Abstract
Lower intraluminal colonic pH is an indication for the development of inflammatory bowel disease including active ulcerative colitis. Involvement of intestinal sulfate-reducing bacteria in decreasing bowel pH by the production of H2S and acetate as well as their sensitivity has never been reported before. The study of the relative pH and survival of Desulfovibrio piger Vib-7 by monitoring sulfate reduction parameters was the aim of this work. Monitoring was done through the measurement of bacterial growth (biomass), dissimilatory sulfate reduction parameters: sulfate consumption, lactate oxidation, hydrogen sulfide and acetate production. According to our results, we observed that lower pH (<5) significantly inhibited D. piger Vib-7 growth. This inhibition was also noticed when alkaline media (>9 pH) was used, though the reduction was not at the rate as in media with pH of 4. The research indicates that the growth of D. piger Vib-7 is inhibited at pH of 4 which is not as low as the pH found in people with severely developed inflammatory bowel diseases such as ulcerative colitis. Certainly the interaction (synergistic effect) between both hydrogen sulfide and acetate accumulation can also play an important etiological role in the development of bowel inflammation in humans and animals.
Keywords: pH gut, Ulcerative colitis, Hydrogen sulfide, Toxicity, Sulfate-reducing bacteria
1. Introduction
Luminal pH plays an important role in the healthy gastrointestinal tract (GIT) and the GIT of patients with ulcerative colitis and with Crohn’s disease [1]. Mucosal bicarbonate, bacterial fermentation of carbohydrates, lactate production and mucosal absorption of short chain fatty acids influence luminal pH [2]. Abnormal pH measurements in inflammatory bowel disease (IBD) can be the result of alterations of these factors [1, 2, 3].
Sulfate-reducing bacteria (SRB) belong to the normal microbiota of human and animal gastrointestinal tract [4, 5, 6, 7, 8]. Consequently, they influence significantly the pH of the gastrointestinal tract since they produce hydrogen sulfide (H2S) and acetate which lower pH. At the same time, the production of H2S is dependent on the growth of SRB which is highly influenced by pH in their environment [9, 10, 11, 12]. The growth of SRB depends on sulfate concentrations in the GIT, which can be influenced by the individual’s daily diet [4, 13]. Sulfate is an electron acceptor in the process of dissimilatory sulfate reduction (DSR) [14, 15, 16, 17]. Fermentation products, especially lactate or other organic compounds can be used as an electron donor in this process. Lactate is incompletely oxidized to acetate by these bacteria, [14, 18]. The final product of SRB metabolism, hydrogen sulfide, can be toxic and affects intestinal cells, especially by blocking their metabolism through inhibition of cytochrome oxidase [19, 20, 21, 22]. A protective epithelial layer may be disrupted by H2S concentrations, especially because hydrogen sulfide can penetrate through the cell membrane without specific receptors. Hydrogen sulfide affects adenosine-5’-triphosphate-de-pendent potassium channels, cytochrome c oxidase, DNA damage and enzyme inactivation in intestinal cells and can be involved in IBD [10]. Butyrate is the product of microbial fermentation and the main substrate for intestinal cells due to its property to serve them as a good energy source. Since there is a positive correlation with H2S and acetate concentration, it is expected that the presence of SRB leads to the decrease of butyrate in the gut. According to the literature to date, this decrease is threefold [9, 14, 21, 22, 23, 24, 25, 26, 27, 28].
The dominant SRB species most often detected in healthy and IBD individuals is the Desulfovibrio genus [11, 12, 29, 30, 31]. According to data in the present literature, the influence of cultivation media of differential acidity on the dissimilatory sulfate reduction by the Desulfovibrio genus has been insufficiently studied. There are data on intestinal luminal pH in inflammatory bowel disease [1] and very low intraluminal colonic pH in patients with active ulcerative colitis [2,3]. However, there is an insufficient amount of data focused on the influence of pH on SRB of Desulfovibrio genus. The relevance of this research is to study the analysis of pH dose-depending SRB growth, including relative pH for growth stimulation and bacterial survival of D. piger, and relative pH for sulfate reduction parameters that has never been reported before. Since the SRB are producers of hydrogen sulfide and acetate, it is very interesting to investigate their sensitivity to pH.
The aim of this work was to study the relative pH and survival of D. piger Vib-7 through monitoring sulfate reduction parameters (sulfate consumption, hydrogen sulfide production, lactate consumption and acetate production) and kinetic parameters of these processes.
2. Material and methods
2.1. Bacterial culture and cultivation
The study focused on the sulfate-reducing bacteria of the Desulfovibrio piger strain Vib-7 (GenBank accession number: KT881309.1.) isolated from the human large intestine. The isolated strain was identified based on physiological and biochemical properties as described previously [32] and sequence analysis of the 16S rRNA gene [33]. The strain was kept in the collection of microorganisms at the Laboratory of Anaerobic Microorganisms of Department of Experimental Biology at Masaryk University (Brno, Czech Republic).
The bacteria were grown in modified liquid Postgate’s C medium [20]. The medium was heated in boiling water for 30 min in order to obtain an oxygen-free medium, and cooled to 37 °C. The final optimal pH 7.5 was obtained by addition of a sterile 1 M solution of NaOH (0.9 ml/l). The bacteria were grown for 72 hours at 37°C under anaerobic conditions. The tubes with bacterial strain were brim-filled with medium and closed to provide anaerobic conditions [34].
2.2. Inoculation of hydrogen sulfide and its determination
A sterile solution of Na2S×9H2O at different concentrations was added to the liquid medium before bacterial seeding. The final concentration of pH in the medium were 4, 5, 6, 7, 8, 9 and 10. The D. piger Vib-7 growth (biomass) and their process of dissimilatory sulfate reduction (consumption of sulfate and lactate and production of hydrogen sulfide and acetate) under the effect of pH were studied.
2.3. Bacterial biomass determination
About 1 mL of liquid medium without Mohr’s salt was transferred into a plastic cuvette and taken to a biophotometer (Eppendorf BioPhotometer®D30) for taring. Subsequently, 1 mL of bacterial suspension was transferred into another cuvette and taken again to the biophotometer for measuring at OD λ=340. Before SRB were used for the experiments, optical density (OD340) was always measured to assure approximately the same amount of bacteria in each experiment [5].
2.4. Sulfate determination
The content of sulfate in the medium was determined by turbidimetric method immediately after seeding and after 24 hours cultivation. The calibration curve was constructed with the same process. Calibration solutions were prepared in distilled water at concentrations of 2, 4, 8, 16, 24, 32, 40 and 48 μM sodium sulfate. A suspension of 40 mg/L BaCl2 was prepared in 0.12 M HCl. The resulting solution was mixed with glycerol in a 1:1 ratio. To the 1 mL of sample supernatant after centrifugation at 5000 × g at 23˚C was added 10 mL of prepared BaCl2:glycerol solution and carefully stirred. The mixture was let to stand 10 minutes and then the absorbance has been measured at 520 nm (Spectrosonic Genesis 5). As a control, the measurement was repeated in the same manner using cultivation medium [35].
2.5. Hydrogen sulfide determination
Hydrogen sulfide was measured spectrophotometrically immediately after seeding and after 72 hours of cultivation. Calibration solutions were prepared in distilled water at concentrations of 12.5, 25, 50, and 100 μM sodium sulfide. The calibration curve was constructed with the same process. 1 mL of the sample was added to 10 mL of 5 g/L aqueous solution of zinc acetate. Then, 2 mL of 0.75 g/mL p-aminodimethylaniline in a solution of sulfuric acid (2 M) was added. The mixture stood for 5 min at room temperature. After that, 0.5 mL of 12 g/ L solution of ferric chloride dissolved in 15 mM sulfuric acid was added. After standing another 5 minutes at room temperature, the mixture was centrifuged 5000 × g at 23˚C. The absorbance of the mixture was determined to measure hydrogen sulfide at a wavelength of 665 nm by a spectrophotometer (Cecil Aquarius CE 7200 Double Beam Spectrophotometer) [36].
2.6. Lactate and acetate determination
Measurements of lactate concentration using theLactate Assay Kit (Sigma-Aldrich, Catalog Number MAK064) were carried out. Accumulation of acetate ions in the process of bacterial growth in the medium was determined using the Acetate Assay Kit (Colorimetric, Catalog Number KA3764).
2.7. Statistical analysis
Using the experimental data, the basic statistical parameters (M – mean, m – standard error, M±m) were calculated. The accurate approximation was when P≤ 0.05 [37].
Overall differences of indicated above parameters were checked by principal component analysis (PCA). The grouping of clusters were done with the usage of PCA. Cross-correlation was used for finding connections between media pH and measured parameters of Desulfovibrio piger Vib-7. All statistical analysis was conducted with the usage of SPSS 20 statistical software (IBM Corporation, Armonk, USA). Plots were built by the software package Origin7.0 (www.origin-lab.com)
3. Results and discussion
Bacterial growth was measured to be the highest in the medium with pH of 7 to 8. The lower pH lower than 6 resulted in 26 % lesser relative survival in comparison with optimal pH (7 – 8). Similar results were found in alkaline medium (> pH 9) (Fig. 1).
Figure 1.
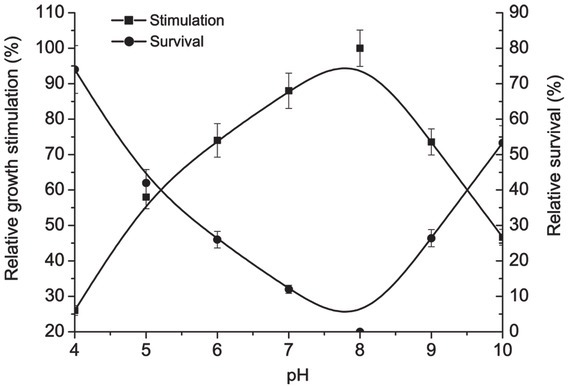
Relative pH for growth stimulation and survival of D. piger Vib-7
The data obtained for bacterial growth under the influence of different pH mediums are in accordance with the process of sulfate reduction by D. piger Vib-7. The optimal pH for sulfate consumption, sulfide production, lactate consumption and acetate production were 7 to 9, 7 to 8, 7 to 9 and 7 to 8, respectively (Fig. 2). The consumption processes (sulfate and lactate consumption) were the highest in pH medium from 7 to 9, while production processes (sulfide and acetate production) were from 7 to 8. Both consumption and production processes were lower in more acidic (< pH 7) or alkaline (> pH 9) media.
Figure 2.
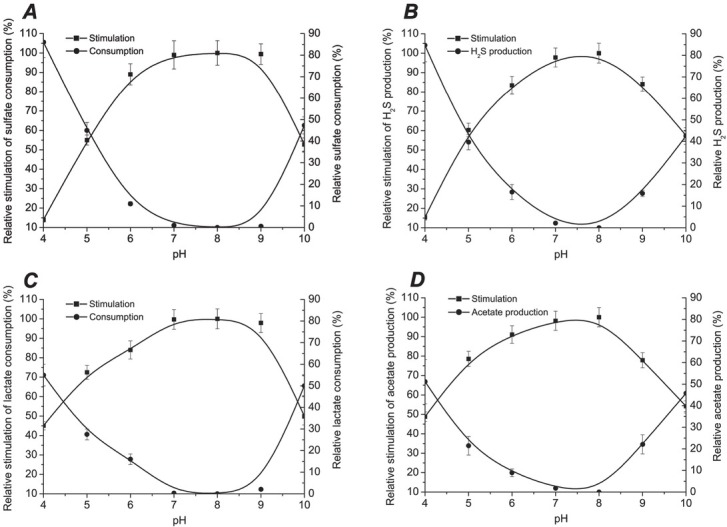
Relative pH for sulfate reduction parameters: sulfate consumption (A), hydrogen sulfide production (B), lactate consumption (C), and acetate production (D)
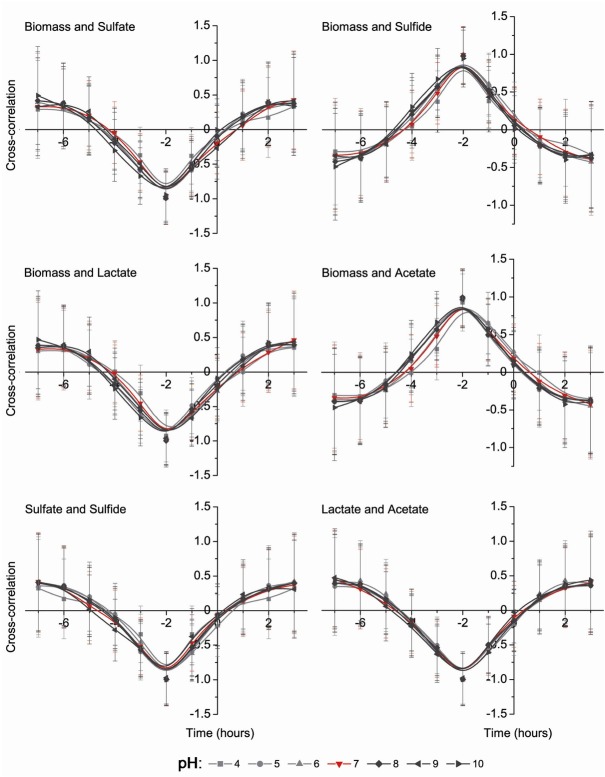
Cross-correlation correlograms are shown in Figs. 3 and 4. Dose dependent pH experiments were done to find dependence of Desulfovibrio growth parameters on different pH media. The results are indicated both negative (between: biomass and sulfate, biomass and lactate, sulfate and sulfide, lactate and acetate, sulfide and lactate) and positive (between: biomass and sulfide, biomass and acetate, sulfate and lactate, sulfate and acetate, sulfide and acetate) correlations. All parameters of positive and negative correlations show that the most influential pH occurred in the media with a pH lower than 5 (Fig. 4).
Figure 3.
Cross-correlation analysis between biomass accumulation and sulfate consumption, sulfide production, lactate oxidation and acetate accumulation as well as between sulfate and sulfide, same as between lactate and acetate
Figure 4.
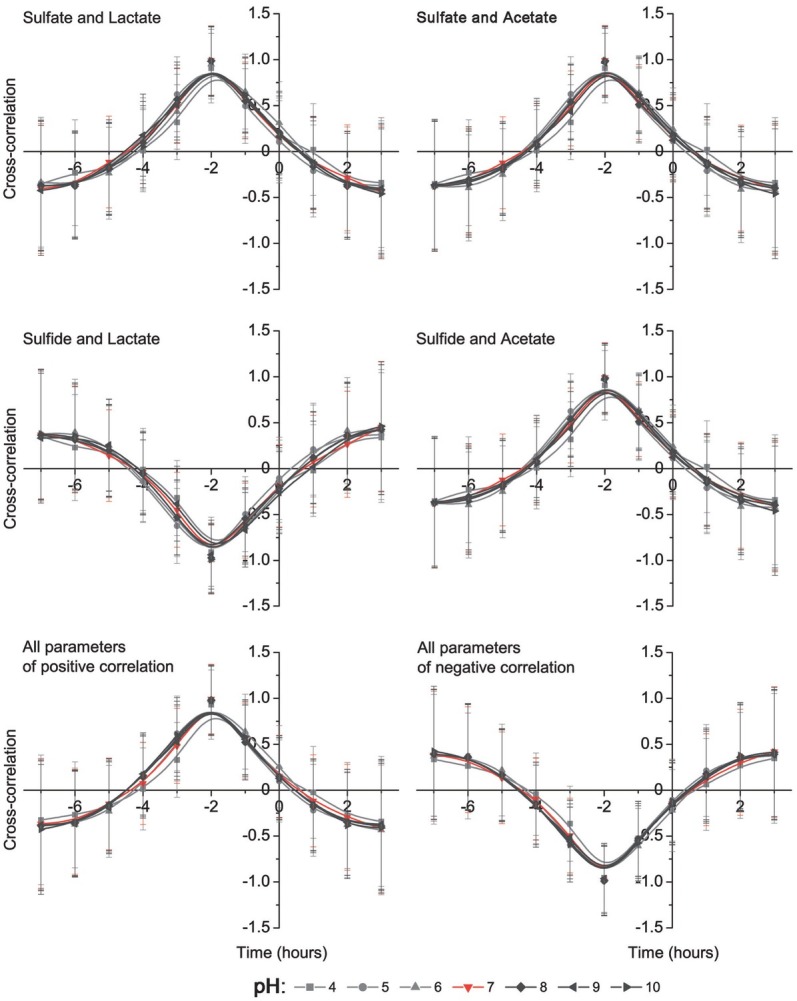
Cross-correlation analysis between sulfate consumption and lactate oxidation, acetate production as well as sulfide and lactate, same as between sulfide and acetate
The results of cross correlation analysis were grouped with the usage of principal component analysis (PCA). All measured parameters were included in the PCA, same as each parameter separately (Fig. 5). PCA analysis that included all measured parameters made three clusters: the first cluster is formed by 7, 6 and 9 pH; the second cluster is formed from 8, 10 and 5 pH; the third cluster consists only of 4 pH. These groups are clearly showing significant (p < 0.05) influence of media pH, especially when the pH was lower than 5 (Fig. 5).
Figure 5.
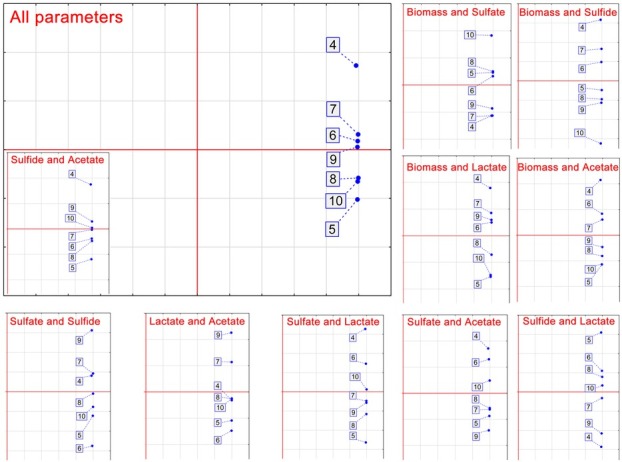
Principal component analysis of the D. piger Vib-7 growth and the parameters of sulfate reduction under the effect of pH
Kinetic parameters (lag phase, generation time, the maximum rate of the bacterial growth and hydrogen sulfide and acetate production) of D. piger Vib-7 affected by different pH during 72 hours of cultivation were calculated and are presented in Table 1. Media with a pH of 4 had high impact on all calculated parameters. The time of lag phase was two times longer in media with a pH of 4, while the generation time (time of division, T d) was almost eight times longer than in all other media with pH higher than 4. The maximum rate of the growth was measured in media with a pH of 7 and 8, corresponding to the maximum rate production of H2S and acetate. Lower and higher pH resulted in bacterial growth inhibition and, accordingly, the process of dissimilatory sulfate reduction.
Table 1.
Kinetic parameters of D. piger Vib-7 growth under the effects of pH during 72 hours of cultivation
| Maximum rate of production (μmax, hour-1) | |||||
|---|---|---|---|---|---|
| pH | Lag phase (hour) | Generation time Td (hour) | Growth (biomass) | Hydrogen sulfide | Acetate |
| 4 | 47±4.5 | 27±2.3 | 0.020±0.0019 | 0.001±0.0001 | 0.065±0.055 |
| 5 | 14±1.3 | 3±0.28 | 0.026±0.0023 | 0.010±0.0015 | 0.092±0.088 |
| 6 | 5.8±0.4 | 2±0.22 | 0.038±0.0034 | 0.024±0.0021 | 0.094±0.091 |
| 7 | 6.6±0.5 | 1.8±0.15 | 0.047±0.0045 | 0.036±0.0033 | 0.102±0.0098 |
| 8 | 6.2±0.5 | 1.7±0.12 | 0.050±0.0049 | 0.035±0.0029 | 0.104±0.0095 |
| 9 | 6.7±0.6 | 2±0.23 | 0.039±0.0033 | 0.032±0.0025 | 0.089±0.0081 |
| 10 | 24±2.3 | 4±0.37 | 0.014±0.0011 | 0.0083±0.0009 | 0.070±0.0062 |
Relative pH values are obtained by our research, where it was found that the growth of D. piger Vib-7 and their process of sulfate reduction, including sulfate consumption, hydrogen sulfide production, lactate consumption, and acetate production, are highly dependent on pH in the GIT. These findings were confirmed by cross-correlation and principal component analysis, same as with kinetic parameters of D. piger Vib-7 growth under the effect of different pH media during 72 hours of cultivation. Lower (< 5 pH) as well as higher (> 9 pH) pH are limiting factors for the growth of SRB, including D. piger Vib-7. Hydrogen sulfide is detected in millimolar concentrations (1.0–2.4 mM) in the lumen of the human large intestine [10, 38]. The concentration of free (unbound) sulfide is in the micromolar range due to a large capacity of fecal components to bind the sulfide [9, 19]. Cytochrome c oxidase, the enzyme important in the processes of intestinal cell respiration, can be severely inhibited by the excessive H2S concentrations. Colonocytes have the ability to metabolize H2S and thus this is an important feature for their resistance toward an excessive concentration of free luminal sulfide [10]. It should be stressed that the data showing prolonged excessive concentrations of hydrogen sulfide, and consequently lower pH, in the luminal content of the large intestine in colon carcinogenesis are scarce. This means that the isolated bacteria D. piger Vib-7 are very promising for further studies. Trans-sulfurization can be also the pathway to the endogenous formation of hydrogen sulfide, which is present in low concentrations in the brain, heart, blood vessels, genitourinary and gastrointestinal tracts [21]. Butyrate oxidation (the main source of energy for intestine colonocytes of humans and animals) is also inhibited by higher concentrations of H2S [21, 23, 28].
The production of H2S in the distal intestine is higher than in the proximal part [11,12,38]. Luminal pH falls from the terminal ileum to the caecum (5.5–7.5), and rises from 6.1 (in the left colon) to 7.5 (rectum). It is important to state that in the colons of people with severely active ulcerative colitis pH values of 2.3 to 3.4 were measured [1, 3] (Fig. 6). SRB are found most often in distal part of the colon to the rectum. SRB form dense biofilms around ulcers [11,38].
Figure 6.
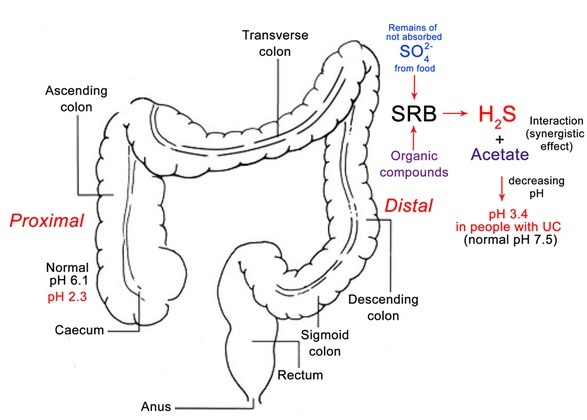
Generalization schema of pH changed in different parts of colon in healthy and not healthy individuals (modified from Whitlock et al., 2008 [39]; pH data according to Nugent et al., 2001 [1])
The immune status of the organism (host), the intestinal lumen pH and the availability of sulfate are all factors influencing the fringe between the ulcer and the colonies of the bacteria [14, 29, 30, 31]. The increased concentrations of H2S have a negative impact on oral mucosa cells since their permeability is also maximized [21]. Advantageous and disadvantageous properties of sulfides correspond with: substrate availability from exogenous (alimentary) and endogenous origins, sulfide concentration inside the colonic lumen, metabolic capacity for the microbiota to produce H2S and other factors which can be influenced for different subjects individually [9, 10].
According to the present literature, there is still a need for a consensus regarding the metabolic pathways involved when sulfide is present in excess, since it is still unknown if H2S is acting as a pro- or antinociceptive agent in the large intestine [22,23].
On the other hand, human diet also plays an important role in the production of H2S by SRB since food types define the concentration of sulfate in diets [13]. Consequently, sulfidogenic SRB can compete with other microorganisms, especially with methanogens [17]. Higher counts of SRB and their H2S production certainly are factors in the development of inflammatory bowel disease [4, 5, 6, 7, 8, 16, 15, 16, 17, 18]. SRB can also be in interaction with Clostridium due to Clostridium species capability to produce H2S, meaning that they can resist certain level of H2S, and also they can decompose complex organic compounds making them available for SRB [14,21,40].
4. Conclusions
SRB are the main producers of H2S and consequently its accumulation highly depends on the presence of these intestinal microorganisms. The production of H2S, same as acetate, leads to the lowering of intestinal pH. The optimal pH for the growth of Desulfovibrio piger Vib-7 and dissimilatory sulfate reduction was in the range from 7 to 8. Acidic pH, same as alkaline pH, resulted in metabolic activity inhibition of these SRB and the rate of the inhibition was higher toward more acidic or alkaline pH. Desulfovibrio genus is one of the main representatives of SRB species and their production of H2S also influences their own growth. Very often higher bacterial counts of SRB are linked to IBD, but at the same time in people with highly developed colitis the pH of the distal lumen is lower than 4 or even close to the pH of 2. Our research showed the highest rate of inhibition of Desulfovibrio in pH media lower than 5. Further in vitro research can be focused on SRB sensitivity toward H2S, different electron donors and acceptors, as well as new synthesized compounds specifically for the inhibition of sulfate reduction process.
Acknowledgements
This study was supported by Grant Agency of the Masaryk University (MUNI/A/0906/2017)
Abbreviations
- SRB
sulfate-reducing bacteria
- DSR
dissimilatory sulfate reduction
- GIT
gastrointestinal tract
- IBD
inflammatory bowel disease
- OD
optical density
- UC
ulcerative colitis
Footnotes
Human and animal rights: This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contribution to Study: Ivan Kushkevych, Dani Dordević, Monika Vítězová wrote the article. All authors contributed to the conception, design and critically revised the manuscript.
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- [1].Nugent S.G., Kumar D., Rampton D.S., Evans D.F. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48(4):571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Press A.G., Hauptmann I.A., Hauptmann L., Fuchs B., Fuchs M., Ewe K., Ramadori G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Alimentary pharmacology & therapeutics. 1998;12(7):673–678. doi: 10.1046/j.1365-2036.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- [3].Fallingborg J., Christensen L.A., Jacobsen B.A., Rasmussen S.N. Very low intraluminal colonic pH in patients with active ulcerative colitis. Digestive diseases and sciences. 1993;38(11):1989–1993. doi: 10.1007/BF01297074. [DOI] [PubMed] [Google Scholar]
- [4].Kováč J., Vítězová M., Kushkevych I.. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med. 2018;13:344–349. doi: 10.1515/med-2018-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kushkevych I., Kollar P., Suchy P., Parak K., Pauk K., Imramovsky A. Activity of selected salicylamides against intestinal sulfate-reducing bacteria. Neuroendocrinol Lett. 2015;36:106–113. [PubMed] [Google Scholar]
- [6].Kushkevych I., Fafula R., Parak T., Bartos M. Activity of Na+/K+-activated Mg2+-dependent ATP hydrolase in the cell-free extracts of the sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Acta Vet Brno. 2015;84:3–12. [Google Scholar]
- [7].Kushkevych I.V. Activity and kinetic properties of phospho-transacetylase from intestinal sulfate-reducing bacteria. Acta Biochemica Polonica. 2015;62:103–108. doi: 10.18388/abp.2014_845. [DOI] [PubMed] [Google Scholar]
- [8].Kushkevych I.V. Kinetic Properties of Pyruvate Ferredoxin Oxidoreductase of Intestinal Sulfate-Reducing Bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Polish J Microbiol. 2015;64:107–114. [PubMed] [Google Scholar]
- [9].Attene-Ramos M.S., Wagner E.D., Plewa M.J., Gaskins H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- [10].Blachier F., Davila A.M., Mimoun S. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39:335–347. doi: 10.1007/s00726-009-0445-2. [DOI] [PubMed] [Google Scholar]
- [11].Gibson G.R., Cummings J.H., Macfarlane G.T. Growth and activities of sulphate-reducing bacteria in gut contents of health subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;86:103–112. [Google Scholar]
- [12].Gibson G.R., Macfarlane S., Macfarlane G.T. Metabolic interactions involving sulphate-reducing and methanogenic bacteria in the human large intestine. FEMS Microbiol Ecol. 1993;12:117–125. [Google Scholar]
- [13].Florin T.H., Neale G., Goretski S., Cummings J.H. Sulfate in food and beverages. Journal of Food Composition and Analysis. 1993;6:140–151. [Google Scholar]
- [14].Barton L.L., Hamilton W.A. Environmental and Engineered Systems. Cambridge University Press; 2010. Sulphate-Reducing Bacteria. [Google Scholar]
- [15].Kushkevych I., Kollar P., Ferreira A.L., Palma D. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J Appl Biomed. 2016;14:125–130. [Google Scholar]
- [16].Kushkevych I., Vítězová M., Fedrová M., Vochyanová Z., Paráková L., Hošek J.. Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet Brno. 2017;86:405–411. [Google Scholar]
- [17].Kushkevych I., Vítězová M., Vítěz T., Bartoš M.. Production of biogas: relationship between methanogenic and sulfate-reducing microorganisms. Open Life Sciences. 2017;12:82–91. [Google Scholar]
- [18].Kushkevych I., Vítězová M., Vítěz T., Kováč J., Kaucká P., Jesionek W., Bartos M., Barton L.. A new combination of substrates: biogas production and diversity of the methanogenic microorganisms. Open Life Sciences. 2018;13:119–128. doi: 10.1515/biol-2018-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pitcher M.C., Cummings J.H. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut. 1996;39:1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Postgate J.R. The sulfate-reducing bacteria. Cambridge University Press; Cambridge: 1984. [Google Scholar]
- [21].Rowan F.E., Docherty N.G., Coffey J.C., O’Connell P.R. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. British J Surgery. 2009;96:151–158. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- [22].Grieshaber M.K., Völkel S. Animal adaptations for tolerance and exploitation of poisonous sulfide. Annual review of physiology. 1998;60:33–53. doi: 10.1146/annurev.physiol.60.1.33. [DOI] [PubMed] [Google Scholar]
- [23].Griesbeck C., Schutz M., Schodl T., Bathe S., Nausch L., Mederer N., Vielreicher M., Hauska G. Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry. 2002;41:11552–11565. doi: 10.1021/bi026032b. [DOI] [PubMed] [Google Scholar]
- [24].Kushkevych I., Dordević D., Vítězová M., Kollár P.. Cross-correlation analysis of the Desulfovibrio growth parameters of intestinal species isolated from people with colitis. Biologia. 2018;73:1137–1143. [Google Scholar]
- [25].Kushkevych I., Kos J., Kollar P., Kralova K., Jampilek J. Activity of ring-substituted 8-hydroxyquinoline-2-carboxanilides against intestinal sulfate-reducing bacteria Desulfovibrio piger. Medicinal Chemistry Research. 2018;27:278–284. [Google Scholar]
- [26].Kushkevych I., Kováč J., Vítězová M., Vítěz T., Bartoš M.. The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch Microbiol. 2018;200:945–950. doi: 10.1007/s00203-018-1510-6. [DOI] [PubMed] [Google Scholar]
- [27].Kushkevych I., Vítězová M., Kos J., Kollár P., Jampílek J.. Effect of selected 8-hydroxyquinoline-2-carboxanilides on viability and sulfate metabolism of Desulfovibrio piger. J. App Biomed. 2018;16:241–246. [Google Scholar]
- [28].Beauchamp R.O., Bus J.S., Popp J.A., Boreiko C.J., Andjelkovich D.A., Leber P. A critical review of the literature on hydrogen sulfide toxicity. CRC Critical Reviews in Toxicology. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- [29].Loubinoux J., Bronowicji J.P., Pereira I.A. Sulphate-reducing bacteria in human feces and their association with inflammatory diseases. FEMS Microbiol Ecol. 2002a;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- [30].Loubinoux J., Valente F.M.A., Pereira I.A.C. Reclassification of the only species of the genus Desulfomonas Desulfomonas pigra as Desulfovibrio piger comb. nov. Int J of System and Evol Microbiol. 2002b;52:1305–1308. doi: 10.1099/00207713-52-4-1305. [DOI] [PubMed] [Google Scholar]
- [31].Loubinoux J., Mory F., Pereira I.A., Le Faou A.E.. Bacteremia caused by a strain of Desulfovibrio related to the provisionally named Desulfovibrio fairfieldensis. J Clin Microbiol. 2000;38:931–934. doi: 10.1128/jcm.38.2.931-934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kushkevych I.V. Identification of sulfate-reducing bacteria strains of human large intestine. Studia Biologia. 2013;7:115–124. [Google Scholar]
- [33].Kushkevych I., Bartos M., Bartosova L. Sequence analysis of the 16S rRNA gene of sulfate-reducing bacteria isolated from human intestine. Int J Curr Microbiol Appl Sci. 2014;3:239–248. [Google Scholar]
- [34].Kováč J., Kushkevych I.. New modification of cultivation medium for isolation and growth of intestinal sulfate-reducing bacteria. Proceeding of International PhD Students Conference MendelNet. 2017:702–707. [Google Scholar]
- [35].Kolmert A., Wikstrom P., Hallberg K.B. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Methods. 2000;41:179–184. doi: 10.1016/s0167-7012(00)00154-8. [DOI] [PubMed] [Google Scholar]
- [36].Cline J.D. Spectrophotometric determination of hydrogen sulfide in natural water. Limnol and Ocean. 1969;14:454–458. [Google Scholar]
- [37].Bailey N.T.J. Statistical Methods in Biology. Cambridge University Press; 1995. [Google Scholar]
- [38].Cummings J.H., Macfarlane G.T., Macfarlane S. Intestinal Bacteria and Ulcerative Colitis. Curr Issues Intest Microbiol. 2003;4:9–20. [PubMed] [Google Scholar]
- [39].Whitlock E.P., Lin J.S., Liles E., Beil T.L., Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Annals of internal medicine. 2008;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- [40].Černý M., Vítězová M., Vítěz T., Bartoš M., Kushkevych I.. Variation in the Distribution of Hydrogen Producers from the Clostridiales Order in Biogas Reactors Depending on Different Input Substrates. Energies. 2018;11(12):3270. [Google Scholar]