Abstract
Background:
The Life Cycle Initiative, hosted at the United Nations Environment Programme, selected human toxicity impacts from exposure to chemical substances as an impact category that requires global guidance to overcome current assessment challenges. The initiative leadership established the Human Toxicity Task Force to develop guidance on assessing human exposure and toxicity impacts. Based on input gathered at three workshops addressing the main current scientific challenges and questions, the task force built a roadmap for advancing human toxicity characterization, primarily for use in life cycle impact assessment (LCIA).
Objectives:
The present paper aims at reporting on the outcomes of the task force workshops along with interpretation of how these outcomes will impact the practice and reliability of toxicity characterization. The task force thereby focuses on two major issues that emerged from the workshops, namely considering near-field exposures and improving dose–response modeling.
Discussion:
The task force recommended approaches to improve the assessment of human exposure, including capturing missing exposure settings and human receptor pathways by coupling additional fate and exposure processes in consumer and occupational environments (near field) with existing processes in outdoor environments (far field). To quantify overall aggregate exposure, the task force suggested that environments be coupled using a consistent set of quantified chemical mass fractions transferred among environmental compartments. With respect to dose–response, the task force was concerned about the way LCIA currently characterizes human toxicity effects, and discussed several potential solutions. A specific concern is the use of a (linear) dose–response extrapolation to zero. Another concern addresses the challenge of identifying a metric for human toxicity impacts that is aligned with the spatiotemporal resolution of present LCIA methodology, yet is adequate to indicate health impact potential.
Conclusions:
Further research efforts are required based on our proposed set of recommendations for improving the characterization of human exposure and toxicity impacts in LCIA and other comparative assessment frameworks. https://doi.org/10.1289/EHP3871
Introduction
Toxicity Impacts: Seeking Harmonization and Global Guidance
Life cycle assessment (LCA) is a standardized method to assess and compare the various potential environmental impacts attributable to chemical emissions and resources used along full product and service life cycles (ISO 2006). LCA aims to comprehensively address potentially adverse environmental outcomes using “characterization factors,” including human toxicity impacts from exposure to chemical substances over the entire product life cycles. By identifying chemical emission and exposure hotspots along product life cycles and the most efficient technologies to address these hotspots, LCA also helps to achieve important targets of the United Nations’ Sustainable Development Goals (http://sustainabledevelopment.un.org/sdgs). To analyze and compare trade-offs among different alternatives or scenarios, LCA works with representative situations, using best estimates rather than conservative assumptions in model and parameter selection (Fantke et al. 2018; Frischknecht and Jolliet 2016). In order to cover the full life cycle with information available from chemical emission inventories, LCA must often work with spatial and temporal averages (Hauschild et al. 2008).
Human health afffected by disease burden attributable to chemical substances is an important area of protection in the life cycle impact assessment (LCIA) phase of LCA, but it is also a key component of other assessment frameworks, including risk assessment, chemical alternatives assessment, and health impact assessment (Fantke and Ernstoff 2018). The Life Cycle Initiative, hosted by the United Nations Environment Programme, is expanding its guidance on human toxicity impacts from exposure to chemical substances, and it convened the Human Toxicity Task Force to address this issue (Frischknecht et al. 2016; Jolliet et al. 2014, 2018; Verones et al. 2017). The task force includes leading experts from academia, industry, and public health institutions who have identified two major challenges—expanding exposure assessments to address near-field exposures and improving dose–response modeling. All authors of the present paper are members of the task force, and all related statements (e.g., “we observe”; “our agreement”) are those of the entire task force (and not only those of the author list or a single set of workshop attendees).
Studies, such as different analyses of U.S. National Health and Nutrition Examination Survey urine biomonitoring samples, reveal the presence of exogenous chemicals (or their metabolites) at detectable levels attributable to consumer products (e.g., Wambaugh et al. 2014). This observation has motivated a call to adequately characterize the constituents of consumer products for potential toxicity (Landrigan et al. 2018). Exposures to chemical substances in consumer products can occur over the entire product life cycle. This includes, for example, exposure to mining wastes during raw material extraction (Hauschild et al. 2018; Hendrickson et al. 2006); worker exposure during the manufacturing of plastics and other materials (Demou et al. 2009); exposure during product use—from personal care products (PCPs), building materials, toys, cleaning products, etc. (Fantke et al. 2016; Shin et al. 2017); and exposure from releases into the environment at the end of product life (Hauschild et al. 2018). Exposure pathways and magnitudes are not only substance but also product specific. For example, dermal exposure varies as a function of the duration of application of washed-off (e.g., shampoo) vs. leave-on PCPs. There have been recent proposals to track cumulative exposures and health impacts for these products (Fantke et al. 2015b; Zimmerman and Anastas 2015). To our knowledge, there are currently no methods available to analyze specific substance–product combinations over entire product life cycles in order to identify trade-offs among exposures at different life cycle stages and compare with other types of impacts, such as ecosystem damage and climate change.
Furthermore, though potentially well suited for addressing chemical impacts of specific products, LCIA has, in practice, often omitted consumer exposure to chemical substances during product use, instead focusing on emissions to the environment during manufacturing and disposal (Fantke et al. 2016). LCIA has also mostly excluded impacts on workers in the supply chain (Demou et al. 2009; Kijko et al. 2015). Since the specific geographic locations of impacts related to different product life cycle stages are often unknown, LCIA models typically have a low spatial resolution (Fantke et al. 2018). There are fate and exposure models available that provide a flexible spatial resolution in any region of interest and that can be applied in LCIA (Wannaz et al. 2018a, 2018b). However, these models require specification of emission locations across modeled chemicals and life cycle stages, which is often not available. The challenge is to address these gaps—for consumer and worker exposures—in an appropriate and consistent manner suitable for substance comparison and prioritization. How well the LCIA assumptions represent reality varies across substances and scenarios, leading to high uncertainties in exposure and toxicity results.
The Human Toxicity Task Force works to improve LCIA methods through evaluation of current practices, discussions with key researchers and stakeholders in relevant areas, and by organizing targeted workshops. An earlier LCIA flagship project provided guidance on assessing impacts from exposure to fine particulate matter, global warming, land use, and water use (Frischknecht and Jolliet 2016). In the context of ongoing task force efforts, the purpose of the present paper is to reflect on the state of the art in exposure and toxicity characterization in terms of a) available approaches for application in LCIA and similar comparative assessment frameworks and their domain coverage, b) strengths of these approaches beyond which currently no major progress has been made, c) limitations of these various approaches and any progress made in exposure science and toxicology across science and policy fields that is suitable for addressing those limitations and for updating current assessment methods, and d) recommendations for guiding further research to operationalize the use of suitable approaches in exposure and toxicity characterization.
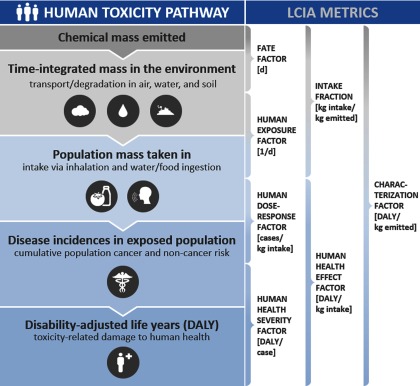
Among available LCIA methods that address health impacts from exposure to toxic chemical substances (referred to as human toxicity impacts), most rely on dose–response data extrapolated from animal toxicity studies and chemical intake estimated from multimedia fate and multipathway exposure models (e.g., using intake fractions; see Figure 1).
Figure 1.
Generalized illustrative representation of the existing life cycle human toxicity source-to-damage characterization framework. Units of metrics and impact pathways considered may differ between methods.
Between 2003 and 2008, the Life Cycle Initiative provided human toxicity guidance for substances emitted to the far-field (i.e., outdoor) environment (Hauschild et al. 2008; Westh et al. 2015). This effort was informed by model comparisons and expert elicitations (Jolliet et al. 2006; McKone et al. 2006), and resulted in the first version of the scientific consensus model USEtox® (Rosenbaum et al. 2008, 2011), which was updated in 2015 with the introduction of a generic indoor air compartment (Rosenbaum et al. 2015). While USEtox® is meant to reflect mature science, our experience reveals that the current toxicity characterization framework in LCIA has limitations that call for further improvement based on new scientific findings. Significant among these improvements are a) addressing spatiotemporal and population-level resolution challenges to estimate impact potential; b) addressing chemical substances in consumer products and in occupational settings, and adding related human exposure pathways that are currently missing; c) extending the limited coverage in available dose–response data and models; and d) improving the coverage and quality in databases on substance physicochemical properties and toxicity information. These limitations drive the need for additional guidance to help practitioners go beyond far-field and indoor emissions to the latest research on near-field (i.e., vicinity of consumers or workers) exposure assessment (e.g., Fantke et al. 2016; Jolliet et al. 2015) and dose–response and severity data (Forouzanfar et al. 2016; Salomon et al. 2015).
Key Questions for Advancing Exposure and Toxicity Characterization
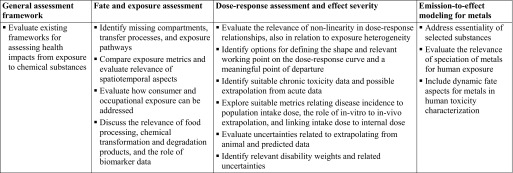
During its scoping phase, the task force enlisted eight leading experts from academia, industry, and public health institutions to develop a roadmap for advancing human toxicity characterization in LCIA. The proposed roadmap included a set of specific questions addressing: a) approaches and data needed to determine human toxicity effect indicators for chemical emissions, b) the validity and maturity of such approaches and data needed to represent human toxicity impacts for currently missing pathways, and c) the relevance and feasibility of considering essentiality and long-term changes in the human toxicity characterization of metal emissions. As a follow-up to this initial effort, there were three workshops that provided an opportunity to discuss these questions, exchange information on research advances among leading practitioners, and consider initial recommendations for action.
The first workshop was the Utrecht Framing Workshop, held at the International Society of Exposure Science (ISES) annual meeting in Utrecht, Netherlands, in October 2016, with 40 exposure and toxicity experts attending from nine countries, who identified and discussed the main scientific questions and challenges. The subsequent Metal Workshop was organized at the Society of Environmental Toxicology and Chemistry annual meeting in Brussels in May 2017. Here, nine researchers associated with the USEtox International Center and 15 experts and representatives from different metal industry associations focused on making use of recent data developed by the metals industry relating to human toxicity characterization of metals and the set of findings from the Eurometaux meeting in 2014 (Eurometaux 2014). Finally, the Research Triangle Park (RTP) Guidance Workshop was organized at the ISES annual meeting in RTP, North Carolina, in October 2017, where 20 toxicity/exposure science experts from industry, government agencies, and academia confronted approaches and data needed to establish improved dose–response and disease severity factors for a large number of hazardous substances. Figure 2 summarizes the key questions addressed during these three scoping-phase workshops, with additional details provided in Table S1. The related outcomes are discussed in detail in the following sections.
Figure 2.
Key points for advancing current exposure and toxicity characterization in life cycle impact assessment (LCIA) and similar comparative assessment frameworks. These key questions were addressed during the scoping phase workshops—the Utrecht Guidance Workshop in October 2016, the Brussels Metals Workshop in May 2017, and the Research Triangle Park (RTP) Guidance Workshop in October 2017.
Discussion
Extending the General Assessment Framework
Participants at all workshops considered the consensus-based framework of Rosenbaum et al. (2008) as a suitable starting point for assessing human toxicity impacts in LCIA within task force recommendations. In this framework, toxicity-related impacts on human health are described by a matrix of characterization factors (disability-adjusted life years, DALY/d per ) expressing impacts on humans via health end points h for emissions into environmental compartments c:
| (1) |
where diagonal matrix contains in its main diagonal the severity factors (DALY/d per case/d) for health effects h with zeros elsewhere, and multiplies matrix of dose–response slope factors (cases/d per ) for health effects h via exposure pathways e. This matrix multiplies the product of matrix of exposure factors () from receiving compartments c via exposure pathways e and square matrix of fate factors () from emission to receiving compartments c. The product of FF and XF is interpreted as matrix of intake fractions expressing the fraction of emissions to compartment c that enters the human population by exposure pathway e. Emissions are expressed as daily equivalent release, based on emission data from other measurement durations (week, month, year) converted to equivalent annual release rates. This approach is considered appropriate for many emission inventories, but has not yet been adapted to releases with a strong temporal character, which requires additional efforts. These emission inventories include those that give rise to acute health effects from localized pulse releases or very long-term releases, such as transfers and discharges to aquifers, both of which may not be readily approximated by long-term steady-state equivalents.
Based on these considerations, we observe the need to extend the existing characterization framework using an approach similar to efforts on expanding health impacts from exposure to fine particulate matter indoors and outdoors (Fantke et al. 2015a, 2017; Hodas et al. 2016; Humbert et al. 2011), and building on recent efforts focusing on coupling near-field consumer and indoor exposures with exposures due to environmental far-field emissions (Fantke et al. 2016; Rosenbaum et al. 2015; Shin et al. 2015).
Extending the Exposure Assessment Framework
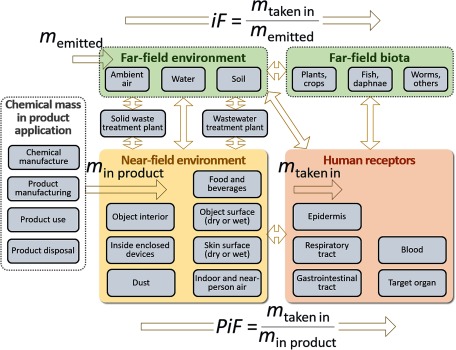
Because chemical substances in consumer products can contribute to exposures in the near field (consumer and occupational environments) and far field (outdoor environment), our workshop and task force efforts concentrated on methods to extend exposure assessments to capture both. To address human exposures during and after product use, exposure of bystanders, and occupational exposure pathways, we selected consistent mass–balance models to link near-field environments to human receptors following the approach of Fantke et al. (2016). This approach combines near-field with far-field exposures into a metric that incorporates the interactions of humans with both environments via dermal, mouthing, inhalation, and oral exposure pathways and potential feedback via, e.g., exhalation. We note that the product intake fraction (PiF) concept developed by Jolliet et al. (2015) provides the metric for consistently linking human intake via all exposure routes to substance mass in products. Figure 3 illustrates how, in contrast to the receptor-oriented perspective followed in risk- and other health-based assessments, the PiF-based framework primarily takes an emitter or product-oriented perspective. This product-oriented approach is not intended to assure safety but provides a basis for product comparisons. Such comparisons provide quantitative estimates with reliability limited by uncertainties that can vary among substances. In contrast to risk and safety assessments, LCIA does not use default uncertainty factors to introduce additional safety levels.
Figure 3.
Proposal for an integrated near-field/far-field human exposure assessment framework, which builds on the intake fraction (iF) linking population-level chemical mass intake () to chemical mass emitted () to the environment (Bennett et al. 2002) covered in the existing framework for general population exposure settings, and on the product intake fraction (PiF) linking population intake to chemical mass in products () for consumer and occupational exposure settings (Jolliet et al. 2015). Arrows connecting boxes denote chemical mass transfer fractions derived from fate and exposure processes, as fully described in Fantke et al. (2016).
Based on the consensus developed at the three task force workshops, we found the combined near-field and far-field exposure assessment framework a viable and operational starting point for extending the capability of LCIA exposure assessments. In moving this approach forward, our consensus findings identified further research to: a) distinguish exposure via initial product use from indirect exposure after initial use; b) capture physiological and functional differences (particularly for children) that can be important for linking exposure to variation of susceptibility; c) explore information needs on substance composition of products, exposure duration, and product application; and d) assess the availability of emission data and the suitability of emission estimates. We agreed that defining a limited number of exposure archetypes can thereby serve as a valuable starting point. Additional work should address exposure through inhalation of suspended particles (McClellan et al. 2016) and hand-to-mouth exposure for toddlers and young children (Xue et al. 2007). For estimating emissions, especially in the consumer and worker environment, there exist approaches using substance flow analysis (Li and Wania 2016; Li et al. 2018) or rapid screening methods (Breivik et al. 2012; McLachlan et al. 2014; Tao et al. 2018). We determined that these tools should be further improved and adapted for providing life cycle emission inventories for exposure modeling in a comparative context following the process described by Huang et al. (2017).
Consumer and occupational exposure.
Our workshop deliberations found that the combined near-field and far-field framework proposed by Fantke et al. (2016), which was originally designed for consumer exposure, constitutes a viable starting point to generally assess product-oriented exposures. This approach has already been vetted through case studies (Csiszar et al. 2017; Ernstoff et al. 2016) and model evaluations (Huang et al. 2017; Shin et al. 2017), and can be integrated into multiple frameworks, such as the stochastic human exposure and dose simulation model, SHEDS-HT (Isaacs et al. 2014) or MERLIN-Expo (Ciffroy et al. 2016).
Although currently excluded from LCIA, occupational chemical exposures during product manufacturing can impose higher risks to workers via exposure at the workplace than to the general population via emissions to the environment. Many countries mandate workplace exposure monitoring, control, and enforcement by governmental agencies, with regional differences. A recently proposed framework to assess impacts on workers exposed along the entire supply chain provides a database to track sector-specific, empirically observed personal airborne chemical concentrations along with associated sector-specific labor hours (Kijko et al. 2015, 2016). Another approach uses reported illnesses for indoor industrial emissions (Scanlon et al. 2014). Our workshop deliberations supported the finding that both approaches provide useful starting points for extending the existing framework for LCIA but require the following additional occupational considerations: a) regionally varying occupational illnesses, accidental injuries, and indirect exposure of worker families to substances originating in the working environment (e.g., mining or farming workers); b) the additional uncertainty in exposure estimates that arise from occupational exposure pathways; and c) potential benefits of employment (e.g., access to health care and income). Health endpoints assessed for occupational injuries in the Global Burden of Disease (GBD) study (Forouzanfar et al. 2016) can provide input for these issues.
Additional fate- and exposure-related components.
Our assessment of workshop outcomes identified several additional components currently absent from characterizing human toxicity in the current LCIA framework (Figure 1) that require consideration for future inclusion. Key among these is the need for more spatial and temporal disaggregation—the current method uses nested generic archetypes (indoor, urban, continental rural, global) and parameterized archetypes for continental and subcontinental regions (Kounina et al. 2014). This limitation excludes differentiation of pulse emissions relevant for, e.g., the application of agricultural pesticides (Fantke et al. 2013) or long-term emissions relevant for, e.g., emissions from landfills (Bakas et al. 2015). Other issues are processing of food (e.g., Kaushik et al. 2009) and treatment of drinking water. A final issue is the need to account for transformation and degradation products of hazardous organic substances in the fate modeling.
We identified through workshop consensus an improvement for spatial variability that can be applied in the short term, which is increasing the set of considered archetypes to, e.g., represent individual urban areas or indoor environments, an approach now used for assessing exposure to fine particulate matter (Fantke et al. 2017). More specificity in urban and indoor environments can address variability of consumer and occupational exposures. We also found that to better capture temporal release patterns requires parameters for pulse emissions and an integration scheme for long-term emissions. The impact on exposure pathways from food processing is relevant but limited by data on food processing activities at different food product life cycle stages, including farm level (e.g., washing), manufacturing (e.g., sterilizing), or during home use (e.g., cooking).
Transformation products are relevant to a number of exposure pathways, especially during food processing, where acrylamide and polycyclic aromatic compounds can be formed (Jägerstad and Skog 2005), but the LCA context for this issue needs to be better defined. Without further research and data to track transformation product generation and when transformation products are toxicologically relevant, focus will continue to be primarily on parent compounds. Biomarkers help to track exposure patterns (Koch et al. 2014; Shin et al. 2013), but the literature on biomarker-based intake fraction calculations is currently still limited.
From Exposure to Dose–Response and Health Effects
As LCA expands to address the large number of substances found in consumer products, there will be a corresponding need for a broader set of dose–response relationships. Workshop presentations identified ongoing research in high-throughput toxicity screening that will be an important tool for addressing this need. Currently, human toxicity impacts for about 3,000 substances in LCIA are derived primarily from chronic or extrapolated acute animal toxicity studies (Rosenbaum et al. 2011). This includes the extrapolation of human cancer risk factors from a few human studies and from roughly 600 animal studies. Noncancer effects are derived from chronic animal studies, where available, and chronic benchmarks estimated from acute studies in other cases. The current starting point for LCIA dose–response modeling is the intake dose, which can eventually be related to human internal dose based on the approach of Wetmore and Thomas (2013), using high-throughput chemical toxicity screening data to define equivalent toxic human intakes. Methods such as this reverse dosimetry approach, may also be applied as a forward dosimetry (i.e., blood concentrations predicted from external doses). We considered other efforts to broaden dose–response relationships (e.g., nonlinear, nonsteady state, etc.), but they require simplifying assumptions, which raised concerns about error and uncertainty around the resulting predictions.
Dose–response shape for human toxicity effects assessment.
Current practice in LCIA toxicity assessment assumes linearity for all dose–response models and additivity of impacts (Fantke et al. 2018; Rosenbaum et al. 2008). The basis for this approach is the typical need for a marginal (rather than absolute) impact estimate in LCA. While likely acceptable for cancer effects that exhibit linearity at population scale, linearity can be questionable for other health endpoints. Recent efforts for fine particulate matter provide an example for addressing nonlinearity by calculating a marginal slope of the dose–response “at a working point,” which is the exposure that the population experiences before an addition or reduction is imposed (Frischknecht and Jolliet 2016). This approach makes the response more site specific, depending on the population vulnerability due to existing exposures.
LCIA currently builds dose–response relationships by extrapolating data from observations of adverse effects over a range of high doses, e.g., administered to rats in standardized tests, to doses in humans at lower, real-world levels (Rosenbaum et al. 2008). For some substances, such as endocrine disruptors, the rate of increase of disease with dose is steeper at low doses and subsides at higher doses (Fagin 2012). Many LCIA practitioners have expressed concern about the current LCA dose–response approach that is based on population intake instead of individual intake and uses a (linear) dose–response extrapolation to zero.
Based on input from the three workshops, it was proposed that the simplest solution to these concerns that could be implemented within the matrix-based consensus framework of Rosenbaum et al. (2008) is to add a modifier to the current linear dose–response slope factor to address potential nonlinearity. This modifier is a dimensionless term that multiplies any exposure pathway x (e.g., food ingestion) and health effect e (e.g., cancer) dose–response slope to arrive at corrected slope factors as input to matrix DRF in Equation 1.
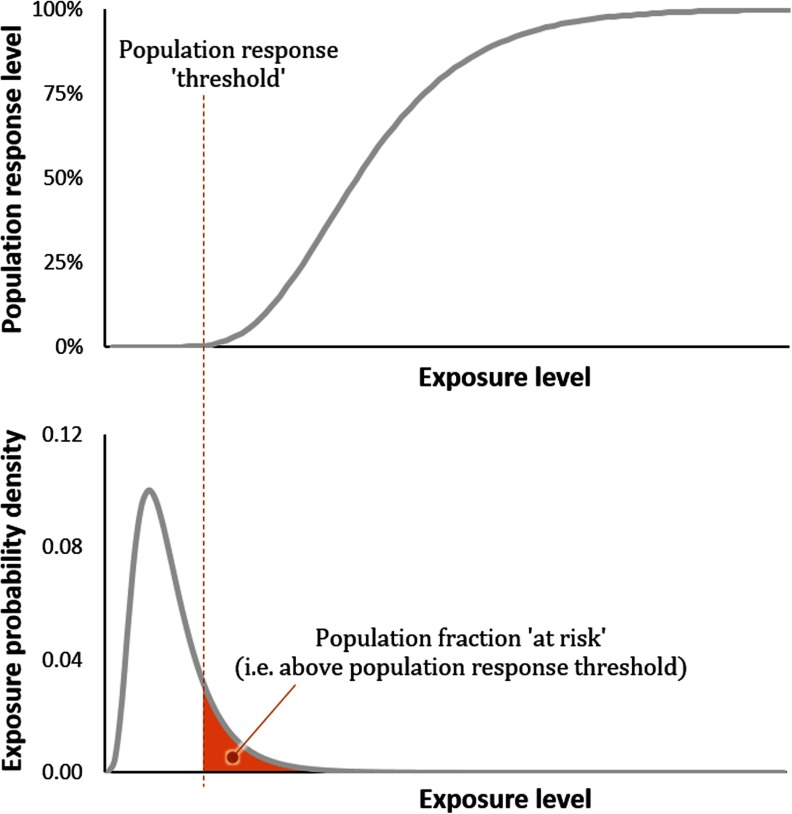
There are multiple potential methods for deriving the dose–response modifier. One method proposed at the workshops is based on the assumption that a population exposure threshold exists below which there is no response. In such case, the multiplier consists of the fraction of the exposed population that is above the population exposure threshold (and therefore “at risk”), as illustrated in Figure 4:
| (2) |
with as a default (no evidence of an exposure threshold) and , where evidence of a population-level exposure threshold is available. In principle, this modifier is the same for pathways that belong to the same exposure route (e.g., fish and drinking water ingestion are both “ingestion”).
Figure 4.
Hypothetical population effect response level as function of population exposure dose indicating that a certain fraction of the exposed population is above a defined threshold for a given exposure distribution. Units of exposure probability density are the inverse of whatever exposure level units are used on the x-axis (e.g., if exposure is in parts per million, exposure probability density has unit 1/ppm).
Population exposure thresholds reflect levels below which there is de minimis risk to any individual. Among many options, Kroes et al. (2005) proposed setting the exposure threshold at a reference dose, a dose corresponding to a cancer risk, or a threshold of toxicological concern. However, because such a population exposure threshold could be potentially derived in additional ways (e.g., essentiality level for certain metals), a significant challenge is that the size of this population can usually not be estimated. An additional concern arises from the use of different metrics as starting points for estimating population exposure thresholds for different substances, resulting in a lack of assurance that effect estimates are consistent. Another challenge is accounting for cumulative exposure to multiple chemical and other stressors that contribute to a given effect. Current approaches for deriving population exposure thresholds cannot actually predict a complete absence of risk with 100% confidence.
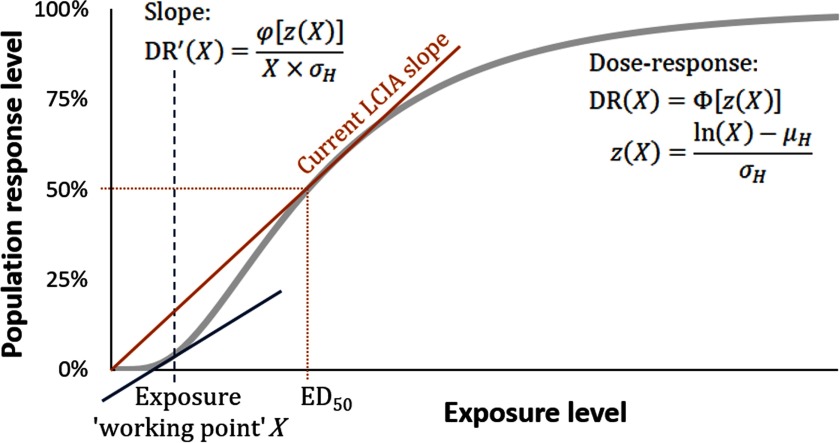
Another approach for deriving a dose–response modifier is based on a recent comprehensive framework from the World Health Organization/International Programme on Chemical Safety (WHO/IPCS) (Chiu and Slob 2015; WHO/IPCS 2014). This framework formally addresses uncertainty and variability using different shapes of dose–response and computes the distribution of the slope at any point. As shown in Figure 5, this approach assumes lognormal sensitivity to provide a dose–response for exposure pathway x and health effect e as a “threshold distribution”:
| (3) |
where X is the working point exposure level (i.e., the exposure level at which we want to evaluate the slope); is the standard normal cumulative distribution; z is the effective z-score of X; is the natural log exposure level affecting 50% of the population (estimated by scaling from experimental animal data); and is the variance of population sensitivity (estimated either from historical human data across chemicals or chemical-specific data). The slope, , which is an element of matrix DRF in Equation 1, is then given by the derivative of the cumulative distribution with respect to X with as standard normal probability density:
| (4) |
Figure 5.
Lognormal threshold distribution–based population effect response level as function of population exposure dose level (based on WHO/IPCS 2014), indicating the exposure dose–response slope as a function of the exposure working point X. is the standard normal cumulative distribution, is the standard normal probability density, is the natural log exposure at which 50% of the population is affected, and is the variance of the population sensitivity distribution. The exposure working point could be estimated directly for the chemical of interest, or alternatively, as suggested by Huijbregts et al. (2005), defined as an “effective” background corresponding to the background incidence rate of the effect of interest.
The key question in this approach is defining the working point exposure. Because LCA is focused on cumulative exposures and aggregate health impacts, a logical evaluation point of X is the background exposure level to which the new exposure is added. This approach can incorporate susceptible populations by increasing or decreasing the variance (shape) of the population dose–response curve (Zeise et al. 2013). One limitation is the increasing uncertainty about the accuracy of the lognormality of sensitivity in cases of low background exposure levels and correspondingly small incidence values (Crump et al. 2010; Pennington et al. 2002). Alternatively, one could set the working point via an “effective” background exposure at the equivalent background incidence rate (Huijbregts et al. 2005).
The field of toxicology is currently undergoing a dramatic change with the introduction of high-throughput computational in vitro mechanistic analyses (e.g., Wambaugh et al. 2015) with associated adverse outcome pathways (Ankley et al. 2010). These approaches provide a useful long-term opportunity for more biologically based derivations of appropriate, possibly nonlinear, modifiers to the current dose–response slope.
Opinions differ about the continued use of linear dose–response relationships in impact assessments. There is evidence that due to population-scale distribution in exposure, potential nonlinearity in dose–response cancels out making linear dose–response applicable to population-level responses (NRC 2009). There is also evidence that a linear dose–response for some carcinogens is likely realistic and sufficient in most cases (NRC 2009). However, for many health endpoints, current slope derivations do not reflect the sigmoidal nature of the exposure–response relationships observed for many substances. Providing dose–response relationships for LCIA toxicity characterization is an important area of continuing research to improve existing LCA practice. The WHO/IPCS framework (Chiu and Slob 2015) is a useful starting point for additional research.
Toxicity-related health effects and endpoints.
LCIA toxicity characterization currently provides two aggregated human health outcomes, cancer and noncancer effects (Rosenbaum et al. 2008), with generic severity factors for both effect types based on global health statistics (Huijbregts et al. 2005). Disaggregating further among different types of health effects within these two categories would provide greater accuracy in the toxicity assessment, but would require additional health endpoint–specific data. Workshop participants recognized this need for greater health endpoint resolution, noting particular interest in cardiovascular diseases, neurotoxicity, reproductive diseases, and endocrine diseases, while also acknowledging that additional work is needed to prioritize endpoints. The current preference for DALY as the severity metric is consistent with the GBD study series (Forouzanfar et al. 2016; Lim et al. 2012), but requires relevant severity weights for the population disease incidence (Verones et al. 2017). There is a need to explore the relevance and availability of data to include additional endpoints and more refined severity weights, for which the GBD and other international health assessment efforts can serve as a useful starting point.
Emission-to-Effect Modeling for Metals
In our workshop discussions on toxicity characterization for metals, we noted that current LCIA practice does not consider essentiality of specific substances to human health or human deficiency in or vulnerability to those substances. Some metal species that play a role in the natural metabolism of humans are essential but may become toxic above a toxicity threshold. This U-shaped dose–response, with deficiency at low dose and toxicity at high dose (e.g., for manganese, see Milton et al. 2017), poses a challenge for LCIA. Populations in several regions are deficient in certain essential metals including zinc, calcium, and iron (Forouzanfar et al. 2016; Lim et al. 2012), whereas workshop participants reported on several LCA studies that have overall toxicity scores dominated by zinc. From these deliberations, we determined that our final recommendations include further investigation of essentiality.
In current LCA, practitioners typically report toxicity (and other) characterization results for different time horizons, e.g., for infinity (steady state) and after 100 years, but current models only allow for steady-state (i.e., time-integrated) calculations (Rosenbaum et al. 2008). This can pose a considerable problem for metals and very persistent organic substances. A lack of temporal flexibility can be addressed with a dynamic multimedia modeling approach as recently proposed by Shimako et al. (2017). This approach, however, is currently not compatible with the existing steady-state matrix framework for LCIA toxicity characterization. This creates the need for an approach that is based on and fully consistent with the current modeling but capable of addressing a dynamic time horizon of 100 y.
Conclusions
In deliberations both during and after the workshops, the task force discussed and evaluated questions addressing key points summarized in Figure 2, with the goal of advancing exposure and toxicity characterization in LCIA and similar comparative assessment frameworks. Task force members developed these questions to focus future research on improving the current toxicity characterization framework in LCIA based on revised practice and the adaptation of models and data from other assessment fields. Workshop discussions, together with subsequent task force evaluations, support both general and specific recommendations. One general recommendation is to develop guidance on the presentation and interpretation of human toxicity characterization results, including a summary of important assumptions and sensitivity of outcome to these assumptions, transparent descriptions of uncertainty and its implications, and consideration of variability among populations and regions. Another general recommendation is informed application, for example, the reality check of comparing for a specific region the cumulative disease burden quantified in LCIA from multiple substances to GBD results. In addition to these general points, there are twelve specific recommendations that are summarized as follows:
The existing Rosenbaum et al. (2008) framework is a suitable starting point for further advancing and harmonizing the assessment of toxicity-related impacts in LCIA.
The combined near-field and far-field framework of Fantke et al. (2016) provides a useful operational foundation for addressing additional consumer and occupational exposure settings but requires additional research to address issues such as exposure through inhalation of suspended particles and hand-to-mouth exposure.
A limited number of archetypes can be used to distinguish exposure for different human receptor groups.
Similar to recent efforts for fine particulate matter, spatial archetypes can be used to increase geographic detail for chemical substances in a form fully compatible with existing LCIA approaches.
The current assumption of additivity of human toxicological effects remains an effective way to handle mixture toxicity when the time and location of emissions is not specified; however, when it is not appropriate to assume additivity, additional research is needed to address mixture toxicity and coexposure effects.
Effects with a strong temporal character, such as acute effects of localized pulse emissions, require further research efforts before they can be operationally included into the existing LCIA framework.
Metal species and very persistent organic substances require dynamic fate factors that can define appropriate time horizons for characterizing related human toxicity.
Food processing can significantly alter exposure and requires additional characterization efforts before incorporation in LCIA.
Because there are no operational approaches or data currently available to fully track transformation products across substances, transformation products, where significant, should currently be characterized as separate chemical substances.
To explore whether and how potential nonlinearity in the dose–response relationships can be addressed in LCIA, two concepts need additional evaluation: a) introducing a modifier for the dose–response relationship based on an assumed population threshold, and b) using recently developed WHO guidance (WHO/IPCS 2014) on population variability, extrapolation, and uncertainty in dose–response relationships and on informing the selection of population threshold distributions.
The current LCIA approach allows for considering additional endpoints of interest, such as endocrine-disrupting compounds, but there are no specific recommendations at this time.
For substances with a U-shaped dose–response relationship, benefits from essential metals and essentiality of nontoxic substances should be assessed.
We captured these summary recommendations from the task force process to propose guidance for efforts to develop a globally harmonized framework and characterization factors for incorporating human toxicity impacts. To ensure that human toxicity impacts are consistently integrated into LCIA, we recommend that the calculation methods and results be harmonized with other LCIA guidance efforts. These efforts include ecosystem toxicity characterization (e.g., the selection of benchmark doses, such as hazardous concentrations), human health impacts from exposure to fine particulate matter (e.g., the selection of considered fate processes), other chemical and nonchemical stressors (e.g., radiation, heat, noise, and accidents), and nutritional status and impacts. We presented and revised the harmonized characterization framework, its related results, and corresponding global guidance at a Pellston expert workshop in summer 2018.
Supplementary Material
Acknowledgments
We thank all participants of the Utrecht Guidance Workshop, the Brussels Metals Workshop, and the RTP Guidance Workshop for their input, and W. Setzer and J. Marshall for their contribution to an earlier manuscript version. W.A.C. was supported, in part, by National Institutes of Health (NIH) grant P42 ES027704.
References
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29(3):730–741, PMID: 20821501, 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Bakas I, Hauschild MZ, Astrup TF, Rosenbaum RK. 2015. Preparing the ground for an operational handling of long-term emissions in LCA. Int J Life Cycle Assess 20(10):1444–1455, 10.1007/s11367-015-0941-4. [DOI] [Google Scholar]
- Bennett DH, McKone TE, Evans JS, Nazaroff WW, Margni MD, Jolliet O, et al. 2002. Defining intake fraction. Environ Sci Technol 36(9):207A–211A, PMID: 12026996, 10.1021/es0222770. [DOI] [PubMed] [Google Scholar]
- Breivik K, Arnot JA, Brown TN, McLachlan MS, Wania F. 2012. Screening organic chemicals in commerce for emissions in the context of environmental and human exposure. J Environ Monit 14(8):2028–2037, PMID: 22785348, 10.1039/c2em30259d. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Slob W. 2015. A unified probabilistic framework for dose-response assessment of human health effects. Environ Health Perspect 123(12):1241–1254, PMID: 26006063, 10.1289/ehp.1409385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciffroy P, Alfonso B, Altenpohl A, Banjac Z, Bierkens J, Brochot C, et al. 2016. Modelling the exposure to chemicals for risk assessment: a comprehensive library of multimedia and PBPK models for integration, prediction, uncertainty and sensitivity analysis - the MERLIN-Expo tool. Sci Total Environ 568:770–784, PMID: 27169730, 10.1016/j.scitotenv.2016.03.191. [DOI] [PubMed] [Google Scholar]
- Crump KS, Chiu WA, Subramaniam RP. 2010. Issues in using human variability distributions to estimate low-dose risk. Environ Health Perspect 118(3):387–393, PMID: 20064772, 10.1289/ehp.0901250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar SA, Ernstoff AS, Fantke P, Jolliet O. 2017. Stochastic modeling of near-field exposure to parabens in personal care products. J Expo Sci Environ Epidemiol 27(2):152–159, PMID: 26758569, 10.1038/jes.2015.85. [DOI] [PubMed] [Google Scholar]
- Demou E, Hellweg S, Wilson MP, Hammond SK, McKone TE. 2009. Evaluating indoor exposure modeling alternatives for LCA: a case study in the vehicle repair industry. Environ Sci Technol 43(15):5804–5810, PMID: 19731680, 10.1021/es803551y. [DOI] [PubMed] [Google Scholar]
- Ernstoff AS, Fantke P, Csiszar SA, Henderson AD, Chung S, Jolliet O. 2016. Multi-pathway exposure modelling of chemicals in cosmetics with application to shampoo. Environ Int 92–93:87–96, PMID: 27062422, 10.1016/j.envint.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Eurometaux (European Association of Metals). 2014. “Leuven Workshop on Environmental and Human Toxicity of metals in LCA: Status, Limitations and New Developments.” Leuven, Belgium: https://www.usetox.org/resources/Eurometaux_2014a.pdf [Accessed 20 April 2018]. [Google Scholar]
- Fagin D. 2012. Toxicology: the learning curve. Nature 490(7421):462–465, PMID: 23099381, 10.1038/490462a. [DOI] [PubMed] [Google Scholar]
- Fantke P, Ernstoff AS, Huang L, Csiszar SA, Jolliet O. 2016. Coupled near-field and far-field exposure assessment framework for chemicals in consumer products. Environ Int 94:508–518, PMID: 27318619, 10.1016/j.envint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Fantke P, Jolliet O, Evans JS, Apte JS, Cohen AJ, Hänninen OO, et al. 2015a. Health effects of fine particulate matter in life cycle impact assessment: conclusions from the Basel guidance workshop. Int J Life Cycle Assess 20(2):276–288, 10.1007/s11367-014-0822-2. [DOI] [Google Scholar]
- Fantke P, Jolliet O, Apte JS, Hodas N, Evans J, Weschler CJ, et al. 2017. Characterizing aggregated exposure to primary particulate matter: recommended intake fractions for indoor and outdoor sources. Environ Sci Technol 51(16):9089–9100, PMID: 28682605, 10.1021/acs.est.7b02589. [DOI] [PubMed] [Google Scholar]
- Fantke P, Weber R, Scheringer M. 2015b. From incremental to fundamental substitution in chemical alternatives assessment. Sustain Chem Pharm 1:1–8, 10.1016/j.scp.2015.08.001. [DOI] [Google Scholar]
- Fantke P, Wieland P, Wannaz C, Friedrich R, Jolliet O. 2013. Dynamics of pesticide uptake into plants: from system functioning to parsimonious modeling. Environ Model Softw 40:316–324, 10.1016/j.envsoft.2012.09.016. [DOI] [Google Scholar]
- Fantke P, Aurisano N, Bare J, Backhaus T, Bulle C, Chapman PM, et al. 2018. Toward harmonizing ecotoxicity characterization in life cycle impact assessment. Environ Toxicol Chem 37(12):2955–2971, PMID: 30178491, 10.1002/etc.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantke P, Ernstoff A. 2018. LCA of chemicals and chemical products. In: Life Cycle Assessment: Theory and Practice . Hauschild M, Rosenbaum RK, Olsen SI, eds. Dordrecht, Netherlands:Springer International Publishing, 783–815. [Google Scholar]
- Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1659–1724, PMID: 27733284, 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Fantke P, Tschümperlin L, Niero M, Antón A, Bare J, et al. 2016. Global guidance on environmental life cycle impact assessment indicators: progress and case study. Int J Life Cycle Assess 21(3):429–442, 10.1007/s11367-015-1025-1. [DOI] [Google Scholar]
- Frischknecht R, Jolliet O. 2016. Global Guidance for Life Cycle Impact Assessment Indicators, Vol. 1. Paris, France:UNEP/SETAC Life Cycle Initiative. [Google Scholar]
- Hauschild MZ, Huijbregts M, Jolliet O, Macleod M, Margni MD, van de Meent D, et al. 2008. Building a model based on scientific consensus for life cycle impact assessment of chemicals: the search for harmony and parsimony. Environ Sci Technol 42(19):7032–7037, PMID: 18939523, 10.1021/es703145t. [DOI] [PubMed] [Google Scholar]
- Hauschild M, Rosenbaum R, Olsen SI. 2018. Life Cycle Assessment: Theory and Practice. Dordrecht, Netherlands:Springer International Publishing. [Google Scholar]
- Hendrickson CT, Lave LB, Matthews HS. 2006. Environmental Life Cycle Assessment of Goods and Services: An Input-Output Approach. Washington, DC:Routledge. [Google Scholar]
- Hodas N, Loh M, Shin H-M, Li D, Bennett D, McKone TE, et al. 2016. Indoor inhalation intake fractions of fine particulate matter: review of influencing factors. Indoor Air 26(6):836–856, PMID: 26562829, 10.1111/ina.12268. [DOI] [PubMed] [Google Scholar]
- Huang L, Ernstoff A, Fantke P, Csiszar S, Jolliet O. 2017. A review of models for near-field exposure pathways of chemicals in consumer products. Sci Total Environ 574:1182–1208, PMID: 27644856, 10.1016/j.scitotenv.2016.06.118. [DOI] [PubMed] [Google Scholar]
- Huijbregts MAJ, Rombouts LJA, Ragas AMJ, van de Meent D. 2005. Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic chemicals for life cycle impact assessment. Integr Environ Assess Manag 1(3):181–244, PMID: 16639884, 10.1897/2004-007R.1. [DOI] [PubMed] [Google Scholar]
- Humbert S, Marshall JD, Shaked S, Spadaro JV, Nishioka Y, Preiss P, et al. 2011. Intake fraction for particulate matter: recommendations for life cycle impact assessment. Environ Sci Technol 45(11):4808–4816, PMID: 21563817, 10.1021/es103563z. [DOI] [PubMed] [Google Scholar]
- Isaacs KK, Glen WG, Egeghy P, Goldsmith M-R, Smith L, Vallero D, et al. 2014. SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ Sci Technol 48(21):12750–12759, PMID: 25222184, 10.1021/es502513w. [DOI] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization). 2006. “Environmental Management - Life Cycle Assessment - Principles and Framework.” ISO 14040 International Standard. Geneva, Switzerland:ISO. [Google Scholar]
- Jägerstad M, Skog K. 2005. Genotoxicity of heat-processed foods. Mutat Res 574(1–2):156–172, PMID: 15914214, 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Jolliet O, Ernstoff AS, Csiszar SA, Fantke P. 2015. Defining product intake fraction to quantify and compare exposure to consumer products. Environ Sci Technol 49(15):8924–8931, PMID: 26102159, 10.1021/acs.est.5b01083. [DOI] [PubMed] [Google Scholar]
- Jolliet O, Frischknecht R, Bare J, Boulay A-M, Bulle C, Fantke P, et al. 2014. Global guidance on environmental life cycle impact assessment indicators: findings of the scoping phase. Int J Life Cycle Assess 19(4):962–967, 10.1007/s11367-014-0703-8. [DOI] [Google Scholar]
- Jolliet O, Rosenbaum R, McKone TE, Scheringer M, van Straalen N, Wania F, et al. 2006. Establishing a framework for life cycle toxicity assessment: findings of the Lausanne Review Workshop. Int J Life Cycle Assessment 11(3):209–212, 10.1065/lca2006.03.002. [DOI] [Google Scholar]
- Jolliet O, Antón A, Boulay A-M, Cherubini F, Fantke P, Levasseur A, et al. 2018. Global guidance on environmental life cycle impact assessment indicators: impacts of climate change, fine particulate matter formation, water consumption and land use. Int J Life Cycle Assess 23(11):2189–2207, 10.1007/s11367-018-1443-y. [DOI] [Google Scholar]
- Kaushik G, Satya S, Naik SN. 2009. Food processing a tool to pesticide residue dissipation - a review. Food Res Int 42(1):26–40, 10.1016/j.foodres.2008.09.009. [DOI] [Google Scholar]
- Kijko G, Jolliet O, Margni M. 2016. Occupational health impacts due to exposure to organic chemicals over an entire product life cycle. Environ Sci Technol 50(23):13105–13114, PMID: 27794595, 10.1021/acs.est.6b04434. [DOI] [PubMed] [Google Scholar]
- Kijko G, Margni M, Partovi-Nia V, Doudrich G, Jolliet O. 2015. Impact of occupational exposure to chemicals in life cycle assessment: a novel characterization model based on measured concentrations and labor hours. Environ Sci Technol 49(14):8741–8750, PMID: 26079305, 10.1021/acs.est.5b00078. [DOI] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, et al. 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: personal care product ingredients. Toxicol Lett 231(2):261–269, PMID: 24956590, 10.1016/j.toxlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Kounina A, Margni M, Shaked S, Bulle C, Jolliet O. 2014. Spatial analysis of toxic emissions in LCA: a sub-continental nested USEtox model with freshwater archetypes. Environ Int 69:67–89, PMID: 24815341, 10.1016/j.envint.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Kroes R, Kleiner J, Renwick A. 2005. The threshold of toxicological concern concept in risk assessment. Toxicol Sci 86(2):226–230, PMID: 15829616, 10.1093/toxsci/kfi169. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, et al. 2018. The Lancet Commission on pollution and health. Lancet 391(10119):462–512, PMID: 29056410, 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Li L, Arnot JA, Wania F. 2018. Towards a systematic understanding of the dynamic fate of polychlorinated biphenyls in indoor, urban and rural environments. Environ Int 117:57–68, PMID: 29727753, 10.1016/j.envint.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Li L, Wania F. 2016. Tracking chemicals in products around the world: introduction of a dynamic substance flow analysis model and application to PCBs. Environ Int 94:674–686, PMID: 27431909, 10.1016/j.envint.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260, PMID: 23245609, 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan GE, Millage KK, Asgharian B. 2016. An improved model of human response to bioaerosol exposure. In: Aerobiology: The Toxicology of Airborne Pathogens and Toxins. Salem H, Katz S, eds. Cambridge, UK:The Royal Society of Chemistry, 400–444. [Google Scholar]
- McKone TE, Kyle AD, Jolliet O, Olsen SO, Hauschild MZ. 2006. Dose-response modeling for life cycle impact assessment: findings of the Portland review workshop. Int J Life Cycle Assessment 11(2):137–141, 10.1065/lca2006.02.005. [DOI] [Google Scholar]
- McLachlan MS, Kierkegaard A, Radke M, Sobek A, Malmvärn A, Alsberg T, et al. 2014. Using model-based screening to help discover unknown environmental contaminants. Environ Sci Technol 48(13):7264–7271, PMID: 24869768, 10.1021/es5010544. [DOI] [PubMed] [Google Scholar]
- Milton B, Krewski D, Mattison DR, Karyakina NA, Ramoju S, Shilnikova N, et al. 2017. Modeling U-shaped dose-response curves for manganese using categorical regression. Neurotoxicol 58:217–225, PMID: 27720796, 10.1016/j.neuro.2016.10.001. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC:The National Academies Press. [PubMed] [Google Scholar]
- Pennington D, Crettaz P, Tauxe A, Rhomberg L, Brand K, Jolliet O. 2002. Assessing human health response in life cycle assessment using ED10s and DALYs: part 2—noncancer effects. Risk Anal 22(5):947–963, PMID: 12442991, 10.1111/1539-6924.00263. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RK, Bachmann TM, Gold LS, Huijbregts MAJ, Jolliet O, Juraske R, et al. 2008. USEtox - the UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 13(7):532–546, 10.1007/s11367-008-0038-4. [DOI] [Google Scholar]
- Rosenbaum RK, Huijbregts MAJ, Henderson AD, Margni M, McKone TE, van de Meent D, et al. 2011. USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: Sensitivity to key chemical properties. Int J Life Cycle Assess 16(8):710–727, 10.1007/s11367-011-0316-4. [DOI] [Google Scholar]
- Rosenbaum RK, Meijer A, Demou E, Hellweg S, Jolliet O, Lam NL, et al. 2015. Indoor air pollutant exposure for life cycle assessment: regional health impact factors for households. Environ Sci Technol 49(21):12823–12831, PMID: 26444519, 10.1021/acs.est.5b00890. [DOI] [PubMed] [Google Scholar]
- Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. 2015. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 3(11):e712–e723, PMID: 26475018, 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- Scanlon KA, Lloyd SM, Gray GM, Francis RA, LaPuma P. 2014. An approach to integrating occupational safety and health into life cycle assessment: development and application of a work environment characterization factor. J Ind Ecol 19(1):27–37, 10.1111/jiec.12146. [DOI] [Google Scholar]
- Shimako AH, Tiruta-Barna L, Ahmadi A. 2017. Operational integration of time dependent toxicity impact category in dynamic LCA. Sci Total Environ 599–600:806–819, PMID: 28499229, 10.1016/j.scitotenv.2017.04.211. [DOI] [PubMed] [Google Scholar]
- Shin HM, Ernstoff AS, Arnot JA, Wetmore B, Csiszar SA, Fantke P, et al. 2015. Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ Sci Technol 49(11):6760–6771, PMID: 25932772, 10.1021/acs.est.5b00498. [DOI] [PubMed] [Google Scholar]
- Shin HM, McKone TE, Bennett DH. 2013. Evaluating environmental modeling and sampling data with biomarker data to identify sources and routes of exposure. Atmos Environ 69:148–155, 10.1016/j.atmosenv.2012.12.027. [DOI] [Google Scholar]
- Shin H, McKone TE, Bennett DH. 2017. Model framework for integrating multiple exposure pathways to chemicals in household cleaning products. Indoor Air 27(4):829–839, PMID: 27859724, 10.1111/ina.12356. [DOI] [PubMed] [Google Scholar]
- Tao M, Li D, Song R, Suh S, Keller AA. 2018. OrganoRelease - a framework for modeling the release of organic chemicals from the use and post-use of consumer products. Environ Pollut 234:751–761, PMID: 29245149, 10.1016/j.envpol.2017.11.058. [DOI] [PubMed] [Google Scholar]
- Verones F, Bare J, Bulle C, Frischknecht R, Hauschild M, Hellweg S, et al. 2017. LCIA framework and cross-cutting issues guidance within the UNEP-SETAC Life Cycle Initiative. J Cleaner Prod 161:957–967, 10.1016/j.jclepro.2017.05.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, et al. 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ Sci Technol 48(21):12760–12767, PMID: 25343693, 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Wetmore BA, Pearce R, Strope C, Goldsmith R, Sluka JP, et al. 2015. Toxicokinetic triage for environmental chemicals. Toxicol Sci 147(1):55–67, PMID: 26085347, 10.1093/toxsci/kfv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannaz C, Fantke P, Jolliet O. 2018a. Multiscale spatial modeling of human exposure from local sources to global intake. Environ Sci Technol 52(2):701–711, PMID: 29249158, 10.1021/acs.est.7b05099. [DOI] [PubMed] [Google Scholar]
- Wannaz C, Fantke P, Lane J, Jolliet O. 2018b. Source-to-exposure assessment with the Pangea multi-scale framework - case study in Australia. Environ Sci Process Impacts 20(1):133–144, PMID: 29261193, 10.1039/C7EM00523G. [DOI] [PubMed] [Google Scholar]
- Westh TB, Hauschild MZ, Birkved M, Jørgensen MS, Rosenbaum RK, Fantke P. 2015. The USEtox story: a survey of model developer visions and user requirements. Int J Life Cycle Assess 20(2):299–310, 10.1007/s11367-014-0829-8. [DOI] [Google Scholar]
- Wetmore BA, Thomas RS. 2013. Incorporating human dosimetry and exposure information with high-throughput screening data in chemical toxicity assessment. In: High-Throughput Screening Methods in Toxicity Testing. Steinberg P, ed. Hoboken, NJ:John Wiley and Sons, 77–95. [Google Scholar]
- WHO/IPCS 2014. Guidance Document on Evaluating and Expressing Uncertainty in Hazard Characterization. Geneva, Switzerland:World Health Organization; http://apps.who.int/iris/bitstream/10665/259858/1/9789241513548-eng.pdf [accessed 30 June 2018]. [Google Scholar]
- Xue J, Zartarian V, Moya J, Freeman N, Beamer P, Black K, et al. 2007. A meta-analysis of children's hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal 27(2):411–420, PMID: 17511707, 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Zeise L, Bois FY, Chiu WA, Hattis D, Rusyn I, Guyton KZ. 2013. Addressing human variability in next-generation human health risk assessments of environmental chemicals. Environ Health Perspect 121(1):23–31, PMID: 23086705, 10.1289/ehp.1205687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JB, Anastas PT. 2015. Chemistry. Toward substitution with no regrets. Science 347(6227):1198–1199, PMID: 25766217, 10.1126/science.aaa0812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.