Abstract
Moderate-to-severe pain is a common problem experienced by patients with cancer. Although analgesic drugs are effective, adverse side effects are common and some analgesic drugs are addictive. Nonpharmacological treatment may be a way to treat cancer pain without causing negative side effects. Mindfulness is used as an effective nonpharmacological treatment to improve quality of life (QoL) and to address psychological problems including distress, anxiety, stress, and depression. However, the effect of mindfulness on pain severity has not been sufficiently investigated. Therefore, a systematic review was undertaken to describe the effectiveness of mindfulness interventions for pain and its underlying pathophysiologic mechanisms. The search was conducted in PubMed, Ovid MEDLINE, and CINAHL and included only empirical studies published from 2008 to 2017. Search terms included mindfulness, mindfulness-based intervention, meditation, cancer, pain, and cancer-related pain. Six studies met the search criteria. These studies tested several types of intervention including mindfulness-based stress reduction, mindfulness-based cognitive therapy, meditation with massage, and mindful awareness practices. Study outcomes include improved pain severity, anxiety, stress, depression, and QoL. However, most studies reviewed were conducted in the United States and Denmark. Further research is needed to test culturally appropriate mindfulness interventions to reduce pain.
Keywords: Cancer, mindfulness, nonpharmacology, pain, unpleasant symptom

Introduction
Globally, between 30% and 50% of the approximately 32 million people living with cancer[1] experience moderate-to-severe pain.[1,2] Cancer pain, which may arise from both cancer and its treatment, leads to both physical and psychological health problems.[3] Many patients with cancer (41.5%) report chronic pain[4] and suffer from psychological symptoms such as depression[5] and lower quality of life (QoL).[6] In a survey study of individuals with cancer (n = 1,800), 25% reported severe pain, 20% other severe symptoms, 45% severe family anxiety, 66% feelings of distress, and 19% severe problems with self-worth.[5] Although analgesic drugs are effective, adverse side effects from pharmacological treatment, such as constipation, nausea, and dizziness, are common. Furthermore, some analgesics, such as opioids, are potentially addictive. Nonpharmacological treatment may be a way to treat cancer pain without inducing negative side effects. In the cancer population internationally, mindfulness is being used as an effective nonpharmacological treatment to improve QoL[7,8,9,10] and to address psychological problems including distress,[11] anxiety,[8,12,13,14,15] stress,[13,14,16,17] and depression.[8,9,12,14,15,18] However, the effect of mindfulness on pain severity has not been sufficiently investigated. This systematic review was undertaken to describe the effectiveness of mindfulness interventions for pain.
Cancer Pain
Cancer pain is a multidimensional experience involving interactions among physical, sensory, affective, cognitive, and behavioral dimensions[19] and is classified into two types: nociceptive and neuropathic.
Nociceptive pain occurs when normal tissue is damaged, after which primary afferent A-delta and C fibers[20,21] are activated or sensitized by noxious thermal, mechanical, and chemical stimuli. Numerous chemicals (potassium,[22] bradykinin, prostaglandin, arachidonic acid, metabolites, adenosine and adenosine phosphates, serotonin, protons, histamine, cytokines, excitatory amino acids, opioid-like substances,[21] adenosine triphosphate, and acetylcholine[20]) are released, which mediate the inflammatory process. The inflammatory mediators interact with the specific receptors on the sensory nerve ending and in turn activate secondary messenger cascades that influence the ion channels in the nerve membrane. Ion channel activation leads to enhanced neuron excitability,[20] increased ion channel transduction, decreased firing threshold, and an exaggerated neuron response.[23] During tissue inflammation, nerve growth factor increases, leading to synthesis and release of substance P and calcitonin gene-related peptide from the neuron sensory ending, which produces neurogenic inflammation and increases pain. Nociceptive pain in cancer can occur from erosion of tumor into adjacent tissue, lymphatic and vascular obstruction, hollow organ distension, edema, and tissue inflammation or necrosis.
Neuropathic pain involves injury of peripheral neuronal tissue such as axonal damage, local neuritis, atrophy, altered Schwann cell activity, and altered signaling of the primary afferent nociceptor neuron.[21] Neuropathic pain also includes damage to central nervous system tissue either through injury, such as stroke, or through changes in nociceptive processing.[21,24] Furthermore, both peripheral and central nociceptive inputs have the potential to alter central nervous system processing (central sensitization), including changes in neurotransmitter release, increased sensitivity to stimuli, anatomical changes, and changes in the genome of neurons. Such changes make the pain difficult to localize and cause unique neuropathic pain characteristics such as burning, electrical shock-like,[20] tingling, numbness, stabbing, or pricking.[25] Neuropathic elements of cancer pain can occur from tumor pressure on either central or peripheral nerves, prolonged nociceptive input from inflammation that alters central pain processing, or from cancer treatment-associated nerve injury. For instance, neuropathic pain results from tumor invasion of nerves are nerve plexuses, radiation therapy-induced inflammation and scarring, and neurotoxic chemotherapy- and surgery-induced peripheral nerve injury.[25]
As a result of nerve injury, neuroplasticity (i.e., permanent changes in structure and function) occurs in peripheral neurons and sprouting emerges in the spinal cord. These neuronal changes produce responses to low threshold input, leading to an expansion of the receptive field[20] in the periphery and increased stimulus response. These mechanisms occur not only in the lower spinal cord but also at upper spinal levels as well.[26] Classic signs of neuropathic pain are spontaneous pain, hyperalgesia, and allodynia. Spontaneous pain occurs without being evoked. Hyperalgesia is an exaggerated pain response to a painful stimulus, and allodynia occurs when a nonnoxious stimulus like a cotton ball or clothing is perceived as painful.[27]
In addition to general pain processing, descending systems from the brain can modulate nociceptive signals in the spinal cord dorsal horn.[28] Neurotransmitters involved include but are not limited to serotonin and norepinephrine from brainstem catecholamine cell groups; orexins from the hypothalamus; and enkephalins, GABA, substance P, and glutamate from neurons in or projecting to the dorsal horn. These endogenous modulatory mechanisms can decrease or increase pain perception.[29]
The Need for Better Cancer Pain Treatments
Cancer pain can be managed with pharmacological and nonpharmacological treatments. Opioids are the most commonly used pharmacological treatment. According to the World Health Organization recommendation, the most commonly used opioid drugs are buprenorphine, codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, tramadol, and tapentadol. Nonsteroidal anti-inflammatory drugs and opioids play an important role in the treatment of nociceptive pain,[20] while a combination of adjuvants (e.g., gabapentin, pregabalin, and duloxetine) and opioids is often used for neuropathic pain.[30] However, these drugs can cause adverse side effects and/or addiction.
For patients who suffer from chronic cancer-related pain, nonpharmacological treatment is another choice. One systematic review[31] provides evidence that nonpharmacological therapies including massage, acupuncture, reflexology, yoga, taichi, hypnotherapy, aromatherapy, and music therapy improve sleep and QoL and decrease stress, anxiety, and pain. In addition to the prior list of techniques, cognitive-behavioral therapy (CBT) and CBT combined with pain education can provide patients with coping skills to deal with pain challenges.[31] CBT techniques have been shown to help patients with cancer change their thoughts about their pain experience.[32] Mindfulness, a specific type of CBT, is a nonpharmacological pain management strategy that includes techniques such as breathing meditation,[16] yoga,[33] mindfulness-based stress reduction (MBSR),[15,17] and mindfulness-based cognitive therapy (MBCT).[34] Two meta-analyses[8,13] provide evidence that both MBSR and MBCT are effective for depression, anxiety, and QoL; however, a critical gap in our scientific knowledge is that the effectiveness of a mindfulness-based intervention for cancer pain as the primary outcome has not been adequately explored.[35]
Theoretical Framework: The Theory of Unpleasant Symptoms
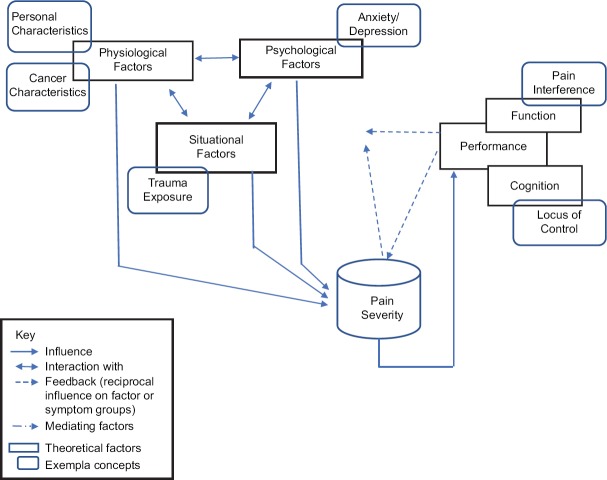
The framework of the middle-range theory of unpleasant symptoms is scientifically relevant and well suited to enhance the understanding of cancer pain,[36] because it outlines the interactions among the physiological, psychological, situational, and performance factors (i.e., physical and cognitive performances) that influence pain outcomes [Figure 1]. Examples of key interacting factors within this theoretical framework are discussed here.
Figure 1.
Theoretical framework
Physiological factors influence pain severity
Physiological factors that influence pain perception include personal (age, gender, and ethnicity) and cancer characteristics (staging and treatments). Several studies provide evidence that older patients report less pain than younger patients[37,38] and that women have higher pain sensitivity than men.[38] African American patients with cancer pain reported greater pain than White Americans.[39] Patients with more advanced-stage cancer report more pain due to metastases.[40] Chemotherapy, surgery, and radiation treatments for cancer also cause acute and chronic pain.[25,41]
Psychological factors influence pain severity
Examples of psychological factors that are associated with increased pain severity include anxiety, depression, and fear, among others. Cancer patients with pain have more anxiety and depression than those without pain.[37,42] Conversely, psychological conditions influence pain severity.[7,8,9,11,12,13,15,16,17,18,43]
Situational factors influence pain severity
A situational factor that is associated with increased pain severity is trauma exposure. Physical and/or sexual abuses, as well as neglect, are associated with higher pain severity.[44,45] For example, female survivors of posttraumatic stress disorder are more likely to develop chronic pain.[45,46]
Pain interference is associated with pain severity
High pain severity is associated with higher pain interference, meaning that patients who are in greater pain are less able to perform activities of daily living. For example, in a study of patients with painful metastatic bone cancer, moderate-to-severe pain interfered with the patients’ ability to walk, stand, and perform other activities.[47]
Maladaptive cognition is associated with pain severity
One type of maladaptive cognition, the belief that the individual has no control over their pain, can influence patients’ participation in pain-relieving activities. Beliefs regarding the ability to control one's pain – one's locus of control – vary across cultures. For example, Thai patients with cancer believe that a cancer diagnosis is associated with bad karma; thus, Thais do not commonly engage in pain management practices because they believe that individuals can do very little to control their own pain.[48]
Mindfulness Works
Definitions of mindfulness
Quoted directly, mindfulness has been defined in various ways:
Bringing attention to the experience that unfolds moment-by-moment in a nonjudgmental way[3]
A nonjudging mental state whereby one attends to and purposefully manages one's awareness of what is happening in the present moment[15]
A mental exercise of regulatory practices intending to attain certain psychological states that involve mind and body interaction of being in the present moment with a nonjudgmental attitude.[49]
Mindfulness may influence pain severity through an integrative process involving neurobiological and psychological systems.[50] Cancer pain impairs cognition, particularly attention, memory, and other psychological functions.[51] Practicing mindfulness meditation has been shown to be associated with increased activity in the anterior cingulate and anterior insular cortices, both of which are involved in cognitive regulation of nociceptive processes, and in the orbitofrontal cortex that is implicated in reframing the contextual evaluation of sensory experiences.[52] The anterior cingulate cortex, the prefrontal cortex (PFC), and the striatum are associated with attention control; the PFC and limbic areas relate to emotional regulation; and the insular cortex, medial PFC, posterior cingulate cortex, and precuneus are involved in self-awareness.[53] One study that revealed specific brain regions involved in pain processing[54] was conducted in eighty healthy participants and showed that mindfulness related to pain relief was associated with cognitive modulation of pain in many brain areas including orbitofrontal, subgenual anterior cingulate, and anterior insular cortices. However, little is known about how mindfulness affects cancer pain.
Methods
Literature search
The integrative literature review methods followed Whittemore and Knafl's guidelines.[55] The search, which was conducted in PubMed, Ovid MEDLINE, and CINAHL, included only empirical studies published in the last 10 years, from 2008 to 2017. The search was conducted using keywords including mindfulness, mindfulness-based intervention, meditation, cancer, pain, and cancer-related pain.
Selection of the studies
Inclusion criteria for the selected studies were as follows: (1) quantitative study, (2) randomized controlled trial (RCT) focusing on a mindfulness intervention in adult patients with cancer, (3) any outcome of pain, and (4) written in English. Figure 2 shows the study selection process.
Figure 2.
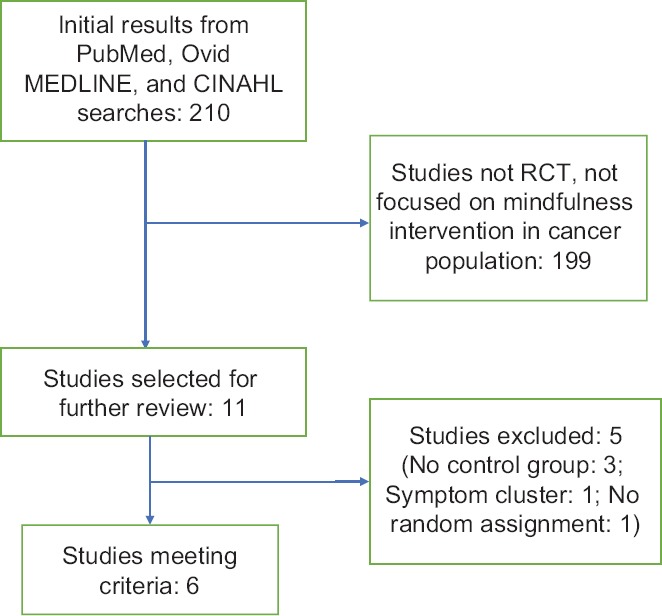
Diagram of the article selection process
Outcome measures
The studies focus on pain as both primary and secondary outcomes. In addition, the studies explore various other secondary outcomes, including pain interference, anxiety and depression, locus of control, and QoL.
Data extraction and quality assessment
The extraction and quality assessment was conducted by the first author (SN). First author and publication year, design, intervention and dose, sample size, measurement, outcomes, and significant results were extracted from each article and are summarized in Table 1.
Table 1.
Mindfulness interventions randomized control trial in cancer patients published in 2008–2017
| Authors (year) | Design | Intervention (dose) | Measure | Sample | Tools | Outcomes |
|---|---|---|---|---|---|---|
| Lengacher et al. (2016) | RCT | MBSR (6 weeks) | Baseline 6 weeks 3 months |
322 BC Intervention group=155 Control group=167 |
BPI FSI CESDS STAIS PSS Medical Outcomes Study Short Form |
Pain severity Pain interference Depression Anxietya QoL |
| Johannsen et al. (2017) | RCT | MBCT (8 weeks) | Baseline Postintervention 3 months 6 months |
129 BC Intervention group=67 Waitlist group=62 |
NRS Axillary lymph node dissection HADS ECR TAS-20 |
Pain intensitya |
| John et al. (2016) | RCT | MBSR Psychoeducation and support (8 weeks) |
Baseline Postintervention 6 months |
71 BC and CC Intervention group=35 Education group=36 |
FSI FSI Severity Subscale SF- 36 Vitality Scale BPI Fatigue global improvement Patients Health Questionnaire 8-item Depression Scale Generalized Anxiety Disorder Scale Insomnia Severity Index Expectancy credibility scale Satisfaction |
Paina Depressiona Anxietya |
| Johannsen et al. (2016) | RCT | MBCT (8 weeks) | Baseline Postintervention 3 months 6 months |
129 BC | MPQ-SF WHO-5 HADS |
Pain (pain intensitya) QoLa Depression and anxiety |
| Intervention group=67 | ||||||
| Waitlist group=62 | ||||||
| Dion et al. (2016) | RCT | Massage | Baseline | 38 BC | VAS | Paina |
| Massage + meditation (3 weeks) | Massage group=19 | Perceived Stress Scale | Stressa | |||
| Postintervention | Massage + meditation group=19 | |||||
| 3 weeks | ||||||
| Bower et al. (2015) | RCT | A MAP (6 weeks) | Baseline | 71 BC | PSS | Pain |
| Postintervention | Intervention group=39 | CESDS | Stressa | |||
| Control group=32 | FSI | Depression | ||||
| 3 months | ||||||
| PSQI | ||||||
| BCPT | ||||||
| QLACS | ||||||
| IES | ||||||
| PANAS-PA | ||||||
| FACIT |
aStatistically significant. RCT: Randomized control trial, MBSR: Mindfulness-based stress reduction, MBCT: Mindfulness-based cognitive therapy, BC: Breast cancer, CC: Colon cancer, BPI: The Brief Pain Inventory, FSI: The Fatigue Symptom Inventory, CESDS: The Center for Epidemiologic Studies Depression Scale, STAIS: The State-Trait Anxiety Inventory-State, NRS: Numerical Rating Scale, HADS: The Hospital Anxiety and Depression Scale, ECR: The Experiences in Close Relationships, TAS-20: The 20-item Toronto Alexithymia Scale, SF-36 Vitality Scale: 36-item Short-Form Survey Vitality Scale, MPQ-SF: The Short-Form McGill Pain Questionnaire, WHO 5 Well-Being Index: The World Health Organization-5 Well-Being Index, VAS: Visual analog scale, QoL: Quality of life, PSQI: Pittsburgh Sleep Quality Index, BCPT: Breast Cancer Prevention Trial Symptom Checklist, QLACS: Quality of life in adult cancer, IES: Impact of Event Scale, PANAS-PA: Positive and Negative Affect Schedule - Positive Affect, FACIT: Functional Assessment of Chronic Illness Therapy, MAP: Mindful awareness practices, PSS: Perceived Stress Scale
Risk of bias assessment
The risk of study bias was assessed and the results are displayed in Table 2. All of the RCT studies were rated based on the following Joanna Briggs Institute criteria:[56] adequate randomization, concealed allocation, adequate power, similarity of groups, blinded, reliable and valid measure, follow-up, fidelity, intention to treat, and conflict of interest. The consideration of risk of bias was divided into three levels based on the percentage of criteria that were met: <50% (high level), 50% to 80% (moderate level), and >80% (low level).
Table 2.
Risk of bias of mindfulness intervention testing for cancer patients
| Authors (year) | Adequate randomization | Concealed allocation | Adequate power | Similar groups | Blinded | Reliable/valid measure | Follow-up | Fidelity | ITT | COI | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lengacher et al. (2016) | Y | Y | Y | Y | Y | Y | Y | Y | Y | No | Low |
| Johannsen et al. (2017) | Y | Y | Y | Y | Y | Y | Y | N | Y | No | Moderate |
| John et al. (2016) | Y | Y | Y | Y | Y | Y | Y | Y | Y | No | Low |
| Johannsen et al. (2016) | Y | Y | Y | Y | N | Y | Y | Y | N | No | Moderate |
| Dion et al. (2016) | Y | Y | N | Y | Y | Y | Y | N | N | No | Moderate |
| Bower et al. (2015) | Y | Y | Y | Y | Y | Y | Y | Y | Y | No | Low |
Y: Criteria were met, N: Criteria were not met, ITT: Intention to treat, COI: Conflict of interest, No: No conflict of interest
Results
Characteristics of included studies
In total, 210 studies were found in PubMed, Ovid MEDLINE, and CINHAL databases by searching for articles containing the aforementioned keywords. After the first review, 199 studies were rejected because they were not empirical studies conducted in the cancer population and/or did not focus on a mindfulness intervention. Six[3,15,17,49,57,58] of the remaining 11 studies met the criteria; five studies were excluded because they were not randomized. MBSR was compared in one study with psychoeducation and support[15] and in another with usual care.[17] Two studies[3,57] compared MBCT to usual care. One[49] examined massage compared to massage plus breathing meditation. The last one[58] tested the mindful awareness practices (MAPs) program compared to the wait-list condition.
Characteristics of the sample
Sample size ranged from 38 to 322 participants. Four studies were conducted in the United States (US)[15,17,49,58] and two in Denmark.[3,57] Study participants had been diagnosed with either breast[3,15,17,49,57,58] or colon cancer.[15]
The type of mindfulness intervention
Two studies used the MBSR intervention,[15,17] two utilized MBCT,[3,57] one used breathing meditation plus massage,[49] and one employed the MAPs.[58]
The MBSR intervention is an 8-week mindfulness program consisting of many meditation exercises including body scan, yoga, brief mindful movement, awareness of breath, sitting and walking meditation, open awareness sitting medication, and loving-kindness practice. During and after these practices, nonreactive and nonjudgmental acceptance of thought, feeling, and body sensation were enhanced and facilitated.[15]
The MBCT intervention is an 8-week course comprising many group sessions involving 8–15 individuals. Participants learn about body scan techniques, mindfulness of breathing, and how to sit while focusing on breathing, thoughts, sounds, body mindful movement, and thinking about something difficult.[57]
In the breathing meditation plus massage study, breathing meditation technique was taught using a 15-min DVD. The massage technique, which followed the breathing meditation, was taught by a therapist during a 20-min session.[49]
The MAPs intervention was tailored by providing information about maintaining health and preventing breast cancer recurrence. The program involved 6 weekly 2-h group sessions that included presentation of theoretical materials on mindfulness, relaxation, and the mind–body connection; experiential practice of meditation and gentle movement exercise (e.g., mindful walking); and a psychoeducational section.[58]
Pain as the primary or secondary outcome
All studies evaluated pain as an outcome; pain was the primary outcome in four studies[3,17,49,57] and the secondary outcome in two.[15,58] After the various interventions, study outcomes were assessed at 3 weeks,[49] 3 months,[3,17,57,58] and 6 months.[3,15,57] Four[3,15,49,57] of the six studies provide evidence that mindfulness interventions result in statistically significant improvements in pain severity.
Other secondary outcomes
Five of six studies were designed to explore psychological factors (anxiety and depression) as secondary outcomes, with the exception of Johannsen's study,[3] which examined psychological factors as moderators of pain intensity. Two studies evaluated QoL, one of which showed that mindfulness led to statistically significant improvements.[57] Only one study[17] evaluated pain interference; it failed to show significant findings. No study measured locus of control as a primary or secondary outcome.
Study quality
Five of the six studies used rigorous research methods and had a low risk of research bias based on true randomization, concealed allocation, similar groups at baseline, and reliable and valid measurement. One study[49] was underpowered and one[57] did not use concealed treatment allocation/blinding methods. External validity was compromised in all the studies because nearly all participants had a breast cancer diagnosis and the studies were conducted only in the US and Danish populations.
Discussion
This systematic review describes various mindfulness interventions that may influence pain perception in patients with cancer. Consistent with the theory of unpleasant symptoms, cancer pain is a dynamic, complex, and subjective experience that is influenced by multiple physiological, psychological, and situational factors. Evidence from the study showed that patients with cancer had low pain control (locus of control), pain endurance, and negative effect beliefs,[59] which means they believed that controlling pain was the duty of the physician.[60] Moreover, since pain symptoms in individuals with cancer may convey that other factors may be worsening (e.g., disease progression and new metastases), the emotional response to how the patient interprets the meaning of the pain can ultimately influence pain severity. Many factors will influence the pain severity perceptions of patients with cancer. Therefore, multidimensional interventions are needed to improve cancer pain.
Mindfulness meditation may be effective for managing pain because it can moderate the patient's emotional appraisal of pain and other stressful events by withdrawing the attention away from the unpleasant symptom.[61] When patients practice mindfulness meditation, they become less cognitively focused on their pain; thus, pain tolerance is enhanced.
From our findings, mindfulness interventions influenced both physical (pain) and psychological variables (anxiety, stress, and depression). However, the evidence does not yet support that mindfulness interventions should be a mainstay treatment for cancer pain. This is because only three studies[15,17,58] had a low risk of bias. Further, studies to date have been conducted in limited study populations, and findings are inconsistent across studies. Therefore, future intervention research is needed to test whether mindfulness interventions will lead to better pain outcomes for a variety of cancer populations, including for Eastern (Asian) populations where mindfulness practices are well accepted and a routine component of daily life.
Limitations
This review has three limitations. First, there are a small number of studies that met the inclusion criteria. Second, only the first author reviewed the studies and decided whether to include them. Third, only three databases were searched, so some high-quality studies may have been missed.
Clinical implementation
The multidimensional and complex nature of the pain experience may not be completely recognized by clinicians who assess and manage cancer-associated pain. Given the increasing number of cancer survivors in the world today who experience pain, ongoing and frequently updated continuing education programs are needed to keep nurses well versed regarding the latest treatment approaches, which include nonpharmacological treatments such as mindfulness meditation. Pharmacological treatments for cancer pain can be very effective, but the adverse effects can cause additional suffering. Based on theory and the current empirical evidence, a nonpharmacological mindfulness intervention may be an effective, safe, and nontoxic treatment option that warrants future consideration in clinical practice settings to improve pain, as well as QoL and other psychological problems. Nurses are perfectly positioned to teach patients about this type of intervention, which could perhaps be reinforced and widely disseminated using innovative technology-based methods.
Conclusion
The preliminary evidence suggests that mindfulness-based interventions may decrease pain severity, anxiety, and depression and can improve QoL. However, before mindfulness interventions can be recommended as mainstay treatment for cancer pain, future intervention research conducted in diverse cancer populations is needed, to expand our current knowledge regarding the potential benefits.
Financial support and sponsorship
This study was supported by The National Institute of Nursing Research (Grant No: 1D43TW009883-01).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
I would like to express my special thanks of gratitude to Dr. Kathleen Potempa, who gave me the opportunity to train in the Fogarty International Training Program for Strengthening Non-Communicable Disease Research and Training Capacity, co-funded by the National Institute of Nursing Research, School of Nursing, University of Michigan, Ann Arbor. Second, I would also like to thank Dr. Roongrutai Mualprasitporn, the director of the Praboromarachanok Institute of Health Workforce Development who gave me 2 years to pursue a postdoctoral program at the University of Michigan School of Nursing in Ann Arbor.
References
- 1.Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain – An overview of cochrane reviews. Cochrane Database Syst Rev. 2017;7:CD012592. doi: 10.1002/14651858.CD012592.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J, et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437–49. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 3.Johannsen M, O’Toole MS, O’Connor M, Jensen AB, Zachariae R. Clinical and psychological moderators of the effect of mindfulness-based cognitive therapy on persistent pain in women treated for primary breast cancer-explorative analyses from a randomized controlled trial. Acta Oncol. 2017;56:321–8. doi: 10.1080/0284186X.2016.1268713. [DOI] [PubMed] [Google Scholar]
- 4.Kurita GP, Sjøgren P. Pain management in cancer survivorship. Acta Oncol. 2015;54:629–34. doi: 10.3109/0284186X.2014.996662. [DOI] [PubMed] [Google Scholar]
- 5.Pidgeon T, Johnson CE, Currow D, Yates P, Banfield M, Lester L, et al. A survey of patients’ experience of pain and other symptoms while receiving care from palliative care services. BMJ Support Palliat Care. 2016;6:315–22. doi: 10.1136/bmjspcare-2014-000748. [DOI] [PubMed] [Google Scholar]
- 6.Melzack R, Perry C. Self-regulation of pain: The use of alpha-feedback and hypnotic training for the control of chronic pain. Exp Neurol. 1975;46:452–69. doi: 10.1016/0014-4886(75)90119-3. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Altern Med 2013. 2013 doi: 10.1155/2013/419496. 419496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. Int J Psychiatry Med. 2006;36:13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- 9.Poulin PA, Romanow HC, Rahbari N, Small R, Smyth CE, Hatchard T, et al. The relationship between mindfulness, pain intensity, pain catastrophizing, depression, and quality of life among cancer survivors living with chronic neuropathic pain. Support Care Cancer. 2016;24:4167–75. doi: 10.1007/s00520-016-3243-x. [DOI] [PubMed] [Google Scholar]
- 10.Reich RR, Lengacher CA, Alinat CB, Kip KE, Paterson C, Ramesar S, et al. Mindfulness-based stress reduction in post-treatment breast cancer patients: Immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage. 2017;53:85–95. doi: 10.1016/j.jpainsymman.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish JA, Ettridge K, Sharplin GR, Hancock B, Knott VE. Mindfulness-based cancer stress management: Impact of a mindfulness-based programme on psychological distress and quality of life. Eur J Cancer Care (Engl) 2014;23:413–21. doi: 10.1111/ecc.12136. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Xu R, Wang B, Wang J. Effects of mindfulness-based therapy for patients with breast cancer: A systematic review and meta-analysis. Complement Ther Med. 2016;26:1–10. doi: 10.1016/j.ctim.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Gotink RA, Chu P, Busschbach JJ, Benson H, Fricchione GL, Hunink MG, et al. Standardised mindfulness-based interventions in healthcare: An overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344. doi: 10.1371/journal.pone.0124344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 15.Johns SA, Brown LF, Beck-Coon K, Talib TL, Monahan PO, Giesler RB, et al. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support Care Cancer. 2016;24:4085–96. doi: 10.1007/s00520-016-3220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson LE. Mindfulness-based interventions for physical conditions: A narrative review evaluating levels of evidence. ISRN Psychiatry 2012. 2012 doi: 10.5402/2012/651583. 651583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lengacher CA, Reich RR, Paterson CL, Ramesar S, Park JY, Alinat C, et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2016;34:2827–34. doi: 10.1200/JCO.2015.65.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobos G, Overhamm T, Büssing A, Ostermann T, Langhorst J, Kümmel S, et al. Integrating mindfulness in supportive cancer care: A cohort study on a mindfulness-based day care clinic for cancer survivors. Support Care Cancer. 2015;23:2945–55. doi: 10.1007/s00520-015-2660-6. [DOI] [PubMed] [Google Scholar]
- 19.McGuire DB. Comprehensive and multidimensional assessment and measurement of pain. J Pain Symptom Manage. 1992;7:312–9. doi: 10.1016/0885-3924(92)90064-o. [DOI] [PubMed] [Google Scholar]
- 20.Schaible HG, Richter F. Pathophysiology of pain. Langenbecks Arch Surg. 2004;389:237–43. doi: 10.1007/s00423-004-0468-9. [DOI] [PubMed] [Google Scholar]
- 21.Kocoglu H, Pirbudak L, Pence S, Balat O. Cancer pain, pathophysiology, characteristics and syndromes. Eur J Gynaecol Oncol. 2002;23:527–32. [PubMed] [Google Scholar]
- 22.Marchand S. The physiology of pain mechanisms: From the periphery to the brain. Rheum Dis Clin North Am. 2008;34:285–309. doi: 10.1016/j.rdc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Moffat R, Rae CP. Anatomy, physiology and pharmacology of pain. Anaesth Intensive Care Med. 2011;12:12–5. [Google Scholar]
- 24.Kashi Y, Ratmansky M, Defrin R. Deficient pain modulation in patients with chronic hemiplegic shoulder pain. Pain Pract. 2018;18:716–28. doi: 10.1111/papr.12658. [DOI] [PubMed] [Google Scholar]
- 25.Smith EM, Bridges CM, Kanzawa G, Knoerl R, Kelly JP, 4th, Berezovsky A, et al. Cancer treatment-related neuropathic pain syndromes – Epidemiology and treatment: An update. Curr Pain Headache Rep. 2014;18:459. doi: 10.1007/s11916-014-0459-7. [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 27.Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22:81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 28.West SJ, Bannister K, Dickenson AH, Bennett DL. Circuitry and plasticity of the dorsal horn – Toward a better understanding of neuropathic pain. Neuroscience. 2015;300:254–75. doi: 10.1016/j.neuroscience.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, et al. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci. 2018;38:465–73. doi: 10.1523/JNEUROSCI.1647-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh P, Chaturvedi A. Complementary and alternative medicine in cancer pain management: A systematic review. Indian J Palliat Care. 2015;21:105–15. doi: 10.4103/0973-1075.150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwekkeboom KL. A model for cognitive-behavioral interventions in cancer pain management. Image J Nurs Sch. 1999;31:151–6. doi: 10.1111/j.1547-5069.1999.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 33.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Libr. 2017;1:CD010802.1–77. doi: 10.1002/14651858.CD010802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington N, Pickles C. Mindfulness and cognitive behavioral therapy: Are they compatible concepts? J Cogn Psychother. 2009;23:315–23. [Google Scholar]
- 35.Knoerl R, Lavoie Smith EM, Weisberg J. Chronic pain and cognitive behavioral therapy: An integrative review. West J Nurs Res. 2016;38:596–628. doi: 10.1177/0193945915615869. [DOI] [PubMed] [Google Scholar]
- 36.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS Adv Nurs Sci. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Kyranou M, Paul SM, Dunn LB, Puntillo K, Aouizerat BE, Abrams G, et al. Differences in depression, anxiety, and quality of life between women with and without breast pain prior to breast cancer surgery. Eur J Oncol Nurs. 2013;17:190–5. doi: 10.1016/j.ejon.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevilacqua LA, Dulak D, Schofield E, Starr TD, Nelson CJ, Roth AJ, et al. Prevalence and predictors of depression, pain, and fatigue in older- versus younger-adult cancer survivors. Psychooncology. 2018;27:900–7. doi: 10.1002/pon.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meghani SH, Thompson AM, Chittams J, Bruner DW, Riegel B. Adherence to analgesics for cancer pain: A comparative study of African americans and whites using an electronic monitoring device. J Pain. 2015;16:825–35. doi: 10.1016/j.jpain.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta M, Sahi MS, Bhargava AK, Talwar V. The prevalence and characteristics of pain in critically ill cancer patients: A prospective nonrandomized observational study. Indian J Palliat Care. 2015;21:262–7. doi: 10.4103/0973-1075.164894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooney MA, Culleton-Quinn E, Stokes E. Current knowledge of pain after breast cancer treatment: A systematic review. Pain Manag Nurs. 2013;14:110–23. doi: 10.1016/j.pmn.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan D, Foo I, O’Shea H, Gillanders D, Williams L, Fallon M, et al. Long-term follow-up of pain and emotional characteristics of women after surgery for breast cancer. J Pain Symptom Manage. 2012;44:608–14. doi: 10.1016/j.jpainsymman.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Carmody J, Reed G, Kristeller J, Merriam P. Mindfulness, spirituality, and health-related symptoms. J Psychosom Res. 2008;64:393–403. doi: 10.1016/j.jpsychores.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Caffo E, Belaise C. Psychological aspects of traumatic injury in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2003;12:493–535. doi: 10.1016/s1056-4993(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 45.Sprang G, Bush HM, Coker AL, Brancato CJ. Types of trauma and self-reported pain that limits functioning in different-aged cohorts. J Interpers Violence. 2017:886260517723144. doi: 10.1177/0886260517723144. [DOI] [PubMed] [Google Scholar]
- 46.Wuest J, Ford-Gilboe M, Merritt-Gray M, Wilk P, Campbell JC, Lent B, et al. Pathways of chronic pain in survivors of intimate partner violence. J Womens Health (Larchmt) 2010;19:1665–74. doi: 10.1089/jwh.2009.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood R, Mitra D, de Courcy J, Iyer S. Patient-reported pain severity, pain interference and health status in HR+/HER2- advanced/metastatic breast cancer. ESMO Open. 2017;2:e000227. doi: 10.1136/esmoopen-2017-000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liamputtong P, Suwankhong D. Living with breast cancer: The experiences and meaning-making among women in southern thailand. Eur J Cancer Care (Engl) 2016;25:371–80. doi: 10.1111/ecc.12321. [DOI] [PubMed] [Google Scholar]
- 49.Dion LJ, Engen DJ, Lemaine V, Lawson DK, Brock CG, Thomley BS, et al. Massage therapy alone and in combination with meditation for breast cancer patients undergoing autologous tissue reconstruction: A randomized pilot study. Complement Ther Clin Pract. 2016;23:82–7. doi: 10.1016/j.ctcp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Guendelman S, Medeiros S, Rampes H. Mindfulness and emotion regulation: Insights from neurobiological, psychological, and clinical studies. Front Psychol. 2017;8:220. doi: 10.3389/fpsyg.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attal N, Masselin-Dubois A, Martinez V, Jayr C, Albi A, Fermanian J, et al. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain. 2014;137:904–17. doi: 10.1093/brain/awt354. [DOI] [PubMed] [Google Scholar]
- 52.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC, et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang YY, Yang L, Leve LD, Harold GT. Improving executive function and its neurobiological mechanisms through a mindfulness-based intervention: Advances within the field of developmental neuroscience. Child Dev Perspect. 2012;6:361–6. doi: 10.1111/j.1750-8606.2012.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J Neurosci. 2015;35:15307–25. doi: 10.1523/JNEUROSCI.2542-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittemore R, Knafl K. The integrative review: Updated methodology. J Adv Nurs. 2005;52:546–53. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 56.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Systematic reviews of effectiveness. In: Aromataris MZ, editor. Joanna Briggs Institute Reviewers Manual. Ch 3. Adelaide: The Joanna Briggs Institute; 2017. [Google Scholar]
- 57.Johannsen M, O’Connor M, O’Toole MS, Jensen AB, Højris I, Zachariae R, et al. Efficacy of mindfulness-based cognitive therapy on late post-treatment pain in women treated for primary breast cancer: A randomized controlled trial. J Clin Oncol. 2016;34:3390–9. doi: 10.1200/JCO.2015.65.0770. [DOI] [PubMed] [Google Scholar]
- 58.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, et al. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2015;121:1231–40. doi: 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X, Luckett T, Wang AY, Lovell M, Phillips JL. Cancer pain management needs and perspectives of patients from chinese backgrounds: A systematic review of the chinese and english literature. Palliat Support Care. 2018 Jan;17:1–15. doi: 10.1017/S1478951517001171. [DOI] [PubMed] [Google Scholar]
- 60.Czerw AI, Religioni U, Deptała A, Fronczak A. Pain, acceptance of illness, adjustment to life with cancer and coping strategies in prostate cancer patients. Arch Med Sci. 2017;13:1459–66. doi: 10.5114/aoms.2016.58458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown CA, Jones AK. Meditation experience predicts less negative appraisal of pain: Electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150:428–38. doi: 10.1016/j.pain.2010.04.017. [DOI] [PubMed] [Google Scholar]