Abstract
Background
Soil salinization is one of the most crucial abiotic stresses that limit the growth and production of eggplant. The existing researches in eggplant were mostly focused on salt-induced morphological, biochemical and physiological changes, with only limited works centered on salt-response genes in eggplant at the transcriptomic level.
Results
Our preliminary work found that Zhusiqie (No.118) is salt-tolerant and Hongqie (No.30) is salt-sensitive. Consequently, they were re-named as ST118 and SS30, respectively. ST118 showed less damaged on growth and higher K+/Na+ ratios in leaves than SS30. Comparative-transcriptome analysis was used as a powerful approach to understand the salt-response mechanisms in the leaves and roots of SS30 and ST118. And it revealed that genotype-specific and organ-specific manners exist in eggplant in response to salt stress. Strikingly, the genotype-specific differentially expressed genes (DEGs) in ST118 were considered crucial to its higher salt-tolerance, because the expression patterns of common DEGs in the leaves/roots of the two eggplant genotypes were almost the same. Among them, some transcription factors have been reported to be in response to elevated external salinity, including the members of C2C2-CO-like, WRKY, MYB and NAC family. In addition, the AKT1, KAT1 and SOS1 were up-regulated only in the leaves of ST118. Furthermore, the complementation assays demonstrated that the salt-tolerances of both yeast and Arabidopsis akt1 mutants were enhanced by heterologous expression of SmAKT1.
Conclusion
The comparative-transcriptome analysis indicated that the salt-tolerance can be increased by higher transcript level of some genotype-specific genes. This work revealed that eggplants seem to be more inclined to absorb K+ rather than to exclude Na+ under salt stress conditions because seven K+ transporters were significantly up-regulated, while only one Na+ transporter was similarly regulated. Finally, the complementation assays of SmAKT1, which is genotype-specific up-regulated in ST118, suggest that the other TFs and K+ transport genes were worthy of future investigation for their functions in salinity tolerance.
Electronic supplementary material
The online version of this article (10.1186/s12870-019-1663-8) contains supplementary material, which is available to authorized users.
Keywords: Eggplant (Solanum melongena L.), Salt stress, Comparative-transcriptome, Genotype-specific expression, SmAKT1
Background
Soil salinity is one of the most important factors that limit plant growth, development, and productivity. According to the data from the FAO (Food and Agriculture Organization of the United Nations), food production should be increased by 70% in the world to meet the requirement of increasing population (http://www.fao.org/documents/card/en/c/a2128b09-361c-5468-9d93-2189cc430234/). In order to develop and utilize the salinized soil as much as possible, it is necessary to understand the salt-response mechanisms of crop plants.
To cope with salt stress, plants developed various protective mechanisms from the physiological and biochemical to the cellular and molecular level. On the molecular level, genes functioning in stress signaling, transcription regulation, ion transport and biosynthesis of specific metabolites are involved in responding to salt stress [1–4]. Transcription factors (TFs) involved in the regulation of salt-response could be activated by multiple signal transduction pathways in plants, such as the ABA-mediated signal [1, 5, 6]. Previous studies reported that the members of TF family genes were differentially expressed in response to elevated external salinity [7], including the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/EREBP) [8], basic leucine zipper (bZIP) [6, 9], NAC [10, 11], basic helix–loop–helix (bHLH) [12], MYB [13–15] and WRKY [16, 17] gene families. In turn, these TFs could amplify the signals for gene regulation and promote the protective mechanisms in plants.
The major damage caused by excess salt was ion toxicity (mainly Na+) except water deficiency that is different from drought stress. The salt-overly-sensitive (SOS) signal transduction pathway has been described as crucial for cellular Na+ detoxification and maintaining intracellular ion homeostasis in plants [18–20]. However, excessive accumulation of Na+ under salt stress would be accompanied by K+ deficiency. Because of the similarity in physicochemical properties between Na+ and K+ (i.e. ionic radius and ion hydration energy), the root cells absorbed excessive Na+ instead of K+ under the saline soil [21], and ion homeostasis in plant cells could be destructed. K+ is one of the most important elements that is required by the key metabolic processes in the cytoplasm, including enzymatic reactions, protein synthesis, and ribosome functions [22]. Thus, K+/Na+ ratio is likely to be one of the key determinants of plant salt tolerance [22] and maintaining a high cytosolic K+/Na+ ratio is very important [23].
Eggplant (Solanum melongena L.) is an important greenhouse crop for out of season production and cultivated on more than 1.5Mha in the world [24]. Eggplant is considered as moderately sensitive to salinity with a very low threshold value [25, 26]. The existing researches in eggplant were focused on salt-induced morphological, biochemical and physiological changes [27–30]. However, there was limited work on salt-response genes in eggplant at the transcription level. Comparative genome and transcriptome have been extensively used as a powerful approach for discovering the genetic information involved in stress tolerance [31–33]. A number of transcriptomic comparisons have been done between salt-sensitive and salt-tolerant genotypes of plant species, such as Arabidopsis [34], Oryza sativa [3] and tomato [35]. Here, comparative transcriptome was used for the first time to explore the molecular mechanisms of salinity tolerance in eggplant.
In this work, the leaves and roots of two eggplant genotypes were exposed to salt-tolerant and comparative-transcriptome analysis under salt conditions. We successfully identified several TFs and ion transporters which might be crucial for the salt-tolerant eggplant genotype ST118 under salt conditions. In particular, a differentially expressed ion transporter was identified and functional verified which is potentially associated with eggplant responses and adaptability to salt conditions.
Methods
Plant material and growth conditions
Uniformly germinated eggplant seeds were selected and transplanted into growing trays with vermiculite and kept in growth chamber with 16/8 h light/dark photoperiod at 25/16 °C, respectively. About 1-months-old eggplant plants with four-true-leaves were treated with 200 mM NaCl. Roots and leaves for RNA extraction and ion content measurement were harvested at 0, 6, 12, 24, 48, 72, 168 h (7d) and 23d after stress treatments, respectively. After salt stress treatment for 23d, the phenotypic and physiological characteristics were inspected and measured. The samples at all the time points were used for ion content analysis. Based on the ion content difference between the two eggplant genotypes, samples collected at 0 h and 12 h were chosen for transcriptomic analysis.
The Arabidopsis (Arabidopsis thaliana) wild-type, mutant and transgenic plants used in this study were Columbia-0 ecotype (Col). The Arabidopsis seeds were germinated on Murashige and Skoog (MS) medium containing 0.8% (w/v) agar and 3% sucrose at 4 °C for 3 days. Then plates were incubated in a controlled-environment growth chamber. 3 days later, uniformly germinated seeds were chosen for low K+ or salt stress tests.
Ion content measurement
All the samples were dried at 105 °C for 30 min, and then kept at 75 °C for 4 days. The grinded samples were digested in 20 ml HNO3, then added 5 mL HClO4 at room temperature. After overnight digestion, HNO3 and HClO4 were removed by heating. The digested samples were diluted with ddH2O. The Na+ and K+ contents were then measured by inductively coupled plasma optical (ICP-AES, iCAP7600).
RNA extraction, library construction and illumina sequencing
Total RNA was extracted by the MiniBEST Universal RNA Extraction Kit (TaKaRa) according to the manufacturer’s instructions. The total RNA sample quality control (QC), library construction and sequencing on BGISEQ-500 was performed at Beijing Genomics Institute (BGI). The Agilent 2100 Bio analyzer (Agilent RNA 6000 Nano Kit) was used to do the total RNA sample QC, including RNA concentration, RIN value, 28S/18S and the fragment length distribution. The mRNA was enriched by magnetic beads with Oligo (dT) and then fragment the RNA and reverse transcription to double-strand cDNA (dscDNA) by N6 random primer. The synthesized cDNA was subjected to end-repair and then was 3′ adenylated. Adaptors were ligated to the ends of these 3′ adenylated cDNA fragments. The ligation products were purified and PCR amplification was performed to enrich the purified cDNA template using PCR primer. Lastly, the PCR products were denatured by heat and the single strand DNA was cyclized by splint oligo and DNA ligase. Then, the libraries were used for sequencing with the sequencing platform BGISEQ-500 (BGI), and the products were called as ‘raw reads’. All the generated raw sequencing reads were filtered to remove the low quality reads by the software SOAPnuke (BGI). After filteration, the remaining reads are called ‘Clean Reads’ and stored in FASTQ format.
Bioinformatics analysis
After QC analysis, the clean reads were assembled into Unigenes and mapped to the eggplant genome sequences (http://eggplant.kazusa.or.jp/) [36] by HISAT (Hierarchical Indexing for Spliced Alignment of Transcripts) [37]. The gene expression level was calculated with RSEM [38]. Pearson’s correlation was exploited to calculate the relevance between all samples [39]. The differentially expressed genes (DEGs) were detected with DEGseq [40], which is based on the Poisson distribution. Combining the strategies described by Y Benjamini and Y Hochberg [41] and JD Storey and R Tibshirani [42], the P-values was adjusted as Q-values. And the threshold of Q-values ≤0.001 and an absolute Log2Ratio value ≥1 among the three biological replicates were used to determine whether a gene was DEG. The sequences of DEGs were compared with the NCBI non-redundant (Nr) database to identify and annotate the obtained DEGs using Blast software [43, 44].
Gene ontology (GO) functional classification of the identified DEGs was performed using Blast2GO [45]. GO enrichment analysis of the DEGs was conducted according to the information from GO databases (http://wego.genomics.org.cn/). Then we calculate the false discovery rate (FDR) for each p-value, in general, the terms which FDR ≤ 0.01 are defined as significant enriched. As for transcription factor prediction, getorf was used to find ORF of each DEG and then ORF was aligned to TF domains (from PlntfDB) using hmmsearch [46].
Quantitative real-time PCR analysis
Total RNA was extracted by the MiniBEST Universal RNA Extraction Kit (TaKaRa). 500 ng RNA was transcribed into cDNA with the PrimeScript™ RT Master Mix (Perfect Real Time) (Takara). According to the manufacturer’s instructions of SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara), qRT-PCR was performed on CFX Real Time PCR Detection System (BioRAD) using the following procedure: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The Smactin (Sme2.5_00072.1_g00003.1) from eggplant and AtACT2 (AT3G18780.1) were amplified in parallel as internal reference genes, respectively. The relative expression levels of the amplified products were analyzed using the comparative CT method based on CT values [47]. All primers used in this study are listed in Additional file 1.
Analysis of the protein structure
The protein sequences of AKT1 from 9 plant species were searched in the Pfam database (http://pfam.xfam.org/) and NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Then the sequences of each putative conserved domains were obtained using ClustalX (version 1.83) [48] and WebLogo 3 (http://weblogo.threeplusone.com/create.cgi). For phylogenetic analysis, ClustalX (version 1.83) and MEGA 6.0 [49] programs were used to construct neighbor-joining (NJ) tree with the following parameters: poission model, complete deletion and bootstrap (1000 replicates; random seed).
Yeast complementation
The coding sequences of SmAKT1 and AtAKT1 were constructed into pYES2.0 vector and transformed into the yeast strain R5421 (trk1△ trk2△), in which the two endogenous K+ transporter genes (TRK1, 2) were deleted. The yeast complementation assay was done as described by J Li, et al. [50]. After 5 days, all the plates were examined and photographed. Three independent experiments were performed.
Generation of Arabidopsis transgenic plants
Full-length coding sequence of SmAKT1 was constructed into the overexpression pHB vector. The construct was transformed into Arabidopsis akt1 mutant. The Arabidopsis was transformed by the floral dip method with Agrobacterium [51]. The T4 homozygous transgenic plants were used to examine the phenotype under low-K+ [50] or salt stress conditions. The expression of targeted genes in complementary plants was detected using qRT-PCR.
Salt tolerance assays of transgenic Arabidopsis
The K+ deficiency assay was done as described previously [52]. The phenotype was observed after low-K+ treatment for 7 d. For salt tolerance assays of transgenic Arabidopsis, 3-week-old wild-type, mutant and complementary plants were subjected to 200 mM NaCl treatment three times a week. The rosette leaves and roots of each Arabidopsis lines were collected for genes expression analysis after NaCl treatment for 0, 12 h and 7 days.
Chlorophyll a fluorescence
Chlorophyll a fluorescence of Arabidopsis leaves was determined with the pulse-amplitude-modulated chlorophyll fluorescence system (PAM; Heinz Walz, GmbH, Effeltrich, Germany). Plants were kept in darkness for 30 min to quantify photosystem II (PSII; Fv/Fm)) maximum efficiency using the saturation pulse method: Fv/Fm = (Fm – F0)/Fm [53, 54]. Data are the means of 6 replicates.
Data analysis
All data were presented as means with standard errors. The data were analyzed using SPSS 17.0 by one-way analysis of variance (ANOVA). Significance statistical analysis was calculated by Duncan’s Multiple Range test at significance levels of P < 0.05 and P < 0.01.
Results
Effect of salt stress on two eggplant genotypes
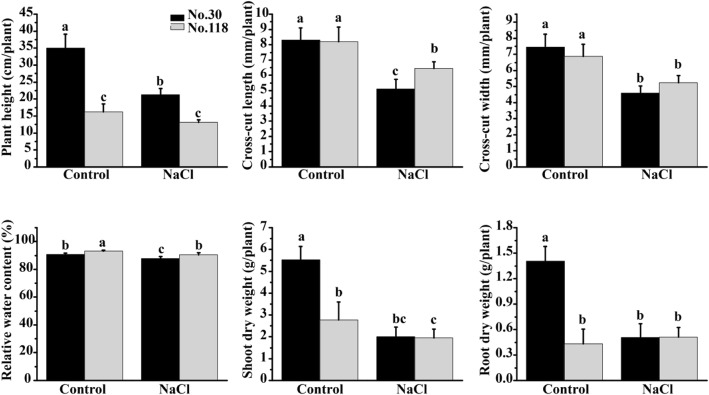
We investigated the salt tolerances of two eggplant genotypes, Hongqie (No.30) and Zhusiqie (No.118), four leaf-stage seedlings were irrigated with 200 mM NaCl. After 23 days, the phenotypic and physiological characteristics of No.30 and No.118 under salt conditions were compared with control. The salt-tolerance related trait values were dramatically reduced, and significant differences were detected between No.30 and No.118. As shown in Fig. 1, the plant height (PH), cross-cut length of stem, shoot dry weight (SDW) and root dry weight (RDW) were much reduced by salt stress in No.30 than No.118. However, no differences in the cross-cut width of stem and relative water content (RWC) between No.30 and No.118 were found.
Fig. 1.
Morphological trait performance of two eggplant genotypes measured under control and salt conditions. The cross-cut length and width represent stem development. Bars represent means ± SD of three biological replicates. Columns with different letters indicate significant differences at P < 0.05 (Duncan’s test)
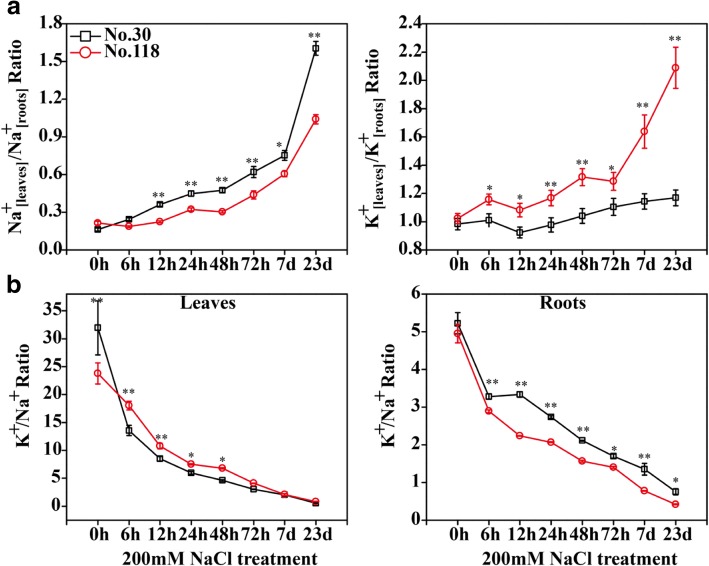
Furthermore, the concentration and distribution of Na+ and K+ were affected along with NaCl stress treatment for 0, 6, 12, 24, 48, 72, 168 h (7 days) and 23 days (Fig. 2 and Additional file 2). The Na+ concentrations increased significantly while K+ concentrations reduced in leaves and roots of both two eggplant genotypes (Additional file 2). As shown in Additional file 2, lower total K+ concentrations (total content covers leaves and roots) but higher total Na+ concentrations were found in No.118 than No.30, and this difference peaked after salt treatment for 12 h. However, the Na+[leaves]/Na+[roots] ratio increased less and the K+[leaves]/K+[roots] ratio increased more in No.118 than in No.30 (Fig. 2a). In addition, the K+/Na+ ratios were gradually reduced in both leaves and roots along with the salt treatment, while a higher decrease found in No.30 leaves and No.118 roots (Fig. 2b).
Fig. 2.
The distribution of Na+ and K+ and the change of K+/Na+ ratio in leaves/roots of two eggplant genotypes along with salt treatment. a The distribution of Na+ and K+ in leaves and roots. b The change of K+/Na+ ratio in leaves and roots. Three replicates were used in each time point, with three seedlings per replicate. Bars represent means ± SD of three biological replicates. Duncan’s Multiple Range test (*P < 0.05 and **P < 0.01) was used to analyze statistical significance
These results suggested that K+ in No.118 was preferentially translocated into leaves, resulting in a higher K+/Na+ ratios. They also indicated that No.30 is more salt-sensitive than No.118. Therefore, we named the two eggplant genotypes as SS30 and ST118, respectively. Taken all together, the 0 h and 12 h time points were chosen for exploring the distribution mechanism of K+ and Na+, which might be a crucial point to explain the salt-tolerant difference between the two eggplant genotypes.
Identification of differentially expressed genes in SS30 and ST118 by RNA-seq
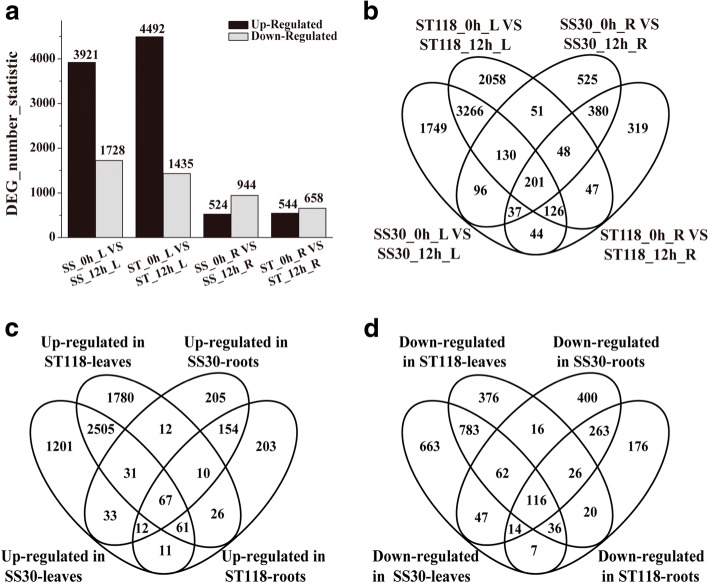
The leaves and roots were harvested from eggplants that have been treated with 200 mM NaCl for 0 h and 12 h, respectively. Using the BGISEQ-500 platform, an average about 24.11 M from 12 leaves samples and 23.77 M reads from 12 roots samples were generated, respectively (Additional file 3). In this project, the average mapping ratios with reference genome were 94.31% (leaves) and 89.85% (roots), the average mapping ratios with genes were 65.27% (leaves) and 58.47% (roots), and total of 50,956 (leaves) and 49,354 (roots) genes were detected. The differentially expressed genes (DEGs) were selected on the basis of DEGseq method with the following parameters: fold change ≥2 and adjusted P-value ≤0.001 [40]. A total of 5649 and 5927 DEGs were obtained in the leaves of SS30 and ST118 (Fig. 3a), while 1468 and 1202 DEGs were obtained in the roots of SS30 and ST118 (Fig. 3a). Subsequently, nine DEGs with different expression pattern were selected to validate the RNA-seq results by qRT-PCR (Additional file 4). Despite some differences, the general expression profiles were conserved between the RNA-seq and qRT-PCR data, which validates the former.
Fig. 3.
Overview and Venn diagrams of up- or down-regulated genes by salt stress in leaves and roots of both two eggplant materials at a level of ≥2-fold and adjusted P-value ≤0.001. a The total number of differentially expressed genes found in the leaves (L) and roots (R) of SS30 and ST118 in the comparison between salt-stressed (12 h) and non-stress treatments (0 h). b Four-way Venn diagram indicating that the DEGs were genotype-specific. The number of salt-up-regulated (c) and -down-regulated (d) genes found exclusively in the roots and leaves of two eggplant genotypes were analyzed
Venn analysis showed that the DEGs identified in both SS30 and ST118 have relatively same expression patterns except 62/3723 (62 out of 3723) in leaves and 4/ 666 in roots (Additional file 5). Among these DEGs, 2664/997 and 243/419 genes were commonly up−/down-regulated in the leaves and roots of both genotypes (Additional file 5). On the other side, only 67/116 genes were commonly up−/down-regulated in both the leaves and roots of the two eggplant genotypes under salt conditions (Fig. 3c, d), indicating that the organ-specific manner adapt to salt stress, observed in rice [2] and Arabidopsis [55], also exists in eggplant.
As shown in Fig. 3b, many DEGs identified under salt conditions were genotype-specific, suggesting that the genotype-specific DEGs might be contributed to the phenotypic differences in salt-tolerance between SS30 and ST118. The Venn diagram in Fig. 3c, d showed that 1201/663 and 205/400 genes were exclusively up−/down-regulated in the leaves and roots of SS30, while 1780/376 and 203/176 genes were exclusively up−/down-regulated in the leaves and roots of ST118. Since ST118 is more salt tolerant than SS30, more attention has been paid to the genotype-specific DEGs in ST118 in the following sections.
Gene ontology analysis of DEGs
The functions of all the DEGs identified in this project were classified by the Gene Ontology (GO) assignments [56]. There were three GO categories including molecular function, biological process and cellular component in leaves and roots of both SS30 and ST118 (Additional file 6). The two largest subcategories found in the three GO categories were consistent, which were ‘metabolic process’ and ‘cellular process’ in the ‘biological process’ category, ‘cell’ and ‘cell part’ in the ‘cellular component’ category, and catalytic activity’ and ‘binding activity’ in the ‘molecular function’ category. Strikingly, the ‘response to stimulus’, ‘transporter activity’ and ‘transcription factor activity, protein binding’ were abundant GO terms.
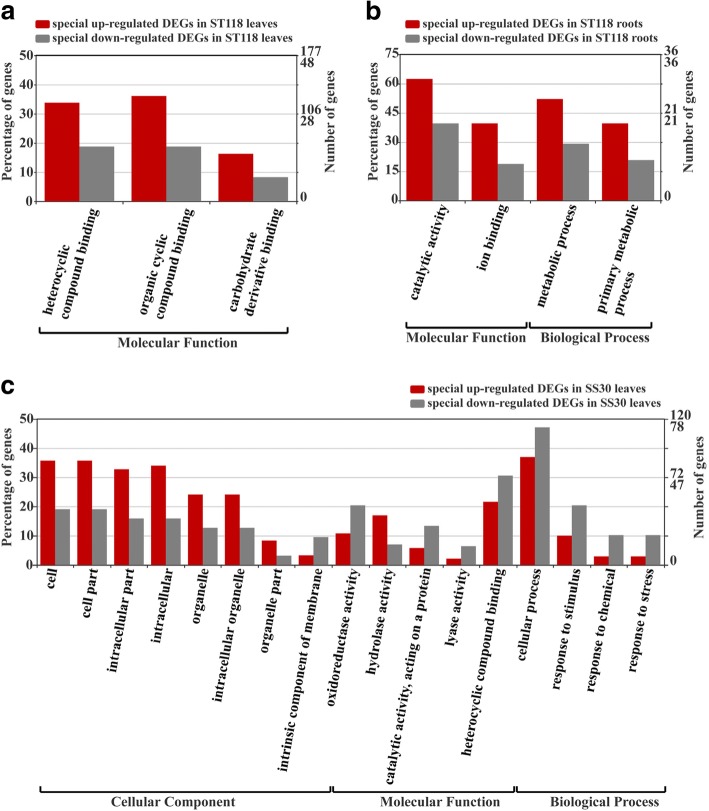
GO terms enriched in the genotype-specific DEGs of SS30 or ST118 were identified using a threshold of P-value < 0.05. In the leaves of ST118, three GO terms were significantly enriched in the ‘molecular function’ category (Fig. 4a). Four GO terms were most abundant in the ‘molecular function’ and ‘biological process’ categories in the roots of ST118 (Fig. 4b). As for SS30, 17 GO terms were distributed in three GO categories including cellular component, molecular function and biological process in the leaves (Fig. 4c), but none GO terms were significantly enriched in the roots. Generally, the up-regulated genes enriched in both leaves and roots ST118 were much more than down-regulated genes, while opposite results showed in the molecular function and biological process categories of SS30. Compared with SS30 leaves, the genes related to ‘organic cyclic compound binding’ and ‘carbohydrate derivative binding’ activities were significantly enriched in ST118 leaves (Fig. 4a). In addition, genes related to ‘ion binding’ activity were exclusively enriched in the roots of ST118. These results suggested that genes with the binding sites for ion, inorganic or organic molecules might play important roles in response to salt stress.
Fig. 4.
GO classification of the genotype-specific DEGs in the leaves/roots of SS30 or ST118. The left y-axis shows the percentages of genes identified, and the right y-axis shows the gene number. The genes were categorized according to the annotation of GO, and the number of every category is displayed based on biological process, cellular components, and molecular functions. The enriched GO terms were identified using a threshold of P-value < 0.05
Differentially expressed transcription factors in SS30 and ST118 caused by salt stress
10 transcription factors (TFs) were found through the analysis of the genes related to ‘heterocyclic compound binding’, ‘organic cyclic compound binding’ and ‘carbohydrate derivative binding’ activities. Considering the crucial role of TFs in response to salt stress, we highlighted the analysis on the TFs that were identified as DEGs in leaves and roots of both SS30 and ST118.
In leaves, a total of 413 TFs were identified as DEGs. Among the 413 TFs, 201 TFs were identified as DEGs in both SS30 and ST118 (named as ST-SS-L-inter), while 120 and 92 TFs were specifically identified as DEGs in SS30 (named as SS30-L-Spe) and ST118 (named ST118-L-Spe), respectively (Additional file 7a). As shown in Additional file 7a, the highest rates of induction by salt stress were observed for genes belonging to the AP2/EREBP, MYB, bHLH, WRKY, NAC, ABI3/VP1, C3H, GRAS and C2C2-Dof families. Strikingly, AP2-EREBP and MYB super-families were the largest in ST118-L-Spe and ST-SS-L-inter, while the WRKY super-family was the largest in the SS30-L-Spe. Members of these identified TFs have been reported to be associated with salt stress responses [8, 17, 57, 58]. Subsequently, the 413 TFs were searched against the Stress Responsive Transcription Factor Database (STIDB) in Arabidopsis [58] for salt-responsive genes. 43 TFs were identified as salt-response genes, including 10 MYBs, 10 NACs, 6 WRKYs, 5 AP2-EREBPs, 3 C2C2-CO-likes (COL), 3 TAZs, 2 bHLHs, 2 Tifys, C3H and G2-like (Table 1). Generally, the expression patterns in majority of TFs were same and most of them were down-regulated by salt stress (Table 1). Strikingly, in ST-SS-L-inter, Sme2.5_00556.1_g00019.1 annotated as WRKY was significantly up-regulated in ST118, but was significantly down-regulated in SS30. In addition, three TFs were slightly up-regulated in ST118 with 0.60~0.99 folds change, but significantly down-regulated in SS30 with − 3.10~ − 1.30 folds change (Table 1), including Sme2.5_03951.1_g00007.1 (annotated as MYB), Sme2.5_03886.1_g00002.1 (annotated as NAC) and Sme2.5_04464.1_g00002.1 (annotated as NAC).
Table 1.
The salt-responsive TFs identified by searching against the Stress Responsive Transcription Factor Database (STIDB) in Arabidopsis in the leaves and roots of the two eggplant materials. SS_0h_L/R-Expression: the mean expression level of each TF after NaCl treatment for 0 h in the leaves/roots of SS30; log2Ratio(s2/s1): Log2(folds of mean expression in two time points); q-value: corrected p-value [41, 42]; Tair10: gene ID in Arabidosis corresponding to the TFs in eggplant
| Gene ID | SS_0h_L-Expression | SS_12h_L-Expression | log2Ratio(SS_12h_L/SS_0h_L) | q-value | ST_0h_L-Expression | ST_12h_L-Expression | log2Ratio(ST_12h_L/ST_0h_L) | q-value | Tair10 | TF family | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ST-SS-L-inter | Sme2.5_05868.1_g00004.1 | 10,615 | 340 | −4.90 | 0.000 | 9160 | 198 | −5.51 | 0.000 | AT5G17300.1 | MYB |
| Sme2.5_02956.1_g00004.1 | 1971 | 82 | −4.52 | 0.000 | 1949 | 53 | −5.18 | 0.000 | AT5G17300.1 | MYB | |
| Sme2.5_02470.1_g00007.1 | 69 | 2 | −5.04 | 0.000 | 26 | 3 | −3.09 | 0.000 | AT1G69490.1 | NAC | |
| Sme2.5_00096.1_g00018.1 | 270.17 | 21.3 | −3.60 | 0.000 | 412.04 | 71.58 | −2.50 | 0.000 | AT5G57550.1 | NAC | |
| Sme2.5_01620.1_g00005.1 | 797 | 204 | −1.90 | 0.000 | 837 | 169 | −2.29 | 0.000 | AT4G37180.1 | G2-like | |
| Sme2.5_08291.1_g00004.1 | 845 | 176 | −2.19 | 0.000 | 728 | 161 | −2.15 | 0.000 | AT1G70000.2 | MYB | |
| Sme2.5_03858.1_g00003.1 | 482 | 133 | −1.79 | 0.000 | 538 | 162 | − 1.71 | 0.000 | AT1G13260.1 | AP2-EREBP | |
| Sme2.5_00276.1_g00022.1 | 788 | 107 | −2.81 | 0.000 | 759 | 229 | −1.71 | 0.000 | AT1G01720.1 | NAC | |
| Sme2.5_11816.1_g00001.1 | 60.47 | 3.95 | −3.87 | 0.000 | 41.65 | 12.75 | −1.69 | 0.000 | AT3G23250.1 | MYB | |
| Sme2.5_01393.1_g00007.1 | 4149 | 1469 | −1.43 | 0.000 | 3623 | 1217 | −1.55 | 0.000 | AT1G05690.1 | TAZ | |
| Sme2.5_15135.1_g00001.1 | 1360 | 291 | −2.16 | 0.000 | 609 | 209 | −1.52 | 0.000 | AT1G69490.1 | NAC | |
| Sme2.5_06280.1_g00002.1 | 3950 | 1499 | −1.33 | 0.000 | 4342 | 1544 | −1.47 | 0.000 | AT5G17300.1 | MYB | |
| Sme2.5_06485.1_g00004.1 | 8176 | 3330 | −1.23 | 0.000 | 8302 | 2979 | −1.46 | 0.000 | AT5G24930.1 | C2C2-CO-like | |
| Sme2.5_01575.1_g00001.1 | 3303 | 881 | −1.84 | 0.000 | 2935 | 1065 | −1.44 | 0.000 | AT3G49530.1 | NAC | |
| Sme2.5_00014.1_g00027.1 | 526.44 | 234 | −1.10 | 0.000 | 723.52 | 281 | −1.34 | 0.000 | AT1G06180.1 | MYB | |
| Sme2.5_04750.1_g00001.1 | 694.26 | 114.48 | −2.53 | 0.000 | 826.05 | 328.84 | −1.31 | 0.000 | AT4G25480.1 | AP2-EREBP | |
| Sme2.5_07791.1_g00001.1 | 7184 | 3294 | −1.06 | 0.000 | 7823 | 3284 | −1.23 | 0.000 | AT3G59060.4 | bHLH | |
| Sme2.5_01200.1_g00003.1 | 215 | 51 | −2.01 | 0.000 | 428 | 192 | −1.13 | 0.000 | AT1G19180.1 | Tify | |
| Sme2.5_00556.1_g00019.1 | 57 | 6 | −3.18 | 0.000 | 55 | 125 | 1.21 | 0.000 | AT1G80840.1 | WRKY | |
| Sme2.5_00912.1_g00004.1 | 380 | 800 | 1.14 | 0.000 | 369 | 965 | 1.41 | 0.000 | AT3G47600.1 | MYB | |
| Sme2.5_00183.1_g00008.1 | 1957.44 | 3883.65 | 1.06 | 0.000 | 1410.45 | 4358.31 | 1.65 | 0.000 | AT5G58620.1 | C3H | |
| Sme2.5_00196.1_g00009.1 | 28 | 4583 | 7.42 | 0.000 | 8 | 4224 | 9.07 | 0.000 | AT3G07650.4 | C2C2-CO-like | |
| ST118-L-Spe | Sme2.5_00956.1_g00005.1 | 3381 | 2072 | −0.64 | 0.000 | 4888 | 1138 | −2.08 | 0.000 | AT3G48360.1 | TAZ |
| Sme2.5_25982.1_g00001.1 | 3698 | 1883 | −0.90 | 0.000 | 3651 | 1404 | −1.36 | 0.000 | AT5G57660.1 | C2C2-CO-like | |
| Sme2.5_08533.1_g00002.1 | 36.34 | 19.06 | −0.86 | 0.037 | 60.75 | 26.25 | −1.19 | 0.000 | AT3G23250.1 | MYB | |
| Sme2.5_00641.1_g00007.1 | 157 | 157 | 0.07 | 0.488 | 295.19 | 129 | −1.17 | 0.000 | AT4G27410.2 | NAC | |
| Sme2.5_06310.1_g00004.1 | 296.78 | 158.58 | −0.84 | 0.000 | 292.67 | 129.1 | −1.16 | 0.000 | AT2G36800.1 | WRKY | |
| Sme2.5_00332.1_g00002.1 | 8112.3 | 4172.0 | −0.89 | 0.000 | 8253.0 | 3671.4 | −1.15 | 0.000 | AT1G19000.2 | MYB | |
| Sme2.5_12868.1_g00001.1 | 6358 | 3737 | −0.70 | 0.000 | 5384 | 2440 | −1.12 | 0.000 | AT3G16770.1 | AP2-EREBP | |
| Sme2.5_00048.1_g00015.1 | 481 | 727 | 0.66 | 0.000 | 1070 | 2124 | 1.01 | 0.000 | AT5G63160.1 | TAZ | |
| Sme2.5_04588.1_g00001.1 | 200 | 213 | 0.16 | 0.240 | 137 | 333 | 1.30 | 0.000 | AT4G28140.1 | AP2-EREBP | |
| Sme2.5_00556.1_g00018.1 | 47 | 71 | 0.66 | 0.017 | 14 | 63 | 2.19 | 0.000 | AT1G80840.1 | WRKY | |
| SS30-L-Spe | Sme2.5_29353.1_g00001.1 | 432.73 | 0 | −9.69 | 0.000 | 24.3 | 19.19 | −0.32 | 0.368 | AT4G27410.3 | NAC |
| Sme2.5_03951.1_g00007.1 | 77 | 8 | −3.20 | 0.000 | 59 | 96 | 0.72 | 0.003 | AT3G23250.1 | MYB | |
| Sme2.5_04750.1_g00004.1 | 112.81 | 13.96 | −2.95 | 0.000 | 93.19 | 46.66 | −0.98 | 0.000 | AT4G25480.1 | AP2-EREBP | |
| Sme2.5_03886.1_g00002.1 | 48 | 6 | −2.93 | 0.000 | 32 | 63 | 1.00 | 0.001 | AT2G43000.1 | NAC | |
| Sme2.5_04190.1_g00001.1 | 110 | 16 | −2.71 | 0.000 | 121 | 92 | −0.37 | 0.064 | AT1G80840.1 | WRKY | |
| Sme2.5_01372.1_g00013.1 | 5293 | 1538 | −1.71 | 0.000 | 4593 | 3439 | −0.40 | 0.000 | AT1G80840.1 | WRKY | |
| Sme2.5_04464.1_g00002.1 | 55 | 22 | −1.25 | 0.000 | 34 | 51 | 0.61 | 0.059 | AT2G43000.1 | NAC | |
| Sme2.5_02104.1_g00004.1 | 4983 | 2298 | −1.05 | 0.000 | 3591 | 3810 | 0.11 | 0.002 | AT1G32640.1 | bHLH | |
| Sme2.5_04924.1_g00003.1 | 3418 | 1595 | −1.03 | 0.000 | 3295 | 1821 | −0.83 | 0.000 | AT1G19180.1 | Tify | |
| Sme2.5_04168.1_g00003.1 | 5649 | 2674 | −1.01 | 0.000 | 3244 | 1645 | −0.96 | 0.000 | AT1G01720.1 | NAC | |
| Sme2.5_15021.1_g00001.1 | 232 | 566 | 1.36 | 0.000 | 352 | 622 | 0.84 | 0.000 | AT2G30590.1 | WRKY | |
| GeneID | SS30_0h-Expression | SS30_12h-Expression | log2Ratio(SS30_12h/SS30_0h) | q-value(Storey et al. 2003) | ST118_0h-Expression | ST118_12h-Expression | log2Ratio(ST118_12h/ST118_0h) | q-value(Storey et al. 2003) | Tair 10 | TF family | |
| ST-SS-R-inter | Sme2.5_02956.1_g00004.1 | 1051 | 128 | −3.04 | 0.000 | 1040 | 51 | −4.43 | 0.000 | AT5G17300.1 | MYB |
| Sme2.5_25982.1_g00001.1 | 1392 | 197 | −2.82 | 0.000 | 1473 | 84 | −4.21 | 0.000 | AT5G57660.1 | C2C2-CO-like | |
| Sme2.5_05868.1_g00004.1 | 937 | 123 | −2.93 | 0.000 | 844 | 88 | −3.34 | 0.000 | AT5G17300.1 | MYB | |
| Sme2.5_06280.1_g00002.1 | 2912 | 512 | −2.51 | 0.000 | 2233 | 478 | −2.30 | 0.000 | AT5G17300.1 | MYB | |
| Sme2.5_06485.1_g00004.1 | 969 | 401 | −1.27 | 0.000 | 1205 | 372 | −1.77 | 0.000 | AT5G24930.1 | C2C2-CO-like | |
| Sme2.5_07791.1_g00001.1 | 415 | 166 | −1.32 | 0.000 | 310 | 148 | −1.14 | 0.000 | AT3G59060.4 | bHLH | |
| Sme2.5_08000.1_g00008.1 | 140 | 392 | 1.49 | 0.000 | 151 | 571 | 1.84 | 0.000 | AT3G22830.1 | HSF | |
| Sme2.5_00196.1_g00009.1 | 72 | 794 | 3.46 | 0.000 | 30 | 915 | 4.85 | 0.000 | AT3G07650.4 | C2C2-CO-like | |
| ST118-R-Spe | Sme2.5_00956.1_g00005.1 | 1900 | 1231 | −0.62 | 0.000 | 1857 | 722 | −1.44 | 0.000 | AT3G48360.1 | TAZ |
| Sme2.5_08291.1_g00004.1 | 1321 | 684 | −0.95 | 0.000 | 1487 | 702 | −1.16 | 0.000 | AT1G70000.2 | MYB | |
| Sme2.5_00374.1_g00013.1 | 1098 | 606 | −0.86 | 0.000 | 889 | 462 | −1.02 | 0.000 | AT1G78080.1 | AP2-EREBP | |
| Sme2.5_06157.1_g00002.1 | 1512 | 2700 | 0.84 | 0.000 | 1314 | 3453 | 1.32 | 0.000 | AT3G16770.1 | AP2-EREBP | |
| Sme2.5_00048.1_g00015.1 | 3223 | 2556 | −0.33 | 0.000 | 2486 | 6901 | 1.39 | 0.000 | AT5G63160.1 | TAZ | |
| SS30-R-Spe | Sme2.5_29353.1_g00001.1 | 218.17 | 7.6 | −4.84 | 0.000 | 0 | 5.73 | 3.44 | 0.061 | AT4G27410.3 | NAC |
| Sme2.5_02470.1_g00007.1 | 160 | 20 | −3.00 | 0.000 | 51 | 27 | −1.00 | 0.009 | AT1G69490.1 | NAC | |
| Sme2.5_15135.1_g00001.1 | 48 | 12 | −2.00 | 0.000 | 14 | 17 | 0.20 | 0.651 | AT1G69490.1 | NAC | |
| Sme2.5_04464.1_g00002.1 | 56 | 16 | −1.81 | 0.000 | 27 | 17 | −0.75 | 0.159 | AT2G43000.1 | NAC | |
| Sme2.5_03858.1_g00003.1 | 491 | 157 | −1.64 | 0.000 | 350 | 301 | −0.30 | 0.023 | AT1G13260.1 | AP2-EREBP | |
| Sme2.5_03434.1_g00003.1 | 77 | 26 | −1.56 | 0.000 | 67 | 51 | −0.47 | 0.139 | AT2G31180.1 | MYB | |
| Sme2.5_24078.1_g00001.1 | 4527 | 2049 | −1.14 | 0.000 | 4664 | 2685 | −0.87 | 0.000 | AT5G67480.2 | TAZ | |
| Sme2.5_09948.1_g00004.1 | 41.98 | 83.96 | 1.00 | 0.001 | 41.92 | 59.97 | 0.44 | 0.208 | AT5G26660.1 | MYB |
In roots, 147 TFs were obtained including 58 ST-SS-R-inter TFs, 56 SS30-R-Spe and 33 ST118-R-Spe TFs (Additional file 7b), and the highest rates of TFs belong to AP2/EREBP, MYB and bHLH families. After searching against STIDB in Arabidopsis [58], 21 TFs were found to be salt-response genes (Table 1), including 6 MYBs, 4 NACs, 3 AP2-EREBPs, 3 COLs, 3 TAZs, bHLH and HSF. Among the 21 TFs, 11 TFs could also be identified as DEGs in leaves with the same expression pattern. Of the 11 TFs, one C2C2-CO-like family member was highly up-regulated by salt-stress with 9.1/3.5 and 7.4/4.9 folds in the leaves/roots of SS30 and ST118, respectively.
These results indicated that the basal salt-resistance mechanism was the same in eggplant varieties, but the specifically up-regulated TFs in SS118 might make a positive contribution to its salt-tolerance.
Identification of the DEGs related to ion transport in SS30 and ST118 under salt condition
K+/Na+ ratio is one of the key determinants of plant salt tolerance, and significant difference was found between SS30 and ST118. Although the ‘transporter activity’ category was enriched in both SS30 and ST118, the number and the members of genes were different. Analysis of these genes involved in the ‘transporter activity’ category showed that 43 DEGs belonged to ST-SS-L-inter, while 24 and 16 DEGs belonged to ST118-L-Spe and SS30-L-Spe, respectively.
In the ST-SS-L-inter category, five DEGs were identified as K+ transporter or K+ channel protein compared with the NCBI non-redundant (Nr) database [43] and all of them were upregulated by salt stress (Table 2). Except the five genes encoding K+ transporters or K+ channel proteins, another K+ transporter gene (AKT1) and K+ channel gene (KAT1) were specifically up-regulated by salt stress in ST118. Strikingly, the ‘salt overly sensitive’ (SOS1) gene was exclusively up-regulated in ST118 but was slightly down-regulated in SS30 (Table 2). However, no more genes related to K+ and Na+ homeostasis were found in SS30-L-spe. The specifically up-regulated expression of AKT1, KAT1 and SOS1 in ST-118 during salinity stress would be expected to stabilize the K+/Na+ ratio in leaves (Fig. 2b).
Table 2.
The different expressed genes related to K+ and Na+ transport in the leaves of the two eggplant materials
| Gene ID | SS_0h_L-Expression | SS_12h_L-Expression | log2Ratio(SS_12h_L/SS_0h_L) | q-value | ST_0h_L-Expression | ST_12h_L-Expression | log2Ratio(ST_12h_L/ST_0h_L) | q-value | Tair10 | Transporter name | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ST-SS-L-inter | Sme2.5_08678.1_g00002.1 | 260.9 | 560.85 | 1.17 | 0.000 | 212.94 | 457.93 | 1.13 | 0.000 | AT2G26650.1 | AKT1-like |
| Sme2.5_09079.1_g00001.1 | 9.1 | 32.15 | 1.89 | 0.000 | 5.06 | 14.07 | 1.50 | 0.000 | AT2G26650.1 | AKT1 | |
| Sme2.5_02726.1_g00002.1 | 306.99 | 698.29 | 1.25 | 0.000 | 344.47 | 971.36 | 1.52 | 0.000 | AT2G40540.2 | potassium transporter 2 | |
| Sme2.5_00325.1_g00013.1 | 102.57 | 949.18 | 3.28 | 0.000 | 111.92 | 1011.9 | 3.20 | 0.000 | AT5G55630.2 | K+ channel protein | |
| Sme2.5_30443.1_g00001.1 | 60.36 | 729.87 | 3.66 | 0.000 | 31.83 | 1026.2 | 5.03 | 0.000 | AT5G55630.2 | K+ channel protein | |
| ST118-L-spe | Sme2.5_00191.1_g00006.1 | 1541 | 2318 | 0.66 | 0.000 | 1140 | 2338 | 1.06 | 0.000 | AT5G46240.1 | KAT1 |
| Sme2.5_00439.1_g00001.1 | 836 | 1349 | 0.76 | 0.000 | 638 | 1452 | 1.21 | 0.000 | AT2G26650.1 | AKT1 | |
| Sme2.5_05879.1_g00004.1 | 202 | 158 | −0.29 | 0.069 | 69 | 169 | 1.31 | 0.000 | AT2G01980.1 | SOS1 | |
| GeneID | SS_0h_R-Expression | SS_12h_R-Expression | log2Ratio(SS_12h_R/SS_0h_R) | q-value | ST_0h_R-Expression | ST_12h_R-Expression | log2Ratio(ST_12h_R/ST_0h_R) | q-value | |||
| In Roots | Sme2.5_08678.1_g00002.1 | 390.71 | 354.69 | −0.14 | 0.289 | 364.86 | 299.73 | −0.36 | 0.004 | ||
| Sme2.5_09079.1_g00001.1 | 64.29 | 44.31 | −0.54 | 0.109 | 32.14 | 38.27 | 0.17 | 0.604 | |||
| Sme2.5_02726.1_g00002.1 | 217.01 | 286.87 | 0.40 | 0.006 | 332.17 | 404.02 | 0.20 | 0.106 | |||
| Sme2.5_00325.1_g00013.1 | 553.43 | 699.6 | 0.34 | 0.000 | 461.18 | 731.5 | 0.59 | 0.000 | |||
| Sme2.5_30443.1_g00001.1 | 266.8 | 388.9 | 0.55 | 0.000 | 286.51 | 463.58 | 0.62 | 0.000 | |||
| Sme2.5_00191.1_g00006.1 | 3 | 2 | −0.58 | 0.642 | 3 | 4 | 0.34 | 0.683 | |||
| Sme2.5_00439.1_g00001.1 | 3863 | 3152 | −0.29 | 0.000 | 3710 | 3041 | −0.36 | 0.000 | |||
| Sme2.5_05879.1_g00004.1 | 238 | 287 | 0.27 | 0.069 | 242 | 305 | 0.26 | 0.080 |
In the roots of both salt-tolerant and salt-sensitive eggplant varieties, none genes related to K+ and Na+ homeostasis was identified as DEG. Further analysis showed that the 8 ion transporter genes identified in leaves remained higher expression level in roots under both control and salt condition comparing with leaves, except for Sme2.5_00191.1_g00006.1 (KAT1) and Sme2.5_02726.1_g00002.1 (a K+ transporter gene) (Table 2). This might be the reason that salinity tolerance is more related to the fine tuning of the ion transporter genes rather than to significant up−/down-regulate these genes by salt stress in roots [3].
It is well known that SOS signaling pathway was the first demonstrated regulator in mediating Na+ extrusion in Arabidopsis and later in other plant species [23, 59–61]. Here, more genes closely related to K+ absorption than those related to Na+ extrusion were found to be up-regulated, indicating that K+ absorption is equally important with Na+ extrusion for maintaining K+ and Na+ homeostasis in plants under salt conditions.
Functional characterization of SmAKT1 in yeast and Arabidopsis under salt conditions
A series of studies showed that AKT1 plays an important role on resisting low-K+ stress in plants [62–64]. However, the function of AKT1 in eggplant under low K+-starvation and salt stress has not been report so far. The full-length amino acid sequences of the two identified AKT1s (Sme2.5_09079.1_g00001.1 and Sme2.5_00439.1_g00001.1) in eggplant together with AKT1 from the other nine plant species were aligned separately and a bootstrapped consensus neighbor-joining (NJ) tree was inferred for SmAKT1 (Additional file 8). As shown in Additional file 8, Sme2.5_00439.1_g00001.1 had the highest degree of similarity with AKT1s from the other plant species. In addition, the typically conserved domains of AKT1 were also found in an 884 amino acid polypeptide of Sme2.5_00439.1_g00001.1 (Additional file 9). Taken together, the Sme2.5_00439.1_g00001.1 could be named as SmAKT1.
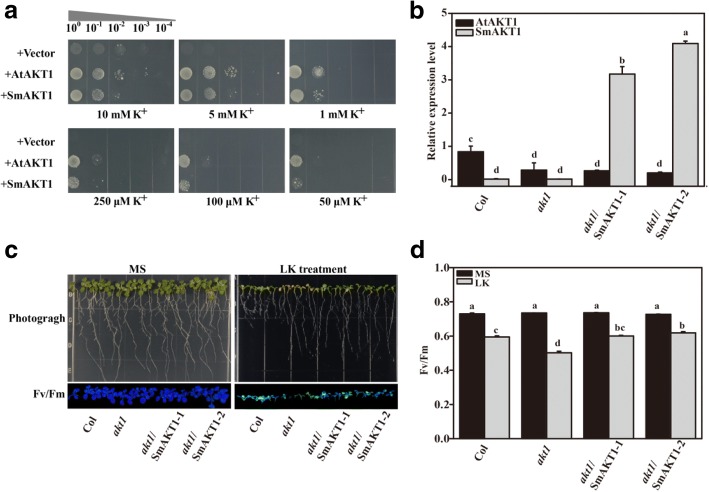
Subsequently, the K+ transport activity of SmAKT1 was tested in the auxotrophic yeast mutant strain R5421 (trk1△, trk2△) [50, 65, 66] and Arabidopsis akt1 mutant [52, 62, 64], respectively. The complementation assays in yeast showed that along with the decline of K+ concentration, the growth of R5421 with empty vector was significantly depressed while both SmAKT1 and AtAKT1 could rescue the growth defect of R5421 mutant (Fig. 5a). In addition, the K+ deficiency symptoms phenotype of akt1 mutant was rescued in the two complementary Arabidopsis lines (akt1/SmAKT1–1 and akt1/SmAKT1–2), which displayed a similar phenotype with wild-type (Col) plants (Fig. 5b-d). These results suggested that SmAKT1 conferred significant K+ uptake in yeasts and Arabidopsis under low K+ concentrations condition.
Fig. 5.
Functional characterization of SmAKT1 in yeast and Arabidopsis under low K+ condition. a SmAKT1 and AKT1 complement the K+ uptake-deficient yeast mutant R5421 on AP medium containing different K+ concentrations. b Real-time PCR verification of SmAKT1 and AKT1 expression in different plant materials. c Phenotype comparison of wild-type Arabidopsis (Col), akt1 mutant and two complementary lines (akt1/SmAKT1–1 and akt1/SmAKT1–2) grown on MS and LK (100 mM K+) medium for 7 d. d Average Fv/Fm values of the whole plant. Bars represent means ± SD of three biological replicates. Columns with different letters indicate significant differences at P < 0.05 (Duncan’s test)
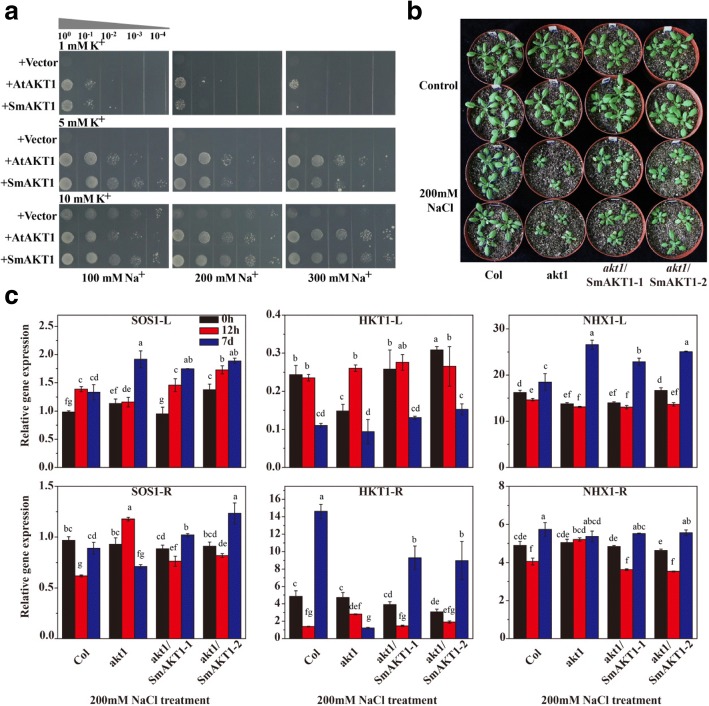
In addition, the transformed yeasts were plated on AP medium containing 1, 5 or 10 mM KCl in combination with 100, 200 or 300 mM NaCl, and the yeasts expressing SmAKT1 and AtAKT1 were able to tolerate higher salt stress than the yeast with empty vector (Fig. 6a). In Arabidopsis, comparing with the WT, the growth of akt1 mutant was inhibited throughout development but was partly recovered in the two complementary lines under control condition (Fig. 6b). These results indicated that SmAKT1 was involved in responding to salt stress.
Fig. 6.
SmAKT1 is involved in response to salt stress in yeast and Arabidopsis under salt condition. a Expression of SmAKT1 and AKT1in yeast mutant strain R5421. Yeast cells were plated on AP medium containing various concentrations of Na+ (100, 200 and 300 mM) with different K+ concentrations (1, 5 and 10 mM). b Phenotype comparison of the four Arabidopsis lines after 200 mM NaCl treatment for 0 and 7 days. c Real-time quantitative PCR analysis of the expression pattern of SOS1, HKT1 and NHX in the four Arabidopsis lines treated without (control) or with 200 mM NaCl for 12 h and 7 days. Bars represent means ± SD of three biological replicates. Columns with different letters indicate significant differences at P < 0.05 (Duncan’s test)
In order to further explore the potential molecular mechanisms underlying the above observations in Arabidopsis, the expression patterns of genes related to Na+ extrusion and transport were analyzed in the four plants under salt conditions with 200 mM NaCl (Fig. 6c). The expression patterns of SOS1, HKT1 and NHX1 in the two complementary lines were all the same with WT in both leaves and roots, while it was completely different with the akt1 mutant, except for NHX1 in leaves after 200 mM NaCl treatment for 0 h, 12 h and 7 days.
Taken together, we speculated that SmAKT1 could enhance the salt tolerance of plants not only through modulating K+ uptake, but also altering Na+ exclusion, transport and homeostasis under salt conditions.
Discussion
Control of the K+ and Na+ distribution is critical for salt-tolerance
In this study, the salt-tolerances of two eggplant genotypes were characterized. By comparison, the SS30 was more significantly affected than ST118 in the phenotypic and physiological attributes by salt stress, including PH, cross-cut length of stem, SDW, RDW and the concentration and distribution of Na+ and K+ (Fig. 1 and Additional file 2). These results were in analogy with previous studies in eggplant [30, 67, 68]. It was well known that K+/Na+ ratio in leaves is an important indicator to measure the salt-tolerance of plants [22, 23]. Here, the K+/Na+ ratio in ST118 was significantly higher in leaves but lower in roots compared with the SS30 (Fig. 2b). Although total K+ concentration was a bit lower in ST118 than in SS30, higher K+[leaves]/K+[roots] ratio were observed in ST118 than SS30. Conversely, higher total Na+ content but lower Na+[leaves]/Na+[roots] were observed in ST118 than SS30 (Fig. 2a and Additional file 2). These results suggested that ST118 preferentially translocated K+ from roots to leaves, but restricted Na+ accumulation in leaves in order to maintain a higher K+/Na+ ratios (Fig. 2b). Taken together, we speculated that the distribution mechanism of K+ and Na+ might be another key factor that determined the different salt-resistance of two eggplant genotypes.
Effect of salt stress on transcriptome changes in SS30 and ST118
Here, the comparative-transcriptome analysis between SS30 and ST118 was carried out in a way similar to previous studies in Arabidopsis [34], rice [3] and tomato [35]. Consistent with earlier studies in rice [2] and Arabidopsis [55], genotype-specific and organ-specific manners also existed in eggplant in response to salt stress (Fig. 3). Since the expression patterns of common DEGs in the leaves/roots of the two eggplant genotypes were almost same (Additional file 5), the genotype-specific DEGs in ST118 were likely responsible for the higher salt-tolerance.
The expressions of genes encoding 2 NACs, WRKY, MYB and COL transcription factors were found different between SS30 and ST118 (Table 1), which were valuable for further investigation in eggplants. Some studies have reported that the members of NAC [10, 11], MYB [13–15] and WRKY [16, 17] were involved in response to elevated external salinity. However, few studies on the function of COL family members in salinity tolerance have been reported so far. Although JH Min, et al. [69] reported that the AtCOL4-overexpressing plants were more tolerant to salt stress than the wild-type, most researches of the COL genes family focused on exploring its function on the flowering time of plants, such as OsCOL10 [70], OsCOL9 [71] and GhCOL1 [72]. In addition, previous studies reported that BTB/TAZ played an essential role during gametogenesis, and probably throughout plant development [73]. Recently, Q Zhao, et al. [74] reported that MdBT1/2 (a BTB/TAZ protein) interact with MdCUL3 to bridge the formation of the MdBTsMdCUL3 complex, which negatively modulates the degradation of the MdbHLH104 protein in response to changes in Fe status to maintain iron homeostasis in plants. And V Araus, et al. [75] reported that BT2 was the most central and connected gene in the nitrogen use efficiency (NUE) network in Arabidopsis and rice. Taken together, we thought that the 6 TFs are good candidates for further investigation of their role in salinity tolerance.
Candidate genes associated with K+ and Na+ homeostasis
Maintaining ion homeostasis is one of the key determinants for the plants survival under salt stress. The finding in this work that the Na+[leaves] /Na+[roots] increased less in ST118 than in SS30 along with salt treatment, indicating that ST118 may possess a mechanism to restrict the accumulation of Na+ in the leaves. The ‘salt overly sensitive’ (SOS) signaling pathway, including SOS1, SOS2 and SOS3 genes, has been proven to be important for plant salt tolerance [76, 77]. Among them, SOS1 was well known to be expressed in root epidermal cells and xylem parenchyma cells and was involved in extruding Na+ into the external medium and loading Na+ into the xylem for long-distance transport to leaves [61, 78, 79]. However, the SOS1 were expressed constitutively at higher levels in the roots of both eggplant genotypes. Strikingly, SOS1 was significantly up-regulated in the leaves of ST118 but was slightly down-regulated in SS30. Previous studies have been reported that SOS1 is also expressed in the xylem parenchyma in leaves but where its function is unclear so far. JK Zhu [18] speculated that the function of SOS1 in leaves may function to extrude Na+ from the xylem parenchyma cells into the apoplastic space of mesophyll cells.
Except SOS1, seven genes encoding K+ transporters or K+ channel proteins were identified in leaves to be up-regulated in response to salt stress (Table 2). It is worth noting that the genes encoding KAT1 and AKT1 were significantly up-regulated only in ST118. These results could partially explain the higher K+ [leaves] /K+ [roots] ratio in ST118 than in SS30 under salt stress. Similar with SOS1, AKT1 were expressed constitutively at higher levels in the roots of both two eggplant genotypes (Table 2). AKT1 was the first inward-rectifying K+ channel identified in Arabidopsis by functional complementation of yeast mutant strains defective in K+ transport system [80]. Moreover, a model of K+ uptake regulated by AKT1 in Arabidopsis and Oryza sativa under low-K+ conditions was proposed [50, 52]. In addition, previous studies showed that the osmotic- and drought-tolerance of rice could be enhanced by overexpression of OsAKT1 [81]. In fact, K+ deficiency would be accompanied by excessive accumulation of Na+ under salt stress. However, extensive researches were directed to the genes related to the influx, extrusion and accumulation of Na+ to improve K+/Na+ ratio in plants. Relatively limited studies focused on investigation of the AKT1 roles in maintaining K+ and Na+ homeostasis in plants under salt stress, especially in eggplants [82–84].
SmAKT1 is not only involved in modulating K+ uptake, but also in altering Na+ exclusion, transportation and homeostasis in Arabidopsis under salt conditions
In this study, more genes related to K+ uptake were identified as DEGs than those related to Na+ regulation (Table 2). Subsequently, the complementation assays in both yeast and Arabidopsis akt1 mutants demonstrated that SmAKT1 was involved in response to both low-K+ condition (Fig. 5) and salt conditions (Fig. 6a, b). Given the phenotype of K+ concentration and distribution in ST118 under salt stress, we speculated that SmAKT1 not only mediates K+ uptake in roots, but is also essential for maintaining long-distance transport and homeostasis of K+ in eggplants, which is similar with Arabidopsis [52] and Z. xanthoxylum [83]. In addition, the expression patterns of SOS1, HKT1 and NHX1, known as Na+ uptake and transport genes, were significantly changed in the Arabidopsis akt1 mutants and recovered in the complementary lines, when compared with the wild type under salt stress.
As described above, the functions of SOS1 in roots were Na+ extrusion and Na+ upload into the xylem [18, 78, 79]. However, the coordination mechanism of these two roles is not well understood. The other important Na+ transporter is HKT1, which acts in the retrieval of Na+ from the xylem to restrict the Na+ amount in the transpiration stream in roots [85] and uploading Na+ into the phloem for recirculation back to roots from the leaves [86]. In this work, the transcription level of SOS1 was significantly increased in roots but no change was observed in leaves of akt1 mutants after short-term salt treatment (12 h) (Fig. 6c). However, the transcription level of HKT1 was significantly decreased in roots but was increased in leaves at the same time (Fig. 6c). Taken together, we speculated that the akt1 mutant transported Na+ into leaves by SOS1, while the leaves restrict Na+ accumulation in leaves by the function of HKT1. And these opposing works by two different genes might be an important reason for its intolerance to salt stress. In contrast to the akt1 mutant, the wild type and the two complementary lines might have developed a mechanism to avoid Na+ from entering into leaves and to transfer Na+ into the apoplastic space of mesophyll cells as soon as possible. In addition, after being exposed to prolonged salt stress (7d), the wild type and the two complementary lines unload the Na+ from xylem by upregulating the expression of HKT1 and extrude it to soil solution by upregulating the expression of SOS1.
As for NHX1, it seems to be not closely to AKT1, and the function of NHX1 in plant leaves has been well studied while it was only partly understood in roots, which could transport the excessive Na+ to vacuole. Here, the NHX1 was down-regulated in roots of the three tolerant Arabidopsis lines at 12 h but up-regulated at 7 days (Fig. 6c). It could be explained as that the vacuole was the ultimate storage space for additional Na+.
Taken together, our results suggested that SmAKT1 is an important determinant for maintaining K+ and Na+ homeostasis in eggplant under salt stress, since it not only mediates K+ uptake, but also modulates Na+ uptake and transport systems.
Conclusion
In order to grow on saline soils, plants developed coordinated physiological traits throughout the lifecycle, among which the K+ and Na+ homeostasis is a key determinant to evaluate salt-tolerance. Here, comparative analysis of transcript levels in response to salt stress between salt-sensitive and salt-tolerant eggplant genotypes provided insights into key candidate genes related to salinity tolerance. The transcriptomic differences between SS30 and ST118 indicated the diversity of approaches to resist the challenge of salt stress. Further, the differently expressed TFs and ion transport genes were selectively analyzed, and the complementation assays demonstrated that SmAKT1 is an important regulator under salt conditions. Objectively, it also suggested that the other TFs and K+ transport genes were also worth further investigation for their functions in salinity tolerance. These data provides a foundation for elucidating the molecular networks underlying salt tolerance in eggplants.
Additional files
Table S1. List of primers sequences used in this study. (DOCX 32 kb)
Figure S1. The K+ (a) and Na+ (b) content in leaves and roots of two eggplant genotypes along with 200 mM NaCl treatment. DW represents dry weight. Three replicates were used in each time point, with three seedlings per replicate. Bars represent means ± SD of three biological replicates. Duncan’s Multiple Range test (*P < 0.05 and **P < 0.01) was used to analyze statistical significance. (DOCX 391 kb)
Table S2. Summary statistics of sequencing and assembly. Tissue: The tissue of eggplant seedling; Samples: Sample names; Total Clean Reads(Mb): The reads amount after filtering, Unit: Mb; Clean Reads Ratio(%): The ratio of the amount of filtered clean reads; Total Mapping Ratio: The percentage of mapped reads; Uniquely Mapping Ratio: The percentage of uniquely mapped reads (%); Expressed Gene No.: The amount of expressed genes; SS represents salt sensitive eggplant SS30; ST represents salt tolerant eggplant ST118; 0 h and 12 h represent the time after NaCl treatment; L: leaves; R: Roots. (DOCX 40 kb)
Figure S2. Validation of RNA-seq data in leaves (a) and roots (b) using qRT-PCR. (DOCX 1923 kb)
Figure S3. Four-way Venn diagram indicating the number of salt-up-regulated and -down-regulated genes found exclusively in the leaves (a) and roots (b) of two eggplant genotypes in the comparison between salt-stressed and non-stress treatments. (DOCX 468 kb)
Figure S4. GO classification of up- and down-regulated genes in leaves or roots of SS30 or ST118. (DOCX 1470 kb)
Figure S5. Overview the salt-up- or down-regulated TFs in the leaves and roots of both two eggplant genotypes at a level of ≥2-fold and adjusted P-value ≤0.001. (DOCX 3480 kb)
Figure S6. Phylogenetic relationships of the two SmAKT1s with AKT1 from other species. Protein sequences of AKT1 were analyzed using MEGA7.0 and the Neighbor-Joining method with 1000 bootstrap replicates. (DOCX 258 kb)
Figure S7. The conserved domains in across AKT1 proteins. The overall height of each stack indicates the conservation of the sequence at that position, whereas the height of letters within each stack represents the relative frequency of the corresponding amino acid. (DOCX 1146 kb)
Acknowledgments
We thank Richard Gaber (Northwestern University) and Weihua Wu (China Agricultural University) for providing yeast strains R5421 that were used in the yeast complementation experiments. We thank Weihua Wu (China Agricultural University) for providing the Arabidopsis akt1 mutant.
Funding
The financial of this research was supported by the National Natural Science Foundation of China (31471870) and the Science Project Supported by Science and Technology Commission of Shanghai Municipality (18391900500). Funding body had no role in the design of the study and collection, analysis, interpretation of data and in writing the manuscript.
Availability of data and materials
The raw sequencing data were deposited in the NCBI Short Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/sra/) under the accession number SRP151507. The other supporting data were included as additional files.
Abbreviations
- FAO
Food and Agriculture Organization of the United Nations
- SS30-L/R-Spe
The DEGs specifically identified in the leaves/roots of SS30
- ST118-L/R-Spe
The DEGs specifically identified in the leaves/roots of ST118
- STIDB
The Stress Responsive Transcription Factor Database
- ST-SS-L/R-inter
The intersection of DEGs identified in the leaves/roots of both SS30 and ST118
Authors’ contributions
H-YC, YL, ZG and JL conceived and designed the experiments; JL and ZG performed experiments and analyzed data; LZ, L-ZL and J-HZ assisted with the experiments; JL wrote the manuscript, and H-YC and YL revised. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable, as this study did not involve human or animal subjects, and the seeds of two eggplant cultivars were stored in School of Agriculture and Biology, Shanghai Jiao Tong University. The seeds of Arabidopsis akt1 mutant for were provided by Prof. Weihua Wu (China Agricultural University).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jing Li, Email: lij19900525@163.com.
Zhen Gao, Email: gaoz89@126.com.
Lu Zhou, Email: zhoulu@sjtu.edu.cn.
Linzhi Li, Email: 1295508518@qq.com.
Junhao Zhang, Email: 719564314@qq.com.
Yang Liu, Phone: +86 21 34206934, Email: liuyangtl@sjtu.edu.cn.
Huoying Chen, Phone: +86 21 34206934, Email: chhy@sjtu.edu.cn.
References
- 1.Kumar K, Kumar M, Kim S-R, Ryu H, Cho Y-G. Insights into genomics of salt stress response in rice. Rice. 2013;6:27. doi: 10.1186/1939-8433-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang WS, Zhao XQ, Li M, Huang LY, Xu JL, Zhang F, Cui YR, Fu BY, Li ZK. Complex molecular mechanisms underlying seedling salt tolerance in rice revealed by comparative transcriptome and metabolomic profiling. J Exp Bot. 2016;67:405–419. doi: 10.1093/jxb/erv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotsaftis O, Plett D, Johnson AAT, Walia H, Wilson C, Ismail AM, Close TJ, Tester M, Baumann U. Root-specific transcript profiling of contrasting Rice genotypes in response to salinity stress. Mol Plant. 2011;4:25–41. doi: 10.1093/mp/ssq056. [DOI] [PubMed] [Google Scholar]
- 4.Walia H, Wilson C, Zeng L, Ismail AM, Condamine P, Close TJ. Genome-wide transcriptional analysis of salinity stressed japonica and indica rice genotypes during panicle initiation stage. Plant Mol Biol. 2007;63:609–623. doi: 10.1007/s11103-006-9112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh K, Foley RC, Onate-Sanchez L. Transcription factors in plant defense and stress responses. Curr Opin Plant Biol. 2002;5:430–436. doi: 10.1016/S1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 6.Todaka D, Nakashima K, Shinozaki K, Yamaguchi-Shinozaki K. Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice (N Y) 2012;5:6. doi: 10.1186/1939-8433-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golldack D, Luking I, Yang O. Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011;30:1383–1391. doi: 10.1007/s00299-011-1068-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang R, Liu J, Lin Z, Sun W, Wu Z, Hu H, Zhang Y. ERF transcription factors involved in salt response in tomato. Plant Growth Regul. 2018;84:1–10. doi: 10.1007/s10725-017-0315-y. [DOI] [Google Scholar]
- 9.Banerjee A, Roychoudhury A. Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma. 2017;254:3–16. doi: 10.1007/s00709-015-0920-4. [DOI] [PubMed] [Google Scholar]
- 10.Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Gen Genomics. 2010;284:173–183. doi: 10.1007/s00438-010-0557-0. [DOI] [PubMed] [Google Scholar]
- 11.Song SY, Chen Y, Chen J, Dai XY, Zhang WH. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta. 2011;234:331–345. doi: 10.1007/s00425-011-1403-2. [DOI] [PubMed] [Google Scholar]
- 12.Mao K, Dong Q, Li C, Liu C, Ma F. Genome wide identification and characterization of apple bHLH transcription factors and expression analysis in response to drought and salt stress. Front Plant Sci. 2017;8:480. doi: 10.3389/fpls.2017.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Ng CK-Y, Fan L-M. MYB transcription factors, active players in abiotic stress signaling. Environ Exp Bot. 2015;114:80–91. doi: 10.1016/j.envexpbot.2014.06.014. [DOI] [Google Scholar]
- 14.Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007;143:1739–1751. doi: 10.1104/pp.106.094532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang A, Dai X, Zhang W-H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot. 2012;63:2541–2556. doi: 10.1093/jxb/err431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Chen J, Wang L, Wang S. Genome-wide investigation of WRKY transcription factors involved in terminal drought stress response in common bean. Front Plant Sci. 2017;8:380. doi: 10.3389/fpls.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59:86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JP, Ishitani M, Halfter U, Kim CS, Zhu JK. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci U S A. 2000;97:3730–3734. doi: 10.1073/pnas.97.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintero FJ, Ohta M, Shi HZ, Zhu JK, Pardo JM. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci U S A. 2002;99:9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozhdestvenskii VI, Vil’iams MV, Tsvetkova IV, Lebedeva EV, Alekhina TP. Control of mineral nutrition of higher plants in biological life support systems. Kosm Biol Aviakosm Med. 1975;9:30–35. [PubMed] [Google Scholar]
- 22.Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiol Plant. 2008;133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–445. doi: 10.1016/S1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 24.Kantharajah A, Golegaonkar P. Somatic embryogenesis in eggplant. Sci Hortic. 2004;99:107–117. doi: 10.1016/S0304-4238(03)00090-6. [DOI] [Google Scholar]
- 25.Jain RK, Dhawan RS, Sharma DR, Chowdhury JB. Salt-tolerance and proline accumulation: a comparative study in salt-tolerant and wild type cultured cells of eggplant. Plant Cell Rep. 1987;6:382–384. doi: 10.1007/BF00269567. [DOI] [PubMed] [Google Scholar]
- 26.Heuer B, Meiri A, Shalevet J. Salt tolerance of eggplant. Plant Soil. 1986;95:9–13. doi: 10.1007/BF02378847. [DOI] [Google Scholar]
- 27.Akinci IE, Akinci S, Yilmaz K, Dikici H. Response of eggplant varieties (Solanum melongena) to salinity in germination and seedling stages. N Z J Crop Hortic Sci. 2004;32:193–200. doi: 10.1080/01140671.2004.9514296. [DOI] [Google Scholar]
- 28.Unlukara A, Kurunc A, Kesmez GD, Yurtseven E, Suarez DL. Effects of salinity on eggplant (Solanum Melongena L.) growth and evapotranspiration. Irrig Drain. 2010;59:203–214. [Google Scholar]
- 29.Yasar F, Ellialtioglu S, Kusvuran S. Ion and lipid peroxide content in sensitive and tolerant eggplant callus cultured under salt stress. Eur J Hortic Sci. 2006;71:169–172. [Google Scholar]
- 30.Abbas W, Ashraf M, Akram NA. Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugarbeet extracts. Sci Hortic. 2010;125:188–195. doi: 10.1016/j.scienta.2010.04.008. [DOI] [Google Scholar]
- 31.Roy NC, Altermann E, Park ZA, McNabb WC. A comparison of analog and next-generation transcriptomic tools for mammalian studies. Brief Funct Genomics. 2011;10:135–150. doi: 10.1093/bfgp/elr005. [DOI] [PubMed] [Google Scholar]
- 32.Ward JA, Ponnala L, Weber CA. Strategies for transcriptome analysis in nonmodel plants. Am J Bot. 2012;99:267–276. doi: 10.3732/ajb.1100334. [DOI] [PubMed] [Google Scholar]
- 33.Xiao N, Gao Y, Qian H, Gao Q, Wu Y, Zhang D, Wang Z, Zhang X, Yu L, Li Y. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant Physiol. 2018;177:1108–1123. doi: 10.1104/pp.18.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YP, Yang L, Zheng ZM, Grumet R, Loescher W, Zhu JK, Yang PF, Hu YL, Chan ZL. Transcriptomic and physiological variations of three Arabidopsis ecotypes in response to salt stress. PLoS One. 2013;8:e69036. doi: 10.1371/journal.pone.0069036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W, Xu XN, Zhu HS, Liu AH, Liu L, Li JM, Hua XJ. Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol. 2010;51:997–1006. doi: 10.1093/pcp/pcq056. [DOI] [PubMed] [Google Scholar]
- 36.Hirakawa H, Shirasawa K, Miyatake K, Nunome T, Negoro S, Ohyama A, Yamaguchi H, Sato S, Isobe S, Tabata S, et al. Draft genome sequence of eggplant (Solanum melongena L.): the representative solanum species indigenous to the old world. DNA Res. 2014;21:649–660. doi: 10.1093/dnares/dsu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. Bmc Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedgwick P. Pearson’s correlation coefficient. Br Med J. 2012;344:e4483. doi: 10.1136/bmj.e4483. [DOI] [Google Scholar]
- 40.Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
- 42.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35:D61–D65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ, Gapped BLAST. PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 46.Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41:e121. doi: 10.1093/nar/gkt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Ren L, Gao Z, Jiang M, Liu Y, Zhou L, He Y, Chen H. Combined transcriptomic and proteomic analysis constructs a new model for light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.) Plant Cell Environ. 2017;40:3069–3087. doi: 10.1111/pce.13074. [DOI] [PubMed] [Google Scholar]
- 48.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Long Y, Qi GN, Li J, Xu ZJ, Wu WH, Wang Y. The Os-AKT1 channel is critical for K+ uptake in Rice roots and is modulated by the Rice CBL1-CIPK23 complex. Plant Cell. 2014;26:3387–3402. doi: 10.1105/tpc.114.123455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clough SJ, Bent AF. Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta (BBA)-Gen Subj. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 54.Kitajima M, Butler WL. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975;376:105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- 55.Begara-Morales JC, Sanchez-Calvo B, Luque F, Leyva-Perez MO, Leterrier M, Corpas FJ, Barroso JB. Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol. 2014;55:1080–1095. doi: 10.1093/pcp/pcu044. [DOI] [PubMed] [Google Scholar]
- 56.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HJ, Nam HG, Lim PO. Regulatory network of NAC transcription factors in leaf senescence. Curr Opin Plant Biol. 2016;33:48–56. doi: 10.1016/j.pbi.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Naika M, Shameer K, Mathew OK, Gowda R, Sowdhamini R. STIFDB2: an updated version of plant stress-responsive transcription factor database with additional stress signals, stress-responsive transcription factor binding sites and stress-responsive genes in Arabidopsis and rice. Plant Cell Physiol. 2013;54:e8. doi: 10.1093/pcp/pcs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi H, Ishitani M, Kim C, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X. The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant. 2013;6:275–286. doi: 10.1093/mp/sst017. [DOI] [PubMed] [Google Scholar]
- 61.Olias R, Eljakaoui Z, Li J, De Morales PA, Marin-Manzano MC, Pardo JM, Belver A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009;32:904–916. doi: 10.1111/j.1365-3040.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 63.Gierth M, Maser P, Schroeder JI. The potassium transporter AtHAK5 functions in K(+) deprivation-induced high-affinity K(+) uptake and AKT1 K(+) channel contribution to K(+) uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137:1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. Potassium uptake supporting plant growth in the absence of AKT1 channel activity - inhibition by ammonium and stimulation by sodium. J Gen Physiol. 1999;113:909–918. doi: 10.1085/jgp.113.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaber RF, Styles CA, Fink GR. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2848–2859. doi: 10.1128/MCB.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura RL, Anderson JA, Gaber RF. Determination of key structural requirements of a K+ channel pore. J Biol Chem. 1997;272:1011–1018. doi: 10.1074/jbc.272.2.1011. [DOI] [PubMed] [Google Scholar]
- 67.Shahbaz M, Mushtaq Z, Andaz F, Masood A. Does proline application ameliorate adverse effects of salt stress on growth, ions and photosynthetic ability of eggplant (Solanum melongena L.)? Sci Hortic. 2013;164:507–511. doi: 10.1016/j.scienta.2013.10.001. [DOI] [Google Scholar]
- 68.Chartzoulakis KS, Loupassaki MH. Effects of NaCl salinity on germination, growth, gas exchange and yield of greenhouse eggplant. Agric Water Manag. 1997;32:215–225. doi: 10.1016/S0378-3774(96)01276-0. [DOI] [Google Scholar]
- 69.Min JH, Chung JS, Lee KH, Kim CS. The CONSTANS-like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J Integr Plant Biol. 2015;57:313–324. doi: 10.1111/jipb.12246. [DOI] [PubMed] [Google Scholar]
- 70.Tan JJ, Jin MN, Wang JC, Wu FQ, Sheng PK, Cheng ZJ, Wang JL, Zheng XM, Chen LP, Wang M, et al. OsCOL10, a CONSTANS-like gene, functions as a flowering time repressor downstream of Ghd7 in Rice. Plant Cell Physiol. 2016;57:798–812. doi: 10.1093/pcp/pcw025. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Gu FW, Dong SY, Liu W, Wang H, Chen ZQ, Wang JF. CONSTANS-like 9 (COL9) delays the flowering time in Oryza sativa by repressing the Ehd1 pathway. Biochem Biophys Res Commun. 2016;479:173–178. doi: 10.1016/j.bbrc.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Cai DR, Liu H, Sang N, Huang XZ. Identification and characterization of CONSTANS-like (COL) gene family in upland cotton (Gossypium hirsutum L.) PLoS One. 2017;12:e0179038. doi: 10.1371/journal.pone.0179038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robert HS, Quint A, Brand D, Vivian-Smith A, Offringa R. BTB and TAZ domain scaffold proteins perform a crucial function in Arabidopsis development. Plant J. 2009;58:109–121. doi: 10.1111/j.1365-313X.2008.03764.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Q, Ren YR, Wang QJ, Wang XF, You CX, Hao YJ. Ubiquitination-related MdBT scaffold proteins target a bHLH transcription factor for Iron homeostasis. Plant Physiol. 2016;172:1973–1988. doi: 10.1104/pp.16.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Araus V, Vidal EA, Puelma T, Alamos S, Mieulet D, Guiderdoni E, Gutiérrez RA. Members of BTB gene family regulate negatively nitrate uptake and nitrogen use efficiency in Arabidopsis thaliana and Oryza sativa. Plant Physiol. 2016;171:1523–1532. [DOI] [PMC free article] [PubMed]
- 76.Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell. 2007;19:1415–1431. doi: 10.1105/tpc.106.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu M, Shabala L, Cuin TA, Huang X, Zhou M, Munns R, Shabala S. Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J Exp Bot. 2016;67:835–844. doi: 10.1093/jxb/erv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad I, Mian A, Maathuis FJM. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J Exp Bot. 2016;67:2689–2698. doi: 10.1093/jxb/erw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duan HR, Ma Q, Zhang JL, Hu J, Bao AK, Wei L, Wang Q, Luan S, Wang SM. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil. 2015;395:173–187. doi: 10.1007/s11104-015-2539-9. [DOI] [Google Scholar]
- 83.Ma Q, Hu J, Zhou XR, Yuan HJ, Kumar T, Luan S, Wang SM. ZxAKT1 is essential for K+ uptake and K+/Na+ homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017;90:48–60. doi: 10.1111/tpj.13465. [DOI] [PubMed] [Google Scholar]
- 84.Ardie SW, Liu SK, Takano T. Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep. 2010;29:865–874. doi: 10.1007/s00299-010-0872-2. [DOI] [PubMed] [Google Scholar]
- 85.Sunarpi HT, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 86.Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of primers sequences used in this study. (DOCX 32 kb)
Figure S1. The K+ (a) and Na+ (b) content in leaves and roots of two eggplant genotypes along with 200 mM NaCl treatment. DW represents dry weight. Three replicates were used in each time point, with three seedlings per replicate. Bars represent means ± SD of three biological replicates. Duncan’s Multiple Range test (*P < 0.05 and **P < 0.01) was used to analyze statistical significance. (DOCX 391 kb)
Table S2. Summary statistics of sequencing and assembly. Tissue: The tissue of eggplant seedling; Samples: Sample names; Total Clean Reads(Mb): The reads amount after filtering, Unit: Mb; Clean Reads Ratio(%): The ratio of the amount of filtered clean reads; Total Mapping Ratio: The percentage of mapped reads; Uniquely Mapping Ratio: The percentage of uniquely mapped reads (%); Expressed Gene No.: The amount of expressed genes; SS represents salt sensitive eggplant SS30; ST represents salt tolerant eggplant ST118; 0 h and 12 h represent the time after NaCl treatment; L: leaves; R: Roots. (DOCX 40 kb)
Figure S2. Validation of RNA-seq data in leaves (a) and roots (b) using qRT-PCR. (DOCX 1923 kb)
Figure S3. Four-way Venn diagram indicating the number of salt-up-regulated and -down-regulated genes found exclusively in the leaves (a) and roots (b) of two eggplant genotypes in the comparison between salt-stressed and non-stress treatments. (DOCX 468 kb)
Figure S4. GO classification of up- and down-regulated genes in leaves or roots of SS30 or ST118. (DOCX 1470 kb)
Figure S5. Overview the salt-up- or down-regulated TFs in the leaves and roots of both two eggplant genotypes at a level of ≥2-fold and adjusted P-value ≤0.001. (DOCX 3480 kb)
Figure S6. Phylogenetic relationships of the two SmAKT1s with AKT1 from other species. Protein sequences of AKT1 were analyzed using MEGA7.0 and the Neighbor-Joining method with 1000 bootstrap replicates. (DOCX 258 kb)
Figure S7. The conserved domains in across AKT1 proteins. The overall height of each stack indicates the conservation of the sequence at that position, whereas the height of letters within each stack represents the relative frequency of the corresponding amino acid. (DOCX 1146 kb)
Data Availability Statement
The raw sequencing data were deposited in the NCBI Short Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/sra/) under the accession number SRP151507. The other supporting data were included as additional files.