Abstract
Pharmacokinetic interactions between natural products and conventional drugs can adversely impact patient outcomes. These complex interactions present unique challenges that require clear communication to researchers. We are creating a public information portal to facilitate researchers’ access to credible evidence about these interactions. As part of a user-centered design process, three types of intended researchers were surveyed: drug-drug interaction scientists, clinical pharmacists, and drug compendium editors. Of the 23 invited researchers, 17 completed the survey. The researchers suggested a number of specific requirements for a natural product-drug interaction information resource, including specific information about a given interaction, the potential to cause adverse effects, and the clinical importance. Results were used to develop user personas that provided the development team with a concise and memorable way to represent information needs of the three main researcher types and a common basis for communicating the design’s rationale.
Introduction
Concomitant use of conventional (prescription and non-prescription) drugs and natural products (NPs)—including vitamins, minerals, herbal medicinal products, and other botanicals—is a frequent occurrence. Approximately 50% of Americans in their midlife are exposed to prescription drugs simultaneously with an NP,1 raising concerns for NP-drug interactions (NPDIs). A 2012 overview of systematic reviews of herbal medicinal products identified 81 interactions between 35 of these NPs and 31 drug classes.2 Several interactions resulted in adverse outcomes, including transplant rejection, cardiovascular collapse, renal and liver toxicity, and death. Estimating the prevalence of potential NPDIs is difficult because patient use is often underreported and unstructured in health records.3,4 One study reported ~34% of patients prescribed warfarin were exposed to an NP that conferred a significant risk for an adverse interaction.5 Often, it is difficult for researchers to answer important questions about a purported NPDI, such as mechanisms and potential clinical impact, either due to a lack of evidence or to variability among evidence sources.
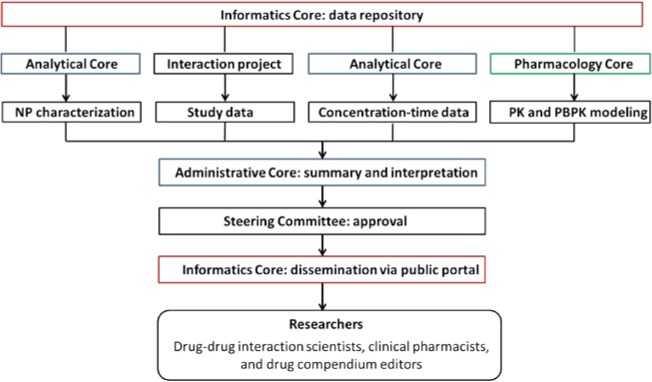
To provide evidence-based information about purported NPDIs, the National Center for Complementary and Integrative Health (NCCIH) established a new Center of Excellence for Natural Product Drug Interaction Research (NaPDI Center) (U54 AT008909). The NaPDI Center has an Administrative Core, which coordinates the efforts of three scientific Informatics (Figure 1). The Informatics Core focuses on creating a publicly accessible database where researchers can access raw data, summarized scientific results, and recommended approaches to optimally assess the clinical significance of pharmacokinetic NPDIs. Prior experience with an existing proprietary repository of human in vitro and clinical drug-drug interaction (DDI) data6 informed the initial design of a new publicly accessible database for NPDI studies. The Informatics Core works with the Analytical and Pharmacology Cores to gather, organize, and archive various types of data that are generated prospectively (Figure 1). In parallel, the Informatics Core is extending the Potential Drug-drug Interaction and Potential Drug-drug Interaction Evidence Ontology (DIDEO)7,8 to provide a semantically-rich resource from which a common set of data elements for the database can be derived.
Figure 1.
The NaPDI Center’s publicly accessible database. PK, pharmacokinetic; PBPK, physiologically-based PK
One of the NaPDI Center’s goals is to make its analytical, in vitro and in vivo (clinical) data accessible to researchers. Toward that goal, the Center is developing a public portal intended to serve a diverse group of researchers interested in assessing NPDIs. Potential users include, but are not limited to:
scientists interested in gaining mechanistic understanding of interactions between conventional medication and natural products;
clinical pharmacists interested in pharmacodynamics, pharmacokinetics, pharmacogenomics, metabolomics, etc. as they relate to drug exposure, response, and interactions; and
drug information compendium editors interested in developing narratives that summarize NPDIs for clinical audiences and for use as
The portal should provide a reliable resource where researchers can access raw data, summarized scientific results, and recommended approaches for investigating NPDIs. To meet these requirements, it is important to first understand what information researchers need to make informed decisions about these NPDIs. Specifically,
What type of information regarding NPDIs do researchers need?
How do researchers perceive the structure of existing NPDI resources?
How do researchers perceive the information available on existing NPDI resources?
Who are the hypothetical users for a NPDI database, and how will they use this database?
This conference paper describes a user information needs survey that was used to improve the understanding of these questions. Results inform the user-centered design process that we are employing for the public portal design (Figure 2).
Figure 2.
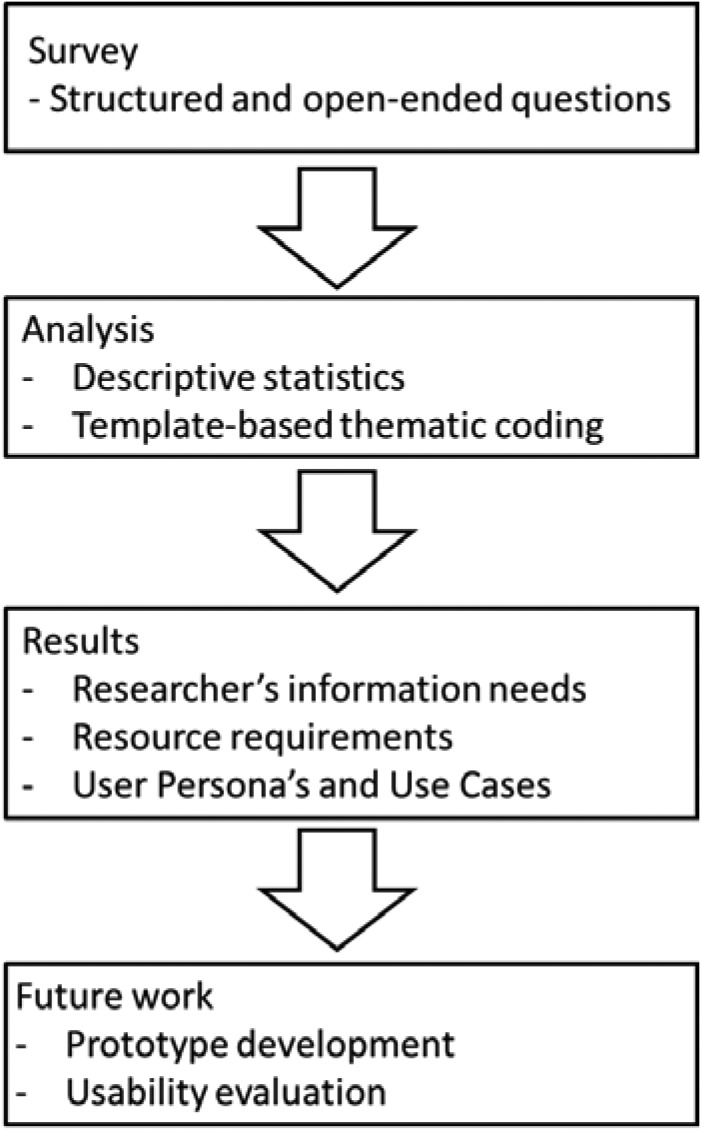
The user-centered design workflow being used to develop a publicly accessible portal for research on pharmacokinetic natural product-drug interactions.
Materials and Methods
Participants and Setting
We developed a new user-needs survey designed to gain a better understanding of researchers’ NPDI information needs and their perceptions of the resources and websites affiliated with these NPDIs. Survey design was a collaborative effort of the Informatics Core team members. Members of the Informatics Core provided input, examples, models, and literature in support of best practices for designing questionnaires when creating user personas and use cases. The initial user-needs survey was pre-tested with seven people who including researchers, clinical pharmacists, and compendia editors. The Washington State University Institutional Review Board provided expedited approval for this survey.
Survey responses were used to create general user profiles (personas) for the user-centered design of a web-based information portal for the NaPDI Center. The survey also informed navigation, site credibility, and workflow process best geared toward the generation of a positive and useful user experience. The pre-test participants were excluded from data analysis.
The final and revised survey was delivered via Qualtrics®, an internet-based research service. Research scientists, clinical pharmacists, and content editors from various organizations and universities were asked to participate in the study. This purposive sample consisted of 23 total participants (12 women and 11 men). They were selected based on their specific roles and areas of expertise to represent a diversity of needs and perspectives associated with NPDI and DDI research. Data were collected from March 20, 2017 to April 21, 2017. Participants completed the survey at venues of their choice; they were not compensated for their time. Participants took approximately 10 minutes to complete the survey consisting of 16 questions. They completed the survey anonymously but self-identified by their areas of expertise (Table 1). Participants could choose more than one area of expertise.
Table 1.
User information needs and seeking behavior questionnaire participant demographics
| Number of participants (N = 17 total) | |
|---|---|
| Area(s) of Expertise1 | |
| DDI scientist | 11 |
| Clinical pharmacist | 8 |
| Drug compendium editor | 4 |
| Bioanalytical research scientist | 3 |
| Systems analyst or content specialist | 2 |
| Physician | 1 |
| Other health care provider | 1 |
| Toxicologist | 1 |
| Public health researcher | 1 |
Because participants may have identified themselves as having multiple areas of expertise, the total number of areas of expertise was 32.
Measures and Procedure
Participants provided 11 responses regarding their knowledge of DDI resources (e.g., “In relation to these web-based resources, how unfamiliar or familiar are you with drug interaction sections in FDA product labels?”).
Responses were measured on a four-point Likert-type scale, from “very unfamiliar” to “very familiar,” coded 0 to 3, respectively. In addition to this scale, participants had a “no opinion” option.
Participants supplied 26 responses regarding perceived importance of drug interaction website capabilities (e.g., “How unimportant or important do you find news sections highlighting new publications on natural product-drug interactions?”). Responses were measured on a four-point Likert-type scale, from “very unimportant” to “very important,” coded 0 to 3, respectively. In addition to this scale, participants had a “no opinion” option.
Open-ended questions allowed participants to elaborate on their information-seeking needs and behaviors (e.g., “What information is most important when looking at potential NPDI or DDI resources?”). Data from open-ended questions were coded using the qualitative data analysis system NVivo. Two research assistants conducted open and axial coding for a total of 106 response paragraphs to identify emergent themes.9 This process included the reading and rereading of participants’ responses for familiarization, identification, and organization of the text into codes. Then, two additional research assistants verified the coding and analysis; they subsequently arranged quotes to answer the proposed research questions.
Results
A total of 23 participants received and began the survey; 17 of the 23 (73.9%) participants completed the survey in its entirety. Results include responses for the 17 completed cases. Table 1 provides frequencies for participants’ responses, in which participants were asked to “check all that apply.” Researchers self-identified with ten of 13 discrete areas of expertise. None of the participants chose the following expertise options: “analytical chemist,” “regulatory scientist,” and “none of the above.” Four participants wrote in additional areas of expertise, which included “computational chemistry,” “pharmacogenetics,” “psychiatric pharmacy” and “quantitative clinical pharmacology.” A majority of participants identified as DDI scientists, clinical pharmacists, or drug compendium editors (Table 1). As such, the data analysis contains three sections that address the research practices, information needs, resource requirements, and resource evaluations for each group.
Three themes emerged based on participants’ discussions of information needs. These themes included drug interaction information, information related to adverse interaction effects, and information concerning the clinical importance of interactions. A summary of these results and representative quotes are provided in Table 2.
Table 2.
General information needs relevant to natural product-drug interactions
| Information Need | N = 17 total | Example Quote |
|---|---|---|
| Category 1: Drug and Interaction Information | ||
| Pharmacokinetics (PK) | 13 | “Include references if a drug interaction between the natural product and the drug may also occur at the pharmacodynamic level (agonist and antagonist effect).” - DDI Scientist |
| Mechanism of action | 9 | |
| Pharmacodynamics (PD) | 5 | “For PK, we like to have area under the curve (AUC) change at therapeutic concentrations that are also connected to a PD effect/result.” - Clinical Pharmacist |
| Dosage | 5 | |
| Relevance to time | 2 | |
| Interaction role | 1 | “I like to have the name of the product used in a study and most importantly, if it is standardized to a certain amount of % of active component.” - Drug Compendium Editor |
| Drug product | 1 | |
| Category 2: Adverse Effects | ||
| Overlapping effects | 2 | “Time of onset, likelihood of overlapping effects, and potential additive nature of side effects.” - Clinical Pharmacist |
| Frequency of adverse effects | 1 | |
| Likelihood of an adverse effect | 1 | |
| Category 3: Seriousness | ||
| Clinical importance | 17 | “Do the results translate to a clinically relevant outcome?” - DDI Scientist |
General Resource and Information Evaluation
Participants were interested in resources that provide links to outside sources, with a specific focus on academic references to literature, case studies, and available data. A majority of participants noted the importance of an NPDI database being created, reviewed, monitored, and updated by DDI scientists and other credible sources. Across all groups, an NPDI database was noted as a useful and a desired tool. Participants stated that they are often frustrated with the lack of credible NPDI information available. Results suggest that perceptions of information in an NPDI database would be positively influenced through the inclusion of human data, reporting of interaction result magnitude, presence of clinical trials, and quantitative data. A summary of these results and representative quotes are provided in Table 3.
Table 3.
Researcher requirements for a natural product-drug interaction information resource
| Requirement | N = 17 total | Example Quote |
|---|---|---|
| Structure of the Resource | ||
| Links to other resources | 3 | “I’m not exactly sure what all is included in the NaPDI Recommended Approaches, but I think case reports can also be important. In my experience, the amount of information about a potential interaction involving a natural product is often limited, and therefore, any and all information can be valuable in such cases.” - Drug Compendium Editor |
| Case studies | 2 | |
| Detailed summaries | 2 | |
| Recommendations | 1 | |
| Perception of the Resource | ||
| Professional organization | 11 | “Authenticity and transparency of the websites (well established by known organizations, agencies or universities, and supervised by known experts in the field). Frequency of the website's updates of content to keep the information provided the most accurate as possible based on the present knowledge on the field.” - DDI Scientist |
| Credible | 8 | |
| Comprehensive | 6 | |
| References provided | 5 | “It’s nice to have links to references embedded within the interaction text so the reader can determine exactly which text came from a specific reference.” - Clinical Pharmacist |
| Regularly updated | 3 | |
| Perception of the Information | ||
| Inclusion of human data | 8 | “Are the results generated from in vitro, in silico, in vivo (animal model), or in vivo (human) methods?” - DDI Scientist |
| Inclusion of result magnitude | 5 | |
| Quantitative data | 2 | “Quantitative information about the interaction.” - Clinical Pharmacist |
User Personas
User Persona 1: DDI Scientist
A majority of surveyed DDI scientists were familiar with drug information search and retrieval methods. These methods included searching MEDLINE using Medical Subject Heading (MeSH) terms, guidelines from the U.S. Food and Drug Administration, European Medicines Agency, and other regulatory agencies for evaluating clinical drug interactions and methodologies for in vitro and clinical evaluation of drug interactions. When using NPDI databases, participants cited the importance of the overall clinical relevance of study results, whether or not the NPDI study results can be extrapolated to other NPs, and background information and literature on potential NPDIs. One participant noted the desire for information on the purity and accuracy of NP constituents and potential contaminants. DDI scientists requested a systematic database of NPDIs that fits into the larger drug interaction research framework. Interaction information also should contain detailed records of pharmacodynamic interactions, such as coagulation parameters and product information. The most important resource attribute noted by DDI scientists was a site’s search capability. Participants also desired links to contact an expert or related researcher, a glossary for the search, and a news section that highlights new publications on pharmacokinetic NPDIs.
PubMed was noted as the top indexed, scientific literature resource on which DDI scientists spend time. The prime subscription-based databases with drugs and NP interaction information were Lexicomp® and Micromedex®. The most cited open-access databases with interaction information were PharmGKB, ClinicalTrials.gov, and Drugs@FDA. Additionally, DDI scientists stated that they use the web resource from the NCCIH. As such, these researchers had varying knowledge of NPDIs. They also reported spending 2 to 40 hours (median 7 hours) per week researching NPDIs or DDIs, and 1 to 40 hours (median 5 hours) per week on related research websites
User Persona 2: Clinical Pharmacist
A majority of surveyed clinical pharmacists were very familiar with drug interaction sections in FDA product labels. Similar to DDI scientists, they often used drug information search and retrieval methods and were very familiar with methodologies for in vitro and clinical evaluation of drug interactions.
When using NPDI databases, quantitative clinical parameters measuring the risk/extent of NPDIs was of highest importance. In addition, clinical pharmacists were interested in the mechanistic understanding of NPDIs and the rationale and validation for the NPDI or DDI studies. As with the DDI scientists, all clinical pharmacists noted the importance of an NPDI database featuring overall clinical relevance of study results. A majority of participants with this expertise were also interested in whether or not the NPDI studies’ results with a probe drug can be extrapolated to other drugs and other NPs. Clinical pharmacists requested a database that offers case studies and information summaries. In line with DDI scientists’ needs, clinical pharmacists noted the need for interaction information with detailed records of pharmacokinetic and pharmacodynamic interactions. One clinical pharmacist cited the need for understanding consequences when NPs are affected by drugs, rather than just viewing NPs as precipitants. As with DDI scientists, the most important resource attribute noted by clinical pharmacists was a website’s search capability. Participants also desired links to contact an expert or related researcher. Unlike DDI scientists, clinical pharmacists noted the importance of a discussion section on an NPDI database.
PubMed and Google Scholar were noted as the top indexed scientific literature resources on which participants spend time. The prime subscription-based databases with drugs and NP interaction information were Lexicomp® and Micromedex®. The most cited databases with interaction information were the University of Washington’s Drug Interaction Database (DIDB), PharmGKB, Drugs@FDA, Indiana University P450 Drug Interaction Table, Natural Standard Integrated Medicine, PubChem, and NCCIH. The clinical pharmacists reported spending less time researching NPDIs than DDI scientists, but more time on websites containing clinical, research, or drug information. The clinical pharmacists reported spending 0 to 40 hours (median 2 hours) per week researching NPDIs or DDIs and 1 to 30 hours (median 4 hours) per week on related research websites
User Persona 3: Drug Compendium Editor
All of the surveyed drug compendium editors were very familiar with drug interaction sections in FDA product labels. A majority of participants were very familiar with guidelines for evaluating clinical drug interactions and methodologies for in vitro and clinical evaluation of drug interactions. A majority of participants were very familiar with the evaluation of mechanisms of action of bioactive constituents in NPs and drug information search and retrieval methods. When using NPDI databases, quantitative clinical parameters measuring the risk/extent of NPDIs were of top importance. Drug compendium editors, like clinical pharmacists, were interested in the mechanistic understanding of NPDIs and the rationale and validation for NPDI or DDI studies. The two main preferences for information resources were overall clinical relevance of study results and whether or not the NPDI study results with a probe drug could be extrapolated to other drugs. The most important resource attributes noted by drug compendium editors were a site’s search capability and acknowledgement of research sponsorship, endorsements, or funding.
All drug compendium editors surveyed reported using PubMed as their top indexed scientific literature resource and Drugs@FDA for interaction information. Drug compendium editors used interaction information to provide clinicians with DDI guidance, crosscheck information for various drugs, and to update current databases with NPDI and DDI information. Drug compendium editors reported spending 1 to 20 hours (median 2 hours) per week researching NPDIs or DDIs and 20 to 30 hours (median 30 hours) per week on websites related to interactions.
Discussion
The researchers who participated in this study reported needing detailed, systematic, and updated information regarding NPDI information. Such information includes but is not limited to the nature of the interaction (pharmacokinetic or pharmacodynamic), mechanism of the interaction, and likelihood that exposed patients will experience specific adverse effects. Participants emphasized the importance of putting NPDIs and DDIs into a larger drug interaction research framework, such as comparing new findings to existing research. Researchers from any of the three expertise areas used to create personas suggested that clinical relevance of the NPDI interaction information in a NPDI database is the most important attribute of this type of resource.
Quality and Content
Our results suggest that participants are interested in a website that offers quantitative data and detailed summaries of NPDIs. For purposes of credibility, participants noted the importance of including human data and citations to studies that support any findings. Generally, participants indicated interest in the full range of relevant study information, including whether or not study results can be extrapolated to other NPs or other drugs. Additional information needs include background information of the NP and its constituents, such as clinical use and sales data. Participants also were interested in the chemical structure of and physicochemical information of NP constituents.
User Personas
User personas have been a useful tool in user-centered design since Alan Cooper reported their use in goal-directed design nearly 20 years ago.10 They form an important complement to other user-centered activities such as contextual inquiry and think-aloud usability studies.11 Personas are a useful tool to focus project development on the needs of hypothetical users. Defining potential users by their characteristics, potential behaviors, and potential experiences through persona development provides a focused approach in concept development. Successful use of user-centered design with personas include personal health record tools, developing an electronic care plan for individuals with chronic kidney disease, informing the design of a communicable disease information systems to improve public health.12-14
Their importance to the NaPDI Center’s public portal and data repository is two-fold: 1) provide a concise and memorable way to represent distinct aspects of the three main researcher types, and 2) provide a common basis for communicating the rationale for the portal design. The three personas we created are grounded in the data collected from the survey and show important differences between the researcher types across the categories of work tasks, information needs, and information seeking behavior. Each persona will serve as a conduit of information about a specific user type that we will modify as the user-centered design process advances. We will define the initial user experience design for the portal by reflecting on the personas and related information needs. For example, the user experience should include an advanced search page that provides a simple interface for finding studies and experiments information based on the information needs reported in each user personas (Tables 3-5).
Table 4.
User Persona 1 - Jessica, DDI Scientist
| Tasks | Jessica is a DDI scientist who spends 24 hours per week researching NPDIs or DDIs and 8 hours per week on websites containing clinical, research, or drug information. |
| Information Needs | Jessica selects three areas of interaction information in a pharmacokinetic NPDI database that she considers to be very important. These included quantitative clinical parameters measuring the risk/extent of NPDIs and DDIs, mechanistic understanding of NPDIs and DDIs, and the rationale and validation for the NPDI or DDI studies. Jessica selects five categories of important background information to include in a pharmacokinetic NPDI database: (1) background information of the NP and its constituents, such as clinical use and sales data; (2) chemical structure of and physicochemical information on NP constituents, such as molecular weight or log P value; (3) whether or not the NPDI study results can be extrapolated to other NPs; (4) overall clinical relevance; and (5) evaluation framework information. |
| Information Seeking | Jessica states that she currently uses information from interaction websites as a guide point. She is cautious about pharmacokinetic NPDI information because “much of the data reported is often fraught with methodological flaws.” Jessica states that there are issues with analyzing pharmacokinetic NPDIs that make the interactions more difficult to research than DDIs. As such, Jessica is interested in the study design of research in the database, including whether or not interaction results are generated from in vitro, in silico, or in vivo (animal model or human) methods. |
Table 5:
User Persona 2 - Carl, Clinical Pharmacist
| Tasks | Carl is a clinical pharmacist who spends 2-3 hours per week researching NPDIs or DDIs and one hour per week on related websites. |
| Information Needs | Carl selects four areas of interaction information in a pharmacokinetic NPDI database that he sees as highly important. These include the quantitative clinical parameters measuring the risk/extent of NPDIs, mechanistic understanding of NPDIs and DDIs, the bioanalytical methodology used for NPDI studies, and the rationale and validation for the NPDI or DDI studies. Carl also requests information pertaining to the “overlapping physiologic reactions with the target patient population.” |
| Carl selects four categories of important background information to include in a NPDI database: (1) whether or not the NPDI study results can be extrapolated to other NPs, (2) whether or not the NPDI study results with a probe drug can be extrapolated to other drugs, (3) overall clinical relevance, and (4) documentation. | |
| Information Seeking | Carl would like a NPDI database to include a search option, search glossary, and news sections highlighting new publications on NPDIs. Carl believes the ideal website would allow for him to analyze if a certain NP will interact with a patient's current or prospective drugs or medical condition. Carl states that the level of risk and clinical relevance are important factors to consider. He notes the prominence of quality research that addresses problems with NPs, such as “the presence of multiple ingredients in the products, and concern for the lack of GMP quality of these products or adulteration with prescription medications or other supplements.” |
Related Data Repositories
A variety of NPDI reviews have been published in the scientific literature.2,15,16 The Natural MedicinesTM database17, developed by the Therapeutic Research Center, claims to provide comprehensive information regarding NPs including uses in treating specific conditions and comparative effectiveness. The database provides a screening tool for possible NPDIs that is oriented to clinicians and does not provide detailed data about the original studies. The Global Natural Products Social Molecular Networking web site18 provides the Mass Spectrometry Interactive Virtual Environment (MassIVE) data repository to enable researchers to analyze and compare Mass Spectrometry datasets. The National Cancer Institute’s Office of Cancer Complementary and Alternative Medicine (OCCAM), whose primary focus on cancer outcomes, provides descriptions called Physician Data Query (PDQ®) on topics that include complementary and alternative medicine.19 Some of the PDQs provide general information on NPDIs written for a clinical or patient audience.
Limitations
The present study has limitations. The first and perhaps most important limitation is the small, convenience sample. Additionally, qualitative data analysis is subject to data interpretation bias. To help ameliorate this bias, data in the present study were independently analyzed and verified by two groups of research assistants. When discrepancies in coding occurred, they were discussed and re-coded based on group consensus.
Conclusion
Americans frequently combine conventional drugs with NPs. As such, there is a need for researchers to have access to credible research and information regarding the risk or safety of NPDIs. The present study analyzed the opinions and information needs of three types of NPDI researchers: DDI scientists, clinical pharmacists, and drug compendium editors. Results will inform the user-centered design of a public information portal that will provide raw data, summarized scientific results, and recommended approaches for the rigorous study of pharmacokinetic NPDIs. This information will facilitate researchers’ access for evidence-based decisions regarding these NPDIs.
Acknowledgement
This research was funded in part by the National Center for Complementary and Integrative Health under grant U54 AT008909.
Figures & Table
Table 6.
User Persona 3 - Joe, Drug Compendium Editor
| Tasks | Joe is a drug compendium editor who rarely conducts research on NPDIs or DDIs, but he spends an average of 30 hours per week on websites containing clinical, research, or drug information. Joe typically uses these resources to characterize drug or NP properties to support and extrapolate DDIs. |
| Information Needs | Joe selects four areas of interaction information in an NPDI database as very important: quantitative clinical parameters measuring the risk/extent of pharmacokinetic NPDIs, mechanistic understanding of NPDIs and DDIs, the bioanalytical methodology used in NPDI studies, bioanalytical characterization of NPs, and the rationale and validation for the NPDI or DDI studies. Additionally, Joe stated, “I like to have the name of the NP used in a study and most importantly, if it is standardized to a certain amount or % of active component.” |
| Joe selects five categories of important background information to include in an NPDI database: (1) whether or not the NPDI study results can be extrapolated to other NPs, (2) whether or not the NPDI study results with a probe drug can be extrapolated to other drugs, (3) overall clinical relevance, (4) an evaluative framework, and (5) documentation. | |
| Information Seeking | Joe believes an incisive data source would include percent change in pharmacokinetic parameters, clinical relevance (e.g., loss of effectiveness, increase in adverse effects), and the mechanism of the effect. Joe desires database attributes that include search capability, recent news, and documentation of funding. |
References
- 1.Kiefer DS, Chase JC, Love GD, Barrett BP. The Overlap of Dietary Supplement and Pharmaceutical Use in the MIDUS National Study. Evid Based Complement Alternat Med. 2014;2014:823853. doi: 10.1155/2014/823853. doi:10.1155/2014/823853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol. 2013;75(3):603–618. doi: 10.1111/j.1365-2125.2012.04350.x. doi:10.1111/j.1365-2125.2012.04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarton LAA, Zeng Q, Bray BE, Shane-McWhorter L. Feasibility and potential benefit of collecting Complementary and Alternative Medicine data through a computerized patient interview. AMIA Annu Symp Proc. 2011;2011:1217–1223. [PMC free article] [PubMed] [Google Scholar]
- 4.Archer M, Proulx J, Shane-McWhorter L, Bray BE, Zeng-Treitler Q. Development of an Alert System to Detect Drug Interactions with Herbal Supplements using Medical Record Data. AMIA Annu Symp Proc. 2014;2014:249–255. [PMC free article] [PubMed] [Google Scholar]
- 5.Leung VWY, Shalansky SJ, Lo MK, Jadusingh EA. Prevalence of use and the risk of adverse effects associated with complementary and alternative medicine in a cohort of patients receiving warfarin. Ann Pharmacother. 2009;43(5):875–881. doi: 10.1345/aph.1L631. doi: 10.1345/aph. 1L631. [DOI] [PubMed] [Google Scholar]
- 6.Hachad H, Ragueneau-Majlessi I, Levy RH. A useful tool for drug interaction evaluation: The University of Washington Metabolism and Transport Drug Interaction Database. Hum Genomics. 2010;5(1):61–72. doi: 10.1186/1479-7364-5-1-61. doi: 10.1186/1479-7364-5-1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judkins J, Tay-Sontheimer J, Boyce RD, Brochhausen M. Extending the DIDEO ontology to include entities from the natural product drug interaction domain of discourse. J Biomed Semantics. 2018;9(1):15. doi: 10.1186/s13326-018-0183-z. doi:10.1186/s13326-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochhausen M, Boyce R. DIDEO/DIDEO: The Potential Drug-drug Interaction and Potential Drug-drug Interaction Evidence Ontology (DIDEO) https://github.com/DIDEO/DIDEO. Published 2017. Accessed February 25, 2017.
- 9.Virginia Braun, Victoria Clarke. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77. [Google Scholar]
- 10.Cooper A. Indianapolis, IN: Sams - Pearson Education; 2004. The Inmates Are Running the Asylum: Why High Tech Products Drive Us Crazy and How to Restore the Sanity. 1 edition. [Google Scholar]
- 11.Pruitt J, Grudin J. Personas: Practice and Theory. Microsoft Research. 2017. Jan, https://www.microsoft.com/en-us/research/publication/personas-practice-theory/. Accessed February 28, 2018.
- 12.Huh J, Kwon BC, Kim S-H, et al. Personas in online health communities. J Biomed Inform. 2016;63:212–225. doi: 10.1016/j.jbi.2016.08.019. doi:10.1016/j.jbi.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.“NIDDKD.” Development of an Electronic CKD Care Plan. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/communication-programs/nkdep/working-groups/health-information-technology/development-electronic-ckd-care-plan. Published 2018. Accessed June 29, 2018.
- 14.Turner AM, Reeder B, Ramey J. Scenarios, personas and user stories from design ethnography: Evidence-based design representations of communicable disease investigations. J Biomed Inform. 2013;46(4):575–584. doi: 10.1016/j.jbi.2013.04.006. doi:10.1016/j.jbi.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutt A, Girard L, Necyk C, et al. Natural health product-drug interaction tool: A scoping review. Can Pharm J (Ott) 2016;149(2):75–82. doi: 10.1177/1715163516629156. doi:10.1177/1715163516629156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher GN, Corbett AH, Hawke RL. Common Herbal Dietary Supplement-Drug Interactions. Am Fam Physician. 2017;96(2):101–107. [PubMed] [Google Scholar]
- 17.“Therapeutic Research Faculty.”. About Natural Medicines Comprehensive Database: Natural Medicines Comprehensive Database. http://naturaldatabase.therapeuticresearch.com/Content.aspx?cs=&s=ND&page=aboutdbhtml&xsl=generic#checker. Published 2018. Accessed June 29, 2018.
- 18.“GNPS.” GNPS - The Future of Natural Products Research and Mass Spectrometry. https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp. Published 2018. Accessed June 29, 2018.
- 19.“National Cancer Institute.”. CAM Therapies: A-Z. https://cam.cancer.gov/health_information/cam_therapies_a-z.htm. Published 2018. Accessed June 29, 2018.