Brito et al., 2015).
In addition to exercise, numerous clinical trials have shown beneficial cardiovascular effects of several different nutrients found in vegetables (Houston, 2010; Ravera et al., 2016; Lennon et al., 2017). More recently, attention has been directed to other possible elements in vegetables that may have a role, including inorganic nitrate (Bondonno et al., 2018), that is present in some vegetables such as spinach, beetroot and celery and have important bioactive phytochemical with cardioprotective properties (Hobbs et al., 2013; Gee and Ahluwalia, 2016). Because the nitrate concentration in vegetables is dependent of different factors such as the nitrate content of the soil where the vegetables are grown or the presence or absence of nitrogen-based fertilizers (Gee and Ahluwalia, 2016), the majority of studies have used either nitrate-rich beetroot juice or sodium nitrate to verify the effects on performance or on health. Oral supplementation with inorganic nitrate or nitrate-containing foods exert pleiotropic, beneficial vascular effects, through mechanisms that involves NO bioavailability restoration in a process known as the nitrate-nitrite-NO pathway (Koch et al., 2017).
A classic systematic review with meta analysis showed reductions in SBP and DBP of - 4.4 mmHg and -1.1 mmHg, respectively, after short-term inorganic nitrate and beetroot juice supplementation (Siervo et al., 2013). Interestingly, one study showed that a single dose of nitrate administered in beetroot juice acutely reduces BP in hypertensive patients, with average reduction of 11.2 mmHg and 9.6 mmHg in systolic and diastolic ambulatory BP, respectively (Ghosh et al., 2013). Despite these reports, no study to date has evaluated whether dietary nitrate in the form of beetroot juice can enhance the reduction of BP that occurs following a single session of exercise. This may be especially relevant in individuals who have an increased cardiovascular risk profile, but have not yet developed hypertension, such as obese individuals.
Thus, the aim of this study was to examine the effects of dietary nitrate supplementation (nitrate-rich beetroot juice) on the post-exercise ambulatory BP in obese males. It was hypothesized that the supplementation with nitrate-rich beetroot juice, prior to an exercise bout, would enhance the post-exercise hypotension effect compared to a low-nitrate drink.
Methods
Design and participants
This study was a prospective single-center, randomized, crossover-controlled trial carried out from February to June 2017 at the nutritional assessment laboratory of the University. The following inclusion criteria were considered in the study: males, aged between 20 and 30 years old, obese (body mass index – BMI – between 30 and 40 kg/m2 with increased body adiposity), free of cardiovascular disease including hypertension, able to perform exercise according to the Physical Activity Readiness Questionnaire (PARQ) (Shephard, 1988), but physically inactive. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by the Human Research Ethics Committee at Federal University of Rio Grande do Norte (Protocol No. 1.808.205, CAAE 59716216.8.0000.5292) and was prospectively registered on a publicly accessible database (http://www.ensaiosclinicos.gov.br/rg/RBR-5gkhhj/). The study design followed CONSORT guidelines (Schulz et al., 2010).
Procedures
Subjects attended the research laboratory on four different occasions before noon and after a 12-hour fasting period. The aim of the first day was to obtain data for sample characterization, to perform the maximum incremental test (to determine the intensity of the exercise on the days of the experimental sessions) and perform a familiarization session. Anthropometric evaluation and body composition assessment were performed after blood collection for biochemical characterization of the sample and fasting nitrite and nitrate concentrations (NOx). After that, subjects received a standardized meal determined by an experienced nutritionist (to avoid perform the exercise test in fast) and performed the maximal exercise test. Finally, group allocation was randomly carried out by means of a lottery in order to determine the order of the experimental sessions for each subject without his knowledge, and with an interval of five days between sessions (washout period). Subjects then underwent controlled experimental trials in the following three visits: 1) juice rich in nitrate (beetroot juice) with exercise (BJE), 2) fruit soda low in nitrate with exercise (FSE), and 3) session with mineral water and without exercise - control (CON).
Subjects were instructed to maintain their habitual diet and activity during the study. At 48-hour pre-assessment day the participants were asked to abstain from any kind of exercise, consuming alcohol, caffeine and nitrate-rich food (Curtis et al., 2015). Subjects were additionally asked to avoid using mouthwash and chewing gum in the 48h period prior to testing, because it can reduce oral bacterial nitrate reductase activity (Govoni et al., 2008).
Anthropometric measures and body composition assessment
Body weight was measured using a digital scale (BKH-200FN, Balmak®, Santa Bárbara D’oeste, Brazil). Height was measured by a portable stadiometer (Personal Caprice Portatil, Sanny®, São Paulo, Brazil) with a precision of 1 mm. Nutritional status was classified according to World Health Organization (WHO, 2014). The waist circumference measurement was performed using inelastic tape (Cescorf®, Porto Alegre, Brazil) at the midpoint between the lower costal margin and the iliac crest (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). Body fat percentage was assessed by dual-energy X-ray absorptiometry (DEXA) (GE, Medical Systems, Madison, WI, USA).
Maximal graded exercise test
Subjects performed a maximal graded exercise test on a motorized treadmill (RT350, Movement®, Pompeia, Brazil) to determine their maximal heart rate. The maximal graded exercise test started at 4 km/h followed by fixed increments of 1 km/h every minute. Heart rate (HR) was monitored throughout the test using a HR monitor (RS800CX, Polar®, Finland) and recorded at the end of each minute. The highest HR value observed during the test was considered as the HRmax. Rating of perceived exertion (RPE) was also monitored and recorded at the end of each minute according to the Borg scale 6-20 (Borg, 1982). The end of the test was determined by the presence of at least one of the following criteria: i) HR ≥ 100% estimated for age; ii) RPE > 18; or iii) when participants voluntarily stopped (Howley et al., 1995). On that day, mean resting heart rate was also measured during a five-minute period in a seated position in order to determine the heart rate reserve (heart rate max – resting heart rate), which was the method used to prescribe the intensity of the aerobic exercise sessions. BP was measured in triplicate by digital sphygmomanometer (Visomat®, comfort 20/40), with intervals of 1 minute between them after 5 minutes of rest in the sitting position with their legs uncrossed, back supported and arm placed on a table with the palm facing up (American College of Sports Medicine, 2009).
Experimental sessions
The experimental pre-session protocol was identical for all three visits, only differing in the drink offered. The volunteers were instructed not to change their diet and avoid foods high in nitrates the day before the experimental sessions. Participants attended the laboratory after a 12-hour overnight fasting. Resting blood pressure (BP) was collected before the standard meal according to the 7th Brazilian Arterial Hypertension Guideline (Malachias, 2016) using the oscillometric method (HEM-7200, OMRON, USA). A standardized meal (cheese sandwich and fruit, 84% carbohydrates, 14% protein and 2% lipids) was offered 70 minutes prior exercise, with an energy value of 15% of the individual daily energy, according to the energy recommendations for physically inactive people (Brazil, 2006). Additionally, 200ml of the drink corresponding to the experimental session was offered 10 minutes after the end of the meal (i.e., 60 minutes prior to exercise session or control session). This amount of food and timing was in accordance with current guidelines (Thomas et al., 2016).
The drinks were: 1) beetroot juice rich in nitrate (two shots of Beet It Sport®, James White Drinks Ltd., Ipswich, UK; 70mL each shot with a total 400mg nitrate) for the BJE condition; 2) fruit soda low in nitrate (Kapo®, Del Valle, Brazil; 200 mL of pasteurized beverage containing a mixture of water, sugar and low quantity of grape and containing only 5.4mg nitrate) for the FSE condition; and 3) mineral water (200 mL, without a significant amount of nitrate) for the CON condition. Sixty (60) mL of mineral water was added in the BJE condition in order to standardize the drink volume. The nutritional composition in 200ml of the drinks was: beetroot juice rich in nitrate (194 calories, 40g of carbohydrate, 8g of protein, 0.4g of fat); fruit soda low in nitrate (78 calories, 19g carbohydrate and other insignificant amounts of protein and fat); and mineral water (no calories and insignificant amounts of these nutrients). All drinks were offered in neutral packaging of a dark color to make it difficult for the subjects to recognize the intervention, and subjects were instructed to drink the full volume.
Subjects then performed the exercise protocol after consuming the drink, except in the CON session in which they ingested water and did not exercise, remaining at rest for the period corresponding to the exercise. Sixty minutes after ingesting the intervention drink, the subjects performed 40 minutes of moderate-intensity aerobic exercise on a treadmill at an intensity of 50% of the heart rate reserve (Garber et al., 2011). The exercise intensity was monitored using a cardiac monitor. The subjects performed a warm-up of three minutes before exercise at 4 km/h, and two minutes of cool down after the session at 3 km/h.
Blood was collected in three different moments after drink ingestion for measuring NOx plasma: pre-intervention (60 minutes after drink ingestion), post-intervention (105 minutes after drink ingestion) and 1-hour post-intervention (165 minutes after drink ingestion). After the last blood collection, an ambulatorial BP monitor (ABPM) was placed on the subject to be used for 24 hours. Figure 1 shows a drawing of the experimental session.
Figure 1.
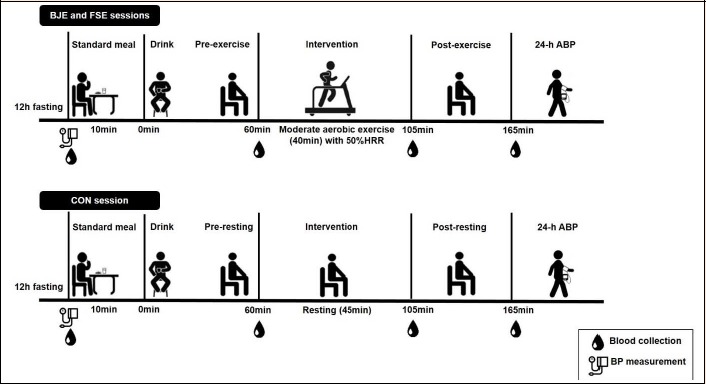
Design of the experimental sessions. BJE = beetroot juice with exercise, FSE = fruit soda with exercise, CON = control without exercise, HRR = heart rate reserve, ABP = ambulatory blood pressure, BP = blood pressure
Ambulatorial blood pressure measurements
Subjects were instructed to shower after each experimental session and then the ABMP device (DynaMAPA, Cardios®, Brazil) was fitted on their non-dominant arm. The participants were fitted with the ABPM device ~60 minutes after the experimental sessions (i.e. between 11:00 am - 12:00 noon) and had it removed on the following day between 11:00 am - 12:00 noon. The device was programmed to measure BP every 15 minutes while the subject was awake (between 07:00 am - 10:59 pm) and every 30 minutes during their periods of sleep (between 11:00 pm - 06:59 am). These periods were defined by the subjects who changed the monitor settings by pressing a button when they went to bed and again when they woke-up (Nobre et al., 2011). Subjects were also instructed to record the hour that they went to bed and the hour that they woke-up using a logbook. All these procedures were previously clarified to the participants in a familiarization session. A minimum of 16 and 8 BP measurements while awake and asleep, respectively, had to be successfully recorded in order to be included in the final analysis. During the 24-hour period after each experimental session, subjects were instructed to avoid physical activity. Subjects came back to the laboratory on the following morning after each experimental session and the data were downloaded to a computer. Data was recorded for a 24-h period, and the awake and asleep periods were defined according to each subject. The average values for each period were considered for data analysis.
Blood samples and biochemical variables
A venous blood sample was obtained by venipuncture during the morning hours after an overnight fast for sample characterization. Triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C) and glycaemia were determined by automated enzymatic methods (Advia, Bayer, USA) and low-density lipoprotein cholesterol (LDL-C) calculated by the Friedewald formula (Friedewald et al., 1972).
Blood (10 ml) was collected for measuring plasma NOx concentration in fasting and during experimental sessions: pre-intervention, immediate post-intervention and 1 hour post-intervention, also by venipuncture. A colorimetric assay was used for measuring NOx in plasma (Elisa technique). The principle of this assay is to reduce nitrate by vanadium (III) combined with detection by the acidic Griess reaction (Backmark Bio Rad-USA, as described by Miranda et al. (Miranda et al., 2001). The plasma sample was initially deproteinized by ultrafiltration using microfilters (Biomax® Membrane, 10 kDa, Millipore, Billerica, MA, USA). Next, an assay was performed and a standard curve was simultaneously made. This methodology was adapted from García-Robledo et al. (García-Robledo et al., 2014). The results were expressed in μM.
Statistical analysis
The Shapiro-Wilk test was used to test normality, and all data presented normal distribution. An ANOVA for repeated measures was used to analyze resting systolic and diastolic BP before the BJE, FSE and CON sessions. A two-way (condition vs. time) ANOVA for repeated measures was used to analyze ambulatory systolic and diastolic BP 1-6 h, 7-13 h, 14-20 h and 21-24 h following the BJE, FSE and CON sessions. The same analysis was used to compare plasma NOx response. The delta was used to show the difference of ambulatory BP between the experimental sessions (i.e. BP values of BJE – BP values of the CON session; and BP values of FSE – BP values of the CON session) at each period following the experimental sessions (i.e. 1-6 h, 7-13 h, 14-20 h and 21-24 h). Data sphericity was verified by the Mauchly’s test. In case of sphericity assumption violation, the degrees of freedom were adjusted and reported using the Greenhouse-Geisser epsilon correction. Bonferroni post hoc was used for comparisons in pairs. The significance level was set at 5% (p ≤ 0.05) for all analyses. The pairwise comparisons are reported as mean difference and confidence interval of 95% (95% CI). All data were analyzed using SPSS® 20.0 for Windows (SPSS, Inc., Chicago, IL). The results are expressed as mean ± standard deviation (SD).
Results
From dissemination in social networks and posters, nineteen subjects volunteered to participate in the study and met the eligibility criteria. These individuals performed the maximal exercise test in the first session and were then randomized for the following three experimental sessions. After randomization, five participants discontinued their participation because they claimed they had no time. Thus, three subjects started their first session in the BJE condition, six subjects in the FSE and five subjects in the CON so that 14 subjects participated in all sessions and had their data analyzed (Figure 2). The first “crossover” line of the flow chart shows the order of the sessions from the visit 1 to the visit 2. The second “crossover” line of the flow chart shows the order of the sessions from the visit 2 to the visit 3.
Figure 2.
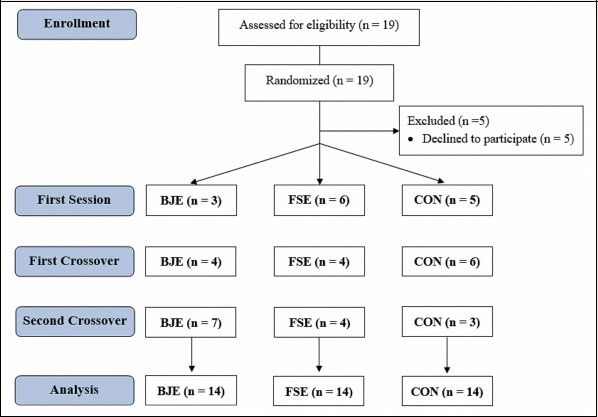
Flow chart of the CONSORT study. BJE = beetroot juice with exercise, FSE = fruit soda with exercise, CON = control without exercise
Table 1 shows the anthropometric, cardiovascular and biochemical characteristics of all subjects. The individuals were non-hypertensive, with an elevated average waist circumference. Increased mean values of total cholesterol and triglycerides allowed us to classify individuals as dyslipidemic – isolated hypertriglyceridemia in 8 individuals (Faludi et al., 2017).
Table 1.
Characterization of the study group (n=14).
| VARIABLES | MEAN ± SD |
|---|---|
| Age (years) | 25.3 ± 4.7 |
| Weight (Kg) | 113.2 ± 15.7 |
| Height (cm) | 177.6 ± 6.9 |
| Body Mass Index (kg/m²) | 35.8 ± 3.3 |
| Body Fat percentage (%) | 40.8 ± 0.1 |
| Fat weight (kg) | 45.2 ± 9.7 |
| Lean body weight (kg) | 64.6 ± 7.1 |
| Waist circumference (cm) | 118.9 ± 10.3 |
| Resting systolic blood pressure (mmHg) | 122.9 ± 9.5 |
| Resting diastolic blood pressure (mmHg) | 82.9 ± 5.7 |
| Resting heart rate (bpm) | 72.9 ± 6.1 |
| Fasting glycaemia (mg/dL) | 83.9 ± 7.4 |
| Total cholesterol (mg/dL) | 196.6 ± 42.4 |
| HDL-cholesterol (mg/dL) | 44.9 ± 10.3 |
| LDL-cholesterol (mg/dL) | 116.6 ± 39.5 |
| Triglycerides (mg/dL) | 175.6 ± 74.9 |
HDL= High-density lipoprotein LDL= Low-density lipoprotein SD = standard deviation
Figure 3 illustrates plasma NOx concentration during the three experimental sessions in the fasting, pre-intervention, post-intervention and 1 hour post-intervention periods. There was a significant condition x time interaction [F(2.399, 31.186) = 24.277, p < 0.001, ŋ2p = 0.651]. The plasma NOx concentration increased significantly after ingestion of beetroot juice from the pre-intervention (pre-I) point (from 9.9 ± 8.4 μM to 47.0 ± 16.9 μM, p < 0.001) and remained elevated until 1 hour post-intervention (54.7 ± 10.1 μM, p < 0.001), while it did not change after ingestion of fruit soda and mineral water. Plasma NOx after BJE in the 1h post-intervention (1h post-I) point was 44.5 μM (IC95%, 36.5 to 52.5, p < 0.001) higher compared to CON and higher 42.1 μM (IC95%, 32.3 to 52.1, p < 0.001) compared to FSE.
Figure 3.
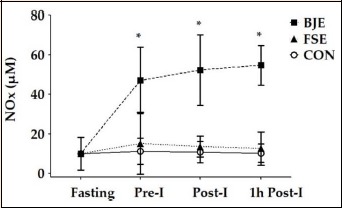
Concentration of fasting NOx plasma, pre-intervention (Pre-I), post-intervention (Post-I) and 1 hour post-intervention (1h Post-I) in normotensive volunteers during acute sessions of beetroot juice with exercise (BJE), fruit soda with exercise (FSE) and control without exercise (CON). Values are expressed as mean ± standard deviation. * Significant difference from the BJE in comparison the other sessions at the same time, p < 0.05
There was no difference in the resting systolic [122.9 ± 9.5 vs. 123.4 ± 10.2 vs. 121.9 ± 9.2; F(2, 26) = 0.323, p = 0.727] and diastolic BP [82.9 ± 5.7 vs. 79.5 ± 6.1 vs. 79.7 ± 6.3; F(2, 26) = 0.2403, p = 0.110] between CON, BJE and FSE sessions, respectively. Table 2 shows the values of ambulatory BP and the deltas (i.e. differences of BP between BJE - CON, and FSE - CON) at 1-6 h, 7-13 h, 14-20 h and 21-24 h following the experimental sessions. There was a condition x time interaction [F(6, 78) = 2.751, p = 0.018, ŋ2p = 0.175] for ambulatory SBP. The post hoc analysis revealed a significant reduction of 5.3 mmHg (IC95%, -10.1 to -0.6, p = 0.025) in the period of 1-6 h after the BJE session compared to the CON session and reduction of 3.8 mmHg (IC95%, -7.5 to -0.007, p = 0.05) compared to the FSE session. The comparison of deltas also revealed a significant condition x time interaction [F(3, 39) = 7.504, p < 0.001, ŋ2p = 0.366] for SBP. The post hoc analysis of deltas revealed significant reduction in BJE – CON (-3.8 mmHg, IC95%, -6.7 to -0.8, p = 0.017) compared to FSE – CON. There was no significant condition x time interaction for DBP [F(6, 78) = 1.730, p = 0.125, ŋ2p = 0.177] and for deltas of DBP [F(3, 39) = 2.664, p = 0.061, ŋ2p = 0.170]. No significant changes in SBP and DBP were observed during sleep between conditions (p > 0.05).
Table 2.
Values of ambulatory blood pressure and the differences of blood pressure between the experimental sessions (i.e. delta) at different periods of post-experimental sessions.
| Awake | Asleep | Awake | P-Value | ||||
|---|---|---|---|---|---|---|---|
| 1-6 h | 7-13 h | 14-20 h | 21-24 h | Interaction | Time | Condition | |
| Systolic blood pressure (mmHg) | |||||||
| Control without exercise (CON) | 125.1 ± 11.1 | 126.3 ± 9.2 | 116.0 ± 8.9 | 125.3 ± 8.4 | 0.018 | <0.001 | 0.035 |
| Beetroot juice with exercise (BJE) | 119.7 ± 7.8* | 123.5 ± 9.5 | 113.9 ± 7.9 | 124.4 ± 7.2 | |||
| Fruit soda with exercise (FSE) | 123.5 ± 8.7 | 123.5 ± 8.8 | 112.4 ± 6.7 | 120.4 ± 7.3 | |||
| Delta BJE | -5.3 ± 6.4 † | -2.8 ± 6.1 | -2.1 ± 6.7 | -0.9 ± 6.2 | <0.001 | 0.988 | 0.789 |
| Delta FSE | -1.6 ± 4.9 | -2.9 ± 4.2 | -3.6 ± 7.6 | -4.9 ± 7.2 | |||
| Diastolic blood pressure (mmHg) | |||||||
| Control without exercise (CON) | 81.4 ± 8.4 | 82.0 ± 6.4 | 67.8 ± 7.0 | 81.0 ± 7.6 | 0.125 | <0.001 | 0.118 |
| Beetroot juice with exercise (BJE) | 78.5 ± 5.4 | 78.5 ± 6.7 | 67.5 ± 7.0 | 80.3 ± 7.3 | |||
| Fruit soda with exercise (FSE) | 82.4 ± 7.0 | 80.1 ± 8.3 | 66.6 ± 6.3 | 78.2 ± 9.5 | |||
| Delta BJE | -2.9 ± 6.9 | -3.5 ± 4.2 | -0.3 ± 5.7 | -0.7 ± 4.4 | 0.061 | 0.671 | 0.578 |
| Delta FSE | 1.0 ± 4.2 | -1.9 ± 4.8 | -1.2 ± 7.2 | -2.8 ± 6.2 | |||
Values are expressed as mean ± standard deviation.
* = Significant difference in comparison to CON and FSE sessions (p ≤ 0.05).
† = Significant difference in comparison to delta FSE - CON (p < 0.05). Delta BJE = means the values of blood pressure of the BJE session – the values of blood pressure of the control session; Delta FSE = means the values of blood pressure of the FSE session – the values of blood pressure of the control session.
Discussion
The results of our trial indicate a positive combined effect of nitrate supplementation and exercise on ambulatory SBP, which was reduced up to 6-hours in obese individuals. To the best of our knowledge, this is the first study to show an interactive effect of a single session of exercise and beetroot juice on post-exercise ambulatory BP in individuals with an elevated cardiovascular risk profile.
Since the first study in human model demonstrating a blood pressure-lowering effect of a dietary nitrate supplement was published (Larsen et al., 2006), dietary nitrate have been one of the most discussed topics in nutrition and exercise communities. Studies have investigated nitrate supplementation in the form of beetroot juice on physical performance in athletes (Betteridge et al., 2016; Lansley et al., 2011; Muggeridge et al., 2014), recreationally active people (Lee et al., 2015; Whitfield et al., 2016), older adults (Kelly et al., 2013) and in some chronic diseases such as chronic obstructive pulmonary disease (Curtis et al., 2015) and type 2 diabetes (Shepherd et al., 2015). Although studies show that beetroot juice lowers BP, the results are still controversial in different populations. This may be attributed to the fact that, in metabolic disorders (such as diabetes), NO bioavailability is compromised and the nitrate supplementation may normalize the concentration of this gas; whereas in healthy people (thus normal levels of NO) the increase in nitrites could exert direct cell effects, in addition to NO enhancement (Affourtit et al., 2015). In the present study, the intake of beetroot juice reduced ambulatory SBP by 5.3 mmHg up to six hours post-exercise. This finding is relevant because a reduction of 3 mmHg in SBP is associated with a reduction of 8% and 5% in mortality due to stroke mortality and cardiovascular disease, respectively (Whelton et al., 2002).
Post-exercise hypotension is well documented in the literature, and the intensity of the exercise training session may impact the magnitude of this effect (Pescatello et al., 2004). A systematic review showed no reductions of ambulatory BP after a single session of moderate-intensity aerobic exercise in normotensive individuals (Cardoso et al., 2010; Sosner et al., 2017). Interestingly, the results of the present study showed that the interaction between moderate-intensity aerobic exercise and beetroot juice reduced ambulatory SBP in non-hypertensive obese individuals. It should be noted that a previous study observed that the interaction of isometric exercise (handgrip exercise) and beetroot juice (Lara et al., 2015) did not reduce the ambulatory BP in healthy normotensive subjects, however, this difference may be explained by the nature of the exercise protocol (isometric/upper body only) and the studied population Taken together, these results suggest that beetroot juice enhances reductions in ambulatory BP in subjects with metabolic disorders, which agrees with studies that observed (Kerley, 2017) the effects of dietary nitrate mainly in people with vascular and/or metabolic diseases. We highlight that the additional effect of the beetroot juice on post-exercise hypotension occurred up to six hours post-exercise under free-living conditions, which increases the external validity of our findings.
Several mechanisms can be suggested to explain the associated effects of beetroot juice supplementation and exercise on BP reductions, such as the reduced peripheral vascular resistance, the release of vasodilator substances such as NO, reduced cardiac output (Forjaz et al., 1998; Halliwill, 2001; Mortensen et al., 2009), increased local muscle histamine system activity (Zafeiridis, 2014) and reduced sympathetic tonus activity (Chen and Bonham, 2010). Dietary nitrate can be enzymatically reduced to nitrite, an important secondary source of NO and other bioactive nitrogen oxides. Formation of nitrite and propagation of its downstream NO-signaling effects depends on the oral bacterial reduction of inorganic nitrate by a set of bacterial nitrate reductase enzymes in a process referred to as the enterosalivary nitrate circulation, or the nitrate-nitrite-NO pathway (Koch et al., 2017). In our study, beetroot juice supplementation significantly increased pre-intervention plasma NOx, which is a highly sensitive marker of NO bioavailability, remaining elevated until the last measurement (1 hour after the exercise session). Interestingly, plasma nitrate concentration peaked 1–2h following the bolus nitrate ingestion, and plasma [nitrite] peaked after 2–3h, after which both gradually fell after about 6h (McIlvenna et al., 2017; Wylie et al., 2013). The maintenance of the high levels of NOx up to 6 hours may explain our findings of lower BP within the same period.
McDonagh et al. (McDonagh et al., 2018) conducted an interesting study with different forms of dietary inorganic nitrate and its effects on nitrate metabolism and blood pressure. Ten healthy males consumed an equimolar dose of NO3- (~5.76 mmol) in three different form: 1) a concentrated beetroot juice drink (55 mL), 2) a non-concentrated beetroot juice drink (456 mL) and a solid beetroot flapjack (60 g). Although plasma NOx was elevated in all conditions, the consumption of a concentrated beetroot juice was the most effective means of reducing BP. Different results were found when compared beetroot juice versus chard gel (McIlvenna et al., 2017), which both interventions increased NOx concentrations and are capable of reducing BP of healthy males with a little difference in the magnitude of these effects. Therefore, the reductions in BP observed in the present study, in the period between 1-6 hours post-exercise, may be influenced by increases in NO bioavailability which remained high one hour after exercise.
Several studies in the literature demonstrated that dietary nitrate significantly reduce BP in healthy individuals (Jonvik et al., 2016; McIlvenna et al., 2017; McDonagh et al., 2018; Wylie et al., 2013). Meta-analyses point to a reduction in BP with values of 4.4/1.1 mmHg (Siervo et al., 2013) and 4.1/2 mmHg (Ashor et al., 2017) for people without vascular disease. This hypotensive effect was greater and more consistent for SBP than for DBP (Ashor et al., 2017; Siervo et al., 2013). However, the effects of nitrate supplementation are likely determined by an interaction between multiple variables, as dose, duration, and source of nitrate and cohorts likely to benefit. For example, in studying older people with overweight/obesity, Lara et al. (Lara et al., 2015) found that beetroot juice does not alter ambulatory BP. According to Kerley (2017), dietary nitrate may be more relevant for people with vascular and/or metabolic impairment, those who engage in short-term and intense exercise, untrained individuals or people with low dietary nitrate intake. It is suggested that increased green vegetables consumption may provide similar benefits to nitrate supplementation in a cheaper, safer, and potentially tastier context (Kerley, 2017).
Finally, recent evidences have shown that dietary nitrate supplementation lowers the oxygen cost of human exercise, as less respiratory activity appears to be required for a set rate of skeletal muscle work in low-intensity sub-maximal exercise and increase the tolerable duration of high-intensity exercise (Larsen et al., 2007; Bailey et al., 2009). In our hands, however, nitrate-rich beetroot juice supplementation did not change any physiological parameters during the sub-maximal exercise set [treadmill speed or Rated Perceived Exertion (RPE) - data not shown). This indicate that nitrate supplementation might improve the economy and performance of exercise in healthy and active people, but not in obese or metabolic compromised people.
Some limitations should be considered in the present study. Our clinical trial was randomized but was not blinded because the flavors of the intervention drinks are striking and impossible to confuse. We did not perform a control session (no exercise) with dietary nitrate supplementation, and we suggest that future studies include this session to clarify whether the results would be similar. Another possible limitation of the study is the different consumption of dietary nitrate in food and beverages in the past 24h of interventions. Although we analyzed the food intake in this period for all conditions, nutritional tables and software are extremely deficient on information about dietary nitrate. Thus, we preferred not to use this information, and we believe this is a limitation of all studies using this dietary intervention. The fact that we only studied men with obesity does not allow us to generalize the results for both genders, nor for other specific populations. Lastly, it was not possible to asses NOx after 60min post-intervention, and previous literature has shown that NOx may last up to 6 h post-ingestion. So, it may be probable that NOx concentration influenced a slight drop in SBP, without data to support NOx concentrations in blood.
Conclusion
The present study showed that supplementation of beetroot juice significantly increased the plasma NOx concentration 1h after its ingestion and decreased the systolic ambulatory blood pressure up to 6 hours following a moderate-intensity aerobic exercise in obese individuals. This finding suggests that inorganic nitrate may have important therapeutic applications to enhance post-exercise BP reductions when individuals have an increased cardiovascular risk. Regarding clinical applications, our findings suggest that there is an interactive effect between aerobic exercise and beetroot juice that contributes to a greater magnitude of post-exercise hypotension when compared to the effects of the exercise alone. Further research is needed to confirm these findings.
Acknowledgements
We would like to thank all the individuals who participated in the study and the GEMEN research group for their support. We also thank the Federal University of Rio Grande do Sul (UFRGS), Department of Physiology of UFRGS, for supporting this work. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Ana Paula Trussardi Fayh, through the Post-Graduation Program of Nutrition, was responsible for grant support for analysis of CRP (FAPERN/CAPES 006/2014, grants #402398/2013-2 and 372373/2013-5). This work was also supported by The State of Rio Grande do Sul Foundation for Research Support (FAPERGS; grant #30791.434.41354.23112017 - CHAMADA FAPERGS/Decit/SCTIE/MS/CNPq/SESRS n. 03/2017 – PPSUS, to M.K.) and The Brazilian National Council for Scientific and Technological Development (CNPq; grants #551097/2007 8, 563870/2010-9, 402626/2012-5 and 402364/2012-0, to P.I.H.B.J.). The experiments comply with the current laws of the country in which they were performed. The authors have no conflicts of interests to declare.
Biographies
Agnes Denise de Lima BEZERRA
Employment
Nutritionist, postgraduate in clinical nutrition and sports nutrition, by University Cruzeiro do Sul (São Paulo/ Brazil)
Degree
MSc
Research interests
Sport nutrition
E-mail: agnes_denise@hotmail.com
Eduardo Caldas COSTA
Employment
Assistant Professor at the Department of Physical Education, Federal University of Rio Grande do Norte, Natal, Brazil. Head of the Research Group on Acute and Chronic Effects of Exercise.
Degree
PhD
Research interests
Clinical exercise physiology with focus on cardiovascular exercise physiology; Exercise interventions to improve health-related physical activity and cardiometabolic health.
E-mail: ecc.ufrn@gmail.com
Daniela Antunes PACHECO
Employment
PhD student at the Health Sciences Program from the Federal University of Goiás, Brazil
Degree
MSc
Research interests
Physiology exercise, Obesity, High-intensity interval training, Psychophysiological response to exercise.
E-mail: danielaap@gmail.com
Luiz Fernando FARIAS-JUNIOR
Employment
Ph.D. student in Health Sciences, Department of Physical Education, University of Rio Grande do Norte, Natal, Brazil
Degree
MPEd
Research interests
Physiology exercise, Post-exercise hypotension, High-intensity interval training, Psychophysiological response to exercise.
E-mail: lfariasjunior@gmail.com
Raphael Mendes RITTI
Employment
Professor at Nove de Julho University
Degree
PhD
Research interests
Exercise physiology area, especially analyzing the cardiovascular effects of exercise in patients with different cardiovascular diseases.
E-mail: raphaelritti@gmail.com
Gisele Bettu GRIGOLO
Employment
Department of Physiology in the Federal University of Rio Grande do Sul
Degree
MSc
Research interest
Exercise physiology
E-mail: gisele.grigolo@hotmail.com
Paulo I.H. de BITTENCOURT Júnior
Employment
Associate Professor of Physiology of the Federal University of Rio Grande do Sul, Brazil.
Degree
PhD
Research interest
Stress response and lipid metabolism in chronic inflammatory disease. Atherosclerosis and diabetes.
E-mail: pauloivo@ufrgs.br
Mauricio KRAUSE
Employment
Professor of Physiology Federal University of Rio Grande do Sul
Degree
PhD
Research interest
The role physical exercise and nutrients in several conditions such as obesity, diabetes, cancer, and exercise.
E-mail: maurcio.krause@ufrgs.br
Ana Paula Trussardi FAYH
Employment
Assistant Professor at the Department of Physical Education, Federal University of Rio Grande do Norte, Natal, Brazil. Head of the Research Group in Nutrition, Exercise and Metabolism.
Degree
PhD
Research interest
Sport nutrition, body composition and clinical exercise physiology with focus on obesity and cardiovascular exercise physiology.
E-mail: apfayh@yahoo.com.br
References
- Affourtit C., Bailey S.J., Jones A.M., Smallwood M.J., Winyard P.G. (2015) On the mechanism by which dietary nitrate improves human skeletal muscle function. Frontiers in Physiology 6, 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2009) ACSM’s guidelines for exercise testing and prescription. 9th edition. New York: Lippincott Williams & Wilkins. [Google Scholar]
- Ashor A.W., Lara J., Siervo M. (2017) Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: a systematic review and meta-analysis. Journal of Hypertension 35, 1353-1359. [DOI] [PubMed] [Google Scholar]
- Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., Dimenna F.J., Wilkerson D.P., Tarr J., Benjamin N., Jones A.M. (2009) Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of Applied Physiology 107, 1144-1155. [DOI] [PubMed] [Google Scholar]
- Betteridge S., Bescós R., Martorell M., Pons A., Garnham A.P., Stathis C.C., McConell G.K. (2016) No effect of acute beetroot juice ingestion on oxygen consumption, glucose kinetics, or skeletal muscle metabolism during submaximal exercise in males. Journal of Applied Physiology 120, 391-398. [DOI] [PubMed] [Google Scholar]
- Bondonno C.P., Blekkenhorst L.C., Liu A.H., Bondonno N.P., Ward N.C., Croft K.D., Hodgson J.M. (2018) Vegetable-derived bioactive nitrate and cardiovascular health. Molecular Aspects of Medicine 61, 83-91. [DOI] [PubMed] [Google Scholar]
- Borg G.A. (1982) Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise 14, 377-381. [PubMed] [Google Scholar]
- Brazil. (2006) Interministerial portal nº 66. Alters the nutritional parameters of the Worker’s Food Program - WFP. Brasília: Official Gazette of the Federative Republic of Brazil. Available from URL: http://www.saude.sp.gov.br/resources/ses/legislacao/2006/agosto/informe-eletronico-de-legislacao-em-saude-n165-29.08.06/legislacaofederal/portariainterministerialmten66de25.08.06.pdf. [Accessed 8 September 2017].
- Brito L.C., Rezende R.A., da Silva Junior N.D., Tinucci T., Casarini D.E., Cipolla-Neto J., Forjaz C.L. (2015) Post-Exercise Hypotension and Its Mechanisms Differ after Morning and Evening Exercise: A Randomized Crossover Study. PLoS One 10, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C.G., Jr., Gomides R.S., Queiroz A.C., Pinto L.G., da Silveira Lobo F., Tinucci T., Mion D., Jr., de Moraes Forjaz C.L. (2010) Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics. 65, 317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Bonham A.C. (2010) Postexercise hypotension: central mechanisms. Exercise and Sport Sciences Reviews 38 122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis K.J., O’Brien K.A., Tanner R.J., Polkey J.I., Minnion M., Feelisch M., Polkey M.I., Edwards L.M., Hopkinson N.S. (2015) Acute Dietary Nitrate Supplementation and Exercise Performance in COPD: A Double-Blind, Placebo-Controlled, Randomised Controlled Pilot Study. PLoS One 10, 1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. (2001) Executive Summary of the Third Report (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). The Journal of the American Medical Association 285, 2486-2497. [DOI] [PubMed] [Google Scholar]
- Faludi A.A., Izar M.C.O., Saraiva J.F.K., Chacra A.P.M., Bianco H.T., Neto A.A., et al. (2017) Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose – 2017. Arquivos Brasileiros de Cardiologia 109, 1-76. Available from URL: http://www.scielo.br/pdf/abc/v109n2s1/0066-782X-abc-109-02-s1-0001.pdf [Accessed 8 July 2017]. [DOI] [PubMed] [Google Scholar]
- Forjaz C.L.M., Santaella D.F., Rezende L.O., Barreto A.C.P., Negrão C.E. (1998) Duração do exercício determina a magnitude e a duração da hipotensão pós-exercício. Arquivos Brasileiros de Cardiologia 70, 99-104. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry 18, 499-502. [PubMed] [Google Scholar]
- Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P., American College of Sports Medicine (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and Science in Sports and Exercise 43, 1334-1359. [DOI] [PubMed] [Google Scholar]
- García-Robledo E., Corzo A., Papaspyrou S. (2014) A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Marine Chemistry 162, 30-36. [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators. (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Obesity Collaborators. (2017) Health Effects of Over weight and Obesity in 195 Countries over 25 Years. The New England Journal of Medicine 377, 13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee L.C., Ahluwalia A. (2016) Dietary Nitrate Lowers Blood Pressure: Epidemiological, Pre-clinical Experimental and Clinical Trial Evidence. Current Hypertension Reports 18, 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.M., Kapil V., Fuentes-Calvo I., Bubb K.J., Pearl V., Milsom A.B., Khambata R., Maleki-Toyserkani S., Yousuf M., Benjamin N., Webb A.J., Caulfield M.J., Hobbs A.J., Ahluwalia A. (2013) Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61, 1091-1102. [DOI] [PubMed] [Google Scholar]
- Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. (2008) The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric oxide 19, 333-337. [DOI] [PubMed] [Google Scholar]
- Halliwill J.R. (2001) Mechanisms and clinical implications of post-exercise hypotension in humans. Exercise and Sport Sciences Reviews 29, 65-70. [DOI] [PubMed] [Google Scholar]
- Hobbs D.A., George T.W., Lovegrove J.A. (2013) The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutrition Research Reviews 26, 210-222. [DOI] [PubMed] [Google Scholar]
- Houston M.C. (2010) The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Therapeutic Advances in Cardiovascular Disease, 4, 165-183. [DOI] [PubMed] [Google Scholar]
- Howley E.T., Bassett D.R., Jr, Welch H.G. (1995) Criteria for maximal oxygen uptake: review and commentary. Medicine and Science in Sports and Exercise 27, 1292-1301. [PubMed] [Google Scholar]
- Jonvik K.L., Nyakayiru J., Pinckaers P.J., Senden J.M., Van Loon L.J., Verdijk L.B. (2016) Nitrate-Rich Vegetables Increase Plasma Nitrate and Nitrite Concentrations and Lower Blood Pressure in Healthy Adults. The Journal of Nutrition 146, 986-993. [DOI] [PubMed] [Google Scholar]
- Joseph P., Leong D., Mckee M., Anand S.S., Schwalm J., Teo K., Mente A., Yusuf S. (2017) Reducing the Global Burden of Cardiovascular Disease, Part 1. Circulation Research 121, 677-694. [DOI] [PubMed] [Google Scholar]
- Kelly J., Fulford J., Vanhatalo A., Blackwell J.R., French O., Bailey S.J., Gilchrist M., Winyard P.G., Jones A.M. (2013) Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. American Journal of Physiology. Regulatory, integrative and comparative physiology 304, 73-83. [DOI] [PubMed] [Google Scholar]
- Kerley C.P. (2017) Dietary nitrate as modulator of physical performance and cardiovascular health. Current Opinion in Clinical Nutrition and Metabolic Care 20, 440-446. [DOI] [PubMed] [Google Scholar]
- Koch C.D., Gladwin M.T., Freeman B.A., Lundberg J.O., Weitzberg E., Morris A. (2017) Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radical Biology and Medicine 105, 48-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley K.E., Winyard P.G., Bailey S.J., Vanhatalo A., Wilkerson D.P., Blackwell J.R., Gilchrist M., Benjamin N., Jones A.M. (2011) Acute dietary nitrate supplementation improves cycling time trial performance. Medicine and Science in Sports and Exercise 43, 1125-1131. [DOI] [PubMed] [Google Scholar]
- Lara J., Ogbonmwan I., Oggioni C., Zheng D., Qadir O., Ashor A., Brandt K., Mathers J.C., Siervo M. (2015) Effects of handgrip exercise or inorganic nitrate supplementation on 24-h ambulatory blood pressure and peripheral arterial function in overweight and obese middle age and older adults: A pilot RCT. Maturitas 82, 228-235. [DOI] [PubMed] [Google Scholar]
- Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. (2006) Effects of dietary nitrate on blood pressure in healthy volunteers. The New England Journal of Medicine 355, 2792-2793. [DOI] [PubMed] [Google Scholar]
- Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. (2007) Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica 191, 59-66. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Stebbins C.L., Jung E., Nho H., Kim J.K., Chang M.J., Choi H.M. (2015) Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. American Journal of Physiology. Regulatory, integrative and comparative physiology 309, 459-466. [DOI] [PubMed] [Google Scholar]
- Lennon S.L., DellaValle D.M., Rodder S.G., Prest M., Sinley R.C., Hoy M.K., Papoutsakis C. (2017) 2015 Evidence Analysis Library Evidence-Based Nutrition Practice Guideline for the Management of Hypertension in Adults. Journal of the Academy of Nutrition and Dietetics 117, 1445-1458. [DOI] [PubMed] [Google Scholar]
- Malachias M.V.B. (2016) 7th Brazilian Guideline of Arterial Hypertension: Presentation. Arquivos Brasileiros de Cardiologia 107, XV-XIX. Available from URL: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0066-782X2016004800001&lng=en&nrm=iso [Accessed 15 August 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh S.T.J., Wylie L.J., Webster J.M.A., Vanhatalo A., Jones A.M. (2018) Influence of dietary nitrate food forms on nitrate metabolism and blood pressure in healthy normotensive adults. Nitric Oxide 72, 66-74. [DOI] [PubMed] [Google Scholar]
- McIlvenna L.C., Monaghan C., Liddle L., Fernandez B.O., Feelisch M., Muggeridge D.J., Easton C. (2017) Beetroot juice versus chard gel: A pharmacokinetic and pharmacodynamic comparison of nitrate bioavailability. Nitric Oxide 64, 61-67. [DOI] [PubMed] [Google Scholar]
- Miranda K.M., Espey M.G., Wink D.A. (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5, 62-71. [DOI] [PubMed] [Google Scholar]
- Mortensen S.P., Nyberg M., Thaning P., Saltin B., Hellsten Y. (2009) Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53, 993-999. [DOI] [PubMed] [Google Scholar]
- Muggeridge D.J., Howe C.C., Spendiff O., Pedlar C., James P.E., Easton C. (2014) A single dose of beetroot juice enhances cycling performance in simulated altitude. Medicine and Science in Sports and Exercise 46, 143-150. [DOI] [PubMed] [Google Scholar]
- Newsholme P., Homem-Bittencourt P.I., O’Hagan C., De Vito G., Murphy C., Krause M.S. (2009) Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clinical Science 118, 341-349. [DOI] [PubMed] [Google Scholar]
- Nobre F., Antonio M., Gomes M., Nobre F. (2011) V Brazilian guidelines for ambulatory monitoring of arterial pressure and III Brazilian guidelines for home monitoring of blood pressure. Jornal Brasileiro de Nefrologia 33, 365-388. [DOI] [PubMed] [Google Scholar]
- Pescatello L.S., Guidry M.A., Blanchard B.E., Kerr A., Taylor A.L., Johnson A.N., Maresh C.M., Rodriguez N., Thompson P.D. (2004) Exercise intensity alters postexercise hypotension. Journal of Hypertension 22, 1881-1888. [DOI] [PubMed] [Google Scholar]
- Ravera A., Carubelli V., Sciatti E., Bonadei I., Gorga E., Cani D., Vizzardi E., Metra M., Lombardi C. (2016) Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 8, 1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., et al. (2017) Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. Journal of the American College of Cardiology 70, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadian F., Dalili N., Jamalian A. (2016) Lifestyle Modifications to Prevent and Control Hypertension. Iranian Journal of Kidney Diseases 10, 237-263. [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D. (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard R.J. (1988) PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Medicine 5, 185-195. [DOI] [PubMed] [Google Scholar]
- Shepherd A.I., Gilchrist M., Winyard P.G., Jones A.M., Hallmann E., Kazimierczak R., Rembialkowska E., Benjamin N., Shore A.C., Wilkerson D.P. (2015) Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled crossover trial. Free Radical Biology and Medicine 86, 200-208. [DOI] [PubMed] [Google Scholar]
- Siervo M., Lara J., Ogbonmwan I., Mathers J.C. (2013) Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. The Journal of Nutrition 143, 818-826. [DOI] [PubMed] [Google Scholar]
- Sosner P., Guiraud T., Gremeaux V., Arvisais D., Herpin D., Bosquet L. (2017) The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta-analysis of selected moderators. Scandinavian Journal of Medicine and Science in Sports 27, 327-341. [DOI] [PubMed] [Google Scholar]
- Thomas D.T., Erdman K.A., Burke L.M. (2016) American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Medicine and Science in Sports and Exercise 48, 543-568. [DOI] [PubMed] [Google Scholar]
- Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D., et al. (2018) 2017 ACC/ AHA/ AAPA/ ABC/ ACPM/ AGS/ APhA/ ASH/ ASPC/ NMA/ PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 15;71(19), e127-e248. [DOI] [PubMed] [Google Scholar]
- Whelton P.K., He J., Appel L.J., Cutler J.A., Havas S., Kotchen T.A., Roccella E.J., Stout R., Vallbona C., Winston M.C., Karimbakas J., National High Blood Pressure Education Program Coordinating Committee (2002) Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. The Journal of the American Medical Association 288, 1882-1888. [DOI] [PubMed] [Google Scholar]
- Whitfield J., Ludzki A., Heigenhauser G.J., Senden J.M., Verdijk L.B., Van Loon L.J., Spriet L.L., Holloway G.P. (2016) Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. The Journal of Physiology 594, 421-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organization. (2014) Obesity and overweight. Available from URL: http://www.who.int/mediacentre/factsheets/fs311/en/ [Accessed 10 August 2017].
- Wylie L.J., Kelly J., Bailey S.J., Blackwell J.R., Skiba P.F., Winyard P.G., Jeukendrup A.E., Vanhatalo A., Jones A.M. (2013) Beetroot juice and exercise: pharmacodynamic and dose-response relationships. Journal of Applied Physiology 115, 325-336. [DOI] [PubMed] [Google Scholar]
- Zafeiridis A. (2014) Mechanisms and Exercise Characteristics Influencing Postexercise Hypotension. British Journal of Medicine and Medical Research 4, 5699-5714. [Google Scholar]


