Abstract
Background:
Until recently, environmental factors in autism spectrum disorder (ASD) were largely ignored. Over the last decade, altered risks from lifestyle, medical, chemical, and other factors have emerged through various study designs: whole population cohorts linked to diagnostic and/or exposure-related databases, large case–control studies, and smaller cohorts of children at elevated risk for ASD.
Objectives:
This study aimed to introduce the MARBLES (Markers of Autism Risk in Babies—Learning Early Signs) prospective study and its goals, motivate the enhanced-risk cohort design, describe protocols and main exposures of interest, and present initial descriptive results for the study population.
Methods:
Families having one or more previous child with ASD were contacted before or during a pregnancy, and once the woman became pregnant, were invited to enroll. Data and biological samples were collected throughout pregnancy, at birth, and until the child’s third birthday. Neurodevelopment was assessed longitudinally. The study began enrolling in 2006 and is ongoing.
Results:
As of 30 June 2018, 463 pregnant mothers have enrolled. Most mothers () were thirty years of age or over, including 7.9% who are fourty years of age or over. The sample includes 22% Hispanic and another 25% nonHispanic Black, Asian, or multiracial participants; 24% were born outside the United States. Retention is high: 84% of participants whose pregnancies did not end in miscarriage completed the study or are still currently active. Among children evaluated at 36 months of age, 24% met criteria for ASD, and another 25% were assessed as nonASD nontypical development.
Conclusion:
Few environmental studies of ASD prospectively obtain early-life exposure measurements. The MARBLES study fills this gap with extensive data and specimen collection beginning in pregnancy and has achieved excellent retention in an ethnically diverse study population. The 24% familial recurrence risk is consistent with recent reported risks observed in large samples of siblings of children diagnosed with ASD. https://doi.org/10.1289/EHP535
Introduction
As recently as 2005, very few environmental factors had been studied in relation to risk for autism or related disorders. A substantial new body of literature suggests that noninherited factors, primarily from the prenatal period, are associated with altered risks for autism spectrum disorder (ASD). After a flurry of ecologic studies with inadequate control for group-level confounders, well-conducted epidemiologic studies emerged with intriguing results on several types of exposures: increased ASD risk associated with prenatal exposures to pesticides (Eskenazi et al. 2007; Roberts et al. 2007; Shelton et al. 2014) or sources of air pollution (Becerra et al. 2013; Raz et al. 2014; Suades-González et al. 2015; Volk et al. 2012; Windham et al. 2006; Yang et al. 2017); a protective relationship from maternal nutrition (Schmidt et al. 2011; Schmidt et al. 2012; Surén et al. 2013) (Wang et al. 2017); a U-shaped relationship with higher risk from interpregnancy intervals in the range of 0–24 and (Durkin et al. 2015) (Cheslack-Postava et al. 2014; Coo et al. 2015; Zerbo et al. 2015); and an elevated risk for children whose mothers experienced infection, fever, metabolic or inflammatory conditions during pregnancy (Atladóttir et al. 2010a, 2012; Krakowiak et al. 2012; Zerbo et al. 2012) (Hornig et al. 2018; Li et al. 2016; Walker et al. 2015); to name a few.
Various designs have proven useful in pursuit of etiologic factors. Whole population cohorts in Scandinavia, California, and New York assembled by linking birth records to developmental diagnoses reported links between ASD and maternal and paternal age (Quinlan et al. 2015; Sandin et al. 2015; Shelton et al. 2010), interpregnancy interval (Cheslack-Postava et al. 2014), maternal but not childhood infection (Atladóttir et al. 2010a, b), and season of conception (Zerbo et al. 2011). These databases also provided the sampling frames for nested case–control designs used to investigate pesticide exposures (Roberts et al. 2007) and air pollution (Becerra et al. 2013). Large sample sizes (thousands to millions of persons; hundreds to tens of thousands with ASD) and complete population enumeration are strengths of these cohorts; however, data are limited or absent for some exposures and potential confounders (e.g., smoking, nutrition, and medical or other factors); standardized confirmation of diagnoses is lacking; diagnostic practices may vary across regions within each database; and generally, biological mechanisms cannot be examined.
Case–control studies have played a major role in both genetic and environmental epidemiology of ASD. The University of California Davis (UC Davis) CHARGE (Childhood ASD Risks from Genetics and Environment) Study, a population-based case–control study nested within California birth cohorts, was launched in 2002 to investigate causes and contributing factors to ASD and developmental delay (DD) (Hertz-Picciotto et al. 2006). Findings from the CHARGE study include evidence of reduced ASD risk in association with maternal prenatal vitamin supplementation during the periconception period (Schmidt et al. 2011, 2012), and evidence of increased risk in association with residential proximity to agricultural pesticide applications (Shelton et al. 2014) or traffic-related and regional air pollution (Volk et al. 2012), parental occupational exposures (McCanlies et al. 2012), maternal metabolic conditions (e.g., obesity) (Krakowiak et al. 2012), preeclampsia (Walker et al. 2015), and fever that went untreated during pregnancy (Zerbo et al. 2012). CHARGE also identified immune dysregulation (Ashwood and Van de Water 2004; Braunschweig et al. 2013; Enstrom et al. 2010) and mitochondrial dysfunction (Giulivi et al. 2010) and mitochondrial DNA damage (Napoli et al. 2013, 2014) as potential underlying mechanisms. The CHARGE Study has also provided population-based data on ASD comorbidities previously reported primarily in clinic-based samples that may overrepresent such conditions; these conditions included an excess of GI symptoms (Chaidez et al. 2014), sleep disturbances (Krakowiak et al. 2008), and minor physical anomalies (Angkustsiri et al. 2011). With each identified environmental, biological, or phenotypic difference between cases and typically developing controls of the same age, the key question was: At what point in early development did the ASD cases diverge from their neurotypically developing counterparts? The case–control design could not answer this question. For example, about 20% of mothers of children with ASD produced antibodies to fetal brain proteins, in comparison with fewer than 2% of mothers of children with typical development (TD) (Braunschweig et al. 2013), but whether this antibody status arose during the pregnancy with the affected child could not be determined. Advancing the field beyond description of differences to investigating etiologic factors, mechanisms, and susceptible windows became an imperative. Thus, the logic of an enriched-risk cohort with enrollment prior to birth emerged organically.
The enriched-risk design is well suited to the study of ASD, given its relatively low prevalence (currently 1 in 59) (Baio et al. 2018). In comparison with a general population cohort, enrollment and follow-up of pregnant women carrying the younger sibling of a child with ASD achieves tremendous efficiencies. This enhanced statistical power is a result of family recurrence risks of ASD, which have long been known to be far greater than incidence in the overall population (Fombonne et al. 1997; Lauritsen et al. 2005). The MARBLES (Markers of Autism Risk in Babies—Learning Early Signs) Study was therefore launched in 2006 by the University of California Davis (UC Davis) Medical Investigations of Neurodevelopmental Disorders (MIND) Institute and Center for Children’s Environmental Health. Shortly thereafter, the similarly designed but multisite Early ASD Risk Longitudinal Investigation was initiated (EARLI) (Newschaffer et al. 2012). Although this design was not new, previous cohorts of younger siblings at high risk for ASD had recruited postnatally and did not address environmental etiology. However, five years after the MARBLES study began recruitment, a pooled analysis of twelve ‘baby sib cohorts’ produced an estimated recurrence risk of 19% in affected families (Ozonoff et al. 2011). Given a ratio of noncases to cases in the high-risk cohort of , in comparison with the population-based ratio at that time of 67:1, the younger sibling approach yielded a 13-fold greater risk.
Contrasting with case–control studies, where the greatest challenge is obtaining accurate exposure information from time periods prior to the onset of symptoms, the MARBLES study collects data prospectively. Effort is concentrated during vulnerable periods: preconception, gestation and infancy. With enrollment before or during pregnancy, monitoring of pregnancy and early childhood years provides an opportunity to accurately measure suspect exposures (both exogenous and endogenous), and simultaneously allows a broad search for early biological and environmental markers (Newschaffer et al. 2012).
This paper describes the design, aims, and protocols of the MARBLES study, and presents preliminary statistics describing the demographics of the study population and the ASD recurrence rate.
Aims
The overarching goals of the MARBLES study are to uncover environmental etiological agents contributing to ASD, investigate their mechanisms of action, and identify early biological markers of elevated risk. Using an integrated approach that includes exposure measurements, biological markers from specimens obtained in the study, and experimental research on tissues not obtained from MARBLES study participants (P01-ES011269; R01-ES020392), our initial aims were: a) to determine whether in utero or infant exposures to pyrethroid pesticides or polybrominated diphenyl ethers (PBDEs) were associated with ASD diagnosis or early indicators of developmental delays; b) to experimentally describe neurotoxicity within these chemical classes; c) to evaluate associations of immune responses with both the environmental chemicals and the neurodevelopmental outcomes; and d) to examine alterations in mitochondrial DNA for relationships with both child outcomes and chemical exposures. The second aim (b) differs from the others in its focus on in vitro neurotoxic outcomes. These molecular toxicology experiments exposed mouse neural cells in vitro to pyrethroids to define the structure–activity relationships for specific compounds (Cao et al. 2014; Kim et al. 2011).
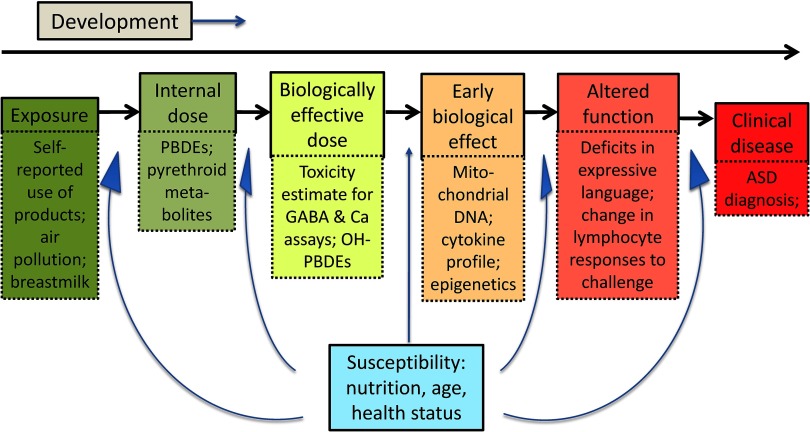
A conceptual framework underlying this study is shown in Figure 1, with the upstream environmental exposures on the left, biological changes in the middle, and clinical outcomes on the right; the examples of each type of variable include many that are measured in the MARBLES study. Additional work recently completed or currently underway is to examine phthalate exposures in relation to ASD and other developmental outcomes (R21ES025551); placental DNA methylation and other epigenetic markers (R01ES029213, DOD AR110194); maternal nutrition (R01ES025574); mitochondrial aberrations (R21HD086769), and the intestinal microbiome (R01ES028089) and builds the MARBLES study to maintain the cohort and facilitate data and sample sharing (R24ES028533). The cohort design provides an efficient framework for assessing, in the future, a broad array of compounds, lifestyle factors, mechanisms, nonchemical stressors, biomarkers, and interactions.
Figure 1.
Conceptual overview of environmental influence on health and development. The upper part of each box indicates a category of measurement, progressing from external exposure to internal dose, to the effective dose based on what reaches the target site—organ or specific tissue/region. If the dose is sufficient, it may result in an early biological effect, which, if strong or persistent enough over time, may lead to altered function. Finally, functional deficits may progress to an outright disease or diagnosable condition. For each box, one or more examples is given, to illustrate the types of measurements that may be feasible in a prospective pregnancy cohort that follows children postnatally, highlighting a few of the variables being assessed in the MARBLES study.
Methods
Study Design
The MARBLES study is a prospective observational study, launched in 2006, that enrolls women who already have a child diagnosed with an ASD and who are pregnant or planning an additional pregnancy. We also enrolled pregnant women whose offspring would be either a half-sibling, or an equivalent or closer blood relative, to a child with ASD. The MARBLES study follows the mother and the child(ren) longitudinally to accurately characterize the early life exposures both pre- and postnatally, to measure prediagnostic biological markers, and to document the development of the child to 36 months of age. Prospective collections encompass: a) extensive lifestyle, home environmental information and samples, behavioral, medical, nutritional and other exposure data; b) biological specimens from the mother, father, and children; and c) detailed psychometric assessments of neurocognitive, social, and behavioral development from birth to 3 years of age.
Study Population, Eligibility, and Recruitment
The study population for MARBLES is defined as mothers who already have a child with autism and who are pregnant or planning a pregnancy. We recruit primarily through the California Department of Developmental Services (DDS), which is the state agency that coordinates services for persons with developmental disabilities, regardless of race, religion, citizenship, or financial status. Estimates from the early 2000s indicate that about 75–80% of affected children are enrolled in DDS services (Croen et al. 2002). Milder cases will be underrepresented because of the state requirement beginning in 2003 that those individuals receiving services must have three significant functional limitations. The UC Davis study team periodically obtains an updated list of families receiving state-funded services for a child with an ASD. Families on the DDS list are mailed a letter notifying them that the MARBLES study will be contacting them, unless they opt out by calling or emailing their response. If, within two weeks, they do not opt out, study staff members call the family and screen for eligibility. The MARBLES study also enrolls a small percent of families who are referred by other research studies at the UC Davis MIND Institute or by health and service providers, or who learn about the study at outreach events, or by word of mouth.
Inclusion criteria.
The inclusion criteria for the full study are: a) mother or father has one or more biological child(ren) with ASD and/or the gestating younger child has an older half-sibling, or an equivalent or closer blood relative, with ASD; b) mother is 18 y or older; c) mother is pregnant; d) mother speaks, reads, and understands English sufficiently to complete the protocol (see Supplemental Material, “Understanding English and Mental Competence” for details) and the younger sibling will be taught to speak English; e) mother lives within 2.5 h of the Davis/Sacramento region at time of enrollment. As part of the recruitment screening phone call, study staff members obtain verbal consent and administer the Social-Communication Questionnaire (SCQ) to confirm the first criterion, i.e., a diagnosis of ASD in the proband (older sibling). Staff members also request the report from the proband’s prior diagnoses, and in particular we seek ASD Diagnostic Observation Schedule (ADOS) scores. If no ADOS has been done or if no scores were included in the report, we schedule a visit to administer the ADOS to the older sibling, for diagnostic confirmation. Ideally, all of the probands would have ADOS scores; however, in a few cases the family did not come in and we defaulted to the SCQ to confirm.
Women who have one or more biological children with ASD but who are not pregnant, are pre-menopausal, and have no known biological condition that would prevent pregnancy, are designated as “active, pre-pregnant” for as long as they are not using contraception and are in a sexual relationship with a nonsterile male partner or are planning assisted reproduction. These women are contacted monthly or bimonthly to check on their pregnancy status. We conduct home visits for a subset of prepregnant women to collect specimens and related blood draw and stress forms (Table 1). Women who could become pregnant but are currently using contraception or not in a sexual relationship with a male partner are scheduled for less frequent recruitment calls ranging from 1 month to 1 y, based on their likelihood of becoming pregnant.
Table 1.
Collection of specimens and Exposure-Related data by time point.
| Specimen or Data | Preconception | Prenatal (trimester) | Perinatal | Postnatal | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | Labor/Delivery | 2wk | 3mo | 6mo | 12mo | 24mo | 36mo | ||
| Maternal | |||||||||||
| Blood and blood draw form | xa | xa | xa | x | x | xb | xb | xb | x | ||
| Urine | xa | xa | xa | x | xb | xb | xb | xb | xb | xb | |
| Hair | xa | xa | x | x | |||||||
| Saliva | xa | xa | xa | x | x | x | |||||
| Vaginal secretions | xa | xa | x | x | |||||||
| Breast milk | xb | xb | xb | xb | xb | xb | |||||
| Exposure diariesc | weeklya | weeklya | weeklya | monthly | monthly | monthly | quarterly | quarterly | |||
| 24h food recallsd | xa | xa | x | x | x | x | x | x | x | ||
| 48h product used | xa | xa | x | x | x | x | x | x | x | ||
| Maternal Block FFQ | xa | x | xe | ||||||||
| EEQ: Current Pregnancyf | xa | xa | x | xg | |||||||
| EEQ: Proband Pregnancyf | xh | ||||||||||
| Younger Sibling | |||||||||||
| Cord blood | x | ||||||||||
| Placenta | x | ||||||||||
| Meconium | x | ||||||||||
| Blood and blood draw form | x | x | x | x | x | ||||||
| Urine | x | x | x | x | x | x | |||||
| Hair | xi | xi | x | x | |||||||
| Saliva | xi | xi | x | x | x | x | x | ||||
| Head circumference | x | x | x | x | x | x | |||||
| Dysmorphology pictures | x | x | x | x | x | x | |||||
| EEQ: postnatal | x | xg | |||||||||
| Diet & supplements | x | ||||||||||
| Paternal | |||||||||||
| Saliva | xj | ||||||||||
| Proband (affected older sibling) | |||||||||||
| Saliva | xj | ||||||||||
| Environmental Exposure | |||||||||||
| Home walkthrough questionnairek | xm | xm | x | x | |||||||
| Dust sample | x | x | |||||||||
Note: EEQ, Environmental Exposure Questionnaire; FFQ, Food Frequency Questionnaire.
If possible, depending on timing of enrollment.
If breastfeeding.
Mother completes Weekly Symptom Diary during pregnancy, Monthly Diary for Babies & Breastfeeding Mothers during the first year, and Quarterly Diary for Children over 1 Year.
Mother completes food and product use recalls to coincide with urine collections. These pertain to her own exposures during the prenatal period, to the younger sibling during the postnatal period, and to her own postnatal exposures if/when breastfeeding.
Administered when mother stops breastfeeding, regardless of when that is, and covers the entire breastfeeding period.
Telephone administered.
Completed at 30 months postpartum.
Environmental exposure questionnaire for the pregnancy with the affected older sibling is completed once by the mother during pregnancy with the younger sibling, timing based on enrollment.
Completed once at the hospital following delivery or at the 2-week postpartum visit.
Collected once at first visit when father/proband are present.
Completed or collected by staff, with additional home walkthrough questions answered by the mother.
Completed once in the first year—generally at the earliest time point during infancy when the baby has hair.
Completed once in pregnancy.
Preenrollment.
Before the first home visit, mothers receive a welcome packet with a detailed description of the study, consent forms for the mother and father, instructions for collection of the 24-h urine specimen and the materials needed, and several self-administered forms. These forms include the Early Developmental Questionnaire (EDQ) (Ozonoff et al. 2005) for the proband, which is a parent reporting instrument that obtains information related to onset of ASD symptoms and potential regression; Social Responsiveness Scale (SRS) (Constantino 2002) for the mother, father, and proband, a standardized instrument for assessing social skills in a general population at any age; and 24-h and 48-h recalls to be completed with the 24-h urine collection kit (see below for details). All of these materials are collected from the mothers at the first visit, after informed consent has been completed.
The MARBLES study complies with all applicable requirements regarding human subjects and is approved by both UC Davis and State of California institutional review boards. No data are collected without informed consent.
Fieldwork: Exposure, Covariate Data, and Specimen Collection
Overview.
Home visits are conducted at enrollment, throughout pregnancy, and during the first year of the younger sibling’s life at 2 wk and 3, 6, and 12 months of age. Visits at 24 and 36 months take place at the UC Davis MIND Institute. Participation involves collection of biological specimens from the younger sibling, the proband (affected older sibling), and both parents (for both proband and younger sibling), as well as environmental samples taken in the home. The mother completes structured self-administered questionnaires, is interviewed by telephone at scheduled time points, and completes diaries related to exposures and illnesses. Maternal and child medical records are obtained and abstracted. Table 1 shows the schedule for specimen collections and data related to exposures, not including medical information.
Biospecimen collection (Table 1).
Prenatally, the first visit occurs as early as possible during pregnancy. During this visit, study staff members collect from all mothers a blood sample, vaginal swabs, hair samples, and saliva samples and instruct mothers on collection of three first-morning spot urine samples and one 24-h sample, each collected a week apart, per trimester. The woman then collects the urine samples on her own, providing them to study staff at subsequent visits. Throughout pregnancy, blood samples are collected at each visit, for up to five, or occasionally six, total samples. We also collect saliva samples from the affected older sibling(s) and biological father of the younger sibling for genetic analyses.
At delivery, nonstudy hospital personnel assist in collecting maternal peripheral blood, vaginal swab, cord blood, placenta, and meconium samples; a MARBLES study staff member attends to ensure all samples are taken, and then transports them to the laboratory for processing and storage. These arrangements are made through our clinician-investigator (CW) outreach to obstetricians and delivery hospitals for all participants, beginning well before the mother’s due date.
Postnatally, maternal biological samples are collected at home visits, including hair (3-month visit) and saliva (3- and 6-month visits); and for breastfeeding mothers, breast milk, urine (first-morning void spot samples) and blood. A single urine sample is collected from mothers who are not breastfeeding at 24 months. Younger sibling biospecimens include blood, saliva, hair, urine (a clean catch sample captured in a pediatric urine bag (U-bag) applied to the infant the day of the visit as well as multiple diapers from infants collected by the parent and stored in the participant’s home freezer), and, for a portion of the study, fecal samples. See Supplemental Material, “Sample collection, containment, processing and storage” for details.
Forms linked to specimen collections.
When blood specimens are collected, information is also obtained using the Blood Draw Form, which is completed by the parent participant, on recent food intake, household (e.g., pesticides, cleaning agents) and personal care products (cosmetics, lotions, shampoo), medication use, illness and stress, and other factors that could affect blood measurements. Other forms are timed with the collection of urine samples: a self-administered 24-h food recall and a 48-h product recall. The food recall was designed by our team to capture consumption of foods potentially contaminated with high levels of pesticides or phthalates, as well as food preparation and cooking methods, and water sources. The product recall focuses on those foods containing chemicals for which the metabolites can be measured in urine. The mother completes these recalls for the younger sibling before each urine or diaper sample and for her own urine collections if she is breastfeeding.
Home walkthrough and environmental sample collection.
Study personnel conduct an observational “Home Walkthrough,” at two home visits—one in pregnancy and one 6 months postnatally—to characterize the building and assess heating and cooling systems, cookware, furnishings, and other sources of environmental exposures. Cleaning and household products are surveyed, and participants are asked about lead testing and other questions regarding home characteristics. Dust samples from the main living area are taken using a standardized vacuum (Eureka Mighty-Mite Model 3670) (Allen et al. 2008; Rudel et al. 2003), and another dust sample is collected from the household vacuum.
Environmental Exposure Questionnaire (EEQ).
Beginning shortly after enrollment, a trained study staff member interviews mothers by telephone in the first part of the EEQ, which documents general demographic, residential, occupational, and lifestyle (e.g., tobacco, drug use) information for both parents, and mother’s pregnancy and medical history. Participants also complete a set of self-administered forms to record their and the child’s residential history and use of a broad range of household (pesticides, cleaning products) and personal care products (shampoos, antibacterial soaps), with a focus on the 6 months before conception of the younger sibling to the time of first interview. The interviews and self-administered EEQ forms are repeated in the third trimester, and at 6 months and 30 months postpartum, each covering the intervening period since prior interview. The mother is also interviewed regarding the pregnancy and early life exposures for the proband, usually around her midpregnancy with the younger sibling. A single interviewer conducts all MARBLES study interviews throughout the pregnancy and at 6 months postnatally, allowing establishment of rapport. Only the 30-month interview is conducted by other interviewers.
Diaries.
During pregnancy, each mother is asked to complete a short Weekly Symptom Diary, covering health symptoms, medication, and supplement usage; interactions with health-care providers: and experiences and environmental exposures from a selected set of household or personal care products for which we want to closely track changes throughout the time period. Following delivery, women complete the Monthly Symptom Diaries for a year, and Quarterly Symptom Diaries thereafter; both of these diaries are focused on the younger sibling. Questions document diaper rash, fever, allergic reactions, medications, what the child is eating, sun exposure, and personal care products used on the child. Participants can choose paper or online completion. The purpose of the diaries is to capture rapidly changing exposures or experiences, whereas the EEQ obtains a broad picture for longer time periods. The three study questionnaires are available at http://marbles.ucdavis.edu/research.html.
Child and Family Health and Development
Table 2 presents the schedule of child developmental evaluations, medical record collection, treatment information, and assessments or reports of health (physical and mental) and developmental conditions in family members. Child developmental batteries include autism-related instruments as well as measures of cognitive and adaptive function and behavioral domains. Additionally, study personnel periodically measure head circumference and photograph the infant’s palm along with frontal and profile views of the head and face for characterizing morphology.
Table 2.
Collection of developmental, behavioral, and medical information on child and family by time point.
| Forms and Source | Prenatal (Trimesters) | Postnatal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Forms | Source | 1st | 2nd | 3rd | 2 Weeks | 3 Months | 6 Months | 12 Months | 24 Months | 36 Months |
| Family Developmental/Mental Health | ||||||||||
| Social Responsiveness Scale | Constantino 2002; Constantino et al. 2003 | mother, father, & probanda | sibling | |||||||
| Early Developmental Questionnaire | Ozonoff et al. 2005 | probanda | siblingb | |||||||
| Social Communication Questionnaire | Rutter et al. 2003 | sibling, proband | ||||||||
| Maternal Perceived Stress Scale | Cohen et al. 1983 | motherc | motherc | mother | mother | mother | mother | mother | mother | mother |
| Family Characteristics Questionnaire | UC Davis study team | all first degree relatives | ||||||||
| Medical | ||||||||||
| Family Medical History (includes physical and mental health) | UC Davis study team | mother | ||||||||
| Autoimmune survey | UC Davis study team | first and second degree relatives | ||||||||
| Medical records | Health care providers | mother, sibling, & probandad | mother, siblinge | sibling | sibling | sibling | ||||
| Autism-Related | ||||||||||
| Autism Observation Scale for Infants | Bryson et al. 2008 | sibling | sibling | |||||||
| Infant Toddler Checklist—Communication and Symbolic Behaviors Scale Developmental Profile | Wetherby et al. 2002 | sibling | sibling | |||||||
| Modified Checklist for Autism in Toddlers | Robins et al. 2001 | sibling | ||||||||
| Autism Diagnostic Observation Schedule | Lord et al. 2000a, b | sibling | sibling | |||||||
| Autism Diagnostic Interview Revised | Lord et al. 1994 | siblingb | ||||||||
| Examiner Ratings of Social Engagement | Ozonoff et al. 2010 | sibling | sibling | sibling | sibling | |||||
| Social Responsiveness Scale | Constantino 2002; Constantino et al. 2003 | sibling | ||||||||
| Cognitive, Adaptive & Behavioral | ||||||||||
| Mullen Scales of Early Learning | Mullen 1995 | sibling | sibling | sibling | sibling | |||||
| Vineland Adaptive Behavior Scales | Sparrow et al. 1984 | sibling | ||||||||
| Child Behavior Checklist | Achenbach 1991, 1994 | sibling | ||||||||
| Children’s Sleep Habits Questionnaire | Owens et al. 2000 | sibling | sibling | |||||||
| Intervention History | UC Davis study team | sibling | sibling | |||||||
| Parent Concerns | Ozonoff et al. 2009 | sibling | sibling | sibling | sibling | sibling | ||||
Note: Shaded items are self-administered by the primary caregiver (generally the mother), others are administered by, or with assistance from staff. Proband is the affected older sibling; Sibling is the younger sibling born during the study.
Administered once at some point during the pregnancy (could be any trimester).
Only for younger siblings showing signs of ASD.
If possible, depending on timing of enrollment.
All records for proband obtained upon study entry.
Maternal is labor and delivery.
Autism-related instruments.
At each visit, beginning at 6 months, ASD symptomatology is assessed in the younger sibling by trained psychologists or other study staff, except where indicated below (see Supplemental Material, “Neurodevelopmental Assessments” for additional details). These instruments vary by the child’s age: the Autism Observation Scale for Infants (AOSI)(Bryson et al. 2008) and parent/caregiver report forms such as the Infant-Toddler Checklist (Wetherby et al. 2002) at 6 and 12 months; the Examiner Ratings of Social Engagement (Ozonoff et al. 2010) at 6, 12, 24, and 36 months; the Modified Checklist for ASD in Toddlers (Robins et al. 2001) at 24 months; and at 24 and 36 months, the gold standard ADOS) (Lord et al. 2000a; 2000b). The ADOS is a semistructured, standardized assessment in which the examiner observes the child’s social interaction, communication, play, and imaginative use of materials. The examiner chooses one of three possible ADOS modules to best match the age and expressive language level of the individual child. At 36 months, parents complete the Social Communication Questionnaire (SCQ) (Rutter et al. 2003) for the younger sibling. If the child shows evidence of ASD on the ADOS, the primary caregiver is interviewed with the ASD Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), a standardized instrument that documents the child’s history of symptoms in social, communication, and repetitive behavior domains required for an ASD diagnosis. Clinicians administering these instruments are trained and have formally attained research reliability on the ADI-R, ADOS, and AOSI.
Other assessment domains for the younger sibling.
Trained staff members test for cognitive, language, and motor development [(Mullen Scales of Early Learning (MSEL)] (Mullen 1995); parents report the child’s adaptive behavior and self-help skills (Vineland Adaptive Behavior Scales) (Sparrow et al. 1984) and internalizing and externalizing behavior problems (Child Behavior Checklist) (Achenbach 2000).
Parental concern.
Parents are asked at each visit, in an open-ended question, if they have concerns about their child’s behavior or development; previous work has demonstrated that parent concerns as early as 6–12 months about sensory behavior or motor, social, or communicative development predict a later ASD outcome, though lack of concerns is not a reliable predictor of later typical development (Ozonoff et al. 2009; Sacrey et al. 2015). When developmental concerns have been reported by a parent, two senior clinicians are present at the next appointment; one to administer the developmental protocol and another to observe and provide clinical support and diagnostic confirmation. If a second clinician is not available during the visit, clinical confirmation is conducted by the second clinician reviewing the videotape. Finally, at the end of each visit, the examiner reports on the child’s overall social–communicative behavior, rating eye contact, shared social affect, and overall social responsiveness using the Examiner Ratings of Social Engagement (Ozonoff et al. 2010).
Outcome classification.
Two methods are used for defining the neurodevelopmental outcome of each participant based on information from the 36-month visit: an algorithmic, data-driven method and a clinical best-estimate (CBE) method.
The outcome algorithm classifies children as ASD, TD, and NonTypical Development (NonTD), based on ADOS and MSEL scores using previously published methods from the Baby Siblings Research Consortium (Ozonoff et al. 2014). Children with ASD have scores over the ADOS cutoff and meet DSM-5 criteria for ASD. NonTD children have low MSEL scores (i.e., two or more MSEL subscales that are more than 1.5 SD below the normative mean or at least one MSEL subscale that is more than 2 SD below the normative mean), elevated ADOS scores (i.e., within 3 points of the ASD cutoff), or both. Children with TD outcomes have all MSEL scores above 2.0 SD below the normative mean, no more than one MSEL subscale that is between 1.5 and 2.0 SD below the normative mean, and scores on the ADOS more than three points below the ASD cutoff.
To determine CBE, two clinicians review the ADOS and ADI-R results, discuss their clinical impressions based on direct observations, and reach a consensus on the final study diagnosis. Initially, nine outcome categories are used: DSM-5 ASD, DSM-5 Social (Pragmatic) Communication Disorder, Broader Autism Phenotype (BAP: social communication difficulties below the ASD threshold), ADHD Concerns (developmentally atypical levels of hyperactivity, inattention, and/or impulsivity), Other Externalizing Behavior Problems (oppositional, defiant, or disruptive behavior that is extreme for the child’s age), Anxiety or Mood Problems (anxious, depressed, or emotionally dysregulated behavior), Learning Difficulties/Global Developmental Delay (low scores across multiple cognitive and motor domains), Speech-Language Problems (immature speech patterns or low language levels), or Typically Developing (TD: none of the above). Children classified into the ASD and Social (Pragmatic) Communication Disorder categories meet full DSM-5 criteria. The CBE outcome categories were not intended to map on to specific DSM categories, but rather to capture categories of clinical concern based on clinician judgment. For analyses using the CBE, we group the six (nonASD nontypical) categories as “Other Developmental Concerns.”
Other health and developmental data.
Each participant gives contact information and signs waivers for MARBLES study staff to obtain her pregnancy and delivery records as well as the child’s neonatal and pediatric charts. Study staff members seek to obtain these four types of records (see Supplemental Material, “Health Information”) and are trained to abstract variables from the records and enter them into a structured electronic system. Supervisors review key aspects of each abstraction, and validation is achieved by blinded reabstraction of over 10% of charts. Because we do not receive 100% of requested records, field staff who attend each MARBLES study birth collect key clinical information from the delivery and neonatal hospital records into structured maternal and infant abstraction forms with the help of hospital personnel. MARBLES staff administer a family medical history, including an autoimmune questionnaire. Staff members systematically attempt to obtain pregnancy, labor and delivery, neonatal, and child’s medical records. If the participant saw multiple providers of a given type, all those providers are contacted. On an as-needed basis, specific variables are abstracted from each of these four main record types and entered into electronic databases. Supervisors review key aspects of each abstraction, and validation is achieved by blinded reabstraction of over 10% of charts.
For the younger sibling, we collect information on treatments and services received, including the type (examples: behavioral therapy, physical therapy, medications), the duration of the treatment, and the age at which the child received it. For information on other family members, MARBLES staff administers a family medical history with an emphasis on some health and developmental disorders and an autoimmune disorders questionnaire in first- and second-degree relatives. Another form that we created documents family members’ developmental and learning disabilities (Family Characteristics Questionnaire). Mothers self-report their level of stress on the abbreviated version of the Perceived Stress Scale (Cohen et al. 1983). The Social Responsiveness Scale (SRS) (Constantino 2002; Constantino et al. 2003), a continuous measure of social impairment in natural social settings for the general population, is completed by each parent for their partner. A parent also completes the EDQ and SRS for the proband, and the SRS for the younger sibling.
Environmental Chemical Exposures
The MARBLES study was designed with a broad goal to evaluate various chemical compounds with potential for influencing brain development, either via direct effects on neural tissue (synapse formation, signaling) (Cao et al. 2014; Vester and Caudle 2016), or through other mechanisms, such as epigenetic (Feinberg and Fallin 2015; Schroeder et al. 2011, 2016; Teh et al. 2014; Tylee et al. 2016), immune (Ashwood et al. 2009; Atladóttir et al. 2010a; Lyall et al. 2014a), endocrine (Adibi et al. 2015; Majewska et al. 2014; Park et al. 2017; Swan et al. 2015), or mitochondrial (Wong and Giulivi 2016) pathways. Table 3 provides a candidate, but not comprehensive, list of chemicals, each with a literature in humans, experimental animals or in vitro systems supporting potential neurodevelopmental toxicity and hence a hypothesized contribution to ASD.
Table 3.
Some chemical classes of interest, biomarkers, and source materials used in MARBLES study.
| Compound Class | Half-lives | Urine | Blood | Dust | Placenta | Cord Blood | Breast Milk | Food Frequency Questionnaires | Food Recalls | Environmental Questionnaires | GIS-Linked California Pesticide Use Report Database | Medical Records |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental xenobiotics | In the human body and environmenta | |||||||||||
| Pyrethroid Pesticides | Hours to a few days in human body (Barr et al. 2010); days to a year in sunlight outdoors; longer indoors (Laskowski 2002; Shin et al. 2013) | x | x | x | x | x | ||||||
| Brominated Flame Retardants (PBDEs) | Typically months in human body; persistent in the environment (Thuresson et al. 2006) | x | x | x | ||||||||
| PBDE Hydroxy-metabolites | Little data found in humans regarding OH-PBDE half-lives, or their variabilityb | x | ||||||||||
| Phthalates | Hours to days in human body (Braun et al. 2012; Koch and Angerer 2007; Koch et al. 2006; Meeker et al. 2012) | x | x | x | x | |||||||
| Nutrients | ||||||||||||
| Folic acid or folate | 1.5 to 31.5 hours for newly absorbed folic acid, and about 100 days for folate pool in human body (Krumdieck et al. 1978; Loew et al. 1987) | x | x | x | x | |||||||
| Vitamin B6 | Minutes to an hour in human body (Zempleni 1995) | x | x | x | ||||||||
| Vitamin B12 | In human body, minutes for holotranscobalamin II (bioavailable form); 2 weeks for transcobalaminI and II (storage protein) (Bauman et al. 2000) | x | x | x | ||||||||
| Iron | Differs by type from hours to days in the human body (Geisser and Burckhardt 2011) | x | x | x | ||||||||
| Vitamin D | 15 hours for calcitriol (active form) and 15 days for calcidiol (Jones 2008) | |||||||||||
| Biomarkers | In stored samples | |||||||||||
| Maternal antibodies to fetal brain | Unknown | x | ||||||||||
| Mitochondrial DNA | : yearsc (Lebedeva et al. 2000) | x | x | |||||||||
| DNA methylation (whole genome bisulfite sequencing) | DNA and its modifications are stable for years if dried or frozen (Kader and Ghai 2015; Shabihkhani et al. 2014) | x | x | |||||||||
| RNA/Gene Expression | RNA is stable for 5 years if human samples are frozen promptly and RNA is isolated directly from frozen samples (Shabihkhani et al. 2014) | x | x | |||||||||
| Metabolomics | Differs by compound and medium (Saude and Sykes 2007; Haid et al. 2018) with about half of compounds stable up to 5 years at in blood (Haid et al. 2018). | x | x | x | x | x |
Note: Table includes analytes for which past or current funding covers measurements in at least one medium; other sources and media are also shown, whether or not funded for assays. Additional media collected in MARBLES include: hair, saliva, maternal vaginal secretions, newborn meconium, and fecal specimens.
Half-lives in both the environment and the body vary by specific compound or congener within a class: ranges are provided.
Some OH-PBDEs in the body are formed through transformation of PBDEs, and therefore temporal variation also depends on half-lives of PBDEs, as well as metabolic rates to form and remove OH-PBDEs.
To ensure the rigor and scientific reproducibility of data, the reported half-life for mtDNA integrity in PBMCs and placenta (expressed as ; ) was experimentally determined in Giulivi’s laboratory assuming a pseudo-first order kinetics. The value is within that reported for mtDNA in hair shafts. Upon receipt of frozen samples, DNA was always extracted within a day. The extracted DNA was stored at in EDTA, Tris-Cl (pH 9.0) at a concentration of . After outcomes are determined by qRTPCR, the mtDNA is stored at .
For example, phthalates, a class of plasticizers used widely in consumer products, building materials, and medical applications, have been associated with a range of behavioral and developmental outcomes in human observational and experimental animal or in vitro studies (Ejaredar et al. 2015; Jeddi et al. 2016; Miodovnik et al. 2014; Zarean et al. 2016). These reviews also discuss potential mechanistic pathways. A second example is the polybrominated diphenyl ethers, which were widely used as flame retardants in household furnishings and electronics during the 1990s and early 2000s, are highly persistent both in the environment and in the human body and have been well studied both in toxicological and epidemiological studies. According to recent reviews and a meta-analysis, neurodevelopmental impacts, and IQ in particular, are among the most consistently observed outcomes, and additional evidence suggests thyroid disruption as one of several potentially contributing biological pathways (Costa et al. 2014; Gibson et al. 2018; Lam et al. 2017; Linares et al. 2015; Vuong et al. 2018). Although fewer studies have directly examined the hydroxylated metabolites (OH-PBDEs), research in experimental animals, humans, and wildlife has supported a greater transplacental transfer than the parent compounds and/or greater binding affinity to neurotransmitter receptors and to thyroid-binding proteins, including transthyretin, as well as thyroid-binding globulin, the primary human thyroid transport protein (Chen et al. 2013; Dingemans et al. 2011).
The pyrethroid and pyrethrin insecticides have received less attention than the above-mentioned chemical classes, despite known neurotoxicity with key targets of dopamine and GABA neurotransmission systems, which are implicated in autism (Meyer et al. 2008; Shelton et al. 2012; Vester and Caudle 2016). Exposure is widespread, resulting from agricultural, household, garden, and pet pest control (U.S. EPA 2010). Previous reviews have emphasized how sparse the research has been, especially for neurotoxic effects from chronic low-level human exposures, i.e., chronic exposures at levels that do not induce acute effects (Koureas et al. 2012; Ray and Fry 2006). In the last few years, associations of pre- or postnatal pyrethroid exposures with suboptimal child development have been reported in cross-sectional (Quirós-Alcalá et al. 2014) (Oulhote and Bouchard 2013; Viel et al. 2015) (Viel et al. 2017); longitudinal (Eskenazi et al. 2018; Watkins et al. 2016) (Furlong et al. 2017); and case–control studies (Roberts et al. 2007) (Shelton et al. 2014), with these latter two specifically addressing ASD risk. Overall, based on this literature, pyrethroids are a candidate exposure for the MARBLES study.
Exposure Assessment
Environmental chemicals.
The sample collection and questionnaires were developed to allow exposure assessment for a wide range of compounds. Chemical exposures occur through inhalation; ingestion of food, water, and nondietary material; and dermal absorption. For many compounds, measurements in biological specimens effectively integrate exposures over all these pathways of intake. For compounds with a long half-life in the body (PBDEs, e.g.), biological samples provide robust exposure measures over substantial time periods. However, for compounds with short half-lives, measurements in biological samples are best supplemented with other information. Because pyrethroid pesticides are rapidly metabolized and excreted, urinary pyrethroid pesticide metabolites, which represent recent exposures, are considered the best biomarkers. However, precisely because of this rapid elimination and our interest in more extended exposures, we additionally collect house dust, a longer-term reservoir for home chemicals; residential address history, which can be linked to commercial pesticide applications through Geographic Information Systems; and participant responses in questionnaires and diaries about household pesticide use and consumption of foods likely to contain pesticide residues. Additionally, repeated sampling of urine specimens can provide integrated metrics of exposures. Table 3 summarizes biological and environmental sampling and other sources of exposure information for a few select chemical classes and some biological markers of potential mechanistic relevance.
Multiple chemicals will be assayed in the course of the MARBLES study. With regard to the chemicals currently under investigation, overall strategies to achieve quality assurance and quality control include analysis of both internal and external standards, as well as duplicates in each batch; participation in the Artic Monitoring and Assessment Program for persistent pollutants, including PBDEs; verification of detection limits; establishing percent recovered for low level compounds; and use of calibration curves. Methods for the PBDE analyses in blood, for pesticide metabolite determinations in urine, and for details on all the quality assurance, quality-control protocols have been published (Lin et al. 2013; Olsson et al. 2004). Additionally, we have described the within-woman variability across pregnancy in pesticide metabolite concentrations (Barkoski et al. 2018).
Food intake and nutritional assessment.
Recall of food intake allows assessment of nutritional status of the mother and child for various time periods, estimation of intake of pesticides and other chemicals in food, and correlation of food intake with nutrient metabolite and environmental toxicant measurements in urine. Mothers are asked to complete food frequency questionnaires (FFQs) to provide a comprehensive history of her usual dietary and supplemental intake across three time-frames: gestational weeks 1–20, gestational weeks 20 through delivery, and the postnatal period from delivery until the end of breastfeeding. The MARBLES study uses the Block 2005 FFQ that was designed to estimate usual and customary intake of a wide array of nutrients and food groups using approximately 110 food items. This Block FFQ underwent extensive validation, including in pregnant women, Californian women, and across multiple ethnicities (Block et al. 1986, 1990) (Block et al. 1992, 2006; Horn-Ross et al. 2008; Johnson et al. 2007). Other questionnaires developed by MARBLES staff and investigators obtain vitamin and supplement intake, including types, brands (open-ended), frequency, dose, and timing for the younger sibling, and for the mother during pregnancies with both younger sibling and proband.
Data Management, Cleaning, and Sharing
All data from child assessments are double-entered, and discrepancies are flagged and reviewed by hand. All scoring that is done prior to data entry is checked by a second study team member. Initial checks of range and distributions, as well as consistency across variables, are done every time new data are entered and added to the main study files. Analysis variables are constructed in consultation with investigators planning to publish using those variables. All data are stored on secure servers in protected files and backed up regularly. Data are deposited in the National Database for Autism Research (NDAR; ndar.nih.gov). Both data and specimens are shared with investigators outside the study team. Outside investigators can make requests using a standardized form. Requests are reviewed by study investigators for relevance of aims, consideration of limited volumes of specimens, and a determination of whether the goals of the proposed project are compatible with, yet not redundant of, those of the MARBLES study investigators.
Results
Enrollment, Retention, Descriptive Statistics, and Diagnoses
Our goal during the current funding cycle was to recruit 450 eligible pregnant mothers, and we have now surpassed that target, with 463 participants enrolled to date. Of these, 40% were enrolled in the first trimester, 36% in the second trimester, and 24% in the third trimester. The vast majority of these were recruited by MARBLES staff contacting households of children who were receiving services for ASD through the California DDS system, with a few also recruited from MIND Institute clinics and other research studies.
As of 30 June 2018, eligible women with 463 separate pregnancies (carrying 471 fetuses) had enrolled in the study; 2 other pregnancies were determined ineligible. Of these 463, 24 pregnancies resulted in loss, 26 dropped out during pregnancy (including one twin pregnancy), and 6 participants are still pregnant. From the remaining 407 pregnancies known to have ended in livebirth(s), 46 families dropped out after delivery (includes 1 set of twins), leaving 361 (87%), which included 6 sets of twins. Thus, 367 children were still actively being followed or have completed the protocol. Of these, 160 (44%) were females and 207 (56%) males. Among families that enrolled and for which the pregnancy did not end in miscarriage (439), the participants from 367 pregnancies (6 still pregnant, 361 already delivered) are currently active in the study. Thus, excluding the miscarriages, who are no longer at risk for ASD, this total corresponds to an overall retention of 84%.
We have also successfully contacted over 2,000 nonpregnant mothers, and for those who are eligible (see eligibility section above), we maintain a call-back list to check for changes in pregnancy status. Additionally, 94 prepregnant women who were actively attempting to become pregnant had a prepregnancy visit. Of these, 44 women did become pregnant and enrolled.
Table 4 presents demographic characteristics of the parents who have completed or are expected to complete the MARBLES study protocol. At conception, three-fourths of the mothers were 30 years of age or older. Fathers were older. Over half the mothers and the fathers were nonHispanic white, 23% were Hispanic, and one-fourth to one-fifth were born in a country other than the United States or Mexico. About half of the mothers and fathers had a bachelor’s or higher degree, and 18% of fathers had a high school diploma or less.
Table 4.
Demographic characteristics of families enrolled and retained through 30 June 2018 in the MARBLES study.
| Characteristic | Mother | Father | ||
|---|---|---|---|---|
| N | % | N | % | |
| Parents’ age at conception of younger sibling (years)ab | ||||
| 12 | 3.3 | 9 | 2.51 | |
| 25–29 | 69 | 18.8 | 40 | 11.14 |
| 30–34 | 129 | 35.2 | 103 | 28.69 |
| 35–39 | 128 | 34.9 | 122 | 33.98 |
| 40–44 | 25 | 6.8 | 65 | 18.11 |
| 4 | 1.1 | 20 | 5.57 | |
| Missing | 8 | |||
| Parents’ Race/Ethnicityc | ||||
| Caucasian/White (nonHispanic) | 184 | 51.5 | 182 | 51.1 |
| Hispanic (any race) | 84 | 23.5 | 82 | 23 |
| Asian | 59 | 16.5 | 59 | 16.6 |
| African American/Black | 18 | 5 | 15 | 4.2 |
| Multiracial or Other Race | 12 | 3.4 | 18 | 5.1 |
| Missing | 4 | 5 | ||
| Parents’ birth locationc | ||||
| United States – California | 200 | 58.5 | 170 | 50.9 |
| United States – other state | 57 | 16.7 | 60 | 18 |
| United States – Unspecified | 3 | 0.8 | 4 | 1.2 |
| Mexico | 14 | 4.1 | 22 | 6.6 |
| Other | 68 | 19.9 | 78 | 23.4 |
| Missing | 19 | 27 | ||
| Parents’ educationc | ||||
| No high school diploma | 9 | 2.5 | 17 | 4.8 |
| High school diploma | 19 | 5.4 | 48 | 13.6 |
| Some college, Associate’s degree or Vocational training | 144 | 40.7 | 105 | 29.8 |
| Bachelor’s degree | 111 | 31.4 | 108 | 30.7 |
| Master’s, PhD, or other professional degree | 71 | 20.1 | 74 | 21 |
| Missing | 7 | 9 | ||
All families actively enrolled (either pregnant or postdelivery) through 30 June 2018. MARBLES Study, for total children (postnatal) or pregnancies not yet delivered (excludes miscarriages, and drop-outs),
Date of conception calculated by taking the child’s date of birth (DOB), subtracting the gestational age (GA) and adding 14 days: . The parent’s age is then calculated on that date.
Among families of younger siblings born alive, and actively enrolled through 30 June 2018. MARBLES Study, .
Among those enrolled, thousands of urine and blood samples have been collected during pregnancy, and of those who delivered, we have obtained placental tissue from 92%, and cord blood from 86%. Beginning at delivery and including postpartum visits at 2 wk, 3, 6, 12, 24, and 36 months, our average visit completion rate is 89%. Of MARBLES younger siblings who passed their third birthday, 93% completed the 36-month visit, providing a final diagnosis for 273 of them using the CBE, or due to missing scores on the MSEL, 266 using the algorithm.
Using the algorithmic diagnosis, 64 met criteria for ASD, for a recurrence risk of 24%, consistent with previous results from similar enhanced-risk sibling cohorts (Ozonoff et al. 2011) (Messinger et al. 2013). An additional 66 (25%) were classified as having nonASD nontypical development, as described above. In a preliminary study of organophosphate pesticides, neither ASD nor nontypical development was associated with prenatal concentrations of these compounds measured in urine. Although statistical power was limited, in stratified analysis we did find a suggestive association between OP pesticides and ASD risk among girls, but not boys (Philippat et al. 2018).
Discussion and Conclusion
The MARBLES study has successfully recruited a cohort of pregnant women at elevated risk to deliver a child who develops ASD and has achieved a high rate of retention (84%). The MARBLES study comprises mother–child pairs with detailed characterizations of both the prenatal and postnatal environmental milieu and the child’s behavioral and developmental profile. Biospecimens collected from mother, father, proband, and younger sibling provide a resource to investigate early exposures, including potentially modifiable factors, that influence the child’s trajectory; to study mechanistic pathways that may compromise neurodevelopment in ASD; and to search for early molecular changes of relevance to autistic behaviors.
Of particular significance is initiation of the cohort in the prenatal period. Neuroanatomic findings, imaging, molecular studies, and epidemiology (Liu 2011; Rapin 1995; Rodier et al. 1996; Schmidt et al. 2012) all place origins of ASD in early gestation. A summary of published time windows of vulnerability to environmental factors suggests, however, that these critical periods of susceptibility to exposure vary by specific agent (Lyall et al. 2014b). Moreover, insults to neurodevelopment could occur not only during, but also before and after gestation.
In comparison with other families, parents participating in MARBLES have a heightened concern about their child’s developmental outcome. Some parents may also be more aware or concerned about environmental factors, which may affect their responses to exposure-related questions, or may alter their behaviors, for instance, to decrease their use of certain products. However, all of the MARBLES study’s mothers are in this category of parents who are concerned because they all have an older child with ASD, and none knows the future outcome for their children, which may reduce the likelihood of reporting bias.
The high-risk pregnancy cohort has a few limitations, the primary one being small sample sizes due to the costs of enrolling, managing specimens, and conducting successful follow-up to the age at which definitive diagnoses are made. For this reason, pooling across studies of similar design would be highly desirable. Secondly, heterogeneity of endophenotypes (genetically related behavioral phenotypes) may translate into wide variation in susceptibility to environmental agents. If environmental influences on ASD do differ for children with versus children without an older affected sibling, findings from the MARBLES study might not be generalizable to children who develop ASD in the population as a whole. For example, if susceptibility to environmental factors that contribute to ASD is determined by the presence of rare, highly penetrant gene variants that are more common in families with a history of ASD, then the influence of the environmental factors might differ in our high-risk population in comparison with the general population. On the other hand, if a contribution from environmental exposures depends on the presence of certain common gene variants that confer greater susceptibility [as was observed for folic acid and one-carbon metabolism genes (Schmidt et al. 2011, 2012)], then we might expect children in high- and low-risk families to be similarly affected. Thus, for both null and positive findings in the MARBLES study, generalizability to families without a history of ASD (the majority of ASD cases) may be difficult to predict. To date, genetic research on ASD has focused primarily on rare gene variants, so the potential influence of common variants, either alone or in combination with environmental exposures, is largely unexplored.
Some research supports a role of common genetic variants interacting with environmental agents in autism etiology, including the MTHFR677A allele, MET-C allele, and total burden of copy number (repeated segments of DNA) (Kim et al. 2017; Schmidt et al. 2011, 2012; Volk et al. 2014). For example, ASD was associated with total copy number burden (summing the number of extra base pairs across the genome), and this association remained significant after excluding known pathogenic (often large and rare) copy number variants (Girirajan et al. 2013). Subsequent work showed an air pollutant that had no impact on risk of ASD when considered alone, but was associated with increased risk of ASD when exposure occurred in those with an elevated copy number burden. This result may indicate an elevated susceptibility from global genetic instability, either because of the interaction with air pollution and/or because increased copy number burden could itself be a consequence of multiple hits from environmental factors. Similarly, the observation that specific de novo single-nucleotide polymorphism (SNP) variants are strongly linked to ASD (Neale et al. 2012; Sanders et al. 2012) raises the possibility that these new mutations may themselves have environmental origins. Either of these mechanisms, depending on prevalence, could lead to either a low or high likelihood that associations with xenobiotic exposures are generalizable beyond the enhanced-risk cohort.
MARBLES and other cohort studies are essential to progress in understanding ASD etiology. With the exception of exposures documented in existing records (such as medical charts or air pollutant monitoring), case–control studies have few sources other than parental reports. Unfortunately, many exposures predominantly depend on individual behaviors and cannot be retrospectively determined from existing records or exposure databases. Additionally, identification of early biological or xenobiotic markers, requires, with few exceptions, prospective specimen collection. Cohort studies fill all these gaps, and when the outcomes are relatively rare, as for ASD, their statistical power can be greatly increased through the design of an “enhanced-risk” population, such as families who already have a child with ASD. The MARBLES study therefore provides an excellent platform for prospectively assessing environmental risk and protective factors, identifying biological markers that may serve as early signs of ASD, and providing clues about underlying mechanistic pathways.
Supplementary Material
Acknowledgments
The MARBLES Study has been supported by NIH grants: P01-ES011269, 1R01-ES020392, R01-ES025574, P30-ES023513, and R01-ES028089 from the National Institute of Environmental Health Sciences; U54-HD079125 from the Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Network; NIH-UL1-TR000002 for the UC Davis Clinical and Translational Sciences Center and 2K12-HD051958; by the following U.S. EPA (U.S. Environmental Protection Agency) grants #R-829388 & R833292; by DOD #AR110194; and by the UC Davis MIND Institute.
Additionally, the authors thank the Whiteley Center for providing subsidized accommodations for a scholarly retreat during which this manuscript was revised. The authors express our gratitude to B. Elms and E. Guerrero-Angel for their data cleaning, management, and analysis, and to the entire MARBLES staff, past and current, for their invaluable and meticulous work in protocol development, recruitment, data collection, and specimen collection, processing, and storage.
I.H.P., C.W., R.S., D.T., and S.O. have received funding for research, travel or meeting costs from Autism Speaks. C.W. has received honoraria and travel support from the Merck Speaker’s Bureau. J.L. has received funding to cover travel costs as a member of the Dup15q Alliance Scientific Advisory Board. D.T. has received research funding from the Merck Investigators Study Program; travel support and consulting fees from Pfizer Consumer Healthcare; and travel support and honoraria from the International Scientific Association of Probiotics and Prebiotics. I.P. has received travel support and compensation for expert testimony from Sher Leff, PC, and funding to cover travel costs as a member of the Scientific Advisory Board of IUF (Leibniz-Institut für Umweltmedizinische Forschung), Düsseldorf, Germany. J.V de W. has received funding for travel to present at the Autism Research Institute.
References
- Achenbach TM. Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994:517–549. [Google Scholar]
- Achenbach TM. 1991. Integrative Guide to the 1991 CBCL/4-18, Ysr, and Trf Profiles. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- Achenbach TM, Rescorla LA. 2000. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT:University of Vermont, Research Center for Children, Youth, & Families; http://www.aseba.org/research/basicreferences.html. [Google Scholar]
- Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, et al. . 2015. Human chorionic gonadotropin partially mediates phthalate association with male and female anogenital distance. J Clin Endocrinol Metab 100(9):E1216–E1224, PMID: 26200238, 10.1210/jc.2015-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. 2008. Critical factors in assessing exposure to PBDEs via house dust. Environ Int 34(8):1085–1091, PMID: 18456330, 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Angkustsiri K, Krakowiak P, Moghaddam B, Wardinsky T, Gardner J, Kalamkarian N, et al. . 2011. Minor physical anomalies in children with autism spectrum disorders. Autism 15(6):746–760, PMID: 21610186, 10.1177/1362361310397620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Van de Water J. 2004. Is autism an autoimmune disease? Autoimmun Rev 3(7-8):557–562, PMID: 15546805, 10.1016/j.autrev.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. 2009. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol 208(1-2):130–135, PMID: 19211157, 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. . 2010a. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40(12):1423–1430, PMID: 20414802, 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Schendel DE, Østergaard L, Lemcke S, Parner ET. 2010b. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch Pediatr Adolesc Med 164:470–477, PMID: 20439799, 10.1001/archpediatrics.2010.9. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Henriksen TB, Schendel DE, Parner ET. 2012. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 130(6):e1447–e1454, PMID: 23147969, 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. . 2018. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 67(6):1–23, PMID: 29701730, 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoski J, Bennett D, Tancredi D, Barr DB, Elms W, Hertz-Picciotto I. 2018. Variability of urinary pesticide metabolite concentrations during pregnancy in the MARBLES Study. Environ Res 165:400–409, PMID: 29860212, 10.1016/j.envres.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Olsson AO, Wong L-Y, Udunka S, Baker SE, Whitehead RD, et al. . 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999-2002. Environ Health Perspect 118(6):742–748, PMID: 20129874, 10.1289/ehp.0901275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. 2000. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 23(9):1227–1231, PMID: 10977010, 10.2337/diacare.23.9.1227. [DOI] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. 2013. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 121(3):380–386, PMID: 23249813, 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. 1986. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124(3):453–469, PMID: 3740045, 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C. 1990. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43(12):1327–1335, PMID: 2254769, 10.1016/0895-4356(90)90099-B. [DOI] [PubMed] [Google Scholar]
- Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. 1992. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92(6):686–693, PMID: 1607564. [PubMed] [Google Scholar]
- Block G, Wakimoto P, Jensen C, Mandel S, Green RR. 2006. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis 3(3):A77, PMID: 16776878. [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. . 2012. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect 120(5):739–745, PMID: 22262702, 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, et al. . 2013. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 3:e277, PMID: 23838888, 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. 2008. The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord 38(4):731–738, PMID: 17874180, 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Cao Z, Cui Y, Nguyen HM, Jenkins DP, Wulff H, Pessah IN. 2014. Nanomolar bifenthrin alters synchronous Ca2+ oscillations and cortical neuron development independent of sodium channel activity. Mol Pharmacol 85(4):630–639, PMID: 24482397, 10.1124/mol.113.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I. 2014. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord 44(5):1117–1127, PMID: 24193577, 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Park JS, Linderholm L, Rhee A, Petreas M, DeFranco EA, et al. . 2013. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol 47(8):3902–3908, PMID: 23506475, 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Suominen A, Jokiranta E, Lehti V, McKeague IW, Sourander A, et al. . 2014. Increased risk of autism spectrum disorders at short and long interpregnancy intervals in Finland. J Am Acad Child Adolesc Psychiatry 53(10):1074–1081. e1074., PMID: 25245351, 10.1016/j.jaac.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. 1983. A global measure of perceived stress. J Health Soc Behav 24(4):386–396, 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Constantino JN. 2002. The Social Responsiveness Scale. Los Angeles, CA:Western Psychological Services. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. . 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 33(4):427–433, PMID: 12959421. [DOI] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lam YM, Brownell M, Flavin MP, Roos LL. 2015. The association between the interpregnancy interval and autism spectrum disorder in a Canadian cohort. Can J Public Health 106:e36–e42, PMID: 25955670, 10.17269/cjph.106.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C. 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230(2):282–294, PMID: 24270005, 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Hoogstrate J, Selvin S. 2002. The changing prevalence of autism in California. J Autism Dev Disord 32(3):207–215, PMID: 12108622, 10.1023/a:1015453830880. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH. 2011. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect 119(7):900–907, PMID: 21245014, 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, DuBois LA, Maenner MJ. 2015. Inter-pregnancy intervals and the risk of autism spectrum disorder: results of a population-based study. J Autism Dev Disord 45(7):2056–2066, PMID: 25636677, 10.1007/s10803-015-2368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. 2015. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res 142:51–60, PMID: 26101203, 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. 2010. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun 24(1):64–71, PMID: 19666104, 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. . 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115(5):792–798, PMID: 17520070, 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, An S, Rauch SA, Coker ES, Maphula A, Obida M, et al. . 2018. Prenatal exposure to DDT and pyrethroids for malaria control and child neurodevelopment: the VHEMBE Cohort, South Africa. Environ Health Perspect 126(4):047004, PMID: 29648420, 10.1289/EHP2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Fallin MD. 2015. Epigenetics at the crossroads of genes and the environment. JAMA 314(11):1129–1130, PMID: 26372577, 10.1001/jama.2015.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E, Du Mazaubrun C, Cans C, Grandjean H. 1997. Autism and associated medical disorders in a French epidemiological survey. J Am Acad Child Adolesc Psychiatry 36(11):1561–1569, PMID: 9394941, 10.1097/00004583-199711000-00019. [DOI] [PubMed] [Google Scholar]
- Furlong MA, Barr DB, Wolff MS, Engel SM. 2017. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62:231–238, PMID: 28811173, 10.1016/j.neuro.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser P, Burckhardt S. 2011. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 3(1):12–33, PMID: 24310424, 10.3390/pharmaceutics3010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA, Siegel EL, Eniola F, Herbstman JB, Factor-Litvak P. 2018. Effects of polybrominated diphenyl ethers on child cognitive, behavioral, and motor development. Int J Environ Res Public Health 15:1636, PMID: 30072620, 10.3390/ijerph15081636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Johnson RL, Tassone F, Balciuniene J, Katiyar N, Fox K, et al. . 2013. Global increases in both common and rare copy number load associated with autism. Hum Mol Genet 22(14):2870–2880, PMID: 23535821, 10.1093/hmg/ddt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivi C, Zhang Y-F, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. . 2010. Mitochondrial dysfunction in autism. JAMA 304(21):2389–2396, PMID: 21119085, 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haid M, Muschet C, Wahl S, Römisch-Margl W, Prehn C, Möller G, et al. . 2018. Long-term stability of human plasma metabolites during storage at -80 °C. J Proteome Res 17(1):203–211, PMID: 29064256, 10.1021/acs.jproteome.7b00518. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 114(7):1119–1125, PMID: 16835068, 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn-Ross PL, Lee VS, Collins CN, Stewart SL, Canchola AJ, Lee MM, et al. . 2008. Dietary assessment in the California Teachers Study: reproducibility and validity. Cancer Causes Control 19(6):595–603, PMID: 18256894, 10.1007/s10552-008-9124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. . 2018. Prenatal fever and autism risk. Mol Psychiatry 23(3):759–766, PMID: 28607458, 10.1038/mp.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddi MZ, Janani L, Memari AH, Akhondzadeh S, Yunesian M. 2016. The role of phthalate esters in autism development: a systematic review. Environ Res 151:493–504, PMID: 27567353, 10.1016/j.envres.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Herring AH, Ibrahim JG, Siega-Riz AM. 2007. Structured measurement error in nutritional epidemiology: applications in the Pregnancy, Infection, and Nutrition (PIN) Study. J Am Stat Assoc 102(479):856–866, PMID: 18584067, 10.1198/016214506000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. 2008. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 88:582S–586S, PMID: 18689406, 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- Kader F, Ghai M. 2015. DNA methylation and application in forensic sciences. Forensic Sci Int 249:255–265, PMID: 25732744, 10.1016/j.forsciint.2015.01.037. [DOI] [PubMed] [Google Scholar]
- Kim D, Volk H, Girirajan S, Pendergrass S, Hall MA, Verma SS, et al. . 2017. The joint effect of air pollution exposure and copy number variation on risk for autism. Autism Res 10(9):1470–1480, PMID: 28448694, 10.1002/aur.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, et al. . 2011. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect 119(4):519–526, PMID: 21106467, 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Angerer J. 2007. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health 210(1):9–19, PMID: 17182279, 10.1016/j.ijheh.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J. 2006. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure – an update and latest results. Int J Androl 29(1):155–165. discussion 181-155, PMID: 16466535, 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- Koureas M, Tsakalof A, Tsatsakis A, Hadjichristodoulou C. 2012. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Lett 210(2):155–168, PMID: 22020228, 10.1016/j.toxlet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. 2008. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res 17(2):197–206, PMID: 18482108, 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. . 2012. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129(5):e1121–e1128, PMID: 22492772, 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumdieck CL, Fukushima K, Fukushima T, Shiota T, Butterworth CE. 1978. A long-term study of the excretion of folate and pterins in a human subject after ingestion of 14C folic acid, with observations on the effect of diphenylhydantoin administration. Am J Clin Nutr 31(1):88–93, PMID: 413430, 10.1093/ajcn/31.1.88. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, et al. . 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect 125(8):086001, PMID: 28799918, 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski DA. 2002. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol 174:49–170, PMID: 12132343, 10.1007/978-1-4757-4260-2_3. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. 2005. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry 46(9):963–971, PMID: 16108999, 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Lebedeva IA, Kulikov EE, Ivanova NV, Poltaraus AB. 2000. [Mitochondrial DNA sequencing of human hair shafts stored for long time]. [In Russian] Sud Med Ekspert 43(3):9–14, PMID: 11186957. [PubMed] [Google Scholar]
- Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. . 2016. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137:1–10, PMID: 26826214, 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Pessah IN, Puschner B. 2013. Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography-tandem mass spectrometry in human serum and plasma. Talanta 113:41–48, PMID: 23708622, 10.1016/j.talanta.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares V, Belles M, Domingo JL. 2015. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 89(3):335–356, PMID: 25637414, 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Liu JS. 2011. Molecular genetics of neuronal migration disorders. Curr Neurol Neurosci Rep 11(2):171–178, PMID: 21222180, 10.1007/s11910-010-0176-5. [DOI] [PubMed] [Google Scholar]
- Loew D, Eberhardt A, Heseker H, Kübler W. 1987. [Plasma kinetics and elimination of folic acid] [In German]. Klin Wochenschr 65(11):520–524, PMID: 3613465, 10.1007/BF01721039. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24(5):659–685, PMID: 7814313, 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. . 2000a. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223, PMID: 11055457, 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. 2000b. The Autism Diagnostic Observation Schedule (ADOS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. 2014a. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord 44:1546–1555, PMID: 24337796, 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I. 2014b. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 43(2):443–464, PMID: 24518932, 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Hill M, Urbanowicz E, Rok-Bujko P, Bienkowski P, Namysłowska I, et al. . 2014. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children. Eur Child Adolesc Psychiatry 23(6):485–498, PMID: 24043498, 10.1007/s00787-013-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCanlies EC, Fekedulegn D, Mnatsakanova A, Burchfiel CM, Sanderson WT, Charles LE, et al. . 2012. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord 42(11):2323–2334, PMID: 22399411, 10.1007/s10803-012-1468-1. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. 2012. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol 22(4):376–385, PMID: 22354176, 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, et al. . 2013. Beyond autism: a Baby Siblings Research Consortium study of high-risk children at 3 years of age. J Am Acad Child Adolesc Psychiatry 52(3):300–308.e1, PMID: 23452686, 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Carter JM, Johnstone AF, Shafer TJ. 2008. Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. Neurotoxicology 29(2):213–225, PMID: 18243323, 10.1016/j.neuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R. 2014. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology 41:112–122, PMID: 24486776, 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen Scales of Early Learning. Circle Pines, MN:American Guidance Services, Inc. [Google Scholar]
- Napoli E, Wong S, Giulivi C. 2013. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism 4(1):2, PMID: 23347615, 10.1186/2040-2392-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Wong S, Hertz-Picciotto I, Giulivi C. 2014. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 133(5):e1405–e1410, PMID: 24753527, 10.1542/peds.2013-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, et al. . 2012. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485(7397):242–245, PMID: 22495311, 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, et al. . 2012. Infant siblings and the investigation of autism risk factors. J Neurodev Disord 4(1):7, PMID: 22958474, 10.1186/1866-1955-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD, et al. . 2004. A liquid chromatography--tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal Chem 76(9):2453–2461, PMID: 15117183, 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Bouchard MF. 2013. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect 121(11–12):1378–1384, PMID: 24149046, 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. 2000. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. Sleep 23(8):1043–1051, PMID: 11145319, 10.1093/sleep/23.8.1d. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. 2005. Parental report of the early development of children with regressive autism: the delays-plus-regression phenotype. Autism 9(5):461–486, PMID: 16287700, 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, et al. . 2009. How early do parent concerns predict later autism diagnosis? J Dev Behav Pediatr 30(5):367–375, PMID: 19827218, 10.1097/DBP.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. . 2010. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 49(3):256–266, PMID: 20410715, 10.1016/j.jaac.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. . 2011. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 128(3):e488–e495, PMID: 21844053, 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, et al. . 2014. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry 53(4):398–407.e2, PMID: 24655649, 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Lee BK, Burstyn I, Tabb LP, Keelan JA, Whitehouse AJO, et al. . 2017. Umbilical cord blood androgen levels and ASD-related phenotypes at 12 and 36 months in an enriched risk cohort study. Mol Autism 8:3, PMID: 28163867, 10.1186/s13229-017-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Barkoski J, Tancredi DJ, Elms B, Barr DB, Ozonoff S, et al. . 2018. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int J Hyg Environ Health 221(3):548–555, PMID: 29478806, 10.1016/j.ijheh.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CA, McVeigh KH, Driver CR, Govind P, Karpati A. 2015. Parental age and autism spectrum disorders among New York City children 0-36 months of age. Matern Child Health J 19(8):1783–1790, PMID: 25776271, 10.1007/s10995-015-1692-3. [DOI] [PubMed] [Google Scholar]
- Quirós-Alcalá L, Mehta S, Eskenazi B. 2014. Pyrethroid pesticide exposure and parental report of learning disability and attention deficit/hyperactivity disorder in U.S. children: NHANES 1999-2002. Environ Health Perspect 122(12):1336–1342, PMID: 25192380, 10.1289/ehp.1308031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I. 1995. Autistic regression and disintegrative disorder: how important the role of epilepsy? Semin Pediatr Neurol 2(4):278–285, PMID: 9422256, 10.1016/S1071-9091(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Ray DE, Fry JR. 2006. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther 111(1):174–193, PMID: 16324748, 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, et al. . 2014. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses' Health Study II Cohort. Environ Health Perspect 123(3):264–270, PMID: 25522338, 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. 2007. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 115(10):1482–1489, PMID: 17938740, 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, Green JA. 2001. The modified checklist for autism in toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord 31(2):131–144, PMID: 11450812, 10.1023/A:1010738829569. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. 1996. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370(2):247–261, PMID: 8808733, . [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. 2003. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37(20):4543–4553, PMID: 14594359, 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. 2003. SCQ: Social Communication Questionnaire. Los Angeles, CA:Western Psychological Services. [Google Scholar]
- Sacrey LA, Zwaigenbaum L, Bryson S, Brian J, Smith IM, Roberts W, et al. . 2015. Can parents' concerns predict autism spectrum disorder? A prospective study of high-risk siblings from 6 to 36 months of age. J Am Acad Child Adolesc Psychiatry 54(6):470–478, PMID: 26004662, 10.1016/j.jaac.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. . 2012. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485(7397):237–241, PMID: 22495306, 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E, et al. . 2015. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry 21(5):693–700, PMID: 26055426, 10.1038/mp.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saude E, Sykes B. 2007. Urine stability for metabolomic studies: effects of preparation and storage. Metabolomics 3:19–27, 10.1007/s11306-006-0042-2. [DOI] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. . 2011. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22(4):476–485, PMID: 21610500, 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. . 2012. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 96(1):80–89, PMID: 22648721, 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DI, Lott P, Korf I, LaSalle JM. 2011. Large-scale methylation domains mark a functional subset of neuronally expressed genes. Genome Res 21(10):1583–1591, PMID: 21784875, 10.1101/gr.119131.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DI, Schmidt RJ, Crary-Dooley FK, Walker CK, Ozonoff S, Tancredi DJ, et al. . 2016. Placental methylome analysis from a prospective autism study. Mol Autism 7:51, PMID: 28018572, 10.1186/s13229-016-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabihkhani M, Lucey GM, Wei B, Mareninov S, Lou JJ, Vinters HV, et al. . 2014. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem 47(4-5):258–266, PMID: 24424103, 10.1016/j.clinbiochem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Tancredi DJ, Hertz-Picciotto I. 2010. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 3(1):30–39, PMID: 20143326, 10.1002/aur.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Hertz-Picciotto I, Pessah IN. 2012. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect 120(7):944–951, PMID: 22534084, 10.1289/ehp.1104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. . 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 122(10):1103–1109, PMID: 24954055, 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, McKone TE, Tulve NS, Clifton MS, Bennett DH. 2013. Indoor residence times of semivolatile organic compounds: model estimation and field evaluation. Environ Sci Technol 47(2):859–867, PMID: 23244175, 10.1021/es303316d. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. 1984. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. Circle Pines, MN:American Guidance Service. [Google Scholar]
- Suades-González E, Gascon M, Guxens M, Sunyer J. 2015. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology 156(10):3473–3482, PMID: 26241071, 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. . 2013. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309(6):570–577, PMID: 23403681, 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. . 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 30(4):963–972, PMID: 25697839, 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, et al. . 2014. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res 24(7):1064–1074, PMID: 24709820, 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K, et al. . 2006. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect 114(2):176–181, PMID: 16451851, 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee DS, Hess JL, Quinn TP, Barve R, Huang H, Zhang-James Y, et al. . 2016. Blood transcriptomic comparison of individuals with and without autism spectrum disorder: a combined-samples mega-analysis. Amer J Med Genet Part B 9999:1–21, PMID: 27862943, 10.1002/ajmg.b.32511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2010. “Reevaluation: Pyrethrins and pyrethroids.” http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html [accessed 24 October 2010].
- Vester A, Caudle WM. 2016. The synapse as a central target for neurodevelopmental susceptibility to pesticides. Toxics 4(3):18, PMID: 29051423, 10.3390/toxics4030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel JF, Warembourg C, Le Maner-Idrissi G, Lacroix A, Limon G, Rouget F, et al. . 2015. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: the PELAGIE mother-child cohort. Environ Int 82:69–75, PMID: 26057254, 10.1016/j.envint.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Viel JF, Rouget F, Warembourg C, Monfort C, Limon G, Cordier S, et al. . 2017. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: The PELAGIE mother-child cohort. Occup Environ Med 74(4):275–281, PMID: 28250046, 10.1136/oemed-2016-104035. [DOI] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. 2012. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70(1):71–77, 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. 2014. Autism spectrum disorder: interaction of air pollution with the met receptor tyrosine kinase gene. Epidemiology 25(1):44–47, PMID: 24240654, 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm Behav 101:94–104, PMID: 29137973, 10.1016/j.yhbeh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. 2015. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr 169(2):154–162, PMID: 25485869, 10.1001/jamapediatrics.2014.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li K, Zhao D, Li L. 2017. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis. Mol Autism 8:51, PMID: 29026508, 10.1186/s13229-017-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Fortenberry GZ, Sánchez BN, Barr DB, Panuwet P, Schnaas L, et al. . 2016. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: distribution and relationships with child neurodevelopment. Environ Res 147:307–313, PMID: 26922411, 10.1016/j.envres.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. 2002. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J Speech Lang Hear Res 45(6):1202–1218, PMID: 12546488, 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Windham G, Zhang L, Gunier R, Croen L, Grether J. 2006. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect 114(9):1438–1444, PMID: 16966102, 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Giulivi C. 2016. Autism, mitochondria and polybrominated diphenyl ether exposure. CNS Neurol Disord Drug Targets 15(5):614–623, PMID: 27071785, 10.2174/1871527315666160413122624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhao W, Deng K, Zhou V, Zhou X, Hou Y. 2017. The association between air pollutants and autism spectrum disorders. Environ Sci Pollut Res Int 24(19):15949–15958, PMID: 28540549, 10.1007/s11356-017-8928-2. [DOI] [PubMed] [Google Scholar]
- Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R. 2016. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ Sci Pollut Res Int 23(24):24642–24693, PMID: 27714658, 10.1007/s11356-016-7648-3. [DOI] [PubMed] [Google Scholar]
- Zempleni J. 1995. Pharmacokinetics of vitamin B6 supplements in humans. J Am Coll Nutr 14(6):579–586, PMID: 8598418, 10.1080/07315724.1995.10718546. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Delwiche L, Walker C, Hertz-Picciotto I. 2011. Month of conception and risk of autism. Epidemiology 22(4):469–475, PMID: 21543984, 10.1097/EDE.0b013e31821d0b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. 2012. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 43(1):25–33, PMID: 22562209, 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Yoshida C, Gunderson EP, Dorward K, Croen LA. 2015. Interpregnancy interval and risk of autism spectrum disorders. Pediatrics 136(4):651–657, PMID: 26371204, 10.1542/peds.2015-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.