Abstract
Background:
Inorganic arsenic (iAs) is a diabetogen. Interindividual differences in iAs metabolism have been linked to susceptibility to diabetes in iAs-exposed populations. Dietary folate intake has been shown to influence iAs metabolism, but to our knowledge its role in iAs-associated diabetes has not been studied.
Objective:
The goal of this study was to assess how folate intake, combined with low-fat (LFD) and high-fat diets (HFD), affects the metabolism and diabetogenic effects of iAs in wild-type (WT) mice and in As3mt-knockout (KO) mice that have limited capacity for iAs detoxification.
Methods:
Male and female WT and KO mice were exposed to 0 or iAs in drinking water. Mice were fed the LFD containing or folate for 24 weeks, followed by the HFD with the same folate levels for 13 weeks. Metabolic phenotype and iAs metabolism were examined before and after switching to the HFD.
Results:
iAs exposure had little effect on the phenotype of mice fed LFD regardless of folate intake. High folate intake stimulated iAs metabolism, but only in WT females. KO mice accumulated more fat than WT mice and were insulin resistant, with males more insulin resistant than females despite similar %fat mass. Feeding the HFD increased adiposity and insulin resistance in all mice. However, iAs-exposed male and female WT mice with low folate intake were more insulin resistant than unexposed controls. High folate intake alleviated insulin resistance in both sexes, but stimulated iAs metabolism only in female mice.
Conclusions:
Exposure to iAs in drinking water resulted in insulin resistance in WT mice only when combined with a HFD and low folate intake. The protective effect of high folate intake may be independent of iAs metabolism, at least in male mice. KO mice were more prone to developing insulin resistance, possibly due to the accumulation of iAs in tissues. https://doi.org/10.1289/EHP3951
Introduction
Chronic exposure to inorganic arsenic (iAs) is a global public health problem. Over 200 million people worldwide are exposed to iAs in drinking water at levels higher than As/L (Naujokas et al. 2013), the limit established by the WHO (2017) and the U.S. EPA (2001). Even more people consume foods with potentially unsafe iAs levels (Stanton et al. 2015). Chronic exposure to iAs has been linked to cancer (Abdul et al. 2015) and other diseases such as type 2 diabetes (T2D) (Maull et al. 2012; Sung et al. 2015; Wang et al. 2014). T2D is a condition of chronic hyperglycemia that affects 8.5% of the world population (WHO 2016). The prevalence of T2D has increased rapidly over the past decade (CDC 2017; WHO 2016). Although obesity is a major risk factor for T2D, normal-weight people displaying the metabolic abnormalities typical for T2D is also a highly prevalent phenomenon (Conus et al. 2007; St-Onge et al. 2004). Growing evidence suggests that environmental diabetogens such as iAs may play a role in the disease etiology (Maull et al. 2012).
The efficiency of iAs metabolism has been associated with differing risks of iAs-associated diseases, including T2D (Antonelli et al. 2014). In humans and in some animal species, iAs is metabolized in a series of methylation steps catalyzed by arsenic ( oxidation state) methyltransferase (AS3MT), forming monomethyl-arsenic (MAs) and dimethyl-arsenic (DMAs), which are excreted primarily in the urine (Thomas et al. 2007). Metabolism of iAs is generally thought to occur primarily in the liver (Thomas et al. 2001). High percentages of DMA (%DMAs) and low percentages of iAs (%iAs) and MAs (%MAs) in the urine are considered indicators of an efficient iAs metabolism. Although low %DMAs and high %MAs in the urine have been positively associated with risk of cancer, hypertension, and skin lesions (Antonelli et al. 2014; Kuo et al. 2017), T2D risk has been linked to high urinary %DMAs and/or low %MAs (Grau-Pérez et al. 2017; Kuo et al. 2017; Mendez et al. 2016), suggesting that full methylation of iAs to DMAs, possibly to its toxic trivalent form (), may enhance the diabetogenic effects of iAs exposure (Del Razo et al. 2011).
S-Adenosylmethionine (SAM) is the methyl group donor for the AS3MT-catalyzed methylation of iAs (Thomas et al. 2007). Synthesis of SAM depends on the one-carbon metabolism pathway and requires folate, an essential micronutrient and vitamin (Selhub 1999). In human studies carried out in arsenicosis-endemic areas of Bangladesh, high folate status has been associated with higher %DMAs and lower %iAs in urine (Gamble et al. 2005). Folate supplementation increased efficiency of iAs metabolism as manifested by increased %DMAs in urine and decreased As levels in blood (Gamble et al. 2006; Peters et al. 2015). High folate intake was also inversely related to precancerous skin lesions in this population (Pilsner et al. 2009). Furthermore, a study in Taiwan found that higher plasma folate and lower urinary %DMAs were associated with decreased risk of urothelial cancers (Huang et al. 2008). Thus, studies in high iAs–exposure areas such as Bangladesh suggest that folate intake could alter the risk of some diseases associated with iAs exposure by modifying iAs metabolism. Results from other human cohorts in other geographical regions; however, have been inconsistent (Spratlen et al. 2017; Steinmaus et al. 2005; Kurzius-Spencer et al. 2017), possibly due to differences in iAs exposures (generally lower than in Bangladesh), ethnicity, or nutritional status of the study populations. Notably, to our knowledge the associations between folate intake, iAs metabolism, and iAs-associated T2D have never been examined in human populations or experimental studies.
The goal of the present study was to determine if varying folate intake modifies iAs metabolism and metabolic phenotype in wild-type (WT) male and female C57BL/6J mice exposed to iAs in drinking water (), with and without a high-fat diet (HFD) as an additional metabolic stressor. This iAs concentration is within the range of concentrations reported in drinking water supplies worldwide, and human exposures to this concentration have been associated with adverse health outcomes (ATSDR 2007). To determine if folate affects the metabolic phenotype by mechanisms independent of iAs methylation, we also used As3mt-knockout (KO) mice, which have a limited capacity to methylate iAs.
Methods
Mice
All procedures involving mice were approved by the University of North Carolina Institutional Animal Care and Use Committee. Male and female C57BL/6J WT mice were obtained from Jackson Laboratories at 3 weeks of age. Male and female As3mt-KO mice on a C57BL/6J background (Drobna et al. 2009) were bred at the UNC animal facilities. Both WT and As3mt-KO mice were housed under controlled conditions with 12-h light/dark cycle at and relative humidity.
Study Design and Treatment
The study design is outlined in Figure 1. Three-week old mice were housed 4 per cage, C57BL/6J and As3mt-KO and male and female mice separately. Mice in all treatment groups were housed in standard-sized seal-safe blue line cages. Mice consumed a purified low-fat AIN-93G diet (LFD) (17% calories from fat; Envigo Teklad) with adequate folate content () and drank deionized water (DIW) for 2 weeks prior to the initial metabolic phenotyping. After 2 weeks on the purified, folate-adequate diet, a baseline intraperitoneal glucose tolerance test (IPGTT) was administered and fasting blood glucose (FBG) levels were measured in all mice. Male and female WT and KO mice were then separated into four groups in such a way that the groups had similar average baseline FBG levels ( for WT and for KO mice). Groups were randomly assigned to treatments: a) iAs, low folate LFD, b) iAs, low folate LFD, c) iAs, high folate LFD, or d) iAs, high folate LFD. The low folate and high folate diets contained and folic acid/kg, respectively. Drinking water containing iAs as arsenite (, pure; Sigma-Aldrich) was prepared weekly using DIW to minimize the oxidation of to . After 24 weeks, mice in all treatment groups were switched from the LFD to a purified AIN-93G diet with high fat content (i.e., HFD) (Envigo Teklad) with the same low or high folate levels. Mice were maintained on this diet for an additional 13 weeks. Diet composition of the LFD and HFD are given in Table S1. Water consumption was measured weekly throughout the study. Food consumption and body weight were monitored per cage biweekly.
Figure 1.
Study design. Male and female C57BL/6J (WT) and As3mt-knockout (KO) mice were fed a purified low-fat diet (LFD) with adequate folate content () and drank deionized water for 4 weeks after weaning. Baseline metabolic characteristics were assessed prior to exposure to inorganic arsenic (iAs) by measuring fasting blood glucose (FBG) and by intraperitoneal glucose tolerance test (IPGTT). Mice were then exposed to 0 or iAs in drinking water and consumed either a low () or high () folate LFD diet for the next 24 weeks. After 24 weeks, all animals were fed a purified high-fat diet (HFD) with the same low and high folate levels and continued iAs exposure for additional 13 weeks, followed by sacrifice and tissue collection. Metabolic phenotyping, including FBG, IPGTT, fasting plasma insulin (FPI) and assessment of body composition by magnetic resonance imaging (MRI), was conducted at Week 6, Week 24, and/or after 8 weeks on the HFD. Spot urine samples and livers for As speciation analysis were collected at Week 6 and/or at sacrifice. aN, number of mice at the beginning of the study.
Metabolic Phenotyping
The phenotyping timeline is shown in Figure 1. All mice were phenotyped at Weeks 6 and 24 while on the LFD and after 8 weeks on the HFD. Tissues were collected at sacrifice after 37 weeks of iAs exposure and 13 weeks on the HFD. The phenotyping included the following:
Body composition assessment using EchoMRI three-in-one Composition Analyzer and Labmaster software (version 3.2.2; Echo Medical Systems) after 24 weeks of the LFD and after 8 weeks on the HFD.
- Measurements of FBG and fasting plasma insulin (FPI) in blood collected via tail cut from mice fasted for 6 h. FBG levels were measured using the OneTouch Ultra 2 glucometer (LifeScan) at Weeks 6 and 24 on the LFD and after 8 weeks on the HFD. FPI was measured at Week 24 on the LFD and after 8 weeks on the HFD. Whole blood was collected via tail cut from mice fasted for 6 h, then centrifuged twice at 600 × g at 4°C for 15 min to isolate plasma. Plasma was stored at until analysis. Plasma insulin was measured using the Ultra Sensitive Mouse Insulin ELISA kit (Cat. No. 90060, Crystal Chem) according to the manufacturer’s directions. FBG (mg/dL) and FPI () were used to calculate the homeostasis model assessment–insulin resistance (HOMA-IR) value:
IPGTT was administered to all mice at Week 6 on the LFD. Mice were fasted for 6 h and injected intraperitoneally with body weight of d-glucose (Sigma-Aldrich) dissolved in Dulbecco’s phosphate-buffered saline (Mediatech) and glucose levels were measured in blood collected via tail cut using the OneTouch Ultra 2 glucometer at 15, 30, 60, and 120 min post-injection. To quantify glucose tolerance, the area under the curve (AUC) was determined for a plot of blood glucose (milligrams per deciliter) versus time (minutes) using Prism 5 (GraphPad).
Plasma Folate Analysis
Folate analysis was conducted on half of the plasma samples from Week 6 on the LFD and half of plasma samples collected at sacrifice (after 13 weeks on the HFD). Samples for folate analysis were chosen at random. As described above, plasma was isolated from blood at the time of collection via centrifugation at 600 × g at 4°C for 15 min. Folate was measured using the Folate Accubind ELISA kits (Cat. No. 7525-300; Monobind Inc.) according to manufacturer directions. For analysis of samples from animals on the high folate diet, plasma was diluted in Dulbecco’s phosphate-buffered saline.
Analysis of Arsenic Metabolites in Urine, Liver, and Diet
Spot urine samples were collected from mice after 6 weeks on the LFD and at sacrifice. After collection, the urine was placed on ice, then stored at . Liver was collected at sacrifice, flash frozen in liquid nitrogen, and stored at . Arsenic (As) species were analyzed in urines and in 10% (w/v) liver homogenates prepared in DIW. The speciation analysis was carried out by hydride generation–cryotrapping–atomic absorption spectrometry (HG-CT-AAS) using a custom-made hydride-generation system coupled with a cryotrap (Hernández-Zavala et al. 2008) and AAnalyst 800 atomic absorption spectrometer (Perkin-Elmer) equipped with a multiatomizer (Matoušek et al. 2008). The HG-CT-AAS analysis determined concentrations of total iAs (), total MAs () and total DMAs (); no trimethyl-As was detected. Total As content in urine and liver was calculated as the sum of all As species detected. HG-CT-AAS was also used to determine the concentrations of arsenic species in mouse diets. Here, five to six pellets from each diet were ground in a ceramic mortar to a fine powder. The powder was digested in ultrapure phosphoric acid (EMD Chemicals, Inc.) using MARS 5 microwave system (CEM Corp.) (Currier et al. 2011).
Statistical Analysis
Four-way analyses of variance (ANOVAs) were performed to assess the effect of iAs exposure, folate, sex, and genotype on all measured end points. Post hoc Student’s t-tests were used to assess end points between two groups of interest (e.g., to determine a significant effect of folate, WT male mice fed the low folate diet exposed to iAs were compared with WT males exposed to iAs fed the high folate diet). A p value of was determined a priori to be significant. The numbers of mice from each treatment group used for specific measurements and analyses in the course of the study are listed in Table 1. All statistical analyses were conducted in JMP (SAS Institute Inc.).
Table 1.
Numbers of WT and As3mt-KO mice in the LFD and HFD groups, with low or high folate intake and 0 or 100 ppb iAs in drinking water at the start and the end of the study, and numbers of mice used for phenotyping (FBG, FPI, body composition) and other analyses carried out in the course of the study.
| Week (n) | Genotype | Low folate | High folate | |||
|---|---|---|---|---|---|---|
| iAs | iAs | iAs | iAs | |||
| Females | ||||||
| Start of study | 0 | WT | 16 | 16 | 16 | 16 |
| KO | 20 | 20 | 16 | 16 | ||
| LFD, urine arsenic | 6 | WT | 16 | 8 | 15 | 10 |
| KO | 10 | 10 | 8 | 8 | ||
| LFD, plasma folate | 6 | WT | 7 | 7 | 8 | 8 |
| KO | 9 | 10 | 8 | 8 | ||
| LFD, FBG | 24 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| LFD, FPI | 24 | WT | 16 | 14 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| LFD, body composition | 24 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| HFD, FBG | 32 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| HFD, FPI | 32 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| HFD, body composition | 32 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| HFD, urine arsenic | 37 | WT | 14 | 16 | 14 | 13 |
| KO | 14 | 10 | 8 | 10 | ||
| HFD, plasma folate | 37 | WT | 8 | 7 | 8 | 8 |
| KO | 9 | 10 | 8 | 8 | ||
| Liver arsenic | 37 | WT | 12 | 10 | 14 | 14 |
| KO | 14 | 8 | 14 | 8 | ||
| End of study | 37 | WT | 16 | 15 | 15 | 15 |
| KO | 19 | 20 | 16 | 16 | ||
| Total deaths | — | WT | 0 | 1 | 1 | 1 |
| KO | 1 | 0 | 0 | 0 | ||
| Males | ||||||
| Start of study | 0 | WT | 16 | 16 | 16 | 16 |
| KO | 20 | 20 | 16 | 20 | ||
| LFD, urine arsenic | 6 | WT | 14 | 11 | 9 | 9 |
| KO | 16 | 12 | 10 | 10 | ||
| LFD, plasma folate | 6 | WT | 7 | 8 | 6 | 7 |
| KO | 10 | 8 | 7 | 11 | ||
| LFD, FBG | 24 | WT | 16 | 16 | 16 | 16 |
| KO | 18 | 17 | 15 | 20 | ||
| LFD, FPI | 24 | WT | 16 | 16 | 15 | 16 |
| KO | 18 | 17 | 15 | 20 | ||
| LFD, body composition | 24 | WT | 16 | 16 | 16 | 16 |
| KO | 18 | 17 | 15 | 20 | ||
| HFD, FBG | 32 | WT | 16 | 16 | 16 | 16 |
| KO | 18 | 16 | 14 | 20 | ||
| HFD, FPI | 32 | WT | 16 | 16 | 16 | 16 |
| KO | 17 | 14 | 13 | 19 | ||
| HFD, body composition | 32 | WT | 16 | 16 | 16 | 16 |
| KO | 18 | 16 | 14 | 20 | ||
| HFD, urine arsenic | 37 | WT | 12 | 16 | 10 | 16 |
| KO | 10 | 12 | 10 | 13 | ||
| HFD, plasma folate | 37 | WT | 7 | 8 | 6 | 7 |
| KO | 10 | 8 | 7 | 11 | ||
| Liver arsenic | 37 | WT | 10 | 11 | 10 | 9 |
| KO | 9 | 12 | 8 | 14 | ||
| End of study | 37 | WT | 16 | 16 | 15 | 16 |
| KO | 18 | 16 | 14 | 20 | ||
| Total deaths | — | WT | 0 | 0 | 1 | 0 |
| KO | 2 | 4 | 2 | 0 | ||
Note: —, not applicable; FBG, fasting blood glucose; FPI, fasting plasma insulin; HFD, high-fat diet; iAs, inorganic arsenic; KO, arsenic () methyltransferase knockout mice; LFD, low-fat diet; WT, wild type.
Results
Survival of Mice in the Study
A total of 13 mice (8.6%) died or had to be euthanized during the course of the study due to injuries suffered during fighting or natural causes. These included 6 KO males fed the low folate diet (2 controls, 4 exposed to iAs), 2 KO males fed the high folate diet (both controls), 1 WT male control fed the high folate diet, 1 KO female control fed the low folate diet, 1 WT female control fed the low folate diet, and 2 WT females fed the high folate diet (1 control and 1 exposed to iAs).
Food and Water Consumption
There were few differences in food or water consumption associated with iAs exposure or folate intake throughout the entire study (see Figures S1 and S2). No significant differences were found in food consumption between the WT and KO mice (see Figure S1), although the KO mice in several groups during LFD or HFD feeding drank less water than their WT counterparts (see Figure S2). Females tended to consume less food than males regardless of treatment.
Arsenic Levels in Diets
The concentration and speciation of As were examined in each of the diets used in the study. Total As in the diets ranged from 36 to and consisted primarily of iAs (89–100%).
Plasma Folate
Plasma folate concentration was measured in half of the mice randomly selected from each treatment group. As expected, mice fed the high folate diet had significantly higher plasma folate levels than mice on the low folate diet, in both LFD (at Week 6) and HFD (at sacrifice) conditions (Table 2). There were no statistically significant differences in plasma folate levels due to iAs exposure and very few due to knockout of As3mt. Significant differences in plasma folate were found between males and females at both time points, mostly among animals consuming the high folate diet. Plasma folate levels were much lower at sacrifice for all groups fed low folate diet, reaching statistical significance in six of eight groups. These differences were statistically significant for all iAs-exposed groups and all KO groups fed the low folate diet.
Table 2.
Folate levels (ng/mL; ) in plasma of male and female, WT and As3mt-KO mice exposed to 0 or 100 ppb iAs in drinking water and consuming low or high folate diets.
| Diet and Exposure | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| WT | KO | WT | KO | |||||
| Week 6a | Sacrificeb | Week 6 | Sacrifice | Week 6 | Sacrifice | Week 6 | Sacrifice | |
| Low folate diet | ||||||||
| iAs | c | d,e | e | |||||
| iAs | e | e | e | d,e | ||||
| High folate diet | ||||||||
| iAs | c,f | f | c,f | c,f | f | f | f | f |
| iAs | f | c,f | c,d,f | c,f | f | f | f | e,f |
Note: Data shown are for ; specific N numbers per group are found in Table 1. HFD, high-fat diet; iAs, inorganic arsenic; KO, arsenic () methyltransferase knockout mice; LFD, low-fat diet; WT, wild type.
Plasma collected after 6 weeks of exposure and LFD.
Plasma collected at sacrifice (37 weeks of iAs exposure and 13 weeks on HFD).
comparing males and females, using post hoc Student’s t-test.
comparing WT and KO mice, using post hoc Student’s t-test.
comparing Week 6 and Sacrifice, using post hoc Student’s t-test.
comparing low folate and high folate diet mice, using post hoc Student’s t-test.
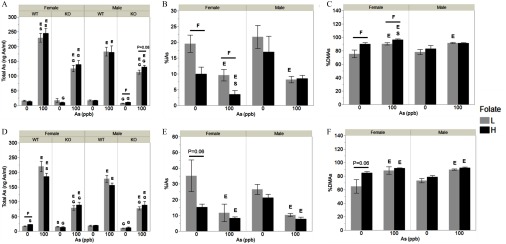
Arsenic Species in the Urine
Spot urine samples for analysis of As species were collected after 6 weeks on the LFD and at sacrifice (Figure 2). Not all mice provided sufficient urine volume, resulting in per treatment group (Table 1). At both time points, total As concentrations were higher in urine of WT and KO mice exposed to iAs in drinking water as compared with controls (Figure 2A,D). Total As concentrations were generally higher in WT than KO mice. Female WT mice exposed to iAs had higher concentrations of total As in urine than their male counterparts. Urine from WT mice contained primarily DMAs (64–96% of total As), followed by iAs (3–35%). iAs was the primary or the only As species found in the urine of As3mt-KO mice. A small amount of DMAs was detected only in the urine of control KO females fed the high folate diet () at sacrifice and in the urine of KO males fed the low folate diet at Week 6, in both control () and exposed groups (). MAs were not detected in the urine of either the WT or the KO mice at either time point.
Figure 2.
Total arsenic (i.e., sum of arsenic species) and percentages of arsenic (As) species in urine of male and female wild-type (WT) and As3mt-knockout (KO) mice fed a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water, before and after switching from the low-fat (LFD) to the high-fat diet (HFD): Total As, in urines of WT and KO mice after 6 weeks on the LFD (A) and at sacrifice (i.e., after 37 weeks of exposure and 13 weeks of the HFD) (D). Percentage inorganic arsenic (%iAs) (B) and percentage dimethylarsenic (%DMAs) (C) in urines of WT mice after 6 weeks on the LFD; %iAs (E) and %DMAs (F) in urines of WT mice at sacrifice. for ; specific N numbers per group are reported in Table 1. using post hoc Student’s t-test unless otherwise noted for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; F, low vs. high folate intake animals of the same genotype, exposure, and sex; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level.
Folate intake had no statistically significant effects on total urinary As in most control and iAs-exposed groups. Only control WT female mice fed the high folate diet had higher total urinary As levels at sacrifice than the control WT females fed the low folate diet (Figure 2D). A similar effect of high folate intake on total urinary As was observed in KO male mice exposed to iAs, but this effect was only marginally significant (; Figure 2A). Effects of folate intake on percentages of urinary As species were more consistent, although not all were statistically significant. Specifically, %iAs was lower and %DMAs was higher in urine of both control and iAs-exposed female WT mice after 6 weeks on high folate LFD as compared with WT females on low folate LFD (Figure 2B,C). At sacrifice, %iAs was still lower and %DMAs was higher in control females with high folate intake than those with low folate intake (Figure 2E,F), but these differences were only marginally significant (). There were little or no statistically significant effects of folate intake on percentages of As species in urine of mice in other treatment groups.
Arsenic Species in the Liver
Livers for As analysis were collected at sacrifice. Concentrations of As species were measured in livers from randomly selected half of the mice in each treatment group (Figure 3; Table 1). In general, total hepatic As concentrations were higher in mice exposed to iAs than in unexposed controls, they and were higher in KO compared with WT mice (Figure 3A). In livers of control WT mice, iAs, MAs, and DMAs represented 31–65%, 17–38%, and 8–46% of total As, respectively (Figure 3B–D). %iAs was lower and %DMAs was higher in the livers of male WT mice exposed to As as compared with controls, regardless of folate intake. WT females exposed to iAs also had lower liver %iAs, but only in the high folate group. Exposure to iAs did not affect %MAs. Livers of KO mice contained mainly iAs (82–97% of total As), with only small percentages of MAs and/or DMAs (average 5% and 2%, respectively). The concentration of total As and percentages of As species in the liver were influenced by folate intake, but only in female WT mice. Livers of female WT mice exposed to iAs and fed the high folate diet contained less total As than livers of iAs-exposed female WT mice fed the low folate diet (Figure 3A). High folate intake had a similar effect in the control female WT mice, but this effect was only marginally significant (). Additionally, high folate intake was associated with lower %iAs and higher %MAs, and marginally higher %DMAs () in female WT mice exposed to iAs (Figure 3B–D). There were no statistically significant differences due to folate in total liver As or percentages of liver As in other treatment groups.
Figure 3.
Total arsenic (i.e., sum of As species) and percentages of arsenic (As) species in livers of male and female wild-type (WT) and As3mt-knockout (KO) mice fed a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water: Total As in livers of WT and KO mice at sacrifice (A). Percentage inorganic arsenic (%iAs) (B), percentage monomethylarsenic (%MAs) (C), and percentage dimethylarsenic (%DMAs) (D) in livers of WT mice at sacrifice. for ; specific N numbers per group are found in Table 1. using post hoc Student’s t-test unless otherwise noted for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; F, low vs. high folate intake animals of the same genotype, exposure, and sex; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level.
Body Weight and Weight Gain
Over the first 24 weeks of iAs exposure, during which the mice were fed the LFD, the growth rates of males and females were similar although males were heavier throughout (see Figure S3). Males gained more weight than females (see Figure S4A,C). There were few differences in weight gain due to iAs exposure or folate intake. KO females gained more weight than WT females over the course of 24 weeks on the LFD; there were no differences in weight gain between WT and KO males (see Figure S4A,C). There were fewer differences in weight gain between males and females after mice were switched from the LFD to the HFD (see Figure S4B,D). KO female mice gained the most weight compared with other groups (see Figure S4B,D). Still, there were few differences in weight gain associated with iAs exposure or folate intake.
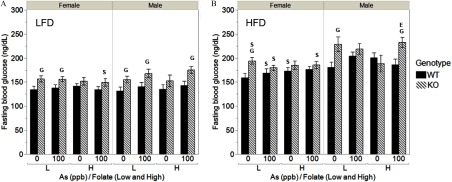
Fasting Blood Glucose
The IPGTT was administered at Week 6 on the LFD after 6-h fasting. FBG levels measured during IPGTT were higher in males compared with females regardless of genotype, and they were higher in KO female mice compared with WT female mice (Figure S5A). Similarly, male mice were more glucose intolerant than female mice (based on greater AUC values), but little or no statistically significant differences due to exposure or folate intake were found (see Figure S5B). FBG was also measured after 24 weeks on the LFD and after 8 weeks on the HFD (Figure 4). At Week 24, FBG levels were higher in KO male and female mice as compared with their WT counterparts, but these differences were statistically significant primarily in the low folate intake groups (Figure 4A). At this time point, little or no differences due to sex or exposure were found. Consumption of the HFD was associated with an increase in FBG across all treatment groups (Figure 4B). However, many of the differences between WT and KO mice seen at Week 24 were no longer significant. In general, FBG levels were lower in WT and KO female mice as compared with males in most treatment groups. Exposure to iAs and folate intake had little or no effects.
Figure 4.
Fasting blood glucose (FBG) of male and female WT and As3mt-KO mice fed a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water, before and after switching from low-fat (LFD) to high-fat diet (HFD): FBG was measured after 24 weeks on the LFD (A) and after 8 weeks on HFD (B). for ; specific N numbers per group are reported in Table 1. using post hoc Student’s t-test for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level. There were no statistically significant differences in body composition between low and high folate intake animals of the same genotype, exposure, and sex.
Body Composition
Body weight was measured biweekly and body composition was assessed via magnetic resonance imaging at Week 24 on the LFD and at Week 8 on HFD (Figure 5). All males were significantly heavier than females at both time points (Figure 5A,B). After 24 weeks of iAs exposure on the LFD, male and female KO mice in most treatment groups had higher body weight and percentage of fat (%fat) mass and lower percentage of lean (%lean) mass compared with WT mice (Figure 5A,C,E). There were little or no significant differences in %fat or % lean mass between males and females in either WT or KO groups. At this time point, no effect of iAs exposure and only random effects of folate intake on body weight and body composition were found. After switching to the HFD, body weight and %fat increased, whereas %lean mass decreased in all treatment groups. Female KO mice remained heavier than female WT mice, whereas few differences were seen between WT and KO males (Figure 5B). KO mice, particularly females, still had higher %fat mass and lower %lean mass than WT mice (Figure 5D,F). Further analysis showed that %fat increase was highest in KO females as compared with any other groups (Figure 5G). On the other hand, the lowest %fat increase was found in KO males. There were no significant differences in %fat mass and %lean mass between sexes, except in the low folate control KO group (Figure 5D,F). Male KO mice fed the low folate diet and exposed to iAs had higher body weight and %fat and lower %lean mass compared with controls. There were no other significant effects related to exposure or folate intake.
Figure 5.
Body composition of male and female WT and As3mt-KO mice fed a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water, before and after switching from low-fat (LFD) to high-fat diet (HFD): Body weight, percentage fat (%fat), and percentage lean (% lean) mass were measured after 24 weeks on a LFD (A,C,E) and after 8 weeks on a HFD (B,D,F). The change in %fat (G) is the difference between %fat at Week 8 on HFD and 24 weeks on the LFD. for ; specific N numbers per group are found in Table 1. using post hoc Student’s t-test for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; F, low vs. high folate intake animals of the same genotype, exposure, and sex; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level.
Fasting Plasma Insulin and Insulin Resistance
Male mice had significantly higher FPI and HOMA-IR compared with female mice in both WT and KO groups at Week 24 on the LFD and after 8 weeks on HFD (Figure 6). After 24 weeks on the LFD, KO mice had significantly higher FPI and HOMA-IR than WT mice in all treatment groups; the differences were statistically significant among males (Figure 6A). There were no significant differences in FPI or HOMA-IR associated with iAs exposure and only one significant effect of folate intake, which was found in control female WT mice (Figure 6C). Consumption of HFD resulted in increases in FPI and HOMA-IR values in all treatment groups and further augmented the differences due to sex and genotype. Notably, after 8 weeks on the HFD, FPI and insulin resistance were significantly influenced by iAs exposure. Specifically, FPI and HOMA-IR values were higher in iAs-exposed WT females and males consuming the low folate diet compared with controls (Figure 6B,D) although the difference in FPI in WT females was only marginally significant (). In contrast, no significant effects of iAs exposure on HOMA-IR or FPI were found among WT males and females fed the high folate diet. WT males on the high folate diet exposed to iAs had lower HOMA-IR and FPI values than the exposed males on the low folate diet, but these differences were only marginally significant (). Similar, but less prominent differences were also found in WT females. In contrast, FPI and HOMA-IR in KO mice were not influenced by either iAs exposure or folate intake.
Figure 6.
Fasting plasma insulin and homeostasis model assessment–insulin resistance (HOMA-IR) values of male and female wild-type (WT) and As3mt-knockout (KO) mice fed a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water, before and after a high-fat diet (HFD), before and after switching from the low-fat diet (LFD) to the HFD: Fasting plasma insulin and HOMA-IR were measured after 24 weeks on a LFD (A,C) and after 8 weeks on a HFD (B, D). for ; specific N numbers are found in Table 1. using post hoc Student’s t-test for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; F, low vs. high folate intake animals of the same genotype, exposure, and sex; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level.
Because insulin resistance is closely linked to adiposity, FPI and HOMA-IR values at Week 8 of HFD were normalized by body fat. Even with the normalized values, FPI and HOMA-IR were still higher in males than females and in male KO as compared with male WT mice (Figure 7). However, the differences between female KO and WT mice and the effects of iAs exposure in WT males and females seen in Figure 6 were eliminated. In fact, after normalization, HOMA-IR in iAs-exposed male KO mice fed the high folate diet was higher compared with respective controls (Figure 7B).
Figure 7.
Fat-adjusted fasting plasma insulin and homeostasis model assessment–insulin resistance (HOMA-IR) values of male and female wild-type (WT) and As3mt-knockout (KO) mice on a low (L) or high folate (H) diet and exposed to 0 or inorganic arsenic (iAs) in drinking water after 8 weeks on high-fat diet (HFD). Fasting plasma insulin (A) and homeostatic model assessment of insulin resistance (HOMA-IR) (B) values were divided by body fat mass (g). for ; specific N numbers are found in Table 1. using Student’s t-test for the following comparisons: S, males vs. females of the same diet, exposure, and genotype; G, WT vs. KO mice of the same sex, diet, and exposure; and E, 0 vs. iAs-exposed mice of the same sex, genotype, and folate level. There were no statistically significant differences in body composition between low and high folate intake animals of the same genotype, exposure, and sex.
Discussion
Effect of iAs Exposure
In humans, chronic exposure to moderate to high levels of iAs in drinking water () has been associated with T2D (Maull et al. 2012). Even lower iAs exposures have been linked to an increased prevalence or incidence of T2D in recent studies (Kuo et al. 2017). In laboratory studies of iAs-associated diabetes, mice and rats developed impaired glucose tolerance and increased FBG after chronic exposure to high (in parts per million) levels of iAs in drinking water (Maull et al. 2012; Paul et al. 2007, 2011; Dávila-Esqueda et al. 2011). However, at lower doses that are more relevant to human exposures (in the parts-per-billion range), the results are inconsistent. For example, Huang and associates have reported that adult female ICR mice exposed to iAs develop diabetic phenotype characterized by fasting hyperglycemia, impaired glucose tolerance, and fasting hypoinsulinemia (Huang et al. 2015). In contrast, our laboratory found only minor impairment of fasting glycemia and glucose tolerance in male and female C57BL/6 mice after exposure to iAs (Douillet et al. 2017). The fact that most laboratories do not control for iAs exposure through diet complicates the comparison. Grain-based chow diets can contain as much as As, with iAs often being the major species (Murko et al. 2018). Thus, the background dietary iAs exposure could be a confounding factor, especially for studies examining effects of low (in parts per billion) levels of iAs in drinking water.
To minimize the background exposure to iAs in the present study, we used a purified LFD and HFD that contained only iAs. We found that a 24-week exposure to an additional iAs in drinking water had no significant effect on glucose homeostasis in male or female WT C57BL/6J mice fed the purified LFD (Figure 6). However, after switching to the HFD for an additional 8 weeks, iAs exposure significantly increased FPI and HOMA-IR in both male and female WT mice fed the low folate diet. This finding suggests that a low-level iAs exposure can elicit a diabetic phenotype in this mouse strain only when combined with the HFD and low folate intake, and that this phenotype is characterized by insulin resistance. The phenotype appears to be driven primarily by increased adiposity, because the effects of exposure were minimized after FPI and HOMA-IR values were normalized by body fat (Figure 7). This result is interesting because iAs is not thought to be an obesogen. In fact, we have previously reported that C57BL/6 mice fed a HFD and exposed to much higher levels of iAs in drinking water (25 and ) had less %fat than control unexposed mice (Paul et al. 2011). Furthermore, exposure to iAs has been shown to inhibit adipocyte differentiation and adipogenesis in in vitro models (Wang et al. 2005; Trouba et al. 2000). Although iAs exposure has not been linked directly to obesity in human studies, recent literature reports some interaction between obesity and adverse effects of iAs exposure. Individuals with high body mass index have been shown to have a higher risk of iAs-associated diseases such as diabetes, nonalcoholic fatty liver disease, and renal cell carcinoma (Spratlen et al. 2018; Castriota et al. 2018; Frediani et al. 2018; Hsueh et al. 2018). Taken together, these data suggest that the diabetic phenotypes associated with iAs exposure are influenced by adiposity or the consumption of a HFD, and that the underlying pathologies may differ between low versus high levels of iAs exposure.
Effect of As3mt-KO
We recently reported that As3mt-KO mice that drank DIW but were exposed to iAs () from a rodent chow diet became obese and insulin resistant (Douillet et al. 2017). In the present study, a similar phenotype was found in control As3mt-KO mice fed a purified diet with lower iAs content (). These mice (particularly males) accumulated more fat and were more insulin resistant than WT mice (Figures 5 and 6). The HFD further exacerbated the adverse phenotype in both female and male KO mice. Results of these two studies suggest that the predisposition of As3mt-KO mice to obesity and development of insulin resistance could be due to the background exposure to iAs from the diets. Indeed, KO mice that could not effectively methylate iAs retained more As in the liver (and probably in other tissues) and excreted less As in urine than WT mice (Figures 2 and 3). We have previously shown that FPI correlates with markers of the inhibition of insulin signaling in the liver of WT and KO mice exposed to 0, 0.1, and iAs in drinking water (Douillet et al. 2017). Thus, iAs (including iAs from diet) that accumulates in the tissues could impair mechanisms that regulate glucose metabolism, specifically the insulin signaling pathway, resulting in insulin resistance. iAs exposure has also been shown to alter epigenetic programing of genes regulating glucose metabolism (Martin et al. 2017; Martin and Fry 2016). Our As3mt-KO mouse colony is bred and maintained on a regular rodent chow, which likely contains high parts-per-billion levels of iAs (Murko et al. 2018). Thus, these mice may be preprogrammed by prenatal iAs exposure to become obese and insulin resistant.
The phenotypic differences between KO and WT mice, particularly females, appeared to be driven by adiposity, but it is unclear what caused the difference in fat accumulation. There were no significant differences in food consumption (i.e., energy intake) between WT and KO mice in this study (see Figure S1). As discussed above, iAs is not known to promote adiposity. However, hyperinsulinemia, which characterizes the metabolic phenotype of KO mice, can drive lipid synthesis even in the presence of insulin resistance (Shimomura et al. 2000; Brown and Goldstein 2008). Thus, whereas adiposity drives the elevated insulin levels, high levels of insulin may also be driving fat accumulation in KO mice. It is also possible that accumulation of iAs in tissues of KO mice in utero or in postnatal life affects other mechanisms involved in metabolic homeostasis, including methylation and/or transcription of genes regulating lipid metabolism.
In the present study, we evaluated iAs metabolism in WT and KO mice using measures of iAs and its metabolites in the urine. This makes it possible to relate our experimental findings to data from epidemiological studies. In addition, we analyzed arsenic metabolites in the liver because our previous work suggested that urinary profiles do not necessarily reflect the concentrations and percentages of arsenic metabolites in the blood (Grau-Pérez et al. 2017) or target tissues (Currier et al. 2014). Indeed, we found that whereas DMAs is the major metabolite in urine of WT mice, iAs is the major arsenic species in the liver. Other indicators of iAs exposure and metabolism have been used in previous population and clinical studies including arsenic content in hair, toenails, or bones (Adeyemi et al. 2010; Dani and Walter 2017; Kapaj et al. 2006; Marchiset-Ferlay et al. 2012; Orloff et al. 2009). Using these indicators in future studies could provide additional insight into the differences in iAs metabolism or toxicity associated with As3mt-KO or with variations in folate intake.
Effect of Folate Intake
Folate, an essential micronutrient, is required for a variety of metabolic pathways, including synthesis of SAM (Selhub 1999), a methyl donor for iAs methylation. Supplementation with folate has been associated with increasing iAs methylation capacity and lowered disease risk in human studies (Hall and Gamble 2012). However, the potentially protective effects of folate via stimulation of iAs metabolism have not been systematically studied in either humans or laboratory animals. In mice that lack a folate transporter (Folbp), iAs metabolism was found to be less efficient than in WT mice, but only when these mice were fed a folate-deficient diet (Spiegelstein et al. 2003, 2005). In our previously published study, dietary folate supplementation significantly decreased iAs concentration and increased the DMAs:MAs ratio in the livers of pregnant CD-1 mice exposed to iAs in drinking water (Tsang et al. 2012).
The standard rodent chow contains folic acid, whereas as low as folate/kg is estimated to be sufficient for adult mice (NRC 1995). Notably, some studies used diets with folate content as low as without major effects on animal growth (Kopp et al. 2017). The lack of a stronger response to the dietary folate withdrawal in mice is associated with the animal’s ability to absorb folate produced by gut microbiota. Thus, the induction of folate deficiency in mice requires the use of an antibiotic to inhibit intestinal folate-producing bacteria (Oleinik et al. 2014). On the other end, folate supplementation studies in mice often used diets with , or even up to folate. In our study, we did not intend to induce folate deficiency or to heavily oversupplement mice with folate, but rather to achieve moderately low or moderately high folate levels in order to investigate the effect of folate intake on iAs metabolism and health effects. Therefore, we used diets with and folate, levels maintaining a large (50-fold) difference between the groups and emulating under- or oversupplemented human populations.
In our study, the difference in plasma folate, the pool most rapidly responding to dietary folate supplementation (Schmitz et al. 1994), was about 2.3-fold lower in mice on low folate diet compared with mice on high folate diet at Week 6 (the averaged difference between all groups; Table 2). This difference increased to 4.5-fold at sacrifice. In general, these data fit well into expected dynamics of animal response to dietary folate supplementation. Because hepatocytes are most susceptible to folate deficiency (Kopp et al. 2017), measurement of folate in the liver or other tissues could reveal more substantial differences in folate levels. We did not use antibiotics to inhibit folate production by the gut bacteria (Rossi et al. 2011) because we observed signs of synergistic toxicity in iAs-exposed mice in our unpublished studies.
We expected to find major differences in the efficiency of iAs metabolism between WT mice fed the low folate diet and WT mice fed the high folate diet containing 50 times more folate. We also expected that high folate intake would reduce the adverse effects of iAs exposure in these mice by stimulating iAs metabolism (methylation) and excretion. Additionally, we predicted that altering folate intake in As3mt-KO mice would not alter diabetogenic effects of iAs exposure because these mice cannot methylate iAs. The results of the study confirm these expectations; however, the effects of folate were relatively minor and were sex dependent. The consumption of a high folate diet altered percentages of As species only in the urine and livers of WT female mice, decreasing %iAs and increasing %DMAs (Figures 2 and 3), but had no effects in male WT mice. Similar shifts in %iAs (decreased by 4%) and %DMA (increased by 7%) have been reported in the urine of Bangladeshi residents after supplementation with folate (Gamble et al. 2006), but sexual differences in the response to folate supplementation have not been noted. Although the folate-related sexual dimorphism has not been extensively investigated, recent publications have indicated sex-specific responses to dietary folate (Winkels et al. 2008; Nilsson et al. 2014). Precise mechanisms underlying these differences are not understood at present. However, the previously reported sex-specific expression patterns of genes involved in folate transport or metabolism (Hashiguchi et al. 2016) may be, at least in part, responsible for the overall differences in the response to dietary folate intake between males and females.
The differences in iAs metabolism between mice on low- and high folate diets were relatively minor, given the differences in the folate intake. It is possible, that even the low folate intake provided sufficient support for methylation of the low dose of iAs delivered in drinking water (). Furthermore, other B-vitamins and other micronutrients involved in one-carbon metabolism have been linked to differences in iAs metabolism in humans (Spratlen et al. 2017; Kurzius-Spencer et al. 2017). It is possible that changes in intakes of these nutrients, in addition to varying the folate intake, may be needed to see more substantial changes in iAs metabolism.
Despite having only modest effects on iAs metabolism and only in female WT mice, high folate intake seemed to rescue the adverse phenotype in both female and male WT mice exposed to iAs (Figure 6B,D). The fact that iAs metabolism was not significantly altered by high folate in male WT mice suggests that the protective effects of folate may be independent of iAs metabolism. It is also possible that higher doses of iAs are needed in mice to observe changes in iAs metabolism due to folate intake because mice metabolize and detoxify iAs more efficiently than humans (Vahter 1999, 2000).
Effect of Sex
Human studies examining the proportion of As species in urine have suggested that women are more efficient methylators of iAs, possibly due to the role of estrogen in the production of choline, which is another donor of methyl groups for SAM synthesis and iAs metabolism (Lindberg et al. 2007; Jansen et al. 2016). In this study, we found that female WT mice fed the low folate LFD and exposed to iAs had more total As, lower %iAs and higher %DMAs in the urine (Figure 2), which is consistent with the human data.
The sexual dimorphism in susceptibility to metabolic disease has been documented in humans and in mice (Kautzky-Willer et al. 2016; Mauvais-Jarvis 2015; Jaworski et al. 2011; Bonaventura et al. 2017; Soares et al. 2017). Differences in adipose tissue storage are thought to underlie some of these sex differences (Kautzky-Willer et al. 2016). However, in our study, the sex differences did not seem to be related to fat accumulation. In KO and WT groups, males and females had similar %fat mass (Figure 5), but males were much more insulin resistant than the females. Normalization of FPI and HOMA-IR by body fat did not eliminate these sex differences. Thus, fat accumulation does not explain the sex difference in insulin resistance among these mice. Differences in energy metabolism and actions of sex hormones may play more of a role. Additionally, prenatal effects could influence sexual differences. Sexual dimorphisms in epigenetic regulation, in the fetus and in the placenta, have been observed and may be an underlying mechanism in the differential susceptibility of males and females to metabolic disease (Kautzky-Willer et al. 2016).
In the present study, the sex difference in insulin resistance was more pronounced in As3mt-KO mice than in WT mice. Notably, normalization of FPI and HOMA-IR by body fat eliminated significant differences due to KO genotype in females but not in males (Figure 7). This observation suggests that factors independent of fat accumulation affect the development of insulin resistance in male KO mice. These factors should be investigated in future studies. Interestingly, in our study sexual dimorphism was also noted in response to dietary folate as discussed above.
Conclusions
Our data indicate that chronic exposure to iAs in drinking water, the exposure level that has been associated with T2D risk in humans (Kuo et al. 2017), in addition to the background exposure to iAs in the diet, had little effect on the metabolic phenotype of WT C57BL/6 male and female mice fed a LFD. However, when combined with an obesogenic HFD and low folate intake, iAs exposure elicited insulin resistance in both male and female WT mice. These findings highlight potential interactions between environmental and dietary factors that also need to be taken into account in human population studies. To our knowledge, this study is the first to show that high folate intake may rescue the diabetic phenotype (insulin resistance) produced in WT mice by low-level iAs exposure combined with obesity, although this effect is probably not related to iAs metabolism. Strengths of this study include the use of low, environmentally relevant concentrations of iAs in drinking water (), while controlling for As content in the diet, and the analysis of As species both in urine and livers, providing information about As species excreted from the body, as well as about As speciation in the organ that plays a key role in iAs metabolism. The fact that we did not monitor As content in other body compartments, especially those containing most of the body As (e.g., skin, hair and bones) can be viewed as a limitation. Another potential weakness of this study is associated with the measurement of folate that was limited to plasma and did not include analysis of folate in the liver or other tissues associated with folate metabolism. As we previously reported (Douillet et al. 2017), As3mt-KO mice were more prone to developing obesity and insulin resistance than WT mice even in the absence of iAs exposure from drinking water. This is likely due to the accumulation of iAs in tissues of KO mice from background dietary iAs exposure, leading to inhibition of insulin signaling. The differences in the susceptibility of male and female mice to developing insulin resistance, despite having similar levels of adiposity and similar levels of iAs in the liver, point to sex as an important factor in the pathways and processes targeted by iAs exposure.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health (NIH) grants R01ES022697 and R01ES022697-03S1 to M.S. and F31ES027743 grant to M.H. Additional support was provided by NIH grant DK 056350 to the UNC Nutrition Obesity Research Center (NORC). The authors would like to thank A. Dunlap for her help in animal care.
References
- Abdul KSM, Jayasinghe SS, Chandana EPS, Jayasumana C, De Silva PMCS. 2015. Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40(3):828–846, PMID: 26476885, 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Adeyemi A, Garelick H, Priest ND. 2010. A biokinetic model to describe the distribution and excretion of arsenic by man following acute and chronic intakes of arsenite/arsenate compounds by ingestion. Hum Exp Toxicol 29(11):891–902, PMID: 20219843, 10.1177/0960327110364912. [DOI] [PubMed] [Google Scholar]
- Antonelli R, Shao K, Thomas DJ, Sams R II, Cowden J. 2014. AS3MT, GSTO, and PNP polymorphisms: impact on arsenic methylation and implications for disease susceptibility. Environ Res 132:156–167, PMID: 24792412, 10.1016/j.envres.2014.03.012. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2007. Toxicological profile for arsenic. https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=22&tid=3 [accessed 26 November 2018]. [PubMed]
- Bonaventura MM, Bourguignon NS, Bizzozzero M, Rodriguez D, Ventura C, Cocca C, et al. 2017. Arsenite in drinking water produces glucose intolerance in pregnant rats and their female offspring. Food Chem Toxicol 100:207–216, PMID: 28017702, 10.1016/j.fct.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. 2008. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7(2):95–96, PMID: 18249166, 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Castriota F, Acevedo J, Ferreccio C, Smith AH, Liaw J, Smith MT, et al. 2018. Obesity and increased susceptibility to arsenic-related type 2 diabetes in Northern Chile. Environ Res 167:248–254, PMID: 30059859, 10.1016/j.envres.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. National Diabetes Statistics Report, 2017. http://www.diabetes.org/assets/pdfs/basics/cdc-statistics-report-2017.pdf [accessed 28 January 2018].
- Conus F, Rabasa-Lhoret R, Péronnet F. 2007. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 32(1):4–12, PMID: 17332780, 10.1139/H07-926. [DOI] [PubMed] [Google Scholar]
- Currier JM, Ishida MC, González-Horta C, Sánchez-Ramírez B, Ballinas-Casarrubias L, Gutiérrez-Torres DS, et al. 2014. Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ Health Perspect 122(10):1088–1094, PMID: 25000461, 10.1289/ehp.1307756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier JM, Svoboda M, Matoušek T, Dědina J, Stýblo M. 2011. Direct analysis and stability of methylated trivalent arsenic metabolites in cells and tissues. Metallomics 3(12):1347–1354, PMID: 22015847, 10.1039/c1mt00095k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani SU, Walter GF. 2017. Chronic arsenic intoxication diagnostic score (CAsIDS). J Appl Toxicol 38(1):122–144, PMID: 28857213, 10.1002/jat.3512. [DOI] [PubMed] [Google Scholar]
- Dávila-Esqueda ME, Morales JMV, Jiménez-Capdeville ME, De la Cruz E, Falcón-Escobedo R, Chi-Ahumada E, et al. 2011. Low-level subchronic arsenic exposure from prenatal developmental stages to adult life results in an impaired glucose homeostasis. Exp Clin Endocrinol Diabetes 119(10):613–617, PMID: 22068553, 10.1055/s-0031-1287782. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Valenzuela OL, Castellanos EH, Sánchez-Peña LC, Currier JM, et al. 2011. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health 10:73, PMID: 21864395, 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillet C, Huang MC, Saunders RJ, Dover EN, Zhang C, Stýblo M. 2017. Knockout of arsenic (+3 oxidation state) methyltransferase is associated with adverse metabolic phenotype in mice: the role of sex and arsenic exposure. Arch Toxicol 7:2617–2627, PMID: 27847981, 10.1007/s00204-016-1890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, et al. 2009. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol 22(10):1713–1720, PMID: 19691357, 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediani JK, Naioti EA, Vos MB, Figueroa J, Marsit CJ, Welsh JA. 2018. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: an association modified by race/ethnicity, NHANES 2005–2014. Environ Health 17:6, PMID: 29334960, 10.1186/s12940-017-0350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, et al. 2005. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 113(12):1683–1688, PMID: 16330347, 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. 2006. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am J Clin Nutr 84(5):1093–1101, PMID: 17093162, 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Pérez M, Kuo C-C, Spratlen M, Thayer KA, Mendez MA, Hamman RF, et al. 2017. The association of arsenic exposure and metabolism with type 1 and type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care 40(1):46–53, PMID: 27810988, 10.2337/dc16-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Gamble MV. 2012. Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol 2012:595307, PMID: 22523489, 10.1155/2012/595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Tanaka T, Shimizu M, Tsuru T, Chiyoda T, Miyawaki K, et al. 2016. Sex differences in mRNA expression of reduced folate carrier-1, folypolyformyl glutamate synthase, and γ-glutamyl hydrolase in a healthy Japanese population. J Clin Pharmacol 56(12):1563–1569, PMID: 27146084, 10.1002/jcph.760. [DOI] [PubMed] [Google Scholar]
- Hernández-Zavala A, Matoušek T, Drobná Z, Paul DS, Walton F, Adair BM, et al. 2008. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer). J Anal At Spectrom 23:342–351, PMID: 18677417, 10.1039/B706144G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh Y-M, Chen W-J, Lin Y-C, Huang C-Y, Shiue H-S, Yang S-M, et al. 2018. Adiponectin gene polymorphisms and obesity increase the susceptibility to arsenic-related renal cell carcinoma. Toxicol Appl Pharmacol 350:11–20, PMID: 29723618, 10.1016/j.taap.2018.04.034. [DOI] [PubMed] [Google Scholar]
- Huang C-F, Yang C-Y, Chan D-C, Wang C-C, Huang K-H, Wu C-C, et al. 2015. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 123(11):1138–1144, PMID: 25859628, 10.1289/ehp.1408663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-K, Pu Y-S, Chung C-J, Shiue H-S, Yang M-H, Chen C-J, et al. 2008. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem Toxicol 46(3):929–938, PMID: 18054417, 10.1016/j.fct.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, Islam MT, et al. 2016. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: demographics, lifestyle, genetics, and toxicity. Cancer Epidemiol Biomarkers Prev 25(2):381–390, PMID: 26677206, 10.1158/1055-9965.EPI-15-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Sideleva O, Stradecki HM, Langlois GD, Habibovic A, Satish B, et al. 2011. Sexually dimorphic diet-induced insulin resistance in obese tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice. Endocrinology 152(4):1300–1313, PMID: 21285317, 10.1210/en.2010-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaj S, Peterson H, Liber K, Bhattacharya P. 2006. Human health effects from chronic arsenic poisoning—a review. J Environ Sci Health A Tox Hazard Subst Environ Eng 41(10):2399–2428, PMID: 17018421, 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- Kautzky-Willer A, Harreiter J, Pacini G. 2016. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37(3):278–316, PMID: 27159875, 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M, Morisset R, Rychlik M. 2017. Characterization and interrelations of one-carbon metabolites in tissues, erythrocytes, and plasma in mice with dietary induced folate deficiency. Nutrients 9(5):462, PMID: 28475162, 10.3390/nu9050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C-C, Moon KA, Wang S-L, Silbergeld E, Navas-Acien A. 2017. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125(8):087001, PMID: 28796632, 10.1289/EHP577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, da Silva V, Thomson CA, Hartz V, Hsu C-H, Burgess JL, et al. 2017. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Sci Total Environ 607–608:381–390, PMID: 28697391, 10.1016/j.scitotenv.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Lindberg A-L, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, et al. 2007. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect 115(7):1081–1086, PMID: 17637926, 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP. 2012. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int 39(1):150–171, PMID: 22208756, 10.1016/j.envint.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Martin EM, Fry RC. 2016. A cross-study analysis of prenatal exposures to environmental contaminants and the epigenome: support for stress-responsive transcription factor occupancy as a mediator of gene-specific CpG methylation patterning. Environ Epigenet 2(1):dvv011, PMID: 27066266, 10.1093/eep/dvv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Stýblo M, Fry RC. 2017. Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: a perspective of the current evidence. Epigenomics 9(5):701–710, PMID: 28470093, 10.2217/epi-2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušek T, Hernández-Zavala A, Svoboda M, Langrová L, Adair BM, Drobná Z, et al. 2008. Oxidation state specific generation of arsines from methylated arsenicals based on L-cysteine treatment in buffered media for speciation analysis by hydride generation – automated cryotrapping – gas chromatography-atomic absorption spectrometry with the multiatomizer. Spectrochim Acta Part B At Spectrosc 63(3):396–406, PMID: 18521190, 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. 2012. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120(12):1658–1670, PMID: 22889723, 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. 2015. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6:14, PMID: 26339468, 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, González-Horta C, Sánchez-Ramírez B, Ballinas-Casarrubias L, Cerón RH, Morales DV, et al. 2016. Chronic exposure to arsenic and markers of cardiometabolic risk: a cross-sectional study in Chihuahua, Mexico. Environ Health Perspect 124(1):104–111, PMID: 26068977, 10.1289/ehp.1408742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murko M, Elek B, Styblo M, Thomas DJ, Francesconi KA. 2018. Dose and diet—sources of arsenic intake in mouse in utero exposure scenarios. Chem Res Toxicol 31(2):156–164, PMID: 29244955, 10.1021/acs.chemrestox.7b00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302, PMID: 23458756, 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson TK, Böttiger AK, Henríquez P, Serra Majem L. 2014. MTHFR polymorphisms and serum cobalamin affect plasma homocysteine concentrations differentially in females and males. Mol Med Rep 10(5):2706–2712, PMID: 25176448, 10.3892/mmr.2014.2521. [DOI] [PubMed] [Google Scholar]
- NRC [National Research Council (U.S.) Subcommittee on Laboratory Animal Nutrition]. 1995. Nutrient Requirements of Laboratory Animals: 3. Nutrient Requirements of the Mouse. 4th Edition Washington, DC:National Academies Press. [Google Scholar]
- Oleinik NV, Helke KL, Kistner-Griffin E, Krupenko NI, Krupenko SA. 2014. Rho GTPases RhoA and Rac1 mediate effects of dietary folate on metastatic potential of A549 cancer cells through the control of cofilin phosphorylation. J Biol Chem 289(38):26383–26394, PMID: 25086046, 10.1074/jbc.M114.569657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff K, Mistry K, Metcalf S. 2009. Biomonitoring for environmental exposures to arsenic. J Toxicol Environ Health B Crit Rev 12(7):509–524, PMID: 20183531, 10.1080/10937400903358934. [DOI] [PubMed] [Google Scholar]
- Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dědina J, Matoušek T, et al. 2007. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 222(3):305–314, PMID: 17336358, 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Stýblo M. 2011. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119(8):1104–1109, PMID: 21592922, 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. 2015. Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect 123(12):1294–1301, PMID: 25978852, 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, et al. 2009. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect 117(2):254–260, PMID: 19270796, 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Amaretti A, Raimondi S. 2011. Folate production by probiotic bacteria. Nutrients 3(1):118–134, PMID: 22254078, 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JC, Grindey GB, Schultz RM, Priest DG. 1994. Impact of dietary folic acid on reduced folates in mouse plasma and tissues. Relationship to dideazatetrahydrofolate sensitivity. Biochem Pharmacol 48(2):319–325, PMID: 8053927. [DOI] [PubMed] [Google Scholar]
- Selhub J. 1999. Homocysteine metabolism. Annu Rev Nutr 19:217–246, PMID: 10448523, 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. 2000. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6(1):77–86, PMID: 10949029. [PubMed] [Google Scholar]
- Soares AF, Paz-Montoya J, Lei H, Moniatte M, Gruetter R. 2017. Sexual dimorphism in hepatic lipids is associated with the evolution of metabolic status in mice. NMR Biomed 30(10):e3761, PMID: 28661066, 10.1002/nbm.3761. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, Melnyk S, et al. 2003. Effects of dietary folate intake and folate binding protein-1 (Folbp1) on urinary speciation of sodium arsenate in mice. Toxicol Lett 145(2):167–174, PMID: 14581169, 10.1016/S0378-4274(03)00307-2. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Lu X, Le XC, Troen A, Selhub J, Melnyk S, et al. 2005. Effects of dietary folate intake and folate binding protein-2 (Folbp2) on urinary speciation of sodium arsenate in mice. Environ Toxicol Pharmacol 19(1):1–7, PMID: 21783456, 10.1016/j.etap.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spratlen MJ, Gamble MV, Grau-Perez M, Kuo C-C, Best LG, Yracheta J, et al. 2017. Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: evidence from the Strong Heart Study. Food Chem Toxicol 105:387–397, PMID: 28479390, 10.1016/j.fct.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlen MJ, Grau-Perez M, Best LG, Yracheta J, Lazo M, Vaidya D, et al. 2018. The association of arsenic exposure and arsenic metabolism with the metabolic syndrome and its individual components: prospective evidence from the Strong Heart Family Study. Am J Epidemiol 187(8):1598–1612, PMID: 29554222, 10.1093/aje/kwy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton BA, Caldwell K, Congdon CB, Disney J, Donahue M, Ferguson E, et al. 2015. MDI Biological Laboratory arsenic summit: approaches to limiting human exposure to arsenic. Curr Environ Health Rep 2(3):329–337, PMID: 26231509, 10.1007/s40572-015-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. 2005. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect 113(9):1153–1159, PMID: 16140620, 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge MP, Janssen I, Heymsfield SB. 2004. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 27(9):2222–2228, PMID: 15333488. [DOI] [PubMed] [Google Scholar]
- Sung T-C, Huang J-W, Guo H-R. 2015. Association between arsenic exposure and diabetes: a meta-analysis. Biomed Res Int 2015:368087, PMID: 26000288, 10.1155/2015/368087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, et al. 2007. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 232(1):3–13, PMID: 17202581, 10.1093/toxsci/kfp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. 2001. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol 176(2):127–144, PMID: 11601889, 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Trouba KJ, Wauson EM, Vorce RL. 2000. Sodium arsenite inhibits terminal differentiation of murine C3H 10T1/2 preadipocytes. Toxicol Appl Pharmacol 168(1):25–35, PMID: 11000097, 10.1006/taap.2000.9012. [DOI] [PubMed] [Google Scholar]
- Tsang V, Fry RC, Niculescu MD, Rager JE, Saunders J, Paul DS, et al. 2012. The epigenetic effects of a high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol Appl Pharmacol 264(3):439–450, PMID: 22959928, 10.1016/j.taap.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2001. “Technical Fact Sheet: Final Rule for Arsenic in Drinking Water.” EPA 815-F-00-016. Washington, DC:U.S. EPA. [Google Scholar]
- Vahter M. 1999. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 82 (pt 1):69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. 2000. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 112–113:209–217, PMID: 10720733. [DOI] [PubMed] [Google Scholar]
- Wang W, Xie Z, Lin Y, Zhang D. 2014. Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Community Health 68(2):176–184, PMID: 24133074, 10.1136/jech-2013-203114. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Jiang CS, Liu L, Wang XH, Jin HJ, Wu Q, et al. 2005. The role of Akt on arsenic trioxide suppression of 3T3-L1 preadipocyte differentiation. Cell Res 15(5):379–386, PMID: 15916724, 10.1038/sj.cr.7290305. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. “Global Report on Diabetes.” http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1 [accessed 26 November 2018].
- WHO. 2017. “Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum.” http://apps.who.int/iris/bitstream/10665/254637/1/9789241549950-eng.pdf?ua=1 [accessed 26 November 2018]. [PubMed]
- Winkels RM, Brouwer IA, Verhoef P, van Oort FVA, Durga J, Katan MB. 2008. Gender and body size affect the response of erythrocyte folate to folic acid treatment. J Nutr 138(8):1456–1461, PMID: 18641191, 10.1093/jn/138.8.1456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.