Abstract
Liver fibrosis is characterised by excessive deposition of extracellular matrix that interrupts normal liver functionality. It is a pathological stage in several untreated chronic liver diseases such as the iron overload syndrome hereditary haemochromatosis, viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and diabetes. Interestingly, regardless of the aetiology, iron-loading is frequently observed in chronic liver diseases. Excess iron can feed the Fenton reaction to generate unquenchable amounts of free radicals that cause grave cellular and tissue damage and thereby contribute to fibrosis. Moreover, excess iron can induce fibrosis-promoting signals in the parenchymal and non-parenchymal cells, which accelerate disease progression and exacerbate liver pathology. Fibrosis regression is achievable following treatment, but if untreated or unsuccessful, it can progress to the irreversible cirrhotic stage leading to organ failure and hepatocellular carcinoma, where resection or transplantation remain the only curative options. Therefore, understanding the role of iron in liver fibrosis is extremely essential as it can help in formulating iron-related diagnostic, prognostic and treatment strategies. These can be implemented in isolation or in combination with the current approaches to prepone detection, and halt or decelerate fibrosis progression before it reaches the irreparable stage. Thus, this review narrates the role of iron in liver fibrosis. It examines the underlying mechanisms by which excess iron can facilitate fibrotic responses. It describes the role of iron in various clinical pathologies and lastly, highlights the significance and potential of iron-related proteins in the diagnosis and therapeutics of liver fibrosis.
Keywords: Iron, Liver pathologies, Liver fibrosis, Hepatic stellate cells, Cirrhosis
Core tip: Excess iron is observed in several liver pathologies, where it can accelerate the progression of liver fibrosis to cirrhosis and hepatocellular carcinoma, regardless of disease aetiology. This review narrates the role of excess iron in liver fibrosis. It examines the mechanisms by which iron enhances fibrogenic responses and describes various iron-related clinical pathologies. Furthermore, it evaluates the significance of iron and iron-related proteins in the diagnosis and therapeutics of liver fibrosis. The review is unique in that it includes both, cellular mechanisms and clinical aspects of liver fibrosis pertaining to iron. This makes it distinct from previous published reviews.
INTRODUCTION
Liver fibrosis is a pathological state, which is attained due to an overactive wound healing response to persistent liver injury. This subsequently disrupts liver architecture and hinders its functions leading to organ failure and death[1]. Fibrotic liver is frequently observed in several untreated chronic liver diseases (CLDs) such as haemochromatosis, viral hepatitis (hepatitis B and hepatitis C infections), alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and diabetes. Elevated iron level is a common feature of all these fibrosis-promoting conditions[2], suggesting that iron loading may pose a risk for disease progression and aggravate liver pathology.
While iron is essential for normal physiology, excess iron is toxic as it can accelerate the Fenton reaction that generates noxious reactive oxygen species (ROS) and severely damage cells and tissues. Thus, maintenance of body iron homeostasis is crucial, particularly because there is no physiological pathway for removal of excess iron from the body[3]. Under normal physiological conditions, systemic iron regulation is mediated via the liver-secreted iron hormone hepcidin[4]. Hepcidin binds to ferroportin (transmembrane iron-exporter protein) on the iron-storing macrophages and hepatocytes, degrades ferroportin and thereby hinders iron-entry into the circulation[5]. Hepcidin also binds to ferroportin on the enterocytes and decreases the expression of divalent metal transporter (DMT)-1 protein on the apical surface of enterocytes that mediates non-haem iron uptake, and thus reduces intestinal iron absorption[6]. Lack of, or resistance to hepcidin due to mutations leads to excessive iron absorption from the duodenum, unregulated iron release from the macrophages into the circulation and excessive iron deposition in various organs. These features manifest as hereditary haemochromatosis[7]. However, in non-hereditary fibrotic CLDs, the basis for iron-loading is not fully understood and whether iron-excess is the cause, a consequence, or a mediator of pathological progression remains unknown. Therefore, it is imperative to understand the role of iron in liver fibrosis and study its mechanism of action to aid in the early diagnosis and therapeutics of myriad of non-hereditary iron-loading CLDs.
HEALTHY FIBROGENESIS TO PATHOLOGICAL FIBROSIS: LOSE CONTROL
Liver fibrogenesis is a normal process of tissue repair. It is mediated via a complex network of interrelated and regulated signalling interactions between the resident parenchymal cells (hepatocytes), non-parenchymal cells [hepatic stellate cells (HSCs), liver sinusoidal endothelial cells, Kupffer cells, biliary epithelial cells, liver associated lymphocytes], and the non-resident infiltrating immune cells. The HSCs located in the space of Disse between the hepatocytes and the liver sinusoids play a pivotal role in liver development and regeneration via fibrogenesis[1]. In addition, the quiescent HSCs store 50%-80% of total vitamin A in the body[8].
Acute liver injury stimulates the non-parenchymal cells to secrete several pro-fibrogenic cytokines including the most potent activator of fibrosis, transforming growth factor beta (TGF-β)[9]. This signals the quiescent HSCs to differentiate into myofibroblasts-like cells to produce components of extracellular matrix (ECM) such as pro-collagen-1 α-1, alpha smooth muscle actin (α-SMA), fibronectin, laminin, elastin and proteoglycans along with mesenchymal proteins like vimentin and desmin, and cause tissue scaring. Upon removal of the stimulus (during recovery), excess ECM is degraded by matrix metalloproteinases (MMPs). In turn, MMP-activity is inhibited and modulated by tissue inhibitors of metalloproteinase (TIMPs) produced by the activated HSCs. Subsequently, the activated HSCs either undergo apoptosis and/or revert to their original quiescent phenotype, thereby terminating a well-regulated and reversible healing process[10].
Prolonged liver injury via chronic inflammation, infection and/or oxidative stress leads to continuous stimulation of the wound healing mechanism whereby the HSCs remain persistently activated. These activated HSCs become the main source and target of TGF-β, which greatly increases the proliferation and dedifferentiation of HSCs into ECM-producing myofibroblasts. Regulatory processes are disregarded leading to excessive deposition of ECM that can rise up to 8-fold higher than normal[11]. This, along with insufficient degradation of ECM gradually distorts the normal architecture of the liver, thereby entering the pathological fibrotic stage.
Removal of stimulus, followed by sufficient time for recovery and treatment can revert the myofibroblasts to an inactive state, reverse fibrosis and restore normal liver functionality[12-14] However, untreated fibrosis often progresses to cirrhosis, which is characterised by further deposition of collagen, nodule formations and restricted blood supply (hypoxia). This increases liver stiffness and portal hypertension, and further distorts hepatic architecture[15]. Unattended, it leads to organ failure and death. As the pathology progresses to cirrhosis, regression becomes increasingly difficult, although possible. Advanced cirrhosis may terminate in hepatocellular carcinoma, where resection or transplantation remain the only curative options.
EXCESS IRON PROMOTES LIVER FIBROSIS
The HSCs
Persistent HSC-activation is the early and key event in fibrosis, and the progression from fibrosis to cirrhosis is a crucial step in determining the fate of liver. In iron loading pathologies, HSC-activation and excessive ECM deposition are cumulative consequences of direct and indirect effect of iron on the HSCs. First, we review the direct effect of iron on HSCs. Normal liver iron concentration (LIC) is lower than 35 μmol/g of dry weight[16]. When LIC crosses a threshold of 60 µmol/g, HSC-functionality begins to derail, and when it exceeds 250 µmol/g, cirrhosis becomes inevitable[17]. Several studies have reported the fibrosis-enhancing effects of iron. For example, iron elevated collagen gene expression in HSCs and increased TGF-β expression in rats[18], induced collagen deposition in gerbil[19] and promoted cirrhosis in mice[20]. For the first time, Ramm et al[21], demonstrated a correlation between LIC and HSC-activation in humans, resulting in increased expression of α-SMA and collagen deposition in patients with haemochromatosis. Similar results were observed in rat HSCs, where iron increased HSC-cell proliferation, selectively increased collagen synthesis without affecting non-collagen proteins[22], and increased expression of α-sma and col-1 α-1[23]. Rat HSCs, when treated with ferritin, demonstrated a pro-inflammatory cascade by nuclear factor kappa-B signalling (NF-k)-B[24]. Likewise, recent studies in murine HSCs showed transferrin-induced elevations in α-sma, collagen secretion and vimentin[25].
Hepatocytes and macrophages
The HSCs do not function independently. Their role in fibrosis is informed by a network of events between other non-parenchymal cells and hepatocytes. Iron-loading in CLDs predominantly occurs in the hepatocytes and Kupffer cells, and this underpins the indirect effect of iron on HSCs whereby iron-damaged hepatocytes and macrophages release humoral factors that activate the HSCs.
Loading begins in the hepatocytes located in Rappaport zone 1 and progresses towards the hepatocytes in zones 2 and 3. Subsequently, when iron is co-loaded in the Kupffer cells, it is believed to trigger fibrosis[17]. The hepatocytes make majority of the liver mass, therefore, iron-loaded hepatocytes substantially affect fibrosis initiation and progression[26]. Wood et al[27] observed that in hereditary haemochromatosis, hepatocyte senescence positively correlated with LIC, serum ferritin and oxidative stress. In the Kupffer cells (largest non-parenchymal cell population in liver), iron deposition causes the secretion of proinflammatory cytokines and thereby promotes fibrosis. Interestingly, phagocytosis of necrotic hepatocytes promotes a pro-inflammatory/pro-fibrotic environment, whereas phagocytosis of collagen-producing cells promotes anti-inflammatory/anti-fibrotic environment. Thus, Kupffer cells play opposing roles; in the progression and regression of liver fibrosis, likely in the early and later stages of fibrosis, respectively.
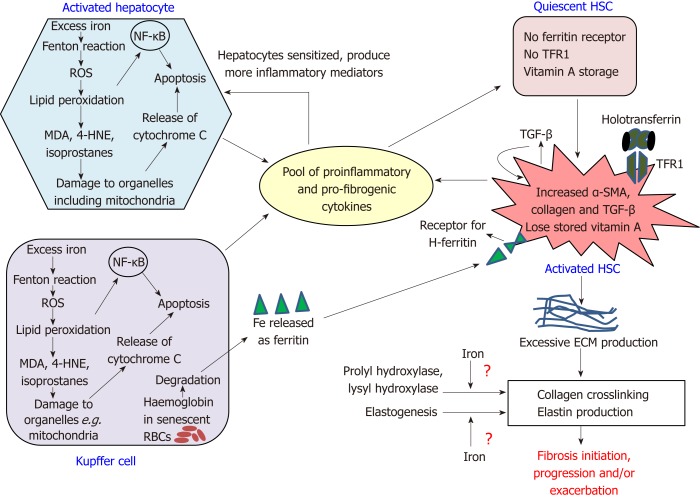
Essentially, these cells collectively produce a pool of elevated levels of proliferative, proinflammatory and profibrogenic mediators including TGF-β[1,28] (Figure 1). While TGF-β ensures a self-sustained HSC-alteration to ECM-producing myofibroblasts[17], other factors sensitize the hepatocytes to produce more proinflammatory factors causing liver inflammation, as seen in haemochromatosis patients[29]. This provokes early HSC-activation in areas of liver that are remote from regions of heavy iron-loading[21] and cause infiltration of circulating immune cells, thereby upholding an inflammatory state. Such an inflammatory liver microenvironment and overexpression of TGF-β is commonly observed in fibrotic livers[1,28].
Figure 1.
Intercellular network of events in fibrosis. The figure shows the interactions between hepatocytes, Kupffer cells and hepatic stellate cells that initiate and drive fibrosis progression. The pool of pro-fibrogenic and pro-inflammatory mediators include C-C motif chemokine ligand 5, macrophage inflammatory proteins 1 and 2, monocyte chemoattractant protein-1, tumor necrosis factor alpha, transforming growth factors alpha and beta, platelet-derived growth factor, interleukin (IL)-1β, IL-6, inducible nitric oxide synthase, and protein adducts of malondialdehyde and 4-hydroxynonenal. HNE: Hydroxynonenal; HSC: Hepatic stellate cell; MDA: Malondialdehyde; NF-κB: Nuclear factor kappa B; RBCs: Red blood cells; ROS: Reactive oxygen species; TFR1: Transferrin receptor 1; αSMA: Alpha smooth muscle actin; ECM: Extracellular matrix; TGF-β: Transforming growth factors beta.
MECHANISMS OF ACTION
The fibrotic responses are collectively mediated by multiple mechanisms involving excess-iron induced Fenton reaction, cell-signalling pathways, contribution to HSC-activation by iron-related proteins, and possibly, iron-mediated ECM remodelling (Figure 1).
Impact of Fenton chemistry on liver biology
The Fenton-Haber-Weiss reaction highlights the ability of iron to freely donate and accept electrons while altering between Fe2+ and Fe3+ states. The reactions encompass iron-catalysed generation of hydroxide ions, along with hydroperoxyl and hydroxyl radicals. Normally, limited amount of excess free-radicals are generated during cellular metabolism, which are quenched by inherent cellular antioxidant mechanisms and electron-donating moieties such as vitamins A, C and E[30]. Moreover, the tight binding of iron to cellular proteins (e.g., ferritin) and circulating proteins (e.g., transferrin) limits the amount of free iron available to feed the Fenton reaction. Hepcidin also offers indirect protection from excess-iron-induced toxic effects by inhibiting iron entry into the circulation[5,6,31]. However, under iron-loading conditions such as haemochromatosis, levels of non-transferrin bound iron (NTBI) (free iron circulating in plasma and iron loosely bound to moieties such as albumin, citrate and acetate) increase[32]. Here, the availability of water-soluble free Fe2+ iron forms the foundation for iron toxicity[33] as it accelerates the Fenton reaction to generate unquenchable levels of ROS, which can saturate the antioxidant systems. These electron-scavenging free radicals attack biomolecules and promote the formation of other free radicals such as thiyl and peroxyl radicals, thereby initiating a perpetual free radical chain reaction[34].
ROS can oxidize lipids, proteins and nucleic acids, thereby promoting fibrosis-initiation and/or fibrosis-progression. ROS-induced lipid peroxidation of cell membranes and the membranes of cellular organelles contributes to hepatocyte apoptosis and necrosis. This also enhances fibrogenic responses; for example, lipid peroxidation stimulated the expressions of col-1 α-1 and TGF-β in iron-loaded rats[18]. The by-products of lipid peroxidation such as malondialdehyde (MDA), isoprostanes and 4-hydroxynonenal (4-HNE), detected in the liver of iron-loaded rats[35], act as pro-fibrogenic stimuli. Isoprostanes, the peroxidation products of arachidonic acid enhanced HSC-proliferation, HSC-collagen-production and TGF-β release from the Kupffer cells[36], while 4-HNE upregulated the expressions of col-1 α-1 and TIMP-1 in HSCs[37].
Cross-connection between iron-related and fibrotic pathways
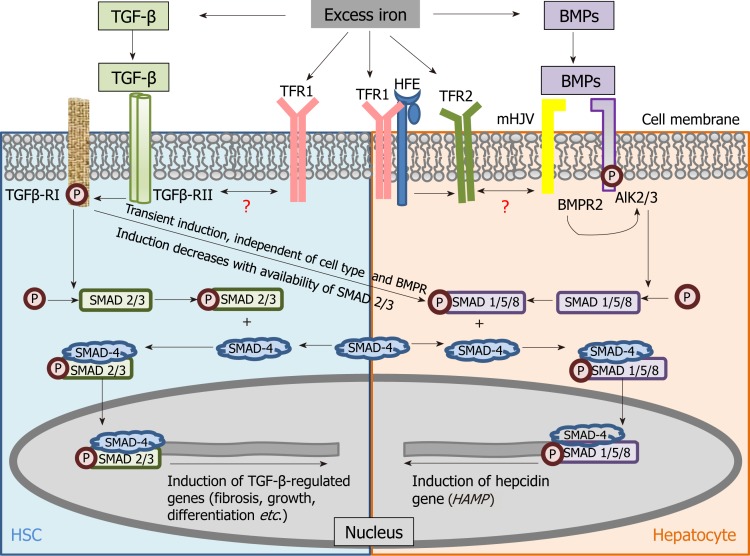
TGF-β signalling is the key fibrosis-mediating pathway and its role in regulating pro-fibrogenic gene expression and ECM deposition is well established[38]. Notably, TGF-β belongs to the TGF-β super-family of molecules, which also includes the bone morphogenetic proteins (BMPs) that induce hepcidin[39], the master regulator of systemic iron homeostasis. These molecules participate in several signalling pathways and function by binding to a complex of receptors (type II and type I serine threonine kinase receptors) and induce phosphorylation of receptor-SMADs (small mothers against decapentaplegic). The phosphorylated receptor-activated SMADs bind to SMAD-4 to form a heterodimer and this complex translocates into the nucleus to modulate the transcription of several genes that determine germ-line specification, embryonic development and cellular differentiation. While TGF-β-mediated activation of TGF-β receptor (R)II/RI -SMAD-2/3-SMAD-4 is the canonical fibrosis pathway, BMP (6)-mediated activation of ALK-2/3 receptor-SMAD-1/5/8-SMAD-4 is central to iron-dependent induction of hepcidin[40,41] (Table 1).
Table 1.
Iron-related characteristics and components of transforming growth factor-β pathway and bone morphogenetic protein signalling
| Stimulant | Pathway | Type II receptors | Type I receptors | Receptor-SMADs phosphorylated | Common SMAD | Significance/feature of pathway |
| BMPs (Belong to TGF-β superfamily) | Canonical | BMPR2, ACVR2A, ACVR2B | ALK 1,2,3,6 | SMAD-1/5/8 | SMAD-4 | Growth, differentiation, and developmental processes |
| BMP-6 induced by iron-loading (Liver specific)[133] | BMPR2, ACVR2A | ALK-2/3 | SMAD-1/5/8 | SMAD-4 | Iron-dependent hepcidin induction, modulated by HJV, HFE and TFR2[44] | |
| TGF-β | Canonical | TGF-β-RII | ALK-5 (TGF-β-RI) | SMAD-2/3 (Stimulation is stable over time)[44] | SMAD-4 | Growth, differentiation, developmental processes and fibrotic responses. |
| Non-canonical TGF-β1 induced by iron-loading[29] | TGF-β-RII | ALK-5 (TGF-β-RI) | SMAD-1/5/8 (Transient stimulation, independent of cell type)[44] | SMAD-4 | Hepcidin induction, independent of modulation by HJV, HFE and TFR2[44] and independent of BMP6-mediated activation of hepcidin | |
| Activins (Belong to TGF-β superfamily) | Canonical[134] | ACVR2A, ACVR2B | ALK-4/7 | SMAD-2/3 | SMAD-4 | Differentiation, proliferation and determine functions of several cell types |
| Non-canonical Activin B induced by inflammation[134] | ACVR2A, ACVR2B | ALK-2/3 with HJV as co-receptor | SMAD-1/5/8 | SMAD-4 | Hepcidin induction during inflammation[135] |
ACVR: Activin receptor; ALK: Activin receptor-like kinase; BMP: Bone morphogenetic protein; BMPR: Bone morphogenetic protein receptor; HFE: High iron protein; HJV: Hemojuvelin protein; SMAD: Small mothers against decapentaplegic protein; TFR: Transferrin receptor; TFR: Transferrin receptor; TGF-β: Transforming growth factor beta; TGF-β-R: Transforming growth factor beta receptor.
Since excess iron in liver induces both, TGF-β[29] and BMP-6[40,42], a connection between the TGF-β-induced fibrosis pathway and the BMP-induced hepcidin induction was envisaged and investigated. Wang et al[43] showed the significance of SMAD-4 in hepcidin induction by iron, TGF-β and BMP, while liver-specific disruption of SMAD-4 abrogated the hepcidin response. This not only demonstrated positive regulation of hepcidin by SMAD-4 and its contribution to iron homeostasis, but also identified overlap between the iron-related and fibrotic pathways based on the common role of SMAD-4 in the two pathways. Moreover, Chen et al[44] showed that TGF-β-induced hepcidin induction occurred via TGF-β-RII/RI and SMAD-1/5/8 phosphorylation, the transient non-canonical TGF-β signalling response[45,46]. This further demonstrated common mediators (TGF-β receptors) between TGF-β signalling and hepcidin induction (iron-regulation). Recently, Mehta et al[25] (2018) demonstrated iron-induced activation of TGF-β signalling in murine HSCs. Collectively, these studies reiterate the connection between the iron-related and fibrotic pathways and highlight the contribution of TGF-β towards hepcidin synthesis, and thereby, potential regulation of iron homeostasis under iron-loaded conditions (Figure 2).
Figure 2.
Schematic of mechanistic cross-connection between the transforming growth factor beta pathway and bone morphogenetic protein signaling. Shared signalling components between transforming growth factor beta (TGF-β) (fibrosis-related) and bone morphogenetic protein (iron-related) pathways have been shown in hepatic stellate cells and hepatocytes. Previous study demonstrated TGF-β-induced hepcidin expression in human macrophages, while Chen et al[44] showed that this occurred through TGF-β-RII/RI in mouse and human hepatocytes via the non-canonical pathway involving small mothers against decapentaplegic protein-1/5/8 phosphorylation. ALK: Activin receptor-like kinase; BMPR: Bone morphogenetic protein receptor; HFE: High iron protein; HSC: Hepatic stellate cell; mHJV: Membrane-bound hemojuvelin protein; P: Phosphorylation; SMAD: Small mothers against decapentaplegic protein; TFR: Transferrin receptor; TGF-β-R: Transforming growth factor receptor.
Signalling pathways such as the Wnt, Hedgehog and Notch that orchestrate the developmental processes during embryogenesis are also active during fibrogenesis to mediate survival, proliferation, differentiation and polarity of their target cells. These pathways function via a cross-talk with each other and with TGF-β pathway[47-49]. Their inhibition has shown to reverse liver fibrosis in vitro and in vivo[50-52]. The effect of iron-induced modulation of these pathways on liver fibrosis was examined in a few studies. Data showed that iron deficiency stimulated Notch signalling, but not TGF-β and Wnt signalling[53]. Recently, in response to iron-loading, a protective role of β-catenin (component of cadherin complex that stimulates Wnt signalling) against liver fibrosis was observed, where hepatocyte-specific β-catenin-knockout mice fed with an iron-overloaded diet developed higher degree of fibrosis and inflammation compared to controls[54]. Further studies are required to better understand the effect of iron on these pathways and how this alters fibrosis.
Iron-related proteins modulate fibrosis
Several iron-related protein-receptor complexes either cause HSC-activation or contribute to iron movement in pre-activated HSCs. One such association is via the ferritin receptor. Unlike quiescent HSCs, activated HSCs express a specific receptor for H-ferritin and thereby internalise ferritin that is supposedly released from Kupffer cells following degradation of haemoglobin from senescent RBCs[55,56]. Ferritin can upregulate the genes involved in HSC-activation via PKCζ and p44/p42-MAP-kinase signalling resulting in activation of NF-κB, which elevates hepatic proinflammatory mediators[24]. H-ferritin from Clonorchis sinensis, which causes liver fibrosis and cholangiocarcinoma, has shown to generate free radicals that activate NF-κB-signalling by promoting nuclear translocation of NF-κB subunits p65 and p50 and increasing the expression of proinflammatory cytokines IL-6 and IL-1β in HSCs[57]. Thus, ferritin and its receptor contribute to both proinflammatory and profibrogenic effects in HSCs. Another iron-related protein-receptor association of interest is between transferrin and transferrin receptor-1 (TFR1). Transferrin is the iron carrier protein that transports iron throughout the body and binds to TFR1 present on cell surfaces to form a complex of transferrin-TFR1. This complex is then internalised into a vesicle and iron is released from this complex into the cytoplasm[58]. Interestingly, only activated HSCs express TFR1[23]. Binding of transferrin to TFR1 contributes to HSC-activation, as demonstrated via increased expressions of α-SMA and procollagen α1(I) mRNA in rat HSCs[23] and supported by similar studies in murine HSCs[25]. Thus, transferrin is an important factor in HSC-activation, and transferrin-bound-iron uptake may be an important route for iron acquisition by activated HSCs. Hepcidin also plays a role in fibrosis modulation, as discussed in the subsequent section.
Iron and ECM remodelling
In addition to excess ECM, fibrosis is characterised by altered composition of ECM, which includes maturation of collagen via crosslinking. Crosslinked collagen is more resistant to proteolytic degradation by MMP-1[59] and is therefore the most challenging therapeutic target for fibrosis resolution. Collagen cross-linking is catalysed by the enzymes prolyl hydroxylase and lysyl hydroxylase that require vitamin C and iron as cofactors. Hence, it is possible that during iron-loading, excess iron may be channelized to promote collagen crosslinking. Along this line, a study showed increased activities of the aforementioned enzymes in rat models of carbon tetrachloride-induced liver injury[60] and in iron-deficient rats, lower levels of procollagen type I N-terminal pro-peptide and increased systemic levels of degradation products from C-terminal telopeptides of type I collagen were reported[61]. However, a previous in vitro study excluded iron as a major participant in collagen crosslinking since the iron chelator deferoxamine did not alter collagen modifications[62]. Thus, the exact effect of iron on collagen maturation is unclear and needs further investigation. Elastin is yet another important component of ECM. Iron appears to modulate elastogenesis in cultured human skin fibroblasts, where it increased the levels of insoluble elastin protein and elastin mRNA levels by 3-fold[63]. Further studies are required to ascertain the role of iron in elastogenesis in the HSCs, as it is a potential target for fibrosis therapy.
IRON LOADING AND FIBROSIS IN DIFFERENT LIVER PATHOLOGIES
In haemochromatosis, iron loading can be very severe. However, in ALD, NAFLD, NASH and viral hepatitis, low to moderate levels of excess iron are sufficient to support the pathological progression. Some iron-related parameters in these CLDs are summarised in Table 2.
Table 2.
Iron-related parameters in various fibrosis-promoting chronic liver diseases
| Normal | Hereditary hemochromatosis | ALD | NAFLD/NASH | Viral hepatitis | Diabetes | |
| Iron level/accumulation | In body: 3-5 g[7]; In RBCs: about 2.5 g; In liver: 300 mg to 1 g[7] | Can be severe; Gradual increase, can reach up to 25-30 g in liver[7] | Moderate | Mild-moderate | Mild-moderate | Mild-moderate |
| Serum ferritin | 24-300 µg/L[109]; 15-200 µg/L[101]; < 300 ng/mL in men, < 200 ng/mL in women[2] | Mostly high, but can be normal[101] | High[69,136] | High[104,105] but 1st/3rd NASH patients can be iron deficient[86] | High[116,137] | High[138], associated with pre-diabetes |
| Serum hepcidin | 0.4-23.3 nmol/L[139] | Low[64] | Low[69,71,140] | High[80,141]; Can be low in iron deficiency[86]; High in obesity, but not in NAFLD[142]; High in obesity with NAFLD[143]; Alterations can occur without iron-overload[111] | Low in hepatitis C infections[144]; High in hepatitis B infections without cirrhosis and normal in those with cirrhosis[145] | No major alteration in type 1[146]; Low in type 2 diabetes[147,148] |
| Transferrin saturation | 20%-45%[101] | > 45%[101,109] | High[69,136] | Slightly raised, but can be normal or sub-normal[7] | Mostly raised[88,149], but occasionally may not statistically differ from the norm[150] | Low[138], associated with pre-diabetes |
Approximate values and percentages for adults have been shown. These include ranges for both genders. ALD: Alcoholic liver disease; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; RBCs: Red blood cells.
Haemochromatosis
Pietrangelo (2010) defined haemochromatosis as a syndrome characterised by excessive deposition of iron in the parenchymal cells of several vital organs, and which is caused by mutation in single or multiple genes that regulate iron import into the circulation. It overarches the mutations in the genes HFE, TFR2, HJV (encoding hemojuvelin), HAMP (encoding hepcidin) and SLC40A1 (encoding ferroportin)[64]. In these cases, insufficient or lack of hepcidin production causes excessive duodenal iron absorption, while mutations in ferroportin reduce cellular iron export or cause hepcidin resistance. Whereas normal hepatic iron ranges from 300 mg to 1 g, haemochromatosis patients can show up to 25-30 g[7], clearly elevating the risk of fibrosis. A study in untreated haemochromatosis showed increased LIC in cirrhotic (378 ± 144 μmol/g) and fibrotic patients (331 ± 168 μmol/g) compared to non-fibrotic patients (237 ± 108 μmol/g)[65]. Interestingly, non-genetic factors like age, gender and alcoholism modulated fibrosis development in these patients. For example, those with fibrosis were significantly older than non-fibrotic patients and alcoholic males demonstrated hepatic fibrosis more frequently than non-alcoholic counterparts[65]. In a study of HFE gene C282Y mutation homozygotes, a higher percentage of men versus women showed increased LIC and biopsy-proven fibrosis and cirrhosis[66].
Normally, liver progenitor cells (LPCs) are activated during chronic liver injury as a backup repair mechanism to generate hepatocytes and cholangiocytes to compensate for the inability of damaged cells to replicate[67]. Activation of LPCs has also been implicated in fibrosis progression. Wood et al[26] suggested that in patients homozygous for the HFE C282Y mutation, LPCs are activated early in disease progression because excessive iron deposition in the hepatocytes hampers their ability to replicate and causes hepatocyte senescence. Reason for the iron-induced derailment of the LPC-repair-mechanism and how it contributes to predisposition to hepatocellular carcinoma in haemochromatosis patients remains unknown.
ALD
ALD exhibits liver iron loading in about half of all patients where serum iron biomarkers are raised in alcohol consumers from an early stage[68]. Alcohol-mediated suppression of hepcidin expression[69-71], upregulation of TFR1 expression in the hepatocytes by habitual alcohol drinking[72] and a concomitant increase in duodenal DMT-1 and ferroportin expression[70] collectively explain the reason for systemic and macrophage iron loading in ALD[68]. In addition, alcohol induces TGF-β expression and phosphorylates SMAD-2[73]. Such an increased availability of activated SMAD-2/3 can reduce TGF-β-induced hepcidin regulation[44]. Also, alcohol inhibits the activation of BMP receptor and SMAD-1,5, and attenuates the binding of SMAD-4 to hepcidin promoter[73]. Together, this reduces hepcidin expression and dysregulates liver iron metabolism.
Since iron and alcohol can independently cause oxidative stress, haemochromatosis patients that consume alcohol show cumulative liver damage, where alcohol-induced damage, together with elevated intestinal iron absorption leads to more deleterious damage to the liver than either condition alone[74]. Resultantly, the pathological progression to cirrhosis is accelerated together with an increased predisposition to hepatocellular carcinoma. Haemochromatosis patients whose daily alcohol intake exceeds more than 60 g are at 9-fold higher risk of cirrhosis than those who consume lesser amount of alcohol[75]. Thus, the British Liver Trust recommends that these patients should completely refrain from alcohol consumption.
NAFLD, NASH and diabetes
While genetic polymorphisms in patatin-like phospholipase domain-containing 3 or transmembrane 6 superfamily member 2 pose a risk for NAFLD[76], high calorie intake combined with a sedentary lifestyle make NAFLD a common liver disease in affluent countries. It is characterised by insulin resistance, high serum triglyceride levels, low serum high-density lipoprotein and excessive fat deposition in the liver. The latter remains undiagnosed in the early stages and quietly progresses to the high-lipid-induced inflammatory state NASH, which can advance to cirrhosis and organ failure[77].
Elevated LIC is observed in about 33% of adult NAFLD patients[2] and it is suggested to be associated with increased fibrosis[78]. LIC can catalyse the pathological progression by causing oxidative and endoplasmic reticulum stress, activation of macrophages and HSCs, reduced export of very low density lipoprotein and increased synthesis of cholesterol[79]. NAFLD patients also exhibit elevations in serum hepcidin (typically)[80], white-adipose-tissue hepcidin and DMT-1 expression. Also, upregulated TFR1 has been observed in mice on high fat diet[79]. Overall, these factors potentiate cellular iron accumulation and can accelerate fibrosis progression. A combination of excess iron and lipids (which initiates an inflammatory cascade via lipid peroxidation[81]), may exacerbate fibrosis, as the excess of both, lipids and iron can distinctly cause oxidative damage. Accordingly, iron-loaded patients with NASH exhibit higher fibrosis grade and more elevated liver function test results compared to those without NASH[81]. Thus, iron has a pathogenic role in NAFLD and is amongst the many factors that determine progression from NASH to fibrosis[79].
However, in some NAFLD/NASH cases, LIC may not be associated with increased fibrosis[82]. Along the same line, in haemochromatosis patients heterozygous for the compound C282Y/H63D HFE mutation, fatty liver and metabolic syndrome were not directly associated with hepatic fibrosis[83]. Such observations are confounded by conflicting opinions on the significance of hepatocellular and macrophagic iron in NAFLD-related fibrosis. Some studies suggest that increased macrophagic iron cause macrophage and HSC activation, and it is primarily responsible for increased risk of advanced fibrosis in NAFLD[2,84]. Others suggest that hepatocellular iron, rather than macrophagic iron, poses a higher risk of fibrosis[85].
Nonetheless, a link between increased iron stores (ferritin), insulin resistance, diabetes and NAFLD is well established, where insulin resistance is central to NAFLD pathogenesis. Iron may promote insulin resistance in the adipose tissue, which in turn may trigger lipolysis of triglycerides; a process that produces most of the free fatty acid influx into the liver[79]. Probably, increased dietary iron in the form of red meat causes predisposition to insulin resistance and type II diabetes[79]. Predictably, type II diabetes is prevalent in iron loading pathologies like HFE-related hereditary haemochromatosis and β-thalassemia major[79]. This partly explains why glucose intolerant patients demonstrate more severe fibrosis than glucose tolerant ones, indicating that glucose intolerance is a risk factor for hepatic fibrosis in C282Y/H63D patients[83]. Also, iron deficiency has been associated with obesity and NAFLD. About 33% of NAFLD patients show transferrin saturation below 20%[79,86]. Thus, the role of iron in NAFLD is multi-dimensional and can differ between NAFLD cases.
Viral hepatitis
Unlike the aforementioned conditions, increased liver iron in viral hepatitis may be a combined consequence of dysregulated liver iron homeostasis and normal defensive processes adopted during infections, which involves sequestration of iron by hepatic cells to limit access to pathogens to inhibit their proliferation. This may explain the differences in LIC during the early and late phases of infection; low in the early stage and gradual increase after 2 wk[87].
About 30%-40% of chronic hepatitis C patients demonstrate elevated levels of serum iron, transferrin saturation and ferritin[88]. In these patients, rapid progression of tissue scarring is observed in those with excess iron, compared to those without[2]. Here, LIC correlates positively with HSC number, where iron could play a crucial role in HSC-activation and fibrosis progression[89]. Although the reason for iron loading in these patients has been attributed to the reduction in hepcidin due to virus-induced oxidative stress, there have been some discrepancies in clinical studies, where no causal relationship between iron overload and hepcidin inhibition was noted[90]. Interestingly, in a case-control study, patients with chronic hepatitis C infection showed lower expression of hepcidin mRNA and more frequent hepatocyte iron deposition than hepatitis B infected patients[91]. Hepatitis B infected patients also show elevated LIC, where high iron is speculated to increase disease severity[92]. Iron can increase hepatitis B virus mRNA expression in HepG2 cells[93], which may contribute to sustenance of infection and inflammation, thereby potentiating fibrosis.
IRON-ASSISTED ASSESSMENT OF LIVER FIBROSIS
Early diagnosis of liver fibrosis is crucial for preventative, prognostic and therapeutic purposes. Liver biopsy is often considered a gold standard for definitive diagnosis, but it presents limitations such as sampling errors variability, invasive nature of the procedure and risk of life-threatening complications[94]. Recent advent of reliable serum-based markers and tools have drastically reduced the need for liver biopsy; for example magnetic resonance imaging (MRI) that accurately measures LIC, and transient elastography and MRI elastography that assess liver stiffness[94,95]. Note that in haemochromatosis, elevated LIC is the main driver of pathology, but in non-hereditary low-moderate iron-loaded CLDs, neither elevated LIC nor the altered iron-related markers are necessarily the main drivers of pathology per se, though these alterations are believed to accelerate the pathological progression to and through fibrosis. Thus, assessment of hepatic iron is not a routine part of CLD evaluation, except for haemochromatosis. However, it is useful to review the iron-related parameters that aid /may aid in prediction, diagnosis, staging and prognosis of liver fibrosis, when used in combination with the routine markers of liver dysfunctionality. Here, we specifically discuss LIC, ferritin, hepcidin and transferrin.
In haemochromatosis patients, LIC correlates significantly with the risk of fibrosis and cirrhosis[66]. Similarly, in chronic hepatitis C infections, hepatic iron accumulation increases with fibrosis stage[96,97]. In NAFLD, hepatocellular siderosis has been associated with higher risk of fibrosis than the absence of siderosis[85]. Thus, regardless of disease aetiology, hepatic iron is considered as a surrogate marker of fibrosis severity and not only a fibrogenic factor[98]. Historically, liver iron was assessed by histological staining of iron granules on samples from liver biopsy. However, presently, serum-based markers are used in combination with MRI, which not only detects and quantifies liver iron, but also helps in the staging of high degree fibrosis (F3-F4)[95]. Although LIC determination is important as it correlates with total body iron, it may not reflect iron deposition in extra-hepatic organs. Likewise, low LIC does not exclude the probability of iron loading in extra-hepatic organs[99].
An iron-related protein of immense clinical significance is ferritin. Serum ferritin is shown to be derived primarily from macrophages in mice models[100]. In C282Y homozygotes, serum ferritin > 1000 μg/L with elevated alanine transaminase (ALT) or aspartate transaminase (AST) predicted cirrhosis[101], and with transient elastography, it accurately classified the severity of fibrosis in more than 50% of patients[102]. Thus, in C282Y homozygotes, serum ferritin proved to be a better predictor of hepatic fibrosis than LIC[103]. In NAFLD, elevated serum ferritin not only acted as an independent predictor of advanced fibrosis, but it was also associated with disease severity. Essentially, serum ferritin greater than 1.5 times the upper limit of normal (> 300 ng/mL in women and > 450 ng/mL in men) was associated with hepatic iron deposition and proved to be a useful marker in identifying NAFLD patients with increased risk for NASH and fibrosis[104]. Also, increased serum ferritin was associated with advanced fibrosis, high NAFLD activity scores and increased mortality in NAFLD patients[105], while it also predicted early mortality in patients with decompensated cirrhosis[106]. Moreover, elevated serum ferritin has been strongly associated with the development of diabetes and increased risk of the metabolic syndrome. It is a marker of histologic damage and has been used in a clinical scoring system for NAFLD patients[79]. However, a few studies could not observe a clear association between serum ferritin and fibrosis. Groups such as Valenti et al[85], Chandok et al[107] and Chitturi et al[108] noted that serum ferritin could not effectively predict fibrosis stage and could not independently predict advanced fibrosis in NAFLD/NASH. Another discrepancy is related to the cell-specific accumulation of iron. While serum ferritin levels were 2-fold higher in NAFLD patients with non-parenchymal iron loading than those with parenchymal iron loading, non-parenchymal siderosis was not found to be associated with moderate-severe fibrosis[85]. Notably, elevated ferritin marks inflammation and can be observed in the absence of iron overload[109].
Like ferritin, hepcidin is also affected by both, inflammation and iron excess[110] and holds diagnostic significance in fibrosis assessment. Its levels decreased in CLD patients and were the lowest in cirrhosis patients[111]. Moreover, the hepcidin:ferritin ratio was lower in CLD patients and further decreased as fibrosis progressed[112]. Similarly, another study showed that in children with CLD, as the severity of fibrosis increased, hepcidin:ferritin ratio decreased, while serum ferritin and transferrin saturation remained high[113]. These studies present hepcidin as a valuable marker of fibrosis progression. Serum hepcidin:ferritin ratio is a potential marker for cirrhosis too[112], where, in addition to the primary insult, oxidative stress may further supress hepcidin synthesis[114].
Yet another iron-related protein of significance in fibrosis evaluation is transferrin. In hepatitis C infection, while ferritin was the only independent predictive factor of severity, transferrin saturation was found to be associated with advanced fibrosis[96]. Also, since the survival estimates were low in patients with transferrin < 180 mg/dL[115], transferrin could act as a predictor of survival in cirrhosis patients. This is in line with observations in chronic hepatitis B infection where serum transferrin reduced as fibrosis progressed from mild to advanced stage and was lower in cirrhotic patients than non-cirrhotic patients[116]. With regards to TFR1, no relationship was observed between its expression and the degree of fibrosis in hepatitis C patients. Levels were upregulated regardless of the degree of liver iron deposition, which suggest that elevated TFR1 may contribute to hepatic iron accumulation in chronic hepatitis C infection[97].
Whether the exclusive usage of such iron-related proteins would be sufficient to predict, diagnose and stage fibrosis/cirrhosis in all liver pathologies remains to be fully answered. However, based on studies hitherto, serum ferritin and hepcidin-ferritin ratio appear to be able to significantly and sufficiently contribute to fibrosis evaluation.
IRON-RELATED THERAPEUTICS FOR LIVER FIBROSIS
Presently, there are no clinically-approved treatments for fibrosis[117]. For decades, several studies have been conducted on animal models and via human clinical trials that evaluated the anti-fibrotic efficacy of herbal and pharmacological agents, but none have translated into established protocols for human use till date. While in clinical settings, fibrosis management is considered holistically, here, we specifically review the iron-related strategies.
Phlebotomy is commonly used as a treatment for haemochromatosis. It not only removes excess systemic iron, but also triggers haematopoiesis that utilises the ongoing high absorption of iron for synthesis of new RBCs, thereby controlling the excess-iron-induced pathology. Thus, it controls the excess iron-induced liver damage in haemochromatosis patients, and has shown to effectively reverse liver fibrosis[14,66], reduce the complications of portal hypertension and restore normal life expectancy in these patients[2]. Long-term phlebotomy along with subsequent maintenance of low iron levels can reverse even cirrhosis, but this data needs to be supported by randomized trials[2]. Similarly, in NASH patients, phlebotomy significantly reduced the staining for 7,8-dihydro-8-oxo-2’ deoxyguanosine, a product of oxidative damage to DNA due to iron excess[118]. It improved glucose tolerance, insulin sensitivity in type II diabetics with hyperferritinemia, and liver histology in majority of NAFLD patients in a randomised controlled trial[79]. It also improved ALT levels and glucose-induced-insulin-response in carbohydrate-intolerant non-iron-overloaded NAFLD patients[119], insulin resistance in NAFLD patients without impaired glucose tolerance[120] and ALT, AST and liver histology in NAFLD with hyperferritinemia[121]. In contrast, phlebotomy showed no effect on liver enzymes, hepatic fat or insulin resistance in a study in NAFLD patients[122]. Also, it was not fully successful in dysmetabolic iron overload syndrome, where there was a subtle increase in iron stores (ferritin) in insulin resistant patients[79]. Here, it did not improve the metabolic features, but improved insulin resistance[123].
Iron chelation has been considered for haemochromatosis patients that could not undergo phlebotomy, where the iron chelator deferoxamine (DFO) successfully removed liver iron[124] and thereby contributed to fibrosis control. However, to tackle fibrosis in non-hereditary mild-moderately iron-loaded CLDs, where phlebotomy is not the norm, the iron chelation strategy is not fully developed yet, although promising results are in sight. Studies in various cell lines and animal models revealed that iron chelation decreased the stability of procollagen mRNA in human foetal fibroblasts[125], and reduced elastin mRNA and elastin deposition in human skin fibroblasts[63]. In another study, DFO inhibited and reversed HSC-activation, induced apoptosis of activated rat HSCs, and reduced the expressions of α-sma, procollagen and TIMPs[126]. More recently, a combination of DFO with pegylated interferon-α showed better antifibrotic effects than either treatment alone and increased hepcidin expression in concanavalin A-induced liver fibrosis in rats[127]. This shows the potential for combining iron-chelation with antifibrotic agents to accelerate fibrosis recovery. Oxidative stress degrades apolipoprotein B100 (apoB100), a major component of very-low density lipo-protein (VLDL) that transports cholesterol throughout the body. This hinders the secretion of VLDL and thereby enhances steatosis. In primary rodent hepatocytes, DFO could restore ApoB100 and increase VLDL secretion[128]. In contrast, deferasirox (another iron chelator) showed some inconsistent anti-fibrotic effects in cell lines and animal models[129]. Thus, the benefits need to be ascertained via clinical trials before drawing final conclusions.
In addition to phlebotomy and iron-chelation, attempts have been made to modulate iron-related proteins to ameliorate fibrosis. Wang et al[130] (2013) demonstrated that inhibition of haem oxygenase-1 (the rate-limiting enzyme in haem catabolism) reduced hepatic iron accumulation, improved portal vein pressure and attenuated rat liver fibrosis. Hepcidin is yet another promising therapeutic agent. Previously, intraperitoneal injections of mini-hepcidin to mice models of hereditary haemochromatosis showed reduced iron loading[131]. Later, Han et al[132] conducted elaborate studies and demonstrated that hepcidin expression inversely corelated with the fibrosis severity in human and rodent models. Also, over-expression of hepcidin in rodents attenuated fibrosis, as demonstrated via reduced expressions of α-SMA, collagen type 1 and other markers. Cell based assays showed a mechanism whereby exogenous hepcidin hindered TGF-β1-induced SMAD-3 phosphorylation in HSCs and inhibited HSC-activation[132]. Thus, hepcidin therapy may be capable of modulating liver fibrosis in the future.
The significance of formulating novel iron-related therapies emerges from the iron-imposed acceleration of fibrosis progression. Even after liver transplantation in CLD patients, iron loading can increase the probability of post-operative infections and can show poor survival, as demonstrated by the HFE-related hereditary haemochromatosis patients following transplantation[2]. Thus, targeting iron metabolism for fibrosis resolution is a valuable and promising adjunctive strategy. Note that all CLDs do not necessarily trigger fibrosis, so fibrosis may not be present in all patients. Likewise, the levels of iron loading and other iron related parameters may differ between patients and between stages of the disease[111].
CONCLUSION
Excess iron is toxic. It is frequently observed in CLDs and can accelerate the progression of liver fibrosis to cirrhosis and hepatocellular carcinoma, regardless of disease aetiology. From an iron-perspective, mechanisms that promote liver fibrosis include the free-radical generating Fenton reaction, direct or indirect HSC-activation by iron or iron-related protein-receptor complexes, iron-induced intercellular interactions that provide an inflammatory milieu, cross-connection between iron and TGF-β signalling, and a putative role of iron in ECM remodelling. Iron-related proteins such as ferritin, hepcidin (hepcidin:ferritin ratio) and transferrin have successfully contributed to disease prognosis and acted as markers of fibrosis severity and progression in certain liver pathologies. Presently, there are no approved antifibrotic protocols for CLDs with mid-moderate iron-loading. Although iron-chelation and modulation of iron-related proteins show potential therapeutic benefits, these need to be tested rigorously in clinical trials before drawing definitive conclusions on their anti-fibrotic effects. The aim would be to design adjunctive strategies to halt, decelerate and/or reverse fibrosis progression, before it reaches the irreversible stages of advanced cirrhosis and hepatocellular carcinoma.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All authors declare no conflict of interest.
Peer-review started: December 3, 2018
First decision: December 28, 2018
Article in press: January 9, 2019
P- Reviewer: Murotomi K, Trinder D S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
Contributor Information
Kosha J Mehta, School of Population Health and Environmental Sciences, Faculty of Life Sciences and Medicine, King’s College London, London SE1 1UL, United Kingdom; Division of Human Sciences, School of Applied Sciences, London South Bank University, London SE1 0AA, United Kingdom. kosha.mehta@lsbu.ac.uk.
Sebastien Je Farnaud, Faculty Research Centre for Sport, Exercise and Life Sciences, Coventry University, Coventry CV1 2DS, United Kingdom.
Paul A Sharp, Department of Nutritional Sciences, School of Life Course Sciences, Faculty of Life Sciences and Medicine, King's College London, London SE1 9NH, United Kingdom.
References
- 1.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowdley KV. Iron Overload in Patients With Chronic Liver Disease. Gastroenterol Hepatol (N Y) 2016;12:695–698. [PMC free article] [PubMed] [Google Scholar]
- 3.Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol. 2007;13:4716–4724. doi: 10.3748/wjg.v13.i35.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 6.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140:1261–1271.e1. doi: 10.1053/j.gastro.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Pietrangelo A. Iron and the liver. Liver Int. 2016;36 Suppl 1:116–123. doi: 10.1111/liv.13020. [DOI] [PubMed] [Google Scholar]
- 8.Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010;34:1247–1272. doi: 10.1042/CBI20100321. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759–768, viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 13.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falize L, Guillygomarc'h A, Perrin M, Lainé F, Guyader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44:472–477. doi: 10.1002/hep.21260. [DOI] [PubMed] [Google Scholar]
- 15.Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–291. doi: 10.1016/j.cld.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrangelo A. Iron in NASH, chronic liver diseases and HCC: how much iron is too much? J Hepatol. 2009;50:249–251. doi: 10.1016/j.jhep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Philippe MA, Ruddell RG, Ramm GA. Role of iron in hepatic fibrosis: one piece in the puzzle. World J Gastroenterol. 2007;13:4746–4754. doi: 10.3748/wjg.v13.i35.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houglum K, Bedossa P, Chojkier M. TGF-beta and collagen-alpha 1 (I) gene expression are increased in hepatic acinar zone 1 of rats with iron overload. Am J Physiol. 1994;267:G908–G913. doi: 10.1152/ajpgi.1994.267.5.G908. [DOI] [PubMed] [Google Scholar]
- 19.Carthew P, Edwards RE, Smith AG, Dorman B, Francis JE. Rapid induction of hepatic fibrosis in the gerbil after the parenteral administration of iron-dextran complex. Hepatology. 1991;13:534–539. [PubMed] [Google Scholar]
- 20.Arezzini B, Lunghi B, Lungarella G, Gardi C. Iron overload enhances the development of experimental liver cirrhosis in mice. Int J Biochem Cell Biol. 2003;35:486–495. doi: 10.1016/s1357-2725(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 21.Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW. Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997;26:584–592. doi: 10.1016/s0168-8278(97)80424-2. [DOI] [PubMed] [Google Scholar]
- 22.Gardi C, Arezzini B, Fortino V, Comporti M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem Pharmacol. 2002;64:1139–1145. doi: 10.1016/s0006-2952(02)01257-1. [DOI] [PubMed] [Google Scholar]
- 23.Bridle KR, Crawford DH, Ramm GA. Identification and characterization of the hepatic stellate cell transferrin receptor. Am J Pathol. 2003;162:1661–1667. doi: 10.1016/S0002-9440(10)64300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, Santambrogio P, Arosio P, Ramm GA. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49:887–900. doi: 10.1002/hep.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta KJ, Coombes JD, Briones-Orta M, Manka PP, Williams R, Patel VB, Syn WK. Iron Enhances Hepatic Fibrogenesis and Activates Transforming Growth Factor-β Signaling in Murine Hepatic Stellate Cells. Am J Med Sci. 2018;355:183–190. doi: 10.1016/j.amjms.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Wood MJ, Gadd VL, Powell LW, Ramm GA, Clouston AD. Ductular reaction in hereditary hemochromatosis: the link between hepatocyte senescence and fibrosis progression. Hepatology. 2014;59:848–857. doi: 10.1002/hep.26706. [DOI] [PubMed] [Google Scholar]
- 27.Li H, You H, Fan X, Jia J. Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets. BMJ Open Gastroenterol. 2016;3:e000079. doi: 10.1136/bmjgast-2016-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houglum K, Ramm GA, Crawford DH, Witztum JL, Powell LW, Chojkier M. Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology. 1997;26:605–610. doi: 10.1002/hep.510260311. [DOI] [PubMed] [Google Scholar]
- 30.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 31.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95:501–504. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Valk B, Addicks MA, Gosriwatana I, Lu S, Hider RC, Marx JJ. Non-transferrin-bound iron is present in serum of hereditary haemochromatosis heterozygotes. Eur J Clin Invest. 2000;30:248–251. doi: 10.1046/j.1365-2362.2000.00628.x. [DOI] [PubMed] [Google Scholar]
- 33.Pietrangelo A. Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis. 1996;16:13–30. doi: 10.1055/s-2007-1007215. [DOI] [PubMed] [Google Scholar]
- 34.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 35.Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest. 1990;86:1991–1998. doi: 10.1172/JCI114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comporti M, Arezzini B, Signorini C, Vecchio D, Gardi C. Oxidative stress, isoprostanes and hepatic fibrosis. Histol Histopathol. 2009;24:893–900. doi: 10.14670/HH-24.893. [DOI] [PubMed] [Google Scholar]
- 37.Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 38.Ranganathan P, Agrawal A, Bhushan R, Chavalmane AK, Kalathur RK, Takahashi T, Kondaiah P. Expression profiling of genes regulated by TGF-beta: differential regulation in normal and tumour cells. BMC Genomics. 2007;8:98. doi: 10.1186/1471-2164-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrow NL, Fleming RE. Bone morphogenetic proteins as regulators of iron metabolism. Annu Rev Nutr. 2014;34:77–94. doi: 10.1146/annurev-nutr-071813-105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 41.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 42.Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54:273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Feng T, Vujić Spasić M, Altamura S, Breitkopf-Heinlein K, Altenöder J, Weiss TS, Dooley S, Muckenthaler MU. Transforming Growth Factor β1 (TGF-β1) Activates Hepcidin mRNA Expression in Hepatocytes. J Biol Chem. 2016;291:13160–13174. doi: 10.1074/jbc.M115.691543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–6902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrighton KH, Lin X, Yu PB, Feng XH. Transforming Growth Factor {beta} Can Stimulate Smad1 Phosphorylation Independently of Bone Morphogenic Protein Receptors. J Biol Chem. 2009;284:9755–9763. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Shen RW, Han B, Li Z, Xiong L, Zhang FY, Cong BB, Zhang B. Notch signaling mediated by TGF-β/Smad pathway in concanavalin A-induced liver fibrosis in rats. World J Gastroenterol. 2017;23:2330–2336. doi: 10.3748/wjg.v23.i13.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Krüger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39–G49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 51.Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn WK, Jung Y, Witek RP, Choi S, Michelotti GA, Rangwala F, Merkle E, Lascola C, Diehl AM. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, Chen G, Liu C, Zern MA, Mu Y, Liu P. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96:350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- 53.Jian J, Yang Q, Shao Y, Axelrod D, Smith J, Singh B, Krauter S, Chiriboga L, Yang Z, Li J, Huang X. A link between premenopausal iron deficiency and breast cancer malignancy. BMC Cancer. 2013;13:307. doi: 10.1186/1471-2407-13-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preziosi ME, Singh S, Valore EV, Jung G, Popovic B, Poddar M, Nagarajan S, Ganz T, Monga SP. Mice lacking liver-specific β-catenin develop steatohepatitis and fibrosis after iron overload. J Hepatol. 2017;67:360–369. doi: 10.1016/j.jhep.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramm GA, Britton RS, O'Neill R, Bacon BR. Identification and characterization of a receptor for tissue ferritin on activated rat lipocytes. J Clin Invest. 1994;94:9–15. doi: 10.1172/JCI117353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibille JC, Kondo H, Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology. 1988;8:296–301. doi: 10.1002/hep.1840080218. [DOI] [PubMed] [Google Scholar]
- 57.Mao Q, Xie Z, Wang X, Chen W, Ren M, Shang M, Lei H, Tian Y, Li S, Liang P, Chen T, Liang C, Xu J, Li X, Huang Y, Yu X. Clonorchis sinensis ferritin heavy chain triggers free radicals and mediates inflammation signaling in human hepatic stellate cells. Parasitol Res. 2015;114:659–670. doi: 10.1007/s00436-014-4230-0. [DOI] [PubMed] [Google Scholar]
- 58.Iacopetta BJ, Morgan EH. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983;258:9108–9115. [PubMed] [Google Scholar]
- 59.van der Slot-Verhoeven AJ, van Dura EA, Attema J, Blauw B, Degroot J, Huizinga TW, Zuurmond AM, Bank RA. The type of collagen cross-link determines the reversibility of experimental skin fibrosis. Biochim Biophys Acta. 2005;1740:60–67. doi: 10.1016/j.bbadis.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Risteli J, Kivirikko KI. Activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in the liver of rats with hepatic injury. Biochem J. 1974;144:115–122. doi: 10.1042/bj1440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Díaz-Castro J, López-Frías MR, Campos MS, López-Frías M, Alférez MJ, Nestares T, Ojeda ML, López-Aliaga I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur J Nutr. 2012;51:241–247. doi: 10.1007/s00394-011-0212-5. [DOI] [PubMed] [Google Scholar]
- 62.Elgawish A, Glomb M, Friedlander M, Monnier VM. Involvement of hydrogen peroxide in collagen cross-linking by high glucose in vitro and in vivo. J Biol Chem. 1996;271:12964–12971. doi: 10.1074/jbc.271.22.12964. [DOI] [PubMed] [Google Scholar]
- 63.Bunda S, Kaviani N, Hinek A. Fluctuations of intracellular iron modulate elastin production. J Biol Chem. 2005;280:2341–2351. doi: 10.1074/jbc.M409897200. [DOI] [PubMed] [Google Scholar]
- 64.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408, 408.e1-408.e2. doi: 10.1053/j.gastro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Loréal O, Deugnier Y, Moirand R, Lauvin L, Guyader D, Jouanolle H, Turlin B, Lescoat G, Brissot P. Liver fibrosis in genetic hemochromatosis. Respective roles of iron and non-iron-related factors in 127 homozygous patients. J Hepatol. 1992;16:122–127. doi: 10.1016/s0168-8278(05)80104-7. [DOI] [PubMed] [Google Scholar]
- 66.Powell LW, Dixon JL, Ramm GA, Purdie DM, Lincoln DJ, Anderson GJ, Subramaniam VN, Hewett DG, Searle JW, Fletcher LM, Crawford DH, Rodgers H, Allen KJ, Cavanaugh JA, Bassett ML. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med. 2006;166:294–301. doi: 10.1001/archinte.166.3.294. [DOI] [PubMed] [Google Scholar]
- 67.Shang H, Wang Z, Song Y. Liver progenitor cells-mediated liver regeneration in liver cirrhosis. Hepatol Int. 2016;10:440–447. doi: 10.1007/s12072-015-9693-2. [DOI] [PubMed] [Google Scholar]
- 68.Milic S, Mikolasevic I, Orlic L, Devcic E, Starcevic-Cizmarevic N, Stimac D, Kapovic M, Ristic S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med Sci Monit. 2016;22:2144–2151. doi: 10.12659/MSM.896494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa-Matos L, Batista P, Monteiro N, Simões M, Egas C, Pereira J, Pinho H, Santos N, Ribeiro J, Cipriano MA, Henriques P, Girão F, Rodrigues A, Carvalho A. Liver hepcidin mRNA expression is inappropriately low in alcoholic patients compared with healthy controls. Eur J Gastroenterol Hepatol. 2012;24:1158–1165. doi: 10.1097/MEG.0b013e328355cfd0. [DOI] [PubMed] [Google Scholar]
- 70.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 71.Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, Suzuki Y, Fujimoto Y, Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007;31:S2–S8. doi: 10.1111/j.1530-0277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto Y, Kohgo Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26:26S–31S. doi: 10.1097/01.ALC.0000026830.27338.23. [DOI] [PubMed] [Google Scholar]
- 73.Gerjevic LN, Liu N, Lu S, Harrison-Findik DD. Alcohol Activates TGF-Beta but Inhibits BMP Receptor-Mediated Smad Signaling and Smad4 Binding to Hepcidin Promoter in the Liver. Int J Hepatol. 2012;2012:459278. doi: 10.1155/2012/459278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fletcher LM, Dixon JL, Purdie DM, Powell LW, Crawford DH. Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology. 2002;122:281–289. doi: 10.1053/gast.2002.30992. [DOI] [PubMed] [Google Scholar]
- 75.Fletcher LM, Powell LW. Hemochromatosis and alcoholic liver disease. Alcohol. 2003;30:131–136. doi: 10.1016/s0741-8329(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 76.Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: a HuGE review and meta-analysis. Sci Rep. 2015;5:9284. doi: 10.1038/srep09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 78.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 79.Britton LJ, Subramaniam VN, Crawford DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8112–8122. doi: 10.3748/wjg.v22.i36.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Senates E, Yilmaz Y, Colak Y, Ozturk O, Altunoz ME, Kurt R, Ozkara S, Aksaray S, Tuncer I, Ovunc AO. Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9:287–290. doi: 10.1089/met.2010.0121. [DOI] [PubMed] [Google Scholar]
- 81.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–1163. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 82.Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 83.Adams LA, Angulo P, Abraham SC, Torgerson H, Brandhagen D. The effect of the metabolic syndrome, hepatic steatosis and steatohepatitis on liver fibrosis in hereditary hemochromatosis. Liver Int. 2006;26:298–304. doi: 10.1111/j.1478-3231.2005.01238.x. [DOI] [PubMed] [Google Scholar]
- 84.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV Nonalcoholic Steatohepatitis Clinical Research Network. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, Fargion S. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Siddique A, Nelson JE, Aouizerat B, Yeh MM, Kowdley KV NASH Clinical Research Network. Iron deficiency in patients with nonalcoholic Fatty liver disease is associated with obesity, female gender, and low serum hepcidin. Clin Gastroenterol Hepatol. 2014;12:1170–1178. doi: 10.1016/j.cgh.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hengeveld P, Zuyderhoudt FM, Jöbsis AC, van Gool J. Some aspects of iron metabolism during acute viral hepatitis. Hepatogastroenterology. 1982;29:138–141. [PubMed] [Google Scholar]
- 88.Georgopoulou U, Dimitriadis A, Foka P, Karamichali E, Mamalaki A. Hepcidin and the iron enigma in HCV infection. Virulence. 2014;5:465–476. doi: 10.4161/viru.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rigamonti C, Andorno S, Maduli E, Morelli S, Pittau S, Nicosia G, Boldorini R, Sartori M. Iron, hepatic stellate cells and fibrosis in chronic hepatitis C. Eur J Clin Invest. 2002;32 Suppl 1:28–35. doi: 10.1046/j.1365-2362.2002.0320s1028.x. [DOI] [PubMed] [Google Scholar]
- 90.Sikorska K. The iron homeostasis network and hepatitis C virus - a new challenge in the era of directly acting antivirals. Virulence. 2016;7:620–622. doi: 10.1080/21505594.2016.1191739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sikorska K, Romanowski T, Stalke P, Izycka Swieszewska E, Bielawski KP. Association of hepcidin mRNA expression with hepatocyte iron accumulation and effects of antiviral therapy in chronic hepatitis C infection. Hepat Mon. 2014;14:e21184. doi: 10.5812/hepatmon.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat. 2012;19:e170–e176. doi: 10.1111/j.1365-2893.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 93.Park SO, Kumar M, Gupta S. TGF-β and iron differently alter HBV replication in human hepatocytes through TGF-β/BMP signaling and cellular microRNA expression. PLoS One. 2012;7:e39276. doi: 10.1371/journal.pone.0039276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567–11583. doi: 10.3748/wjg.v21.i41.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castiella A, Zapata E, Alústiza JM. Non-invasive methods for liver fibrosis prediction in hemochromatosis: One step beyond. World J Hepatol. 2010;2:251–255. doi: 10.4254/wjh.v2.i7.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99:286–291. doi: 10.1111/j.1572-0241.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 97.Saito H, Fujimoto Y, Ohtake T, Suzuki Y, Sakurai S, Hosoki Y, Ikuta K, Torimoto Y, Kohgo Y. Up-regulation of transferrin receptor 1 in chronic hepatitis C: Implication in excess hepatic iron accumulation. Hepatol Res. 2005;31:203–210. doi: 10.1016/j.hepres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Guyader D, Thirouard AS, Erdtmann L, Rakba N, Jacquelinet S, Danielou H, Perrin M, Jouanolle AM, Brissot P, Deugnier Y. Liver iron is a surrogate marker of severe fibrosis in chronic hepatitis C. J Hepatol. 2007;46:587–595. doi: 10.1016/j.jhep.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 99.Sirlin CB, Reeder SB. Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am. 2010;18:359–381, ix. doi: 10.1016/j.mric.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 101.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328–343. doi: 10.1002/hep.24330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Legros L, Bardou-Jacquet E, Latournerie M, Guillygomarc'h A, Turlin B, Le Lan C, Désille Y, Lainé F, Moirand R, Brissot P, Deugnier Y, Guyader D. Non-invasive assessment of liver fibrosis in C282Y homozygous HFE hemochromatosis. Liver Int. 2015;35:1731–1738. doi: 10.1111/liv.12762. [DOI] [PubMed] [Google Scholar]
- 103.Wood MJ, Crawford DHG, Wockner LF, Powell LW, Ramm GA. Serum ferritin concentration predicts hepatic fibrosis better than hepatic iron concentration in human HFE-Haemochromatosis. Liver Int. 2017;37:1382–1388. doi: 10.1111/liv.13395. [DOI] [PubMed] [Google Scholar]
- 104.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hagström H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, Stål P. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int. 2016;36:1688–1695. doi: 10.1111/liv.13144. [DOI] [PubMed] [Google Scholar]
- 106.Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, Rastogi A, Bihari C, Vashisht C, Sarin SK. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol. 2014;61:43–50. doi: 10.1016/j.jhep.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 107.Chandok N, Minuk G, Wengiel M, Uhanova J. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis. 2012;21:53–58. [PubMed] [Google Scholar]
- 108.Chitturi S, Weltman M, Farrell GC, McDonald D, Kench J, Liddle C, Samarasinghe D, Lin R, Abeygunasekera S, George J. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology. 2002;36:142–149. doi: 10.1053/jhep.2002.33892. [DOI] [PubMed] [Google Scholar]
- 109.Koperdanova M, Cullis JO. Interpreting raised serum ferritin levels. BMJ. 2015;351:h3692. doi: 10.1136/bmj.h3692. [DOI] [PubMed] [Google Scholar]
- 110.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vela D. Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol Med. 2018;24:5. doi: 10.1186/s10020-018-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan TC, Crawford DH, Franklin ME, Jaskowski LA, Macdonald GA, Jonsson JR, Watson MJ, Taylor PJ, Fletcher LM. The serum hepcidin:ferritin ratio is a potential biomarker for cirrhosis. Liver Int. 2012;32:1391–1399. doi: 10.1111/j.1478-3231.2012.02828.x. [DOI] [PubMed] [Google Scholar]
- 113.Cakir M, Erduran E, Turkmen ES, Aliyazicioglu Y, Reis GP, Cobanoglu U, Demir S. Hepcidin levels in children with chronic liver disease. Saudi J Gastroenterol. 2015;21:300–305. doi: 10.4103/1319-3767.166205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trinder D, Ayonrinde OT, Olynyk JK. HCV, iron, and oxidative stress: the new choreography of hepcidin. Gastroenterology. 2008;134:348–351. doi: 10.1053/j.gastro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 115.Viveiros A, Finkenstedt A, Schaefer B, Mandorfer M, Scheiner B, Lehner K, Tobiasch M, Reiberger T, Tilg H, Edlinger M, Zoller H. Transferrin as a predictor of survival in cirrhosis. Liver Transpl. 2018;24:343–351. doi: 10.1002/lt.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mao W, Hu Y, Lou Y, Chen Y, Zhang J. Abnormal serum iron markers in chronic hepatitis B virus infection may be because of liver injury. Eur J Gastroenterol Hepatol. 2015;27:130–136. doi: 10.1097/MEG.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prakash J, Pinzani M. Fibroblasts and extracellular matrix: Targeting and therapeutic tools in fibrosis and cancer. Adv Drug Deliv Rev. 2017;121:1–2. doi: 10.1016/j.addr.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, Iwasa M, Watanabe S, Takei Y. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424–432. doi: 10.1158/1055-9965.EPI-08-0725. [DOI] [PubMed] [Google Scholar]
- 119.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 120.Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866; author reply 866–866; author reply 867. doi: 10.1053/gast.2003.50130. [DOI] [PubMed] [Google Scholar]
- 121.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. 2014;20:3002–3010. doi: 10.3748/wjg.v20.i11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adams LA, Crawford DH, Stuart K, House MJ, St Pierre TG, Webb M, Ching HL, Kava J, Bynevelt M, MacQuillan GC, Garas G, Ayonrinde OT, Mori TA, Croft KD, Niu X, Jeffrey GP, Olynyk JK. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology. 2015;61:1555–1564. doi: 10.1002/hep.27662. [DOI] [PubMed] [Google Scholar]
- 123.Lainé F, Ruivard M, Loustaud-Ratti V, Bonnet F, Calès P, Bardou-Jacquet E, Sacher-Huvelin S, Causse X, Beusnel C, Renault A, Bellissant E, Deugnier Y Study Group. Metabolic and hepatic effects of bloodletting in dysmetabolic iron overload syndrome: A randomized controlled study in 274 patients. Hepatology. 2017;65:465–474. doi: 10.1002/hep.28856. [DOI] [PubMed] [Google Scholar]
- 124.Nielsen P, Fischer R, Buggisch P, Janka-Schaub G. Effective treatment of hereditary haemochromatosis with desferrioxamine in selected cases. Br J Haematol. 2003;123:952–953. doi: 10.1046/j.1365-2141.2003.04708.x. [DOI] [PubMed] [Google Scholar]
- 125.Ikeda H, Wu GY, Wu CH. Evidence that an iron chelator regulates collagen synthesis by decreasing the stability of procollagen mRNA. Hepatology. 1992;15:282–287. doi: 10.1002/hep.1840150218. [DOI] [PubMed] [Google Scholar]
- 126.Jin H, Terai S, Sakaida I. The iron chelator deferoxamine causes activated hepatic stellate cells to become quiescent and to undergo apoptosis. J Gastroenterol. 2007;42:475–484. doi: 10.1007/s00535-007-2020-5. [DOI] [PubMed] [Google Scholar]
- 127.Darwish SF, El-Bakly WM, El-Naga RN, Awad AS, El-Demerdash E. Antifibrotic mechanism of deferoxamine in concanavalin A induced-liver fibrosis: Impact on interferon therapy. Biochem Pharmacol. 2015;98:231–242. doi: 10.1016/j.bcp.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 128.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sobbe A, Bridle KR, Jaskowski L, de Guzman CE, Santrampurwala N, Clouston AD, Campbell CM, Subramaniam VN, Crawford DH. Inconsistent hepatic antifibrotic effects with the iron chelator deferasirox. J Gastroenterol Hepatol. 2015;30:638–645. doi: 10.1111/jgh.12720. [DOI] [PubMed] [Google Scholar]
- 130.Wang QM, Du JL, Duan ZJ, Guo SB, Sun XY, Liu Z. Inhibiting heme oxygenase-1 attenuates rat liver fibrosis by removing iron accumulation. World J Gastroenterol. 2013;19:2921–2934. doi: 10.3748/wjg.v19.i19.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880–4888. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han CY, Koo JH, Kim SH, Gardenghi S, Rivella S, Strnad P, Hwang SJ, Kim SG. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat Commun. 2016;7:13817. doi: 10.1038/ncomms13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica. 2011;96:199–203. doi: 10.3324/haematol.2010.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Canali S, Core AB, Zumbrennen-Bullough KB, Merkulova M, Wang CY, Schneyer AL, Pietrangelo A, Babitt JL. Activin B Induces Noncanonical SMAD1/5/8 Signaling via BMP Type I Receptors in Hepatocytes: Evidence for a Role in Hepcidin Induction by Inflammation in Male Mice. Endocrinology. 2016;157:1146–1162. doi: 10.1210/en.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanamori Y, Sugiyama M, Hashimoto O, Murakami M, Matsui T, Funaba M. Regulation of hepcidin expression by inflammation-induced activin B. Sci Rep. 2016;6:38702. doi: 10.1038/srep38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 137.Vagu C, Sultana C, Ruta S. Serum iron markers in patients with chronic hepatitis C infection. Hepat Mon. 2013;13:e13136. doi: 10.5812/hepatmon.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheung CL, Cheung TT, Lam KS, Cheung BM. High ferritin and low transferrin saturation are associated with pre-diabetes among a national representative sample of U.S. adults. Clin Nutr. 2013;32:1055–1060. doi: 10.1016/j.clnu.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 139.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, Wetzels JF, Kiemeney LA, Sweep FC, den Heijer M, Swinkels DW. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117:e218–e225. doi: 10.1182/blood-2011-02-337907. [DOI] [PubMed] [Google Scholar]
- 140.Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106–112. doi: 10.1111/j.1530-0277.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 141.Hoki T, Miyanishi K, Tanaka S, Takada K, Kawano Y, Sakurada A, Sato M, Kubo T, Sato T, Sato Y, Takimoto R, Kobune M, Kato J. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology. 2015;62:751–761. doi: 10.1002/hep.27774. [DOI] [PubMed] [Google Scholar]
- 142.Vuppalanchi R, Troutt JS, Konrad RJ, Ghabril M, Saxena R, Bell LN, Kowdley KV, Chalasani N. Serum hepcidin levels are associated with obesity but not liver disease. Obesity (Silver Spring) 2014;22:836–841. doi: 10.1002/oby.20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demircioğlu F, Görünmez G, Dağıstan E, Göksügür SB, Bekdaş M, Tosun M, Kızıldağ B, Kısmet E. Serum hepcidin levels and iron metabolism in obese children with and without fatty liver: case-control study. Eur J Pediatr. 2014;173:947–951. doi: 10.1007/s00431-014-2268-8. [DOI] [PubMed] [Google Scholar]
- 144.Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J, Dong A, Liu G, Anderson GJ, Hu TY, Shi J, Hu Y, Nie G. Correlation of serum hepcidin levels with disease progression in hepatitis B virus-related disease assessed by nanopore film based assay. Sci Rep. 2016;6:34252. doi: 10.1038/srep34252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sam AH, Busbridge M, Amin A, Webber L, White D, Franks S, Martin NM, Sleeth M, Ismail NA, Daud NM, Papamargaritis D, Le Roux CW, Chapman RS, Frost G, Bloom SR, Murphy KG. Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet Med. 2013;30:1495–1499. doi: 10.1111/dme.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pechlaner R, Weiss G, Bansal S, Mayr M, Santer P, Pallhuber B, Notdurfter M, Bonora E, Willeit J, Kiechl S. Inadequate hepcidin serum concentrations predict incident type 2 diabetes mellitus. Diabetes Metab Res Rev. 2016;32:187–192. doi: 10.1002/dmrr.2711. [DOI] [PubMed] [Google Scholar]
- 148.Suárez-Ortegón MF, Moreno M, Arbeláez A, Xifra G, Mosquera M, Moreno-Navarrete JM, Aguilar-de Plata C, Esteve E, Ricart W, Fernández-Real JM. Circulating hepcidin in type 2 diabetes: A multivariate analysis and double blind evaluation of metformin effects. Mol Nutr Food Res. 2015;59:2460–2470. doi: 10.1002/mnfr.201500310. [DOI] [PubMed] [Google Scholar]
- 149.Martinelli AL, Filho AB, Franco RF, Tavella MH, Ramalho LN, Zucoloto S, Rodrigues SS, Zago MA. Liver iron deposits in hepatitis B patients: association with severity of liver disease but not with hemochromatosis gene mutations. J Gastroenterol Hepatol. 2004;19:1036–1041. doi: 10.1111/j.1440-1746.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- 150.Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40:834–841. doi: 10.1086/428062. [DOI] [PubMed] [Google Scholar]