Abstract
Decision support system designs often do not align with the information environments in which clinicians work. These work environments may increase Clinicians’ cognitive workload and harm their decision making. The objective of this study was to identify information needs and decision support requirements for assessing, diagnosing, and treating chronic noncancer pain in primary care. We conducted a qualitative study involving 30 interviews with 10 primary care clinicians and a subsequent multidisciplinary systems design workshop. Our analysis identified four key decision requirements, eight clinical information needs, and four decision support design seeds. Our findings indicate that clinicians caring for chronic pain need decision support that aggregates many disparate information elements and helps them navigate and contextualize that information. By attending to the needs identified in this study, decision support designers may improve Clinicians’ efficiency, reduce mental workload, and positively affect patient care quality and outcomes.
Introduction
A significant body of evidence has shown that clinical decision support systems can positively impact care delivery, especially process outcomes1–4. Yet, decision support benefits are not automatic and decision support may provide little value or even cause harm5–8. This is unsurprising given that a majority of health information technology projects fail in some way9, 10. EHRs and clinical decision support systems are part of complex sociotechnical systems that involve interacting technology, people, and clinical processes. If systems are not designed and implemented with the relevant people and processes in mind, the likelihoods of system non-use, slow-downs, workarounds, and unintended negative effects increase. Recommendations for implementing clinical decision support emphasize system speed, timely information, simplicity, usability, and fit with clinical workflow11. Similarly, the “5 Rights” of clinical decision support focus on delivering the right information to the right people in the right format through the right channels at the right points in workflow12. Unfortunately, these best practices for decision support are inconsistent with the EHRs and information environments in which many clinicians regularly work.
Primary care clinicians often work in environments described as “information chaos”. Information chaos is characterized by patient information that is missing, scattered, erroneous, or conflicting13. Such conditions can increase mental workload and inhibit accurate understanding of patients and clinical scenarios (i.e., sensemaking). This may lead to inefficiencies, patient safety risks, and poor clinician and patient experiences13. Today, these risks may be worsened by the fact that electronic health records (EHRs) increasingly incorporate new types of information, such as genetic data and patient-reported outcomes14–16. These risks may also be exacerbated by increased health information exchange adding to the volume and scope of patient information available to clinicians17. Therefore, to accommodate the growth and variation in health information and to combat information chaos, it is important for decision support research and development activities to incorporate users’ needs and decision making processes18, 19.
The objective of this study was to identify information needs and decision support requirements for assessing, diagnosing, and treating chronic noncancer pain in primary care. Tens of million Americans suffer from chronic pain20, 21. Primary care clinicians care for the majority of chronic pain despite the fact that they typically receive minimal training in pain, and many pain conditions have complex biopsychosocial roots22–25. Furthermore, safe and effective chronic pain care is challenging because commonly-prescribed opioid analgesics pose serious individual and public health risks26. Not surprisingly, clinicians often report dissatisfaction and uncertainty when managing patients with chronic pain22–25, 27. Thus, the prevalence, etiological complexities, health risks, and negative experiences associated with chronic pain care make it an important area for understanding information needs and decision support designs. Well-designed decision support may reduce clinicians’ mental workload, improve sensemaking, and lead to more efficient and effective care processes. The findings from this study highlight key requirements for developing EHR-based decision support for chronic pain. The findings from this study may also generalize to decision support for other conditions, especially those for which assessment, diagnosis, and/or treatment are not routinized, and thus clinicians need to frequently gather, review, and assess disparate clinical information.
Methods
Overview
We conducted a multi-step qualitative observational study (Figure 1). First, we conducted and analyzed critical decision method interviews28 with primary care clinicians. Each interview focused on a specific patient with chronic noncancer pain and their recent office visit. The interviews identified key decision requirements associated with the patient and their pain care. Second, we conducted a multidisciplinary design workshop that built on the key decision requirements to identify and describe specific information needs and decision support design seeds that may aid clinicians in delivering efficient and effective pain care. The study described here is part of a larger, ongoing study analyzing clinician interviews, patient-clinician interactions, and EHR data to understand decision making and decision support for chronic pain. The Indiana University Institutional Review Board approved this study.
Figure 1.
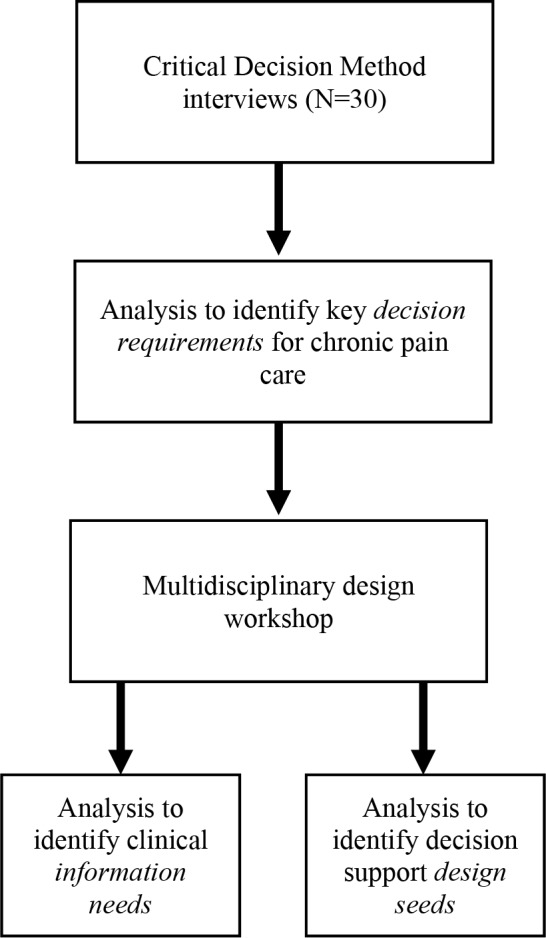
Overview of methods
Critical Decision Method Interviews
We recruited ten adult primary care clinicians (general internal medicine or family medicine) who actively see patients with chronic noncancer pain. To increase heterogeneity in our sample, we recruited from four clinics affiliated with two health systems spanning rural, suburban, and urban areas of Indiana and Illinois. Over the course of the study, clinicians in the two health systems used EHRs from three different vendors. Prior to participation, clinicians received a presentation describing the study and provided written informed consent. Participating clinicians were eligible to participate in up to five interviews about different adult patients with chronic noncancer pain. Clinicians were compensated up to $500 for their time.
Interviews lasted approximately 60-90 minutes and were audio-recorded and transcribed. In order to aid clinicians’ recall, we conducted interviews within 72 hours of a visit by an eligible patient. The patient visit was also audio-recorded, transcribed, and analyzed, with patient consent. However, we present results of those analyzes elsewhere. Also, to maximize clinicians’ available time, each clinician was allowed to give one interview about a salient (non-identified) patient even if that patient’s visit had not been audio-recorded. Three interviewers worked together to conduct the first six interviews to support common process across the research team and to help identify refinements to the interview guide. One or two interviewers conducted each subsequent interview. Each clinician’s first interview included general questions about their patient population and approach to chronic pain treatment, including tools or aids they use when delivering care. The remainder of the interview used an adapted critical decision method interview technique28.
The critical decision method part of the interview began with the clinician recalling the patient of interest, and often involved the clinician reviewing the patient’s EHR. Together, the clinician and interviewer(s) created a timeline of critical events in the patient’s care history. Such events included things like changes in the patient’s diagnosis, treatment, or outcomes. When creating this timeline, we also asked the clinician to describe information, actions, goals, and decision making strategies they used. Finally, we concluded the interview with a series of questions about the clinicians’ EHR and how it helps and/or hinders information use and decision making in chronic pain care.
We used a modified grounded theory approach to analyze deidentified transcripts from each interview29. Rather than focusing on inter-rater reliability, our analysis process focused on exploring differences in interpretation of the data and then reaching consensus30. First, we created a codebook by having eight researchers (five behavioral or informatics researchers, two primary care clinicians, and one pain specialist physician) review two transcripts and identify topics or themes of interest. Next, four members of the research team compiled the topics and themes into a draft codebook. The same four researchers then iteratively coded three transcripts using the draft codebook, discussing and refining the codebook between each coding. Each remaining transcript was coded individually by two researchers who met after coding and reached consensus on all codes. Once the coders judged they had reached thematic saturation, we analyzed the codes to identify key decision requirements.
Using the coded data, we extracted higher-level concepts that represented key decision requirements31. We conceptualized decision requirements as challenging decision making tasks and/or cognitive demands that clinicians encountered when managing chronic noncancer pain. First, three researchers explored the codes, both within and across categories, to identify and describe a list of key decision requirements. Next, each member of our team (behavioral researchers, informatics researchers, and clinicians) categorized each requirement according to their perception of its frequency of occurrence in practice, existence of related evidence or clinical practice guidelines, feasibility of decision support, and clinician acceptability of related decision support. Last, we compiled and discussed our categorizations to create a prioritized list and descriptions of key decision requirements for potential decision support.
Design Workshop
Based on the data collection and analysis described above, we arrived at four decision requirements related to assessing, diagnosing, and treating chronic pain. We conducted a half-day systems design workshop to refine these decision requirements, and to identify information needs and related design seeds for clinical decision support. For this study, we conceptualized information needs as specific clinical information elements that clinicians perceive as necessary to help assess, diagnose, and treat chronic pain. We conceptualized design seeds as approaches to organizing information, visually displaying information, and navigating between information elements.
Our research team of behavioral and informatics researchers, two primary care physicians, and one pain specialist participated in the workshop. We also recruited five primary care physicians from outside the research team to participate in the workshop. Four of the five physicians had previously participated in the critical decision making interview portion of the study. The participants completed written informed consent, and were compensated with a $10 gift card for their participation.
Guided by the four decision requirements, participants worked in four small groups of three to five members each. Each group included one or two non-research team clinicians and research team members from different disciplinary backgrounds. Prior to the small group work, we introduced participants to the four key decision requirements and to the concept of rapidly sketching low-fidelity decision support concepts. In their small groups, participants then sketched and described visual designs of possible EHR-based decision support features to meet the key decision requirements. Next, each small group presented their designs in a video-recorded large group session that included feedback and discussion. Finally, the small groups reconvened for additional discussion and refinements to their designs.
Following the workshop, we qualitatively analyzed the design sketches (including associated notes) and the video-recorded presentations and discussions. Two researchers jointly analyzed the designs and video recordings. The researchers coded the content for clinical information needs and design seeds. Next, the two researchers separately analyzed the coded information needs and design seeds to identify commonalities within and across codes. Finally, they merged their analysis by consensus to arrive at a final list and descriptions of information needs and decision requirements.
Results
Key Decision Requirements
In the interviews, nine participating clinicians were physicians and one was a nurse practitioner. Seven worked in federally qualified health centers affiliated with a large, urban safety net health system. Three worked in health system-affiliated practices in rural Indiana or Illinois. Participants’ practice experience ranged from two to 30 years. All participants reported receiving limited or no formal training in pain care. The analysis presented here includes three interviews from each participant, for a total of 30 interviews.
Our interview analysis identified four priority decision requirements related to to assessing, diagnosing, and treating chronic pain. First, clinicians struggle to efficiently understand current treatment plans and medications. This primarily refers to clinicians’ need and ability to quickly identify a patient’s current treatment plan, and specific medications being taken. This includes the often challenging task of obtaining other context related to treatment planning, such as logically connecting an individual treatment to a rationale for use, identifying how and why the plan has changed over time, and understanding whether a treatment is providing health benefits to the patient. Second, clinicians are challenged to identify treatment options. This primarily refers to clinicians’ need and ability to understand treatments not currently being used that may be effective in treating a patient’s particular pain condition. Similar to the first requirement, this task involves the ability to efficiently access and incorporate relevant context, such as an accurate diagnosis, an accurate history of treatments and their effects, and access to treatments based on factors such as community resources and patient insurance status. Third, clinicians struggle to manage complex cases involving physical and mental health co-morbidities. This broad requirement refers to clinicians’ need and ability to care for chronic pain conditions in patients who have co-morbid conditions. These conditions may reduce possible treatment options or increase the risks posed to patients by some treatments. Fourth, clinicians struggle to manage complex cases involving social needs. This requirement refers to clinicians’ need and ability to care for chronic pain conditions in patients who have unmet social needs, such as lack of housing or transportation. These unmet social needs may limit patients’ ability to access or adhere to some prescribed treatments.
Information Needs
Building on the identified decision requirements, our analysis of the design workshop data identified specific clinical information elements needed to support chronic pain care (Table 1). This included information that is often found in an EHR, including past and current medication information, imaging, and urine drug screen results. Information needs also extended to harder-to-access data, such as prescription drug monitoring program data, which is less often integrated within an EHR. We also identified a need for better information about specialty care, including referrals, appointments, and indication as to whether referred or scheduled visits actually occurred. We also identified social needs (e.g., information about transportation, housing, food access, and language preferences) and pain-related goals and outcomes (e.g., physical function and pain intensity) as important to clinicians. Finally, our analysis identified a need for better information about treatment options, including context that describes past and current treatments and their rationale for use as well as treatments that have not been tried. Desired treatment option information encompassed a range of treatment types, including oral and injectable medications, physical therapy, chiropractic, nutritional, acupuncture, mindfulness, and transcutaneous electrical nerve stimulation (TENS).
Table 1.
Information needs to support chronic pain treatment
| Information Needs | Description |
|---|---|
| Medications | Past and current medications relevant to pain treatment and related comorbidities |
| Imaging | Recent imaging (e.g., over the last 6 months) related to pain; organized by body part |
| Specialty utilization | Referrals to pain-related specialist; recent specialist appointments; indication of whether referrals and appointments led to actual encounters |
| Social determinants | Social determinants of health, such as insurance status, transportation options, housing, food access, and patients’ preferred language |
| Outcomes and goals | Current pain-related health outcomes (e.g., pain intensity, physical function, sleep disturbance); Patient-clinician goals for pain-related outcomes |
| Treatment options | Listing of pain treatment options (e.g., medications, physical therapy, chiropractic, transcutaneous electrical nerve stimulation, nutrition, acupuncture, mindfulness); Context describing rationale for use and discontinuation for past treatments. |
| Urine drug screen results | For patients prescribed opioids, date and results of most recent urine drug screen; interpretation to identify potential medication misuse, abuse, or diversion |
| Prescription drug monitoring database results | For patients prescribed opioids, date and report of controlled substances dispensed to patient; Interpretation of results to identify potential medication misuse, abuse, or diversion. |
Design Seeds
Building on the identified decision requirements, our analysis also identified several design seeds for clinical decision support (Table 2). Design seeds are early design ideas that represent potential decision support concepts and may guide detailed design of decision support solutions32. First, rather than being organized by information type, such as medications or results, pain-related information could be aggregated in a single view within the EHR. Second, needed information may be organized in tabular form. Third, needed information should be summarized briefly, but system functionality should support the user in drilling down for detailed information. For example, a view could summarize treatment options in a list, and provide drill-down functionality to review a history of that treatment’s use. Fourth, visual cues should be used to focus clinicians’ attention on clinically relevant changes or items of concern. For example, a list summarizing treatment options could use color, icons, or other cues to indicate which options had previously been tried.
Table 2.
Clinical decision support design seeds to support chronic pain treatment
| Design Seed | Description |
|---|---|
| Information accessible in a single EHR location | Pain-related information aggregated and organized in a single view in the EHR (e.g., a patient-level chronic pain dashboard) |
| Information organized in tables | Pain-related information organized in tables (e.g., a treatment options table or medication table) |
| Hierarchical information organization | Pain-related information summarized briefly with interactive capability to drill down for more details as required (e.g., clicking on a specialist appointment date to display a visit note, hovering over a physical function outcome score displays an outcome trend over time) |
| Visual cues to focus attention | Cues focus clinicians’ attention on relevant changes, risks, or needed action (e.g., a urine drug screen result suggesting medication misuse, an overdue check of the prescription drug monitoring report, or a missed appointment for physical therapy) |
Discussion
Our study identified and described several information needs and decision making challenges regularly faced by primary care clinicians when managing patients with chronic noncancer pain. Our study also described several design seeds that designers of EHRs or clinical decision support may consider when designing their systems. In particular, the design seeds may help to support care for conditions characterized by lengthy histories, comorbidities, multiple treatment options with different risks and benefits, and coordination with multiple health care specialties.
Our findings indicate that primary care clinicians are frequently challenged by tasks that Osheroff and colleagues refer to as “data review” or “assessment and understanding”12. To conduct these tasks thoroughly, clinicians may spend significant amounts of time “data foraging”, a term used in domains such as intelligence analysis33. Thus, data review tasks may benefit from information summaries that reduce the need to search for and integrate key data, ensure relevant data are considered, and ensure that the best actions are taken. Indeed, three of the four design seeds we identified relate to aggregating information in a single location and organizing it in formats that are cognitively manageable. In the case of chronic pain, important data to summarize include past and current treatment as well as potential future treatments. These summaries may be more valuable if they provide outcomes-related context, such as if and how past and current treatments affected health outcomes. These summaries could also be improved by contextual information related to comorbidities and unmet social needs. For example, clinicians may consider different treatments based on context that describes transportation barriers that prevent regular physical therapy visits, or context that a patient’s insurance benefits do not cover certain interventional pain treatments.
Assessment and understanding tasks refer to clinicians’ needs for reference information or knowledge resources as they assess, diagnose, and treat patients12. In the case of chronic pain, opioid prescribing rates have recently decreased, perhaps due to policy changes and increased knowledge about the risks associated with opioids26, 34. Notably, while opioid-related risk assessment did not emerge explicitly as a decision requirement or as an information need, it was clearly a driver of several identified decision requirements (e.g., identify treatment options) and information needs (e.g., urine drug screen results and prescription drug monitoring database results). However, as they reduce opioid use, primary care clinicians struggle to fully understand alternate pain treatment options that are safe and effective. Thus, decision support that offers data review and incorporates up-to-date knowledge resources, such as clinical guidelines for non-opioid pain care may also be useful. To prevent cognitively overloading clinicians, such information would need to be carefully incorporated into workflows. Along the lines of avoiding cognitive overload, designers may consider systems that organize information hierarchically, such as through visual overviews and then provide interactive functionality to drill down when details are needed.35
An overarching theme in our findings is clinicians’ desire for context related to the many clinical information elements needed for effective pain care. Clinicians’ treatment decisions may vary significantly based on context that describes things like past treatment adherence (e.g., opioid misuse), past treatment failure (e.g., no improvement in physical function), side effects (e.g., sedation from opioids), comorbidities (e.g., behavioral health conditions), or unmet social needs (e.g., food insecurity inhibiting nutrition). Unfortunately, there are barriers to efficient access and communication about these contextual factors. Details on treatment adherence, failures, and side effects are often maintained only in unstructured clinical notes, which cannot be easily reviewed during short primary care visits. Health systems are increasingly documenting and leveraging EHR-based social determinants data for decision support, but this practice is not widespread36–38. Also, key information created during behavioral health or other specialty care may be difficult to share, not exchanged between organizations, or only available in unstructured clinical note format. As a consequence, meeting information needs and effectively supporting clinical sensemaking may be difficult until data are efficiently processed, synthesized, and shared widely. Thus, policy and technology changes that incentivize data standardization and sharing may be needed to advance decision support design.
Several of the information needs we identified relate to the types of information that clinicians require to deliver guideline-based chronic pain care. For example, the Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain39 recommends regular review of urine drug screens, prescription drug monitoring databases, and patients’ functional goals and outcomes. Thus, meeting Clinicians’ information needs with decision support may also increase delivery of guideline-based care.
This study has several strengths and limitations. We focused on decision support for a prevalent and clinically challenging condition, chronic pain. Effective decision support for chronic pain care has the potential to help millions of patients, and help combat the risks of opioid misuse and abuse. Still, our results may not generalize to other conditions, especially outside of the primary care setting. That said, our findings are relevant to the broader literature examining clinical information needs, especially the literature on primary care management of chronic conditions. Similar to other analyses of primary care13, we observed that clinicians struggle to obtain high quality information and efficiently care for chronic conditions. At the same time, the need for high quality information and decision support may be uniquely critical for chronic pain given the intense scrutiny and legal and public health implications currently surrounding opioid prescribing.
Methodologically, we employed a heterogeneity sampling approach to interview clinicians from three health systems using different EHRs across urban and rural areas. We also confirmed and extended our understanding of key decision making challenges and requirements through a multidisciplinary design workshop that used an iterative design process. Still, our data collection was confined to two states and ten clinicians, thus our results may not transfer to other areas and providers. Future studies should continue to explore and refine the identified information needs and decision support requirements. Future studies should also be conducted to further understanding of the effect of decision support systems that incorporate user-centered design concepts, such as those identified here. Some of the design seeds we identified may not actually improve process or outcomes, and this should be formally tested. Biomedical informatics research would benefit from more well-designed evaluation studies that carefully measure the ability of decision support to reduce cognitive load, improve sensemaking, and subsequently impact care quality and outcomes. Our own ongoing and future studies are translating findings from this study into prototype designs and decision support implementations in EHRs of several primary care practices. We plan to study the effect of these implementations on clinical decisions and patient outcomes.
In conclusion, we identified several key decision making challenges, information needs, and design seeds that may inform decision support design and implementation for chronic pain care. By attending to these needs and requirements when designing and implementing clinical decision support, health systems may improve Clinicians’ efficiency, reduce mental workload, and positively affect patient care quality and outcomes.
References
- 1.Garg AX, Adhikari NJ, McDonald H. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA. 2005;293(10):1223–38. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobach D, Sanders GD, Bright TJ. Enabling health care decision making through clinical decision support and knowledge management. Evid Rep Technol Assess. 2012;(203):1–784. [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy EV. Clinical decision support: Effectiveness in improving quality processes and clinical outcomes and factors that may influence success. Yale J Biol Med. 2014;87(2):187–97. [PMC free article] [PubMed] [Google Scholar]
- 5.Fihn S, McDonell M, Vermes D. A computerized intervention to improve timing of outpatient follow-up. J Gen Intern Med. 1994;9(3):131–9. doi: 10.1007/BF02600026. [DOI] [PubMed] [Google Scholar]
- 6.Han YY, Carcillo JA, Venkataraman ST. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005;116(6):1506–12. doi: 10.1542/peds.2005-1287. [DOI] [PubMed] [Google Scholar]
- 7.Koppel R, Metlay JP, Cohen A. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 8.Spetz J, Keane D. Information technology implementation in a rural hospital: A cautionary tale. J Healthc Manag. 2009;54(5):337–47. [PubMed] [Google Scholar]
- 9.Littlejohns P, Wyatt JC, Garvican L. 2003. Evaluating computerised health information systems: Hard lessons still to be learnt. BMJ; p. 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wears R, Berg M. Computer technology and clinical work: Still waiting for Godot. JAMA. 2005;293(10):1261–3. doi: 10.1001/jama.293.10.1261. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Kuperman GJ, Wang S. Ten commandments for effective clinical decision support: Making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–30. doi: 10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osheroff JA TJ, Levick D, Saldana L, Velasco FT, Sittig DF, Rogers KM, Jenders RA. 2nd ed. Chicago, IL: Health Information and Management Systems Society (HIMSS); 2012. Improving outcomes with clinical decision support: An implementer’s guide. [Google Scholar]
- 13.Beasley JW, Wetterneck TB, Temte J. Information chaos in primary care: Implications for physician performance and patient safety. J Am Board Fam Med. 2011;24(6):745–51. doi: 10.3122/jabfm.2011.06.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harle CA, Listhaus A, Covarrubias CM. Overcoming barriers to implementing patient-reported outcomes in an electronic health record: A case report. J Am Med Inform Assoc. 2016;23(1):74–9. doi: 10.1093/jamia/ocv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen RE, Rothrock NE, DeWitt EM. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care. 2015;53(2):153–9. doi: 10.1097/MLR.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen-Torvik LJ, Stallings SC, Gordon AS. Design and anticipated outcomes of the eMERGE-PGx project: A multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmaco Ther. 2014;96(4):482–9. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa MF, Patel V, Charles D, Swain M, Mostashari F. Hospital electronic health information exchange grew substantially in 2008–12. Health Aff. 2013;32(8):1346–54. doi: 10.1377/hlthaff.2013.0010. [DOI] [PubMed] [Google Scholar]
- 18.Middleton B, Bloomrosen M, Dente MA. Enhancing patient safety and quality of care by improving the usability of electronic health record systems: Recommendations from AMIA. J Am Med Inform Assoc. 2013;20(e1):e2–e8. doi: 10.1136/amiajnl-2012-001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne TH, Corley S, Cullen TA. Report of the AMIA EHR-2020 task force on the status and future direction of EHRs. J Am Med Inform Assoc. 2015;22(5):1102–10. doi: 10.1093/jamia/ocv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–24. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: Results of an internet-based survey. J Pain. 11(11):1230–9. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: A national survey. South Med J. 2010;103(8):738–47. doi: 10.1097/SMJ.0b013e3181e74ede. [DOI] [PubMed] [Google Scholar]
- 23.Committee on Advancing Pain Research Care Education, Institute of Medicine. Washington (DC): The National Academies Press; 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. [PubMed] [Google Scholar]
- 24.Leverence RR, Williams RL, Potter M. Chronic non-cancer pain: A siren for primary care – A report from the primary care multiethnic network (PRIME Net). J Am Board Fam Med. 2011;24(5):551–61. doi: 10.3122/jabfm.2011.05.110030. [DOI] [PubMed] [Google Scholar]
- 25.O’Rorke JE, Chen I, Genao I, Panda M, Cykert S. Physicians’ comfort in caring for patients with chronic nonmalignant pain. Am J Med Sci. 2007;333(2):93–100. doi: 10.1097/00000441-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Schuchat A, Houry D, Guy GP., Jr New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425–6. doi: 10.1001/jama.2017.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upshur CC, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21(6):652–5. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crandall B, Klein G, Hoffman RR. Cambridge, MA: MIT Press; 2006. Working minds: A practitioner’s guide to cognitive task analysis. [Google Scholar]
- 29.Corbin J, Strauss AC. 3rd ed. Thousand Oaks, CA: Sage Publications, Inc; 2008. Basics of qualitative research: Techniques and procedures for developing grounded theory. [Google Scholar]
- 30.Barbour RS. Checklists for improving rigour in qualitative research: A case of the tail wagging the dog? BMJ. 2001;322(7294):1115. doi: 10.1136/bmj.322.7294.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Militello LG, Klein G. Decision-centered design. In: Lee JD, Kirlik A, editors. The Oxford handbook of cognitive engineering. Oxford: Oxford University Press; 2013. pp. 261–71. [Google Scholar]
- 32.Patterson E, Woods D, D T, Roth E. Los Angeles, CA: Sage Publications; 2001. Using cognitive task analysis (CTA) to seed design concepts for intelligence analysts under data overload. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. [Google Scholar]
- 33.Pirolli P, Card SK. The sensemaking process and leverage points for analyst technology as identified through cognitive task analysis. Proceedings of the International conference on intelligence analysis.2005. [Google Scholar]
- 34.Guy GP, Zhang K, Bohm MK. Vital signs: Changes in opioid prescribing in the United States, 2006-2015. MMWR. 2017;66:697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shneiderman B. IEEE Computer Society; 1996. The eyes have it: A task by data type taxonomy for information visualizations. Proceedings of the 1996 IEEE Symposium on Visual Languages; p. 336. [Google Scholar]
- 36.Gottlieb LM, Tirozzi KJ, Manchanda R, Burns AR, Sandel MT. Moving electronic medical records upstream: Incorporating social determinants of health. Am J Prev Med. 2015;48(2):215–8. doi: 10.1016/j.amepre.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Vest JR, Grannis SJ, Haut DP, Halverson PK, Menachemi N. Using structured and unstructured data to identify patients’ need for services that address the social determinants of health. Int J Med Inform. 2017;107:101–6. doi: 10.1016/j.ijmedinf.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Vest JR, Harle CA, Schleyer T. Getting from here to there: Health IT needs for population health. Am J Manag Care. 2016;22(12):827–9. [PubMed] [Google Scholar]
- 39.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–45. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]


