Abstract
Context
The NRLP3 inflammasome is a multiprotein danger-sensing complex that serves as a critical link between obesity-related adipose inflammation and insulin resistance and has been shown in animal models to be inhibited by fish oil-derived long chain omega-3 polyunsaturated fatty acids (n-3 PUFA).
Objective
We conducted a clinical trial and in vitro experiments to test our hypothesis that n-3 PUFA suppress NLRP3 inflammasome in human obesity through downregulation of inflammasome gene expression in adipocytes and macrophages.
Design
Placebo-controlled clinical trial and in vitro coculture experiments with primary human adipocytes (from biopsy specimens) and human THP-1 monocyte-derived macrophages treated with eicosapentaenoic acid (EPA) and/or docosahexaenoic acid (DHA) vs vehicle control.
Setting
General community, research laboratory.
Patients and Other Participants
Obese (body mass index ≥ 30 kg/m2), nondiabetic males and females age 18 to 50. N = 25.
Interventions
Clinical trial: Eight-week treatment with 4 g Lovaza (EPA and DHA) or placebo. Cells culture: EPA and/or DHA at 100 µg/mL or vehicle control in culture medium.
Main Outcome Measures
Adipose tissue or adipocyte/macrophage mRNA expression of IL-1β and IL-18 and circulating IL-18 levels.
Results
Treatment of obese human subjects with fish oil supplements reduced expression of adipose inflammatory genes including inflammasome-associated IL-18 and IL-1β and circulating IL-18 levels. Both EPA and DHA reduced inflammasome gene expression in obese human adipose and human adipocyte and macrophages.
Conclusions
N-3 PUFA reduce NLRP3 inflammasome in human adipose through downregulation of gene expression in adipocytes and monocytes/macrophages and has potential as nutritional therapeutic agent in prevention of obesity-related inflammation.
Keywords: obesity, inflammation, adipose, nutrition
Obesity is well-recognized as a low-grade inflammatory state; inflammation in metabolically active tissues, including adipose tissue, can contribute to obesity-related metabolic dysfunction and cardiometabolic disease. Factors including expansion of the adipose tissue depot and free fatty acid excess lead to activation of inflammatory pathways that recruit proinflammatory macrophages to adipose tissue. Together, adipocytes and activated macrophages interact to increase secretion of inflammatory cytokines (including TNFα and IL-1β) that directly and indirectly act upon insulin signaling pathways to promote insulin resistance [1–13].
A critical link between obesity-induced adipose inflammation and insulin resistance is the NLRP3 [nucleotide-binding and oligomerization domain-like receptor, leucine–rich repeat and pyrin domain-containing 3] inflammasome—a multiprotein danger-sensing complex composed of NLRP3, adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and the cysteine protease caspase-1 [14]. The NRLP3 inflammasome is a sensor of metabolic stress “danger signals,” which include exogenous and endogenous signals, including free fatty acids and fatty acid derivatives [15], reactive oxygen species, and ATP. Activation of the inflammasome is a two-step process. Phase 1 (priming) occurs when toll-like receptor 4 is stimulated via saturated fatty acids, lipopolysaccharide, or inflammatory cytokines (e.g., TNFα and IL-6) and triggers the nuclear factor κB (NF-κB) pathway. This results in increased production of pro-IL-1β and pro-IL-18, as well as increased NLRP3 expression. The second phase is initiated by recognition of danger signals, upon which NLRP3 interacts with ASC, which then binds procaspase-1 forming the inflammasome complex [16]. This formation leads to cleavage of procapose-1 to active caspase-1, which subsequently cleaves the prointerleukins to active IL-1β and IL-18 [17–19]. Inflammasome activation has been implicated in a multitude of inflammatory, metabolic, and neoplastic diseases [20].
IL-1β results in insulin resistance through activation of NF-κB and JNK signaling [21], which promotes serine phosphorylation of IRS1, targeting it from destruction instead of the tyrosine-phosphorylation required for insulin signaling [22]. Although it is less clear how IL-18 impacts insulin signaling, a human study showed that macrophages from subjects with type 2 diabetes had higher IL-18 secretion than healthy controls, and that treatment with the insulin-sensitizing agent metformin reduced IL-18 secretion [23].
Expression of NRLP3 components have been found to be increased in adipose tissue of obese humans and mice [24, 25] and weight loss in humans resulted in reduced expression in white adipose tissue [25]. Several animal models have demonstrated that absence of inflammasome components is associated with protection from obesity-related insulin resistance [25–27]; though there is inconsistency in this response as another group has shown that obesity-related inflammation was not associated with increased caspase-1 cleavage and Nlrp3 null mice were not protected from adipose tissue inflammation [28]. Lending further credence to a likely role of NRLP3 in obesity and metabolic dysregulation, genome wide association studies (thus far reported in Chinese Han populations) have shown association of an NLRP3 single nucleotide polymorphism with obesity [29] and with type 2 diabetes and insulin resistance [30, 31].
Several studies utilizing animal and in vitro models have demonstrated that saturated fatty acids are potent activators of the NLRP3 inflammasome [15, 16, 32–34]. In contrast, unsaturated fatty acids, particularly fish oil-derived long chain omega-3 polyunsaturated fatty acids (n-3 PUFA), may inhibit the inflammasome [35]. A general anti-inflammatory effect of fish oils in obesity-induced adipose inflammation has been shown repeatedly in animal and human models [36–39]. In 3T3-L1 adipocytes, coculture with macrophages from mice fed a high-fat diet (HFD) with fish oil had reduced IL-1β, IL-18, and caspase-1 expression, as well as reduced caspase-1 activity compared with HFD without fish oil [35]. To date, there have been no published studies on the impact of n-3 PUFA on the inflammasome in human or human cell culture models.
We conducted a clinical trial of n-3 PUFA supplementation on adipose tissue NLRP3 inflammasome activity in obesity as well as ex vivo adipose and coculture experiments with human primary adipocytes and THP-1 monocyte-derived macrophages to demonstrate the effects of n-3 PUFA on the NLRP3 inflammasome.
1. Materials and Methods
A. Clinical Trial
All clinical studies described were performed with approval of the University of Pennsylvania (UPenn) Institutional Review Board after written informed consent was obtained from all research participants and was registered at clinicaltrials.gov (NCT02010359). We recruited healthy obese subjects age 18 to 50 with body mass index ≥30 mg/m2 from the general community for the Fish Oils and Adipose Inflammation Reduction study. A total of 25 subjects (13 Lovaza, 12 placebo) completed the study and are included in these analyses.
Exclusions included diagnosis of diabetes (glucose fasting > 126 mg/dL, or random > 200 mg/dL, or use of any antidiabetic agent), use of any lipid-lowering medications, inflammatory disease or use of anti-inflammatory agents (including over the counter analgesics, and inhaled/topical/nasal steroids), habitual fish intake of >three servings/month and/or unwilling to eliminate all fish intake during the study, fish oil supplement intake within 6 months, conditions that would contraindicate use of Lovaza (liver dysfunction, anemia, arrhythmia, coagulopathy), pregnancy, and breastfeeding.
Participants who met initial criteria by telephone/E-mail interview underwent a screening visit with medical history, physical examinations, electrocardiogram, urine pregnancy test in females, and laboratories for fasting glucose, complete blood count, liver function panel, and lipids. If eligible, they returned for a randomization visit that included fasting blood draw and gluteal adipose biopsy, as previously reported [40, 41]. Briefly, subcutaneous adipose samples were collected by core needle aspiration through a 4-mm gluteal incision. Subjects were assigned a subject ID and randomized to Lovaza 4 g/d or placebo in a double-blind manner (managed via UPenn Investigational Drug Service). Subjects returned for study completion visit scheduled after 8 to 10 weeks of study drug, which again involved fasting blood draw and adipose biopsy from contralateral gluteal site. Adipose tissue and blood samples were aliquoted and stored at −80°C until further analysis.
B. Measurement of Circulating Metabolites and Inflammatory Markers
Fasting glucose insulin and lipid measurements were performed by the Translational Core Laboratory of the University of Pennsylvania using the Roche Cobas c311 (Roche, Basel, Switzerland). HOMA-IR was calculated using the following formula: fasting insulin level (µIU/mL) × fasting glucose level (in mM)/22.5. Inflammatory markers [IL-6, monocyte chemoattractant protein-1 (MCP-1), IL-18, and IL-1β] and high molecular weight (HMW) adiponectin were quantified by enzyme-linked immunosorbent assay run in duplicate according to manufacturer’s directions (R&D Systems, Minneapolis, MN) [42–45] and read on an Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT). Coefficients of variance were <15% for all samples and assays run.
C. Human Adipose Ex Vivo Experiments
Human abdominal visceral and subcutaneous adipose tissue collected from bariatric surgery performed on obese subjects was supplied by the Penn Human Adipose Resource Center under a protocol approved by the UPenn Institutional Review Board. The tissue was dissected into 1-g samples and plated on a 12-well plate. The tissue was then incubated in adipocyte growth media containing 100 µM eicosapentaenoic acid (EPA), 100 µM docosahexaenoic acid (DHA), a combination of 50 µM EPA and 50 µM DHA, or vehicle [dimethyl sulfoxide (DMSO)] for 48 hours.
D. Primary Human Adipocyte Isolation
Primary adipocytes were isolated from fresh abdominal adipose tissue collected from bariatric surgery provided by the Penn Human Adipose Resource. The adipose tissue was digested with collagenase (1 mg/mL) in serum free DMEM/F12 culture media using the protocol described with minor modifications [27]. The digested tissue was then filtered and resuspended in 10 mL adipocyte growth media [DMEM/F12 with Primocin (InvivoGen, San Diego, CA)], biotin (4 mg/L), pantothenate (8 mg/L), epidermal growth factor (5 g/L), fibroblast growth factor (5 µg/L), and 10% fetal bovine serum (Sigma-Aldrich Corp., St. Louis, MO). The suspension was then centrifuged and the cell pellet was resuspended in OF media and plated at 40,000 cells/cm2. The cells were grown to 80% confluence before being differentiated as previously described [41].
E. THP1 Macrophage Differentiation and Polarization
THP1 monocytes commercially purchased (Sigma-Aldrich Corp.) were maintained in culture at 1 × 106 cells/mL in THP1 culture media [RPMI medium supplemented with l-glutamine (2 mM), HEPES (10 mM), sodium pyruvate (1 mM), d-glucose (625 mg/L), betamercaptaethanol (100 µL/L), and 10% fetal bovine serum]. Differentiation was achieved by plating 3 × 103 cells/cm2 in THP1 culture media with 200 µM phorbol 12-myristate 13-acetate (PMA) for 72 hours. At this point the PMA was removed and the cells were cultured in PMA-free THP1 culture media for 5 days before being polarized. Preliminary time course and dose response experiments determined these culture conditions to produce neutral, nonpolarized (M0) macrophages (data not shown). The M0 macrophages were then polarized to M1 classically activated, proinflammatory macrophages by culturing the cells in THP1 culture media with lipopolysaccharide (100 ng/mL) and INF-γ (20 ng/mL) for 24 hours.
F. Primary Adipocyte/Macrophage Co-Culture Experiments
Primary adipocytes were cultured with classically activated THP1 macrophages using the Costar Transwell coculture system (Corning Life Sciences, Corning, NY). Prior to coculture, primary adipocytes were plated in the lower compartment of a 12-well plate, at 1 × 106 cells/mL and differentiated. In a separate 12-well plate, without any cells in the lower compartment, 1 × 105 THP1 cells were plated, differentiated, and polarized in the trans well insert upper compartment. Once both primary adipocytes and THP1 macrophages were differentiated and polarized, the trans well inserts containing classically activated macrophages were placed inside of the lower compartment containing adipocytes.
Primary adipocyte and THP1 macrophage cocultures were treated with fish oil derived LC n-3 PUFA EPA and DHA dissolved in DMSO. Both cell types were treated with 100 µM EPA, 100 µM DHA, a combination of 50 µM EPA and 50 µM DHA, or vehicle (DMSO) for 48 hours. Treatment timing and concentration was determined based upon preliminary dose response and time course experiments (data not shown).
G. RNA Extraction, cDNA Synthesis, and Quantitative PCR
Whole adipose tissue was homogenized, or, for in vitro experiments adipocytes and monocytes were washed and lysed for RNA isolation using TRIzol reagent (Life Technologies, Foster City, CA). RNA concentration and quality were determined with an Epoch Microplate Spectrophotometer (BioTek Instruments, Inc.), and cDNA was prepared using the High Capacity cDNA Reverse transcription kit (Life Technologies). Expression of genes was determined by quantitative real-time PCR using TaqMan Universal PCR MasterMix and primers and probes from Life Technologies and the QuantStudio6 quantitative PCR system. Gene expression levels were normalized to the housekeeping gene GAPDH, and relative expression was determined using the 2-ΔΔCt method [46].
H. Statistical Analysis
Statistical analysis was preformed using GraphPad Prism 6 software. The statistical significance of difference between control and cocultured cells was determined by Student t test or Mann-Whitney U test depending on whether normality could be assumed. When measuring the statistical difference between EPA and DHA treated cells, one-way ANOVA was used. A two-way ANOVA was used to determine the statistical significance between gene expression in visceral and subcutaneous tissue in ex vivo experiments.
2. Results
A. Fish Oil Supplements Reduced Adipose Tissue Expression of NLRP3 Inflammasome Genes and Circulating IL-18 in Human Obesity
Demographic information and measurements of circulating metabolic and inflammatory markers for all subjects at randomization and study completion is presented in Table 1. Both groups had similar age and sex. Although the placebo group had a greater number of subjects self-identified as black, and less as white, the proportions in these racial groups by treatment arm did not reach statistical significance between treatment groups. Body mass index and metabolic parameters (glucose, lipids, HMW adiponectin) were similar between groups and did not change significantly during study. Notably, while the classic inflammatory marker IL-6 and the inflammatory adipochemokine MCP-1 were not altered by Lovaza, there was a modest reduction in the NRLP3 inflammasome-associated markers IL-18 (36.2 +/− 16.7 to 34.3 +/− 16.9, P < 0.0001). IL-1β levels in plasma were below the lower limits of detection in our assay and thus could not be compared.
Table 1.
Demographic, Metabolic, and Inflammatory Parameters at Baseline and After 8 Weeks of Study Drug
|
|
Lovaza (n = 13) |
Placebo (n = 12) |
||
|---|---|---|---|---|
| Randomization | Completion | Randomization | Completion | |
| Age (y) | 35.9 (9.41) | — | 37.4 (8.36) | — |
| % Female | 62 | — | 67 | — |
| % White | 69 | — | 23 | — |
| % African American | 23 | — | 42 | — |
| Body mass index (kg/m2) | 36.3 (5.61) | 35.9 (5.12) | 35.7 (6.42) | 36 (5.9) |
| Glucose, mg/dL | 86.5 (11.6) | 79.3 (11.8) | 86.8 (10.2) | 84.3 (7.62) |
| Insulin, uIU/mL | 17.6 (4.97) | 17.0 (6.82) | 16.2 (5.31) | 17.8 (4.91) |
| HOMA-IR | 3.14 (0.9) | 3.53 (1.52) | 3.3 (1.17) | 3.45 (1.22) |
| Triglycerides, mg/dL | 139 (108) | 106 (62.9) | 92.9 (35.1) | 85.4 (37) |
| LDL, mg/dL | 111 (24.5) | 116 (21.6) | 119 (26.8) | 115 (37.4) |
| HDL, mg/dL | 47.3 (20) | 49.2 (22) | 49.1 (9.21) | 50.2 (7) |
| HMW adiponectin, µg/mL | 1.79 (1.56) | 1.86 (1.71) | 2.44 (2.07) | 2.5 (2.21) |
| IL-6, pg/mL | 1.72 (1.17) | 2.55 (2) | 2.03 (1.51) | 2.02 (1.91) |
| MCP-1, pg/mL | 128 (28.2) | 133 (29.1) | 134 (52.2) | 123 (54.9) |
| IL-18, pg/mL | 36.2 (16.7) | 34.3 (16.9)a,b | 34 (9.83) | 38.5 (17.2) |
Data are presented as mean (SD) for continuous variables or as a percentage for categorical variables.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 between groups.
P < 0.05 within group over time.
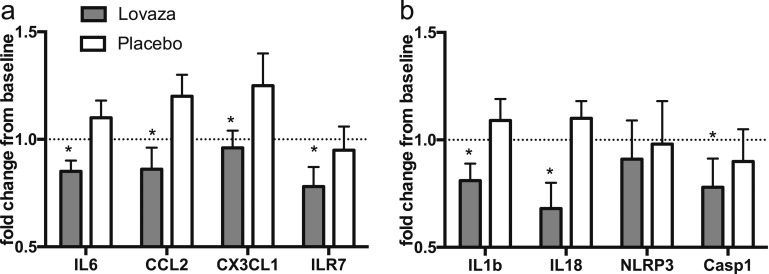
In contrast, comparison of adipose tissue pre- and posttreatment demonstrated a marked decrease in expression of IL-6, MCP-1 (CCL2), IL-7R, and CX3CL1 (adipose tissue cytokines and chemokines associated with obesity) in the Lovaza group (Fig. 1a). Lovaza also resulted in significant reduction in adipose tissue expression of the NLRP3 inflammasome genes IL-18, IL-1β, and CASP-1 though NLRP3 did not change (Fig. 1b).
Figure 1.
Lovaza treatment of 8 weeks reduced inflammatory and inflammasome gene expression in obese adipose. Eight-wk treatment of obese subjects with Lovaza (n = 13), but not placebo (n = 12), led to decrease in relative adipose tissue mRNA expression of (a) classic obesity-associated inflammatory genes IL-6, CCL2, CX3CL1, and IL-7R and (b) inflammasome associated genes IL-18, IL-1β, and Casp1, though not NLRP3. *P < 0.05.
B. Both EPA and DHA Reduce NRLP3 Gene Expression in Human Adipose From Obese Subjects
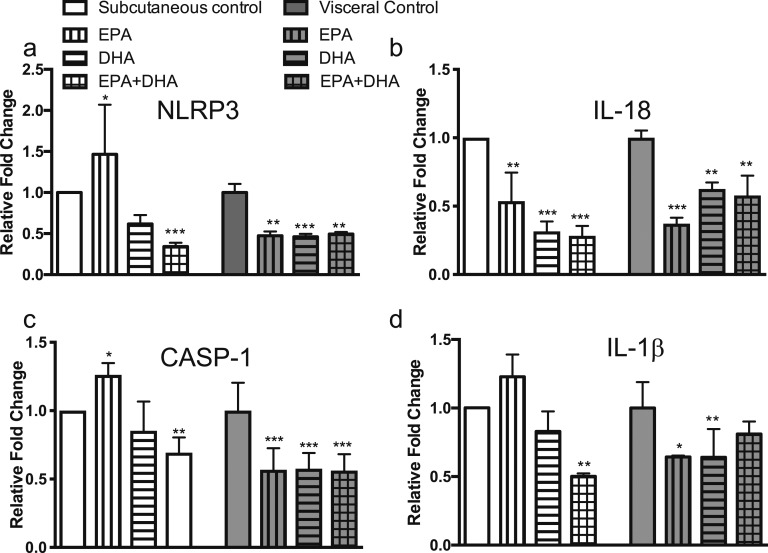
To determine whether inflammasome gene expression was suppressed by EPA or DHA, we designed ex vivo experiments treating obese human subcutaneous and visceral adipose tissue obtained during bariatric surgery with 100 µM DHA, 100 µM EPA, combination of 50 µM DHA + 50 µM EPA or DMSO vehicle control. Both EPA and DHA treatment of adipose tissue diminished gene expression of IL-18, IL-1β, NLRP3, and caspase 1 as shown in Fig. 2, though effects were overall more pronounced in subcutaneous adipose tissue. Expression changes were more likely with DHA as compared with EPA and were not additive, suggesting at least partial overlap of mechanistic pathways.
Figure 2.
EPA and DHA reduced inflammasome gene expression in human visceral and subcutaneous adipose from obese subjects. (a) NLRP3 expression was significantly decreased in subcutaneous tissue by EPA and DHA, and trended toward lower levels by DHA in visceral tissue. (b) IL-18 expression was significantly decreased by both EPA and DHA in both tissue types. (c) CASP-1 and (d) IL-1β expression trends showed reduction in visceral tissue with DHA only and with both in subcutaneous tissue. The results represent three experiments repeated in tissue isolated from three separate human subjects. *P < 0.05; **P < 0.01; ***P < 0.001. Data plotted as mean fold change (relative to control visceral) and SD.
C. Pretreatment With EPA and DHA Reduces NLRP3 Inflammasome Gene Expression in Both Adipocytes and Macrophages in Coculture
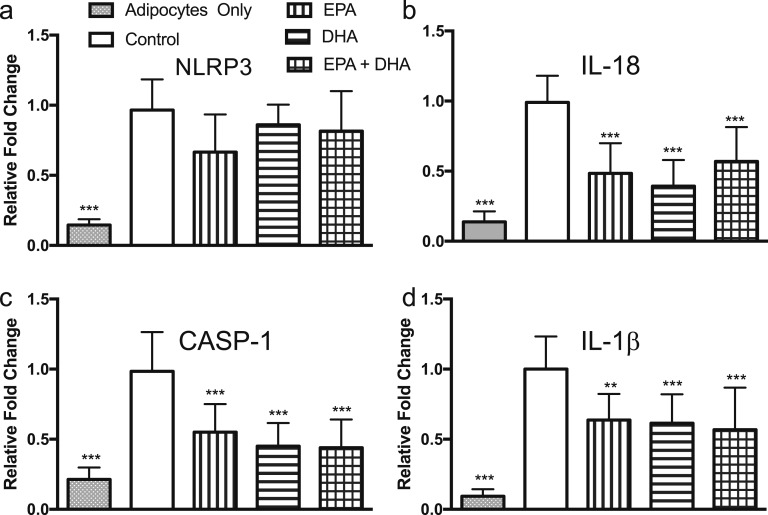
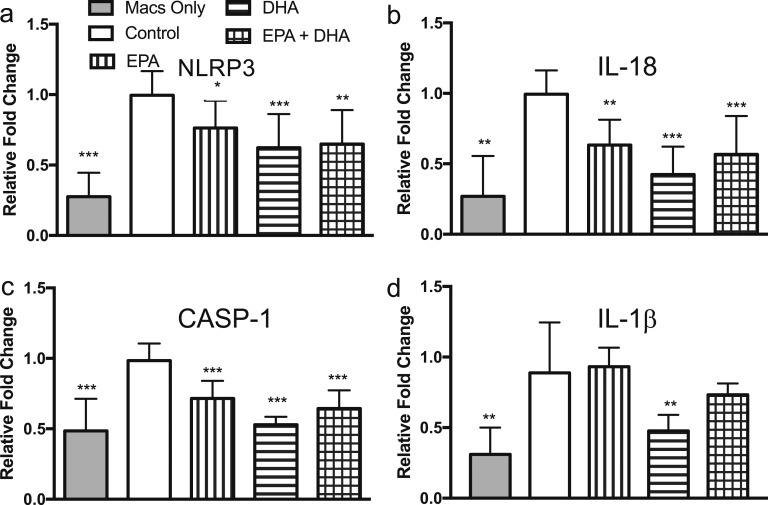
To determine contribution of cell types found in adipose tissue to our observed effects on the inflammasome, we designed a coculture system with primary human adipocytes and human THP-1 monocyte-derived macrophages. Differentiated adipocytes and M1-activated macrophages were cultured together using the Transwell system for 48 hours and treated with 100 µM EPA, 100 µM DHA, 50 µM EPA + 50 µM DHA, and vehicle control and gene expression evaluated in both cell types. Coculture led to increased expression of the NLRP3 inflammasome genes IL-18, IL-1β, CASP-1, and NLRP3 in both cell types. In primary adipocytes, both EPA and DHA led to significant reduction in IL-18, CASP-1, and IL-1β expression, though NLRP3 expression was unchanged (Fig. 3). Similarly, M1 macrophage gene expression of inflammasome genes was reduced by EPA and DHA, though effects of DHA were larger in this cell type (Fig. 4). These results suggest involvement of adipocytes and adipose-tissue macrophages in the response to N-3 PUFA.
Figure 3.
EPA and DHA reduce expression of NLRP3 inflammasome genes in primary human adipocytes in coculture with classically activated THP-1 macrophages. Coculture with macrophages stimulated expression of inflammasome genes in adipocytes. Although NLRP3 expression was unchanged (a) treatment with EPA and DHA during the 48-h coculture led to reduced expression of (b–d) IL-18, CASP-1, and IL-1β. *P < 0.05; **P < 0.01; ***P < 0.001. Data plotted as mean fold change (relative to vehicle control in coculture) and SD.
Figure 4.
EPA and DHA reduce expression of NLRP3 inflammasome genes in classically activated THP-1 macrophages in coculture with primary human adipocytes. Coculture with primary human adipocytes stimulated expression of inflammasome genes in macrophages. Treatment with DHA, and to a lesser extent EPA, during the 48-h coculture reduced expression of (a–d) NLRP3, IL-18, CASP-1, and IL-1β in M1 macrophages. *P < 0.05; **P < 0.01; ***P < 0.001. Data plotted as mean fold change (relative to vehicle control in coculture) and SD.
3. Discussion
These studies, which assess the role of n-3 PUFA on the NLRP3 inflammasome in human adipose, demonstrate inhibition of NRLP3-associated gene expression by EPA and DHA in adipocytes and macrophages. In a placebo-controlled clinical trial of obese participants, there was a decrease in adipose tissue NRLP3 gene expression and circulating levels of IL-18, which is a downstream product of NRLP3 signaling.
In recent years, n-3 PUFA fish oil supplements have been widely used and recommended for prevention and treatment of disease. In addition to the well-known clinical utility of n-3 PUFA on lowering triglyceride levels, there is also evidence, such as the PREDIMED study of the Mediterranean diet, demonstrating protection from cardiovascular mortality with increased intake of marine n-3 PUFA [47] and the American Heart Association supports addition of fish to the diet for cardiovascular prevention in recent guidelines [48]. Yet, over several decades, overall data from clinical trials assessing effect of n-3 PUFA on primary and secondary prevention of cardiovascular disease is conflicting [49–56]. Thus, better understanding of the pleiotropic effects of n-3 PUFA to guide evidence-based recommendations on their use is critical.
Mechanisms by which n-3 PUFA exert their anti-inflammatory effects is also not fully clarified. Recent evidence has highlighted mechanisms involving EPA-derived resolvin E1 and DHA-derived protectin D1 [57]. In high-fat–fed mice, there are decreased levels of protectin in adipose and skeletal muscle, whereas transgenic restoration prevents the HFD–induced insulin resistance [58]. EPA and DHA also bind to G protein-coupled receptor (GPR) 120 and GPR40, and inhibit NF-κB and JNK through which effects on inflammasome signaling may also be mediated [59]. One study in monocyte cell culture models found that treatment with a GPR120 and GPR40 agonist decreased caspase-1 activation and secretion of IL-1β and IL-18 and short hairpin RNA targeting GPR40 and GPR120 mRNA partially abolished DHA and EPA effects on NLRP3 inflammasome activation. Further, deficiency of beta arrestin 2 (Arrb2), a downstream product of GPR120 signaling, using Arrb2 −/− mice only partially inhibited DHA activity suggesting other pathways may be involved [60]. There is minimal data on mechanisms by which n-3 PUFA interact with the NLRP3 inflammasome in adipose, though one study in murine 3T3-L1 adipocytes suggested that inhibition of IL-18, IL-1β, and caspase-1 by DHA and EPA were predominantly dependent upon adiponectin, as they were largely attenuated by use of an adiponectin-neutralizing antibody [35].
Our studies highlight the utilization of human adipocytes and macrophages and the experimental design of the coculture model, which better replicates the complex interactions in adipose tissue than isolated cell types. Further, we acquired adipose tissue from the natural state of human obesity, to increase applicability to real-world situations in which fish oil supplements would be clinically used. Finally, we incorporated a placebo controlled clinical trial with pre- and postexposure measurements to attempt to isolate the differences attributable to the EPA and DHA supplement.
We acknowledge that there are limitations to our studies, including the lack of mechanistic data. Further, our focus on adipocytes and macrophages specifically may have missed contribution from the myriad other cell types populating the adipose depot (vascular and neural cells, fibroblasts, stem cells, monocytes, T lymphocytes, and other immune cells) [61]. Conversely, as the stromal vascular fraction from adipose biopsy was used to create the primary adipocyte cell lines, there may be some contribution of immune cells to these models. The fish oil preparation in the clinical trial was a mixture of EPA and DHA that did not allow exploration of their individual effects, though the in vitro studies were designed to test specific effects of each agent. Finally, in this short-term 8-week study, determination of long-term effects and outcomes was not feasible.
Despite these limitations, our studies add to the emerging body of knowledge regarding potential beneficial effects of fish oils and their derivatives. Further exploration of mechanism is needed, such as experimental models of fatty acid receptor deficiencies and polymorphisms and characterization of systemic and tissue-specific metabolites with fish oil supplementation to map involved pathways. Further studies into the effects of specific agents, inflammasome signaling in other metabolically active tissues (muscle, liver), and longer term studies looking at chronic effects are needed.
Acknowledgments
The clinical trial was supported by the University of Pennsylvania Center for Human Phenomic Science (supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001878). We thank the University of Pennsylvania Diabetes Research Center (DRC) for the use of the Human Adipose Resource (P30-DK19525).
Financial Support: This work was supported by the National Institutes of Health (DK095913-01A1).
Clinical Trial Information: ClinicalTrials.gov no. NCT02010359 (registered 9 December 2013).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- DHA
docosahexaenoic acid
- DMSO
dimethyl sulfoxide
- EPA
eicosapentaenoic acid
- GPR
G protein-coupled receptor
- HFD
high-fat diet
- HMW
high molecular weight
- MCP-1
monocyte chemoattractant protein-1
- n-3 PUFA
fish oil-derived long chain omega-3 polyunsaturated fatty acids
- NF-κB
nuclear factor κB
- PMA
phorbol 12-myristate 13-acetate
- UPenn
University of Pennsylvania
References and Notes
- 1. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clément K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94(11):4619–4623. [DOI] [PubMed] [Google Scholar]
- 4. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenès C, Lafontan M, Galitzky J, Bouloumié A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29(10):1608–1614. [DOI] [PubMed] [Google Scholar]
- 8. Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206(13):3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15(8):846–847. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. [DOI] [PubMed] [Google Scholar]
- 12. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842(3):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pavillard LE, Marín-Aguilar F, Bullon P, Cordero MD. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol Res. 2018;131:44–50. [DOI] [PubMed] [Google Scholar]
- 16. Ralston JC, Lyons CL, Kennedy EB, Kirwan AM, Roche HM. Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu Rev Nutr. 2017;37(1):77–102. [DOI] [PubMed] [Google Scholar]
- 17. Liu D, Zeng X, Li X, Mehta JL, Wang X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2017;113(1):5. [DOI] [PubMed] [Google Scholar]
- 18. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265(1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Próchnicki T, Mangan MS, Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000 Res. 2016;5:F1000 Faculty Rev-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075–1079. [DOI] [PubMed] [Google Scholar]
- 22. McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism. 2017;74:1–9. [DOI] [PubMed] [Google Scholar]
- 25. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108(37):15324–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152(11):4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ringling RE, Gastecki ML, Woodford ML, Lum-Naihe KJ, Grant RW, Pulakat L, Vieira-Potter VJ, Padilla J. Loss of Nlrp3 does not protect mice from Western diet-induced adipose tissue inflammation and glucose intolerance. PLoS One. 2016;11(9):e0161939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Zhang D, Zheng Y, Hu Z, Zeng Y, Yue F. [Association of NLRP3 gene single nucleotide polymorphisms with metabolic syndrome]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;33(4):530–534. [DOI] [PubMed] [Google Scholar]
- 30. Wang S, Fang F, Jin WB, Wang X, Zheng XS. Investigation into the association between NLRP3 gene polymorphisms and susceptibility to type 2 diabetes mellitus. Genet Mol Res. 2015;14(4):17447–17452. [DOI] [PubMed] [Google Scholar]
- 31. Zheng Y, Zhang D, Zhang L, Fu M, Zeng Y, Russell R. Variants of NLRP3 gene are associated with insulin resistance in Chinese Han population with type-2 diabetes. Gene. 2013;530(1):151–154. [DOI] [PubMed] [Google Scholar]
- 32. Legrand-Poels S, Esser N, L’homme L, Scheen A, Paquot N, Piette J. Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochem Pharmacol. 2014;92(1):131–141. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KH, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res. 2012;56(8):1212–1222. [DOI] [PubMed] [Google Scholar]
- 34. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Boer AA, Monk JM, Liddle DM, Hutchinson AL, Power KA, Ma DW, Robinson LE. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J Nutr Biochem. 2016;34:61–72. [DOI] [PubMed] [Google Scholar]
- 36. Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140(11):1915–1922. [DOI] [PubMed] [Google Scholar]
- 37. Pérez-Echarri N, Pérez-Matute P, Marcos-Gómez B, Baena MJ, Marti A, Martínez JA, Moreno-Aliaga MJ. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: effects of EPA ethyl ester treatment. Eur J Nutr. 2008;47(7):380–386. [DOI] [PubMed] [Google Scholar]
- 38. Puglisi MJ, Hasty AH, Saraswathi V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J Nutr Biochem. 2011;22(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Todoric J, Löffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhäusl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49(9):2109–2119. [DOI] [PubMed] [Google Scholar]
- 40. Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59(1):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah R, Lu Y, Hinkle CC, McGillicuddy FC, Kim R, Hannenhalli S, Cappola TP, Heffron S, Wang X, Mehta NN, Putt M, Reilly MP. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes. 2009;58(10):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RRID:AB_354659.
- 43.RRID:AB_358545.
- 44.RRID:AB_2071559.
- 45.RRID:AB_2221612.
- 46. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 47. Sala-Vila A, Guasch-Ferré M, Hu FB, Sánchez-Tainta A, Bulló M, Serra-Mir M, López-Sabater C, Sorlí JV, Arós F, Fiol M, Muñoz MA, Serra-Majem L, Martínez JA, Corella D, Fitó M, Salas-Salvadó J, Martínez-González MA, Estruch R, Ros E, B; PREDIMED Investigators . Dietary α-linolenic acid, marine ω-3 fatty acids, and mortality in a population with high fish consumption: findings from the Prevención con Dieta Mediterránea (PREDIMED) Study. J Am Heart Assoc. 2016;5(1):e002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rimm EB, Appel LJ, Chiuve SE, Djousse L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou Y, Tian C, Jia C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies. Br J Nutr. 2012;108(3):408–417. [DOI] [PubMed] [Google Scholar]
- 50. Siegel G, Ermilov E.. Omega-3 fatty acids: benefits for cardio-cerebro-vascular diseases [published online ahead of print 4 October 2012]. Atherosclerosis. 10.1016/j.atherosclerosis.2012.09.006. PubMed PMID: 23031363. [DOI] [PubMed] [Google Scholar]
- 51. Sasaki J, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Itakura H, Hishida H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y; JELIS Investigators . Relationship between coronary artery disease and non-HDL-C, and effect of highly purified EPA on the risk of coronary artery disease in hypercholesterolemic patients treated with statins: sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). J Atheroscler Thromb. 2012;19(2):194–204. [DOI] [PubMed] [Google Scholar]
- 52. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; JELIS Investigators, Japan . Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200(1):135–140. [DOI] [PubMed] [Google Scholar]
- 53. Oikawa S, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; JELIS Investigators, Japan . Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2009;206(2):535–539. [DOI] [PubMed] [Google Scholar]
- 54. Marchioli R, Schweiger C, Tavazzi L, Valagussa F. Efficacy of n-3 polyunsaturated fatty acids after myocardial infarction: results of GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Lipids. 2001;36(S1, Suppl):S119–S126. [DOI] [PubMed] [Google Scholar]
- 55. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 56. Respondek F, Gerard P, Bossis M, Boschat L, Bruneau A, Rabot S, Wagner A, Martin JC. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS One. 2013;8(8):e71026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59(12):3066–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–1163. [DOI] [PubMed] [Google Scholar]
- 61. Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, Stathopoulos EN, Tsapis A, Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol. 2009;183(9):5948–5956. [DOI] [PubMed] [Google Scholar]