Abstract
Background
Mesenchymal stem cells have immense potential in stem cell-based therapies, however there is a pre-requisite to develop a curative cell dose. Adipose tissue-derived mesenchymal stem cells are promising mainly due to their potential abundance, immunomodulatory effect and remarkable differentiation potential. Nevertheless, senescence may develop during their in vitro expansion due to the incidence of genetic instability. Hence, it is important to attain an ideal balance between mesenchymal stem cell growth, quality and genetic integrity before their clinical use.
Methods
Stromal vascular fraction was obtained from omentum tissue of patients undergoing liposuction procedures for morbid obesity. This study standardized a closed system protocol which can be utilized for clinical grade stem cell derivation. Stages of cell growth and characterization of human adipose tissue-derived mesenchymal stem cells were also assessed along with the chromosomal stability in these in vitro cultures.
Results
Human adipose tissue-derived mesenchymal stem cells maintained their spindle-shaped morphology and were able to proliferate and renew, confirming their suitability for in vitro cultivation and generate clinical grade mesenchymal stem cells. Immunophenotyping indicates that the cells expressed cluster of differentiation (CD)73/CD90/CD105, mesenchymal stem-cell markers, while lacked CD34/CD45/ Human Leukocyte antigen-antigen D related (HLA-DR) expression (hematopoietic cell markers). A cell cycle study demonstrated growth kinetics under in vitro culture conditions. Human adipose tissue derived mesenchymal stem cells expressed normal cell karyotype by chromosomal G-banding indicating their genetic stability at Passage 5. Mesenchymal stem cells also demonstrated trilineage differentiation.
Conclusions
Availability of adipose tissue in abundance is a major advantage for clinical applications. Furthermore, detailed characterization of human adipose tissue-derived mesenchymal stem cells, their genomic stability and differentiation potential from stromal vascular fraction of human adipose tissue would help assist in tissue regeneration and repair.
Keywords: Differentiation, Genetic stability, Human adipose derived stem cell, Mesenchymal stem cells, Proliferation
Introduction
Stem cells are characterized by their self-renewal properties and by their capacity to generate differentiated cell lineages providing prospects for cell-based tissue regeneration. Stem cells are maintained during the life of the organism to help in lineage-specific differentiation.1, 2 Although stem cells are maintained in their adult tissues, some of the cells undergo division without differentiation, such that one of the progeny remains undifferentiated, while the other proliferates and differentiates to generate new tissue mass.3
Mesenchymal stem cells (MSCs) are present in tissues or organs from which they exit into the circulation to home into sites of injury or to other tissues. The MSCs are well characterized based on their surface markers and also have the ability to regenerate into various cells and tissues.4, 5 Human MSCs (MSCs) are a unique progenitor cell that can be recovered from most vascularized tissues. The most salient features of MSCs also include their immunomodulation properties and trophic capabilities. MSCs act as immune-conductors of tissue repair and regeneration based on their ability to secrete trophic factors that stimulate neighboring parenchymal cells to start repairing damaged tissues.6
The clinical use of MSCs has been growing enormously in both human and animal research. MSCs exhibit a remarkable feature of transdifferentiation into ectodermal, neuroectodermal and endodermal cells, phenomena referred to as ‘stem cell plasticity’. This knowledge opened the possibility of clinical applications of MSCs in the regeneration of other tissues such as corneal reconstruction, treatment of acute lung injury, oral mucosal regeneration, homing of MSCs for regeneration at sites of injury, etc. Though evidence has accrued demonstrating this phenomenon, there is still a gap in understanding the molecular mechanism of transitions that will be important to control the process efficiently. Studies also demonstrated the utilization of diverse organ-resident perivascular MSC-like cells and bone marrow-derived MSCs in tissue regeneration.7, 8 These cells can be cultured to create homogenous populations under controlled culture conditions. Moreover, MSCs also possess immunosuppressive effects, implying their possible clinical applications in regenerative medicine and therapies for treatment-resistant immune disorders.
MSCs are described as immature cells within the bone marrow, peripheral blood, menstrual blood, and nearly all adult tissues and solid organs.9, 10 MSCs have also been shown to possess immunogenic properties and a powerful immunosuppressive potential, which make them attractive for allogeneic cell therapy.11
Adipose tissue has evolved as another potential alternative source of stem cells. Adipose tissue-derived populations of cells from the stromal vascular fraction (SVF) are harvested by digesting the fat and concentrating the remaining cells. Earlier, Young et al.12 reported that these stem cells from the SVF of fat had similar morphology to MSCs. Subcutaneous adipose deposits are accessible, abundant, and replenishable, thereby providing a potential adult stem cell reservoir for every individual.
Human adipose tissue-derived stem cells (ADSCs), when compared with bone marrow-derived stem cells (BMSCs), can be easily cultured and proliferate rapidly with a high cellular activity.13 Izadpanah et al. reported that human ADSCs can maintain their chromosomal stability for more passages (30) compared to human BMSCs (20) before becoming senescent.14 All these qualities make adipose tissue a useful source of MSCs. Jurgens et al. showed that the yield, proliferation and differentiation of MSCs also depend on the tissue harvesting site.15 Thus, it is also important to monitor the processes to expand MSCs in a robust and reproducible way, while assuring their purity and quality for clinical applications.
Although the current work is not incremental, it is very important to reproduce clinical grade MSCs for clinical purposes and demonstrate the same phenotypic and genotypic characteristics. Hence, the present study deals with the isolation and propagation of human MSCs derived from the SVF of adipose tissue. The growth pattern, long-term chromosomal stability and trilineage differentiation potential of human ADSCs was also studied in order to employ these cells for chronic ailments.
Methods
Low-glucose Dulbecco's modified eagle medium (L-DMEM; 4.5 mmol/L glucose), type I collagenase and fetal bovine serum (FBS) were purchased from Gibco (BRL, USA). All antibodies were purchased from BD Bioscience (San Jose, USA). All the fine chemicals were purchased from Sigma (St. Louis, USA).
Prior written informed consent was obtained from donors aged from 26 to 57 years undergoing liposuction procedures for morbid obesity. The study was approved by the Global Hospitals, Institution Ethics Committee (IEC – Ref no. GMERF/BS/SAC/IEC/IC_SCR2014/01). The adipose tissue for the study was collected in sterile Dulbecco's modified eagle medium (DMEM) supplemented with antibiotics in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki).
Stromal vascular fraction isolation from omentum tissue
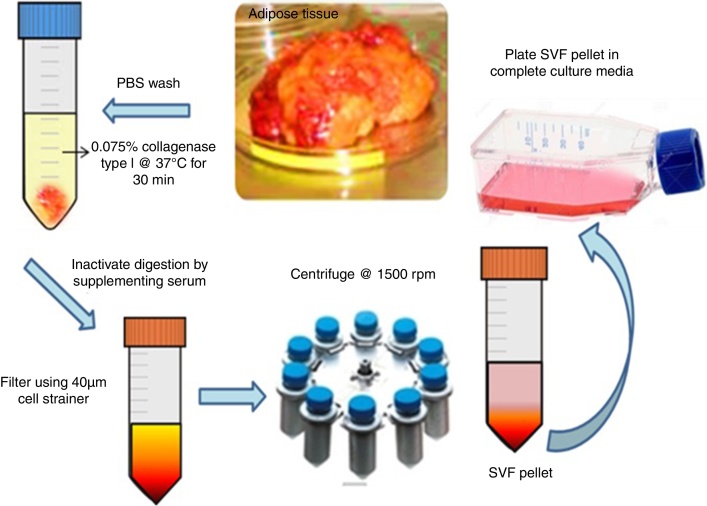
Cells were isolated from 2–3 g of adipose tissue using a modified procedure of Zuk et al.16 The collected tissues were washed two or three times with phosphate buffer saline (PBS). The tissue was minced into pieces of 1–3 mm in diameter and treated with pre-warmed collagenase type I (2 mg/mL) at 37 °C with intermittent vortexing for 1–2 h. The digested tissue was filtered using a 40-μm cell strainer, the filtrate was centrifuged at 600 g for 10 min. The supernatant containing the mature adipocytes was discarded. The pellet was identified as the human adipose tissue-derived MSCs consisting a heterogeneous mixture of cells.
The cells were seeded at a density of 4000 cells/cm2 in complete MSC culture media for 3–5 days for initial attachment. The SVF was characterized for various hematopoietic, mesenchymal and side population cell surface markers using flow cytometry. The cultured cells were washed and counted using Trypan blue to distinguish viable and dead cells, and then frozen in freezing medium consisted of DMEM with 90% FBS and 10% dimethyl sulfoxide (DMSO).
Assessment of cell viability
The cell viability of cryopreserved human ADSCs (n = 5) after revival was determined by incubating with 7-amino-actinomycin D (7-AAD, BD Pharmingen, USA) for 20 min in the dark. 7-AAD viability assays were carried out by flow cytometry using Cell Quest software (Becton Dickinson, USA) at each passage level. Approximately, 1 × 106 cells/mL were incubated with 20 μL of the dye for 10 min before analysis. This reagent is used as a viability probe for methods to exclude dead cells based on light scatter and uptake of 7-AAD as detected in the fluorescence 3 (FL3) channel.
Characterization of human adipose tissue-derived stem cells
Morphological characterization-staining
Human ADSCs were maintained in growth media till they reached 60% confluency. Giemsa staining was done to assess the basic morphology of human ADSCs. Approximately 1 × 103 cells/cm2 were cultured and later fixed with 3.7% paraformaldehyde for 20 min at room temperature. Cells were washed and stained with Giemsa stain followed by hematoxylin & eosin (H&E) staining for morphological evaluation.
Marker expression by confocal microscopy
Human ADSCs were further characterized by immunocytochemistry to detect the presence or absence of the CD90, CD73 and HLA-DR, CD45, and CD34 markers. Human ADSCs (1 × 103 cells/cm2) were grown on cover slips in a humidified atmosphere. After 18 h, the cover slips were fixed with 3.7% paraformaldehyde (Sigma–Aldrich, St. Louis, MO, USA) for 20 min at room temperature. The cover slips were incubated at 4 °C overnight with primary antibodies against the following surface markers: CD90 (1:100), CD73 (1:100), HLA-DR (1:50), CD34 (1:100), and CD45 (1:100). The cover slips were subsequently incubated with Alexa Fluor 488 (Molecular probes, Life technologies, USA) and labeled with secondary antibodies at room temperature for 1 h. Further, they were mounted on slides with mounting medium (Vectashield) containing 4′,6-diamidino-2-phenylindole (Abcam, Cambridge, UK) and incubated for 2–3 min at room temperature in the dark. The imaging was performed using a confocal microscope (Leica TCS, CLSM) and the images were processed using Leica software.
Immunophenotyping by flow cytometry
The cells were trypsinized and the pellet was re-suspended in PBS. A cell suspension was made with 1 × 106 cells/mL and incubated in the dark for 30 min at 4 °C with the antibodies. Cell surface antigen expression of human ADSCs was detected at each passage using flow cytometry. Epitopes, such as CD90 (FITC), CD34 (PE), CD73 (APC), CD45 (FITC), and HLA-DR (PE), were evaluated for positive versus negative expression by flow cytometry using Cell Quest software (Becton Dickinson). All these anti-human antibodies were raised in mouse and were obtained from BD Pharmingen, USA.
Proliferation kinetics of human adipose tissue-derived stem cells
ADSCs were seeded at a density of 1 × 104 cells/cm2 to study the growth kinetics at each passage (n = 5). Periodically, after 24 h, 48 h, 72 h, 96 h, 120 h and 144 h the cells were trypsinized, harvested and counted. A graph was plotted of the number of cells against timepoint indicating the growth phases of stem cells.
Cell cycle analysis
Cell cycle demonstrates the stages of cell growth. Proliferating human ADSCs (n = 5) were harvested and fixed in cold 70% ethanol. Ethanol was added dropwise to the pellet while vortexing, ensuring fixation of all the cells. The pellet was allowed to fix for 30 min at 4 °C. The cell pellet was stained with 200 μL propidium iodide from 50 μg/mL stock solution.
Karyotyping of human adipose tissue-derived stem cells
The cytogenetic analysis of adipose tissue-derived MSCs helps in assessing their stability and tumorigenicity. Human ADSCs (n = 5) were seeded at a density of 8.0 × 103 cells/cm2 in complete basal media. The actively dividing cells were arrested at the mitotic phase after 48 h. The cells were treated with colchicine (0.1 μg/mL) (Sigma–Aldrich, USA) for 3–4 h prior to terminating culture and harvested using 0.25% trypsin-EDTA. The cells were washed and re-suspended in pre-warmed hypotonic solution (0.075 M KOH) for 20 min at 37 °C. The pellet was fixed and incubated in chilled methanol and glacial acetic acid (3:1) solution overnight at 4 °C. The fixed cells were dropped on to a pre-warmed slide by holding the slide at a 60° angle. Images of 5 metaphase spreads for each sample were karyotyped using spectral imaging software at a resolution of 550 bands per haploid set resolution.
Trilineage differentiation potential
Adipogenic differentiation
Exponentially growing human ADSCs (n = 5) were cultured as a monolayer for 21 days in the presence of high-glucose DMEM, 10% FBS, and adipogenic supplements (1 μM dexamethasone, 1 μg/mL insulin and 0.5 mM 3-isobutyl-1-methylxanthine) (Sigma–Aldrich, USA). To visualize adipocytes, cultures were fixed in 10% neutral buffered formalin solution (Sigma–Aldrich, USA) for 30 min, then washed and stained with Oil Red O (3 mg/mL in H2O) for 10 min.
Osteogenic differentiation
The human ADSCs were differentiated into osteogenic lineages by growing them in osteogenic induction media. Human ADSCs (n = 5) were seeded in the culture media at a seeding density of 200 cells/cm2 in culture dishes. When the plates reached 90% confluency, human ADSC culture media was replaced with osteogenic induction media containing 100 nM dexamethasone, 10 mM β-glycerophosphate and 0.1 mg/mL ascorbate-2-phosphate in the presence of 10 ng/mL bone morphogenetic protein 2 (BMP-2). After three weeks, the cultures were fixed in 10% neutral buffered formalin solution (Sigma–Aldrich, USA) and were evaluated for the presence of calcium deposits by von Kossa staining.
Chondrocyte differentiation
The confluent human ADSCs (n = 5) were removed from the culture flask and a cell pellet was obtained by centrifuge at 100 g for 5–10 min. The cells were suspended in chondrogenic induction media containing high-glucose DMEM supplemented with 10% FBS, 50 μg/mL ascorbic acid, 1% ITS-premix, and 0.1 μM dexamethasone in the presence of 10 ng/mL transforming growth factor beta (TGF-β – Sigma–Aldrich, USA). The cells were cultured in a pellet system for 21 days. The differentiated cells were stained with Alcian blue.
Statistical analysis
All quantitative data are represented as means ± standard error (SE).
Results
Isolation of human adipose tissue-derived stem cells from stromal vascular fraction
Human MSCs were successfully isolated from adipose tissue (n = 5). Figure 1 depicts the isolation process of SVF from adipose tissue. The SVF was plated in culture flasks. An average yield of 1 × 106 cells/mL was obtained from 2–3 g adipose tissue. MSCs were seen to attach to culture dishes sparsely and most cells displayed a spindle-like shape. These cells began to proliferate at about Day 4 and gradually grew to form small colonies.
Figure 1.
Isolation of mesenchymal stem cells from adipose tissue. Schematic representation of enzymatic digestion of adipose tissue using collagenase type I. Isolation and expansion of the mesenchymal stem cells population from the stromal vascular fraction.
Characterization of human adipose tissue-derived stem cells
Culture of human adipose tissue-derived stem cells
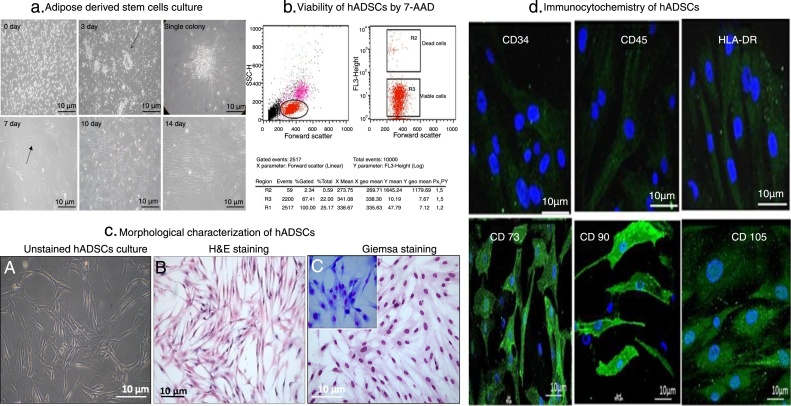
In large colonies, cells were more densely distributed and showed a spindle shape. The colonies continued to grow, gradually expanding in size and reached confluency by Day 10. The plate reached 80–85% confluency ready for passaging 14 days after seeding (Figure 2A).
Figure 2.
Human adipose tissue-derived stem cells culture, viability and characterization. (A) Human mesenchymal stem cells were isolated from adipose tissue in low-glucose Dulbecco's modified eagle medium. The cells formed small colonies within three days of culture as shown by the arrows. The plate showed adherent, spindle-shaped fibroblast-like cells coming out of the colonies. Human mesenchymal stem cells isolated in low-glucose Dulbecco's modified eagle medium reached 80–85% confluency by Day 10 after seeding (magnification 10×). (B) Viability of adipose tissue-derived stem cells after freeze/thaw cycles (n = 5). Forward scatter versus log fluorescence 3 (FL3) channel (7-AAD fluorescence) dot plot shows the mean percentage of viable and dead cells. (C) The adhered mesenchymal stem cells showed spindle-shaped morphology by hematoxylin & eosin staining. Giemsa staining also confirmed the spindle-shaped morphology of adipose tissue-derived stem cells. (D) CD34/45 and HLA-DR expressions were negative, while CD90/CD73/CD105 expression was observed on the cell surface (shown in green). The nucleus was stained with 4′,6-diamidino-2-phenylindole (blue).
Viability of human adipose tissue-derived stem cells
The cells were trypsinized and passaged further. The isolated adherent population showed thin, long spindle shaped fibroblast like cells. The freeze-thawed cells also showed more than 87% viability by 7-AAD staining (Figure 2B).
Morphological evaluation
Morphologically, human ADSCs showed their spindle shape and fibroblast-like appearance on Day 14 of culture using H&E and Giemsa staining. Cells exhibited high nucleus to cytoplasmic ratio on Giemsa staining. The adhered MSCs showed spindle shaped morphology on H&E staining (Figure 2C).
Immunocytochemistry of human adipose tissue-derived stem cells
Further, to characterize human ADSCs, an immunocytochemical procedure was carried out using several cell surface markers. They were recognized for their immunoreactivity to MSC-specific cell type markers. As shown, MSCs were marked by CD 90, CD105 and CD73, but were not immunopositive for CD45, CD34 and HLA DR (Figure 2D).
Immunophenotyping of human adipose tissue-derived stem cells
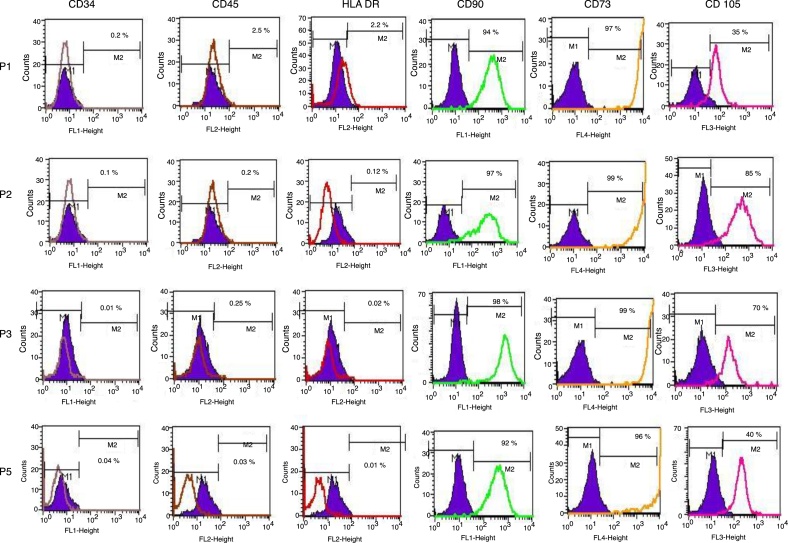
The isolated SVF showed positive expression of CD34/45 indicating the heterogeneous hematopoietic cell population. Upon culture, at each passage human ADSCs represented only 1–3% expression of CD34/45 and HLA DR. The percentage expression of CD90/CD73/CD105 increased up to 90–99% at every passage (P1–P5). More than 90% of the SVF expressed surface markers for stem cells (n = 5). The data suggested that the third passage human ADSCs exhibit a MSC origin, and that they are not intermingling with hematopoietic cells (Figure 3).
Figure 3.
Characterization of the cell surface markers for human adipose tissue-derived stem cells by flow cytometry. CD34-PE, CD45-FITC, HLA-DR-PE, CD90-FITC, CD105-PerCP and CD73-APC. The shaded histograms represent unstained negative control cells. Passage 1 did not express CD34/45 markers (0.2% and 2.5%, respectively), while low levels of HLA-DR expression (2.2%) were observed. Human adipose tissue-derived stem cells were positive for CD90 (98%) and CD73 (99%) expression, indicating a stem cell immunophenotype (n = 5).
Proliferation of human adipose tissue-derived stem cells
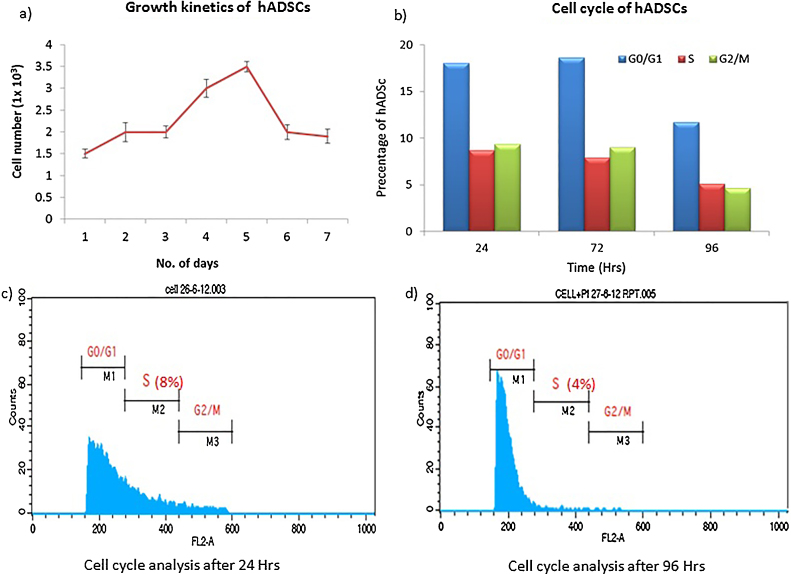
The growth rate of adipose tissue-derived MSCs was defined by plotting a growth curve. On an average, human ADSCs exhibited a short lag time in their growth curve at each passage. The growth curve for these human ADSCs (n = 5) included a lag phase but cell numbers increased after two days. The cells were at log phase for 48 h and later the cell number decreased indicating their death phase, representing a sigmoid growth curve of human ADSCs (Figure 4A).
Figure 4.
Growth profile of human adipose tissue-derived stem cells. (A) Growth curve obtained for the human adipose tissue-derived stem cells (n = 5) shows an exponential growth phase. The decline phase started after Day 5 and cell number decreased to 1.5 × 103 cells/mL. (B) The graph represents mean percentage of human adipose tissue-derived stem cells at different stages of the cell cycle. The percentage of cells in the S phase decreased significantly after 72 h. (C and D) All the stages of cell cycle of human adipose tissue-derived stem cells were tested after 24 h and 96 h using flow cytometry. hADSC: human adipose tissue-derived stem cells.
DNA content of cultured human adipose tissue-derived stem cells
The phases of cell cycle and the exponentially growing human ADSCs were also studied (Figure 4B). The cell cycle analysis emphasizes the need for earlier subculturing to optimize growth rate and non-differentiation. Data show that after 72 h, the percentage in S phase is reduced, as evident from the mean channel shift (MFI) values from the flow cytometry based Cell Quest analysis report and thus, revealing a decrease in DNA replication and indicating cells should be subcultured according to recommended criteria. Cells were also analyzed at 96 h without subculturing. A significant decrease in the percentage of cells in S phase was observed as the cells remain in culture and become more confluent over time (Figure 4C and D). Thus, subculturing prior to 80% confluency is required for an optimized growth rate without differentiation.
Genomic stability of human adipose tissue-derived stem cells
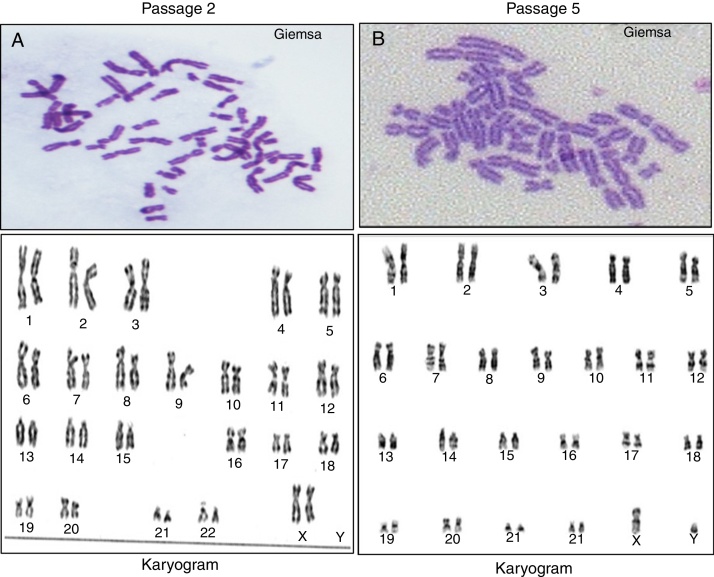
The primary screening of the chromosomal spreads was done by Giemsa staining. The karyogram of cultured human ADSCs (n = 5) from a female donor revealed a normal female (46, XX) at Passage 2 (n = 5) as shown in Figure 5A. Whereas, human ADSCs isolated and cultured from the SVF fraction of a male donor also conserved the normal male karyotype (46, XY) at Passage 5 (n = 5) as shown in Figure 5B. The chromosome numbers and structure remained stable after expansion by serial passaging. Chromosomes were paired based on banding patterns and position of their respective centromere.
Figure 5.
Karyotypic analysis of a representative stem cell isolated from human adipose tissue. (A) Karyotypic analysis of Passage 2 human adipose tissue-derived stem cells from a female donor with a normal female Karyotype (46, XX). (B) At Passage 5, human adipose tissue-derived stem cells from a male donor expressed normal a male karyotype (46, XY).
Trilineage differentiation potential
Differentiation into adipogenic lineage
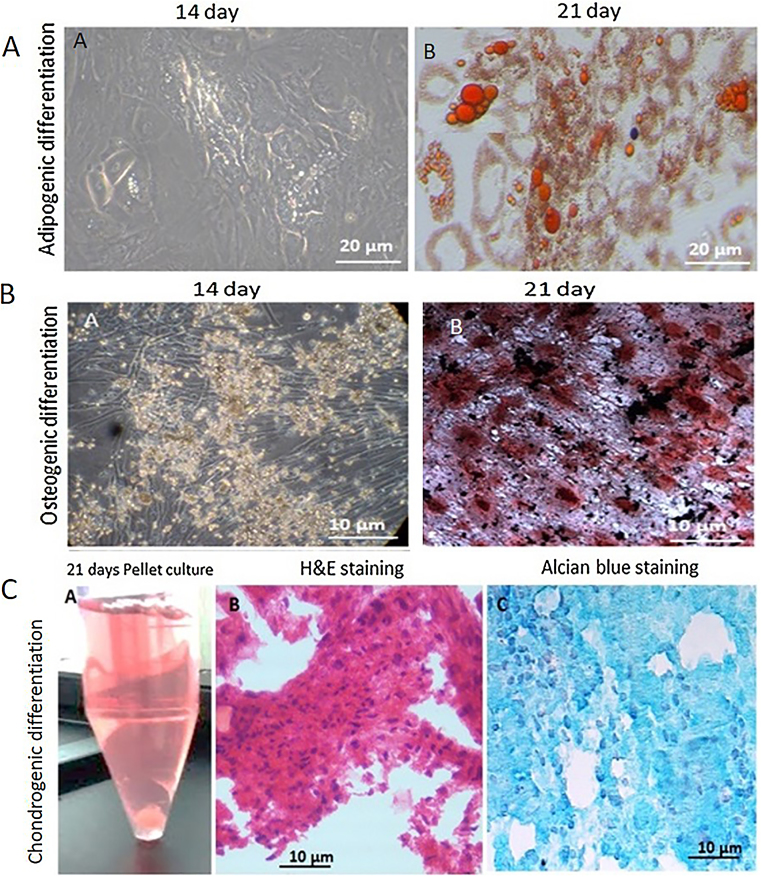
The human ADSCs (n = 5) were differentiated into adipocytes when grown in adipogenic induction media. Once the adipogenic induction media was added, cell proliferation stopped and the cells appeared flattened and increased in size. Oil droplets started accumulating in the differentiating cells, which increased in number and size as the differentiation progressed (Figure 6A.a). Oil Red O staining showed oil droplets stained red, confirming the differentiation of human ADSCs into adipocytes. Although there was change is cell morphology in the uninduced cultures, there was no visible staining of lipid droplets.
Figure 6.
Trilineage differentiation potential of human adipose tissue-derived stem cells. (A) Phase contrast micrographs showing the different stages of differentiation of human adipose tissue-derived stem cells to adipocytes. Adipocytes with oil droplets are visible on Day 14 of adipogenic induction; the size and number increased as the differentiation progressed. Oil Red O stained the lipid droplets red on Day 21 (magnification 20×). (B) Phase contrast micrographs showing different stages of the differentiation of human adipose tissue-derived stem cells to osteoblasts. Day 14 of osteogenic induction showed visible mineral deposits. There was a significant increase in the mineralization on Day 21 of osteogenic induction (magnification 20×). (C) Pellet culture of human adipose tissue-derived stem cells on Day 21. Hematoxylin & eosin staining showed homogenous cell distribution. Alcian blue staining of acid showed mucopolysaccharide aggregates in the differentiated chondrocytes.
Differentiation into osteogenic lineage
When human ADSCs (n = 5) were grown in osteogenic induction media, they differentiated into osteogenic lineage and showed mineralization. Rapid cell proliferation was observed in the early phase of differentiation, which was gradually reduced at the onset of mineralization; von Kossa staining for calcium demonstrated the secreted calcium containing mineral deposits (Figure 6B.b) thereby confirming osteoblast differentiation on Day 21 of differentiation. Plates with L-DMEM media did not show any mineralization even after 21 days of culture.
Differentiation into chondrogenic lineage
Pellet culture was used for chondrogenic differentiation. The human ADSCs (n = 5) cell pellet was supplemented with chondrogenic differentiation media. A spherical cell pellet was observed after seven days of culture. There was a progressive increase in the size of the cell pellet mass after 21 days (Figure 6C.a). The paraffin fixed pellets were sectioned and stained with H&E and Alcian blue stains. The differentiated cells in chondrogenic media were observed to have large nuclei that became progressively bigger and more rounded over 21 days of culturing. Clear pericellular lacunae were also observed in the induced chondrogenic culture. The deposits of acid mucopolysaccharides were confirmed by Alcian blue staining as shown in Figure 6C.c. An uninduced hADSC pellet was used as a control.
Discussion
The stromal component of mesenchymal tissues is thought to harbor stem cells that display extensive proliferative capacity and multilineage potential. Stromal cells isolated from various mesodermal tissues share key characteristics, including the ability to adhere to plastic to form fibroblastic like colonies (called CFU-F), extensive proliferative capacity and ability to differentiate into several mesodermal lineages. Considering these characteristics, stromal cells may potentially be useful in the field of regenerative medicine.
A study has found that the SVF from adipose tissue provides 500 times more stem cells than bone marrow (only 0.01–0.001%).17 Adipose tissue is considered the main energy source of the organism. Adipose tissue contains a large number of stromal stem cells.18 In 2003, van Harmelen et al. showed that the ratio of adipocytes to MSCs is constant in humans, independent of body mass index and age.19 This allows a greater harvest of MSCs from patients or subjects with a higher BMI.
This work focused on the standardization of the isolation procedure of human stem cells from the SVF of adipose tissue. The type of culture media used for isolation and propagation greatly influence the viability, growth characteristics and the differentiation potential of human MSCs.20, 21 Primary cultures maintained for 5–10 days in L-DMEM media supplemented with 10% FBS, l-glutamine and antibiotics showed enriched, adherent cells having fibroblast-like morphology. In this study, the surface marker expression of these human ADSCs from Passages 1 to 5 was observed. Ideally, Passage 3 expressed a higher percentage of stem cell-related surface markers. The adipose tissue-derived SVF cells isolated and propagated in L-DMEM had positive MSC surface markers and were negative for the hematopoietic markers. However, the endoglin (CD105) expression was altered at later passages owing to the inclination of human ADSCs toward osteogenic differentiation. Mitchell et al. also observed similar expression patterns of CD105 and CD166 markers.22 The isolated cells also had CD90, CD73 protein expressions on their surface as observed by confocal imaging.
The phenotype of human ADSCs is quite similar to BMSCs.23 The stages of the cell cycle in proliferating human ADSCs were studied at different timepoints. The growth kinetics of cultured human ADSCs consistently represented a sigmoidal growth curve in all the 5 passages demonstrating an exponential pattern of growth. The cell cycle pattern also revealed that after 72 h the cell division is slowed down in the cultures. Myung et al. reported that the integrity of the genome and genetic stability is protected in the S phase of the cell cycle.24
In vitro expanded human ADSCs were chromosomally stable without any clonal alterations. Their genetic integrity was maintained without any chromosomal abnormalities or structural aberrations until Passage 5. These results are beneficial to determine the quality of the ADSCs for therapeutic translation. Unaltered karyotypes can be considered one of the important quality indicators of MSCs in clinical implications. Similar to Borgonovo et al., the data of this study also suggest that conventional karyotyping can be useful as a preliminary tool to determine the chromosomal stability during in vitro expansion of MSCs.25
This study also showed that MSCs are capable of forming osteoblasts, chondrocytes and adipocytes in vitro. Furthermore, our previous studies demonstrated similar trilineage differentiation potential of human ADSCs on various biopolymers.26 Thus, the SVF from adipose tissue holds great promise in tissue engineering and regeneration along with its effective utilization in cell based therapeutics.
Conclusion
In conclusion, advances in cell biology have led to innovative and new therapeutic potentials of using fat tissue for regenerative medicine. The SVF from adipose tissue has a sufficiently large population of MSCs. Particularly, in vitro MSC culture conditions must be validated properly to avoid chromosomal abnormalities. The well characterized autologous human ADSCs could have clinical applicability for cell-based therapies.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kuznetsov S.A., Mankani M.H., Gronthos S., Satomura K., Bianco P., Robey P.G. Circulating skeletal stem cells. J Cell Biol. 2001;153(5):1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Tamer M.K., Reis R.L. Progenitor and stem cells for bone and cartilage regeneration. J Tissue Eng Regen Med. 2009;3(5):327–337. doi: 10.1002/term.173. [DOI] [PubMed] [Google Scholar]
- 3.Sherley J.L. Asymmetric cell kinetics genes: the key to expansion of adult stem cells in culture. Stem Cells. 2002;20(6):561–572. doi: 10.1634/stemcells.20-6-561. [DOI] [PubMed] [Google Scholar]
- 4.Ghaneialvar H., Soltani L., Rahmani H.R., Lotfi A.S., Soleimani M. Characterization and classification of mesenchymal stem cells in several species using surface markers for cell therapy purposes. Indian J Clin Biochem. 2018;33(1):46–52. doi: 10.1007/s12291-017-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelluri L.K., Kancherla R., Turlapati N., Vemuri S., Debnath T., Kumar M.P. Improved differentiation protocol of rat bone marrow precursors to functional islet like cells. Stem Cell Stud. 2011;1:e5. [Google Scholar]
- 6.Fu Y., Karbaat L., Wu L., Leijten J., Both S.K., Karperien M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B: Rev. 2017;23(6):515–518. doi: 10.1089/ten.TEB.2016.0365. [DOI] [PubMed] [Google Scholar]
- 7.Regulski M.J. Mesenchymal stem cells: “guardians of inflammation”. Wounds. 2017;29(1):20–27. [PubMed] [Google Scholar]
- 8.Barui A., Chowdhury F., Pandit A., Datta P. Rerouting mesenchymal stem cell trajectory towards epithelial lineage by engineering cellular niche. Biomaterials. 2018;156:28–44. doi: 10.1016/j.biomaterials.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z., Zhang Y., Hao L., Wang F., Liu D., Su Y. More insight into mesenchymal stem cells and their effects inside the body. Expert Opin Biol Ther. 2010;10:215–230. doi: 10.1517/14712590903456011. [DOI] [PubMed] [Google Scholar]
- 10.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Baer P.C. Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6(3):256–265. doi: 10.4252/wjsc.v6.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young C., Jarrell B.E., Hoying J.B., Williams S.K. A porcine model for adipose tissue derived endothelial cell transplantation. Cell Transplant. 1992;1(4):293–298. doi: 10.1177/096368979200100406. [DOI] [PubMed] [Google Scholar]
- 13.Miyagi-Shiohira C., Kurima K., Kobayashi N., Saitoh I., Watanabe M., Noguchi Y. Cryopreservation of adipose-derived mesenchymal stem cells. Cell Med. 2015;8(1–2):3–7. doi: 10.3727/215517915X689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izadpanah R., Kaushal D., Kriedt C., Tsien F., Patel B., Dufour J. Long term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68(11):4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurgens W.J., Oedayrajsingh-Varma M.J., Helder M.N., Zandiehdoulabi B., Schouten T.E., Kuik D.J. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332(3):415–426. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser J.K., Wulur I., Alfonso Z., Hedrick M.H. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Boquest A.C., Shahdadfar A., Brinchmann J.E., Collas P. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol. 2006;325:35–46. doi: 10.1385/1-59745-005-7:35. [DOI] [PubMed] [Google Scholar]
- 19.van Harmelen V., Skurk T., Röhrig K., Lee Y.M., Halbleib M., Aprath-Husmann I. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27(8):889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- 20.Pal R., Hanwate M., Jan M., Totey S. Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2009;3(3):163–174. doi: 10.1002/term.143. [DOI] [PubMed] [Google Scholar]
- 21.Nekanti U., Rao V.B., Bahirvani A.G., Jan M., Totey S., Ta M. Long-term expansion and pluripotent marker array analysis of Wharton's jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19(1):117–130. doi: 10.1089/scd.2009.0177. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd Z.E., Kloster A. Immunophenotype of human adipose derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 23.Gokhale A.G., Chelluri L.K., Kumaresan K., Subramanyam G., Sudhakar K., Vemuri S. Evaluation of the autologous bone marrow mononuclear therapy and functional restoration in the scarred myocardium by imaging analysis. J Cardiovasc Dis Res. 2011;2(2):133–136. doi: 10.4103/0975-3583.83037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myung K., Datta A., Kolodner R.D. Suppression of spontaneous chromosomal rearrangements by S phase checkpoint functions in Saccharomyces cerevisiae. Cell. 2001;104(3):397–408. doi: 10.1016/s0092-8674(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 25.Borgonovo T., Vaz I.M., Senegaglia A.C., Rebelatto C.L., Brofman P.R. Genetic evaluation of mesenchymal stem cells by G-banded karyotyping in a Cell Technology Center. Rev Bras Hematol Hemoter. 2014;36(3):202–207. doi: 10.1016/j.bjhh.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debnath T., Ghosh S., Potlapuvu U.S., Kona L., Kamaraju S.R., Sarkar S. Proliferation and differentiation potential of human adipose-derived stem cells grown on chitosan hydrogel. PLOS ONE. 2015;10(3):e0120803. doi: 10.1371/journal.pone.0120803. [DOI] [PMC free article] [PubMed] [Google Scholar]