Abstract
We aimed to compare the effects between non-vibration foam rolling (NVFR) and vibration foam rolling (VFR) on visual analogic scale (VAS), pressure pain threshold (PPT), oxygen saturation (SmO2), countermovement jump (CMJ) and hip and knee range of movement (ROM) after eliciting muscle damage through eccentric acute exercise using an inertial flywheel. Thirty-eight healthy volunteers (32 men, 6 women; aged 22.2±3.2 years) were randomly assigned in a counter-balanced fashion to either a VFR or NVFR protocol group. All participants performed a 10x10 (sets x repetitions) eccentric squat protocol to induce muscle damage. The protocols were administered 48-h post-exercise, measuring VAS, PPT, SmO2, CMJ and ROM, before and immediately post-treatment. The treatment technique was repeated on both legs for 1 minute for a total of five sets, with a 30-s rest between sets. The VFR group showed substantially greater improvements (likely to very likely) in the passive VAS (VFR -30.2%, 90% CI -66.2 to -12.8) with chances for lower, similar or greater VAS compared with the NVFR group of 82%, 14% and 4%, respectively and passive extension hip joint ROM (VFR 9.3%, 90% CI 0.2–19.2) with chances for lower, similar or greater ROM compared with the NVFR group of 78%, 21% and 1%, respectively. For intragroup changes, we observed substantial improvements in VAS (p=.05), lateral vastus, rectus femoris and medial vastus PPT. The results suggest that the VFR group achieved greater short-term benefits in pain perception and passive extension hip joint ROM. Both protocols were effective in improving PPT, SmO2, CMJ and knee joint ROM. The enhanced improvement in VAS and hip ROM measures could have significant implications for VFR treatment.
Key points.
The vibration foam rolling group showed substantially greater improvements in pain perception and passive hip extension ROM.
Both groups of foam rolling improve lateral vastus, rectus femoris and medial vastus pressure pain threshold, oxygen saturation and countermovement jump.
Future studies and clinical practice should consider these data with delayed-onset muscle soreness.
Key words: Foam roller, vibration foam roller, pressure pain threshold, oxygen saturation, countermovement jump, delayed onset muscle soreness
Introduction
The effects of myofascial treatment have been investigated more thoroughly in recent years (Pearcey et al., 2015). This physical therapy approach has two forms of application: as manual treatment or as self-myofascial technique (Healey et al., 2014; MacDonald et al., 2013; Romero-Moraleda et al., 2017). In the former, the treatment is applied by a therapist, in contrast, the latter, the individual uses their own body weight to apply pressure on a foam roller (FR) to produce friction, increasing muscular temperature and decreasing pain associated with delayed-onset muscle soreness (DOMS) (Macdonald et al., 2014; Pearcey et al., 2015).
The FR technique potentially improves recovery from demanding exercise (Cheatham et al., 2015). Recent studies have shown that FR improves range of motion (ROM) in various joints. MacDonald et al. (2014) reported that following exercise with induced muscle damage (EIMD), FR improved knee joint ROM compared to the control group. Hip-flexion/extension ROM changes have also been observed after FR treatment (Mohr et al., 2014; Pearcey et al., 2015; Su et al., 2017). Mohr et al. (2014) reported changes in passive hip-flexion ROM in individuals receiving FR and static stretching in comparison with individuals that received static stretching alone, FR alone and control (wait and see). Moreover, Su et al., (2017) designed a cross-over study in which participants improved significantly after foam rolling in comparison with static and dynamic stretching. Similarly, FR on calf muscles was shown to significantly improve ankle joint ROM (Halperin et al., 2014).
Perception of muscle soreness after exercise measured with the visual analogue scale (VAS) or pressure pain threshold (PPT) are important variables to consider when studying FR effects on recovery. Pearcey et al. (2015) observed no significant differences in the PPT when comparing FR treatment with a control group. In contrast, Jay et al., (2014) reported that FR increased PPT in comparison with massage. Some authors have also observed a significant difference between FR treatment and control groups in VAS scores, which is in agreement with MacDonald et al. (2013) findings.
However, FR treatment appears to have led to controversial results in terms of muscular performance. MacDonald et al. (2013) found no beneficial effects when muscular performance was measured on a maximal voluntary contraction. Another study showed that FR compared with planking had no effect on isometric force (Healey et al., 2014). No significant differences were found between FR and static-stretching for electromyography (EMG) (Halperin et al., 2014). In contrast, when FR was added to a warm-up protocol, results showed improvements in muscle performance testing (power, speed, strength and agility) (Peacock et al., 2014). In a recent study, maximal voluntary contraction in the rectus femoris improved after FR treatment compared with manual therapy techniques as neurodynamic mobilization (Romero-Moraleda et al., 2017).
Vibration therapy (VT) could be an alternative method to enhance recovery (Fagnani et al., 2006; Cronin, Nash and Whatman, 2007; Imtiyaz, Veqar and Shareef, 2014). Vibrating foam rollers (VFRs) have recently emerged from the designs of traditional therapeutic apparatus where VFR combines FR with local vibration that targets a specific muscle group. However, only a few studies have examined the effectiveness of VFRs. Cheatham et al. (2017) obtained greater improvements in knee ROM as well as improvements in a pain perception scale with a VFR compared with a non-vibration foam roller (NVFR). Additionally, Han et al., (2017) observed improvements in hip ROM and pain measurements through PPT when comparing both approaches. However, the aforementioned interventions were conducted on participants without exercise induced muscle damage EIMD. Furthermore, to the best of our knowledge, no study has compared the effect of vibration rolling with non-vibration rolling in individuals with EIMD in both the knee and hip joint to analyze its effects on training recovery. Therefore, the purpose of this study was to compare the effects of VFR and NVFR on perceived pain, muscle oxygen saturation (SmO2), vertical jump and hip and knee ROM, after EIMD.
Methods
Participants
Based on previous related studies (Cavanaugh et al., 2017; MacDonald et al., 2013; 2014; Pearcey et al., 2015) we conducted a pilot study with 5 participants in each group to determine the effect size and statistical power. The analysis indicated that a minimum of 12 participants would be needed to attain an alpha of 0.05 with a power of 0.8.
Thirty-eight healthy individuals (32 men, 6 women; aged 22.2 ± 3.2 years) participated in the study. All the participants had been free from musculoskeletal disorders and considered active (more then 600 Mets/week of moderate or vigorous exercise) in the last year. The participants were asked to abstain from unaccustomed exercise, as well as all medications and dietary supplements, for 72 hours before baseline measurements, during the experimental period and post-treatment (Figure 1). Informed consent was obtained from all participants after the researchers explained the study design and requirements. The study and informed consent procedures were approved by the Camilo José Cela Ethics Committee in agreement with the latest version of the Declaration of Helsinki. The trial was registered with the United States National Institutes of Health Clinical Trials Registry, with the registration number NCT03662152.
Figure 1.
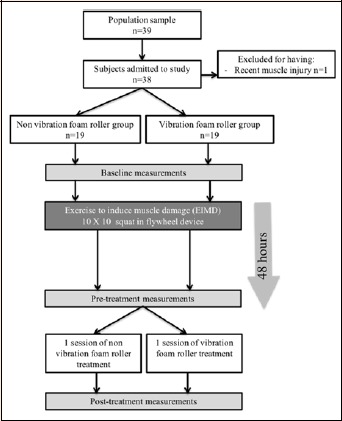
Flow chart.
Procedures: Description of muscle damage protocol
Muscle damage was induced with overload eccentric training using an inertial flywheel (2.7-kg flywheels with a moment inertia of 0.07 kg·m-2). Immediately after baseline measurements, the participants performed 10 sets x 10 repetitions of parallel squats using a gravity-free training device flywheel (Kbox squat, Exxentric, Sweden) with 2 minutes of recovery between sets; similar to the protocol used by Coratella et al. (2016). The squat exercise until 90º of knee flexion with 2:1 tempo was chosen as a basic movement because of its similar muscle recruitment to that of many athletic movement patterns. Furthermore, the squat exercise is one of the main exercises used to improve lower-body strength. All the participants practiced before beginning the eccentric session, and they exerted maximal effort in each repetition. Participants warmed up for 5 minutes on a treadmill at 50-60% of the heart rate reserve and dynamic stretching before starting the protocol.
Description of the foam roller intervention
The intervention technique was identical to a previously published protocol (Romero-Moraleda et al., 2017). The timing for both techniques (NVFR and VFR) was fixed at 3:4 using a metronome (free Iphone app; Gismar).
NVFR Group: participants performed the FR protocol using a regular foam roller.
VFR Group: participants performed the same protocol using a Hyperice foam roller with vibration (frequency provided by the device: 18 Hz).
Primary outcome
The primary outcome was the VAS used to measure DOMS and to measure the intensity of perceived pain. In order to standardize the scaling instructions, the endpoints were described for the participant as 0 = no sensation and 10 = the most intense sensation imaginable. The VAS was measured in four different conditions: (1) passive; (2) during isometric quadriceps contraction (holding the back in the wall); (3) performing a squat; and (4) during quadriceps stretching. Participants that do not elicited DOMS were excluded (at least 3 on the VAS scale).
Secondary outcomes: Pressure pain threshold
The PPT was measured using a digital pressure algometer (Microfet3, Hoggan Health Industries). The digital dynamometer with a 1-cm probe was used to measure three points of the quadriceps: vastus lateralis (VL), rectus femoris (RF) and vastus medialis (VM) all of them measured in the center of each muscle. Participants lay on the stretcher, the algometer was positioned perpendicularly to the skin at each point and gradual pressure was applied until the participant reported a change from the sensation of pressure to pain. At that point, the pressure was stopped immediately and the value was recorded representing the pain threshold (Fingleton et al., 2014). The mean of the 3 measurements was calculated. Algometry has been shown to be highly reliable (intraclass correlation coefficient [ICC] = 0.91) (Chesterton et al., 2007).
Muscle oxygen saturation
Muscle oxygen saturation (SmO2) was recorded during rest, during 10 squat repetitions (body weight load) and immediately afterwards, using a commercially available portable noninvasive near infrared spectroscopy (NIRS) device (Moxy, Fortiori Design LLC, MN, USA). The use of a NIRS during muscle contraction has previously been studied (Perrey and Ferrari, 2017). In this study, muscle oxygenation was used for measuring the VL of the dominant leg (Ferrari et al., 2004; Wang et al., 2012). The probe weighed 40 g, with dimensions of 61×44×21 mm. The NIRS measures muscle tissue saturation by emitting near-infrared light (wavelength, 630–850 nm) into the muscle tissue. The reflected near-infrared light is collected by two optical detectors positioned at a distance of 12.5 and 25 mm from the light source. The light is processed by an algorithm that combines a tissue light propagation model and the Beer–Lambert law to calculate the amount of light absorbed at wavelengths pertaining to oxygenated and deoxygenated hemoglobin, allowing us to determine the percentage of hemoglobin containing O2 (SmO2).
To ensure the correct SmO2 data, VL skinfold thickness was measured (average of three measurements) to place the NIRS device, using a Holtain caliper (Crymych, UK). Using the previous protocol (van Beekvelt et al., 2001). Adequate adipose tissue thickness (ATT) is between 2.0 and 11.7 mm (Van Der Zwaard et al., 2016).
Countermovement jump
Infrared Optojump photoelectric cells (Microgate Corporation, Bolzano, Italy) were used to measure explosive strength in the lower limbs (Glatthorn et al., 2011). Optojump bars were connected to a personal computer, and Microgate software (Optojump software, version 3.01.0001) allowed jump height quantification. The participants performed vertical jumps with hands on hips, knees straight in the flight phase and flexed 90º in the landing phase, while jumping as high as possible. The rest period was 1 minute between jumps. The participants followed these technical instructions, rigorously controlled by the practitioner during each test session. A total of three jumps were recorded, and the highest jump was used for evaluation.
Range of motion
Passive and active hip extension and knee flexion ROM of the dominant leg were measured with a Microfet3 inclinometer placed in the middle of the thigh and tibia respectively (Norkin and White, 2004). Hip extension ROM was assessed in a prone position with knees extended in a treatment table. To achieve the hip extension measurement, the participant extended the hip to the maximum extent possible, then a research assistant extended the hip passively to the point of pain or physiological limitation. Knee flexion ROM was also measured the in the prone position, with the hip in a neutral position. To measure active knee flexion, the participant flexed until the voluntary maximal point. A research assistant then flexed the knee passively until the point of pain or anatomical limitation. Each ROM test was measured three times, and the average measurement of these trials was used in the analysis.
Reliability of the measurements
A researcher blinded to the group allocation completed the evaluations. The reliability and precision were calculated with ICC model 3.1, using PPT. The ICC for test-retest trials was 0.93 (95% CI 0.71–0.95). The standard error of measurement (SEM) was calculated using the following formula: S x (√1 – ICC), in which S corresponded to the pooled standard deviation and ICC was the reliability coefficient. The SEM value was 1.15. The minimum difference was also calculated at a 95% CI employing the formula 1.96 x (√2 x SEM) = 2.98.
Statistical analyses
Data are presented as mean ± standard deviation (SD). To determine the magnitude of the protocol effect, effect size (ES) was determined by converting partial eta-squared to Cohen’s d. According to Cohen, ES can be classified as trivial (d ≤ 0.19), small (0.20 ≤ d ≤ 0.49), medium (0.50 ≤ d ≤ 0.79) or large (d ≥ 0.80) (Cohen J., 1988). Test-retest reliability was assessed using ICCs and SEM. Quantitative chances of beneficial/better or detrimental/poorer effect were assessed qualitatively as follows: <1%, almost certainly not; 1% to 5%, very unlikely; 5% to 25%, unlikely; 25% to 75%, possibly; 75% to 95%, likely; 95% to 99%, very likely; and >99%, almost certainly. A substantial effect was set at >75%. If the chances of having beneficial/better and detrimental/poorer performances were both >5%, the true difference was assessed as unclear. Otherwise, we interpreted that change as the observed chance (Hopkins et al., 2009). To determine differences between measurement moments (baseline, pretreatment, post-treatment) analyses of variance (ANOVA) were used (time x group), with the post hoc Bonferroni test used for analyzing changes in the intragroup results. P-values less than 0.05 were considered statistically significant. All the data analyses were performed with the Statistical Package for Social Sciences software, version 21.0 for Mac (SPSS Inc., Chicago, IL, USA).
Results
The participants’ data revealed no significant differences in age, height, weight, ATT or training days per week (Table 1), except PPT.
Table 1.
Characteristics at baseline. Data are shown as mean (±SD).
| NVFR (n = 19) | VFR (n = 19) | |
|---|---|---|
| Age | 22.2 (3.2) | 21.9 (3.7) |
| Height (m) | 1.74 (.007) | 1.77 (.07) |
| Weight (kg) | 69.7 (11.4) | 75.26 (8.0) |
| ATT (mm) | 5.8 (2.1) | 5.4 (1.9) |
| Training days per week | 4.21 (1.08) | 4.63 (1.01) |
NVFR, non-vibration foam roller group; VFR, vibration foam roller group; ATT, adipose tissue thickness; SD, standard deviation.
Between treatment group analysis
The treatment x time interaction was significant only for passive VAS (F2,35 = 3.76; p = 0.033) and during the quadriceps contraction (F2,35 = 2.741; p = 0.01). The VFR group produced substantially better results in the passive VAS VFR -30.2%, 90% CI -66.2 to -12.8) with chances for lower, similar or greater VAS compared with the NVFR group of 82%, 14% and 4%, respectively and the passive hip joint extension ROM VFR 9.3%, 90% CI 0.2–19.2) with chances for lower, similar or greater ROM compared with the NVFR group of 78%, 21% and 1%, respectively. The VAS data are shown in Figures 2 and 3.
Figure 2.
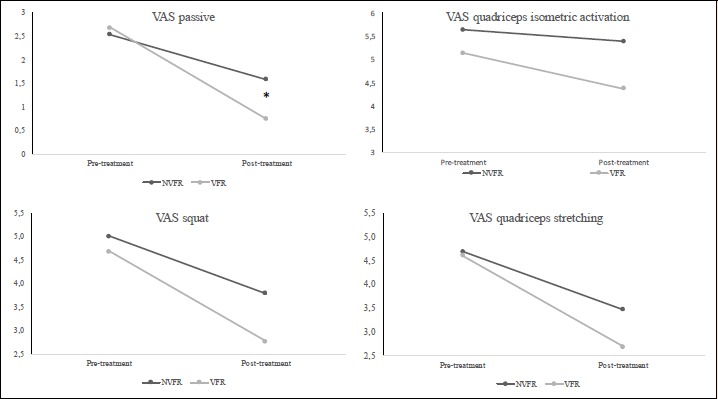
Comparison of the effects of EIMD and treatments: pre-treatment and post-treatment values in visual analogic scale (VAS). * significant differences between treatments p ≤ 0.05. NVFR, non-vibration foam roller group; VFR, vibration foam roller group; VAS, visual analogic scale.
Figure 3.
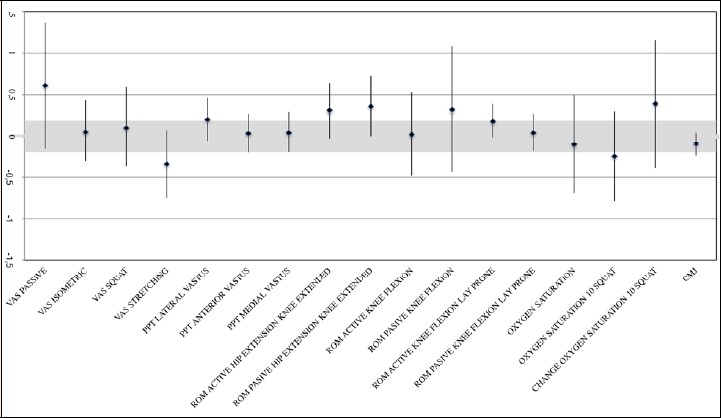
Forest plot with standardized mean differences (SMD) and 90% confidence interval (CI) for dependent variables. Lower scores (i.e., to the bottom in the X-axis) means lower scores in the VFR group. NVFR, non-vibration foam roller group; VFR, vibration foam roller group; VAS, visual analogic scale; ROM, range of movement; PPT, pressure pain threshold.
Within treatment group comparison
The intragroup changes are shown in Tables 2 and 3. Substantial improvements were obtained in both groups compared with the pre-test in terms of VAS, vastus lateralis, rectus femoris and medial vastus PPT; in oxygen saturation during rest, after ten squat repetitions and between these conditions; in countermovement jump (CMJ); and in passive and active hip extension ROM and knee flexion ROM. In addition, the VFR group also showed very likely substantial improvements in PPT, whereas the NVFR group showed possible changes. Both groups revealed a highly beneficial effect on O2 saturation after 10 squats (NVFR group ES = 1.23, 90% CI 0.44–2.01; VFR group ES = 0.96, 90% CI 0.36–1.55) (Table 4 and 5).
Table 2.
Data of pain (VAS score) at baseline, pretreatment and post-treatment. Data are shown as mean (± SD).
| Baseline | Pretreatment | Posttreatment | |||
|---|---|---|---|---|---|
| VAS | Passive | NVFR | 0.00 | 2.53 (2.55) a | 1.58 (1.43) a,b |
| VFR | 0.00 | 2.68 (2.81) a | 0.74 (1.14) * | ||
| Quadriceps isometric activation | NVFR | 0.00 | 5.63 (2.63) a | 5.38 (2.52) a,b | |
| VFR | 0.00 | 5.13 (2.45) a | 4.37 (2.31) a,b | ||
| Squat | NVFR | 0.00 | 5.00 (2.40) a | 3.79 (2.68) a,b | |
| VFR | 0.00 | 4.68 (2.03) a | 2.76 (2.15) a,b | ||
| Quadriceps Stretching | NVFR | 0.00 | 4.68 (2.45) a | 3.47 (2.55) a,b | |
| VFR | 0.00 | 4.61 (2.13) a | 2.68 (2.00) a,b | ||
| PPT | Lateral vastus | NVFR | 11.27 (2.37) | 8.03 (3.96) a | 9.17 (4.15) |
| VFR | 14.80 (4.38) * | 10.32 (4.12) a | 12.06 (5.24) a,b | ||
| Rectus femoris | NVFR | 12.68 (3.54) | 9.42 (4.63) a | 10.36 (4.64) a | |
| VFR | 16.60 (6.20) * | 12.05 (4.88) a | 13.82 (5.34) a,b* | ||
| Vastus medialis | NVFR | 10.90 (3.02) | 7.71 (4.16) a | 8.23 (3.42) a | |
| VFR | 15.17 (5.56) | 10.51 (4.50) a* | 12.53 (5.27) a,b** | ||
NVFR, non-vibration foam roller group; VFR, vibration foam roller group; VAS, visual analogic scale; PPT, pressure pain threshold.
* p <0.05
**p < 0.01 compared with NVFR.
a, difference (p < 0.05) with baseline
b, difference (p < 0.05) with pretreatment.
Table 3.
Data of pain at pretreatment and post-treatment.
| Pretreatment post-treatment intragroup comparison | ||||||||
|---|---|---|---|---|---|---|---|---|
| ES standardized (90% CL) intragroups | Chances | QA | ES standardized (90% CL) between groups | Chances | QA | |||
| VAS | Passive | NVFR | -0.5 (-0.91;-0.10) | 90/10/0 | Likely | 0.61 (1.37;-0.15) | 82/14/4 | Likely |
| VFR | -1.22 (-1.93;-0.51) | 99/1/0 | Very likely | |||||
| Quadriceps isometric activation | NVFR | -0.79 (-1.12;-0.46) | 100/0/0 | Very likely | 0.06 (0.44;-0.31) | 27/61/12 | Unclear | |
| VFR | -0.6 (-0.79;-0.4) | 100/0/0 | Very likely | |||||
| Squat | NVFR | -0.77 (-1.04;-0.51) | 100/0/0 | Very likely | 0.11 (0.59;-0.37) | 38/48/14 | Unclear | |
| VFR | -0.95 (-1.37;-0.53) | 100/0/0 | Very likely | |||||
| Quadriceps Stretching | NVFR | -0.97 (-1.27;-0.66) | 100/0/0 | Very likely | -0.34 (0.08;-0.75) | 71/27/2 | Unclear | |
| VFR | -0.73 (-1.07;-0.39) | 99/1/0 | Very likely | |||||
| PPT | Lateral vastus | NVFR | 0.23(0.05;0.41) | 61/39/0 | Possibly | 0.21 (-0.05;0.46) | 51/48/1 | Unclear |
| VFR | 0.41 (0.19;0.63) | 95/5/0 | Very likely | |||||
| Rectus femoris | NVFR | 0.23 (0.07;0.38) | 62/38/0 | Possibly | 0.04 (-0.2;0.28) | 14/81/5 | Unlikely | |
| VFR | 0.35 (0.13;0.56) | 87/13/0 | Likely | |||||
| Vastus medialis | NVFR | 0.24 (0.03;0.45) | 63/37/0 | Possibly | 0.05 (-0.19;0.29) | 14/81/4 | Unlikely | |
| VFR | -0.5 (-0.91;-0.10) | 90/10/0 | Likely | |||||
NVFR, non-vibration foam roller group; VFR, vibration foam roller group; VAS, visual analogic scale; PPT, pressure pain threshold; ES, standardized differences; QA, quality assessments.
Table 4.
Data of oxygen saturation, neuromuscular strength and range of motion in baseline, pretreatment and posttreatment moments. Data are shown as mean (± SD).
| Baseline | Pretreatment | Posttreatment | |||
|---|---|---|---|---|---|
| SmO2 | Rest | NVFR | 62.00 (12.82) | 60.95 (9.90) | 66.74 (13.86) |
| VFR | 65.37 (10.20) | 62.00 (15.1) | 65.74 (14.42) | ||
| After ten squats | NVFR | 37.74 (14.66) | 36.58 (12.7) | 48.47 (17.49) a,b | |
| VFR | 36.11 (16.80) | 42.26 (15.6) | 50.53 (16.47) a | ||
| CMJ | NVFR | 34.63 (4.97) | 31.40 (4.97) a | 33.05 (5.53) a,b | |
| VFR | 37.09 (5.89) | 33.78 (6.01) a | 36.23 (6.18) b | ||
| Hip joint ROM | Active | NVFR | 27.16 (4.41) | 22.63 (5.21) a | 26.47 (3.95) a,b |
| VFR | 28.21 (5.65) | 22.21 (5.69) a | 27.21 (5.44) a,b | ||
| Passive | NVFR | 30.63 (4.22) | 26.11 (5.31) a | 29.21 (3.90) a,b | |
| VFR | 31.89 (5.79) | 26.42 (5.98) a | 30.79 (6.21) a,b | ||
| Knee joint ROM | Active | NVFR | 124.95 (3.58) | 121.8 (3.26) a | 123.58 (3.31) a,b |
| VFR | 125.63 (6.59) | 120.8 (5.14) a | 124.26 (6.31) a,b | ||
| Passive | NVFR | 129.95 (5.67) | 125.0 (4.13) a | 126.11 (3.86) a | |
| VFR | 129.95 (6.65) | 126.0 (6.08) a | 128.26 (6.59) b | ||
SmO2, muscle oxygen saturation; CMJ, countermovement jump; ROM, range of motion; SD, standard deviation.
a. Significant (p ≤ 0.05) difference with baseline measure.
b Significant (p ≤ 0.05) difference with pretreatment group.
Table 5.
Data of oxygen saturation, neuromuscular strength and range of motion in baseline, pretreatment and posttreatment moments.
| Pretreatment post-treatment intragroup comparison | ||||||||
|---|---|---|---|---|---|---|---|---|
| ES standardized (90% CL) intragroups | Chances | QA | ES standardized (90% CL) between groups | Chances | QA | |||
| SmO2 | Rest | NVFR | 0.28 (-0.09;0.65) | 64/43/2 | Possibly | -0.10 (0.49;-0.70) | 20/41/39 | Unclear |
| VFR | 0.41 (-0.08;0.91) | 77/21/2 | Likely | |||||
| After ten squats | NVFR | 1.23 (0.44;2.01) | 98/1/0 | Very likely | 0.21 (0.74;-0.33) | 10/39/51 | Unclear | |
| VFR | 0.96 (0.36;1.55) | 98/2/0 | Very likely | |||||
| CMJ | NVFR | 0.42 (0.3;0.54) | 100/0/0 | Very likely | -0.09 (-0.24;0.05) | 0/89/11 | Unclear | |
| VFR | 0.29 (0.2;0.38) | 95/5/0 | Very likely | |||||
| Hip joint ROM | Active | NVFR | 0.53 (0.32;0.73) | 99/1/0 | Very likely | 0.31 (-0.02;0.64) | 71/28/1 | Unclear |
| VFR | 0.91 (0.63;1.19) | 100/0/0 | Very likely | |||||
| Passive | NVFR | 0.34 (0.14;0.53) | 88/12/0 | Likely | 0.36 (0.01;0.72) | 78/22/1 | Likely | |
| VFR | 0.71 (0.45;0.97) | 100/0/0 | Very likely | |||||
| Knee joint ROM | Active | NVFR | 0.62 (0.48;0.76) | 100/0/0 | Very likely | 0.19 (-0.01;0.39) | 47/52/0 | Unclear |
| VFR | 0.48 (0.33;0.62) | 100/0/0 | Very likely | |||||
| Passive | NVFR | 0.43 (0.23;0.63) | 97/3/0 | Very likely | 0.05 (-0.18;0.28) | 14/82/4 | Unlikely | |
| VFR | 0.26 (0.1;0.42) | 74/26/0 | Possibly | |||||
ES, effect size; CL, confidence limits; QA, qualitative assessment; CMJ, countermovement jump; SmO2, muscle oxygen saturation; ROM, range of motion.
Discussion
The present study assessed whether a VFR could enhance recovery more than NVFR application after EIMD. To our knowledge, this is the first study to explore the influence of adding vibration to an FR during recovery. The first important finding is that the VFR group achieved greater short-term benefits in pain perception and passive hip extension ROM, after EIMD. The second important finding is that both the vibration and non-vibration FR protocols achieved similar short-term results in PPT, CMJ, oxygen saturation, active hip extension ROM and knee flexion ROM.
This study provided the first data verifying that VFR could improve individual tolerance to pain more than a traditional FR when measuring only post treatment. Our FR protocol decreased 0.95 points (ES: 0.61) in muscle soreness measured with the VAS, in accordance with a decrease of ~ 0.8 points reported by MacDonald et al. (2013), when measured 48 h after EIMD. Further, the significant improvement of the VFR group in comparison with the NVFR group (-1.95 points; ES: 0.61) could be due to the mechanism underlying the positive effects after vibration. The vibration protocol also has already been shown to reduce pain perception (Aminian-Far et al., 2011; Weerakkody et al., 2003a; 2003b). A study by Ayles et al. (2011) has reported hypoalgesia after applying the vibration protocol after DOMS was provoked in the anterior tibialis muscle in 15 healthy males, using the contralateral leg as control. Pressure (without controlling the pressure) placed on the muscle through the FR and vibration are effective to attenuate pain perception; this finding is supported in the broader concept of the gate control theory (Melzack and Wall, 1965). An incremental increase of analgesia could be induced by selectively activating, through pressure and vibration, the large rapid-conduction A fibers; these could provoke the liberation of inhibitory neurotransmitters to block the nociceptive impulses transmitted by C fibers and by the lateral spinothalamic tract in the spinal cord to keep them from reaching the pain center in the thalamus, as postulated by the gate control theory (Melzack and Wall, 1965).
Following these results, it is reasonable to speculate that VFR could also enhance PPT more than NVFR. However, in our study, NVFR and VFR were effective in increasing PPT in the vastus lateralis, medialis and rectus femoris after treatment (NVFR group ES: 0.23, 0.24, 0.23; VFR group ES: 0.41, 0.29, 0.35, respectively). The clinical trial conducted by Cheatham et al., (2017) concluded that PPT showed a significant difference between vibrating roller use and the other groups (non-vibrating roller and control group) in healthy individuals under normal circumstances (without soreness or fatigue). These authors also showed an increase in PPT for both groups; however, greater PPT was found for the vibration group (ES for vibration group: 0.79, ES for non-vibration group: 0.50; p = 0.001 for both groups) (Cheatham et al., 2017). The lower ES found in our study in comparison with Cheatham et al. (2017) could be due to our participants having EIMD.
The physiological mechanism behind the influence of foam rolling on recovery from EIMD remains unclear and warrants further investigations (D’Amico and Gillis, 2017). In the present study, an oximeter device was used in order to provide information about performance and muscle oxygen function and to evaluate previous response during and after exercise (Perrey and Ferrari, 2017). Several NIRS studies have shown that the muscle reoxygenation rate after either static (Fryer et al., 2015) or dynamic (McLean et al., 2016) exercise could be an important determinant of both muscle fatigue and training status. To our knowledge, this is the first study to measure SmO2 after a VFR or NVFR protocol. The SmO2 measured in the vastus lateralis through NIRS had similar changes for both protocols when measured during rest and after ten body squat repetitions. Both groups increased SmO2 in rest, 5.79% for NVFR (ES: 0.28) and 3.75% for VFR (ES: 0.41). The most remarkable change in SmO2 was after completion of 10 repetition squats: the NVFR group showed 11.89% more SmO2 (ES: 1.23) and VFR group 8.27% more (ES: 0.96). These data indicate that in both groups the SmO2 in the vastus lateralis was greater, demonstrating an impact of FR application in the recovery period and after 10 repetition squats. Therefore, the FR pressure promotes vasodilation and O2 delivery, as well as an increased blood volume, which could stimulate mitochondrial to accelerate adenosine-triphosphate and phosphocreatine repletion, which are O2 dependent (Kime et al., 2003). This process aids the removal of metabolic waste products during recovery periods between efforts. A previous pilot study showed acute physiological responses after FR application on vascular tissue function. Nitric oxide increase and pulse wave velocity decreased after one FR treatment session (Okamoto et al., 2014). These data might suggest that external compression is related to increased SmO2, promoting recovery.
In neuromuscular performance, the current study showed an average decrease of 9% in CMJ after EIMD, which subsequently improved an average of 7.49% (ES: 0.29) in the VFR group and 5.18% (ES: 0.42) in the NVFR group. The decrease in CMJ after an eccentric protocol to induce muscle soreness was similar to that reported in high-level middle- and long-distance runners post-competition (Balsalobre-Fernández et al, 2014) or in squat jumps due to neuromuscular fatigue induced by a soccer game (Robineau et al., 2012). Nevertheless, both protocols had similar effects in restoring neuromuscular performance measured through CMJ, and differences were not observed between them. In agreement with our results, Pearcey et al. (2015) showed that 20 min of FR protocol improved quadriceps muscle tenderness, sprint time, power and dynamic strength-endurance, attenuating the decrements of performance after DOMS.
Researchers have suggested that FR prior to training and competition and as a recovery tool is an optimal way to increase ROM, without the potential performance decrements associated with static stretching (MacDonald et al., 2013; Su et al., 2017). In the present investigation, the VFR group achieved an average 18.5% hip joint ROM, whereas the NVFR group obtained 15%, which shows a small improvement in the VFR group for passive hip joint ROM in comparison with the NVFR group (ES: 0.36). Both the NVFR and VFR interventions produced similar short-term results in knee ROM. A growing body of literature suggests that FR acutely increases ROM (Bradbury-Squires et al., 2015; Couture et al., 2015; Cheatham et al., 2017). MacDonald et al. (2013) showed an increment in knee joint ROM of 7°–10º greater in comparison with controls after two rounds of 1 minute FR application. Halperin et al. (2014) reported increases in ankle ROM after 3 rounds of 30 seconds’ FR application in the plantar flexor muscles (triceps surae muscle). The modulation of pain inducing analgesia and the change in tissue properties suggest increases in ROM. Vibration therapy leads to an increase in temperature and blood flow, which could provoke ROM improvements (Veqar and Imtiyaz, 2014). The reasons for the similar neuromuscular changes that occurred could be due to the effects of FR pressure on the viscoelastic properties of tissues. In accord with prior studies, the FR-induced modulation of pain (Cavanaugh et al. 2017) inducing analgesia and the change in tissue properties suggest increases in ROM (Aboodarda et al., 2015; Cavanaugh et al., 2017).
This study has several limitations. First, it measured healthy and active participants, which limits the generalizability of these results to other populations. Second, the vibrating roller was used at only one frequency (18 Hz), without comparing other frequencies. Third, only the dominant leg was measured to study the effects of each intervention. Fourth, measurements were performed short-term; it would be interesting to retest to 24 and 48 hours in future research. Finally, no participants were tested in a blinded condition, due to its impossibility with the sensation of vibration.
Conclusion
The results suggest that VFR achieved greater short-term effects on pain perception and in passive hip extension ROM. Both protocols were effective in improving PPT, SmO2, CMJ and knee joint ROM. These results must be considered with caution, and future research is needed to deepen our understanding of the effects of this combination of techniques.
Acknowledgements
The experiments comply with the current laws of the country in which they were performed. The authors have no conflicts of interests to declare.
Biographies

Blanca ROMERO-MORALEDA
Employment
Lecturer in Camilo José Cela University and she is Strength and Conditioning coach in a professional female soccer team.
Degree
PhD
Research interests
Female soccer, performance and recovery in sport and field hockey.
E-mail: blancaromeromoraleda@gmail.com

Jaime GONZÁLEZ-GARCÍA
Employment
Spanish Anti-Doping Agency (ESP-NADO).
Degree
Bachelor
Research interests
Human performance development.
E-mail: jaime33gonzalez@gmail.com

Ángel CUÉLLAR
Employment
Rehabilitation Fitness Coach and Physical Trainer
Degree
BSc
Research interests
Rehabilitation and sport physiology
E-mail: angelc.rayo@gmail.com

Carlos BALSALOBRE-FERNÁNDEZ
Employment
Consultant of elite athletes and develops apps to measure performance
Degree
PhD
Research interests
Strength and conditioning, biomechanics and technology
E-mail: carlos.balsalobre@icloud.com

Daniel MUÑOZ-GARCÍA
Employment
Lecturer, Centro Superior de Estudios Universitarios La Salle (CSEU). He is currently researcher in the Institute of Neuroscience and Science of Movement (INCIMOV) research group.
Degree
PhD
Research interests
Neuroscience, neurorehabilitation and exercise in chronic pain patients.
E-mail: danimgsan@gmail.com

Esther MORENCOS
Employment
Prof., Exercise and Sport Sciences, Education and Humanities Faculty, Francisco de Vitoria University, Madrid.
Degree
PhD
Research interest
Field hockey; Team analysis; Training load; strength and conditioning
E-mail: esther.morencos@ufv.es
References
- Aboodarda S., Spence A., Button D.C. (2015) Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskeletal Disorders 16(1), 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminian-Far A., Hadian M.-R., Olyaei G., Talebian S., Bakhtiary A.H. (2011) Whole-Body Vibration and the Prevention andTreatment of Delayed-Onset Muscle Soreness. Journal of Athletic Training 46(1), 43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayles S., Graven-Nielsen T., Gibson W. (2011) Vibration-induced afferent activity augments delayed onset muscle allodynia. Journal of Pain 12(8), 884-891. [DOI] [PubMed] [Google Scholar]
- Balsalobre-Fernández C., Ma Tejero-González C., Del Campo-Vecino J. (2014) Hormonal and neuromuscular responses to high-level middle- and long-distance competition. International Journal of Sports Physiology and Performance 9(5), 839-844. [DOI] [PubMed] [Google Scholar]
- van Beekvelt M.C., Borghuis M.S., van Engelen B.G., Wevers R.A., Colier W. N. (2001) Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clinical Science (London, England : 1979), 101(1), 21-28. [DOI] [PubMed] [Google Scholar]
- Bradbury-Squires D. J., Noftall J. C., Sullivan K. M., Behm D. G., Power K. E., Button D. C. (2015) Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. Journal of Athletic Training 50(2), 133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh M. T., Döweling A., Young J. D., Quigley P. J., Hodgson D. D., Whitten J. H. D., Reid J. C., Aboodarda S. J., Behm D. G. (2017) An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. European Journal of Applied Physiology 117(1), 109-117. [DOI] [PubMed] [Google Scholar]
- Cheatham S. W., Kolber M. J., Cain M., Lee M. (2015) The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: a systematic review. International Journal of Sports Physical Theraphy 10(6), 827-838. [PMC free article] [PubMed] [Google Scholar]
- Cheatham S. W., Stull K. R., Kolber M. J. (2017) Comparison of a Vibrating Foam Roller and a Non-vibrating Foam Roller Intervention on Knee Range of Motion and Pressure Pain Threshold: A Randomized Controlled Trial. Journal of Sport Rehabilitation 12(2), 1-23. [DOI] [PubMed] [Google Scholar]
- Chesterton L. S., Sim J., Wright C. C., Foster N. E. (2007) Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. The Clinical Journal of Pain 23(9), 760-766. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical Power Analysis for the Behavioural Science (2nd Edition). Statistical Power Anaylsis for the Behavioural Science (2nd Edition). [Google Scholar]
- Coratella G., Chemello A., Schena F. (2016) Muscle damage and repeated bout effect induced by enhanced eccentric squats. The Journal of Sports Medicine and Physical Fitness 56(12), 1540-1546. [PubMed] [Google Scholar]
- Couture G., Karlik D., Glass S. C., Hatzel B. M. (2015) The effect of foam rolling duration on hamstring range of motion. The Open Orthopaedics Journal 9(May), 450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J., Nash M., Whatman C. (2007) The effect of four different vibratory stimuli on dynamic range of motion of the hamstrings. Physical Therapy in Sport 8(1), 30-36. [Google Scholar]
- D’Amico A. P., Gillis J. (2017) The influence of foam rolling on recovery from exercise-induced muscle damage. Journal of Strength and Conditioning Research September 6, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fagnani F., Giombini A., Di Cesare A., Pigozzi F., Di Salvo V. (2006) The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. American Journal of Physical Medicine and Rehabilitation 85(12), 956-962. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Mottola L., Quaresima V. (2004) Principles, techniques, and limitations of near infrared spectroscopy. Canadian Journal of Applied Physiology 29(4), 463-487. [DOI] [PubMed] [Google Scholar]
- Fingleton C. P., Dempsey L., Smart K., Doody C. M. (2014) Intraexaminer and interexaminer reliability of manual palpation and pressure algometry of the lower limb nerves in asymptomatic subjects. Journal of Manipulative and Physiological Therapeutics 37(2), 97-104. [DOI] [PubMed] [Google Scholar]
- Fryer S. M., Stoner L., Dickson T. G., Draper S. B., McCluskey M. J., Hughes J. D., How S. C., Draper N. (2015) Oxygen recovery kinetics in the forearm flexors of multiple ability groups of rock climbers. Journal of Strength and Conditioning Research 29(6), 1633-1639. [DOI] [PubMed] [Google Scholar]
- Glatthorn J. F., Gouge S., Nussbaumer S., Stauffacher S., Impellizzeri F. M., Maffiuletti N. A. (2011) Validity and reliability of optojump photoelectric cells for estimating vertical jump height. Journal of Strength and Conditioning Research 25(2), 556-560. [DOI] [PubMed] [Google Scholar]
- Halperin I., Aboodarda S. J., Button D. C., Andersen L. L., Behm D. G. (2014) Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. International Journal of Sports Physical Therapy 9(1), 92-102. [PMC free article] [PubMed] [Google Scholar]
- Han S.-W., Lee Y., Lee D.-J. (2017) The influence of the vibration form roller exercise on the pains in the muscles around the hip joint and the joint performance. Journal of Physical Therapy Science 29(10), 1844-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey K. C., Hatfield D. L., Blanpied P., Dorfman L. R., Riebe D. (2014) The effects of myofascial release with foam rolling on performance. Journal of Strength and Conditioning Research 28(1), 61-68. [DOI] [PubMed] [Google Scholar]
- Hopkins W. G., Marshall S. W., Batterham A. M., Hanin J. (2009) ‘Progressive statistics for studies in sports medicine and exercise science. Medicine and Science in Sports and Exercise 41(1), 3-12. [DOI] [PubMed] [Google Scholar]
- Imtiyaz S., Veqar Z., Shareef M. Y. (2014) To compare the effect of vibration therapy and massage in prevention of delayed onset muscle soreness (DOMS). Journal of Clinical and Diagnostic Research 8(1), 133-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay K., Sundstrup E., Søndergaard S. D., Behm D., Brandt M., Særvoll C. A., Jakobsen M. D., Andersen L. L. (2014) Specific and cross over effects of massage for muscle soreness: randomized controlled trial. International Journal of Sports Physical Therapy 9(1), 82-91. [PMC free article] [PubMed] [Google Scholar]
- Kime R., Hamaoka T., Sako T., Murakami M., Homma T., Katsumura T., Chance B. (2003) Delayed reoxygenation after maximal isometric handgrip exercise in high oxidative capacity muscle’, European Journal of Applied Physiology 89(1), 34-41. [DOI] [PubMed] [Google Scholar]
- Macdonald G. Z., Button D. C., Drinkwater E. J., Behm D. G. (2014) Foam rolling as a recovery tool after an intense bout of physical activity. Medicine and Science in Sports and Exercise 46(1), 131-142. [DOI] [PubMed] [Google Scholar]
- MacDonald G. Z., Penney M. D. H. H., Mullaley M. E., Cuconato A. L., Drake C. D. J. J., Behm D. G., Button D. C. (2013) An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force’, Journal of Strength and Conditioning Research 27(3), 812-821. [DOI] [PubMed] [Google Scholar]
- McLean S., Kerhervé H., Lovell G. P., Gorman A. D., Solomon C. (2016) The effect of recovery duration on vastus lateralis oxygenation, heart rate, perceived exertion and time motion descriptors during small sided football games. PLoS ONE 11(2), p. e0150201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. (1965) Pain mechanisms: a new theory. Science (New York, N.Y.), 150(3699), 971-979. [DOI] [PubMed] [Google Scholar]
- Mohr A. R., Long B. C., Goad C. L. (2014) Foam Rolling and Static Stretching on Passive Hip Flexion Range of Motion. Journal of Sport Rehabilitation 23(4), 296-299. [DOI] [PubMed] [Google Scholar]
- Norkin C. C., White D. J. (2004) Measurement of joint motion : a guide to goniometry. 5th edition. F.A. Davis, Philadelphia. [Google Scholar]
- Okamoto T., Masuhara M., Ikuta K. (2014) Acute effects of self-myofascial release using a foam roller on arterial function.’, Journal of Strength and Conditioning Research 28(1), 69-73. [DOI] [PubMed] [Google Scholar]
- Peacock C. A., Krein D. D., Silver T. A., Sanders G. J., von Carlowitz K. P. A. (2014) An acute bout of self-myofascial release in the form of foam rolling improves performance testing.’, International Journal of Exercise Science 7(3), 202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcey G. E. P., Bradbury-Squires D. J., Kawamoto J. E., Drinkwater E. J., Behm D. G., Button D. C. (2015) Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures’, Journal of Athletic Training 50(1), 5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey S., Ferrari M. (2017) Muscle Oximetry in Sports Science: A Systematic Review. Sports Medicine 1(11(4)), 606-613. [DOI] [PubMed] [Google Scholar]
- Robineau J., Jouaux T., Lacroix M., Babault N. (2012) Neuromuscular fatigue induced by a 90-minute soccer game modeling. Journal of Strength and Conditioning Research 26(2), 555-562. [DOI] [PubMed] [Google Scholar]
- Romero-Moraleda B., La Touche R., Lerma-Lara S., Ferrer-Peña R., Paredes V., Peinado A. B., Muñoz-García D. (2017) Neurodynamic mobilization and foam rolling improved delayed-onset muscle soreness in a healthy adult population: a randomized controlled clinical trial. PeerJ 5, e3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Chang N.-J., Wu W.-L., Guo L.-Y., Chu I.-H. (2017) Acute Effects of Foam Rolling, Static Stretching, and Dynamic Stretching During Warm-ups on Muscular Flexibility and Strength in Young Adults. Journal of Sport Rehabilitation. 26(6), 469-477. [DOI] [PubMed] [Google Scholar]
- Veqar Z., Imtiyaz S. (2014) Vibration Therapy in Management of Delayed Onset Muscle Soreness (DOMS). Journal of Clinical and Diagnostic Research 8(6), LE01-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Xu G., Tian Q., Sun J., Sun B., Zhang L., Luo Q., Gong H. (2012) Differences between the vastus lateralis and gastrocnemius lateralis in the assessment ability of breakpoints of muscle oxygenation for aerobic capacity indices during an incremental cycling exercise. Journal of Sports Science and Medicine 11(4), 606-613. [PMC free article] [PubMed] [Google Scholar]
- Weerakkody N. S., Percival P., Hickey M. W., Morgan D. L., Gregory J. E., Canny B. J., Proske U. (2003a) Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain 105(3), 425-435. [DOI] [PubMed] [Google Scholar]
- Weerakkody N. S., Percival P., Hickey M. W., Morgan D. L., Gregory J. E., Canny B. J., Proske U. (2003b) Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain 105(3), 425-435. [DOI] [PubMed] [Google Scholar]
- Van Der Zwaard S., Jaspers R. T., Blokland I. J., Achterberg C., Visser J. M., Den Uil A. R., Hofmijster M. J., Levels K., Noordhof D. A., De Haan A., De Koning J. J., Van Der Laarse W. J., De Ruiter C. J. (2016) Oxygenation threshold derived from near- Infrared spectroscopy: Reliability and its relationship with the first ventilatory threshold. PLoS ONE 11(9), e0162914. [DOI] [PMC free article] [PubMed] [Google Scholar]


