Abstract
This study investigates the safety and efficacy of a large-dose, needle-based epidural technique in obstetric anesthesia. The technique differs from a standard, catheter-based approach in that the anesthetic dose is administered through an epidural needle prior to insertion of the epidural catheter. Using a data-driven informatics and machine learning approach, our findings show that the needle-based technique is faster and more dose-effective in achieving sensory level. We also find that injecting large doses in the epidural space through the epidural needle is safe, with complication rates similar to those reported in published literature for catheter-based technique. Further, machine learning reveals that if the needle dose is kept under 18 ml, the resulting hypotension rate will be significantly lower than published results. The machine learning framework can predict the incidence of hypotension with 85% accuracy. The findings from this investigation facilitate delivery improvement and establish an improved clinical practice guideline for training and for dissemination of safe practice.
1. Introduction
The potential consequences of failed or misplaced epidural needles are well known to obstetric anesthesiologists. A well-documented epidural complication, a “wet tap,” results in a headache and possible total spinal anesthesia/block, requiring immediate maintenance of the patient’s airway and blood pressure. The inadvertent intravenous injection of local anesthetic into a vein in the epidural space leads to seizures and fatal cardiac arrhythmias. Equally worrisome is the inadequate epidural block leading to complications during a caesarian section. These complications include an emergency general anesthetic, resulting in airway loss, hypoxemia, hypercarbia and death1-3. However, to date, limited research has been performed regarding standardization of the epidural anesthesia procedure to avoid practice variance with minimal complications.
Traditionally, the epidural catheter is placed, aspirated, and a test dose of medication is given to detect the possibility of an intravascular (IV) or intrathecal (IT) catheter prior to administering additional doses of local anesthetic and opioids. More rapid injection is often possible through the epidural needle given the relatively larger gauge and shorter length compared to a catheter,4 which could potentially enhance the spread of medication within the epidural space. However, there have been very few studies in which anesthesia providers have initiated labor analgesia by injecting medications through the epidural needle immediately after loss of resistance in order to achieve faster onset of pain relief.5 The rationale for potentially improved analgesia onset with epidural needle injection is uncertain. In addition to faster onset of analgesia, it has been reported that dosing through the epidural needle may result in improved quality of epidural anesthesia compared to dosing through the catheter.6 However, other investigations in obstetric7 and non-obstetric8 patients receiving epidural anesthesia have observed similar onset and quality of surgical anesthesia as well as similar level of sensory blockade when dosing through the needle versus the catheter. In a small double-blinded prospective investigation (n=60), Ristev et al. 20179 directly compared needle and catheter injection of epidural medications for the initiation of labor analgesia. Their results observed that epidural needle and catheter injection of medications result in similar onset of analgesia and sensory blockade, quality of labor analgesia, patient satisfaction, and complication rates. To date little is known regarding practice and patient outcome related to large doses of local anesthetic injected through the epidural needle.
In this paper, we perform an in-depth study of epidural process to capture practice variance and quantify the time and dose required to achieve the desired sensory level. In particular, using a data-driven and machine learning approach, we establish a safe and quickly effective epidural dose that can be administered through the epidural needle prior to the insertion of the epidural catheter. Based on clinical results, we quantify complications for doses as large as 20 ml that is injected through the epidural needle. We contrast the proficiency of physician practice and provide insights on their preference in medication and dosage. Understanding the causes and effects of such variation can help providers avoid practices that negatively impact outcomes. The machine learning analysis reveals practice characteristics that result in the best outcome with the least complications. Our findings facilitate establishment of improved clinical practice guidelines (CPG) for care outcome and delivery improvement.
2. Materials and Methods
This study employs a broad range of informatics techniques to facilitate improvement in patient care. It aims to capture practice variance, quantify dose-sensory achievement characteristics, and evaluate the safety and utility of injecting large doses (up to 20 ml) in the epidural space through the epidural needle for elective caesarian sections in the hands of experienced anesthesiologists at a large urban obstetric hospital. The study involves five major steps: 1) Develop process maps of patient and epidural service workflow via objective process observations and structured interviews. 2) Perform time-motion studies of epidural processes, record complications and practice variance, and analyze hospital data. 3) Perform statistical analysis of collected data, conduct system analysis on practice variance, quantify effective dose-sensory level achievement, 4) Develop a machine-learning predictive analytic model to predict patient/outcome characteristics. 5) Develop a computerized simulation-optimization system to simulate current performance, optimize systems and estimate anticipated global improvement. 6) Report findings and establish practice guideline recommendations for improved quality of care.
Epidural Workflow and Services
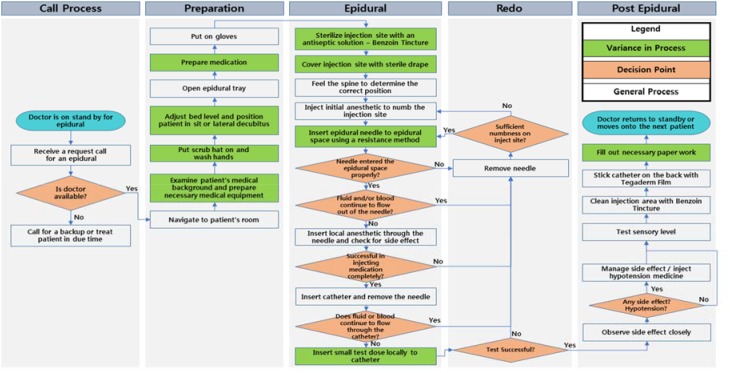
Figure 1 summarizes the epidural process performed by anesthesiologists observed by medical informaticians and engineers. The green denotes practice variance that we observed. Anesthesiologists choose one of three basic techniques of loss of resistance (or a combination thereof) to identify the proper epidural space: air, saline and local anesthetic. Medication dosages vary by providers with most injecting as much as 20 ml through the epidural needle prior to inserting the epidural catheter.
Figure 1.
This figure summarizes the anesthesiologist epidural procedure workflow process. Green highlights those processes with variance among providers.
Observations, Time-Motion Studies, and Chart Review
From January 2014 through December 2014, eight trained observers collected epidural process data via a standardized checklist through shadowing of the epidural team. Data collected includes patient demographics, vital signs, medication type and dosage, time to achieve sensory level, outcomes and response to medication. The observers simultaneously conducted time-motion studies, and measured service time for each step of the epidural workflow. Variability of practitioners and processes were also captured. In addition to observation, a random sample of clinical charts was gathered and reviewed to serve as a validation set for our machine learning and system simulationoptimization analysis. Two types of epidural approaches were defined based upon the primary delivery mechanism of the majority dose. If the majority of the dose is delivered through a needle, it is defined as a needle-based approach. Likewise, a catheter-based approach delivers the primary dose through a catheter.
Statistical Analysis
Statistical analysis was conducted to quantify variations and their associated outcome. Specifically, variations on delivery types, complications caused by delivery types, time to sensory level, medications and dosage, epidural approaches (needle-based versus catheter-based), and practitioner performance were noted.
Statistical analyses were performed using MATLAB10. Statistical significance was assessed at the 0.05 level unless otherwise noted. Descriptive statistics were calculated for all variables of interest including median and 25th to 75th percentiles, and counts and percentages when appropriate. Two-sample t-test and Wilcoxon rank-sum tests were used to compare continuous variables between groups and Chi-square tests were used for comparing categorical variables between groups.
Next, a machine learning predictive framework was designed to uncover key factors that influence and predict hypotension. Specifically, we used discriminant analysis via mixed integer program (DAMIP) as our classifier11, 12 and contrasted its performance to other popular classification methods.
Machine-Learning Predictive Analytic Framework: Discriminant Analysis via Mixed Integer Program (DAMIP)
Suppose we have n entities from K groups with m features. Let = {1, 2, …, K} be the group index set, = {1, 2, … n} be the entity index set, and = {1, 2, …, m} be the feature index set. Also, let k, k ∈ and k ⊆ , be the entity set which belong to group k. Moreover, let j, j ∈ , be the domain of feature j, which could be the space of real, integer, or binary values. The ith entity, i ∈ , is represented as (yi, xi) = {yi, xi1, …, xim) ∈ × 1 × … × m, where yi is the group to which entity i belongs, and (xi1, …, xim) is the feature vector of entity i. The classification model finds a function f: (1 × … × m) → to classify entities into groups based on a selected set of features.
Let πk be the prior probability of group k and fk (x) be the conditional probability density function for the entity x ∈ ℝm of group k, k ∈ . Also let αhk ∈ (0,1), h, k ∈ , h ≠ k, be the upperbound for the misclassification percentage that group h entities are misclassified into group k. DAMIP seeks a partition {P0, P1, …, PK} of ℝRK, where Pk, k ∈ , is the region for group k, and P0 is the reserved judgement region with entities for which group assignment are reserved (for potential further exploration).
Let uki be a 0/1 variable to denote if entity i is classified to group k or not. Mathematically, DAMIP11, 13-15 can be formulated as
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
DAMIP has many appealing characteristics including: 1) the resulting classification rule is strongly universally consistent, given that the Bayes optimal rule for classification is known16, 17, 2) the misclassification rates using the DAMIP method are consistently lower than other classification approaches in both simulated data and real-world data; 3) the DAMIP classifiers appear to be insensitive to the choice of prior probabilities, yet capable of reducing misclassification rates when the number of training entities from each group is different; 4) the DAMIP model generates robust classification rules on imbalanced data, regardless of the proportions of training entities from each group12, 16, 18.
In this study, the entities correspond to the patients. The features are patient demographics, health conditions and clinical history, epidural workflow (processes, medication, and dosage), and provider experience and delivery characteristics. The goal is to undercover discriminatory features that can predict which patients will have a higher likelihood for complications. Ten-fold cross-validation is performed on the training set to obtain an unbiased estimate. To gauge the predictive power of the rule, we perform blind prediction on an independent set of subjects. We compared the performance of DAMIP against well-known classifiers: classification tree, logistic regression, Naive Bayes, random forest k-nearest neighbors, and support vector machine.
Development of a Computerized Simulation-Optimization System
A computer simulation-optimization model was then established as a framework for modeling and optimizing the entire epidural workflow. This allows for development of improved CPGs. Parameters in the simulation include the entire epidural workflow as shown in Figure 1. The model captures delivery characteristics, service time, types and probabilities for each provider; response, risk factors, and outcome characteristics (including complication) of each patient; and overall throughput of processes. The model was fitted using the data collected from our time-motion studies and observations to simulate the annual hospital patient visits and treatment performance. The computer simulation model captures practice variations statistically, and allows us to investigate improvement strategies. We first fine-tuned the model to reflect the hospital regular performance. Then using the validation set from chart review, we further fine-tuned and cross-validated the accuracy of our model. The system was then optimized to identify areas of improvement.
3. Results
Northside Hospital delivers the highest number of newborns in the United States (the Centers for Medicare & Medicaid Services). During the study period, 19,651 deliveries were performed with 55.3% vaginal birth and 44.7% C-section. Among these, 75.1% received epidural labor analgesia (epidural anesthesia for C-section and epidural analgesia for vaginal birth). A total 750 parturition cases under routine epidural analgesia were observed in full detail. This included 667 C-section, 76 vaginal birth, and 7 unlabeled cases. Majority of them (94%) were performed with patients in the sitting position. The observations covered 44 anesthesiologists.
The two groups of patients have similar distributions in weight (p < 0.3138), height (p < 0.5784), and weeks of pregnancy (p < 0.3082). The occurrence of allergies was similar (C-section group: 13.75%, vaginal birth group: 16.44%). 73.04% of the C-section patients had previous deliveries. And 29.41% of the vaginal deliveries were primigravidas. The systolic blood pressure of C-section and vaginal birth patients was similar (p < 0.5700). However, the diastolic pressure of vaginal birth patients (73.0714 mmHg) was lower than that of the C-section patients (78.0315 mmHg) (p < 0.0074).
We selected an independent set of patients through chart review for validation of findings. This consisted of 1,398 cases that used epidural procedures, which included 892 C-section, 505 vaginal births, and 1 unlabeled case. This represented roughly 10% of the newborns delivered between January to September 2015.
Vaginal Birth and C-section
Age: It is well-documented that advancing maternal aged women are more likely to have cesarean delivery without labor.19 The hospital data echoed this trend. The average age for the C-section group was 32.72 versus 30.77 of the vaginal birth group (p-value < 0.0089). The age range for vaginal birth and C-section groups are 18 – 39, and 17 – 49, respectively.
Medication: More than 40 combinations of medication are used. In vaginal birth, the most common medicine combinations are: 1. Lidocaine 2%, NaHCO3, Epinephrine, Fentanyl (15.58%), 2. Lidocaine 1.5%, Ropivacaine 0.5%, Epinephrine (14.29%), 3. Lidocaine 2%, NaHCO3, Epinephrine (11.69%), 4. Ropivacaine 0.5% (11.69%), 5. Lidocaine 2%, Epinephrine (10.39%). In C-section, they are: 1. Lidocaine 2%, NaHCO3, Epinephrine, Fentanyl (28.98%), 2. Lidocaine 2%, NaHCO3, Epinephrine (20.23%), 3. Lidocaine 2%, Epinephrine, Fentanyl (8.90%), 4. Lidocaine 2%, Epinephrine (8.61%), 5. Marcaine 0.5%, Fentanyl (5.74%). Ropivacaine is used for vaginal birth patients since Ropivacaine is less lipophilic than bupivacaine and less likely to penetrate large myelinated motor fibers, resulting in a relatively reduced motor blockade. It is preferred in vaginal birth where motor blockage is undesirable.20
Sensory level achievement: Sensory level is an important indicator to measure the effect of anesthesia. A higher than desired anesthesia level (high block) can cause motor block, dyspnea, apnea and even loss of consciousness. Both groups achieve similar sensory level, with T4 (vaginal: 61.02%, C-section: 61.07%) and T6 (vaginal: 23.73%, C-section: 22.29%) being the most frequently achieved. However, the average time it takes to achieve sensory level between the vaginal birth and the C-section groups is significantly different (13.51 minutes versus 15.95 minutes, p < 0.0126).
Complications: Our observations identified six complication symptoms: hypotension, epidural replacement, wet tap, blood in the catheter/needle, high block, and nausea and vomiting. Only two of these, hypotension (11.84%) and blood in the catheter/needle (2.63%), were observed in the vaginal birth group. For C-section group, we observed the following incidence percentage: hypertension 52.32%, blood in the catheter/needle 2.10%, epidural replaced 3.60%, wet tap 0.15%, high block 0.30% and nausea/vomit 0.45%. Since hypotension was the most common complication, we present detailed analysis on hypotension in a dedicated section.
Needle-based vs Catheter-based Approach
We seek to quantify effective dose to achieve desired sensory level and evaluate the safety and utility of injecting large doses (up to 20 ml) in the epidural space through the epidural needle.
Among the 750 observed cases, 717 cases (95.6%) were needle-based and 33 cases (4.4%) were catheter-based. In the needle-based approach, in almost all cases over 90% of the dose was delivered through the needle; whereas for the catheter-based approach, an average of 60% of dose was delivered via the catheter.
Sensory level achievement: Needle-based approach achieved higher sensory level (average T4.79) than catheter-based approach (average T5.3) (p < 0.0265). Figure 2 shows that the most frequent sensory levels were T4 (needle-based: 62.35%, catheter-based 36.36%) and T6 (needle-based: 21.80%, catheter based: 30.30%).
Figure 2:
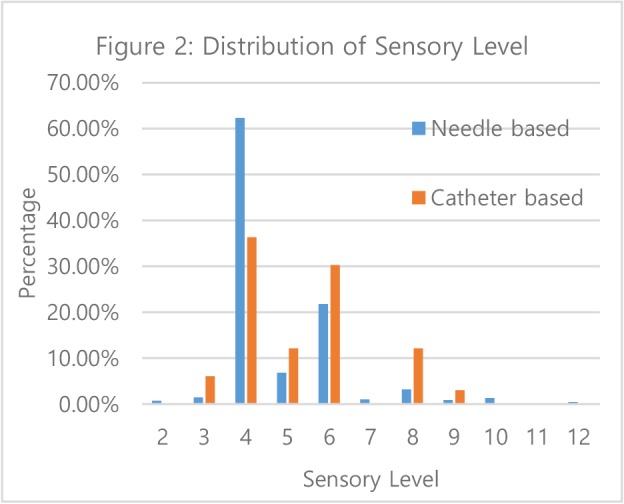
Distribution of Sensory Level
Time to achieve: The average time to achieve similar sensory level (p < 0.7789) in needle-based approach was 15.63 minutes versus 20.00 minutes for catheter-based (p < 0.0037). Specifically the time difference is significant for both vaginal birth and C-section deliveries: 13.02 minutes vs 26.00 minutes for vaginal birth, p < 0.0181, and 15.80 minutes vs 18.97 minutes for C-section deliveries, p < 0.0390. Hence, this study reports that needle-based approach is faster and more effective in achieving the required sensory result.
Furthermore, the needle-based approach uses less dose than catheter-based approach (mean 24.99 ml versus 30.27 ml, p < 6.0e-05). These findings support that needle-based approach is more dose-effective, achieving better sensory level faster than the traditional catheter-based approach.
Complications
General Statistics: Table 1 contrasts this hospital’s complication incidence to published results.21-32 We note that these published results are all catheter-based since they did not inject dose via the epidural needles. Compared to published results, the complication incidence of needle-based approach appears to be comparable to the traditional catheter-based approach.
Table 1.
This table contrasts the hospital’s complication rate against published results.
| Type of Complications | Epidural replace | Nausea/V omit | Wet tap | High Block | Blood in the catheter/needle | Re-do local anesthetic | Re-do loss of resistance |
| Known literature | 4.7% - 17.8% (n=181-10995)21-26 |
1% (n=388)27 |
0.43% - 3.2% (n=141-29749)21-24, 26-31 |
0.02%-0.07% (n=10995-145550)22, 32 |
N/A | N/A | N/A |
| This study observed cases (n=750) | 3.20% | 0.40% | 0.13% | 0.27% | 2.13% | 4.00% | 6.53% |
| This study: needlebased (n=717) | 3.07% | 0.42% | 0.00% | 0.28% | 1.81% | 4.04% | 6.56% |
| This study: catheter-based (n=33) | 6.06% | 0.00% | 3.03% | 0.00% | 9.09% | 3.03% | 6.06% |
Hypotension: The most frequent complication for spinal, epidural and combined spinal and epidural anesthesia (CSE) is hypotension. Depending on different labor analgesia methods and definitions of hypotemsion, hypotension rates reported by previous studies can be as high as 70%.33, 34 In this study, the reported hypotension rate was 51.73% across all approaches and delivery types. Specifically, it was 52.32% for C-section and 11.54% for vaginal birth. The average age was 33 for the hypotension group and 32 for the non-hypotension group (p < 0.034).
Table 2 contrasts the hypotension rates of our patients to published results. We caution that since all previously reported results except one involved very small sample sizes, one can only meaningfully compare the hypotension results based on the “30% Drop of mean arterial pressure (MAP)” definition.
Table 2.
Comparison of hypotension rates.
| Hypotension Definition | Percentage and sample size from literature | Percentage and sample size in our study | ||||||
|---|---|---|---|---|---|---|---|---|
| Observed cases (n=750) | Chart Review cases (n=1398) | |||||||
| Vaginal birth | C-section n=10735 |
Vaginal birth n=76 |
C-section n=667 |
Overall n=750 |
Vaginal birth n=505 |
C-section n=892 |
Overall n=1398 |
|
| <90 mm Hg, systolic | 23.1% , n = 6536; 4.0%, n=37527 | 16.3%35 | 17.04% | 23.73% | 21.62% | 13.86% | 23.54% | 20.03% |
| <90 mm Hg or a 20% decrease from baseline, systolic | 0%, n = 4037; 5.2%, n=1938 | 54.8%35 | 35.37% | 48.91% | 44.64% | 33.07% | 48.65% | 42.99% |
| <100 mm Hg | 7.5%, n=37527; | 41.5%35 | 39.87% | 50.66% | 47.35% | 38.22% | 50.56% | 46.14% |
| <100 mm Hg or >20% reduction from baseline, systolic | 24%, n = 2539; 48% n=2540 | 59.3%35 | 49.20% | 60.99% | 57.36% | 47.72% | 60.65% | 56.01% |
| <100 mm Hg or >30% reduction from baseline, systolic | 9%, n = 5341 | 46.7%35 | 40.51% | 53.42% | 49.45% | 39.21% | 53.14% | 48.14% |
| 30% Drop of MAP | N/A | 46.5%, n=91942 | 16.40% | 28.53% | 24.72% | 13.66% | 29.48% | 23.75% |
Table 2 reports first the complication statistics for the observed cases. In the reporting, we do not separate the needle-based versus catheter-based cases since statistically, there is no significant difference in the resulting hypotension rate. To validate that the observed cases are representative of the overall hospital practice, we also report the complication statistics for the 1,398 charts reviewed. The study reveals that the needle-based approach requires less dose for faster and effective epidural anesthesia and epidural analgesia without increasing the incidence of hypotension.
Uncovering Features for Predicting Hypotension
Machine learning is employed to uncover clinical and patient features that can predict hypotension. This allows for potential clinical practice guideline modification and/or early provider intervention to mitigate the effect. Our study consists of three folds. First, we used 561 observations from the first nine months (January – September 2014) and partitioned them randomly into two sets for training and blind prediction. Next, we used the established predictive rules to blind predict the future three months of 189 patients (October to December 2014). And finally, we blind predicted 1,398 patients from the period January to September 2015. This allows us to measure the accuracy in predicting status of future patients. It also sheds light on the consistency of the physicians’ hypotension definition.
Inputs to the computational model consist of patient demographics, physical and allergy characteristics and overall health, weeks of pregnancy, number of redo epidurals, number of reboluses and dose, test dose, epidural needle and catheter doses, total dose, duration of injection, sensory level and time achieved, delivery type, position, medication type, and provider. Using the DAMIP machine learning algorithm, we seek to uncover a small subset of discriminatory features that can predict hypotension. DAMIP returns 27 predictive rules each achieving greater than 82% 10-fold cross-validation accuracy and greater than 85% blind prediction accuracy for predicting hypotension and non-hypotension in patients for the period January – September 2014. The discriminatory features selected include weeks of pregnancy, number of redos, epidural needle/catheter dose, number of reboluses and dosage, and patients’ allergy. When blind predicting against new patients from October – December 2014, the predictive accuracy reaches 89%. Further, it reaches > 85% when blind predicting patients from January – September 2015. Table 3 contrasts the performance of DAMIP against other well-known classifiers. Compared to other classifiers, we note the consistently good predictive accuracy of DAMIP in both hypotension and non-hypotension patients. The better performance of DAMIP over other classifiers may be due to the fact that its resulting classification rule is strongly universally consistent, given that the Bayes optimal rule for classification is known. In addition, it handles imbalanced data very well.
Table 3.
DAMIP classification results for predicting hypotension and comparison against other classifiers.
| Classifier | 10-fold cross validation (424 cases) unbiased prediction estimate | Blind Prediction on 137 cases (January - September 2014) | Blind Prediction on 189 cases (October - December 2014) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall Accuracy | Hypotension | Normal | Overall Accuracy | Hypotension | Normal | Overall Accuracy | Hypotension | Normal | |
| Classification Tree | 65.60% | 71.61% | 51.76% | 51.09% | 73.85% | 30.56% | 59.79% | 79.05% | 35.71% |
| Logistic Regression | 74.69% | 88.24% | 43.53% | 47.45% | 61.54% | 34.72% | 57.67% | 67.62% | 45.24% |
| Naive Bayes | 73.80% | 87.47% | 42.35% | 43.07% | 78.46% | 11.11% | 58.20% | 89.52% | 19.05% |
| Random forest | 76.83% | 98.21% | 27.65% | 46.72% | 96.92% | 2.78% | 56.08% | 100.00% | 1.19% |
| k-nearest neighbors | 66.31% | 86.19% | 20.59% | 38.69% | 80.00% | 15.28% | 56.08% | 86.67% | 17.86% |
| Support vector machine | 60.61% | 68.29% | 42.94% | 49.64% | 69.23% | 31.94% | 52.38% | 72.38% | 27.38% |
| DAMIP | 82.30% | 82.50% | 82.00% | 89.00% | 90.70% | 87.50% | 90.40% | 89.20% | 91.40% |
Identified provider practice features offer an opportunity for CPG improvement, whereas patient characteristics allow for target/personalized care intervention. In the simulation study below, we used the identified predictive rules and their associated discriminatory features to construct care / delivery redesign experiments in an attempt to reduce hypotension incidence.
System Simulation and Clinical Practice Improvement
We first ran the computer simulation model using parameters from the 750 observed cases. The simulation was run for 19,651 patients to reflect the total number of babies delivered during a calendar year. We focused on highly revealing issues based on our outcome findings: re-do epidural procedure, hypotension and other complications such as blood in catheter/needle, wet tap, high block, and nausea and vomiting. Expected time for completing the entire epidural workflow was 9.26 minutes under current conditions.
Guided by the results from machine learning and the identified discriminatory features, we optimized the needle-base epidural dose administration process. Table 4 summarizes briefly the anticipated changes from the current practice on three scenarios. These scenarios focus on physician variations on administering medication, test dosage and total dosage. Each scenario is characterized by physician’s individual epidural technique. In our simulation model, each scenario reflects actual physicians’ characteristics such as selecting medications, loss of resistance technique and injecting durations.
Table 4.
Simulation scenarios performed to investigate potential reduction in complications.
| Scenario 1: Needle dosage of 15-18 ml | Scenario 2: Needle dosage 20-25 ml (Scenario 2) | Scenario 3: Diverse dose range | |
| Test dosage | 2 ~ 5 ml | 0 ~ 5 ml | 0 ~ 5 ml |
| Epidural needle dose | 15 ~ 18 ml | 20 ~ 25 ml | 5 ~ 20 ml |
| Total dosage | 15 ~ 25 ml | 20 ~ 30 ml | 10 ~ 30 ml |
Scenario 1 reflects a moderate needle dose with a tight total needle dose across all practitioners (15-18 ml). Scenario 2 allows for higher needle dose up to 25 ml. Scenario 3 offers broader dose variance reflecting current practice while limiting needle dose to 20 ml. Table 5 shows that Scenario 1 results in the lowest re-do rate, hypotension rate and total procedure time compared to the other two scenarios; whereas Scenario 3 shows acceptable results on hypotension. The hospital delivers roughly 19,651 newborns annually, with 75.1% receiving epidural labor analgesia. Hence the reduction in patient complication cases, reflected in the last two columns in Table 5, is substantial. Overall, all three scenarios improve the procedure time. Using high epidural needle dose, Scenario 2 performs worse than the current practice. For the 1,398 chart review cases, the hypotension rate is 21% among patients satisfying Scenario 1 criteria.
Table 5.
Contrast of complication rates using 3 scenarios of needle-based approach.
| Complication Reduction in number of patient cases | |||||||
| Complication | Occurrence rate per year: Current performance |
Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 - Scenario 2 | Scenario 1 - Scenario 3 | |
| Re-do epidural process | Needle | 5.14% | 4.18% | 4.75% | 4.92% | - 84 | - 109 |
| Catheter | 5.15% | ||||||
| Replace epidural | 2.80% | 2.40% | 2.88% | 3.02% | - 71 | - 91 | |
| *Hypotension | 50.89% | 31.82% | 55.43% | 49.47% | - 3484 | - 2605 | |
| Blood in catheter/needle | 0.32% | 0.31% | 0.32% | 0.33% | - 1 | - 3 | |
| Wet tap | 0.17% | 0.16% | 0.18% | 0.17% | - 3 | - 1 | |
| High block | 0.33% | 0.33% | 0.33% | 0.33% | 0 | 0 | |
| Nausea/Vomit | 0.35% | 0.34% | 0.35% | 0.35% | - 1 | - 1 | |
| Faint | 0.17% | 0.17% | 0.16% | 0.17% | 1 | 0 | |
| Procedure time | 9.26 minutes | 5.12 minutes | 5.98 minutes | 5.78 minutes | |||
*Based on definition used by hospital providers (Table 6).
Practice Variance among Providers
Forty-four physicians were observed. The years of practice ranges from 6 to 30 years. All physicians report using needle-based approach with over 68% acquiring this skill at this hospital.
The top five medications are used in over 50% of the patient cases. We categorized physicians by years of practice: greater than 25 years (long), between 10 to 25 years (medium), and fewer than 10 years (short). Analysis shows that there is no significant difference in prescribed epidural dosage for C-section (long vs medium: p < 0.8590, short vs medium: p < 0.6623, long vs short: p < 0.8245).
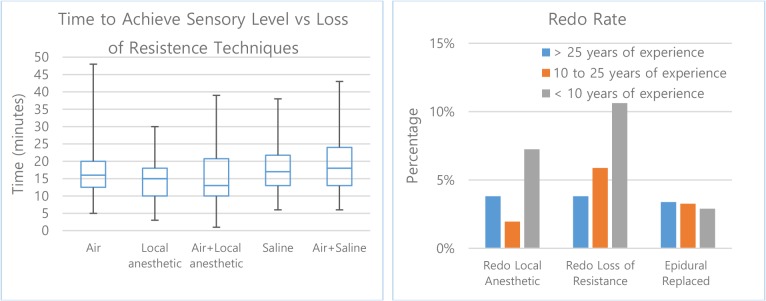
43.52% of providers favor the use of air in the loss of resistance technique, followed by 28.9% for local anesthetic, 11.9% for saline, 11.5% for air+local anesthetic and 4.3% for air+saline. When comparing the time to sensory level versus different loss of resistance techniques, a significant difference is observed while the height, weight, and age of patients are similar across the preference techniques. When the loss of resistance utilizes air with local anesthetics, the average time to sensory level and frequency of re-bolus are lowest among all other techniques (Figure 3, left and Table 7).
Figure 3:
Loss of resistance techniques versus the time to achieve desired sensory level.
Table 7.
Frequency of re-bolus with different loss of resistance techniques.
| Loss of Resistance Technique | Air | Local anesthetic | Air+Local anesthetic | Saline | Air+Saline |
| Frequency of Re-bolus | 27.66% | 32.05% | 17.74% | 25.00% | 37.50% |
While there is marginal difference in epidural replaced rate, the overall redo rate appears to be lowest among physicians with medium years of experience. This may be explained that they have adequate experience and knowledge and are in prime condition to deliver high quality service. The statistics also show that experienced physicians have the lowest redo rate in loss of resistance (Figure 3, right).
4. Discussion
This study demonstrates the use of broad range of informatics techniques can help to uncover practice characteristics that are critical for care improvement. Of the 3,988,076 documented births in the United States in 2014, 32.2% were delivered via cesarean delivery43; and among women delivering vaginally, as high as 61% received epidural or spinal anesthesia44. The potential consequences of a failed or misplaced epidural needle are well known to anesthesiologists who practice obstetric anesthesia. While much has been analyzed regarding complications, especially hypotension, there has been limited research regarding the dose-sensory response and standardization of the epidural analgesia procedure to reduce practice variance and maintain low rates of complication. Provision of neuraxial labor analgesia in a timely manner has been shown to be important to many parturients on open-ended patient surveys.45 Thus, it is important to examine the efficacy and safety of epidural dosing techniques that may shorten analgesic onset.
The rationale for potentially improved analgesia onset with epidural needle injection is uncertain. Dosing prior to placement of the catheter, such as with a combined spinal and epidural approach or through the epidural needle, may have the additional benefit of allowing labor analgesia to commence in instances which catheter placement is in an epidural vein and additional procedure time is necessary. In addition to faster onset of analgesia, it has been reported that dosing through the epidural needle may result in improved quality of epidural anesthesia compared to dosing through the catheter.6 However, other studies in obstetric7, 9 and non-obstetric8 patients receiving epidural anesthesia have observed similar outcome. Recently, Ristev et al. 20179 performed the first study in comparing needle and catheter injection of epidural medications for the initiation of labor analgesia. There remains a serious lack of research examining the potential benefits and risks of initiating labor analgesia with injection of anesthetic medications through the epidural needle. Moreover, little is known regarding practice and patient outcome related to large dose injected through the epidural needle.
We hypothesized that needle injection directly into the epidural space would shorten analgesic onset and improve the quality of subsequent labor analgesia compared to catheter injection. With increasing demand on quality of medical service and evidence of outcome, the medical providers seek to work collaboratively with medical informaticians and systems engineers to comprehensively analyze the performance of the epidural anesthesia service. Leveraging the unique clinical practice at this hospital, we evaluate the safety of a needle-based epidural technique for elective caesarian sections and establish evidence of a safe-level of epidural needle dose. We also analyze and quantify the dose-sensory response evidence and the associated complications in the hands of experienced anesthesiologists. For objective comparison, we contrast our findings to published results. To the best of our knowledge, there is no previous comparative effectiveness study analyzing dosage delivered via needle versus catheter.
Our findings indicate that needle-based approach is faster and more dose-effective in achieving comparable sensory level than the traditional catheter-based approach. Injecting large doses (up to 20 ml) in the epidural space through the epidural needle is safe, with complication rates similar to those reported in published literature. Further, if the needle dose is kept under 18 ml, the resulting hypotension rate will be significantly lower than current catheter delivery practice.
We identified a small subset of discriminatory features, including weeks of pregnancy, patient allergies, number of redos, epidural needle/catheter dose, number of reboluses and dosage that can predict hypotension with 85% confidence using our DAMIP machine learning approach. The identified patient characteristics allow for precautionary care intervention for at-risk patients during the epidural procedure. The provider practice features offer an opportunity for clinical practice guideline development and process improvement. Using system simulation and optimization, we investigated scenarios to reduce the hypotension incidence. Our results suggest that the hypotension rates can be driven down to 31% while the needle dose can be as high as 18 ml. A cohort of 1,398 patients obtained via chart review is used to validate our findings to ensure that they are representative of the hospital clinical practice.
We contrasted the proficiency of physician practice and provided insights on their preference in medication and dosage. Understanding the causes and effects of variation can help providers and healthcare organizations avoid practices that negatively impact outcomes. Our results establish evidence of safe and effective epidural needle dosage. They confirm that needle injection directly into the epidural space shortens analgesic onset, reduces medication dosage, and improves the quality of subsequent labor analgesia when compared to catheter injection. This facilitates evidence-based dose delivery to patients that results in safer and more effective pain control during child delivery. The new CPG results in fewer complications and helps with training of anesthesiologists based on evidence-based best practice.
Currently there are very few reported studies in which anesthesia providers have initiated labor analgesia by injecting medications through the epidural needle immediately after loss of resistance in order to achieve faster onset of pain relief.5 This practice site offers a unique opportunity for in-depth study in the efficacy and quality of a needle-based epidural approach due to the practice and the large volume of newborns delivered annually. While design of a direct clinical comparison may be desirable, because the expertise of these physicians is mostly needle-based approach, it seems better suited to compare outcome of this site to reported published results. While this study was performed in a single hospital location, the practice of injecting dose via the epidural needles has been used and reported in obstetric7, 9 and non-obstetric8 patients. The results thus are applicable to broad epidural practice in other types of surgeries that required localized anesthesia.
Table 6.
Self-reported hypotension definition used by anesthesiologists in the studied hospital.
| Self-reported hypotension definition | % of anesthesiologists |
| 20% off baseline, systolic | 34.09% |
| Below 90mmHg, systolic | 18.18% |
| Below 90mmHg, systolic or 20% off baseline, systolic | 9.09% |
| Below 100mmHg, systolic or 20% off baseline, systolic | 4.55% |
| 15% off baseline, MAP<55 | 2.27% |
| 30% off baseline, systolic | 2.27% |
| Below 80mmHg, systolic or 20% off baseline, systolic | 2.27% |
| 20% off baseline, MAP<55 | 2.27% |
| Non-Quantitative Standard | 9.09% |
| Not Reported | 15.91% |
Acknowledgement
This work is partially supported by grants from the National Science Foundation, IIP-0832390 and IIP-1361532. Findings and conclusions in this paper are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Dunham CM, Hileman BM, Hutchinson AE, Chance EA, Huang GS. Perioperative hypoxemia is common with horizontal positioning during general anesthesia and is associated with major adverse outcomes: a retrospective study of consecutive patients. BMC anesthesiology. 2014;14(1):43. doi: 10.1186/1471-2253-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Q, Zhang J, Wang H, Zhang R, Yue Y, Li L. Effect of Acute Hypercapnia on Outcomes and Predictive Risk Factors for Complications among Patients Receiving Bronchoscopic Interventions under General Anesthesia. PloS one. 2015;10(7):e0130771. doi: 10.1371/journal.pone.0130771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesthesia & Analgesia. 2005;101(6):1634–42. doi: 10.1213/01.ANE.0000180829.70036.4F. [DOI] [PubMed] [Google Scholar]
- 4.Omote K, Namiki A, Iwasaki H. Epidural administration and analgesic spread: comparison of injection with catheters and needles. Journal of anesthesia. 1992;6(3):289–93. doi: 10.1007/s0054020060289. [DOI] [PubMed] [Google Scholar]
- 5.Gambling D, Berkowitz J, Farrell TR, Pue A, Shay D. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesthesia & Analgesia. 2013;116(3):636–43. doi: 10.1213/ANE.0b013e31827e4e29. [DOI] [PubMed] [Google Scholar]
- 6.Cesur M, Alici HA, Erdem AF, Silbir F, Yuksek MS. Administration of local anesthetic through the epidural needle before catheter insertion improves the quality of anesthesia and reduces catheter-related complications. Anesthesia & Analgesia. 2005;101(5):1501–5. doi: 10.1213/01.ANE.0000181005.50958.1E. [DOI] [PubMed] [Google Scholar]
- 7.Husain F, Herman N, Karuparthy V, Knape K, Downing J. A comparison of catheter vs needle injection of local anesthetic for induction of epidural anesthesia for cesarean section. International journal of obstetric anesthesia. 1997;6(2):101–6. doi: 10.1016/s0959-289x(97)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Yun M, Yong-Chul K, Lim Y, Choi G. The differential flow of epidural local anaesthetic via needle or catheter: a prospective randomized double-blind study. Anaesthesia and intensive care. 2004;32(3):377. doi: 10.1177/0310057X0403200313. [DOI] [PubMed] [Google Scholar]
- 9.Ristev G, Sipes AC, Mahoney B, Lipps J, Chan G, Coffman JC. Initiation of labor analgesia with injection of local anesthetic through the epidural needle compared to the catheter. Journal of pain research. 2017;10:2789–2796. doi: 10.2147/JPR.S145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MATLAB and Statistics Toolbox Release 2012b. Natick, Massachusetts, United States: The MathWorks Inc. 2012.
- 11.Lee EK. Large-scale optimization-based classification models in medicine and biology. Annals of biomedical engineering. 2007;35(6):1095–109. doi: 10.1007/s10439-007-9317-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee EK, Yuan F, Hirsh DA, Mallory MD, Simon HK. AMIA Annual Symposium Proceedings. American Medical Informatics Association; 2012. A clinical decision tool for predicting patient care characteristics: patients returning within 72 hours in the emergency department. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EK, Gallagher RJ, Patterson DA. A linear programming approach to discriminant analysis with a reserved-judgment region. INFORMS Journal on Computing. 2003;15(1):23–41. [Google Scholar]
- 14.Lee EK, Wu T-L. Handbook of optimization in medicine. Springer; 2009. Classification and disease prediction via mathematical programming; pp. 1–50. [Google Scholar]
- 15.Lee EK. Integration of AI and OR Techniques in Constraint Programming for Combinatorial Optimization Problems. Springer; 2009. Machine learning framework for classification in medicine and biology; pp. 1–7. [Google Scholar]
- 16.Brooks JP, Lee EK. Analysis of the consistency of a mixed integer programming-based multi-category constrained discriminant model. Annals of Operations Research. 2010;174(1):147–68. [Google Scholar]
- 17.Brooks JP, Lee EK. Solving a multigroup mixed-integer programming-based constrained discrimination model. INFORMS Journal on Computing. 2014;26(3):567–85. [Google Scholar]
- 18.Lee EK, Wu T-L, Goldstein F, Levey A. Optimization and Data Analysis in Biomedical Informatics. Springer; 2013. Predictive Model for Early Detection of Mild Cognitive Impairment and Alzheimer’s Disease; pp. 83–97. [Google Scholar]
- 19.Ecker JL, Chen KT, Cohen AP, Riley LE, Lieberman ES. Increased risk of cesarean delivery with advancing maternal age: indications and associated factors in nulliparous women. Am J Obstet Gynecol. 2001;185(4):883–7. doi: 10.1067/mob.2001.117364. [DOI] [PubMed] [Google Scholar]
- 20.Eledjam JJ, Ripart J, Viel E. Clinical application of ropivacaine for the lower extremity. Curr Top Med Chem. 2001;1(3):227–31. doi: 10.2174/1568026013395317. [DOI] [PubMed] [Google Scholar]
- 21.Pan P, Bogard T, Owen M. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. International journal of obstetric anesthesia. 2004;13(4):227–33. doi: 10.1016/j.ijoa.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Paech M, Godkin R, Webster S. Complications of obstetric epidural analgesia and anaesthesia: a prospective analysis of 10 995 cases. International Journal of Obstetric Anesthesia. 1998;7(1):5–11. doi: 10.1016/s0959-289x(98)80021-6. [DOI] [PubMed] [Google Scholar]
- 23.Eappen S, Blinn A, Segal S. Incidence of epidural catheter replacement in parturients: a retrospective chart review. Int J Obstet Anesth. 1998;7(4):220–5. doi: 10.1016/s0959-289x(98)80042-3. [DOI] [PubMed] [Google Scholar]
- 24.Crawford JS. The second thousand epidural blocks in an obstetric hospital practice. Br J Anaesth. 1972;44(12):1277–87. doi: 10.1093/bja/44.12.1277. [DOI] [PubMed] [Google Scholar]
- 25.Thangamuthu A, Russell IF, Purva M. Epidural failure rate using a standardised definition. International Journal of Obstetric Anesthesia. 2013;22(4):310–5. doi: 10.1016/j.ijoa.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. International Journal of Obstetric Anesthesia. 2010;19(4):373–8. doi: 10.1016/j.ijoa.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Norris MC, Grieco WM, Borkowski M, et al. Complications of labor analgesia: epidural versus combined spinal epidural techniques. Anesthesia and analgesia. 1994;79(3):529–37. doi: 10.1213/00000539-199409000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Sprigge J, Harper S. Accidental dural puncture and post dural puncture headache in obstetric anaesthesia: presentation and management: A 23-year survey in a district general hospital. Anaesthesia. 2008;63(1):36–43. doi: 10.1111/j.1365-2044.2007.05285.x. [DOI] [PubMed] [Google Scholar]
- 29.Van de Velde M, Schepers R, Berends N, Vandermeersch E, De Buck F. Ten years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia department. International Journal of Obstetric Anesthesia. 2008;17(4):329–35. doi: 10.1016/j.ijoa.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Verstraete S, Walters MA, Devroe S, Roofthooft E, Van De Velde M. Lower incidence of post-dural puncture headache with spinal catheterization after accidental dural puncture in obstetric patients. Acta Anaesthesiologica Scandinavica. 2014;58(10):1233–9. doi: 10.1111/aas.12394. [DOI] [PubMed] [Google Scholar]
- 31.Sadashivaiah J, Wilson R, McLure H, Lyons G. Double-space combined spinal-epidural technique for elective caesarean section: a review of 10 years’ experience in a UK teaching maternity unit. International Journal of Obstetric Anesthesia. 2010;19(2):183–7. doi: 10.1016/j.ijoa.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins JG. Some immediate serious complications of obstetric epidural analgesia and anaesthesia: a prospective study of 145 550 epidurals. International Journal of Obstetric Anesthesia. 2005;14(1):37–42. doi: 10.1016/j.ijoa.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Rout C, Akoojee S, Rocke D, Gouws E. Rapid administration of crystalloid preload does not decrease the incidence of hypotension after spinal anaesthesia for elective caesarean section. British Journal of Anaesthesia. 1992;68(4):394–7. doi: 10.1093/bja/68.4.394. [DOI] [PubMed] [Google Scholar]
- 34.Cascio M, Pygon B, Bernett C, Ramanathan S. Labour analgesia with intrathecal fentanyl decreases maternal stress. Can J Anaesth. 1997;44(6):605–9. doi: 10.1007/BF03015443. [DOI] [PubMed] [Google Scholar]
- 35.KlÖHR S, Roth R, Hofmann T, Rossaint R, Heesen M. Definitions of hypotension after spinal anaesthesia for caesarean section: literature search and application to parturients. Acta anaesthesiologica Scandinavica. 2010;54(8):909–21. doi: 10.1111/j.1399-6576.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 36.Kopacz DJ, Sharrock NE, Allen HW. A comparison of levobupivacaine 0.125%, fentanyl 4 ug/mL, or their combination for patient-controlled epidural analgesia after major orthopedic surgery. Anesthesia & Analgesia. 1999;89(6):1497. doi: 10.1097/00000539-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DC, Zwack RM, Crone LA, Yip RW. Ambulatory labor epidural analgesia: bupivacaine versus ropivacaine. Anesthesia and analgesia. 2000;90(6):1384–9. doi: 10.1097/00000539-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Campbell DC, Banner R, Crone L-A, Gore-Hickman W, Yip RW. Addition of epinephrine to intrathecal bupivacaine and sufentanil for ambulatory labor analgesia. The Journal of the American Society of Anesthesiologists. 1997;86(3):525–31. doi: 10.1097/00000542-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Meister GC, D’Angelo R, Owen M, Nelson KE, Gaver R. A comparison of epidural analgesia with 0.125% ropivacaine with fentanyl versus 0.125% bupivacaine with fentanyl during labor. Anesthesia and analgesia. 2000;90(3):632–7. doi: 10.1097/00000539-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Sia ATH, Chong JL, Tay DHB, Lo WK, Chen LH, Chiu JW. Intrathecal sufentanil as the sole agent in combined spinal-epidural analgesia for the ambulatory parturient. Canadian journal of anaesthesia. 1998;45(7):620–5. doi: 10.1007/BF03012089. [DOI] [PubMed] [Google Scholar]
- 41.Breen TW, Shapiro T, Glass B, Foster-Payne D, Oriol NE. Epidural anesthesia for labor in an ambulatory patient. Anesthesia and analgesia. 1993;77(5):919–24. doi: 10.1213/00000539-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Maayan-Metzger A, Schushan-Eisen I, Todris L, Etchin A, Kuint J. Maternal hypotension during elective cesarean section and short-term neonatal outcome. American journal of obstetrics and gynecology. 2010;202(1):56–e1e5. doi: 10.1016/j.ajog.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Mathews TJ. Births: Final Data for 2014. National Center for Health Statistics. 2015. Contract No.: 12. [PubMed]
- 44.Osterman MJK, Martin JA. Epidural and Spinal Anesthesia Use During Labor: 27-state Reporting Area 2008. National Center for Health Statistics. 2011. Contract No.: 5. [PubMed]
- 45.Attanasio L, Kozhimannil KB, Jou J, McPherson ME, Camann W. Women’s experiences with neuraxial labor analgesia in the listening to mothers II survey: a content analysis of open-ended responses. Anesthesia and analgesia. 2015;121(4):974. doi: 10.1213/ANE.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]