Abstract
Clinical informatics makes use of anatomical representation—particularly in the form of anatomical terms. But differences and ambiguities in naming anatomical structures and partitioning the body can complicate efforts to interlink anatomical resources and integrate clinical data. To better understand differences in representations of human anatomy, we compare five digital resources: a formal ontology, a terminology, and three 3D graphics applications. Because the graphics applications offer explicit representation of the boundaries and partitions of anatomical structures, they reveal the differences in modeling of anatomy that may not be apparent through text-based representations. The variations in these resources allow us to categorize differences in representations of anatomy and to highlight the importance of this topic in the context of clinical informatics.
Introduction
Communicating about human anatomy is fundamental to the delivery and documentation of clinical care. Anatomical terms are used in many contexts —discussions between care providers, narratives within medical records, and as labels for standardized codes. The precision with which these terms transmit the intended meaning is important for human-to-human communications, documentation with electronic systems, and “secondary use” of clinical data for research.
Consider these examples:
If “septal perforation” is entered into a medical record in reference to a hole in the nasal septum that separates the nasal cavities, it may later be interpreted as a hole in the septum that separates the chambers of the heart. Both entities are commonly referred to clinically as “septal perforations”, and the meaning is unclear without explicitly naming the domain (nasal vs. cardiac).
“Sinus” is commonly used as a shorthand for “paranasal sinus”, although there are other sinuses in the body.
“Oral cavity” is a common clinical term. But instead of referring to the air-filled space of the mouth (as “cavity” implies), in clinical use it refers to the internal mouth.
Resources that present knowledge of human anatomy play an essential role in medical education and mediating communications about anatomy. To better understand differences in representations of human anatomy, we compare examples of representation in a formal ontology (the Foundational Model of Anatomy) to a terminology (ICD-O) and three 3D graphics applications. These resources serve different purposes and are used in different contexts. Therefore, the variations in these resources allow us to categorize differences in representations of anatomy, provide examples that demonstrate these differences, and highlight the importance of this topic for efforts to integrate clinical data.
Background
Ontologies and terminologies play a crucial role in standardizing the representation of knowledge and data, facilitating interoperability of systems, and expressing domain-specific conceptual frameworks. Formal representation of anatomy presents challenges because the representations need to support multiple uses, and clinical conventions often do not conform to logical modeling principles (1).
Due to the variety of ontologies and terminologies used in clinical practice and research, data integration often requires that equivalent concepts within different representation schemes are identified and “mapped” to each other. Work to align representations of anatomy is based on lexical (string-based), structural (relation-based), and semantic matching techniques (2,3). Projects to produce alignments have been conducted on clinically-oriented representations of human anatomy and phenotypes (4–6) as well as between different vertebrate species (for example, (7)). This paper contributes to alignment efforts by providing examples of matches and mismatches between representations of adult human anatomy for which alignment projects have not been previously reported.
Methods
Five resources describing whole-body adult human anatomy were examined:
1. Foundational Model of Anatomy, version 4.6. The FMA is an ontology of canonical human anatomy that represents over 100,000 anatomical structures as classes. The purpose of the FMA is to provide a representation of human anatomy that will allow computers to reason about anatomy, while providing a form that is also human-readable. Each class is labeled with a preferred term, and many classes also have synonyms. In addition to the class hierarchy, the FMA encodes additional types of relationships between anatomical structures. The most common relationships are regional parts (partitions based on divisions in space) and constitutional parts (partitions based on type of substance).
2. International Classification of Diseases for Oncology, third edition. The ICD-O is used for coding the site (topography) and pathological description (morphology) of neoplasms for cancer registries. Only the topography codes were examined in this project. These codes have the format C[site].[subsite] and are accompanied by a preferred term. For some codes, the documentation provides synonyms and anatomical subdivisions to be subsumed by the code.
3. BodyParts3D, version 4.3. Segments of this 3D graphical model are annotated with terms from version 3.0 of the FMA. The online tool allows users to search, browse, create views that combine the segments, and download the models (8). Available at http://lifesciencedb.jp/bp3d/?lng=en.
4. Human Anatomy Atlas, version 7.4.01, by Visible Body. This is a downloadable 3D graphics application for medical education. Anatomical structures are organized using a single hierarchy.
5. ZygoteBody, by Zygote Media Group.This 3D graphics web application was originally known as GoogleBody. Anatomical structures are organized using a single hierarchy. Access to the hierarchy is available only through the subscription-based premium version. Available at https://www.zygotebody.com.
The process of examining the resources was performed manually by M.D.C. Work began with a list of known discrepancies based on our previous work. Examination of the ICD-O codes was biased toward head anatomy due to previous work of M.D.C and expertise of M.E.W. Over a dozen example were collected for each resource. Examples presented in this paper were selected to demonstrate categories of inconsistencies, show differences across the 3D modeling applications, and for ease of explanation. FMA version 4.9 was used in this work.
Results: Comparing ICD-O codes and FMA classes
Analysis of potential mappings between ICD-O codes and FMA classes revealed three types of issues:
1. Preferred terms and synonyms: As shown in Figure 1, there are five categories of matches and mismatches based on preferred terms and synonyms. Of particular interest are cases in which preferred terms in the ICD-O and FMA are the same, but it is a mismatch because the FMA class is an anatomical space or two-dimensional anatomical surface—which does not contain tissue and cannot be the site of a neoplasm (Figure 1, part D). The other category of mismatch occurs when an ICD-O preferred term and its synonym map to different FMA classes (Figure 1, part E).
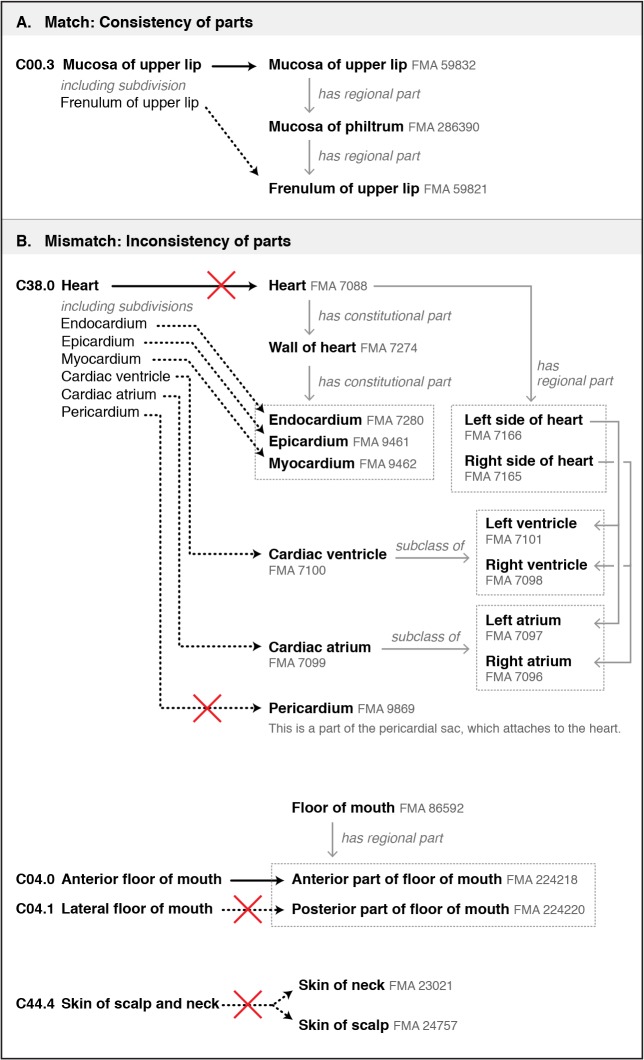
Figure 1.
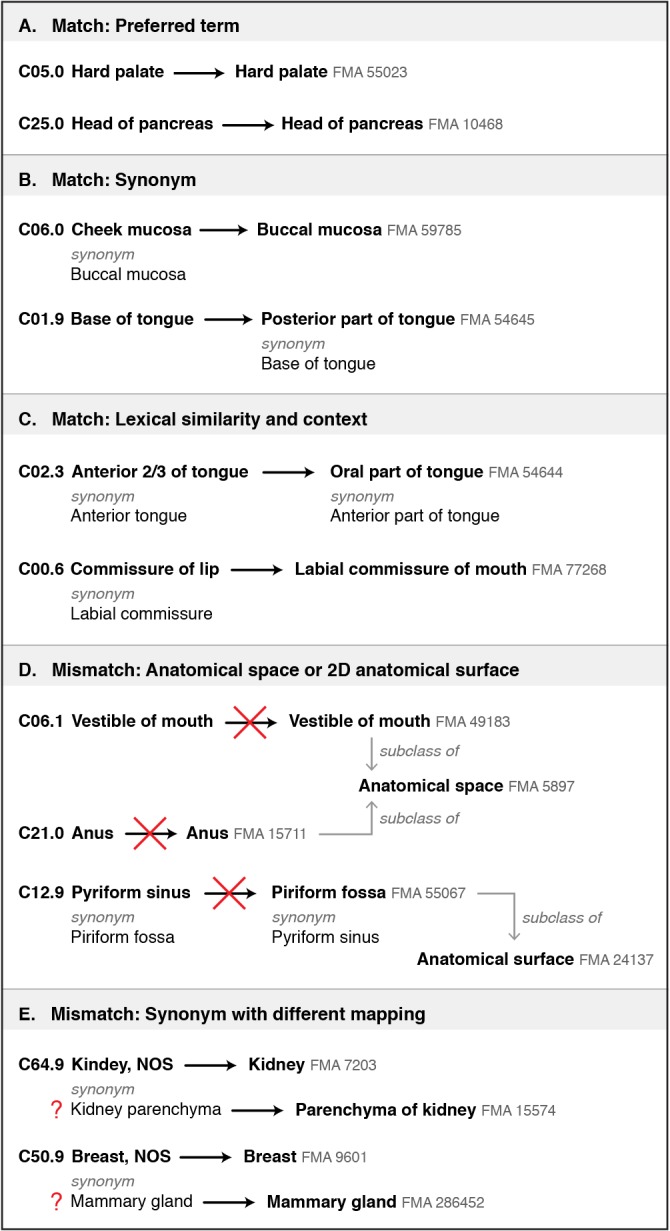
Five categories of term-based matches and mismatches found in mapping ICD-O topography codes to classes within the FMA. Preferred terms for both the ICD-O and FMA are in bold. Synonyms of preferred terms are shown when relevant. (A) Matches on preferred terms, with no apparent discrepancies. (B) Matches using synonyms, with no apparent discrepancies. (C) Matches based on lexical similarity of terms and context. (D) Mismatches resulting from matching on preferred terms. In the FMA these terms refer to anatomical spaces and 2D surfaces, not structures from which neoplasms can arise. Note that the “subclass of” (“is a”) relationships shown for the FMA classes condense several layers of the class hierarchy. (E) Examples where ICD-O preferred terms and their synonyms map to different classes within the FMA. “NOS” stands for “not otherwise specified”.
2. Different partitions of structures: Specifying the parts of an anatomical structure helps authors to communicate how they define a structure. But as Figure 2 shows, these partitions may vary among different resources. Differences may arise in the boundaries and components of a structure (Figure 2, part B; Heart) or in the way a structure is partitioned (Figure 2, part B; Floor of mouth, Skin of scalp and neck).
Figure 2.
Examples showing how partitions of anatomical structures affect mappings between ICD-O topography codes and classes within the FMA. (A) An example in which the subdivision listed for an ICD-O code is consistent with the modeling within the FMA. (B) Three examples of inconsistencies in the modeling of parts. (B, top) The structure “Heart” is defined differently in the ICD-O and FMA, with the ICD-O definition including the pericardium. In the FMA, the pericardium is modeled as part of the pericardial sac, which attaches to the heart (instead of being part of the heart). (B, middle) The ICD-O divides the floor of mouth into anterior and lateral regions, while the FMA partitions it into anterior and posterior regions. (B, bottom) The ICD-O has a single code for skin of the scalp and neck. The FMA has separate classes for skin of neck and skin of scalp. Therefore, data labeled with this ICD-O code cannot be translated to classes in the FMA (but the FMA to ICD-O translation is possible).
3. Specificity: The ICD-O codes and the FMA ontology were designed to serve different purposes, and this is reflected in the levels of specificity they offer (Figure 3).
Figure 3.
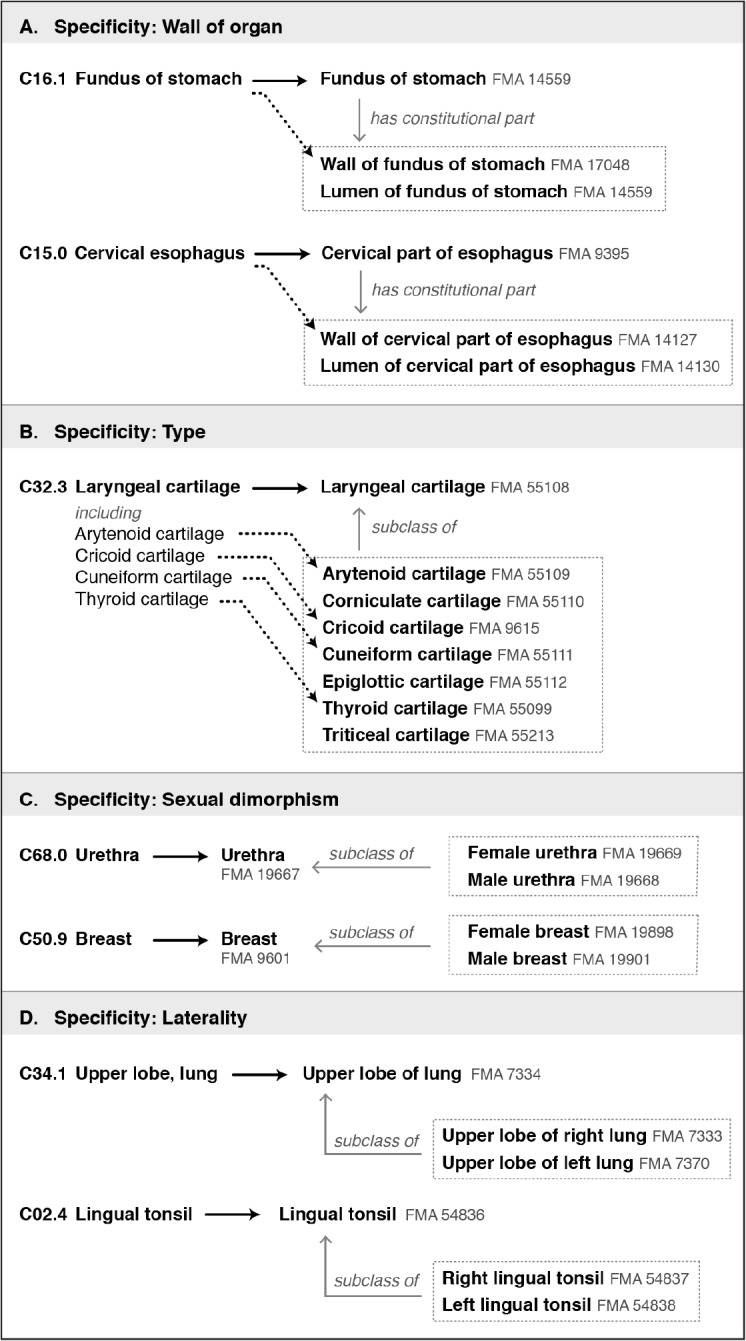
Four ways in which the FMA differs in specificity from ICD-O codes. (A) Examples of ICD-O codes that correspond to FMA classes consisting of a wall and lumen (an anatomical space). Neoplasms can arise only from the wall. (B) Several ICD-O codes refer to general types of structures, such as laryngeal cartilages. The FMA provides classes for both the type of structure and the specific structures. (C) Some structures such as the urethra and breast display significant sexual dimorphism. The FMA provides separate female and male classes for these structures. (D) For bilateral structures, the FMA provides classes for left and right. (In ICD-O this information is coded separately, allowing right, left, unilateral but unknown, and bilateral sites to be specified.)
Results: Comparing the 3D models and FMA classes
Because the authors of 3D graphical models of anatomy must segment anatomical structures and then apply labels to the segments, they offer a much more explicit representation of the boundaries and partitions of anatomical structures than terminologies and ontologies.
We compared three graphical models with the FMA. Results are shown for analysis of structures associated with the rib cage (Figure 4), bones and ligaments of the pelvis (Figure 5), gallbladder and extrahepatic bile ducts (Figure 6), and systemic arterial tree (Figure 7). This analysis highlights four issues:
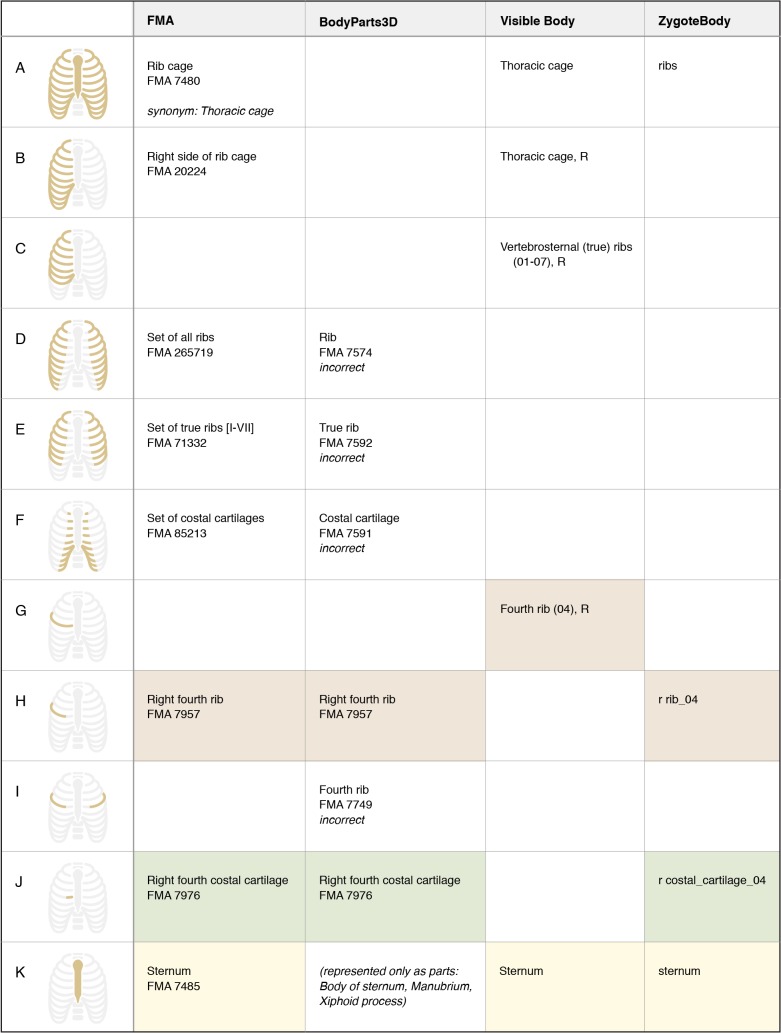
Figure 4.
Representation of structures associated with the rib cage. For FMA classes, preferred terms and FMA IDs are given. For 3D graphics resources, the label of the model is given. Colored boxes highlight terms with lexical similarity. Empty boxes indicate that the structure (or set of structures) is not represented in that resource. Notice that authors disagree on whether a rib includes costal cartilage. BodyParts3D incorrectly labels the pair of right and left fourth ribs with the FMA class “Fourth rib”.
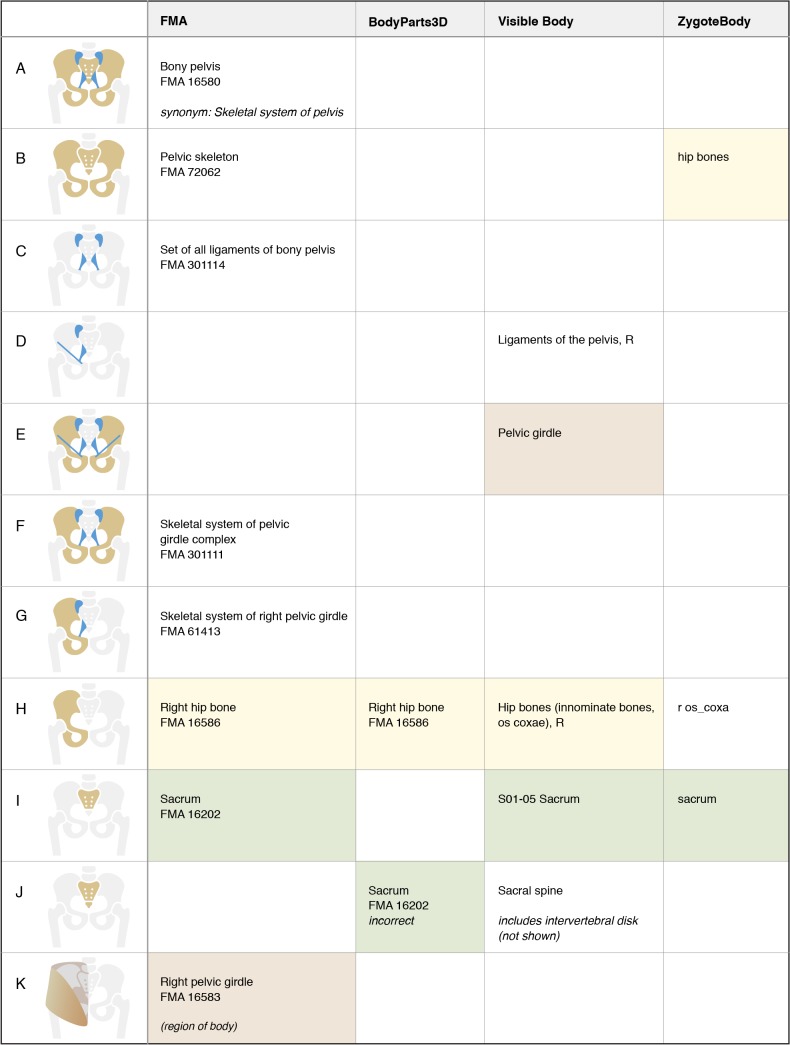
Figure 5.
Representation of structures associated with the bones and ligaments of the pelvis. As shown in the bottom row, FMA class “Right pelvic girdle” refers to the region of the body containing the “Skeletal system of right pelvic girdle”. Notice that BodyPart3D incorrectly labels the combination of sacrum and coccyx (tailbone) with the FMA class “Sacrum”.
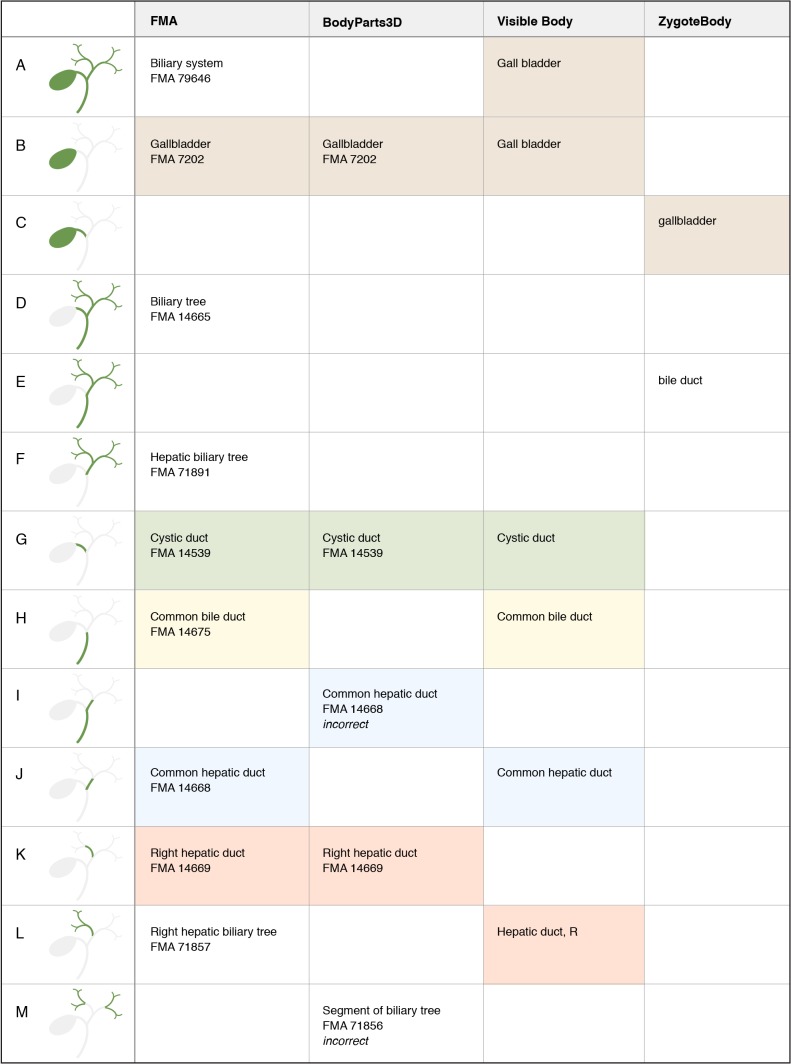
Figure 6.
Representation of structures associated with gallbladder and extrahepatic bile ducts. Notice that “Gall bladder” is applied to both a structure and its part in the Visible Body model. BodyPart3D shows incorrect segmentation for “Common hepatic duct” and uses the high-level FMA class “Segment of biliary tree” to label segments of the right and left biliary trees.
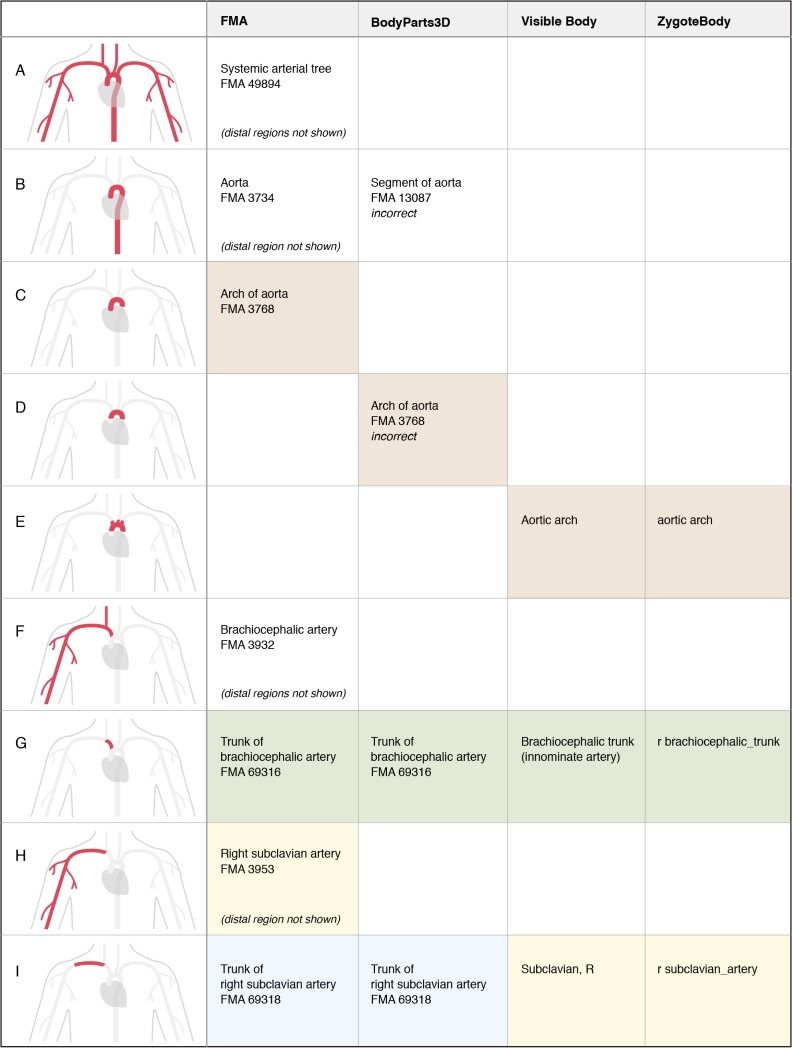
Figure 7.
Representation of structures associated with the systemic arterial tree, aorta, and right subclavian artery. Notice that the arch of aorta has three different boundaries in these representations. Authors also disagree on whether the right subclavian artery is an entire tree or only the trunk of the tree.
1. Authors often disagree on the boundaries and partitions of structures. As shown in Figure 4 (rows G and H), authors disagree on whether a rib includes the costal cartilage. The arch of aorta (or aortic arch) has been defined with three different boundaries in these resources (Figure 7, rows C, D, and E).
2. A term may have different meanings in different resources. The subclavian artery refers to the entire arterial tree in the FMA modeling scheme, but only the trunk of this tree in two of the graphical models (Figure 7, rows H and I). A term may also have different meanings within the same resource, as demonstrated with the term “gall bladder” in the Visible Body model. (Figure 6, rows A and B).
3. Different resources partition anatomy in different ways. The blank cells in Figures 4–7 indicate that a resource does not represent that partition. This likely reflects the different purposes of the resources and varying levels of development.
4. Terms from an ontology may be misapplied when labeling graphical representations. The authors of the BodyParts3D model have used labels from the FMA in an effort to standardize the representation of anatomy. However, some of their segmentation does not match the definition of the FMA class (such as the sacrum, Figure 5, row I). In other cases a partition represented in their model does not exist in the FMA, and they have selected a similar class (the pair of ribs in Figure 4, row I) or a general superclass (segments of the biliary tree, Figure 6, row M). We note in Figures 4–7 where incorrect mappings occur in our examples. We have not declared the other modeling applications to have incorrect term assignment, because we are not aware of any standard used in their development.
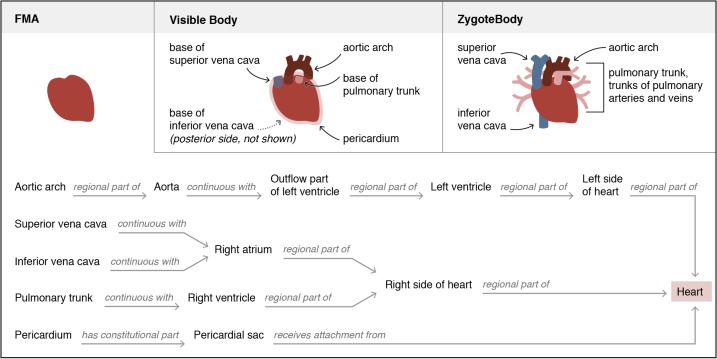
Some variation in the partitioning and labeling of the 3D graphical models may be due to authors not having formal training in human anatomy. This may explain the different representations of “heart” shown in Figure 8.
Figure 8.
Comparison of the representation of “heart” in the FMA and two 3D graphic resources. Visible Body and ZygoteBody use the term “heart” in their hierarchies to subsume the set of structures diagrammed here. In the FMA, structures such as the aortic arch and pericardium are parts of structures that have relationships to the heart (continuous with the heart, or attaching to the heart) but are not modeled as parts of the heart.
Results: Comparing organizational schemes of the FMA and 3D graphic models
Resources for anatomical knowledge provide not only the names of anatomical structures, but also schemes for organizing the structures. The ICD-O codes, Visible Body, and Zygote Body use a single hierarchy for organization, with the purpose of helping users to quickly locate the structure they are seeking. Visible Body and ZygoteBody are organized around organ systems and designed so that every structure is subsumed by one of a small number of hierarchies. This approach allows users to hide or reveal entire organ systems.
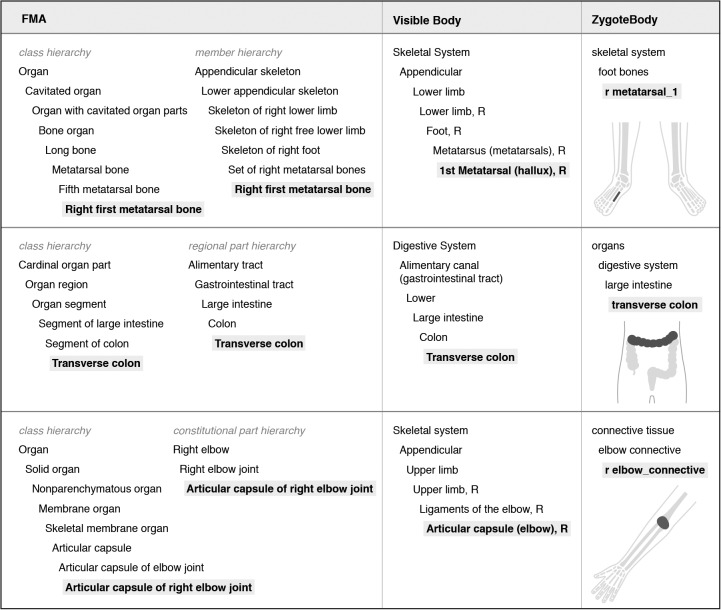
In contrast, the FMA is organized by the class hierarchy and part hierarchies (regional parts, constitutional parts, and membership within sets). The purpose of the FMA is also different—it provides a standardized representation of human anatomy that can be applied across different contexts and used for computational purposes. Examples of differences in hierarchies are shown in Figure 9 for the right first metatarsal bone, transverse colon, and articular capsule of right elbow.
Figure 9.
Comparison of class and part-based hierarchies within the FMA with the system-based approach of two 3D graphics resources, for each of the three anatomical structures graphically depicted on the right.
Conclusion
Many circumstances do not require precisely-defined anatomical modeling schemes. Face-to-face conversations accommodate imprecision by relying on context and interaction with the speaker, and clinicians communicating with others in their specialty can rely on a common understanding of their field. But for clinical informatics applications where representations must be well-defined and stable, differences and ambiguities in naming anatomical structures and partitioning the body present an obstacle to efforts to link anatomical resources and integrate clinical data.
This examples in this paper provide evidence that text-based representations of anatomy may obscure differences in how people understand and apply anatomical terms. Thus, the role of domain experts in recognizing these differences is crucial for developing applications that use anatomical terms and resources that provide modeling schemes.
Different anatomical representations may be appropriate for different tools and systems. For stand-alone tools such as 3D modeling applications, these differences are unlikely to have negative effects. But as efforts to integrate knowledge resources proceeds, our work highlights how variations in modeling will complicate accurate integration and linking of resources. Therefore, quality-assurance efforts during the development of integrated or linked resources should consider the sources of anatomical terms, their level of ambiguity, and their original context of use.
This work provides examples of FMA classes misapplied to some of the BodyPart3D models. Mismatches such as this have the potential to be propagated within resources and datasets. For example, the BodyPart3D models have been used to visualize gene expression data (9). Finally, this work demonstrates the value of graphical representations (both 3D graphical models and the stylized graphics in this paper) for making the definitions of anatomical terms salient.
References
- 1.Rector AL. Clinical terminology: Why is it so hard? Methods of Information in Medicine. 1999;38(4):239–52. [PubMed] [Google Scholar]
- 2.Zhang S, Bodenreider O. 2007. Identifying mismatches in alignments of large anatomical ontologies. In: AMIA Annual Symposium Proceedings; pp. 851–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Dragisic Z, Ivanova V, Li H, Lambrix P. Experiences from the anatomy track in the ontology alignment evaluation initiative. Journal of Biomedical Semantics. 2017 Dec;8:56. doi: 10.1186/s13326-017-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodenreider O, Songmao Z. 2006. Comparing representation of anatomy in the FMA and SNOMED CT. In: AMIA Annual Symposium Prodeedings; pp. 46–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Burgun A, Bodenreider O. 2007. Issues in integrating epidemiology and research information in oncology. In: AMIA Annual Symposium Proceedings; pp. 85–89. [PubMed] [Google Scholar]
- 6.Dhombres F, Bodenreider O. Interoperability between phenotypes in research and healthcare terminologies—Investigating partial mappings between HPO and SNOMED CT. J of Biomedical Semantics. 2016 Dec;7:3. doi: 10.1186/s13326-016-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayamizu TF, de Coronado S, Fragoso G, Sioutos N, Kadin JA, Ringwald M. The mouse-human anatomy ontology mapping project. Database. 2012 Mar 20;2012(0):bar066. doi: 10.1093/database/bar066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsuhashi N, Fujieda K, Tamura T, Kawamoto S, Takagi T, Okubo K. BodyParts3D: 3D structure database for anatomical concepts Nucleic Acids Research. 2009 Jan 1;37(Database):D782–5. doi: 10.1093/nar/gkn613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono H, Ogasawara O, Okubo K, Bono H. RefEx, a reference gene expression dataset as a web tool for the functional analysis of genes. Scientific Data. 2017 Aug 29;4:170105. doi: 10.1038/sdata.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]