Abstract
Purpose
Limited data exist on intensifying chemotherapy regimens in the treatment of adult acute lymphoblastic leukemia (ALL) outside the setting of a clinical trial.
Materials and Methods
Retrospectively, data from 507 consecutive adults (age ≥ 15 years) with a diagnosis of ALL treated at our center were analyzed. Standard-risk (SR) patients were offered treatment with a modified German Multicenter ALL (GMALL) regimen, whereas high-risk (HR) patients were offered intensification of therapy with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HCVAD). Because of resource constraints, a proportion of HR patients opted to receive the same treatment regimen as used for SR patients.
Results
There were 344 SR patients (67.8%) and 163 HR patients (32.2%) at diagnosis. Among the HR patients, 53 (32.5%) opted to receive intensification with the HCVAD regimen. The SR cohort showed a superior 5-year event-free survival rate compared with the HR cohort (47.3% v 23.6%, respectively; P < .001). Within the HR subgroup, there was no statistically significant difference in overall survival or event-free survival between patients who received the modified GMALL regimen (n = 59) and patients who received HCVAD (n = 53).
Conclusion
Intensified therapy in the HR subset was associated with a significant increase in early treatment-related mortality and cost of treatment. A modified GMALL regimen was found to be cost-effective with clinical outcomes comparable to those achieved with more intensive regimens.
INTRODUCTION
Current established regimens in adult acute lymphoblastic leukemia (ALL) result in a long-term overall survival (OS) rate of 40% to 50%.1-4 Over the years, improvement in OS has been attributed to more intensive combination therapies using high-dose methotrexate, cytarabine, monoclonal antibodies, and nelarabine, and, where appropriate, the optimal use of an allogeneic hematopoietic stem-cell transplantation (allo-SCT).1-6 Most of these results reflecting improvement in outcomes are from developed countries and were largely generated in the clinical trial setting. There has been a paucity of real-world data and even less data from developing nations, where good single-center registry or population-based registry data are usually not available.1-7 Available information from developing countries has been derived from small, retrospective, and often single-center studies, with the reported OS for ALL reaching up to 40% in a select few reports.8-16 Buyukasik et al9 retrospectively compared the commonly used regimen of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HCVAD; n = 68) with the Cancer and Leukemia Group B 8811 regimens (n = 65) and reported an inferior OS rate (26.3% v 44.2% at 5 years, respectively; P = .05) and a higher nonrelapse mortality rate (29.7% v 5.9%, respectively; P = .003) with the HCVAD regimen. This study had limitations as a result of its retrospective nature, small numbers, and a selection bias for different treatment regimens.17 However, similar to the report by Buyukasik et al,9 Alacacioglu et al14 also reported a superior 5-year OS rate using a Berlin-Frankfurt-Münster regimen (n = 20) compared with the more intensive HCVAD regimen (n = 30; 59% v 34%, respectively). A common theme in these reports was the significant challenge in intensifying the chemotherapy regimens in adult patients with ALL in a developing country. The challenges were related to limited resources to manage treatment-related toxicities and prolonged cytopenia with subsequent infections leading to substantial morbidity and mortality.
Cancer care in India faces several economic challenges.18-20 As reported previously by us and others, challenges to administering intensive chemotherapy regimens include resource constraints, a predominantly self-paying system of medical care, and a high incidence of multidrug-resistant infections.18,19,21,22 Long interruptions and abandonment of therapy after successful initial induction remission also lead to more resistant disease, as a result of delays in cancer care.13,23 An earlier study from our center reporting on the outcomes of 202 adults (age ≥ 15 years) using a modified German Multicenter ALL (GMALL) regimen demonstrated a complete remission (CR) rate of 82.2% and a 5-year OS rate of 38%.15 To further improve results, we adopted a strategy of intensifying therapy for high-risk (HR) patients. The current analysis aimed to evaluate whether this strategy yielded improved results.
MATERIALS AND METHODS
Study Population
This was a retrospective, single-center study approved by our institutional review board (No. 7903, dated April 7, 2012). All consecutive adult patients (age ≥ 15 years) with a diagnosis of ALL from January 2004 to November 2014 were included in our study. Data for this analysis were frozen as of December 31, 2015. Diagnosis was made as per the WHO 2008 classification.24,25 Patients diagnosed before 2008 were reclassified to fit the WHO 2008 criteria.24,25 Prognostic risk stratification of cytogenetic abnormalities was performed based on the Medical Research Council UKALLXII/Eastern Cooperative Oncology Group 2993 trial and a population-based cytogenetic study of 349 adults (age ≥ 15 years) with ALL.26,27 Patients with t(8;14) were excluded from the current study. Molecular screening for BCR-ABL, TEL-AML, E2A-PBX, and MLL gene mutations was performed using conventional molecular techniques.28,29 CNS involvement at diagnosis was defined as reported previously.15,30,31
Clinical Risk Stratification
Patients were stratified at diagnosis into either the standard-risk (SR) subgroup or HR subgroup (HR) to aid in the treatment regimen decision. HR disease was defined as presence of at least one of the following four criteria: poor prednisolone response (peripheral blood blast count ≥ 1,000/μL on day 8 of initiating corticosteroids)3; HR cytogenetics, including t(9;22) or t(4;11)2,4,32 (complex cytogenetics [≥ five chromosomal abnormalities] and low hypodiploidy or near triploidy [chromosomes 30 to 39 and 60 to 78] were not considered as high risk for the purpose of deciding initial therapy after diagnosis]; residual disease at end of induction (> 5% blasts on bone marrow [BM] or persistent extramedullary disease); and early precursor T-cell ALL immunophenotype.33 Early precursor T-cell ALL was included in the HR category beginning in October 2012.33 Patients with none of these risk factors were stratified as being SR for the purpose of this analysis.
Treatment Strategy
Patients stratified as SR received the modified GMALL protocol, as reported previously by us15 (Data Supplement). Given the available financial resources, patients in the HR subgroup were offered the following treatment options: patients with financial limitations received our modified GMALL regimen, similar to our SR patients, whereas patients without financial limitations received the standard HCVAD-based therapy.34 A myeloablative allo-SCT (conventional cyclophosphamide and total-body irradiation) was offered to all HR patients in the first CR provided they had an HLA-matched donor35,36 and after they had received three to four cycles of HCVAD. Patients who did not have a donor or opted not to have an allo-SCT were scheduled to receive six to eight cycles of HCVAD followed by maintenance therapy for 2 years.34 Patients with CNS disease at diagnosis received six doses of triple intrathecal therapy (methotrexate 12.5 mg, cytarabine 40 mg, and hydrocortisone 50 mg) during the initial induction, in addition to assigned treatment protocol.31,37
Definitions
CR was defined as the absence of blasts in the peripheral blood and CNS, an absolute neutrophil count > 1.5 × 109/L, an unsupported platelet count of > 100 × 109/L, and < 5% blasts in the BM at the end of phase I induction.38 Relapse was defined as the reappearance of lymphoblasts at any site after achieving remission.39 The time points for relapse were defined as follows: very early relapse, < 18 months from initiation of therapy; early relapse, > 18 months from initiation of treatment but < 6 months after completion of final maintenance therapy; and late relapse, > 6 months after completion of final maintenance therapy.39 OS was defined as time from beginning of treatment to either death or the last follow-up date if alive. Event-free survival (EFS) was defined as time from beginning of treatment until the first event (relapse or death).
Statistical Methods
A comparison between the quantitative parameters was performed using a Mann-Whitney U test or a t test as appropriate, whereas differences in the qualitative parameters were evaluated using the χ2 statistics or the Fisher’s exact test. OS and EFS survival probabilities were estimated using the Kaplan-Meier method. A log-rank (Mantel-Cox) comparison was used to assess any statistically significant difference in the OS or EFS between the different subgroups. The prognostic significance of clinical and biologic factors in all of the patients was tested first using a univariate Cox regression analysis and then a subsequent multivariate Cox regression analysis as required. For all of the tests, a two-sided P ≤ .05 indicated statistical significance. All analysis was done using SPSS version 16.0 (SPSS, Chicago, IL).
RESULTS
Patient Accrual and Baseline Characteristics
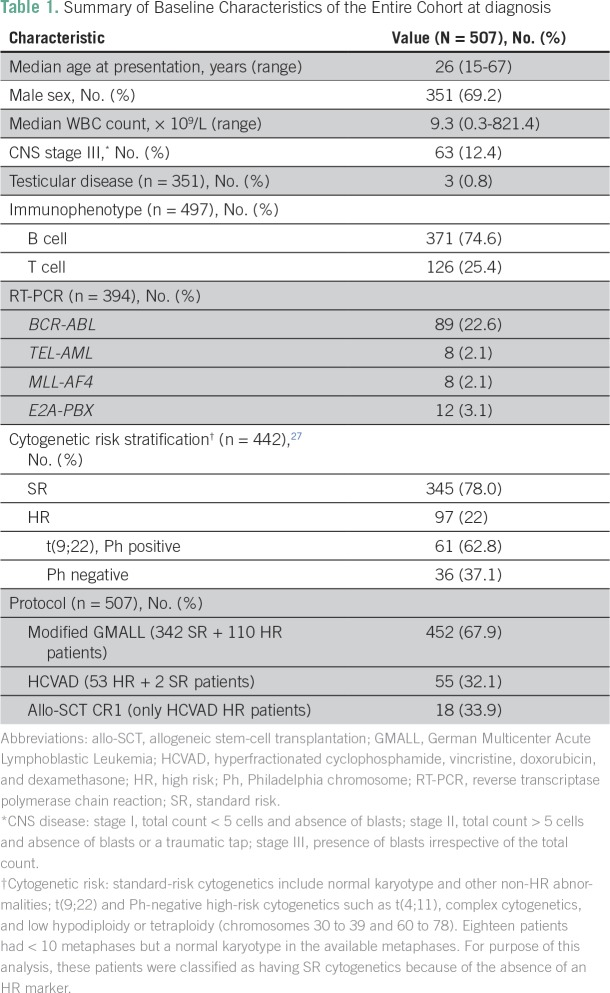
Over the study period, 507 adults (age ≥ 15 years) were diagnosed and treated for ALL at our institution. The median age was 26 years (range, 15 to 67 years), with 334 patients (65.8%) ≤ 35 years old. Three hundred fifty-one patients (69.2%) were male. A total of 117 patients (23.07%) presented with WBC counts ≥ 50 × 109/L. A total of 344 patients (67.8%) were identified as SR, and 163 patients (32.2%) were identified as HR. The baseline demographic characteristics of the entire cohort are listed in Table 1.
Table 1.
Summary of Baseline Characteristics of the Entire Cohort at diagnosis
Cytogenetic and Molecular Genetics
BM karyotype results were available in 442 patients (87.1%). Three hundred forty-five patients (78%) had SR cytogenetics (normal karyotype and other non-HR chromosomal abnormalities), and 97 patients (21.9%) had HR cytogenetics (Table 1). Of these 97 patients with HR cytogenetics, 61 (62.8%) were Philadelphia chromosome positive [t(9;22)] and 36 (37.1%) were Philadelphia chromosome negative [complex cytogenetics, t(4;11), and low hypodiploidy or near triploidy (chromosomes 30 to 39 and 60 to 78)]. Of the 394 patients (77.7%) in whom the leukemia-specific fusion transcript data were available, 89 (22.6%), eight (2.1%), eight (2.1%), and 12 (3.1%) tested positive for BCR-ABL, TEL-AML, MLL-AF4, and E2A-PBX transcripts, respectively (Table 1).
Risk Stratification and Distribution of Patients
Of the 344 SR patients (67.8%), two had received one cycle of HCVAD-based therapy before coming to our center and hence were continued on the same regimen. The modified GMALL regimen was offered to the remaining 342 SR patients (99.4%). None of these SR patients opted for palliation.
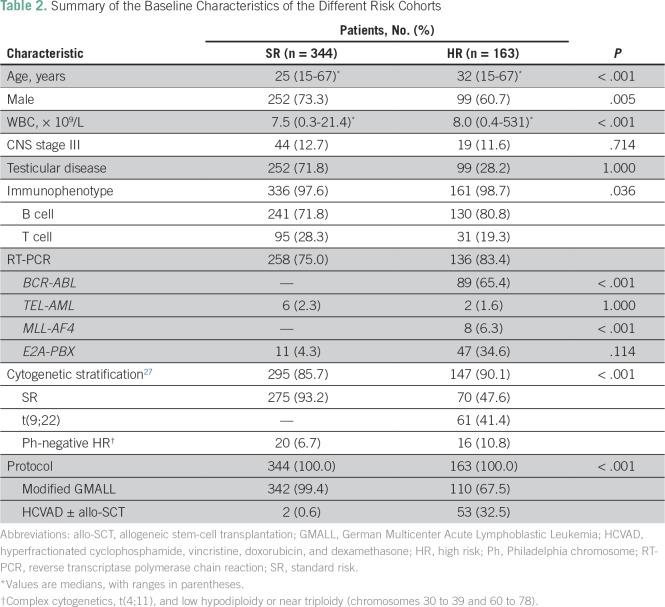
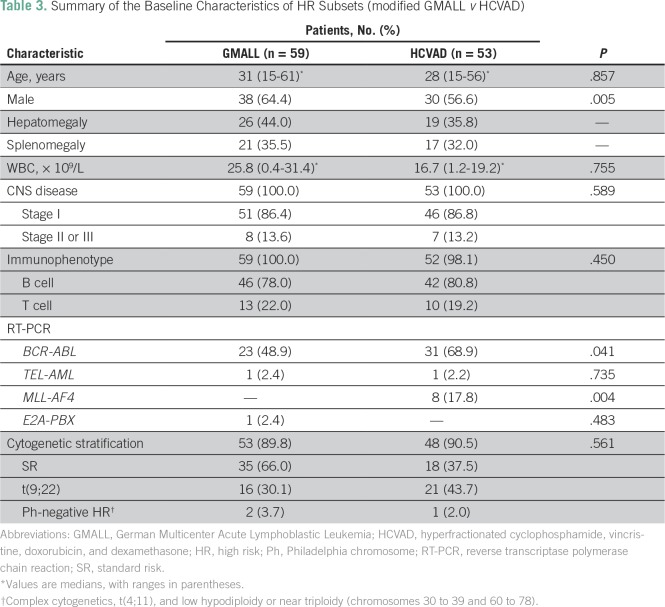
Of the 163 patients (32.2%) who were stratified at diagnosis as being HR, 53 (32.5%) opted to receive the intensified HCVAD-based regimen and an allo-SCT was performed in 18 of these patients (33.9%). Of the remaining 110 HR patients (67.5%), 59 (53.6%) opted to receive a modified GMALL regimen, whereas 51 (46.3%) opted for chemotherapy with palliative intent (individualized at treating physician’s discretion). The baseline characteristics of the two different risk cohorts (SR and HR) and the two HR subsets treated with two different regimens are listed in Tables 2 and 3, respectively.
Table 2.
Summary of the Baseline Characteristics of the Different Risk Cohorts
Table 3.
Summary of the Baseline Characteristics of HR Subsets (modified GMALL v HCVAD)
Induction Outcomes
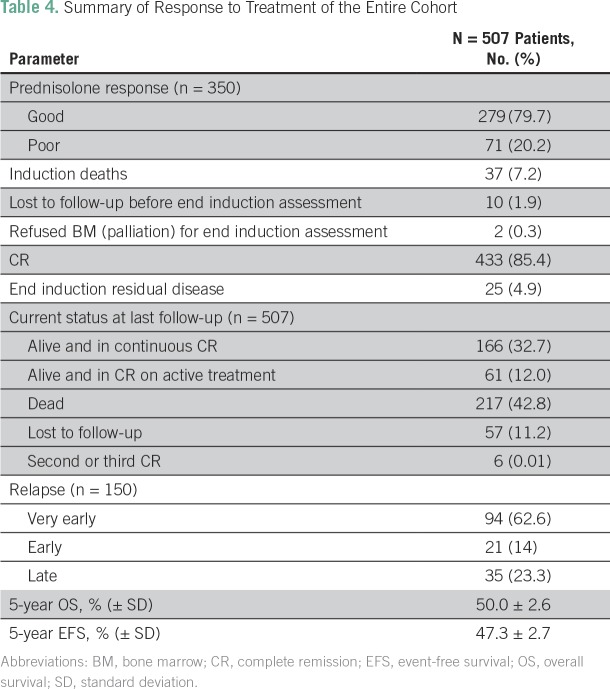
Forty-nine patients (9.6%) did not have an assessment at the end of induction chemotherapy. Ten patients (1.9%) were lost to follow-up before their end-of-induction assessment, two patients (0.3%) refused a BM assessment, and a further 37 patients (7.2%) suffered induction deaths (Data Supplement and Table 4). A total of 433 patients (85.4%) achieved a CR, and 25 patients (4.9%) remained refractory at the end of induction (Table 4).
Table 4.
Summary of Response to Treatment of the Entire Cohort
Outcomes of Patients Refractory to the Initial Induction
Of the 25 refractory patients (4.9%), five (20.0%) opted for palliation at a local place. Eleven patients (44%) continued therapy with the same protocol. Of these patients, four (36.6%) achieved transient remission but died later as a result of relapse and seven (63.6%) remained refractory and died later as a result of progressive disease (PD). Nine (36%) of the 25 refractory patients opted for intensified therapy with the HCVAD regimen. Of these nine patients, three (33.3%) achieved remission but experienced relapse later and died. Five patients (55.5%) remained refractory to therapy and died as a result of PD. One patient (11.1%) was lost to follow-up.
Profile of Disease Relapse
A total of 150 patients (29.5%) experienced relapse, with a median time to relapse of 14 months (range, 2 to 81 months; Table 4). Isolated medullary and extramedullary relapse was seen in 120 patients (80.0%) and 19 patients (12.6%), respectively. CNS was the most frequently involved extramedullary site (n = 14; 9.3%). Combined medullary and extramedullary disease was seen in 11 patients (7.3%). Of the 150 patients who experienced relapse, six patients (4.0%) continue to remain in second or third CR, and 144 patients (96.0%) have died as a result of PD after opting for palliation.
Current Status
At the time of study closure, 166 patients (32.7%) were in continuous CR and 61 patients (12%) were in CR on active treatment. Fifty-seven patients (11.2%) were identified as being lost to follow-up. Of a total of 217 deaths (42.8%), 163 deaths (75.1%) occurred as a result of PD and 54 deaths (24.8%) were secondary to infection and sepsis. Six patients achieved a second or third CR and were alive at the last documented follow-up (Table 4).
Univariable and Multivariable Analysis
On univariable analysis, older age at diagnosis was associated with an inferior EFS (age > 20 but ≤ 40 years: hazard ratio [HR], 1.4; 95% CI, 1.04 to 1.97; P = .028; and age > 40 years: HR, 1.8; 95% CI, 1.26 to 2.64; P = .001). In addition, the following parameters were also identified as indicators of an inferior EFS: presence of BCR-ABL1 fusion abnormality (HR, 1.8; 95% CI, 1.34 to 2.51; P < .001), poor prednisolone response on day 8 (HR, 2.1; 95% CI, 1.53 to 2.99; P < .001), and no achievement of CR at the end induction (HR, 7.4; 95% CI, 4.78 to 11.66; P < .001). On multivariable analysis, only a higher age at diagnosis (age > 20 but ≤ 40 years: HR, 1.6; 95% CI, 1.0 to 2.7; P = .044; and age > 40 years: HR, 2.2; 95% CI, 1.2 to 3.9; P = .008) and no achievement of CR at the end of induction (HR, 4.2; 95% CI, 2.2 to 7.9; P < .001) were identified as independent prognostic factors.
Survival Statistics
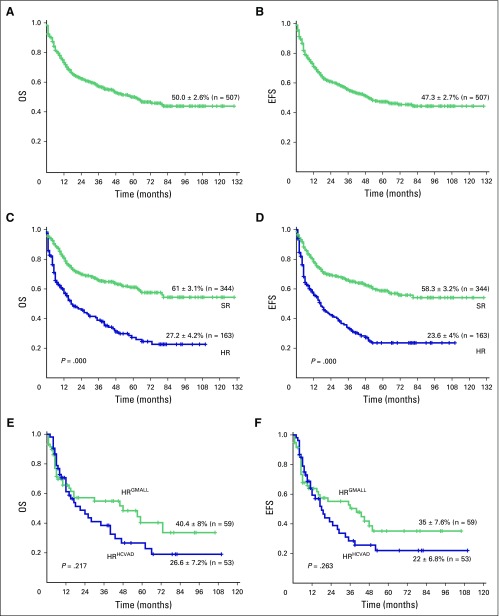
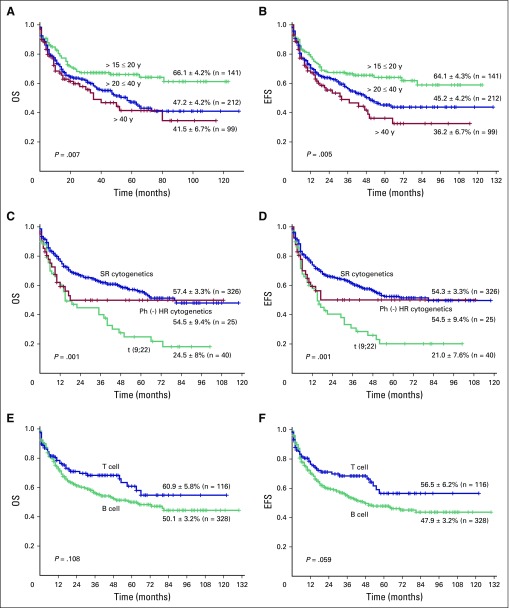
The 5-year OS and EFS rates (± standard deviation) of the entire cohort were 50.0% ± 2.6% and 47.3% ± 2.7% (Figs 1A and 1B), respectively, at an actuarial median follow-up time of 59 months. The 5-year OS and EFS rates of the SR cohort were 61% ± 3.1% and 58.8% ± 3.2% (Figs 1C and 1D), respectively, whereas OS and EFS rates of the HR cohort were 27.2% ± 4.2% and 23.6% ± 4%, respectively (Figs 1C and 1D). The OS and EFS rates between these two groups were statistically significantly different (OS, P < .001; and EFS, P < .001). Among the HR subsets, the group that received the modified GMALL protocol (n = 59) had 5-year OS and EFS rates of 40.4% ± 8.1% and 35.1% ± 7.6%, respectively, whereas the group that received HCVAD with or without an allo-SCT based on availability of donor (n = 18) had 5-year OS and EFS rates of 26.6% ± 7.2% and 22.0% ± 6.8%, respectively (Figs 1E and 1F). However, there was no statistically significant difference in survival between these two groups (OS, P = .217; and EFS, P = .263). The survival analysis of the subset of patients who received only the modified GMALL regimen, which included SR and HR patients (n = 452) with different additional variables such as age, cytogenetic risk group, and immunophenotype, is shown Figure 2 and the Data Supplement.
Fig 1.
(A) Five-year Kaplan-Meier product-limit estimates of overall survival (OS) of the entire cohort (N = 507). (B) Event-free survival (EFS) of the entire cohort (N = 507). (C) OS and (D) EFS of the standard-risk group (n = 344) and the high-risk (HR) group (n = 163). (E) OS and (F) EFS of the two HR subsets that received either the German Multicenter Acute Lymphoblastic Leukemia protocol (n = 59) or the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen (n = 53).
Fig 2.
Five-year Kaplan-Meier product-limit estimates in the cohort treated with the German Multicenter Acute Lymphoblastic Leukemia regimen (n = 452). (A) Overall survival (OS) and (B) event-free survival (EFS) in the different age groups (15 to 20 years, n = 141; > 20 to 40 years; n = 212; and > 40 years, n = 99). (C) OS and (D) EFS of the following three cytogenetic risk groups: standard-risk cytogenetics (n = 326) include normal karyotype and other non–high-risk abnormalities; Philadelphia chromosome–negative high-risk cytogenetics (n = 25) include t(4;11), complex cytogenetics, and low hypodiploidy or tetraploidy (chromosomes 30 to 39 and 60 to 78); and Philadelphia chromosome–positive high-risk cytogenetics (n = 40) include t(9;22). (E) OS and (F) EFS of the following two immunophenotypic subsets: B cell (n = 328) and T cell (n = 116).
Cost Analysis of Different Regimens
For cost analysis purposes, 15 HR patients were selected randomly from both subgroups (modified GMALL and HCVAD-based regimens). For the modified GMALL regimen subgroup (chemotherapy only; n = 15), the total cost incurred over 2.5 years from the date of first contact, which including all incurred outpatient and inpatient costs during initial induction, consolidation, and the subsequent 2 years of maintenance therapy, was included. For the group that received the HCVAD-based regimen (chemotherapy with or without maintenance [n = 8] or allo-SCT in first CR [n = 7]), the total cost incurred from the date of first contact, which included all incurred outpatient and inpatient costs during the initial induction, consolidation, and either 2 years of maintenance (n = 8) or up to 3 months after a successful allo-SCT (n = 7), was included. All cost-related data were the data captured comprehensively on the computerized hospital information and payment system. For the purpose of this analysis, only HR patients with an uneventful course and who had successfully completed their entire course of treatment were included. The average cost of treatment of patients in the modified GMALL subgroup and the HCVAD subgroup was 0.32 ± 0.16 million Indian rupees (US $3,800 ± $2,412.32) and 2.13 ± 1.10 million Indian rupees (US $32,000 ± $16,556.43), respectively (US $1 = 67 Indian rupees).
DISCUSSION
This single-center retrospective study over 10 years broadly illustrates the challenges and long-term outcomes of treating adult ALL (patients age ≥ 15 years) in India, although we do recognize that only a prospective multicenter study can definitively address the issue of dose intensification and improved clinical outcomes. With 5-year OS and EFS rates of 50.0% and 47%, respectively, the overall outcomes from our study cohort are comparable to several published studies.2,40,41 Our induction mortality rate of 7.2% is similar to that from previously published studies.2,42,43 Long distances of travel leading to delays before initiating treatment, environmental factors, and an increased risk of bacterial and fungal infections during the initial induction could partly explain our high induction mortality rate, as reported in this analysis and previously by us.15,22,44
In this study, 11.2% of patients were lost to follow-up after an initial remission. In another study from India, Malhotra et al13 had reported an abandonment rate of 14.9%. Although several reasons have been attributed to the discontinuation of the therapy, limited finances often remain the most significant factor leading to abandonment of treatment, as has been previously reported from our center in the context of treatment of acute myeloid leukemia.22 The cost of intensified chemotherapy in HR subsets, which has been reported to be successful in developed countries, is often not feasible because of the high cost of such therapy, as illustrated in this study (10-fold more expensive than GMALL regimen in this study). In a predominantly self-pay system such as exists in India, only 32.5% of the HR patients could afford and opted for the HCVAD regimen. The relatively low-cost modified GMALL protocol used at our center offers an acceptable long-term survival rate, which has been previously reported by us and further validated in this analysis.7 An interesting observation in our study was that we did not find a significant difference in outcomes between the two HR patient subgroups treated with either the low-cost modified GMALL regimen or the HCVAD-based regimen (with or without maintenance therapy or allo-SCT). Although not statistically significant, the lower incidence of PD in the HCVAD subgroup was offset by its higher nonrelapse mortality rate. It is important to note that this comparison was limited by its small numbers and the retrospective nature of the study.
This study illustrates the caution required in implementing dose-intensive regimens in resource-constrained environments or where additional challenges exist in their implementation. One cannot assume that the excellent results with these regimens, often in the context of a clinical trial, will be duplicated in different economic and social settings. Attention also needs to be given to the additional cost required to administer such regimens, and the cost-effectiveness of such approaches needs to be carefully addressed.
Footnotes
Supported by senior fellowship programs of Wellcome DBT India Alliance, New Delhi, India (IA/S/11/2500267 [V.M.] and IA/S/15/1/501842 [P.B.]) and Fluid Research Grant No. FG/7903/07/2012 (IRB Min No. 7903; P.J.).
AUTHOR CONTRIBUTIONS
Conception and design: Punit Jain, Biju George, Vikram Mathews
Financial support: Vikram Mathews
Administrative support: Alok Srivastava, Vikram Mathews
Provision of study material or patients: Nisham PN, Aby Abraham, Alok Srivastava, Biju George, Vikram Mathews
Collection and assembly of data: Punit Jain, Anu Korula, Nisham PN, Ansu Abu Alex, Poonkuzhali Balasubramanian, Biju George, Vikram Mathews
Data analysis and interpretation: Punit Jain, Anu Korula, Prashant Deshpande, Aby Abraham, Nancy Beryl Janet, Kavitha M. Lakshmi. Biju George, Vikram Mathews
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authorsAUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Punit Jain
No relationship to disclose
Anu Korula
No relationship to disclose
Prashant Deshpande
No relationship to disclose
Nisham PN
No relationship to disclose
Ansu Abu Alex
No relationship to disclose
Aby Abraham
Travel, Accommodations, Expenses: Novoseven
Alok Srivastava
Consulting or Advisory Role: Bayer
Research Funding: Bayer (Inst), Alnylam (Inst)
Travel, Accommodations, Expenses: Merck, Novo Nordisk
Nancy Beryl Janet
No relationship to disclose
Kavitha M. Lakshmi
No relationship to disclose
Poonkuzhali Balasubramanian
No relationship to disclose
Biju George
No relationship to disclose
Vikram Mathews
No relationship to disclose
REFERENCES
- 1.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 2.Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin Hematol. 2009;46:64–75. doi: 10.1053/j.seminhematol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): Long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 4.Fielding AK. The treatment of adults with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2008:381–389. doi: 10.1182/asheducation-2008.1.381. [DOI] [PubMed] [Google Scholar]
- 5.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: The GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Thomas D, Wayne AS, et al. Monoclonal antibody-based therapies: A new dawn in the treatment of acute lymphoblastic leukemia. J Clin Oncol. 2012;30:3876–3883. doi: 10.1200/JCO.2012.41.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeoh AE, Tan D, Li CK, et al. Management of adult and paediatric acute lymphoblastic leukaemia in Asia: Resource-stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol. 2013;14:e508–e523. doi: 10.1016/S1470-2045(13)70452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 9.Buyukasik Y, Acar K, Kelkitli E, et al. Hyper-CVAD regimen in routine management of adult acute lymphoblastic leukemia: A retrospective multicenter study. Acta Haematol. 2013;130:199–205. doi: 10.1159/000351172. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Li JY, Qian SX, et al. Outcome of treatment with hyper-CVAD regimen in Chinese patients with acute lymphocytic leukemia. Leuk Res. 2008;32:930–935. doi: 10.1016/j.leukres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Charafeddine KM, Hatoum HA, Otrock ZK, et al. Long-term outcome of adult acute lymphoblastic leukemia in Lebanon: A single institution experience from the American University of Beirut. Hematol Oncol Stem Cell Ther. 2009;2:333–339. doi: 10.1016/s1658-3876(09)50021-0. [DOI] [PubMed] [Google Scholar]
- 12.Abbasi S, Maleha F, Shobaki M. Acute lymphoblastic leukemia experience: Epidemiology and outcome of two different regimens. Mediterr J Hematol Infect Dis. 2013;5:e2013024. doi: 10.4084/MJHID.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra P, Varma S, Varma N, et al. Outcome of adult acute lymphoblastic leukemia with BFM protocol in a resource-constrained setting. Leuk Lymphoma. 2007;48:1173–1178. doi: 10.1080/10428190701343255. [DOI] [PubMed] [Google Scholar]
- 14.Alacacioglu I, Medeni SS, Ozsan GH, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? A retrospective analysis of the outcomes of BFM and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60:219–223. doi: 10.1159/000375258. [DOI] [PubMed] [Google Scholar]
- 15.Bajel A, George B, Mathews V, et al. Adult ALL: Treatment outcome and prognostic factors in an Indian population using a modified German ALL (GMALL) protocol. Leukemia. 2007;21:2230–2233. doi: 10.1038/sj.leu.2404785. [DOI] [PubMed] [Google Scholar]
- 16.Fogliatto L, Bittencourt H, Nunes AS, et al. Outcome of treatment in adult acute lymphoblastic leukemia in southern Brazil using a modified German multicenter acute lymphoblastic leukemia protocol. Acta Haematol. 2002;107:203–207. doi: 10.1159/000058315. [DOI] [PubMed] [Google Scholar]
- 17.Douer D. Adult acute lymphoblastic leukemia: A cancer with no standard of care. Acta Haematol. 2013;130:196–198. doi: 10.1159/000351601. [DOI] [PubMed] [Google Scholar]
- 18.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet. 2011;377:505–515. doi: 10.1016/S0140-6736(10)61894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 20.Magrath I, Shanta V, Advani S, et al. Treatment of acute lymphoblastic leukaemia in countries with limited resources: Lessons from use of a single protocol in India over a twenty year period [corrected] Eur J Cancer. 2005;41:1570–1583. doi: 10.1016/j.ejca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Horton R, Das P. Indian health: The path from crisis to progress. Lancet. 2011;377:181–183. doi: 10.1016/S0140-6736(10)62179-4. [DOI] [PubMed] [Google Scholar]
- 22.Philip C, George B, Ganapule A, et al. Acute myeloid leukaemia: Challenges and real world data from India. Br J Haematol. 2015;170:110–117. doi: 10.1111/bjh.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostert S, Sitaresmi MN, Gundy CM, et al. Influence of socioeconomic status on childhood acute lymphoblastic leukemia treatment in Indonesia. Pediatrics. 2006;118:e1600–e1606. doi: 10.1542/peds.2005-3015. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow SH, Campo E., Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, International Agency for Research on Cancer; 2008 [Google Scholar]
- 25.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorman AV, Chilton L, Wilkinson J, et al. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115:206–214. doi: 10.1182/blood-2009-07-232124. [DOI] [PubMed] [Google Scholar]
- 27.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 28.van Dongen JJ, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: Investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 29.Mullighan CG. Molecular genetics of B-precursor acute lymphoblastic leukemia. J Clin Invest. 2012;122:3407–3415. doi: 10.1172/JCI61203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutch Childhood Oncology Group. te Loo DM, Kamps WA, et al. Prognostic significance of blasts in the cerebrospinal fluid without pleiocytosis or a traumatic lumbar puncture in children with acute lymphoblastic leukemia: Experience of the Dutch Childhood Oncology Group. J Clin Oncol. 2006;24:2332–2336. doi: 10.1200/JCO.2005.03.9727. [DOI] [PubMed] [Google Scholar]
- 31.Del Principe MI, Maurillo L, Buccisano F, et al. Central nervous system involvement in adult acute lymphoblastic leukemia: Diagnostic tools, prophylaxis, and therapy. Mediterr J Hematol Infect Dis. 2014;6:e2014075. doi: 10.4084/MJHID.2014.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks DI, Moorman AV, Chilton L, et al. The clinical characteristics, therapy and outcome of 85 adults with acute lymphoblastic leukemia and t(4;11)(q21;q23)/MLL-AFF1 prospectively treated in the UKALLXII/ECOG2993 trial. Haematologica. 2013;98:945–952. doi: 10.3324/haematol.2012.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 35.Goldstone AH. Transplants in adult ALL--? Allo for everyone. Biol Blood Marrow Transplant. 2009;15(suppl 1):7–10. doi: 10.1016/j.bbmt.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Weisdorf DJ, Woods WG, Nesbit ME, Jr, et al. Allogeneic bone marrow transplantation for acute lymphoblastic leukaemia: Risk factors and clinical outcome. Br J Haematol. 1994;86:62–69. doi: 10.1111/j.1365-2141.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomas X, Le QH. Central nervous system involvement in adult acute lymphoblastic leukemia. Hematology. 2008;13:293–302. doi: 10.1179/102453308X343374. [DOI] [PubMed] [Google Scholar]
- 38.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: Results of more than 1500 patients from the international ALL trial—MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 39.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: Results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 40.Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: Analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 41.Stock W, Johnson JL, Stone RM, et al. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: Results of Cancer and Leukemia Group B Study 19802. Cancer. 2013;119:90–98. doi: 10.1002/cncr.27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribera JM, Oriol A, Bethencourt C, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia: Results of the PETHEMA ALL-93 trial. Haematologica. 2005;90:1346–1356. [PubMed] [Google Scholar]
- 43.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation: A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1366. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 44.Abraham A, George B, Ahmed R, et al. Outcome of treatment with a low cost protocol in adults with T cell acute lymphoblastic leukemia in a tertiary care center in India. Leuk Lymphoma. 2014;55:947–949. doi: 10.3109/10428194.2013.814130. [DOI] [PubMed] [Google Scholar]