Abstract
Background:
Limited evidence is available regarding the association between heat exposure and morbidity in Brazil and how the effect of heat exposure on health outcomes may change over time.
Objectives:
This study sought to quantify the geographic, demographic and temporal variations in the heat–hospitalization association in Brazil from 2000–2015.
Methods:
Data on hospitalization and meteorological conditions were collected from 1,814 cities during the 2000–2015 hot seasons. Quasi-Poisson regression with constrained lag model was applied to examine city-specific estimates, which were then pooled at the regional and national levels using random-effect meta-analyses. Stratified analyses were performed by sex, 10 age groups, and 11 cause categories. Meta-regression was used to examine the temporal change in estimates of heat effect from 2000 to 2015.
Results:
For every 5°C increase in daily mean temperature during the 2000–2015 hot seasons, the estimated risk of hospitalization over lag 0–7 d rose by 4.0% [95% confidence interval (CI): 3.7%, 4.3%] nationwide. Estimated 6.2% [95% empirical CI (eCI): 3.3%, 9.1%] of hospitalizations were attributable to heat exposure, equating to 132 cases (95% eCI: 69%, 192%) per 100,000 residents. The attributable rate was greatest in children and was highest for hospitalizations due to infectious and parasitic diseases. Women of reproductive age and those had higher heat burden than men. The attributable burden was greatest for cities in the central west and the inland of the northeast; lowest in the north and eastern coast. Over the 16-y period, the estimated heat effects declined insignificantly at the national level.
Conclusions:
In Brazil’s hot seasons, 6% of hospitalizations were estimated to be attributed to heat exposure. As there was no evidence indicating that thermal adaptation had occurred at the national level, the burden of hospitalization associated with heat exposure in Brazil is likely to increase in the context of global warming. https://doi.org/10.1289/EHP3889
Introduction
Brazil is the fifth most populous country and has the ninth largest economy in the world (The World Bank 2017a, 2017b). Paralleling Brazil’s significant economic development over recent decades has been the substantial gains in health outcomes: Over the past thirty years, Brazil’s age-standardized mortality rate and years of life lost have fallen by 34% and 41%, respectively (IHME 2016). However, the increase in life expectancy and unchanged morbidity rate in Brazil’s population has been accompanied by an enormous burden on health care, particularly among the older population (IHME 2016).
Traditional and lifestyle risk factors (such as cigarette smoking, diabetes, and high blood pressure levels) explain a substantial proportion of Brazil’s growing burden of disease (Prince et al. 2015). However, increasing evidence highlights the role that climatic factors, such as high temperature, can play in the determination of health outcomes. For example, in the United States, extreme heat has been associated with a 23% increase in the risk of hospitalization for asthma in the summer months (Soneja et al. 2016). Similarly, in China, the risk of all-cause emergency room visits has been reported to increase by 15% following exposure to extreme heat (Zhao et al. 2017).
In Brazil, the mean temperature has been increasing 25% faster than the global average since 1910, implying that the population may be particularly exposed to the effects of increasing temperature relative to other populations (NOAA 2017). To date, most of the evidence regarding the health effects associated with heat exposure has been derived from populations in East Asia, Europe, and North America, with comparatively little information from Brazil (Bai et al. 2014; Gronlund et al. 2014; Michelozzi et al. 2009). However, Brazil’s unique geographic and climatic features and substantial regional economic disparity may mean that any previously observed heat–morbidity associations may differ significantly within and across the Brazilian population. In addition, most previous studies tended to report on only a single or few diseases within the same population (Basu et al. 2012; Fletcher et al. 2012; Ha and Kim 2013). This reporting precludes a more sensitive assessment of the relationship between heat exposure and disease incidence, which may vary according to disease outcomes (Bai et al. 2016; Michelozzi et al. 2009).
In this study, we used a national dataset to quantify the geographic, demographic, and temporal variations in the association between heat exposure and risk of hospitalization from all causes and cause-specific hospitalization in the Brazilian population during the hot seasons from 2000 to 2015.
Methods
Data Collection
City-specific data on hospitalization were collected between 1 January 2000 and 31 December 2015 through the Brazilian Unified Health System. Only cities that had complete data for the entire 16 y of study duration were included in this analysis. Overall, 1,814 cities ( of national population) contributed to the analysis. The cities were located in five regions: the north, northeast, central west, southeast, and south (Figure S1). Information included date of admission, sex, age, and primary diagnosis, which was then divided into 11 main cause categories according to the 10th Revision of International Classification of Diseases (ICD-10) (see Table S1), and 10 age groups (0–4, 5–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ).
Daily maximum and minimum temperatures during the study period were downloaded from a Brazilian meteorological dataset (Xavier et al. 2016). City-specific data were collected from the grid overlaying the centroid of each city (Zhao et al. 2018). Daily mean temperature, the mean of maximum and minimum temperatures, was applied as the thermal index in this study to analyze the heat–hospitalization association. We also collected observed data on daily mean temperature and relative humidity from 193 city-specific meteorological stations between 2000 and 2012 from the Brazilian National Institute of Meteorology. Data on city-specific population were downloaded from the Brazilian Census 2000 and 2010 (http://www.ibge.gov.br/censo/, http://www.censo2010.ibge.gov.br/). In 2010, the size of population for the cities included in this study ranged from 7,903 to 11,253,503 people.
In this study, to estimate the effect of heat exposure on risk of hospitalization, analyses were restricted to the hot season, which was defined as the hottest four consecutive months for each city during 2000–2015. By using this definition for the hot season, we were able to take into consideration the wide climatic variation across Brazil’s large geographic area (Alvares et al. 2013; Guo et al. 2017).
Statistical Analysis
Heat-hospitalization association.
A two-stage approach was performed to assess the associations between heat exposure and risk of hospitalization for all-causes and cause-specific. First, a quasi-Poisson regression with constrained lag model was applied for the time-series data for each city:
| (1) |
where is the daily counts of hospitalization in city i on day t during hot seasons. is the intercept; is the cross-basis function to fit the lagged effect of daily mean temperature. is the stratum combining the year and calendar month to control for the intraseasonal pattern and long-term trend. As a time-stratified case–crossover design, it provides a flexible alternative to control for temporal trends in the interrupted time-series analysis (Armstrong et al. 2014; Guo et al. 2011). is a categorical variable to control for day of the week. is a binary variable to control for public holidays. , , and are the coefficients. Our analysis indicated that the relationship between heat exposure and hospitalization was linear and lagged for 0–7 d. Therefore, a linear function was used for the temperature dimension, with 0–7 lag d and three degrees of freedom (df) for lag dimension in the cross-basis function.
In the second stage, the city-specific estimates were pooled at the regional and national levels using a random-effect meta-analysis with maximum likelihood estimation. This method has been used to estimate the average exposure–response association by assuming a normal distribution of city-specific parameters (Gasparrini et al. 2012a; Gasparrini and Armstrong 2013). Stratified analyses were performed by sex, age, and cause category. The heat–hospitalization association was described as the percentage change in the estimated risk of hospitalization [with 95% confidence intervals (CIs)] over lag 0–7 d per 5°C increase in daily mean temperature during hot seasons. The differences in estimated associations across regions, population subgroups, and cause categories were tested using the random-effect meta-regression, respectively (Guo 2017). For example, when testing for the sex difference, the city-specific estimates for men and women were entered as the dependent variable, and the binary predictor (men and women) was entered as the independent variable.
Attributable hospitalizations.
For each city, the number of hospitalizations attributable to heat exposure in city i on day t () was estimated using the method described by Gasparrini and Leone (2014), with empirical CIs (eCIs) estimated using Monte Carlo simulation (5,000 random samples):
| (2) |
where is the cumulative risk of hospitalization in the following 0–7 d associated with the daily mean temperature in city i on day t, in comparison with the minimum temperature value during the hot seasons. is the moving average of daily hospitalization counts in city i in the following 0–7 d since day t, considering that the heat–hospitalization lasted for up to seven days. The total number of attributable hospitalizations in city i () was calculated by summing during 2000–2015. The attributable fraction () in city i was calculated by dividing by the total number of hospitalizations during the study period.
Some of the smaller cities had a relatively low number of daily hospitalizations, which might result in spurious exposure–response associations. To mitigate this issue, city-specific associations were predicted by the best linear unbiased prediction (BLUP). This method has been widely used in multicountry studies (Gasparrini et al. 2015b; Guo et al. 2018). In summary, the heat–hospitalization association in each city was estimated using Equation 1. Then, city-specific cumulative effect estimates were applied as the dependent variable in a meta-regression, whereas city-specific average temperatures of hot seasons and temperature ranges of hot seasons and a categorical variable representing regions were included as predictors. The BLUP of the cumulative association in each city was derived by the meta-regression. When calculating attributable hospitalizations in the elderly, we combined the three older age-groups (60–69, 70–79, and ) into due to the grouping limitation of the Brazilian Census 2000 and 2010 datasets.
Long-term change in the heat–hospitalization association.
Temporal change in the heat–hospitalization association during the 16-y period was assessed using a two-stage strategy. In the first stage, the city-year–specific effects were estimated using Equation 1. In the second stage, a random-effect meta-regression model was performed to examine the annual variation in the heat effect. The years were entered as the independent variable (linear), and the city-year–specific coefficients were entered as the dependent variable (weighted by variances) (Bauters et al. 2016; Zhao et al. 2018).
Sensitivity analyses.
Sensitivity analyses were performed to test the robustness of the results by changing the lag of temperature from 0–7 to 0–9 d and the df of lag days from three to five. We compared the performance of gridded and station-based temperature data and assessed the confounding effect of relative humidity using data from 193 city-specific meteorological stations between 2000 and 2012. We also tested the linearity of the heat–hospitalization association using a natural cubic spline with two df for the temperature dimension.
All data analyses were conducted using R software (version 3.4.1; R Core Team), with “dlnm” and “mvmeta” packages used for the first-stage and second-stage analyses, respectively (Gasparrini et al. 2010; Gasparrini 2011). The residual heterogeneity in the meta-analysis was quantified using the Cochran Q test and the statistic. P value (two-sided) was considered as statistically significant.
Results
The average daily mean temperature of the 1,814 cities was 25.3°C (range: 18.1°C–30.3°C) in the hot seasons between 2000 and 2015 (Table 1). Cities in northern Brazil had higher daily mean temperatures than those in the south. During the study period, there were 49,145,997 hospitalizations (sex ratio: 1.4 women:1 men), equating to 2.1% of all residents being hospitalized during every hot season. Children aged 0–4 y and adults had the highest hospitalization rates (3.1% and 3.9%, respectively), whereas children aged 5–9 y had the lowest rate (0.9%). The mean number of daily hospitalizations in the smallest and largest cities were 4 (median: 4; interquartile range: 2–6) and 1,597 (median: 1,530; interquartile range: 1,227–1,787) cases, respectively.
Table 1.
Distribution of enrolled hospitalizations and temperature features in the 1,814 Brazilian cities during the 2000–2015 hot seasons.
| No. of enrolled city | Population coverage (%) | No. of cases | Crude (age-standardized) hospitalization rate (%) | Average city-specific daily mean temperature (°C) | |||
|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | |||||
| National | 1,814 | 78.4 | 49,145,997 | 2.1 (2.1) | 25.3 | 18.1 | 30.3 |
| Region | |||||||
| North | 28 | 26.3 | 1,271,435 | 2.0 (2.1) | 27.7 | 20.8 | 32.1 |
| Northeast | 662 | 78.0 | 13,823,251 | 2.1 (2.2) | 27.1 | 22.0 | 30.9 |
| Central West | 128 | 80.7 | 3,847,427 | 2.2 (2.3) | 26.3 | 17.4 | 31.8 |
| Southeast | 622 | 87.0 | 22,077,029 | 2.0 (2.0) | 24.2 | 16.4 | 29.5 |
| South | 374 | 83.2 | 8,126,855 | 2.2 (2.3) | 23.4 | 14.0 | 30.0 |
| Sex | |||||||
| Men | — | — | 20,232,358 | 1.8 (1.8) | — | — | — |
| Women | — | — | 28,912,721 | 2.5 (2.4) | — | — | — |
| Age (years) | |||||||
| 0–4 | — | — | 5,456,097 | 3.1 (−) | — | — | — |
| 5–9 | — | — | 1,693,045 | 0.9 (−) | — | — | — |
| 10–19 | — | — | 5,141,581 | 1.2 (−) | — | — | — |
| 20–29 | — | — | 9,621,821 | 2.3 (−) | — | — | — |
| 30–39 | — | — | 6,835,872 | 1.9 (−) | — | — | — |
| 40–49 | — | — | 5,402,941 | 1.8 (−) | — | — | — |
| 50–59 | — | — | 4,907,819 | 2.3 (−) | — | — | — |
| 60–69 | — | — | 3,969,895 | 3.9 (−) | — | — | — |
| 70–79 | — | — | 3,253,568 | 3.9 (−) | — | — | — |
| — | — | 2,144,441 | 3.9 (−) | — | — | — | |
Note: —, data not available. The hot season was defined as the hottest four consecutive months for each Brazilian city during 2000–2015. Data on hospitalization were extracted via the Brazilian Unified Health System. Data on population were collected from Brazilian Census 2000 and 2010. The temperature features in the 1,814 cities were calculated by averaging the mean, minimum and maximum daily mean temperatures in each city during the 2000–2015 hot seasons.
Heat–Hospitalization Association
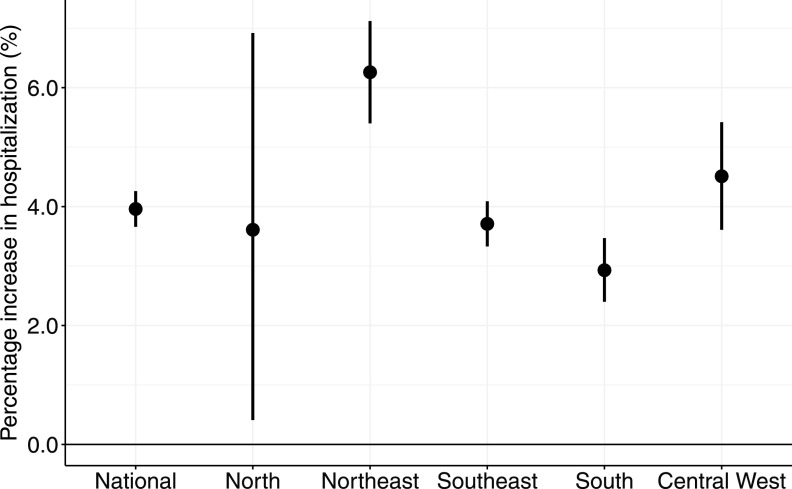
At the national level, the estimated risk of hospitalization increased by 4.0% (95% CI: 3.7%, 4.3%) per 5°C increase in daily mean temperature over lag 0–7 d, ranging from the lowest in the south [2.9% (95% CI: 2.4–3.5%)] to the highest in the northeast [6.3% (95% CI: 5.4–7.1%)] (Figure 1; for results of the significance tests see Table S2). The value of the statistic was 29% at the national level and varied by region: 14% in the southeast, 20% in the central west, 25% in the northeast, 41% in the south, and 43% in the north. Despite the geographic difference in the cumulative effect estimates, the heat–hospitalization associations showed similar lag patterns across the five Brazilian regions (Figure S2): The estimates were highest during the first day of exposure and declined thereafter.
Figure 1.
Cumulative association between heat exposure (5°C increase in daily mean temperature) and hospitalization over lag 0–7 d by region during 2000–2015 hot seasons. Data on hospitalization were extracted from 1,814 cities via the Brazilian Unified Health System. City-specific heat–hospitalization associations were estimated using quasi-Poisson regression with constrained lag model, which were then pooled at the regional and national levels using random-effect meta-analyses. Long-term trend and intra-seasonal variation, day of the week and holidays were controlled for.
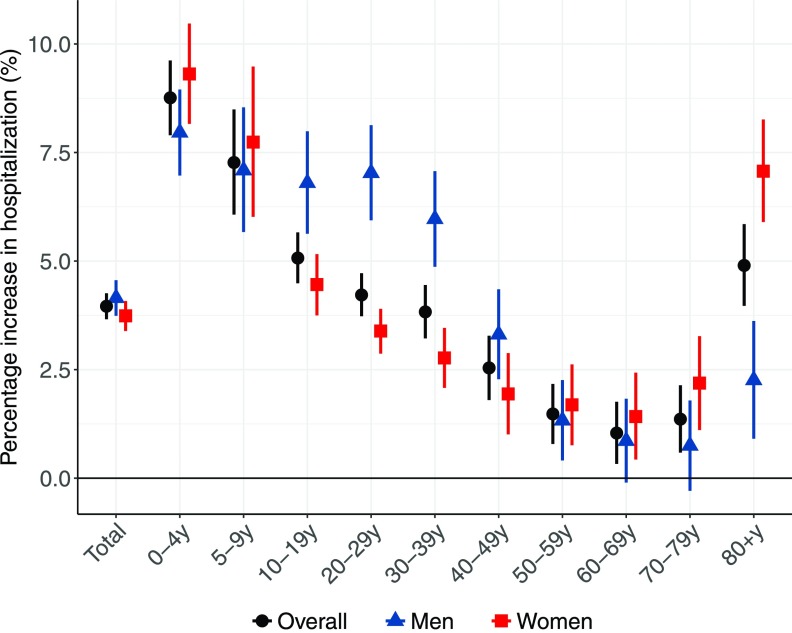
Associations between heat exposure and hospitalization were not significantly different for men [4.2% (95% CI: 3.7–4.6%)] and women [3.7% (95% CI: 3.4–4.1%)] over lag 0–7 d (Figure 2, Table S2). However, an age-sex difference was apparent: on the whole, the estimated cumulative effect was highest in children 0–4 y [8.8% (95% CI: 7.9–9.6%)], followed by 5–19 y, and in the elderly . By contrast, the effect estimate was lowest in adults 50–79 y (1.3% on average). The estimated effect of heat was stronger for older women (particularly those ) than for men (for results of significance tests, see Table S3). By comparison, the estimated risk of hospitalization in men 10–39 y was more substantial than in women. Estimates for men, women, and all age groups indicated positive associations between heat and hospitalization during lag 0–2 d, followed by a decrease in hospitalization () thereafter (Figure S3).
Figure 2.
Cumulative association between heat exposure (5°C increase in daily mean temperature) and hospitalization over lag 0–7 d by sex and age-group during 2000–2015 hot seasons. Data on hospitalization were extracted from 1,814 cities via the Brazilian Unified Health System. City-specific heat–hospitalization associations were estimated using quasi-Poisson regression with constrained lag model, which were then pooled at the national level using random-effect meta-analyses. Long-term trend and intra-seasonal variation, day of the week and holidays were controlled for.
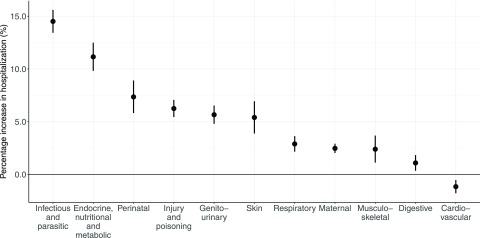
Cause-specific analysis indicated that hospitalizations for ten cause categories were positively associated with heat exposure over lag 0–7 d, with the exception of cardiovascular admissions (Figure 3). Specifically, the estimated effects were highest in hospitalizations for infectious and parasitic diseases, and for endocrine, nutritional, and metabolic diseases (Figure 3 and Table S2). The effect estimates in most cause categories appeared positively on lag 0–2 d and were followed by a decrease in hospitalization () thereafter (Figure S4). For cardiovascular admissions, there was an immediate increase in the estimated heat effect after exposure and a substantial hospitalization decline in the following days.
Figure 3.
Cumulative association between heat exposure (5°C increase in daily mean temperature) and hospitalization over lag 0–7 d by cause category during 2000–2015 hot seasons. Data on hospitalization were extracted from 1,814 cities via the Brazilian Unified Health System. City-specific heat–hospitalization associations were estimated using quasi-Poisson regression with constrained lag model, which were then pooled at the national level using random-effect meta-analyses. Long-term trend and intra-seasonal variation, day of the week and holidays were controlled for.
Attributable Hospitalizations
During the 16-y hot seasons, assuming a causal relationship, an estimated 3,070,360 (95% eCI: 1,600,393, 4,468,577) cases could be attributable to heat exposure over lag 0–7 d (Table 2). This accounted for 6.2% (95% eCI: 3.3–9.1%) of the total hospitalizations, equating to 132 cases (95% eCI: 69–192%) per 100,000 residents nationwide. The estimated age-standardized rate (ASR) of attributable hospitalizations was the highest in the central west and followed by the northeast, with no substantial difference in the other three regions. The majority of cities with attributable rates were located in the central west and inland of the northeast, whereas cities along the eastern coast and in the north had the lowest attributable rates (Figure S5).
Table 2.
Hospitalizations (with 95% empirical confidence intervals) attributable to heat exposure over lag 0–7 days in the 1,814 Brazilian cities during 2000–2015 hot seasons by region, sex and age.
| Attributable cases | Attributable fraction (%) | Annual attributable rate (per 100,000 population) | ||
|---|---|---|---|---|
| Crude | Age-standardized | |||
| National | 3,070,360 (1,600,393, 4,468,577) | 6.2 (3.3, 9.1) | 132 (69, 192) | 132 (54, 205) |
| Region | ||||
| North | 64,315 (6,738, 118,833) | 5.1 (0.5, 9.3) | 102 (11, 188) | 124 (, 307) |
| Northeast | 867,155 (269,494, 1,434,051) | 6.3 (1.9, 10.4) | 135 (42, 223) | 146 (51, 235) |
| Central West | 316,024 (138,043, 481,681) | 8.2 (3.6, 12.5) | 182 (80, 278) | 180 (43, 305) |
| Southeast | 1,313,757 (841,652, 1,765,259) | 6.0 (3.8, 8.0) | 120 (77, 162) | 117 (62, 169) |
| South | 496,648 (384,860, 605,893) | 6.1 (4.7, 7.5) | 140 (108, 170) | 123 (55, 189) |
| Sex | ||||
| Men | 1,381,501 (725,616, 2,004,986) | 6.8 (3.6, 9.9) | 120 (63, 175) | 121 (35, 200) |
| Women | 1,682,369 (857,792, 2,468,353) | 5.8 (3.0, 8.5) | 143 (73, 209) | 141 (52, 225) |
| Age (years) | ||||
| 0–4 | 686,394 (368,162, 972,421) | 12.6 (6.7, 17.8) | 393 (211, 556) | — |
| 5–9 | 180,592 (76,158, 274,766) | 10.7 (4.5, 16.2) | 98 (41, 149) | — |
| 10–19 | 385,259 (281,882, 485,903) | 7.5 (5.5, 9.5) | 92 (67, 116) | — |
| 20–29 | 600,819 (199,159, 982,670) | 6.2 (2.1, 10.2) | 143 (47, 233) | — |
| 30–39 | 436,899 (187,161, 675,904) | 6.4 (2.7, 9.9) | 119 (51, 185) | — |
| 40–49 | 265,505 (84,602, 437,748) | 4.9 (1.6, 8.1) | 88 (28, 145) | — |
| 50–59 | 154,272 (19,231, 284,036) | 3.1 (0.4, 5.8) | 71 (9, 130) | — |
| 60–69 | 47,951 (, 142,711) | 1.2 (, 3.6) | 90 (, 201) | — |
| 70–79 | 45,603 (, 130,920) | 1.4 (, 4.0) | 90 (, 201) | — |
| 122,564 (28,364, 210,802) | 5.7 (1.3, 9.8) | 90 (, 201) | — | |
Note: Heat exposure for calculating attributable burden was defined as the increase in daily mean temperature during 2000–2015 hot seasons (the hottest four consecutive months for each city), compared with the minimum daily mean temperature. The best linear unbiased prediction of the cumulative association in each city was used to calculate the attributable burden.
Although there was no substantial sex difference on the whole, the age difference was observed (Table 2 and Table S4); for both sex groups, the estimated attributable rate was much higher in children 0–4 y in comparison with adults. The attributable rates in women 20–29 y and were much higher than those in men of the same age groups. Of all cause categories, the attributable crude rate and ASR were highest in hospitalizations for infectious and parasitic diseases, both approximating to 40 cases per 100,000 residents nationwide during the hot season per year (Figure S6).
Long-Term Change in Heat–Hospitalization Association
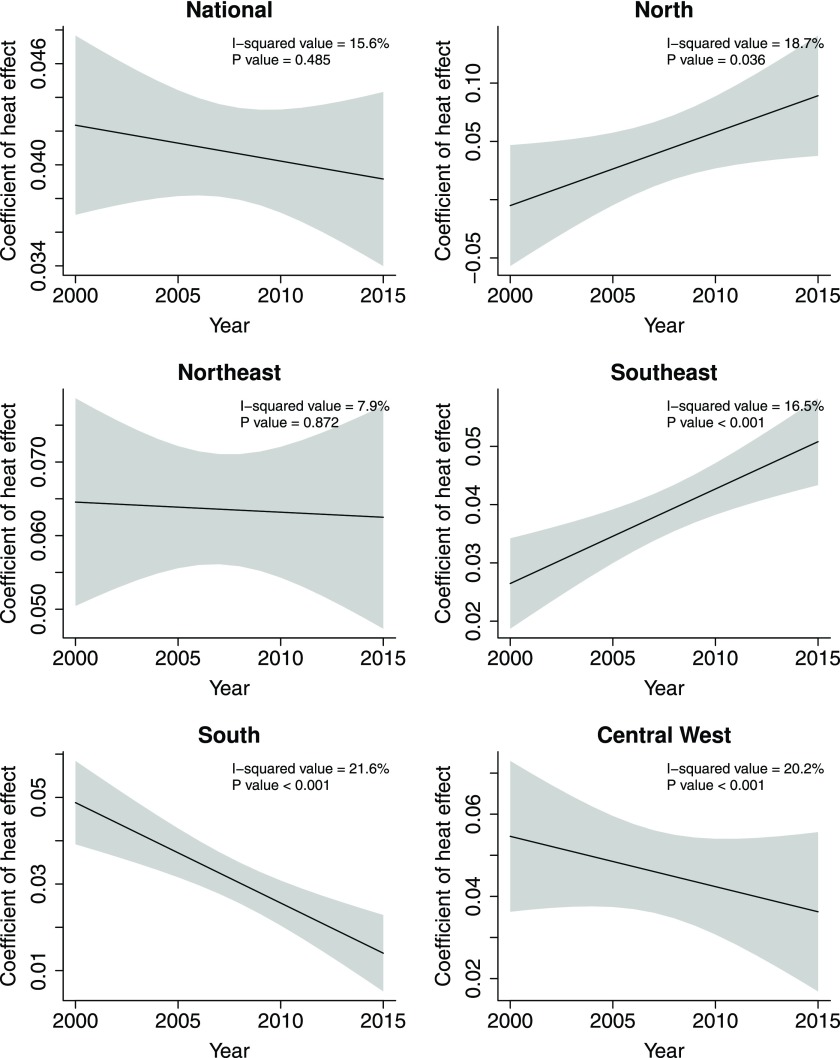
Figure 4 shows the change in coefficient of the estimated association between heat exposure and hospitalization over lag 0–7 d between 2000 and 2015. There was a nonsignificant declining trend in the heat effect on the risk of hospitalization at the national level. However, at the regional level, significant increases were observed in the southeast and the north, whereas significant declines were observed in the central west and the south.
Figure 4.
Annual change in coefficient of the cumulative association between heat exposure (5°C increase in daily mean temperature) and hospitalization over lag 0–7 d by region during 2000–2015 hot seasons. Data on hospitalization were extracted from 1,814 cities via the Brazilian Unified Health System. Random-effect meta-regression models were conducted, with “years” as the independent continuous variable (linear) and city-year-specific estimates as the dependent variable.
Results of Sensitivity Analyses
Sensitivity analysis showed that our results were robust after changing the lag of temperature from 0–7 d to 0–9 d and the df of lag days from three to five, and adding data on relative humidity (Table S5). The difference between gridded and station-based temperature data was also minimal. The result of the linear test indicated that the heat–hospitalization association was linear (Figure S7).
Discussion
This paper reports the first nationwide study to comprehensively examine the geographic, demographic, and temporal variations in the association between heat exposure and risk of hospitalization in the Brazilian population. We observed adverse effects of heat on a range of health outcomes in the Brazilian population that were greatest in children and the elderly. Assuming a causal exposure pathway, approximately 6% of Brazilian hospitalizations (equivalent to 132 cases per 100,000 residents) could be attributable to heat exposure during every hot season, with the attributable ASR greatest for infectious and parasitic admissions. Temporal change was negligible at the national level but varied by region over the 16-y study period.
Despite using different methodologies and exposure definitions, the relationship between heat exposure and hospitalization that we observed in Brazil is similar to findings from populations in other countries. A Vietnamese study reported that for every 1°C rise in daily mean temperature, the risk of hospitalization increased by 1.3% over lag 0–6 d in 13 tropical provinces during 2002–2014 (Phung et al. 2016). Another study conducted in 114 American cities reported an association between exposure to extreme heat and a 3% increase in the risk of all-cause hospitalization over lag 0–7 d during the 1992–2006 hot seasons (Gronlund et al. 2014). In Australia and China, 6% and 4% of current day’s emergency admissions were attributable to daily heat exposure during summer, respectively (Cheng et al. 2018).
Substantial evidence exists to support a causal relationship between heat exposure and impaired functioning of many physiological systems. Both human and animal studies have demonstrated that exposure to high temperatures may disturb the secretion, transportation, and targeting of hormones, that could lead to endocrine, nutritional, and metabolic disorders (Faure et al. 2016; Kang et al. 2017). Some researchers have found that dehydration and hyperthermia compromise kidney function (Hansen et al. 2008; Williams et al. 2012). Heat-induced release of Interleukin-1 or Interleukin-6 has been shown to trigger an inflammatory response, resulting in respiratory distress syndrome (Kaldur et al. 2016; Michelozzi et al. 2009). Exposure to high temperatures has also been associated with a range of maternal and perinatal conditions, such as preterm birth and psychological stress during pregnancy (Carolan-Olah and Frankowska 2014; Lin et al. 2017). The associations observed in the current study between heat exposure and hospitalization for skin and muscle problems or injury are also in agreement with previous reports on heat rash and edema, or increased violent crime during hot days (Caspani et al. 2004; Grubenhoff et al. 2007; Raleigh et al. 2014).
Numerous studies have examined the impact of heat exposure on cardiovascular disease (Goldie et al. 2018; Tsangari et al. 2016). Potential physiopathologic mechanisms are that exposure to extreme heat is potentially associated with adverse changes in blood viscosity, plasma cholesterol, heart rate, and blood pressure, and increased oxidative damage to the arteries (Halonen et al. 2011; Kaldur et al. 2016; Radin et al. 2018). In our study, cardiovascular hospitalization was positively associated with heat on the first exposure day, but the cumulative association was negative due to the large hospitalization displacement on subsequent days. Moreover, this negative cumulative association may reflect high rates of mortality subsequent to prolonged heat exposure, particularly among the most vulnerable population subgroups, such as the elderly and individuals with established cardiovascular disease. In support of this finding, a study covering 12 European cities reported a negative association for cardiovascular admissions but a positive association for cardiovascular deaths in the same population (Michelozzi et al. 2009).
Previously, the health impact associated with heat exposure has been attributed to an insufficient response of the body’s thermoregulatory capacity (Gasparrini et al. 2012b; Guo et al. 2014). However, the high effect estimates for infectious, parasitic, and digestive diseases in this study imply that some ecological and behavioural factors may also contribute to the heat–health pathways. For example, evidence indicates that a variety of pathogens and vectors have advanced proliferation or survival in warmer environments, contributing to the epidemics of water- and food-borne diseases during the hot season (Bennet et al. 2006; Bentham and Langford 2001). Greater consumption of cold, contaminated water and uncooked food during hot days may stimulate gastrointestinal perturbation or facilitate the transmission of bacteria (Farthing et al. 2013; Thompson et al. 1983).
Findings from previous studies and our observations have shown that some health outcomes are more associated with high temperature than are other diseases. However, the attributable heat burden at the population level may exhibit a heterogeneous pattern. For example, although with similar estimated risks of hospitalization, the attributable ASR was higher for infectious and parasitic diseases than for endocrine, nutritional, and metabolic diseases in Brazil. This evidence should not be overlooked when developing protective strategies against high temperatures.
Demographic factors are important effect modifiers for the association between heat exposure and human health, such that women, children, and the elderly may be at greater risk during hot days (Basu 2009; Phung et al. 2016). In line with these studies, we found that for the entire Brazilian population, the youth and the elderly had higher heat susceptibility than adults below age 80 did, possibly due to immature or impaired physiological systems against heat exposure (Committee on Sports Medicine Fitness 2000; Landrigan and Garg 2005). Our analyses indicated that the heat–hospitalization association and the associated attributable fraction were lowest for individuals at or around retirement age (50–79 y). This finding matches some mental–behavioural changes in this population that may benefit their health, such as less occupational stress and more time for healthier lifestyles (e.g., balanced diet) (Laranjeira et al. 2010; Monteiro et al. 2007).
Overall, no substantial sex difference was found in the heat effect and the attributable fraction of hospitalization. However, a marked sex–age difference was observed. Similar to findings in Australia and the United Kingdom (Hajat et al. 2007; Tong et al. 2014), older women in Brazil were more vulnerable to heat exposure than were men, possibly due to the effects of menopause on their thermoregulatory capacity. In comparison, the higher estimated risk in adolescent boys and middle-age men than in women may be explained by the males’ higher levels of outdoor activities. The exception was among women of reproductive age, for whom, although they had low estimated risk of heat-related hospitalization, the attributable rate of hospitalization was still high due to the vast number of hospitalizations in this population subgroup.
Geographic variation in the relationship between temperature and health has been previously reported in other countries (Baccini et al. 2008; Phung et al. 2016). In Brazil, we found that the heat–hospitalization association varied by region, with individuals in the northeast most susceptible, whereas those in the south were least susceptible to heat. This finding likely reflects the fact that southern Brazil has a higher proportion of adults aged 50–79 y than does northern Brazil who are less affected by high temperatures relative to other population subgroups (IBGE 2013). However, imbalance in the population distribution within Brazil distorted the comparison of regional difference in attributable heat burden on the population level. This imbalance is also true for other countries with high levels of population migration. To address this issue, we calculated the ASRs of hospitalization attributable to heat. Our results indicated that the estimated heat-related burden indeed varied across Brazil after population adjustment; although it was broadly comparable in the north, southeast, and south, the ASR was highest in the central west, followed by the northeast. This variation suggests that there are region-specific characteristics that influence the rate of hospitalization due to heat exposure. For example, because the main occupation in the central west is agriculture, the average time spent outdoors per capita (and hence, exposure to heat) is likely to be high in this region (The Brazil Business 2013). In contrast, the northeast region has a more constant temperature relative to other regions, which mitigates the local attributable hospitalization burden over the hot season (Alvares et al. 2013).
Evidence from studies conducted in high-income countries, including the United States, Japan, and Spain, suggests that there has been a substantial decline in the effect of heat exposure on major health outcomes (such as mortality) during the 20th century (Barreca et al. 2016; Gasparrini et al. 2015a). Researchers speculated that this decline is a result of various factors, such as physiological adaptation to heat and improvements in infrastructure (e.g., building insulation, air-conditioning) associated with increased economic development (Anderson and Bell 2011; Gasparrini et al. 2015a; Vicedo-Cabrera et al. 2018). Despite Brazil’s impressive economic growth and significant improvements in health outcomes over recent decades (IBGE 2013; The World Bank 2017c), the association between heat exposure and hospitalization has remained temporally unchanged at the national level during the 2000–2015 hot seasons. This finding suggests that minimal adaptation to heat has occurred within the Brazilian population at the national level. However, the regional variations (i.e., temporal increases in the north and southeast, declines in the central west and south, and nonsignificant change in the northeast) indicate a complicated geographic pattern in thermal adaptation. Additional studies are needed to explore whether this regional variation is modified by a range of local characteristics relevant to behavior, culture, and geography.
This study has several strengths. First, to the best of our knowledge, ours is the largest research study to characterize the relationship between heat exposure and cause-specific hospitalization and to examine the geographic, demographic, and temporal variations in the association. Second, this study included information on more than three-quarters of the Brazilian population, and thus our findings, particularly the city-specific estimates, are likely to be representative of the general population. Third, considering the geographic location and climatic diversity of Brazil, our results are also likely to be relevant to populations from other countries in South America. However, several limitations warrant brief discussion. First, the use of gridded temperature data rather than personal measurement may have introduced measurement error, resulting in an underestimation of the size of the observed associations. Second, we were unable to assess the modification effect of air pollution because of the lack of data from most Brazilian cities. However, previous findings have indicated that the heat–health associations from the effects of air pollution on health outcomes are robust (Guo et al. 2016; Zhao et al. 2017). Third, we were unable to provide the sex-specific ASRs for the 11 cause-specific categories because of the small sample sizes for some of the categories. Finally, we were unable to make any inference as to the possible mechanistic pathways governing the relationship between heat exposure and cause-specific outcomes because of the lack of data on clinical information and other sociodemographic and health-related factors.
Conclusions
In the Brazilian population, exposure to high temperature was associated with a broad range of cause-specific hospitalizations between 2000 and 2015, in particular those hospitalizations due to infectious and parasitic diseases. The burden of hospitalization associated with heat exposure was not uniformly distributed across the population, but varied by age, sex, and region, as well as by underlying disease or health condition. The lack of evidence for demonstrable thermal adaptation at the national level over the study period implies that any future increases in mean temperature, as predicted by various climate change scenarios, will result in an increase in the attributable hospitalization burden in the Brazilian population. This increase is particularly true for residents dwelling in the north and southeast.
Supplementary Material
Acknowledgments
The authors thank the Brazilian Ministry of Health and Brazilian National Institute of Meteorology for providing hospitalization and meteorological data.
Q.Z. was supported by a Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship. S.L. was supported by an Early Career Fellowship of the Australian National Health and Medical Research Council (APP1109193). Y.G. was supported by a Career Development Fellowship of the Australian National Health and Medical Research Council (APP1107107). M.A. holds investigator-initiated grants from Pfizer and Boehringer-Ingelheim for unrelated research.
References
- Alvares CA, Stape JL, Sentelhas PC, de Moraes G, Leonardo J, Sparovek G. 2013. Köppen's climate classification map for Brazil. Meteorologische Zeitschrift 22(6):711–728, 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- Anderson GB, Bell ML. 2011. Heat waves in the United States: mortality risk during heat waves and effect modification by heat wave characteristics in 43 U.S. communities. Environ Health Perspect 119(2):210–218, 10.1289/ehp.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BG, Gasparrini A, Tobias A. 2014. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC Med Res Methodol 14:122, PMID: 25417555, 10.1186/1471-2288-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M, Biggeri A, Accetta G, Kosatsky T, Katsouyanni K, Analitis A, et al. . 2008. Heat effects on mortality in 15 European cities. Epidemiology 19(5):711–719, PMID: 18520615, 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Bai L, Ding G, Gu S, Bi P, Su B, Qin D, et al. . 2014. The effects of summer temperature and heat waves on heat-related illness in a coastal city of China, 2011–2013. Environ Res 132:212–219, PMID: 24815333, 10.1016/j.envres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Bai L, Li Q, Wang J, Lavigne E, Gasparrini A, Copes R, et al. . 2016. Hospitalizations from hypertensive diseases, diabetes, and arrhythmia in relation to low and high temperatures: population-based study. Sci Rep 6:30283, PMID: 27456033, 10.1038/srep30283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca A, Clay K, Deschenes O, Greenstone M, Shapiro JS. 2016. Adapting to climate change: the remarkable decline in the US temperature-mortality relationship over the twentieth century. J Political Econ 124(1):105–159, 10.1086/684582. [DOI] [Google Scholar]
- Basu R. 2009. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health 8:40, PMID: 19758453, 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Pearson D, Malig B, Broadwin R, Green R. 2012. The effect of high ambient temperature on emergency room visits. Epidemiology 23(6):813–820, PMID: 23007039, 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Bauters C, Lemesle G, de Groote P, Lamblin N. 2016. A systematic review and meta-regression of temporal trends in the excess mortality associated with diabetes mellitus after myocardial infarction. Int J Cardiol 217:109–121, PMID: 27179900, 10.1016/j.ijcard.2016.04.182. [DOI] [PubMed] [Google Scholar]
- Bennet L, Halling A, Berglund J. 2006. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur J Clin Microbiol Infect Dis 25(7):426–432, PMID: 16810531, 10.1007/s10096-006-0167-2. [DOI] [PubMed] [Google Scholar]
- Bentham G, Langford IH. 2001. Environmental temperatures and the incidence of food poisoning in England and Wales. Int J Biometeorol 45(1):22–26, PMID: 11411411, 10.1007/s004840000083. [DOI] [PubMed] [Google Scholar]
- Carolan-Olah M, Frankowska D. 2014. High environmental temperature and preterm birth: a review of the evidence. Midwifery 30(1):50–59, PMID: 23473912, 10.1016/j.midw.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Caspani M, Savioli M, Crotti S, Bruzzone P, Gattinoni L. 2004. Heat stress: characteristics, pathophysiology and avoidable mistakes. Minerva Anestesiol 70(7–8):617–624, PMID: 15252373. [PubMed] [Google Scholar]
- Cheng J, Zhang Y, Zhang W, Xu Z, Bambrick H, Hu W, et al. . 2018. Assessment of heat-and cold-related emergency department visits in cities of China and Australia: population vulnerability and attributable burden. Environ Res 166:610–619, PMID: 29982149, 10.1016/j.envres.2018.06.026. [DOI] [PubMed] [Google Scholar]
- Committee on Sports Medicine Fitness. 2000. Climatic heat stress and the exercising child and adolescent. Pediatrics 106:158–159, PMID: 10878169, 10.1542/peds.106.1.158. [DOI] [PubMed] [Google Scholar]
- Farthing M, Salam MA, Lindberg G, Dite P, Khalif I, Salazar-Lindo E, et al. . 2013. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol 47(1):12–20, PMID: 23222211, 10.1097/MCG.0b013e31826df662. [DOI] [PubMed] [Google Scholar]
- Faure C, Charlot K, Henri S, Hardy-Dessources MD, Hue O, Antoine-Jonville S. 2016. Impaired glucose tolerance after brief heat exposure: a randomized crossover study in healthy young men. Clin Sci (Lond) 130(12):1017–1025, PMID: 26980346, 10.1042/CS20150461. [DOI] [PubMed] [Google Scholar]
- Fletcher BA, Lin S, Fitzgerald EF, Hwang SA. 2012. Association of summer temperatures with hospital admissions for renal diseases in New York State: a case-crossover study. Am J Epidemiol 175(9):907–916, PMID: 22455834, 10.1093/aje/kwr417. [DOI] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43(8):1–20, PMID: 22003319, 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B. 2013. Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol 13:1, PMID: 23297754, 10.1186/1471-2288-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non‐linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2012a. Multivariate meta‐analysis for non‐linear and other multi‐parameter associations. Stat Med 31(29):3821–3839, PMID: 22807043, 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kovats S, Wilkinson P. 2012b. The effect of high temperatures on cause-specific mortality in England and Wales. Occup Environ Med 69(1):56–61, PMID: 21389012, 10.1136/oem.2010.059782. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Kinney PL, Petkova EP, Lavigne E, et al. . 2015a. Temporal variation in heat–mortality associations: a multicountry study. Environ Health Perspect 123(11):1200–1207, PMID: 25933359, 10.1289/ehp.1409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. . 2015b. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386(9991):369–375, PMID: 26003380, 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Leone M. 2014. Attributable risk from distributed lag models. BMC Med Res Methodol 14:55, PMID: 24758509, 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie J, Alexander L, Lewis SC, Sherwood SC, Bambrick H. 2018. Changes in relative fit of human heat stress indices to cardiovascular, respiratory, and renal hospitalizations across five Australian urban populations. Int J Biometeorol 62(3):423–432, PMID: 28965155, 10.1007/s00484-017-1451-9. [DOI] [PubMed] [Google Scholar]
- Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O'Neill MS. 2014. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006. Environ Health Perspect 122(11):1187–1192, PMID: 24905551, 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubenhoff JA, du Ford K, Roosevelt GE. 2007. Heat-related illness. Clin Pediatr Emerg Med 8(1):59–64, 10.1016/j.cpem.2007.02.006. [DOI] [Google Scholar]
- Guo Y. 2017. Hourly associations between heat and ambulance calls. Environ Pollut 220(Pt B):1424–1428, PMID: 27825842, 10.1016/j.envpol.2016.10.091. [DOI] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Pan X, Yu W, Tong S. 2011. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag non-linear model. Environ Health Perspect 119(12):1719–1725, PMID: 21827978, 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gasparrini A, Armstrong B, Li S, Tawatsupa B, Tobias A, et al. . 2014. Global variation in the effects of ambient temperature on mortality: a systematic evaluation. Epidemiology 25(6):781–789, PMID: 25166878, 10.1097/EDE.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gasparrini A, Armstrong B, Tawatsupa B, Tobias A, Lavigne E, et al. . 2016. Temperature variability and mortality: a multi-country study. Environ Health Perspect 124(10):1554–1559, PMID: 27258598, 10.1289/EHP149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gasparrini A, Armstrong B, Tawatsupa B, Tobias A, Lavigne E, et al. . 2017. Heat wave and mortality: a multicountry, multicommunity study. Environ Health Perspect 125(8):087006, PMID: 28886602, 10.1289/EHP1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gasparrini A, Li S, Sera F, Vicedo-Cabrera AM, de Sousa Zanotti Stagliorio Coelho M, et al. . 2018. Quantifying excess deaths related to heatwaves under climate change scenarios: a multicountry time series modelling study. PLoS Med 15(7):e1002629, PMID: 30063714, 10.1371/journal.pmed.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Kim H. 2013. Changes in the association between summer temperature and mortality in Seoul, South Korea. Int J Biometeorol 57(4):535–544, PMID: 22872184, 10.1007/s00484-012-0580-4. [DOI] [PubMed] [Google Scholar]
- Hajat S, Kovats RS, Lachowycz K. 2007. Heat-related and cold-related deaths in England and Wales: who is at risk? Occup Environ Med 64(2):93–100, PMID: 16990293, 10.1136/oem.2006.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. 2011. Outdoor temperature is associated with serum HDL and LDL. Environ Res 111(2):281–287, PMID: 21172696, 10.1016/j.envres.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, Bi P, Ryan P, Nitschke M, Pisaniello D, Tucker G. 2008. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int J Epidemiol 37(6):1359–1365, PMID: 18710886, 10.1093/ije/dyn165. [DOI] [PubMed] [Google Scholar]
- IBGE (Brazilian Institute of Geography and Statistics). 2013. Population Projection of Brazil by sex and age: 2000–2060. http://www.ibge.gov.br/english/estatistica/populacao/projecao_da_populacao/2013/default.shtm [accessed 2 September 2017].
- IHME (Institute for Health Metrics and Evaluation). 2016. GBD Compare Data Visualization. http://vizhub.healthdata.org/gbd-compare [accessed 22 January 2018].
- Kaldur T, Unt E, Ööpik V, Zilmer M, Eha J, Paapstel K, et al. . 2016. The acute effects of passive heat exposure on arterial stiffness, oxidative stress, and inflammation. Medicina (Kaunas) 52(4):211–216, PMID: 27697238, 10.1016/j.medici.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Piao MY, Lee IK, Kim HJ, Gu MJ, Yun C-H, et al. . 2017. Effects of ambient temperature and dietary glycerol addition on growth performance, blood parameters and immune cell populations of Korean cattle steers. Asian-Australas J Anim Sci 30(4):505, PMID: 27608638, 10.5713/ajas.16.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P, Garg A. 2005. “Children are not little adults.” Children’s Health and the Environment—A Global Perspective: A Resource Manual for the Health Sector Pronczuk-Garbino J, ed. Geneva: World Health Organization:3–16. [Google Scholar]
- Laranjeira R, Pinsky I, Sanches M, Zaleski M, Caetano R. 2010. Alcohol use patterns among Brazilian adults. Braz J Psychiatr 32(3):231–241, PMID: 19918673, 10.1590/S1516-44462009005000012. [DOI] [PubMed] [Google Scholar]
- Lin Y, Hu W, Xu J, Luo Z, Ye X, Yan C, et al. . 2017. Association between temperature and maternal stress during pregnancy. Environ Res 158:421–430, PMID: 28689033, 10.1016/j.envres.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Michelozzi P, Accetta G, De Sario M, D'Ippoliti D, Marino C, Baccini M, et al. . 2009. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 179(5):383–389, PMID: 19060232, 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Cavalcante TM, Moura EC, Claro RM, Szwarcwald CL. 2007. Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989-2003). Bull World Health Organ 85(7):527–534, PMID: 17768501, 10.2471/BLT.06.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration). 2017. Climate at a glance: Time series. http://www.ncdc.noaa.gov [accessed 11 May 2017].
- Phung D, Guo Y, Nguyen HT, Rutherford S, Baum S, Chu C. 2016. High temperature and risk of hospitalizations, and effect modifying potential of socio-economic conditions: a multi-province study in the tropical Mekong Delta region. Environ Int 92:77–86, PMID: 27060418, 10.1016/j.envint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- Prince MJ, Wu F, Guo Y, Robledo LMG, O'Donnell M, Sullivan R, et al. . 2015. The burden of disease in older people and implications for health policy and practice. Lancet 385(9967):549–562, PMID: 25468153, 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- Radin JM, Neems D, Goglia R, Siddiqui K, Steinhubl SR. 2018. Inverse correlation between daily outdoor temperature and blood pressure in six US cities. Blood Press Monit 23(3):148–152, PMID: 29677012, 10.1097/MBP.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh C, Linke A, O'Loughlin J. 2014. Extreme temperatures and violence. Nature Clim Change 4(2):76–77, 10.1038/nclimate2101. [DOI] [Google Scholar]
- Soneja S, Jiang C, Fisher J, Upperman CR, Mitchell C, Sapkota A. 2016. Exposure to extreme heat and precipitation events associated with increased risk of hospitalization for asthma in Maryland, USA. Environ Health 15:57, PMID: 27117324, 10.1186/s12940-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Brazil Business. 2013. Brazilian Regions. http://thebrazilbusiness.com/article/brazilian-regions [accessed 23 November 2017].
- The World Bank. 2017a. GDP ranking (2016) [Data file]. World Development Indicators 2017 ed.
- The World Bank. 2017b. Population ranking (2016) [Data file]. World Development Indicators 2017 ed.
- The World Bank. 2017c. GINI index (World Bank estimate) [Data file]. World Development Indicators 2017 ed.
- Thompson D, Richelson E, Malagelada J. 1983. Perturbation of upper gastrointestinal function by cold stress. Gut 24(4):277–283, PMID: 6832623, 10.1136/gut.24.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Wang XY, Yu W, Chen D, Wang X. 2014. The impact of heatwaves on mortality in Australia: a multicity study. BMJ open 4(2):e003579, PMID: 24549159, 10.1136/bmjopen-2013-003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsangari H, Paschalidou A, Kassomenos A, Vardoulakis S, Heaviside C, Georgiou K, et al. . 2016. Extreme weather and air pollution effects on cardiovascular and respiratory hospital admissions in Cyprus. Sci Total Environ 542(Pt A):247–253, PMID: 26519584, 10.1016/j.scitotenv.2015.10.106. [DOI] [PubMed] [Google Scholar]
- Vicedo-Cabrera AM, Sera F, Guo Y, Chung Y, Arbuthnott K, Tong S, et al. . 2018. A multi-country analysis on potential adaptive mechanisms to cold and heat in a changing climate. Environ Int 111:239–246, PMID: 29272855, 10.1016/j.envint.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Williams S, Nitschke M, Weinstein P, Pisaniello DL, Parton KA, Bi P. 2012. The impact of summer temperatures and heatwaves on mortality and morbidity in Perth, Australia 1994–2008. Environ Int 40:33–38, PMID: 22280925, 10.1016/j.envint.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Xavier AC, King CW, Scanlon BR. 2016. Daily gridded meteorological variables in Brazil (1980–2013). Int J Climatol 36(6):2644–2659, 10.1002/joc.4518. [DOI] [Google Scholar]
- Zhao Q, Coelho MSZS, Li S, Saldiva PH, Hu K, Abramson MJ, et al. . 2018. Spatiotemporal and demographic variation in the association between temperature variability and hospitalizations in Brazil during 2000–2015: a nationwide time-series study. Environ Int 120:345–353, PMID: 30114624, 10.1016/j.envint.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Zhang Y, Zhang W, Li S, Chen G, Wu Y, et al. . 2017. Ambient temperature and emergency department visits: Time-series analysis in 12 Chinese cities. Environ Pollut 224:310–316, PMID: 28222977, 10.1016/j.envpol.2017.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.