Abstract
Primary central nervous system lymphoma (PCNSL) is a group of extranodal non-Hodgkin lymphoma that exhibits specific biological characteristics and clinical behavior, with an aggressive disease course and unsatisfactory patient outcomes. It is of great importance to identify aberrant genetic loci and important molecular pathways that might suggest potential targets for new therapeutics and provide prognostic information. In this review, we listed various genetic and epigenetic alterations that are involved in PCNSL pathogenesis. In the aspect of treatment, we summarized the related literatures and evaluated the efficacy of surgery, induction chemotherapy, radiotherapy, intrathecal chemotherapy, and autologous stem cell transplantation in PCNSL. We also proposed the possible new agents for recurrent and relapse PCNSL based on the result of recent clinical researches.
Introduction
Primary central nervous system lymphoma (PCNSL) represents a rare form of extranodal, malignant non-Hodgkin lymphoma. It is an aggressive type of cancer confined to the craniospinal axis without evidence of systemic involvement (brain>eyes>leptomeninges>spinal cord), with more than 90% of cases histologically classified as diffuse large B-cell lymphoma (DLBCL) [1].
While, clearly, high-dose methotrexate (HD-MTX) alone with additional agents is the mainstay of first-line therapy, it is often inadequate to achieve a complete response and requires treatment consolidation. The most challenging conundrum is which consolidation therapy has the optimal therapeutic index for balancing lasting cure with minimal early mortality and long-term neurotoxicity risk. The typical options for consolidation seem to be dose-reduced whole-brain radiotherapy (dr-WBRT) and high-dose chemotherapy with autologous stem cell transplantation (HDC-ASCT). Consolidation with dr-WBRT is simple to deliver and now has an adequate long-term record of efficacy and safety. The latter may be suitable for younger patients with adequate performance status. However, treatment outcomes are still unsatisfactory for patients with relapsed/refractory PCNSL, and further clinical trial data are needed to guide the therapeutic management for this group of patients.
Epidemiology
PCNSL accounts for 4%-6% of all extranodal lymphomas, up to 1% of all lymphomas, and about 2% of all central nervous system tumors [2]. Although the incidence of PCNSL increased by three-fold from 1973 to 1984, recent data from the Surveillance, Epidemiology, and End Results (SEER) database demonstrates that an incidence plateau has been reached [3]. The median age at diagnosis is 65 years old. PCNSL has been observed to occur with increased frequencies in individuals with acquired immunodeficiencies [acquired immune deficiency syndrome (AIDS) or posttransplant conditions] and/or congenital immunodeficiencies (X-linked lymphoproliferative syndrome, Wiskott-Aldrich syndrome, or ataxia telangectasia) [4]. PCNSL is one of the most common AIDS-related malignancies in individuals with low CD4 cell counts (<50 cells/mL) and Epstein-Barr virus (EBV) infection [5], [6]. However, since the discovery and implementation of combined antiretroviral therapy (the highly active antiretroviral therapy, HAART), a decreasing incidence of PCNSL has been reported among AIDS patients [7]. By contrast, epidemiological data have shown a progressively increasing PCNSL incidence among elderly individuals [8].
Molecular Pathogenesis
Pathology and Histogenetic Origin
PCNSL represents a histologically and immunohistochemically homogeneous type of lymphoma. Typical histological features include a vasocentric growth pattern and high lymphocyte proliferation, explaining its diffuse infiltration in the central nervous system (CNS). DLBCLs account for most PCNSLs (>90%), and the remainder comprise of Burkitt's lymphomas, low-grade lymphomas, or T-cell lymphomas (peripheral T-cell lymphomas and anaplastic large T-cell lymphomas) [1], [5]. EBV early RNA transcripts are often detectable by in situ hybridization in immunocompromised patients.
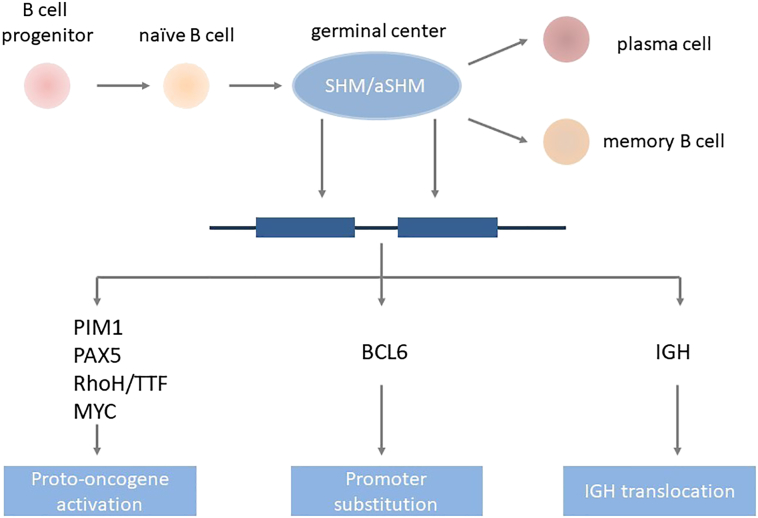
The B cell differentiation process may provide clues to the understanding of the histogenetic origins of PCNSL. The first step is the assembly of the V, D, and J gene segments of the heavy and light chains of immunoglobulin (Ig) genes in the bone marrow [9], [10]. Upon successful gene segment assembly, naive B cells leave the bone marrow and start their next maturation step, where they encounter antigens in the germinal centers (GCs) of secondary lymphoid organs, such as the spleen and lymph nodes, to improve the binding affinity of their B cell receptors (BCRs). The process of somatic hypermutation (SHM) in the first 1.5-2.0 kb of the V region genes of BCR heavy and light chains is activated in the GCs [11]. The processes of SHM and affinity maturation require the presence of the specific antigens, antigen-presenting cells and T cells, and BCL6 [12]. The SHM process may either increase or decrease the affinity of BCR and results in the selection of B cell clones for further rounds of SHM and, finally, to either go through apoptosis or exit the GCs [13]. After SHM, B cells can undergo Ig class switch recombination, which replaces the μ constant region of the BCR with other constant regions located downstream to generate diverse antibodies. Differentiation into memory or plasma cells completes B cell's differentiation [14] (Figure 1).
Figure 1.
Histogenetic origin of PCNSL. The CD10−BCL6+IRF4/MUM1+ phenotype of PCNSL cells indicates that they have participated in GC reactions and that further B cell maturation is impaired, which corresponds to the late GC B cell phenotype.
Abbreviations: aSHM, aberrant somatic hypermutation; SHM, somatic hypermutation, IGH, immunoglobulin heavy locus.
PCNSL cells morphologically resemble centroblasts, and the introduction of SHMs into rearranged Ig segments proves that they have participated in a GC reaction [15]. Expression of B cell markers, including CD19, CD20, and CD79a, is detectable in almost all PCNSLs. CD10 is present in 10%-20% of PCNSLs, and plasma cell markers (CD38, CD138) are generally absent. BCL6 and BCL2 are expressed in 60%-80% and 56%-93% of PCNSLs, respectively [16]. BCL6 is the main regulator of the GC reaction and represses the exit of B cells from GCs [17], [18]. The strong IRF4/MUM1 expression is observed in about 90% of PCNSLs, which indicates that the tumor cells are transitioning to leave the GC. The IRF4/MUM1 expression is usually associated with memory B cells rather than GC-B cells. This CD10−BCL6+IRF4/MUM1+ phenotype indicates that further B cell maturation is impaired, which corresponds to the late germinal center B cell phenotype [6] and correlates with a poor prognosis [14] (Figure 1).
It is still unclear whether PCNSL truly originates within the CNS or whether it is part of a systemic lymphoma that escapes from the immune system and grows in the “sanctuary” of the CNS. B cells recruited to the brain in the case of an immune reaction may stay for extended periods and eventually transform while residing inside the CNS. On the other hand, B cells might also have transformed to a malignant state outside the CNS, i.e., during a GC reaction in a secondary lymphoid organ. It is assumed that each of these two mechanisms is probable. However, homing of a malignant B cell exclusively to the brain is hard to explain and difficult to confirm experimentally. To date, no cell adhesion molecule or a chemokine predicting B cell homing selectively to the brain has been identified in the development of PCNSL [14].
Genomic alterations
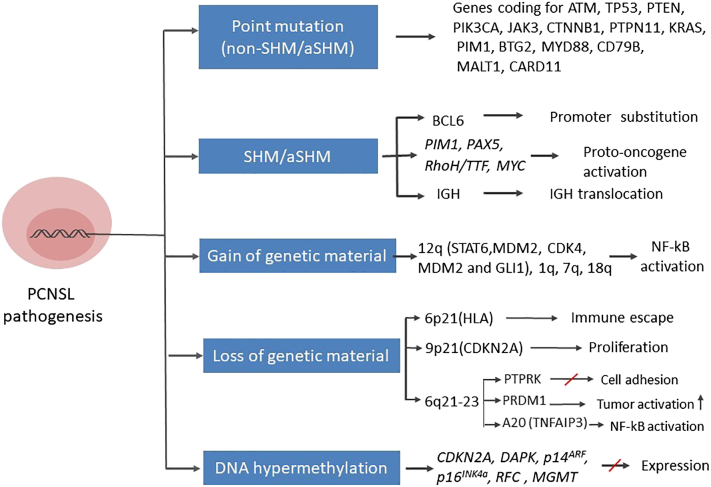
As all steps of B cell differentiation, especially SHM, require DNA double-strand breaks, the failure of DNA double-strand breaks may lead to the formation of malignant cells. PCNSL cells often carry translocations affecting Ig and Ig-related genes, especially Bcl6 [18]. A substitution in the promoter of the Bcl6 gene results in constitutive BCL6 activity, which can have tumorigenic effects. The Cancer and Leukemia Group B (CALGB) 50202 trial demonstrated that BCL6 overexpression is associated with poorer survival and refractory PCNSL condition [19]. While some other studies have confirmed this finding [20], [21], several small retrospective analyses have provided conflicting results [22], [23], [24]. Variation in treatment regimens, sample sizes, and analytical procedures may explain this discrepancy. Aberrant SHM (aSHM) can target proto-oncogenes including Myc, Pim1, Pax5, and Rhoh/Ttf; these genes are often involved in the modulation of B cell activity, proliferation, and apoptosis (Figure 1). Other recurrent targets of aSHM include genes coding for TBL1XR1, TRDM1, BTG2, and PRDM1 [25], [26].
Next-generation sequencing (NGS) analyses have shown that over 80% of nonconservative mutations are introduced at loci encoding eight proteins (ATM, TP53, PTEN, PIK3CA, JAK3, CTNNB1, PTPN11, and KRAS) [27]. Mutations in genes encoding PTEN and SMO may correlate with poorer survival and earlier relapse, and mutations in genes encoding TP53 and ATM could be involved in the molecular pathophysiology of PCNSL. Nonsynonymous somatic mutations in im1, Btg2, and MYD88 have also been detected at high frequency by whole-exome sequencing in PCNSL samples, which are in agreement with previous studies [28] (Figure 2).
Figure 2.
Molecular pathogenesis of PCNSL. Different mechanisms of genetic alteration are involved in the pathogenesis of PCNSL. These mechanisms target a variety of genes, which become dysregulated, ultimately leading to uncontrolled B cell proliferation and differentiation or impairment of B cell apoptosis. Genetic changes may also interfere with the sensitivity of tumor cells to immune responses in the CNS.
Abbreviations: aSHM, aberrant somatic hypermutation; SHM, somatic hypermutation, IGH, immunoglobulin heavy locus.
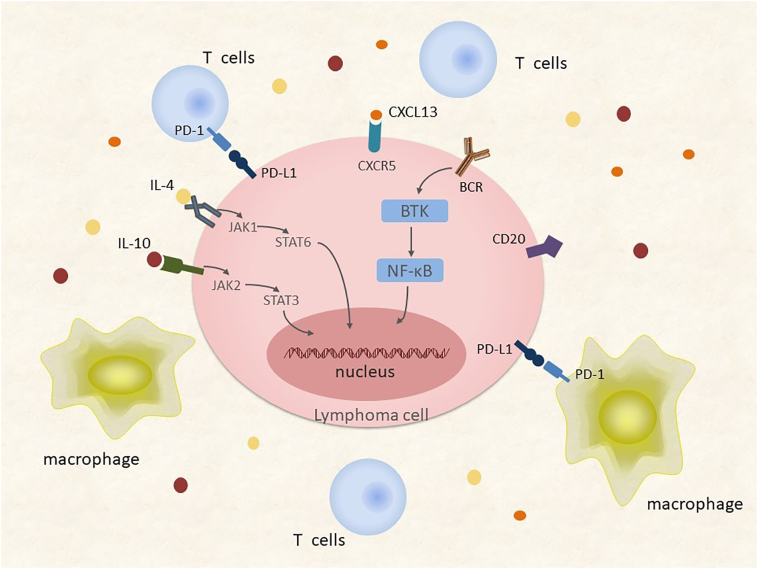
Insertions and deletions of genetic material are also very common in PCNSL. The most frequent genomic alteration in PCNSL involves the deletion of a region of chromosome 6p21 harboring the HLA locus [29]; this lesion occurs in immune-privileged sites and potentially represents a DLBCL immune escape mechanism. Chromosome 6q deletions occur frequently in PCNSL, in particular, deletions at the 6q21-23 [30] region containing: i) PTPRK, a protein tyrosine phosphatase involving in cell adhesion signaling; ii) PRDM1, a suppressor of tumor activity and regulator of B cell differentiation; and iii) A20 (TNFAIP3), which downregulates nuclear factor-kB (NF-kB) signaling. Recurrent chromosomal losses have also been detected at the 9p21 region [30], which encodes loci involved in cell cycle regulation including CDKN2A. Chromosome 12 insertions are very common, especially in the 12q region harboring genes encoding STAT6, MDM2, CDK4, and GLI1. Recurrent insertions also occur on the long arms of chromosomes 1, 7, and 18 [31] (Figure 2). Copy number alterations and translocations at chromosome 9p24, involving the genes coding for programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), appear to be frequent in PCNSL. This finding suggests that immune escape may be important in the PCNSL pathophysiology [32] (Figure 3).
Figure 3.
Tumor microenvironment and aberrant activation pathways of PCNSL. Amplification of 9p24 and resulting gene expression increase the dosage of PD-L1/2 and contribute to the immune escape of PCNSL cells. High concentrations of cytokines such as IL-4, IL-10, and CXCL13 correlate with adverse prognosis in PCNSL patients, suggesting that related pathways are aberrantly activated and result in tumorigenesis.
Abbreviations: NF-kB, nuclear factor-kB; BCR, B cell receptor; PD-L1, programmed death-ligand 1; PD-1, programmed cell death-protein 1; IL-4, interleukin-4; IL-10, interleukin-10; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
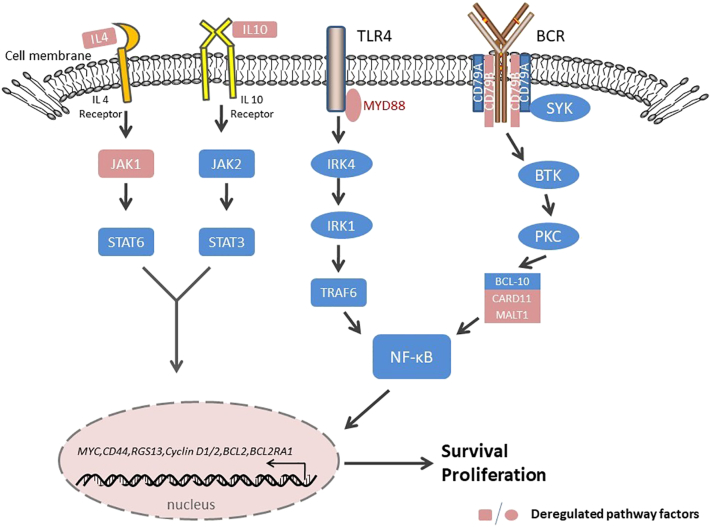
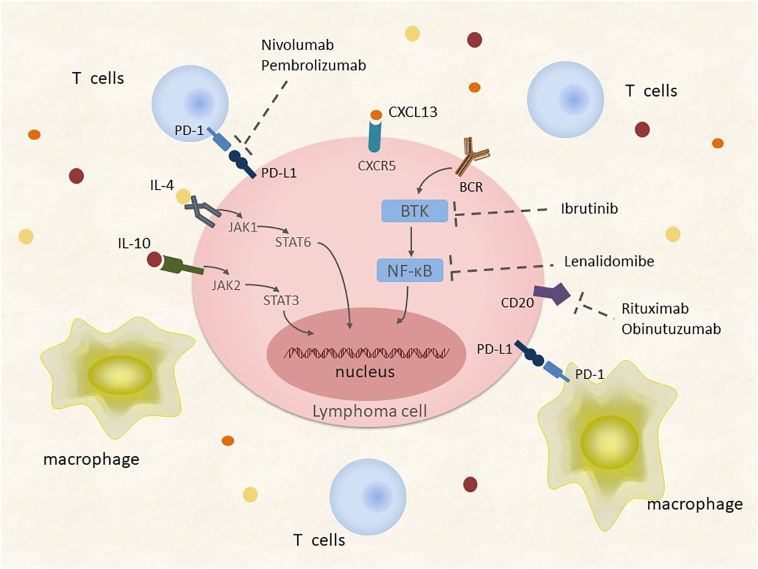
Molecular investigations have uncovered evidence suggesting that the Janus kinase (JAK)/STAT signaling pathway mediates the PCNSL biology. Transcript and protein levels of interleukin (IL)-4 and IL-10, which are mediators of the JAK/STAT intracellular signaling pathway and B cell proliferation, are upregulated in the microenvironment of tumor vessels, which are correlated with tumor response and progression [33], [34], [35] (Figure 3). Importantly, the upregulation of IL-4 and IL-10 and downstream JAK/STAT signaling correlate with aberrant activation of MYD88, which is involved in the Toll-like receptor (TLR) signaling pathway [36]. Elevated concentrations of intratumoral JAK1 transcripts have also been identified in PCNSL [23], [33], [37] (Figure 4). The BCR and TLR signaling pathways, along with their target NF-kB, are influenced by common mutations introduced by aSHM, especially in genes encoding MYD88 and CD79B. NF-kB signaling may be the core pathway involved in the regulation of PCNSL [25], [32], [38], [39], [40]. An L265P substitution in MYD88 occurs in 38%-50% of PCNSL patients, and CD79B is mutated in approximately 20% of patients [23], [29], [41]. MYD88 encodes a signaling adaptor protein that induces activation of NF-κB and the JAK/STAT3 pathway after stimulation of Toll-like receptors, interferon-β production, and IL-1/IL-18 receptors. The CD79B gene encodes a BCR subunit that is essential for BCR signaling, resulting in NF-κB activation. The BCR pathway transmits its signals to the CBM signalosome complex composed of BCL10, CARD11, and MALT1. Less frequent mutations and overexpression of MALT1 [42] and CARD11 [43] have also been demonstrated in PCNSL (Figure 4).
Figure 4.
Components of oncogenic survival signaling in PCNSL. Activation of TLR4/MYD88 and the BCR complex may contribute to prosurvival signaling via NF-κB. Enhanced production of IL-4/10 also contributes to survival signals along with the JAK/STAT pathway.
Abbreviations: BCR, B cell receptor; IL-4, interleukin-4; IL-10, interleukin-10; TLR, Toll-like receptor; NF-kB, nuclear factor-kB; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
Epigenetic Alterations in PCNSL
Epigenetic silencing by DNA methylation also contributes to PCNSL pathogenesis. DNA hypermethylation was observed in several loci including CDKN2A, DAPK, p14ARF, p16INK4a, RFC, and MGMT [44], [45]. Using array-based DNA methylation profiling, 194 differentially methylated genes have been identified comparing PCNSL to control patients; a significantly enriched CpG content was detected in these differentially methylated genes. However, no differences between the methylation patterns of PCNSL and systemic DLBCL patients was identified [46]. The presence of methylated MGMT promoter sequences was demonstrated to correlate with a better overall survival (OS) among patients who received high-dose chemotherapy. Also, elderly PCNSL and patients with recurrent PCNSL bearing a methylated MGMT promoter have been shown to have a superior response to temozolomide [47], [48], [49] (Figure 2).
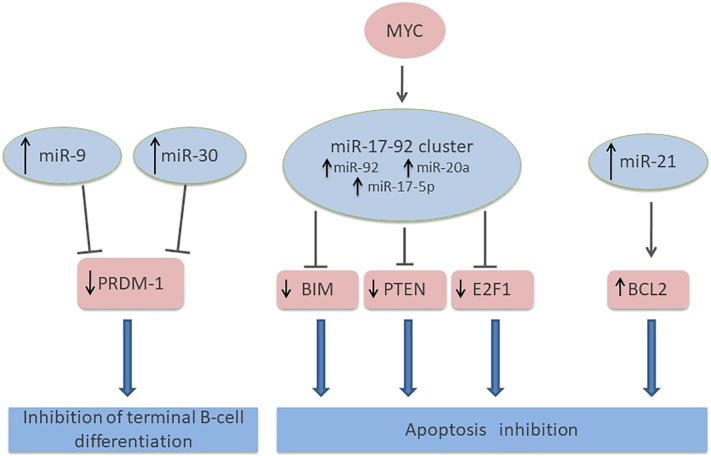
As in other malignancies, microRNA may also play an important role in the PCNSL pathogenesis. MiR-17-5p, which targets the proapoptotic gene E2F1, was shown to be significantly upregulated in nine PCNSL patients as compared to nodal DLBCL patients [50]. Upregulation of miRNAs associated with overexpression by inflammatory cytokines (miR-155), inhibition of terminal B cell differentiation (miR-30b/c, miR-9), or the MYC pathway (miR-92, miR-17-5p, miR-20a) has also been demonstrated [51]. Notably, the results by Robertus et al. were contradictory, in which they reported that miR-155 showed the lowest expression level compared with other miRNAs involved in PCNSL [50]. Analysis of cerebrospinal fluid (CSF) from PCNSL patients showed that miR-19, miR-21, and miR-92 were expressed at significantly higher levels compared to controls with inflammatory CNS disorders, suggesting the usefulness of these miRNAs as clinical biomarkers [52]. In a study of PCNSL miRNA associated with short- and long-term survival, 12 annotated miRNAs were detected to be significantly dysregulated between the short- and long-term survival groups. Among these miRNAs, miR-151a-5p and miR-151b showed the most significant differences in expression [53] (Figure 5).
Figure 5.
Relevant miRNA regulation and signaling pathways in PCNS. The major miRNAs involved in PCNSL pathogenesis: the miR-9, miR-30, miR-17-92 clusters, and miR-21. The arrows indicate the expression level of each miRNA in PCNSL. Solid lines indicate suppression of the target gene. Blue arrows lead from the gene to the final effect of the miRNA intervention.
Tumor microenvironment
The mechanisms of intracerebral tropism and dissemination of lymphoma cells are important in PCNSL pathogenesis. These mechanisms might be related to the expression of chemokines CXCL12 (SDF-1) and CXCL-13 (Figure 3). The impact of IL-10 and CXCL-13 concentration in the diagnosis of CNS lymphoma has been demonstrated [35], [54]. High CXCL-13 and IL-10 levels in CSF also correlate with adverse prognosis in PCNSL patients [55], [56].
Under normal physiological conditions, the brain is immunologically quiescent, while some PCNSL specimens show evidence of inflammatory responses, with activated macrophage and reactive T cell infiltration (Figure 3). In the perivascular space of CNS, T cells residing in the perivascular space may interact with perivascular antigen-presenting macrophages. The subsequent invasion of the CNS parenchyma requires the stimulation of antigen. If the antigen is absent, T cells may remain confined to the perivascular space [57]. Activated perivascular CD8 T cell infiltration may correlate with favorable outcomes, suggesting the potential efficacy of immunotherapy in enhancing T cell–mediated immunosurveillance [58]. Inflammatory activation may precede or accompany PCNSL. These “sentinel” inflammatory lesions may represent the first immune responses generated against PCNSL. Therefore, demyelination or neuroinflammation should be considered as radiographic features for some PCNSL cases [59].
Clinical Features
Patients of PCNSL develop neurologic symptoms over weeks including focal neurologic deficits (70%), neuropsychiatric symptoms (~43%), symptoms of increased intracranial pressure (~33%), and seizures (~14%). Clinical presentation is determined by the neuroanatomical location of the lymphoma [60]. Leptomeningeal involvement occurs in 11%-20% of PCNSL cases, usually without any clinical manifestations. Intraocular involvement occurs in 15%-25% of PCNSL patients, often with insidious onset and delayed diagnosis. Ocular symptoms are represented by floaters, blurred vision, eye pain, and photophobia due to the involvement of the retina and/or vitreous [61]. Systemic B symptoms are uncommon in PCNSL.
Diagnostic Procedure and Prognostic Factors
Imaging
Radiologic evaluation is crucial to define the location and extension of the disease. Cranial magnetic resonance imaging (MRI) using fluid-attenuated inversion recovery (FLAIR) and T1-weighted sequences before and after contrast injection are the preferred methods [62]. Advanced imaging techniques, including diffusion-weighted imaging (DWI), perfusion and permeability imaging, magnetic resonance spectroscopy (MRS), susceptibility-weighted imaging (SWI), are helpful for differential diagnosis and to increase the diagnostic accuracy [63]. Positron emission tomography–computed tomography (PET/CT) is also a useful tool but in the assessment of accompanying systemic disease [64].
Histopathology
The gold standard diagnosis is stereotactic brain biopsy or a subtotal resection if deemed to be safe. Steroid pretreatment should be avoided before biopsy [62] since it may alter the sensitivity of histopathological diagnosis. For patients with corticosteroids pretreatment, in case of inconclusive biopsy or disease remission, a second biopsy is recommended when serial MRIs indicate evident tumor progression. Flow cytologic analysis of CSF lymphoma cells in patients with leptomeningeal involvement and vitrectomy in patients with intraocular involvement might be helpful to establish the diagnosis. Bivariate elevated CXCL13 plus IL-10 is demonstrated to be highly specific for the diagnosis of CNS lymphoma [35].
Extent-of-Disease Evaluation
Staging evaluation aims to rule out systemic lymphoma and eye involvement. A comprehensive physical, neurological, and cognitive evaluation should be conducted in all newly diagnosed PCNSL patients. Laboratory evaluation includes renal and hepatic function in patients who will receive HD-MTX, HIV, hepatitis B and C, and lactate dehydrogenase (LDH) testing. Computed tomography (CT) scan of the chest, abdomen, and pelvis, as well as testicular ultrasound in elderly males, is also essential. Whole-body fluorodeoxyglucose PET may be an optimal choice. Lumber puncture for CSF cytology and bone marrow biopsy should be performed for systemic staging. Ophthalmologic evaluation with a fundoscopy and a slit lamp examination in all patients (even without ocular symptoms) is also recommended [62], [65].
Prognostic Factors
Several clinical factors may influence the survival of PCNSL patients. Age and performance status have been consistently acknowledged as the baseline prognostic variables [66]. Two scoring systems have been established to stratify PCNSL patients into several risk groups to predict prognosis [67], [68] (Table 1.). A complete response on neuroimaging after two courses of chemotherapy has also been found to be predictive for improved OS and progression-free survival (PFS) [69].
Table 1.
Prognostic Models for PCNSL
| IELSG Prognostic Score for PCNSL [67] | |||
|---|---|---|---|
| Variable | Favorable Feature (Value 0) | Unfavorable Feature (Value 1) | |
| Age (years) | <60 | >60 | |
| ECOG PS | 0-1 | >1 | |
| LDH serum level | Normal | Elevated | |
| CSF protein level | Normal | Elevated | |
| Involvement of deep regions of the CNS | No | Yes | |
| MSKCC Prognostic Model for PCNSL[68] | |||
| Variable | Good Risk | Intermediate Risk | High Risk |
| Age | <50 | ≥50 | ≥50 |
| ECOG PS | ≥70 | <70 | |
PCNSL, primary central nervous system lymphoma; ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; CSF, cerebrospinal fluid; MSKCC, Memorial Sloan-Kettering Cancer Center.
Treatment Advances
Surgery
Surgery is considered to have no role in PCNSL treatment, and its use is limited to stereotactic biopsy for histopathologic diagnosis. PCNSL has a multifocal and infiltrating nature and tends to extend beyond visible margins, contributing to the poor efficacy of surgical interventions [70]. The high radiosensitivity and chemosensitivity of PCNSL, as well as the high risk of surgical complications in PCNSL patients, have resulted in the limited application of surgical resection. However, this clinical consensus is based on small retrospective analyses, which have shown that surgical treatment alone has no survival advantage compared with supportive care [71] and postoperative radiotherapy or chemotherapy [72], [73]. The phase 3 trial of the German Primary CNS lymphoma study group-1 (G-PCNSL-SG-1) [74] which enrolled a high proportion of postoperative PCNSL patients has demonstrated that the OS and PFS were significantly improved in patients with subtotal or gross total resection compared with patients who received biopsies, which were independent of performance status and age. Since patients who had a biopsy more often had multiple deeply seated CNS lesions than resected patients, this difference may contribute to the unfavorable outcome in biopsied patients. When adjusted based on the number of lesions (site of the lesions was not analyzed in the study), the survival benefit remained significant for PFS but not for OS. Anyway, surgical resection may be crucial in patients suffering from large occupying lesions and symptoms of brain herniation [75]. In conclusion, there is insufficient clinical evidence to advise surgical resection in PCNSL patients.
Systemic Chemotherapy
High-dose methotrexate–based regimen is the first-line induction therapy for newly diagnosed PCNSL. The most effective dose of HD-MTX has not been established. A dose range of 1-8 g/m2 is sufficient to cross the blood-brain barrier (BBB), and evidence of a dose-response association is unclear [76], [77]. Doses of HD-MTX ≥ 3.5 g/m2 administered by rapid intravenous infusion (within 2-3 hours) are thought to have cytotoxic levels in the CSF [78]. A minimum of four to six injections at an interval of 14-21 days is delivered in most induction protocols, especially in the absence of subsequent consolidation treatment. Patients achieving only partial response (PR) after four or five courses of HD-MTX are recommended to receive additional courses of chemotherapy [79]. Infusion of HD-MTX requires pretreatment and posttreatment hyperhydration, urine alkalinization, leucovorin rescue, and serum methotrexate level monitoring. Significant variations in MTX metabolism exist among PCNSL patients. However, the individualized dosing schedule for HD-MTX based on pharmacokinetic analysis instead of body surface area is not well established in the current clinical practice, only in a few clinical trials [80].
Rituximab has been shown to effectively improve clinical outcomes in systemic lymphoma, which is suggestive of its potential efficacy in PCNSL (Figure 6). Single-arm trials have reported encouraging survival outcomes achieved with rituximab at doses of 375-500 mg/m2 as induction or salvage chemotherapy [79], [81], [82], [83], [84], [85], [86], [87]. Results from the recent International Extranodal Lymphoma Study Group (IELSG) 32 trial [88] have shown that patients treated with HD-MTX in combination with cytarabine and rituximab had a complete remission (CR) rate of 30% compared with 23% for those not receiving rituximab. The efficacy of single-agent rituximab has also been reported in refractory and relapsed PCNSL patients [89]. However, encouraging outcomes were not observed in a recent randomized phase III trial (HOVON 105 PCNSL/ALLG NHL24 trial) [90], and despite such, the routine use of rituximab has been incorporated in initial treatment regimens for PCNSL in most centers. Several ongoing clinical trials are evaluating the effectiveness of other CD20 antibodies such as obinutuzumab in PCNSL (NCT02498951) (Table 5).
Figure 6.
Therapeutic agents and targets in PCNSL. Novel therapeutic agents affect tumor pathways directly but also influence the tumor microenvironment, DNA transcription, translation of antiapoptotic factors, and immune modulation of tumor cells.
Abbreviations: NF-kB, nuclear factor-kB; BCR, B cell receptor.
Table 5.
Selected Ongoing Phase 2 and 3 Randomized Clinical Trials in PCNSL
| Trial | Phase | N Patients | Eligibility | Control arm | Intervention Arm | Primary Endpoint |
|---|---|---|---|---|---|---|
| Radiation Therapy Oncology Group (RTOG 1114) (NCT01399372) | Phase 2 | 91 | >18 years, KPS ≥50 | Sequential R-MPV and HD-AraC | Sequential R- MPV, lower-dose WBRT(23.4 Gy) and HD-AraC | PFS |
| ANOCEF/GOELAMS (PRECIS) (NCT00863460) |
Phase 2 | 140 | 18 to 60 years | Sequential R-MBVP and R-HD-AraC→WBRT(40 Gy) | Sequential R-MBVP and R-HD-AraC→HDC-ASCT with thiotepa, busulfan, cyclophosphamide | 2 year PFS |
| Alliance for Clinical Trials in Oncology (NCT01511562) |
Phase 2 | 113 | 18 to 75 years, KPS: ≥30 (≥50 for patients aged 60-70). | Induction therapy for five cycles as defined in the protocol→consolidation chemotherapy. | Patients undergo induction therapy for five cycles as defined in the protocol→ stem cell transplant | 2 year PFS and up to 10 years |
| International Extranodal Lymphoma Study Group (IELSG43) (NCT02531841) |
Phase 3 | 250 | 18-65 years irrespective of ECOG or 66-70 years (with ECOG PS< 2) | MATRix induction for 4 cycles→R-DeVIC consolidation for 2 cycles | MATRix induction for 4 cycles→BCNU-thiotepa-conditioned ASCT as consolidation | PFS |
| OHSU Knight Cancer Institute (NCT02498951) | Phase 2 | 120 | ≥ 18 years, CD20+ PCNSL, CR after first line treatment | Observation | Obinutuzumab | CR duration |
PCNSL, primary central nervous system lymphoma; CR, complete response; EFS, event-free survival; KPS, Karnofsky performance status; ECOG, Eastern Cooperative Oncology Group; PS, performance status; Gy, gray; HD-AraC, high-dose cytarabine; HD-MTX, high-dose methotrexate; BCNU, carmustine; HDC-ASCT, high-dose chemotherapy with autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival; R-MBVP, rituximab, HD-MTX, carmustine, etoposide, HD-AraC; R-MPV, rituximab, HD-MTX, procarbazine, vincristine, WBRT, whole brain radiotherapy; -, not mentioned; MATRix, methotrexate, cytarabine, thiotepa, and rituximab, R-DeVIC, rituximab, dexamethasone, etoposide, ifosfamide and carboplatin.
Currently, the combination of HD-MTX with other chemotherapeutic agents has been shown to improve therapeutic responses as compared to the use of HD-MTX alone (Table 2). Chemotherapeutic agents used in combination with HD-MTX should be active drugs known to cross the blood-brain barrier, such as high-dose cytarabine, ifosfamide, vincristine, procarbazine, temozolomide and thiotepa. Combination regimens currently used are R-MT, R-MPV, and MATRix.
Table 2.
Studies of Combination Chemotherapy in PCNSL
| Study | N | Median Age (Range) | Therapy | CR (%) | PFS | OS | Neurotoxicity |
|---|---|---|---|---|---|---|---|
| Ferreri et al. (IELSG20) [91] | 40 | 58(27-72) | Methotrexate | 18 | 3 years 21% | 3 years 32% | - |
| 39 | 59 (25-74) | Methotrexate-cytarabine | 46 | 3 years 38% | 3 years 46% | 3% | |
| Rubenstein et al. (CALGB 50202) [19] | 44 | 61 (12-76) | R-MT (induction)-EA (consolidation) | 66 (after R-MT) |
2 years 57% | - | none |
| Glass et al. (RTOG 0227) [93] |
53 | 57 (24-73) | R-MT (induction)-WBRT (consolidation) | 51 (after R-MT) |
2 years 63.6% (R-MT + WBRT) |
2 years 80.8% (R-MT + WBRT) |
5/45 |
| Morris et al. [79] | 52 | 60 (30-79) | R-MPV (induction)-rdWBRT (consolidation) | 60 (after R-MPV) |
2 years 77% (R-MPV + rdWBRT) |
Median 6.6 years | none |
| Omuro et al. [94] | 32 | 57 (23-67) | R-MPV (induction)-ASCT (consolidation) | 44 (after R-MPV) |
2 years 81% (R-MPV + ASCT) |
2 years 81% (R-MPV + ASCT) |
- |
| Ferreri et al.(IELSG32) [88] | 75 | 58 (50-64) | Methotrexate-cytarabine | 23 | 2 years 36% | 2 years 42% | - |
| 69 | 57 (53-63) | Methotrexate-cytarabine-rituximab | 30 | 2 years 46% | 2 years 56% | - | |
| 75 | 57 (53-62) | MATRix | 49 | 2 years 61% | 2 years 69% | - |
PCNSL, primary central nervous system lymphoma; CR, complete response; EA, etoposide and cytarabine; R-MT, methotrexate, temozolomide, and rituximab; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; MATRix, methotrexate, cytarabine, rituximab and thiotepa; rd, reduced-dose; WBRT, whole-brain radiation therapy; ASCT, autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival; -, not mentioned.
The IELSG20 trial evaluated the role of HD-MTX combined with cytarabine [91]. This study demonstrated a better CR rate and improvements in PFS but not OS in PCNSL patients receiving combination chemotherapy. Although the study consisted of a relatively small population (79 patients in two groups), it was the first randomized trial of combination chemotherapy in PCNSL. The IELSG32 trial [88] recruited a larger control group (75 patients) who received the HD-MTX and cytarabine combination regimen. However, this control group showed lower response rates than patients in the IELSG20 trial. This finding may be related to the differences in patient populations. In fact, unfavorable prognostic features were more common among patients enrolled in the IELSG32 trial. The addition of ifosfamide to HD-MTX was evaluated in the G-PCNSL-SG-1 phase 3 trial, which demonstrated an improvement of response rate, but not PFS and OS [92].
The CALGB 50202 multicenter study used induction therapy with rituximab, HD-MTX, and temozolomide (R-MT) followed by high-dose consolidation with etoposide plus cytarabine (EA) without WBRT. A CR to R-MT of 66% and PFS at 2 years of 57% were observed [19]. Similarly, the Radiation Therapy Oncology Group (RTOG) 0227 trial employed the R-MT regimen followed by WBRT consolidation. Only 66% of patients were assessable for radiographic response. The CR rate was 51% and PR rate was 34%, with a median PFS of 90 months [93].
Several single-arm phase 2 trials have evaluated the combination of methotrexate, alkylating agents, and rituximab [79], [94]. The efficacy of rituximab, methotrexate, procarbazine, and vincristine (R-MPV) followed by dose-reduced WBRT was investigated in 52 newly diagnosed PCNSL patients, for which a CR rate of 47% and a PR rate of 49% were observed [79]. The R-MPV regimen followed by consolidative ASCT was assessed in 33 patients in another phase 2 trial, in which 42% of the patients achieved CR and 48% achieved PR after the R-MPV induction chemotherapy [94]. The efficacy of MT and MPV combination was compared in an elderly population in a multicenter phase 2 trial, and the result favored the MVP regimen [95]. Future randomized trials are expected to evaluate the therapeutic difference between R-MT and R-MPV regimens.
The IELSG32 phase 2 trial assessed the combination of methotrexate, cytarabine, thiotepa, and rituximab (MATRix) in 78 PCNSL patients. At a median follow-up of 30 months, patients treated with the MATRix regimen had significantly higher CR rate (49%) as compared to a CR of 23% and 30% in those treated with methotrexate-cytarabine alone and methotrexate-cytarabine plus rituximab, respectively. This new combination has also shown significant improvement in the PFS and OS of these patients. This MATRix regimen was proved to be a new standard chemoimmunotherapy for patients aged up to 70 years with newly diagnosed PCNSL [88].
The BBB disruption by intra-arterial infusion of hypertonic mannitol followed by intra-arterial methotrexate has been identified to increase the drug concentrations in the CNS [96], [97], [98]. This procedure demonstrated a good safety profile and neurocognitive tolerance in newly diagnosed PCNSL. Active drugs for lymphoma with a poor BBB infiltration should be evaluated with this procedure in PCNSL. Notably, patients should be carefully selected for this approach since safety depends on the extent of intracranial mass effect and contraindications, to general anesthesia should be ruled out. We suggest that only teams highly trained in BBB disruption could provide this procedure as it requires cannulation of the intracranial vessels.
Intrathecal Chemotherapy
The clinical role of intrathecal chemotherapy in PCNSL is still under debate. Several single-arm studies using identical HD-MTX in combination with cytarabine regimens have reported an additional benefit of intrathecal therapy (1-year PFS of 40% and median OS of 14.3 months) and a higher risk of early relapse without intrathecal therapy [99], [100]. The addition of rituximab to HD-MTX regimens with intraventricular administration also showed encouraging treatment efficacy [82]. However, encouraging outcomes have not been replicated in other studies [101], [102]. The efficacy and neurotoxicity of such treatments remain unclear. In conclusion, there is a lack of strong evidence supporting the routine use of intrathecal chemotherapy in PCNSL.
Radiotherapy
Radiotherapy alone as a first-line treatment for PCNSL was investigated in a phase 2 trial by the RTOG 8315 [103]; it was found that patients receiving radiotherapy had a poor survival and tumor relapse occurred in areas receiving the highest doses of radiation.
The G-PCNSL-SG-1 phase 3 trial [92] further assessed the role of radiotherapy combined with chemotherapy. In this trial, 318 patients were randomly allocated to receive HD-MTX–based chemotherapy with or without WBRT. No significant benefit in median OS or PFS was observed in patients receiving WBRT. Moreover, neuropsychological evaluation showed inferior cognitive function and quality of life after combination therapy [104]. Therefore, it may be supposed that WBRT has no role in patients achieving CR after induction chemotherapy. However, contradictory results were demonstrated in other studies [105], [106]. Retrospective analyses [105] have shown improved PFS but no OS benefit in patients receiving WBRT in addition to chemotherapy. A systematic review [106] has also suggested that consolidation WBRT confers significantly prolonged survival in younger patient (<60 years).
Given the neurotoxicity of WBRT, dr-WBRT combined with immune chemotherapy has been taken under consideration. Morris et al. reported encouraging disease control in 31 patients given dr-WBRT (23.4 Gy) as consolidation therapy following a regimen of R-MPV (rituximab, methotrexate, procarbazine, and vincristine); these patients achieved CRs, with a 2-year PFS rate of 77%, a 3-year OS of 87%, and PFS of 7.7 years [79]. Comprehensive neuropsychiatric tests have also demonstrated improvement in verbal memory and baseline executive function with no evidence of a significant cognitive decline in 12 patients. However, this promising result represents a small and single-institution experience, and PCNSL relapse and late neurotoxicity effects can occur many years after treatment. Thus, longer follow-up is necessary to clarify long-term oncologic outcomes. There has also been interest in assessing whether immune-chemotherapy alone would be able to provide similar benefits; a randomized study comparing R-MPV-rd-WBRT to R-MPV alone has been started, and the results are highly anticipated (RTOG1114, NCT01399372) (Table 5).
In summary, the value of consolidation WBRT, as well as the optimal dose of radiotherapy, remains controversial, especially for patients achieving CR after induction chemotherapy.
HDC-ASCT
The efficacy of HDC-ASCT as a first-line treatment for PCNSL has been reported in several studies. In a phase 2 trial [107], 30 patients <65 years of age were treated with HDC-ASCT following WBRT, achieving a 5-year OS of 69%. Further, another trial conducted by the same group which used a similar regimen in 13 patients <70 years of age [108] demonstrated a 3-year OS of 77%. Long-term follow-up data were reported for a subset of the patients involved in these two trials [109]. Thirty-four of the 43 patients proceeded to ASCT, and the resulting 5-year OS and event-free survival (EFS) for this cohort were observed to be 82% and 79%, respectively. After a 10-year follow-up of the patients who had completed the HDC-ASCT regimen, those with or without WBRT demonstrated excellent health and cognitive function in seven of the eight living patients [110]. In a single-center phase 2 study [94], PCNSL patients who achieved CR or PR after R-MPV chemotherapy were prescribed consolidation HDC-ASCT without radiotherapy in 32 patients, and excellent disease control rates (overall response rate of 97% and 2-year PFS of 79%) were obtained. Outcomes from the largest reported cohort (105 patients) treated with HDC-ASCT were also encouraging, with 2-year and 5-year OS rates of 82% and 79%, respectively [111], and a median PFS and OS of 85 and 121 months, respectively (Table 3).
Table 3.
Selected Studies of Autologous Stem Cell Transplantation in PCNSL
| Authors/ Study | N | Median Age (Range) |
Therapy Line | Therapy (Induction) | Reaction to Induction | Conditioning Regimen | WBRT | OS | Neurotoxicity |
|---|---|---|---|---|---|---|---|---|---|
| Abrey et al. [139] | 28 | 53 (25-71) |
First | HD-MTX- ARAC |
OR 50% CR 8/28 |
BEAM | No | 2 years 25% |
None |
| Illerhaus et al.(2006) [107] | 30 | 54 (27-64) |
First | HD-MTX- ARAC/TT |
OR 24/30 CR 10/30 |
BCNU/TT | Yes | 5 years 69% |
None |
| Illerhaus et al.(2008) [108] | 13 | 54 (38-67) |
First | HD-MTX- ARAC/TT |
OR 8/13 CR 4/13 |
BCNU/TT | Yes | 3 years 77% |
None |
| ∗Kasenda et al. [109] | 43 | First | HD-MTX- ARAC/TT |
BCNU/TT | Yes† | 5 years 82% |
None | ||
| Soussain et al. [112] | 43 | 52 (23-65) |
Salvage | CYVE | OR 20/43 CR 15/43 |
TT/BU/ ARAC | No | 2 years 45% |
None |
| Kiefer et al. [110] |
23 | 54 (18-69) |
First | HD-MTX | - | BU/ TT | Yes† | 10 years 35% |
10/23 |
| Schorb et al. [111] |
105 | 54 (23-70) |
First | HD-MTX based protocols | OR 84/105 CR 43/105 |
BCNU/TT or BEAM |
Yes† | 5 years 79% | None |
| Omuro et al. [94] | 32 | 57 (23-67) |
First | R-MPV | OR 31/32 CR 21/32 |
TT/BU/ ARAC | No | 2 years 81% | None |
PCNSL, primary central nervous system lymphoma; ARAC, cytarabine; BCNU, carmustine; BEAM, carmustine, etoposide, cytarabine, and melphalan; BU, busulfan; CYVE, cytarabine and etoposide; HD, high dose; MTX, methotrexate; OS, overall survival; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; TT, thiotepa; WBRT, whole brain radiation therapy; -, not mentioned.
This study was based on two studies above [Illerhaus et al. (2006) and Illerhaus et al. (2008)].
Only for patients not achieving a complete remission.
HDC-ASCT is a common therapeutic strategy in relapsed systemic DLBCL after chemotherapy. The efficacy of HDC-ASCT has also been assessed in relapsed/refractory PCNSL [112]. The median OS was 58.6 months among patients who completed HDC-ASCT compared with 18.3 months in the overall PCNSL population.
In summary, HDC-ASCT is a promising consolidation strategy in PCNSL, especially in younger (<60-65 years) and sufficiently fit patients. Different conditioning regimens across different studies have demonstrated varying results. Among these regimens, thiotepa-based chemotherapy has conferred superior outcomes, possibly because of the thiotepa's small and lipophilic nature which allows it to efficiently penetrate the BBB. However, this intervention should be reserved for centers with a high level of experience with the application of HDC-ASCT. Ongoing multicenter randomized trials in the United States (Alliance 5110u1, NCT01511562) and Europe (ANOCEF/GOELAMS, NCT00863460) are assessing the roles of WBRT or chemotherapy versus HDC-ASCT for consolidation.
The significance of consolidation therapy in patients who achieved CR with HD-MTX–based induction chemotherapy is currently controversial in PCNSL. Generally, nearly half of these patients will relapse [113], and from this perspective, subsequent consolidation is crucial. However, there are neither conclusive data showing whether there is an overall benefit from additional consolidation nor clear data demonstrating how much additional therapy is needed for those patients who already achieved CR. Thus, in clinical practice, the benefit and risk of each of the consolidation regimens (conventional chemotherapy, radiotherapy, HDC-ASCT, or their combinations) should be balanced as per individual cases. Prognostic models should be developed through multicenter collaborations and to help in deciding the optimal therapeutic schedule.
The significantly high 5-year OS reported with the HDC-ASCT consolidation suggests that PCNSL patients could potentially be cured. However, these data were obtained from either clinical trials comprising of small cohort of patients or retrospective studies. Moreover, the improvement of survival depends on both the optimal induction and consolidation treatment. The combination of the new standard induction regimen (MATRix) with consolidation HDC-ASCT has not been investigated. In an ongoing randomized phase 3 trial conducted by the IELSG group (NCT02531841) (Table 5), newly diagnosed PCNSL patients are prescribed the MATRix regimen for induction, and those achieving partial or complete response are randomly assigned to receive high-dose chemotherapy with BCNU (or busulfan) and thiotepa followed by autologous stem cell transplantation (HDC-ASCT), or conventional chemotherapy for consolidation. These combination approaches could be promising and may improve the long-term survival of PCNSL patients.
Salvage Treatment
Although the response rates to multimodality treatments are high, nearly half of the responders will relapse, and about one-third of the patients with PCNSL are primary refractory [62], [114]. The median time to relapse is 10-18 months, and relapse occurs within 2 years after initial diagnosis in most CR patients [114]. Moreover, relapse may also be observed in some patients even after more than 5 years following treatment [115], [116]. The prognosis of primary refractory or relapsed PCNSL remains poor, with a median survival of 2 months without additional treatment [117].
Salvage treatment is dependent on age, performance status, site of relapse within the CNS, previous treatments, and duration of response (Table 4). Currently, no consensus on treatment for relapsed and refractory PCNSL has been established. Retreatment with HD-MTX–based regimens is probably the most commonly used approach by many clinicians if the time interval from initial diagnosis is considerably long. Rechallenge with HD-MTX led to a significant OR rate of 85%-91% in a retrospective analysis, with median OS of 41-62 months [118], [119]. HDC-ASCT is another typical therapeutic option. Data from the largest cohort of relapsed PCNSL patients (n = 79) reported an excellent result in patients treated with thiotepa-based HDC-ASCT, with a 5-year EFS and OS rates of 37.8% and 51.4%, respectively [120]. The efficacy of WBRT as a salvage treatment seems to be equivalent to that of many salvage chemotherapy regimens, with overall radiographic response rates of 74%-79% and OS of 10-16 months [121].
Table 4.
Recent Studies of Salvage Treatment in PCNSL
| Study | N | Median Age (Range) | Therapy | OR | PFS | OS |
|---|---|---|---|---|---|---|
| Kim et al. [130] | 8 | 56.5 (36-72) |
Procarbazine/ Lomustine/Vincristine |
50% (CR: 37.5%) |
Median 7 months |
Median 8 months |
| Korfel et al. [131] | 37 | 70 (22-83) |
Temsirolimus | 54% (CR: 13.5%) |
Median 2.1 months 1 year 5.4% |
Median 3.7 months 2 year 16.2% |
| Grommes et al. [135] | 25 | 68 (21-85) |
Ibrutinib | 68% (CR: 10/25) |
Median 4.6 months |
- |
| Tun et al. | 21 | - | Pomalidomide | 43% (CR: 4/21) |
- | - |
| Nayak et al. [136] | 5 | 64 (54-85) |
Nivolumab (PD-1 blockade) |
5/5 (CR: 4/5) |
1 year 3/5 |
- |
CR, complete response; OR, overall response; OS, overall survival; PFS, progression-free survival; -, not mentioned.
Salvage chemotherapy regimens containing rituximab and temozolomide, either in combination or as a single agent, have resulted in response rates of 14%-53% and 1-year OS of 31%-55% in both prospective and retrospective studies [89], [122], [123], [124]. The efficacy of topotecan has been evaluated in two studies of relapsed PCNSL patients, with OR rates of 33%-40% and OS of 8.4 and 35 months [125], [126]. Single-agent pemetrexed has also shown therapeutic activity in recurrent and relapsed PCNSL, with an OR rate of 55.0%-71.4% and median PFS of 5.8 months observed in prospective and retrospective studies [127], [128], [129]. In a retrospective study assessing the effects of a combination regimen of procarbazine, vincristine, and CCNU (lomustine), an OR rate of 86% was reported [130]. The utility of single-agent temsirolimus as salvage treatment has been reported in a recent phase 2 study (NCT00942747), with an OR rate of 54% and median PFS of 2.1 months in 37 patients. However, responses were usually short-lived [131]. The role of an ifosfamide-etoposide combination as a second-line salvage treatment has also been studied, together with rituximab (R-IE) or cytarabine (VIA). In patients receiving the R-IE regimen, an OR rate of 41% was reported, and 8 of 22 patients did not experience relapse during the median 24-month follow-up [132]. In patients receiving the VIA regimen, a CR rate of 37% and a 12-month OS of 41% were observed [133].
The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib has shown efficacy in PCNSL with mutations altering the BCR subunit CD79B and MYD88. In one trial, 86% of patients achieved CR with dose-adjusted temozolomide, etoposide, doxil, dexamethasone, ibrutinib, and rituximab (DA-TEDDi-R) [134]. The efficacy of single-agent ibrutinib in refractory and recurrent PCNSL is also being studied. Recent results from a trial investigating single-agent ibrutinib showed an OR rate of 68% and median PFS of 4.6 months in 25 patients [135] (Figure 6).
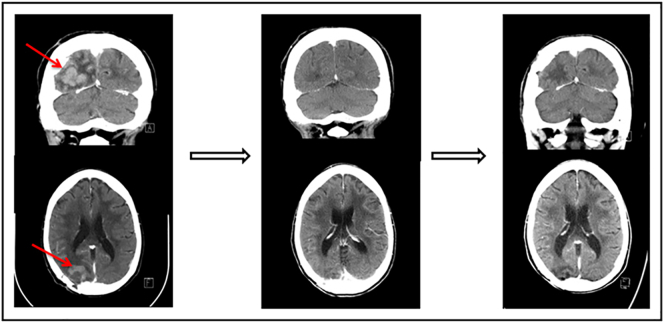
The efficacy of immunotherapy for salvage therapy of PCNSL is also being considered. Pomalidomide has shown some therapeutic activity with an OR rate of 43% in 21 patients. Several ongoing clinical trials are evaluating the effectiveness of lenalidomide as salvage treatment (NCT01956695 and NCT01542918). The result from one of these trials (NCT01542918) has demonstrated that lenalidomide is effective in relapsed CNS DLBCL and that maintenance lenalidomide significantly prolongs time to progression after salvage therapy and delays WBRT (Figure 6). The activity of PD-1 blockade has been demonstrated in other lymphomas with 9p24.1 alteration. Nayak et al. recently reported a retrospective study of salvage therapy with nivolumab. Clinical and radiographic responses to PD-1 blockade were observed in all five patients studied, and three patients remained progression-free after >13-17 months [136] (Figure 7). Several ongoing clinical trials are evaluating the efficacy of other PD-1 antibodies such as pembrolizumab (NCT02498951 and NCT02779101).
Figure 7.
Nivolumab pre- and posttreatment head CTs with contrast in a patient with primary refractory PCNSL. (Left) The contrast-enhancing lesion before treatment with nivolumab. (Middle) Complete response following 2 months of therapy with nivolumab. (Right) Continued complete response 13 months following initiation of therapy. This figure is reprinted from Nayak et al.
Histone deacetylase inhibitors (HDAC inhibitors) have recently been investigated as possible cancer therapies and have shown promising outcomes in several types of tumors. Durable clinical remission using romidepsin has been achieved in a refractory peripheral T-cell lymphoma case with CNS involvement [137]. Patients with cerebral metastasis of non–small-cell lung cancer were given chidamide combined with paclitaxel and carboplatin, and complete disappearance of the metastatic tumor after chemotherapy was observed [138]. This evidence suggests the potential efficacy of HDAC inhibitors in CNS tumors.
As there are still a few remaining long-term survivors and the toxicity of multiple courses of systemic therapy and WBRT is high, there is still ample interest in evaluating the efficacy of other agents targeting the various molecules involved in PCNSL pathogenesis.
Conclusion
Improvements in our understanding of PCNSL genomics, optimal drug dosing, sequence and timing of therapies, and patient care strategies over recent years will continue to influence the management of PCNSL. Biological studies will refine our knowledge of PCNSL pathogenesis and provide potential biomarkers for diagnosis, prognosis, or treatment with novel agents. Prospective randomized clinical trials will offer further evidence for clinicians to establish optimal doses or combinations of induction chemotherapy and consolidation strategies. Results of ongoing and future trials incorporating immunological agents currently under investigation in systemic lymphomas will continue to change the disease landscape and treatment options for patients with PCNSL.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81672686), Natural Science Foundation of Guangdong Province, China (2015A030313020), and Sister Institution Network Fund of the MD Anderson Cancer Center. We thank Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
Footnotes
Declarations of interest: none.
Contributor Information
Qingqing Cai, Email: caiqq@sysucc.org.cn.
Ken H. Young, Email: khyoung@mdanderson.org.
References
- 1.Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol. 2017;35:2410–2418. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D.M. Kluin PM, Ferry JA. Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Harris NL, editors. World Health Organization classification of tumours pathology and genetics of tumours of the haematopoietic and lymphoid tissues. IARC Press; Lyon: 2008. pp. 240–241. [Google Scholar]
- 3.O'Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin's lymphoma. Am J Hematol. 2013;88:997–1000. doi: 10.1002/ajh.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdag N, Bhorade RM, Alberico RA, Yousuf N, Patel MR. Primary lymphoma of the central nervous system: typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. 2001;176:1319–1326. doi: 10.2214/ajr.176.5.1761319. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri-Broet S, Martin A, Moreau A, Angonin R, Henin D, Gontier MF, Rousselet MC, Caulet-Maugendre S, Cuilliere P, Lefrancq T. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d'etude des Leucenies et Autres Maladies du Sang (GOELAMS) Am J Clin Pathol. 1998;110:607–612. doi: 10.1093/ajcp/110.5.607. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A, Kujas M, Raphael M, Iraqi W, Sautes-Fridman C. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 7.Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32:984–992. doi: 10.3174/ajnr.A2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlissel MS. Regulating antigen-receptor gene assembly. Nat Rev Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 10.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 11.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 12.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 13.McHeyzer-Williams MG, McLean MJ, Nossal GJ, Lalor PA. The dynamics of T cell-dependent B cell responses in vivo. Immunol Cell Biol. 1992;70(Pt 2):119–127. doi: 10.1038/icb.1992.17. [DOI] [PubMed] [Google Scholar]
- 14.Deckert M, Montesinos-Rongen M, Brunn A, Siebert R. Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice. Acta Neuropathol. 2014;127:175–188. doi: 10.1007/s00401-013-1202-x. [DOI] [PubMed] [Google Scholar]
- 15.Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M. Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood. 2004;103:1869–1875. doi: 10.1182/blood-2003-05-1465. [DOI] [PubMed] [Google Scholar]
- 16.Montesinos-Rongen M, Brunn A, Bentink S, Basso K, Lim WK, Klapper W, Schaller C, Reifenberger G, Rubenstein J, Wiestler OD. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia. 2008;22:400–405. doi: 10.1038/sj.leu.2405019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 18.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, Kaplan LD. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy O, Deangelis LM, Filippa DA, Panageas KS, Abrey LE. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2008;112:151–156. doi: 10.1002/cncr.23149. [DOI] [PubMed] [Google Scholar]
- 21.Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, Harris NL, Batchelor TT. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063–1069. [PubMed] [Google Scholar]
- 22.Cady FM, O'Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C, Porter AB, Kurtin PJ, Johnston PB, Dogan A. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol. 2008;26:4814–4819. doi: 10.1200/JCO.2008.16.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122:791–792. doi: 10.1007/s00401-011-0891-2. [DOI] [PubMed] [Google Scholar]
- 24.Kreher S, Johrens K, Strehlow F, Martus P, Borowiec K, Radke J, Heppner F, Roth P, Thiel E, Pietsch T. Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro Oncol. 2015;17:1016–1021. doi: 10.1093/neuonc/nov046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braggio E, Van Wier S, Ojha J, McPhail E, Asmann YW, Egan J, da Silva JA, Schiff D, Lopes MB, Decker PA. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21:3986–3994. doi: 10.1158/1078-0432.CCR-14-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori K, Sakata-Yanagimoto M, Okoshi Y, Goshima Y, Yanagimoto S, Nakamoto-Matsubara R, Sato T, Noguchi M, Takano S, Ishikawa E. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br J Haematol. 2017;177:492–494. doi: 10.1111/bjh.14080. [DOI] [PubMed] [Google Scholar]
- 27.Todorovic Balint M, Jelicic J, Mihaljevic B, Kostic J, Stanic B, Balint B, Pejanovic N, Lucic B, Tosic N, Marjanovic I. Gene mutation profiles in primary diffuse large B cell lymphoma of central nervous system: next generation sequencing analyses. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukumura K, Kawazu M, Kojima S, Ueno T, Sai E, Soda M, Ueda H, Yasuda T, Yamaguchi H, Lee J. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016;131:865–875. doi: 10.1007/s00401-016-1536-2. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A, Bruno A, Jouvet A, Polivka M, Adam C. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18:5203–5211. doi: 10.1158/1078-0432.CCR-12-0845. [DOI] [PubMed] [Google Scholar]
- 30.Braggio E, McPhail ER, Macon W, Lopes MB, Schiff D, Law M, Fink S, Sprau D, Giannini C, Dogan A. Primary central nervous system lymphomas: a validation study of array-based comparative genomic hybridization in formalin-fixed paraffin-embedded tumor specimens. Clin Cancer Res. 2011;17:4245–4253. doi: 10.1158/1078-0432.CCR-11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenstein JL, Treseler P, O'Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:705–717. doi: 10.1016/j.hoc.2005.05.012. vii. [DOI] [PubMed] [Google Scholar]
- 32.Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127:869–881. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, Karrim J, Damon L, Treseler P, Kunwar S, Shuman MA. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol. 2008;26:96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M, Prados M. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121:4740–4748. doi: 10.1182/blood-2013-01-476333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung CO, Kim SC, Karnan S, Karube K, Shin HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. 2011;117:1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- 38.Bruno A, Boisselier B, Labreche K, Marie Y, Polivka M, Jouvet A, Adam C, Figarella-Branger D, Miquel C, Eimer S. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5:5065–5075. doi: 10.18632/oncotarget.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura T, Tateishi K, Niwa T, Matsushita Y, Tamura K, Kinoshita M, Tanaka K, Fukushima S, Takami H, Arita H. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42:279–290. doi: 10.1111/nan.12259. [DOI] [PubMed] [Google Scholar]
- 40.Vater I, Montesinos-Rongen M, Schlesner M, Haake A, Purschke F, Sprute R, Mettenmeyer N, Nazzal I, Nagel I, Gutwein J. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29:677–685. doi: 10.1038/leu.2014.264. [DOI] [PubMed] [Google Scholar]
- 41.Montesinos-Rongen M, Schafer E, Siebert R, Deckert M. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol. 2012;124:905–906. doi: 10.1007/s00401-012-1064-7. [DOI] [PubMed] [Google Scholar]
- 42.Schwindt H, Vater I, Kreuz M, Montesinos-Rongen M, Brunn A, Richter J, Gesk S, Ammerpohl O, Wiestler OD, Hasenclever D. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia. 2009;23:1875–1884. doi: 10.1038/leu.2009.120. [DOI] [PubMed] [Google Scholar]
- 43.Montesinos-Rongen M, Schmitz R, Brunn A, Gesk S, Richter J, Hong K, Wiestler OD, Siebert R, Kuppers R, Deckert M. Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol. 2010;120:529–535. doi: 10.1007/s00401-010-0709-7. [DOI] [PubMed] [Google Scholar]
- 44.Chu LC, Eberhart CG, Grossman SA, Herman JG. Epigenetic silencing of multiple genes in primary CNS lymphoma. Int J Cancer. 2006;119:2487–2491. doi: 10.1002/ijc.22124. [DOI] [PubMed] [Google Scholar]
- 45.Mrugala MM, Rubenstein JL, Ponzoni M, Batchelor TT. Insights into the biology of primary central nervous system lymphoma. Curr Oncol Rep. 2009;11:73–80. doi: 10.1007/s11912-009-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter J, Ammerpohl O, Martin-Subero JI, Montesinos-Rongen M, Bibikova M, Wickham-Garcia E, Wiestler OD, Deckert M, Siebert R. Array-based DNA methylation profiling of primary lymphomas of the central nervous system. BMC Cancer. 2009;9:455. doi: 10.1186/1471-2407-9-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toffolatti L, Scquizzato E, Cavallin S, Canal F, Scarpa M, Stefani PM, Gherlinzoni F, Dei Tos AP. MGMT promoter methylation and correlation with protein expression in primary central nervous system lymphoma. Virchows Arch. 2014;465:579–586. doi: 10.1007/s00428-014-1622-6. [DOI] [PubMed] [Google Scholar]
- 48.Kurzwelly D, Glas M, Roth P, Weimann E, Lohner H, Waha A, Schabet M, Reifenberger G, Weller M, Herrlinger U. Primary CNS lymphoma in the elderly: temozolomide therapy and MGMT status. J Neurooncol. 2010;97:389–392. doi: 10.1007/s11060-009-0032-0. [DOI] [PubMed] [Google Scholar]
- 49.Adachi J, Mishima K, Wakiya K, Suzuki T, Fukuoka K, Yanagisawa T, Matsutani M, Sasaki A, Nishikawa R. O(6)-methylguanine-DNA methyltransferase promoter methylation in 45 primary central nervous system lymphomas: quantitative assessment of methylation and response to temozolomide treatment. J Neurooncol. 2012;107:147–153. doi: 10.1007/s11060-011-0721-3. [DOI] [PubMed] [Google Scholar]
- 50.Robertus JL, Harms G, Blokzijl T, Booman M, de Jong D, van Imhoff G, Rosati S, Schuuring E, Kluin P, van den Berg A. Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol. 2009;22:547–555. doi: 10.1038/modpathol.2009.10. [DOI] [PubMed] [Google Scholar]
- 51.Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol. 2011;13:1090–1098. doi: 10.1093/neuonc/nor107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, Maghnouj A, Zollner H, Reinacher-Schick A, Schmiegel W. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- 53.Roth P, Keller A, Hoheisel JD, Codo P, Bauer AS, Backes C, Leidinger P, Meese E, Thiel E, Korfel A. Differentially regulated miRNAs as prognostic biomarkers in the blood of primary CNS lymphoma patients. Eur J Cancer. 2015;51:382–390. doi: 10.1016/j.ejca.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Sasayama T, Nakamizo S, Nishihara M, Kawamura A, Tanaka H, Mizukawa K, Miyake S, Taniguchi M, Hosoda K, Kohmura E. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL) Neuro Oncol. 2012;14:368–380. doi: 10.1093/neuonc/nor203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk HD, Cakiroglu H, Widmann T, Thiel E. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15:5968–5973. doi: 10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- 56.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 57.Pesic M, Bartholomaus I, Kyratsous NI, Heissmeyer V, Wekerle H, Kawakami N. 2-photon imaging of phagocyte-mediated T cell activation in the CNS. J Clin Invest. 2013;123:1192–1201. doi: 10.1172/JCI67233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ponzoni M, Berger F, Chassagne-Clement C, Tinguely M, Jouvet A, Ferreri AJ, Dell'Oro S, Terreni MR, Doglioni C, Weis J. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol. 2007;138:316–323. doi: 10.1111/j.1365-2141.2007.06661.x. [DOI] [PubMed] [Google Scholar]
- 59.Lu JQ, O'Kelly C, Girgis S, Emery D, Power C, Blevins G. Neuroinflammation preceding and accompanying primary central nervous system lymphoma: case study and literature review. World Neurosurg. 2016;88 doi: 10.1016/j.wneu.2015.11.099. [692.e691–698] [DOI] [PubMed] [Google Scholar]
- 60.Josephson SA, Papanastassiou AM, Berger MS, Barbaro NM, McDermott MW, Hilton JF, Miller BL, Geschwind MD. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106:72–75. doi: 10.3171/jns.2007.106.1.72. [DOI] [PubMed] [Google Scholar]
- 61.Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR, Batchelor TT. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16:1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C, Abacioglu U. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16:e322–e332. doi: 10.1016/S1470-2045(15)00076-5. [DOI] [PubMed] [Google Scholar]
- 63.Nabavizadeh SA, Vossough A, Hajmomenian M, Assadsangabi R, Mohan S. Neuroimaging in central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30:799–821. doi: 10.1016/j.hoc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Zou Y, Tong J, Leng H, Jiang J, Pan M, Chen Z. Diagnostic value of using 18F-FDG PET and PET/CT in immunocompetent patients with primary central nervous system lymphoma: a systematic review and meta-analysis. Oncotarget. 2017;8:41518–41528. doi: 10.18632/oncotarget.17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 66.Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013;9:317–327. doi: 10.1038/nrneurol.2013.83. [DOI] [PubMed] [Google Scholar]
- 67.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 68.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 69.Pels H, Juergens A, Schirgens I, Glasmacher A, Schulz H, Engert A, Schackert G, Reichmann H, Kroschinsky F, Vogt-Schaden M. Early complete response during chemotherapy predicts favorable outcome in patients with primary CNS lymphoma. Neuro Oncol. 2010;12:720–724. doi: 10.1093/neuonc/noq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59:1557–1562. doi: 10.1212/01.wnl.0000034256.20173.ea. [DOI] [PubMed] [Google Scholar]
- 71.Henry JM, Heffner RR, Jr., Dillard SH, Earle KM, Davis RL. Primary malignant lymphomas of the central nervous system. Cancer. 1974;34:1293–1302. doi: 10.1002/1097-0142(197410)34:4<1293::aid-cncr2820340441>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 72.Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 73.Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31:100–105. doi: 10.1016/j.ejso.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Weller M, Martus P, Roth P, Thiel E, Korfel A. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14:1481–1484. doi: 10.1093/neuonc/nos159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoang-Xuan K, Taillandier L, Chinot O, Soubeyran P, Bogdhan U, Hildebrand J, Frenay M, De Beule N, Delattre JY, Baron B. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 76.Ferreri AJ, Guerra E, Regazzi M, Pasini F, Ambrosetti A, Pivnik A, Gubkin A, Calderoni A, Spina M, Brandes A. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer. 2004;90:353–358. doi: 10.1038/sj.bjc.6601472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joerger M, Huitema AD, Krahenbuhl S, Schellens JH, Cerny T, Reni M, Zucca E, Cavalli F, Ferreri AJ. Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: a pharmacokinetic-pharmacodynamic analysis from the IELSG no. 20 trial. Br J Cancer. 2010;102:673–677. doi: 10.1038/sj.bjc.6605559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joerger M, Huitema AD, Illerhaus G, Ferreri AJ. Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leuk Lymphoma. 2012;53:1867–1875. doi: 10.3109/10428194.2012.676177. [DOI] [PubMed] [Google Scholar]
- 79.Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31:3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joerger M, Ferreri AJ, Krahenbuhl S, Schellens JH, Cerny T, Zucca E, Huitema AD. Dosing algorithm to target a predefined AUC in patients with primary central nervous system lymphoma receiving high dose methotrexate. Br J Clin Pharmacol. 2012;73:240–247. doi: 10.1111/j.1365-2125.2011.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM, Abrey LE. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 82.Rubenstein JL, Li J, Chen L, Advani R, Drappatz J, Gerstner E, Batchelor T, Krouwer H, Hwang J, Auerback G. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121:745–751. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fritsch K, Kasenda B, Hader C, Nikkhah G, Prinz M, Haug V, Haug S, Ihorst G, Finke J, Illerhaus G. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol. 2011;22:2080–2085. doi: 10.1093/annonc/mdq712. [DOI] [PubMed] [Google Scholar]
- 84.Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C, Bajel A, Cher L, Grigg A, Ritchie D. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol. 2013;15:1068–1073. doi: 10.1093/neuonc/not032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, Grossman SA, Ye X. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83:235–239. doi: 10.1212/WNL.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chamberlain MC, Johnston SK. High-dose methotrexate and rituximab with deferred radiotherapy for newly diagnosed primary B-cell CNS lymphoma. Neuro Oncol. 2010;12:736–744. doi: 10.1093/neuonc/noq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wieduwilt MJ, Valles F, Issa S, Behler CM, Hwang J, McDermott M, Treseler P, O'Brien J, Shuman MA, Cha S. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18:1146–1155. doi: 10.1158/1078-0432.CCR-11-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferreri AJM, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosée PL, Schorb E. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 89.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76:929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doorduijn JK. 2018. https://ep70.eventpilot.us/web/page.php?page=Inthtml&project=ASH17&id=103102P
- 91.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 92.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T, Hundsberger T. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 93.Glass J, Won M, Schultz CJ, Brat D, Bartlett NL, Suh JH, Werner-Wasik M, Fisher BJ, Liepman MK, Augspurger M. Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG Oncology RTOG 0227. J Clin Oncol. 2016;34:1620–1625. doi: 10.1200/JCO.2015.64.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C, Pentsova E, Grommes CC. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Omuro A, Chinot O, Taillandier L, Ghesquieres H, Soussain C, Delwail V, Lamy T, Gressin R, Choquet S, Soubeyran P. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2:e251–e259. doi: 10.1016/S2352-3026(15)00074-5. [DOI] [PubMed] [Google Scholar]
- 96.Neuwelt EA, Goldman DL, Dahlborg SA, Crossen J, Ramsey F, Roman-Goldstein S, Braziel R, Dana B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 97.McAllister LD, Doolittle ND, Guastadisegni PE, Kraemer DF, Lacy CA, Crossen JR, Neuwelt EA. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery. 2000;46:51–60. discussion 60-51. [PubMed] [Google Scholar]
- 98.Angelov L, Doolittle ND, Kraemer DF, Siegal T, Barnett GH, Peereboom DM, Stevens G, McGregor J, Jahnke K, Lacy CA. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R, Haas A, Helke K, Theilig A, Lotze C. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18:665–671. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 100.Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, Zellner A, Schackert G, Reichmann H, Kroschinsky F. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 101.Sierra Del Rio M, Ricard D, Houillier C, Navarro S, Gonzalez-Aguilar A, Idbaih A, Kaloshi G, Elhallani S, Omuro A, Choquet S. Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol. 2012;106:143–146. doi: 10.1007/s11060-011-0649-7. [DOI] [PubMed] [Google Scholar]
- 102.Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, Aondio GM, Ferrarese F, Gomez H, Ponzoni M. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 103.Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, Thomson JW, Murray KJ. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 104.Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, Thaler HT. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14:101–108. doi: 10.1093/neuonc/nor186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Omuro A, Taillandier L, Chinot O, Sierra Del Rio M, Carnin C, Barrie M, Soussain C, Tanguy ML, Choquet S, Leblond V. Primary CNS lymphoma in patients younger than 60: can whole-brain radiotherapy be deferred? J Neurooncol. 2011;104:323–330. doi: 10.1007/s11060-010-0497-x. [DOI] [PubMed] [Google Scholar]
- 106.Prica A, Chan K, Cheung MC. Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system lymphoma: a decision analysis. Br J Haematol. 2012;158:600–607. doi: 10.1111/j.1365-2141.2012.09208.x. [DOI] [PubMed] [Google Scholar]
- 107.Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, Frickhofen N, Feuerhake F, Volk B, Finke J. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 108.Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 109.Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma—a long-term follow-up study. Ann Oncol. 2012;23:2670–2675. doi: 10.1093/annonc/mds059. [DOI] [PubMed] [Google Scholar]
- 110.Kiefer T, Hirt C, Spath C, Schuler F, Al-Ali HK, Wolf HH, Herbst R, Maschmeyer G, Helke K, Kessler C. Long-term follow-up of high-dose chemotherapy with autologous stem-cell transplantation and response-adapted whole-brain radiotherapy for newly diagnosed primary CNS lymphoma: results of the multicenter Ostdeutsche Studiengruppe Hamatologie und Onkologie OSHO-53 phase II study. Ann Oncol. 2011;23:1809–1812. doi: 10.1093/annonc/mdr553. [DOI] [PubMed] [Google Scholar]
- 111.Schorb E, Kasenda B, Atta J, Kaun S, Morgner A, Hess G, Elter T, von Bubnoff N, Dreyling M, Ringhoffer M. Prognosis of patients with primary central nervous system lymphoma after high-dose chemotherapy followed by autologous stem cell transplantation. Haematologica. 2013;98:765–770. doi: 10.3324/haematol.2012.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, Casasnovas O, Dupriez B, Souleau B, Taksin AL. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 113.Paydas S. Primary central nervous system lymphoma: essential points in diagnosis and management. Med Oncol. 2017;34:61. doi: 10.1007/s12032-017-0920-7. [DOI] [PubMed] [Google Scholar]
- 114.Jahnke K, Thiel E, Martus P, Herrlinger U, Weller M, Fischer L, Korfel A. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80:159–165. doi: 10.1007/s11060-006-9165-6. [DOI] [PubMed] [Google Scholar]
- 115.Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13:525–529. doi: 10.1093/neuonc/nor014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ambady P, Holdhoff M, Bonekamp D, Wong F, Grossman SA. Late relapses in primary CNS lymphoma after complete remissions with high-dose methotrexate monotherapy. CNS Oncol. 2015;4:393–398. doi: 10.2217/cns.15.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reni M, Ferreri AJ, Villa E. Second-line treatment for primary central nervous system lymphoma. Br J Cancer. 1999;79:530–534. doi: 10.1038/sj.bjc.6690083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117:161–165. doi: 10.1007/s11060-014-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plotkin SR, Betensky RA, Hochberg FH, Grossman SA, Lesser GJ, Nabors LB, Chon B, Batchelor TT. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 120.Soussain C, Choquet S, Fourme E, Delgadillo D, Bouabdallah K, Ghesquieres H, Damaj G, Dupriez B, Vargaftig J, Gonzalez A. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97:1751–1756. doi: 10.3324/haematol.2011.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nayak L, Pentsova E, Batchelor TT. Primary CNS lymphoma and neurologic complications of hematologic malignancies. Continuum (Minneap Minn) 2015;21:355–372. doi: 10.1212/01.CON.0000464175.96311.0a. [DOI] [PubMed] [Google Scholar]
- 122.Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–903. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- 123.Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M, Bordonaro R, Ilariucci F, Faedi M, Corazzelli G. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makino K, Nakamura H, Hide T, Kuratsu J. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol. 2012;106:155–160. doi: 10.1007/s11060-011-0652-z. [DOI] [PubMed] [Google Scholar]
- 125.Fischer L, Thiel E, Klasen HA, Birkmann J, Jahnke K, Martus P, Korfel A. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 126.Voloschin AD, Betensky R, Wen PY, Hochberg F, Batchelor T. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neurooncol. 2008;86:211–215. doi: 10.1007/s11060-007-9464-6. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y, Wang X, Zhao Y, Tao R, Zhu Y, Zhao R, Xu J. Curative effect of pemetrexed on the treatment of relapsed primary central nervous system lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2014;35:46–49. doi: 10.3760/cma.j.issn.0253-2727.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 128.Zhang JP, Lee EQ, Nayak L, Doherty L, Kesari S, Muzikansky A, Norden AD, Chen H, Wen PY, Drappatz J. Retrospective study of pemetrexed as salvage therapy for central nervous system lymphoma. J Neurooncol. 2013;115:71–77. doi: 10.1007/s11060-013-1196-1. [DOI] [PubMed] [Google Scholar]
- 129.Raizer JJ, Rademaker A, Evens AM, Rice L, Schwartz M, Chandler JP, Getch CC, Tellez C, Grimm SA. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118:3743–3748. doi: 10.1002/cncr.26709. [DOI] [PubMed] [Google Scholar]
- 130.Kim YJ, Choe JH, Park JH, Hong YK. Efficacy of procarbazine, lomustine, and vincristine chemotherapy for recurrent primary central nervous system lymphomas. Brain Tumor Res Treat. 2015;3:75–80. doi: 10.14791/btrt.2015.3.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korfel A, Schlegel U, Herrlinger U, Dreyling M, Schmidt C, von Baumgarten L, Pezzutto A, Grobosch T, Kebir S, Thiel E. Phase II Trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34:1757–1763. doi: 10.1200/JCO.2015.64.9897. [DOI] [PubMed] [Google Scholar]
- 132.Mappa S, Marturano E, Licata G, Frezzato M, Frungillo N, Ilariucci F, Stelitano C, Ferrari A, Soraru M, Vianello F. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2013;31:143–150. doi: 10.1002/hon.2037. [DOI] [PubMed] [Google Scholar]
- 133.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, Nomdedeu B, Montserrat E, Graus F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 134.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31 doi: 10.1016/j.ccell.2017.04.012. [833–843.e835] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi: 10.1158/2159-8290.CD-17-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MG, Chapuy B, Armand P, Rodig SJ, Shipp MA. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan KL, van der Weyden C, Khoo C, Lade S, Blombery P, Westerman D, Khot A, Melo B, Johnstone RW, Prince HM. Durable clinical remission induced by romidepsin for chemotherapy-refractory peripheral T-cell lymphoma with central nervous system involvement. Leuk Lymphoma. 2017;58:996–998. doi: 10.1080/10428194.2016.1222375. [DOI] [PubMed] [Google Scholar]
- 138.Hu X, Wang L, Lin L, Han X, Dou G, Meng Z, Shi Y. A phase I trial of an oral subtype-selective histone deacetylase inhibitor, chidamide, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer. Chin J Cancer Res. 2016;28:444–451. doi: 10.21147/j.issn.1000-9604.2016.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, Paleologos N, Correa DD, Anderson ND, Caron D. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]