Abstract
Background:
The Minamata Convention on Mercury provided a mandate for action against global mercury pollution. However, our knowledge of mercury exposures is limited because there are many regions and subpopulations with little or no data.
Objective:
We aimed to increase worldwide understanding of human exposures to mercury by collecting, collating, and analyzing mercury concentrations in biomarker samples reported in the published scientific literature.
Method:
A systematic search of the peer-reviewed scientific literature was performed using three databases. A priori search strategy, eligibility criteria, and data extraction steps were used to identify relevant studies.
Results:
We collected 424,858 mercury biomarker measurements from 335,991 individuals represented in 312 articles from 75 countries. General background populations with insignificant exposures have blood, hair, and urine mercury levels that generally fall under , , and , respectively. We identified four populations of concern: a) Arctic populations who consume fish and marine mammals; b) tropical riverine communities (especially Amazonian) who consume fish and in some cases may be exposed to mining; c) coastal and/or small-island communities who substantially depend on seafood; and d) individuals who either work or reside among artisanal and small-scale gold mining sites.
Conclusions:
This review suggests that all populations worldwide are exposed to some amount of mercury and that there is great variability in exposures within and across countries and regions. There remain many geographic regions and subpopulations with limited data, thus hindering evidence-based decision making. This type of information is critical in helping understand exposures, particularly in light of certain stipulations in the Minamata Convention on Mercury. https://doi.org/10.1289/EHP3904
Introduction
Mercury is a pollutant of global concern largely because of its adverse effects on human health. The current state of knowledge concerning the human health impacts of mercury has been extensively reviewed by international agencies (IPCS 1990, 2003; JECFA 2007a, 2007b, 2011; EFSA CONTAM Panel 2012) as well as by national agencies and other authors (ATSDR 1999; NRC 2000; U.S. EPA 1997, 2001; Clarkson and Magos 2006; Eagles-Smith et al. 2018; Ha et al. 2017; Karagas et al. 2012).
All populations worldwide are likely exposed to some amount of mercury (UNEP/WHO 2008). Human exposures to elemental and inorganic mercury may occur in occupational settings [e.g., in artisanal and small-scale gold mining (ASGM) and dentistry], from contact with certain products (e.g., dental amalgams, some skin-lightening creams, broken fluorescent bulbs and other waste products), and from environmental contamination (Mergler et al. 2007; ATSDR 1999; Clarkson and Magos 2006; UNEP/WHO 2008; Eagles-Smith et al. 2018; Ha et al. 2017). Mercury released into the environment may be converted by microorganisms to methylmercury, which bioaccumulates and biomagnifies through the food web, particularly in aquatic systems (Obrist et al. 2018). Seafood is the main source of protein for about 1 billion people worldwide (FAO 2014). Sampling of seafood has found widespread methylmercury contamination (Sioen et al. 2009; GEMS/Food Contaminants 2018), with some widely consumed predatory species (e.g., tuna, swordfish, grouper, mackerel) being among the most highly contaminated (GEMS/Food Contaminants 2018; Groth 2010). Therefore, for many communities, dietary consumption of contaminated fish, shellfish, and marine mammals is an important source of exposure. Other foods, such as rice grown in sites heavily contaminated with mercury, may also represent a source of exposure for some communities (Rothenberg et al. 2014).
The entry into force of the Minamata Convention on Mercury on 16 August 2017 signaled the global commitment by governments to reduce the use and environmental release of mercury in order to protect human health and the environment (Article 1) (UNEP 2017). Notably, Convention Article 16 is titled “Health Aspects,” and Article 19 (“Research, Development and Monitoring”) emphasizes a need to focus on vulnerable populations (19.1c) and to follow harmonized methods (19.1d). Article 22 describes the process for evaluating the effectiveness of the convention, which includes monitoring trends in human exposure. The onus is now on parties to the convention to develop and implement strategies and programs to identify and protect populations at risk of exposure, particularly vulnerable populations; to set targets for mercury exposure reduction; and to develop means for assessing the effectiveness of control measures, for example, by monitoring human exposure to mercury.
Human exposures can be assessed through the measurement of mercury concentrations in a number of different biological sample types, and key approaches for mercury biomonitoring have been reviewed by UNEP/WHO (2008) and the U.S. EPA (1997). The most commonly used biomarkers are the concentrations of mercury in hair, urine, blood, and cord blood, and their selection can depend on factors such as the potential source of exposure, chemical form, and exposure life stage. Here we briefly elaborate on these biomarkers.
Analysis of hair is commonly used to assess exposure to methylmercury, which accounts for 80–90% of the total mercury content within this matrix (Clarkson and Magos 2006; UNEP/WHO 2008; NRC 2000). Once incorporated, the mercury remains in the hair, and this biomarker can therefore provide an integrated measurement of internal exposure to methylmercury. Because hair grows at approximately per month, exposure can be tracked over time by careful sampling. Hair has the advantage that it is easy to collect and transport, although, based on the authors’ experiences in several countries, there may be cultural objections to taking hair samples in some communities. In highly contaminated areas, however, there is a danger of external contamination of the hair, which can confound interpretation of the measurements. For example, external contamination of hair by elemental mercury has been demonstrated in ASGM communities (Sherman et al. 2015). Therefore, when conducting studies in contaminated sites, care is needed in the interpretation of total mercury levels in hair. In such settings analyzing the hair for methylmercury rather than total mercury gives a better measure of dietary exposure (Sherman et al. 2015), especially when coupled with quality survey instruments.
Urine analysis primarily provides information about exposure to inorganic and elemental mercury, although in people with high seafood consumption methylmercury may also contribute to the mercury content (Sherman et al. 2013). Because the concentration of the analyte may depend on the dilution of the urine, which can vary, the measurement of mercury is often expressed in terms of its concentration per unit of creatinine or in relation to the specific gravity of the urine sample. Urine is a relatively easy and noninvasive sample to collect.
Mercury is measured in whole blood and this provides information about recent () exposures to both methylmercury and inorganic mercury (Clarkson and Magos 2006). In most communities, the measurement of blood total mercury is an accepted biomarker for methylmercury exposure because it correlates relatively well to seafood consumption (Sheehan et al. 2014). The use of speciation can provide an indication of potential mercury sources but requires careful sample preparation and sophisticated instrumentation. The measurement of mercury in cord blood provides information about developmental exposure (UNEP/WHO 2008). Blood collection, storage, and transport pose certain logistical and financial barriers, however.
Each biomarker can provide pertinent exposure information on the type of mercury (organic vs. inorganic) and timeline of exposure (acute vs. chronic). When multiple biomarker measurements are taken from a given individual and combined with surveys, a deeper exposure assessment can be performed. In general, careful measurement of mercury content in hair and urine offers the most convenient and cost-effective scheme to monitor mercury in a given population, particularly those situated in resource-limited settings (UNEP/WHO 2008). Although we can conclude that human biomonitoring of mercury provides reliable information on exposures to mercury and that it is relatively straightforward (especially compared with other chemical pollutants), there remain major gaps in our knowledge of mercury exposures around the world. Recent comprehensive reviews of biomonitoring data on mercury exposure among seafood consumers (Sheehan et al. 2014) and the ASGM sector (Gibb and O’Leary 2014) as well as a regional assessment across Europe (Višnjevec et al. 2014) and the Arctic (Donaldson et al. 2016) suggest that there are gaps in data from many geographic regions and subpopulations. Assessing the effectiveness of the Minamata Convention on Mercury in terms of its impact on human exposure to mercury will, therefore, necessitate worldwide cooperation and coordination to fill these data gaps (Evers et al. 2016).
This study aims to increase the understanding of human exposures to mercury worldwide by collating and analyzing data on mercury concentrations in biomarker samples by using a systematic approach to review published studies. This paper focuses on biomarkers of mercury exposure in human tissue (i.e., hair, urine, blood, cord blood) for which there is a reasonably large body of knowledge with well-validated methods of measurement and interpretation. The impetus for this review was the decision to include a chapter on human biomonitoring of mercury exposure in the 2018 UN Global Mercury Assessment, an effort that was coordinated by the World Health Organization (WHO). The 2018 UN Global Mercury Assessment is being developed at the request of the Governing Council of the UN Environment Assembly (Decision 27/12), rather than at the request of the Conference of the Parties of the Minamata Convention. Nevertheless, it is anticipated that the exposure data collected here will provide a useful foundation for work to come on assessing the effectiveness of the convention as well as identifying data gaps requiring attention.
Methods
Search Strategy
A protocol for the search strategy was developed by the authors prior to conducting the work, and it was refined based on a variety of external reviews. The protocol was developed through consulting key resources [Preferred reporting items for systematic reviews and meta-analyses (PRISMA); http://www.prisma-statement.org/; OHAT 2015] including a recent systematic review concerning methylmercury exposure from seafood consumption (Sheehan et al. 2014). The protocol defined the biomarkers and study populations that would be considered, the criteria for selecting studies, and the data that would be extracted for each study. The protocol was initially reviewed by the larger group working on the 2018 UN Global Mercury Assessment project and was later commented on during an external review of the preliminary draft chapters organized by the UN Environment Programme (UNEP). This external review included input from representatives of UN member states and scientific experts.
We decided to focus our review on data from three key study types, as follows. National human biomonitoring programs (A): These programs are usually sponsored and/or operated by government agencies. Longitudinal birth cohort studies (B): These studies tend to provide high quality data on fetal exposures. Cross-sectional studies (C): These studies focus on four target groups a) general background populations, that is, those with no particular or significant exposure to mercury; b) populations who are vulnerable because of point source exposures to inorganic or elemental mercury sources (for this review: populations involved in the ASGM sector and community members, people living and working in former mercury-contaminated sites, and dental workers); c) populations who are vulnerable because of relatively high exposure to methylmercury through their diet (i.e., groups with a high consumption of fish and other aquatic animals including, for example, Indigenous Peoples and coastal and island communities); and d) populations vulnerable because of fetal exposures. For fetal exposures, we drew on biomarker measurements taken from the pregnant mother, cord blood at delivery, and measurements in infants. For each included cross-sectional study, we assigned the population to the most appropriate group (based on our expert understanding of the study population and design), realizing that the categories are not mutually exclusive.
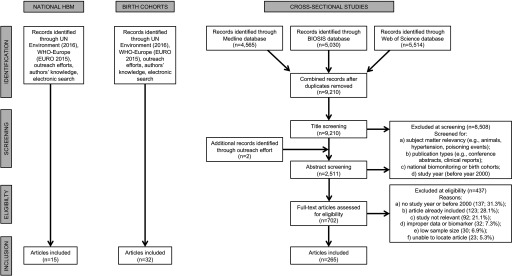
The national biomonitoring studies and the birth cohort studies were initially identified using lists compiled by the UNEP (2016) and WHO-Europe (2015) that was supplemented by a search of government websites for relevant data and through an outreach effort for the 2018 UN Global Mercury Assessment involving a web-based consultation with UN Member States and experts (Figure 1). A systematic electronic literature search was used to identify the cross-sectional studies (i.e., four target groups) and helped supplement the identification of other national biomonitoring studies and birth cohort studies. The electronic literature search was carried out on three databases (Medline, Biosis, and Web of Science Core Collection), with the last search carried out on 23 January 2018 as outlined in the PRISMA flow diagram (Figure 1). The search strategy included two Boolean search phrases: #1—“mercury OR methylmercury OR (methyl AND mercury) OR MeHg”; and #2—“blood OR hair OR urine.” As an example, the Medline search strategy was as follows: ((“mercury” [MeSH Terms] OR “mercury” [All Fields]) OR (“methylmercury compounds” [MeSH Terms] OR (“methylmercury” [All Fields] AND “compounds” [All Fields]) OR “methylmercury compounds” [All Fields] OR “methylmercury” [All Fields])) OR (methyl[All Fields] AND (“mercury”[MeSH Terms] OR “mercury” [All Fields]))) AND (((“blood” [Subheading] OR “blood” [All Fields] OR “blood” [MeSH Terms]) OR (“urine” [Subheading] OR “urine” [All Fields] OR “urine” [MeSH Terms])) OR (“hair” [MeSH Terms] OR “hair” [All Fields])).
Figure 1.
PRISMA flow diagram indicating the number of articles that were identified, screened, and included in the current review from the three main study types. Note: HBM, human biomonitoring of mercury programs; UNEP, UN Environment Programme; WHO-Europe, WHO Regional Office for Europe.
Study Selection Criteria
Data from national biomonitoring programs were expected to be of good quality because these studies are usually designed to be nationally or regionally representative (Haines et al. 2017; CDC 2017). Therefore, these studies tend to use random sampling of an adequate population size and use reference laboratories for mercury analysis. The studies are also required to have ethical committee approval. All national biomonitoring programs that measured mercury in hair, blood, urine, or cord blood were included (i.e., no exclusion criteria were applied).
Data from longitudinal birth cohort studies were also expected to be of good quality given that these are resource-intensive, usually well-designed studies that aim to answer questions about the longer-term health impacts of early life exposures (Vrijheid et al. 2012). To be included, however, there had to be at least two discrete sampling periods, one of which was an exposure biomarker measured during pregnancy or birth. Studies that did not have these two discrete exposure measures could be considered for inclusion as a cross-sectional study under the fetal exposures category.
For cross-sectional studies, the studies were identified through the electronic search (Figure 1). Scientific papers were reviewed through a two-stage process: first, the title and abstract fields were searched to ascertain relevancy, and second, the full text was reviewed on papers that were deemed potentially relevant. We applied an a priori sample size cutoff (ranging between 50 and 200) that was differentially applied to high-income countries versus low- and middle-income countries as well as across the different cross-sectional study types (Table 1). This was in order to ensure that the studies were more likely to be representative of the population studied. Because we anticipated fewer studies in low- and middle-income countries, we reduced the minimum sample size so as to be able to report at least some data. We then restricted our search to populations who were sampled in the year 2000 onward because a) it matched the approach used by Višnjevec et al. (2014) in their assessment of mercury exposures across Europe; b) it represented an inflection point in the mercury exposure literature (i.e., number of publications and types of exposures) as observed by Sheehan et al. (2014); c) it represented a period in which the quality of analytical approaches to characterize mercury exposure started to increase, based on our professional judgment; and d) it represented a relatively recent period with ample data from which an understanding of current global exposures could be established.
Table 1.
Method of identification and criteria for selection of studies.
| Study type | Studies identified by expert panel | Studies identified from country submissions to UNEP or WHO-Europe | Studies identified by electronic search | Sampling year | Minimum sample size |
|---|---|---|---|---|---|
| A. National biomonitoring | Yes | Yes | Yes | Any | N/A |
| B. Longitudinal birth cohort | Yes | Yes | Yes | Any; but a minimum of 2 sampling periods | N/A |
| C. Cross-sectional studiesa | |||||
| General populations (HIC) | No | N/A | Yes | 2000–present | |
| General populations (LMIC) | Yes | N/A | Yes | 2000–present | |
| Vulnerable populations (HIC) | No | N/A | Yes | 2000–present | |
| Vulnerable Populations (LMIC) | Yes | N/A | Yes | 2000–present |
Note: All studies provided a point estimate of central tendency (median, geometric mean) and a measure of variation from which an upper bound could be determined. HIC, high-income countries; LMIC, low- and middle-income countries; N/A, not applicable; UNEP, UN Environment Programme; WHO-Europe, World Health Organization Regional Office for Europe.
HIC and LMIC were as categorized by the World Bank (Fantom and Serajuddin 2016).
In all cases, to be included, studies had to provide a point estimate of the central tendency and a measure of variation from which an upper bound value could be determined. When a study was reported upon in multiple articles, we chose the article with the most complete data set to serve as a representative piece.
Data Extraction and Analysis
For all included studies we extracted the following data: population characteristics (age, age group, sex), geography (country, city or sub-country region, WHO region), mercury exposure measurements [sample size, mercury biomarker and speciation information, analytical quality control (including reported detection limit, accuracy, and precision)], and measures of central tendency (geometric mean or median or mean) and high-end (95th or 90th or 75th percentile or maximum value) biomarkers. Information on fish consumption and proximity to water bodies was also noted. Papers were also checked for mention of ethical approval.
To summarize the mercury biomarker data, we adopted the approach of Sheehan et al. (2014), and thus reported upon two summary distributions (central and upper) for each biomarker for a given category by pooling the data across relevant studies. Because studies often provide multiple measures of central or high-end exposures, we favored certain measures of central exposures () and high-end () as indicated in the brackets in terms of decreasing order of preference.
We evaluated study quality by examining risk of bias in three areas based on guidance from the U.S. National Toxicology Program’s (NTP) Office of Health Assessment and Translation (OHAT 2015): a) Selection Bias (selection method, demographics, exposure characteristics), b) Exposure Detection Bias (quality of the methods used to measure the mercury biomarkers), and c) Statistical and Other Bias (biomarker distribution, reporting Hg exposure sources, ethics approval). These were not used to exclude studies but rather to help understand the state of the science worldwide from which conclusions could be drawn and recommendations made.
Throughout our report, we refer to total mercury as “mercury” measurements in a given sample type. We present data on each biomarker type but to maximize the use of the data, we also converted results across biomarker types by converting between hair and blood measurements and between cord blood and maternal blood. For the conversions, there are two notable conventions for exposures that largely involve methylmercury (applicability to elemental and inorganic mercury is not known). First, the Joint Food and Agriculture Organization (FAO) and WHO Expert Committee on Food Additives (JECFA) established a methylmercury hair-to-blood ratio of 250 that is now commonly used by the research community (JECFA 2004). Second, cord blood levels are, on average, 70% higher (ranging from 10% to over 200% higher) than maternal blood as discussed by Stern and Smith (2003), and thus a conversion factor of 1.7 was used here. Although we used these conversions to derive blood mercury equivalents from hair and cord blood values, we acknowledge on-going debate in the literature concerning the validity of these approaches, in particular relating to heterogeneity across individuals with respect to influential factors such as sex, age, and ethnicity (Stern and Smith 2003; Bartell et al. 2000; JECFA 2007a; EFSA CONTAM panel 2012). In addition, there is a growing awareness that interindividual differences in the toxicokinetics of mercury and the resulting biomarker measurements may be influenced by genetic polymorphisms (Basu et al. 2014) although at this time such information cannot be put into practice. Nonetheless, biomarker conversions facilitate comparability across studies, and they have been effective at helping derive large, regional biomonitoring assessments (e.g., in Europe by Višnjevec et al. 2014; in the Arctic by Donaldson et al. 2016).
Results
Overview of Studies
In total, we collected 424,858 mercury biomarker measurements taken from 335,991 individuals represented in 312 articles from 75 countries (Table 2; see also Excel Table S1). For the national biomonitoring programs, we obtained mercury exposure data from nine countries (Belgium, Canada, Czech Republic, France, Germany, Republic of Korea, Slovenia, Sweden, and the United States) (see Tables S1–S3). The total sample population of these surveys, which sometimes span multiple years, comprised 121,413 people from whom 192,651 biomarker measurements of mercury exposure were extracted (see Table S2).
Table 2.
Count of studies, countries, individuals, and measurements by the type of population included in this review.
| Population type | Study type | Studies (n) | Countries (n) | Individuals (n) | Biomarker measurements (n) |
|---|---|---|---|---|---|
| Background general population | National biomonitoring | 15 | 9 | 121,413 | 192,651 |
| Cross-sectional | 201 | 53 | 96,141 | 101,606 | |
| Methylmercury exposure from high consumption of fish and other aquatic animals | Cross-sectional | 71 | 18 | 29,751 | 33,814 |
| Point source exposures to inorganic and elemental mercury | Cross-sectional | 79 | 28 | 16,673 | 22,257 |
| Fetal exposure to methylmercury | Birth cohorts | 32 | 17 | 46,748 | 47,699 |
| Cross-sectional | 90 | 31 | 25,265 | 26,831 | |
| TOTALS | 312 | 75 | 335,991 | 424,858 |
Note: Column totals for number of studies and number of countries do not reflect column count data because some cross-sectional studies were broken into multiple population types (e.g., one article could include data from one or more different population types).
For the birth cohorts, we identified 32 studies (many of which had multiple sub-cohorts) from 17 countries in which there was at least one mercury exposure measurement during pregnancy or birth as well as a follow-up time period in which a mercury exposure-associated health outcome measurement was taken in the child (Table 2). The total sample population of these birth cohort studies was 23,374 mother–child pairs from which 47,699 biomarker measurements were taken.
For the cross-sectional studies, the initial database search retrieved 9,210 non-duplicate articles from which 265 articles were deemed eligible for data extraction, as outlined in the PRISMA flow chart (Figure 1) and listed in Excel Table S2. From these articles, we organized the data into 441 subpopulations from which we had 184,510 mercury biomarker measurements taken from 167,830 individuals from 73 countries (see Tables S4 and S5).
In the analysis of the cross-sectional data, we largely examined the study populations according to three variables (see Table S4): by WHO geographic region, by population type and source of exposure (point source, dietary, fetal), and by proximity to water bodies. This enabled a deeper assessment of key drivers that may help explain exposure biomarker levels. Briefly, and as further outlined below (percentages that follow are a fraction of the number of cross-sectional subpopulations, ), most cross-sectional studies were situated, according to WHO region classifications, in Europe (29.5%), Western Pacific (28.6%), and the Americas (25.6%) with far fewer studies from the Eastern Mediterranean (6.6%), Africa (5.2%), and South-East Asia (4.5%). Of the population types and sources of exposure, 45.6% were assigned to the general background group that had no known significant sources of mercury exposure, 20.4% were assigned to the fetal exposure group, 17.9% were assigned to the point source exposures to inorganic or elemental mercury group, and 16.1% were assigned to the dietary exposure to methylmercury group. In terms of proximity to water bodies, 55.1% of the groups were situated inland (40.6% with no link to water and 14.5% were associated with inland lakes or rivers), and 38.5% were associated with marine (oceanic) ecosystems. Finally, the average sample size across the cross-sectional studies was 249 individuals with a range of 50 to 1,910.
Study Quality
As explained earlier, national biomonitoring and birth cohort studies were taken to be of good quality. For the cross-sectional studies, 62% of the articles that were read were excluded, with the most common reasons for exclusion being no study year listed or study was before year 2000, study already included, and study not relevant (see the PRISMA diagram in Figure 1 for more information). We did not attempt to characterize the excluded studies in any manner.
For the cross-sectional studies, we evaluated study quality by examining risk of bias in three categories as detailed earlier (below, percentages are a fraction of the number of cross-sectional subpopulations, ). In terms of Selection Bias categories, a) participants were randomly selected in 8.6% of the studies (1 study each from Africa and Eastern Mediterranean; 9 from the Americas; 12 from Western Pacific; 15 from Europe); b) both sexes were studied in 66.4% of the studies, whereas 26.3% focused only on females, 3.0% focused only on males, and 4.3% did not report on a participant’s sex; c) age was reported in 59.2% of the studies, and of the remaining studies (180 subpopulations) all but 6 gave some indication of a participant’s life stage; and d) the studies provided enough information on sources of mercury exposure for us to assign each study to one of the four target groups described earlier. In terms of Exposure Detection Bias categories, the most commonly used instruments to quantify mercury were “direct” analyzers that combine thermal decomposition of the sample with mercury–gold amalgamation and atomic absorption detection (32.7%), followed by atomic absorption spectroscopy (28.8%) and Inductively coupled plasma mass spectrometry (ICPMS) (22.7%) on digested samples. Most (72.3%) studies considered analytical quality control measures by reporting on three key items (accuracy, precision, and detection limit), whereas 21.3% did not report any quality control measure taken (although these were carefully read and deemed appropriate). In terms of Statistical and Other Biases categories, we note that all studies included needed to have a point estimate of the central tendency of a mercury biomarker and a measure of variation from which an upper bound value could be determined. Of the estimates of central exposure that were retained here, 22.2% of the biomarker values were geometric means, 26.8% were means, and 50.6% were medians. Of the estimates of high-end exposure, 25.6% of the biomarker values were 95th percentiles, 6.5% were 90th percentiles, 4.3% were 75th percentiles, 54.1% were maximum values, and the rest were computed by adding twice the value of the standard deviation (SD) to the mean. We did not further examine a paper’s statistical quality (e.g., biomarker distribution, choice of tests). A food frequency questionnaire (FFQ) was used to calculate fish intake in 7.9% of the studies, whereas a crude survey was reported to be used in 13.4% of the studies. The remaining studies did not gauge fish intake. An evaluation of the quality of the methods used in studies to assess fish consumption was outside the scope of this review. In terms of ethical conduct of research, 16.3% of the studies had no mention of either consent being obtained from the participants or the study having received institutional review board (IRB) approval.
General Background Population Group
Blood mercury levels.
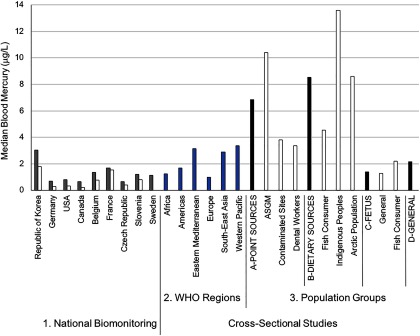
Data on background exposure in the general population were obtained from national biomonitoring studies and from cross-sectional studies (Table 2). Across the national biomonitoring studies, the majority of participants had blood mercury levels that fell below , especially when reviewing the median (Figure 2) and upper bound values (see Table S3). Blood mercury levels in both adults and children were higher in the Republic of Korea than in the other eight countries. These values were, however, similar to those in neighboring countries (see WHO Western Pacific region data in Figure 2). In all countries, blood mercury levels in adults were approximately 2.1-fold higher than in children (Figure 2; see also Table S3), and this varied across age groups. For example, median blood mercury levels in Canadians from the Canadian Health Measures Survey (CHMS), second cycle (2009–2011), increased with age as follows: for 6- to 11-y-olds, for 12- to 19-y-olds, for 20- to 39-y-olds, for 40- to 59-y-olds, and for 60- to 79-y-olds (Health Canada 2017). Similar trends were observed in the United States and Korean national data sets (Burm et al. 2016; Seo et al. 2015; CDC 2017).
Figure 2.
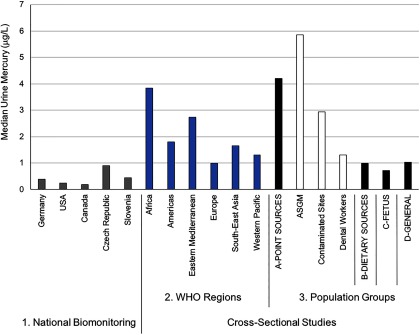
Median blood mercury levels across different population groups. In the National Biomonitoring studies (#1; reflect country representative information on exposures), the gray shaded bar refers to measures in adults, whereas the white shaded bar reflects measures in children. For the Cross-Sectional Studies (that cover all ages), all data are organized according to WHO geographic region (#2) and Populations Groups (#3) that were set a priori. Under the four main Population Groups categories, the first bar (black shade) represents the pooled biomarker data from the respective subgroups that follow (indicated in white shade, and labels prefaced with letters A–D). Subgroups that fall under the Point Sources group (A) include ASGM (individuals engaged in artisanal and small-scale gold mining); Contaminated Sites (individuals living at contaminated sites); and Dental Workers (individuals exposed from working in dental settings). Subgroups that fall under the Dietary Sources group (B) include Fish Consumer (non-Indigenous or non-Arctic groups that were identified in the study as being ones who consume relatively high amounts of seafood); Indigenous Peoples (self-identified group by study authors and not including those from the Arctic and mainly composed of populations from the Amazonian region); and Arctic Population (populations living in the Arctic or Subarctic region). Subgroups that fall under the Fetus group (C) include General (background population without specific exposures to mercury) and Fish Consumer, see above under “Dietary Sources.” The General group (D) refers to the background population without specific exposures to mercury. Note: WHO, World Health Organization. For source data, see Excel Table S3.
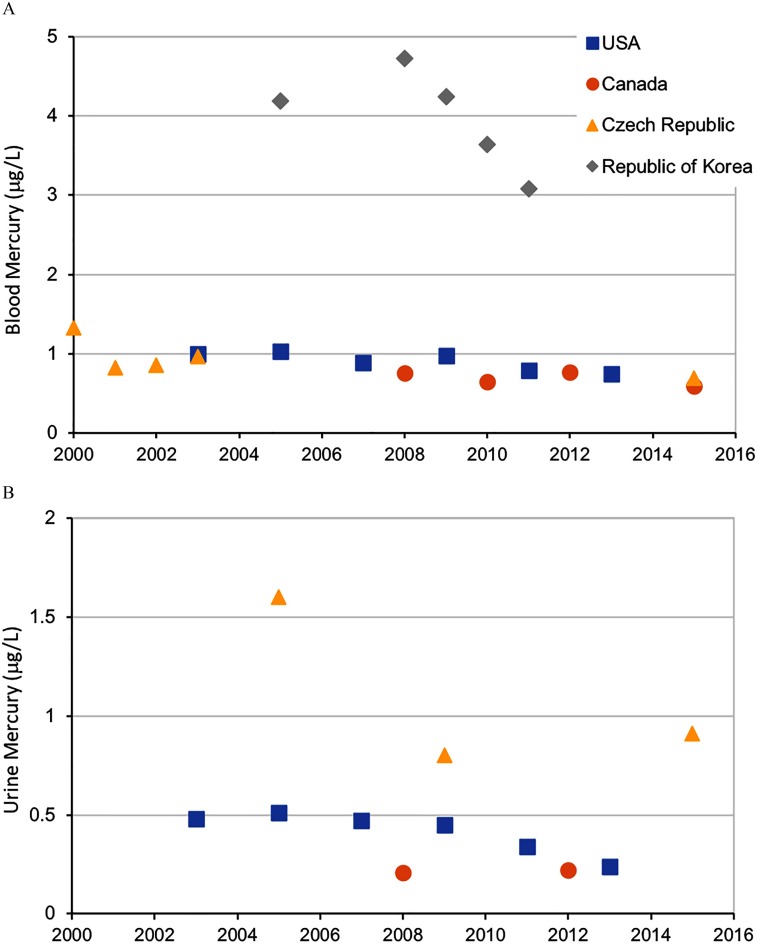
The representative data from these national biomonitoring programs can be used to gauge temporal trends, especially when there are two or more comparable sampling periods. For blood mercury, combining the data from the United States, Canada, and the Czech Republic into a linear regression model showed annual decreases of approximately or 2.25% (i.e., over 10 y, this would be a decrease of or ) with median blood mercury levels leveling around (Figure 3).
Figure 3.
Temporal changes in blood (A) and urine (B) mercury levels across national biomonitoring programs in which these measures were taken in multiple cycles. Median values are reported. For source data, see the respective references listed in Table S1.
From the cross-sectional studies, general background exposures were characterized from 201 subpopulations from 53 countries that included 52,136 and 39,035 mercury biomarker measures taken in blood and hair, respectively (Table 2; see also Table S4). The hair mercury values were converted to blood mercury equivalents as described earlier, and from this the pooled central median blood mercury concentration was [interquartile range (IQR): ] with the upper bound median value being (see Excel Table S3). These findings compare well to those of the national biomonitoring studies in which most blood mercury levels were below .
The cross-sectional data show geographic differences in exposure with pooled central median blood mercury concentrations being higher in general background populations from the Western Pacific (, IQR 2.2–6.6), Eastern Mediterranean (, IQR 1.0–8.8), and South-East Asia (, IQR 2.1–6.5) regions versus those in the Americas (, IQR 1.1–2.1), Africa (, IQR 0.7–2.8), and Europe (, IQR 0.6–2.2) (see Excel Table S3). Table S5 provides examples of reference values for mercury concentrations from different countries and organizations, which gives some context for the above results.
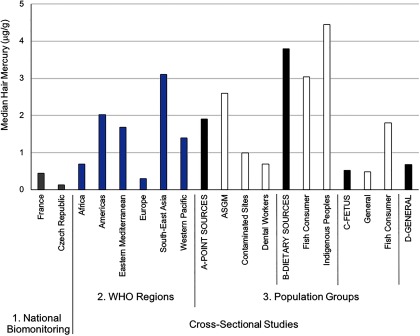
Hair mercury levels.
Across all studies, the majority of participants had hair mercury levels that fell below (Figure 4). From the national biomonitoring data, only hair mercury levels in adults were reported from Belgium and France (total of 2,264 samples), while values in children were also reported from the Czech Republic (total of 3,470 samples from 1996 to 2008) (Croes et al. 2014; Dereumeaux et al. 2016; Puklová et al. 2010). Hair mercury results were more meaningful from the cross-sectional studies given that 69,910 measurements were captured (see Table S6). The overall pooled central median mercury concentration from the cross-sectional studies was (IQR: ) with the upper bound median value being . Focusing on hair mercury levels from those categorized as coming from a general background population (39,035 measures), the overall pooled central median mercury concentration was (IQR: ) with the upper bound median value being . As with blood, hair mercury levels (median values shown) were higher in individuals sampled from the Western Pacific (), Eastern Mediterranean (), and South-East Asia () regions versus those in the Americas (), Europe (), and Africa ().
Figure 4.
Median hair mercury levels across different population groups. The National Biomonitoring Studies (#1) provide country representative information on exposures. For the Cross-Sectional Studies, all data are organized according to WHO geographic region (#2) and Populations Groups (#3) that were set a priori. Under the four main Population Groups categories, the first bar (black shade) represents the pooled biomarker data from the respective subgroups that follow (indicated in white shade). Subgroups that fall under the Point Sources group include ASGM (individuals engaged in artisanal and small-scale gold mining); Contaminated Sites (individuals living at contaminated sites); and Dental Workers (individuals exposed from working in dental settings). Subgroups that fall under the Dietary Sources group include Fish Consumer (non-Indigenous or non-Arctic groups that were identified in the study as being ones who consume relatively high amounts of seafood) and Indigenous Peoples (self-identified group by study authors and mainly composed of populations from the Amazonian region). Subgroups that fall under the Fetus group include General (background population without specific exposures to mercury) and Fish Consumer, see above under “Dietary Sources.” The General group refers to the background population without specific exposures to mercury. Note: WHO, World Health Organization. For source data, see Table S6.
Urinary mercury levels.
Urine mercury levels were consistent across the countries from which national biomonitoring data were obtained, with a majority of the values falling under when reviewing the median (Figure 5) and upper bound values (see Table S7). Urine mercury levels were higher in adults than in children, with many of the children’s values falling below analytical detection limits. The urine data from national biomonitoring programs, as with blood, can be used to gauge temporal trends; over time decreases are observed, particularly when examining the U.S. NHANES data set in which the population median mercury levels in adults ( y of age) from the latest data set is 50% lower than that measured 10 y earlier ( vs. comparing 2013 with 2003; Figure 3) (CDC 2017).
Figure 5.
Median urine mercury levels across different population groups. The National Biomonitoring Studies (#1) provide country representative information on exposures. For the Cross-Sectional Studies, all data are organized according to WHO geographic region (#2) and Populations Groups (#3) that were set a priori. Under the four main Population Groups categories, the first bar (black shade) represents the pooled biomarker data from the respective subgroups that follow (indicated in white shade and labels prefaced with letters A–D). Subgroups that fall under the Point Sources group (A) include ASGM (individuals engaged in artisanal and small-scale gold mining); Contaminated Sites (individuals living at contaminated sites); and Dental Workers (individuals exposed from working in dental settings). The Dietary Sources group (B) includes populations who consume relatively high amounts of seafood. The Fetus group (C) includes populations who are vulnerable because of early life exposures. The General group (D) refers to the background population without specific exposures to mercury. Note: WHO, World Health Organization. For source data, see Table S7.
For urine measurements from the cross-sectional studies, the pooled central median mercury concentration in the general population was (IQR: ), with the upper bound median value being (see Table S8). These urine mercury values corresponded well with the national biomonitoring studies, with most values falling under (Figure 5). Compared with blood, there were fewer urinary mercury data spread across geographical regions. Some geographic differences were apparent from the available data, with pooled central median urine mercury concentrations among those sampled from the general population being higher in the Eastern Mediterranean (, IQR 1.0–4.4) and Western Pacific (, IQR 1.2–1.6) compared with Europe (, IQR 0.5–1.1) and the Americas (, IQR 0.3–1.0) (see Table S8).
Target Population Groups
Dietary exposure from consumption of fish and other aquatic animals.
Within the cross-sectional studies, we identified 71 subpopulations from 18 countries that were specifically studied because of concerns associated with the consumption of fish and other aquatic animals. These studies included 29,751 individuals from which 33,814 mercury biomarker measures taken. The pooled central median blood mercury concentration across these studies was (IQR: ) with the upper bound median value being (Figure 2; see also Excel Table S3). Exposures in this group (both the central and upper bound median group values) were approximately four times higher than in general background populations. This is not surprising given that the consumption of fish and other aquatic animals (i.e., high consumption amounts or regular consumption of relatively highly contaminated items) is widely considered to be the main source of methylmercury exposure for most populations worldwide.
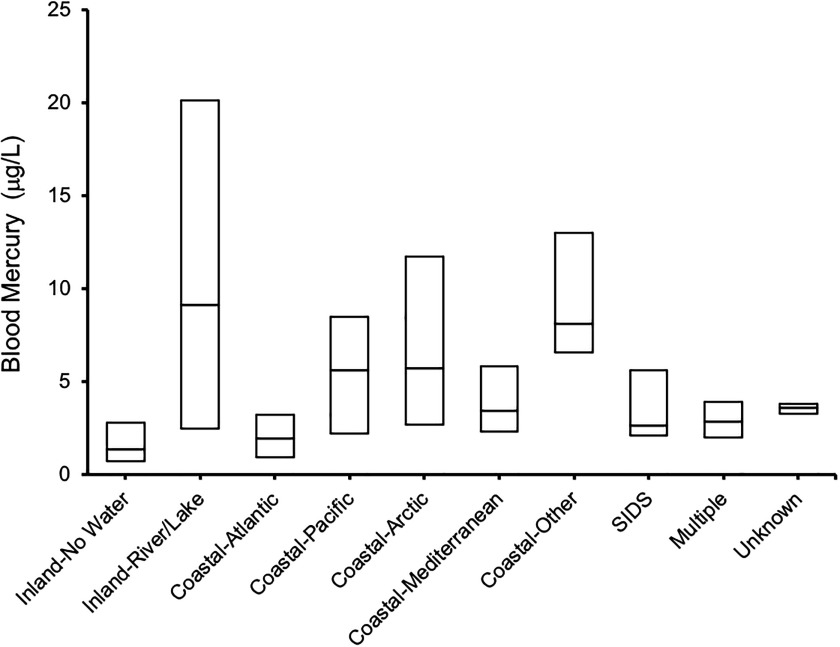
Populations associated with water bodies tended to have higher blood mercury (Figure 6; see also Excel Table S3) and hair mercury (see Table S6) levels. This was a major conclusion of the systematic review by Sheehan et al. (2014) in which they analyzed 164 studies from 43 countries to relate seafood consumption and mercury biomarkers. We make a similar observation here. Whereas groups that were situated inland with no strong link to water had a central median blood mercury concentration of (IQR: ; 55,088 individuals from 179 subpopulations); inland groups that were linked with rivers or lakes had 6.7 times higher central median blood mercury concentrations (, IQR 2.5–20.1; 24,813 individuals from 64 subpopulations). Exposures were also higher in groups associated with marine ecosystems, with central median blood mercury concentrations being highest among groups situated along the Arctic (, IQR 2.7–11.7), Pacific (, IQR 2.2–8.5), Mediterranean (, IQR 2.3–5.8), and Atlantic (, IQR 0.9–3.2) seas. Another highly exposed, but relatively understudied, group comprises people living within Small Island Developing States (SIDS). We found 16 subpopulations from SIDS that included 1,325 individuals, and within this group the central median blood mercury concentration was (IQR: ), with the upper bound median value being . The Seychelles is an example of a particularly well-known SIDS within the mercury research community (Strain et al. 2015).
Figure 6.
Box plots of blood mercury levels across different population groups in association with water bodies. The mid bar of the box plot refers to the median, and the upper and lower boundaries refer to the 75th and 25th percentile values, respectively. Coastal, populations living on the coast of various oceans from where they might take seafood; Inland, populations living away from coastal regions, either living close to rivers or lakes from where they might take fish (Inland-River/Lake) or not associated with any water (Inland-No Water); Multiple, studies in which the populations were associated with different categories; SIDS, Small Island Developing States; Unknown, not enough information was provided in the study to assign a particular location. For source data, see Excel Table S3.
Data from within this section on cross-sectional populations also provides strong evidence that Indigenous Peoples in many areas of the world (but especially the Inuit from the Arctic and groups within the Amazonian region) generally experience high exposures to mercury. Many from these communities are reliant upon traditional and locally caught foods such as fish and marine mammals for sustenance. For example, Cisneros-Montemayor et al. (2016) compiled data from more than 1,900 coastal Indigenous groups (27 million people from 87 countries) to show that per capita seafood consumption in these communities is 15 times higher than in non-Indigenous groups. In addition, such traditional foods also form a strong basis for the culture, spirituality, recreation, and economy of many of these communities, and so contamination of food by mercury presents an issue of environmental justice (Nriagu et al. 2012).
The Inuit in the Arctic are exposed to some of the highest methylmercury levels globally, largely due to their reliance on fish and marine mammals as culturally important food staples. Here we identified 15 Arctic subpopulations from which 7,472 individuals were studied, with the resulting central median blood mercury concentration being (IQR: ) with an upper bound median concentration of (Figure 2; see also Excel Table S3). The Artic Monitoring and Assessment Program (AMAP) Human Health Report (Donaldson et al. 2016) is exemplary in its review of several human biomonitoring programs across the circumpolar region, many of which are large and complex undertakings. As an example, in Canada as part of the 2007–2008 International Polar Year study of 2,172 individuals, the geometric mean of blood mercury across four study regions ranged from 2.8 to , with individual values ranging from 0.1 to (Donaldson et al. 2016). In another example, the Greenlandic Inuit Health and Transition Study of 3,105 participants from all geographic areas and community sizes (9 towns, 13 villages) reported blood mercury levels to range from 0.1 to (Donaldson et al. 2016).
Indigenous Peoples residing within the Amazon have also been documented to be highly exposed to mercury through both fish consumption and proximity to gold mining (Berzas Nevado et al. 2010), and thus it is not surprising to find elevated mercury levels in both blood and urine. Here we identified 46 subpopulations (from three countries) from which 18,509 individuals (and 20,344 measures) were studied, with the resulting central median blood mercury concentration being (IQR: ) and an upper bound median concentration of (IQR: 54.3–170.3). For urine, the central median urinary mercury concentration was (IQR: ) with an upper bound median concentration of (IQR: 63.8–178.8). Exposures to mercury within this group are approximately 7.5 times higher for both urine and blood when compared with the general background populations identified in the cross-sectional studies.
Point source exposures to inorganic and elemental mercury.
In this group, 79 subpopulations from 28 countries were identified, including 16,673 individuals from which 22,257 mercury biomarker measures taken. The pooled central median blood mercury concentration was (IQR: ) with the upper bound median value being (Figure 2; see also Excel Table S3). For urine, which is the primary biomarker to gauge inorganic and elemental mercury exposures, the pooled central median mercury concentration was (IQR: ) with the upper bound median value being (Figure 5; see also Table S8). These values are higher than those found in the general populations, as discussed previously.
Exposures in this group were driven by the ASGM sector (pooled central median urine concentration was , IQR: ). For example, the upper bound median urinary mercury levels among ASGM workers and community members (, IQR 79–374) is approximately 10- and 20-fold higher than the same upper measurement calculated from studies based in the two other “point source” groups (contaminated sites and dental workers, respectively); this value is also 30- and 200-fold higher than in the general populations (as identified through the cross-sectional studies), with upper () and central () bound urinary mercury levels, respectively. The ASGM sector is rapidly growing worldwide with upward of 15 million miners estimated to be directly involved with mining and potentially 100 million people living in ASGM communities (WHO 2016; UNEP 2012). There are a number of public health concerns in ASGM communities (Basu et al. 2015; WHO 2016) as well as a growing number of human biomonitoring studies to showcase the high mercury exposures (reviewed by Gibb and O’Leary 2014). Our values from 3,463 individuals are comparable with an earlier meta-analysis of data from 1,245 miners (many of which data are included here) from across Indonesia, Philippines, Tanzania, Zimbabwe, and Mongolia. This meta-analysis reported median urine mercury values of (95th percentile ), with maximum values in excess of (Baeuml et al. 2011).
A number of studies were performed in areas classified as mercury-contaminated sites. Of the 45 subpopulations identified, most were based in Europe (18) and Asia-Pacific (17), and most represented sites with industrial (non-mining) facilities (e.g., chlor-alkali industry, waste incinerators, smelters), whereas 8 represented mercury-mining sites. Mercury exposures among this group was approximately 3- and 1.5 times higher (for urine and blood mercury, respectively) than in general background populations (from across all regions) at both the central and upper-bound levels.
Four subpopulations of dental workers were identified here (see Excel Table S3). The pooled central median blood mercury concentration in this group was (IQR: ), with the upper bound median value being . For urine, the pooled central median mercury concentration was (IQR: ), with the upper bound median value being (see Table S8).
Fetal exposures.
The presence of methylmercury in fish and other aquatic animals poses particular risk–benefit dilemmas to all populations because there are health benefits (e.g., from selenium and polyunsaturated fatty acids), while, conversely, there may be risks from the same food items because of the amounts of methylmercury they contain (FAO/WHO 2010). This is especially true for pregnant women and fetuses, groups that have particular susceptibility to exposure to mercury (as well as other chemical pollutants). Not surprisingly, birth cohort studies concerning methylmercury have received tremendous scientific, regulatory, and societal attention. We identified 32 studies that included 23,374 mother–child pairs from which 47,699 biomarker measurements were taken (see Excel Table S4). Of these studies, 53% measured mercury in cord blood, 28% measured mercury in maternal blood during pregnancy, and 59% measured mercury in maternal hair. In general, these birth cohort studies focused on methylmercury exposures.
There are some noteworthy observations from the birth cohort data set (see Excel Table S4). First, groups consuming large amounts of seafood (Seychelles, Spain), freshwater fish (Brazil) and/or marine mammals (Faroe Islands, Inuit communities of the Arctic) have the highest mercury exposures that often exceed in cord blood. There has, however, been a decrease in mercury exposures in the Faroe Islands of nearly 5-fold from to (blood mercury from 22.3 to ), and in the Seychelles of approximately 2-fold from to (hair mercury from 5.9 to ) (Donaldson et al. 2016; Strain et al. 2015). Elsewhere, cord blood mercury levels range between 5 and across several Mediterranean populations, are approximately in Asia, and generally less than across communities in North America and Europe (excluding Indigenous Peoples and the Mediterranean).).
We also identified a number of cross-sectional studies in which cord blood mercury measures as well as other indices of fetal exposure (e.g., biomarker measures in the pregnant mother, hair measures in infants) were taken. Specifically, 90 subpopulations were identified from which 18,651 biomarker measures were taken from 25,265 individuals. The pooled central median blood mercury concentration in this group was (IQR: ), with the upper bound median value being (Figure 2; see also Excel Table S3). These values are generally lower than those reported from the birth cohort studies, recognizing that the focus of the aforementioned birth cohorts has often been biased toward studying the most highly exposed groups.
Discussion
This review suggests that all populations are exposed to some amount of mercury and that there is great variability in exposures around the world. We conclude that individuals in select general background populations worldwide with insignificant exposures to mercury sources have blood mercury levels that generally fall below , hair mercury levels that generally fall below , and urine mercury levels that fall below (Figures 2, 4, and 5), although these general background values can vary across certain geographic regions, as outlined earlier. Further, there are a number of vulnerable populations with relatively high mercury exposures, which we discuss below. This type of information is critical in helping understand exposures, particularly in light of the Minamata Convention on Mercury and certain stipulations within the convention text that, for example, can inform the effectiveness evaluation under Article 22, especially for vulnerable populations; gauge changes over geographic space and time.
Despite a relatively large data set to work from (i.e., 424,858 mercury biomarker measurements taken from 335,991 individuals), there remain some challenges. Foremost is that there are a number of countries and geographic regions for which data are lacking, including populations residing in SIDS and in Asia and Africa who are exposed to mercury in their diets and from point-source emissions (e.g., from ASGM and chlor-alkali industries). In our review we were able to draw from data from 75 countries, meaning that most of the world’s 194 countries do not have data of the required standard. Further, within these 75 countries, and focusing strictly on the cross-sectional studies as an example, nearly 50% (48.6%) of the data set in terms of the number of individuals studied, was represented by 4 countries (in decreasing order of contribution: Republic of Korea, China, Japan, the United States). The addition of Brazil, Saudi Arabia, Canada, and the Russian Federation takes this number to nearly 70% (69.2%). Other reviews of specific exposure groups have similarly noted these gaps (Sheehan et al. 2014; Gibb and O’Leary 2014; Višnjevec et al. 2014; Donaldson et al. 2016). As documented here, very few countries have national biomonitoring programs that yield data that is nationally representative.
We were also struck by the general lack of quality in many studies. For example, many studies did not indicate the year in which their population was sampled and thus these were not included. In terms of ethics, 56% of the included studies indicated having had IRB approval, with 27.2% noting that consent was obtained (but no indication of IRB approval) and the rest (16.3%) having neither. We characterized risk of bias in three areas based on guidance from OHAT (2015) and found a number of issues across the studies in terms of selection bias (e.g., need more information on how populations were sampled, including more randomization and details on exposure sources; need more information on age and other demographic variables), exposure detection bias (e.g., need studies to carefully report on quality control steps taken including the use of reference materials and participation in quality assurance programs), and statistical and other biases (e.g., need studies to have IRB approvals, and for more detailed exposure surveys, such as FFQs). Moving forward, we advocate that checklists, such as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), be strictly followed so that observational study findings can be suitably repurposed to contribute to a larger body of knowledge.
The Minamata Convention is motivated by human health concerns (i.e., Articles 1, 16, and 19) and one means for evaluating the effectiveness of the convention (Article 22) is to determine temporal and geographic trends in mercury exposure as reflected by human biomonitoring data (Evers et al. 2016). The significant data gaps and problems of data quality identified in this review highlight the need for more and better studies to be carried out in a wider range of countries.
There is general agreement concerning the methods to assess mercury exposure (UNEP/WHO 2008). Measurements of mercury in hair and urine are particularly suitable because they provide a relatively low-cost and noninvasive scheme to gauge exposure to the main forms of mercury (Evers et al. 2016). Further, with some basic training, sampling and handling procedures are easy to implement, and quality assurance programs and suitable reference materials are also in place to help ensure comparability of measurement results. Biomarker measures can be further improved by also including quality survey instruments that collect pertinent information on the study population and exposure sources.
Programs to harmonize mercury biomonitoring and to scale activities across regions are now being pursued. For example, the DEMOnstration of a study to COordinate and Perform Human biomonitoring on a European Scale (DEMOCOPHES) project showed that hair mercury could be measured in 1,799 mother–child pairs from 17 European countries to yield comparable values (Castaño et al. 2015). Such an approach is now being realized across six low- and middle-income countries (China, Ghana, India, Kyrgyzstan, Mongolia, and the Russian Federation) through a WHO program that is assessing prenatal exposures (via 3,631 mercury measurements in hair, cord blood, and urine) in women. This WHO program is being pursued in response to a 2014 World Health Assembly resolution (WHA67.11) on the role of the WHO and Ministries of Health in the implementation of the Minamata Convention on Mercury. The development of harmonized and standardized biomonitoring programs may enable spatial and temporal trends to be better realized.
Exposure to mercury in vulnerable groups who are sensitive because of extrinsic (e.g., high exposures) and intrinsic (e.g., genetics) factors remains of utmost concern (Eagles-Smith et al. 2018). In terms of “high exposures,” here we focused our attention on two key groups: populations exposed to inorganic and elemental mercury from point sources and populations exposed to methylmercury from the high consumption of fish and other aquatic animals. From our work, we identified four populations of concern for which there exist a relatively robust data set: a) Arctic populations (mainly Inuit) who consume fish and marine mammals; b) tropical riverine communities (especially Amazonian) who consume fish, and in some cases may be exposed to mining operations; c) coastal and/or small-island communities who rely substantially on seafood; d) individuals who either work or reside among ASGM sites. In addition to these identified and relatively well-studied groups, there are other highly exposed groups for which there is growing awareness but relatively little data to draw firm conclusions. These include individuals living in mercury-contaminated sites, particularly in low- and middle-income countries (Trasande et al. 2016), consumers of rice from contaminated sites (Rothenberg et al. 2014), and users of mercury-containing skin-lightening creams (UNEP/WHO 2008). In addition, there are certain ecosystems sensitive to mercury loading and methylation, and these may represent hotspots of biologically available methylmercury that warrant attention for those who consume local aquatic food items (Evers et al. 2016).
Mercury risk is not just driven by high exposures. There are concerns about mercury susceptibility during early life, which we reviewed here. In the birth cohort studies we identified, a range of health outcomes were measured in the newborn, infant, toddler, or child, including, for example, birth weight, motor function, and intelligence (see reviews by Ha et al. 2017, Karagas et al. 2012). We flagged the cohorts in which a methylmercury-associated health outcome was observed and, in doing so, we observed that these associations spanned a range of exposures and were not restricted to the most highly exposed groups or particular geographic regions (see Excel Table S4). In addition to susceptibility during certain life stages, there is an increasing awareness that: a) multiple physiological systems may be targeted by mercury, not just the nervous system (Karagas et al. 2012); b) complex interactions between mercury and other chemical and nonchemical stressors exist, and that understood situations of mercury exposure and risk are further exacerbated under the context of global change drivers (Eagles-Smith et al. 2018); and c) genetic differences in subpopulations can influence exposure biomarkers and exposure–outcome relationships (Basu et al. 2014). Although we do not relate the exposure biomarker levels to any reference values, we do provide as a resource a summary table of key reference values for mercury (see Table S5).
The concerns over mercury pollution and human health risks are firmly established, although there are success stories to be noted. Through our review, there were some studies that illustrated that steps to limit mercury exposures—intentional or otherwise—may be effective. First, the approximately 2-fold reduction in urinary mercury levels measured over the past decade across the U.S. population is likely due to a combination of the development of encapsulated amalgams, the increasing use of composite resins, and the overall awareness of occupational and environmental risks associated with mercury use. Similar trends have been observed elsewhere, such as in German children (Link et al. 2007) and among U.S. dental professionals (Anglen et al. 2015; Goodrich et al. 2016). Second, across Arctic circumpolar regions, mercury exposures remain elevated even though these levels have dropped over the past two decades, probably as a result of local dietary advisories and changing consumption patterns. According to Donaldson et al. (2016), these decreases may be a sign that risk management efforts are having a beneficial effect, but there remain concerns about changing consumption patterns and how this may affect indigenous culture, identity and spirituality, recreational opportunities, and human nutrition. In other jurisdictions, there have been cases of decreased mercury exposures as a result of dietary consumption advisories (e.g., Kirk et al. 2017; Knobeloch et al. 2011), and we note that decreases have also been observed in both the Faroe Islands and the Seychelles (see Excel Table S4). Moving ahead, there is a need to better plan and coordinate studies so that temporal trends can be gauged, particularly in relationship to an intervention or, more broadly, the Minamata Convention on Mercury.
Conclusion
The motivation for this study was the decision to include a chapter on human biomonitoring of mercury exposure in the 2018 UN Global Mercury Assessment. To achieve this, we aimed to increase worldwide understanding of human exposures to mercury by collecting, collating, and analyzing mercury concentrations in biomarker samples using systemic review methodologies. From this work we are able to conclude that all populations worldwide are likely exposed to some amount of mercury and that there is great variability in exposures within and across countries and regions. This type of information is critical in helping understand exposures, particularly in light of certain stipulations in the Minamata Convention on Mercury. Notably, the entry into force of the Minamata Convention on Mercury in 2017 signaled the global commitment by governments to take action against mercury in order to protect human health and the environment (Article 1) (UNEP 2017). The parties to the convention now have a responsibility to develop schemes to evaluate the effectiveness of the convention (Article 22), which includes monitoring trends in human populations (vulnerable ones in particular, Article 19.1c) through harmonized methods (Article 19.1d). The state-of-the-science review performed here concerning mercury exposures worldwide results in a database that will be further developed and shared with the global community through future-planned WHO and UNEP outreach efforts and thus help to address several convention articles (e.g., Article 17, Information Exchange; Article 18, Public Information, Awareness and Education; and Article 19, Research, Development and Monitoring, among others). In doing so here, we provide an evidence-based foundation for work to come on assessing the effectiveness of the convention as well as for identifying data gaps requiring attention.
Supplementary Material
Acknowledgments
The authors acknowledge support from the UN Environment Programme (UNEP), especially J. Munthe and S. Wilson for their coordination of the 2018 Global Mercury Assessment. We thank the anonymous peer reviewers for their constructive insights. We also thank C. Achar, J. Griffin, J. Eng, and J. Snoj Tratnik for their technical support. Financial support for aspects of this work were received from the World Health Organization (WHO) through funds provided by the European Union (European Centre for Environment and Health, World Health Organization Regional Office for Europe, Bonn, Germany). In addition, funding to N.B. [the Canada Research Chairs program, the Natural Sciences and Engineering Research Council of Canada (NSERC), the International Development Research Centre/National Institutes of Health Global Environmental and Occupational Health (IDRC/NIH GEOHealth) Program Project 1U2RTW010110-01; the McGill Global Health Programs] and M.H. [EU projects: Spreading Excellence and Widening Participation in Support of Mass Spectrometry and Related Techniques in Health, the Environment and Food Analyis (MASSTWIN), the Health Effects of Arsenic Longitudinal Study (HEALS), the European Human Biomonitoring Initiative (HBM4EU), and the European Research Area Chair in Isotope Techniques in Food (ERAChair IsoFood)] are acknowledged. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
References
- Anglen J, Gruninger SE, Chou HN, Weuve J, Turyk ME, Freels S, et al. . 2015. Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists. J Am Dent Assoc 146(9):659–668.e1, PMID: 26314975, 10.1016/j.adaj.2015.05.016. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry. 1999. “Toxicological Profile for Mercury.” Atlanta, GA:Centers for Disease Control and Prevention. [Google Scholar]
- Baeuml J, Bose-O’Reilly S, Matteucci Gothe R, Lettmeier B, Roider G, Drasch G, et al. . 2011. Human biomonitoring data from mercury exposed miners in six artisanal small-scale gold mining areas in Asia and Africa. Minerals 1(1):122–143, 10.3390/min1010122. [DOI] [Google Scholar]
- Bartell SM, Ponce RA, Sanga RN, Faustman EM. 2000. Human variability in mercury toxicokinetics and steady state biomarker ratios. Environ Res 84(2):127–132, PMID: 11068925, 10.1006/enrs.2000.4104. [DOI] [PubMed] [Google Scholar]
- Basu N, Clarke E, Green A, Calys-Tagoe B, Chan L, Dzodzomenyo M, et al. . 2015. Integrated assessment of artisanal and small-scale gold mining in Ghana–part 1: human health review. Int J Environ Res Public Health 12(5):5143–5176, PMID: 25985314, 10.3390/ijerph120505143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Goodrich JM, Head J. 2014. Ecogenetics of mercury: from genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem 33(6):1248–1258, PMID: 24038486, 10.1002/etc.2375. [DOI] [PubMed] [Google Scholar]
- Berzas Nevado JJ, Rodríguez Martín-Doimeadios RC, Guzmán Bernardo FJ, Jiménez Moreno M, Herculano AM, do Nascimento JL, et al. . 2010. Mercury in the Tapajós River basin, Brazilian Amazon: a review. Environ Int 36(6):593–608, PMID: 20483161, 10.1016/j.envint.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Burm E, Song I, Ha M, Kim YM, Lee KJ, Kim HC, et al. . 2016. Representative levels of blood lead, mercury, and urinary cadmium in youth: Korean Environmental Health Survey in Children and Adolescents (KorEHS-C), 2012–2014. Int J Hyg Environ Health 219(4–5):412–418, PMID: 27107843, 10.1016/j.ijheh.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Castaño A, Cutanda F, Esteban M, Pärt P, Navarro C, Gómez S, et al. . 2015. Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ Res 141:58–68, PMID: 25667172, 10.1016/j.envres.2014.10.029. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. “Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables.” Vol. 1 Atlanta, GA:CDC. [Google Scholar]
- Cisneros-Montemayor AM, Pauly D, Weatherdon LV, Ota Y. 2016. A global estimate of seafood consumption by coastal indigenous peoples. PLoS One 11(12):e0166681, PMID: 27918581, 10.1371/journal.pone.0166681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. 2006. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662, PMID: 16973445, 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Croes K, De Coster S, De Galan S, Morrens B, Loots I, Van de Mieroop E, et al. . 2014. Health effects in the Flemish population in relation to low levels of mercury exposure: from organ to transcriptome level. Int J Hyg Environ Health 217(2–3):239–247, PMID: 23920476, 10.1016/j.ijheh.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Dereumeaux C, Saoudi A, Pecheux M, Berat B, de Crouy-Chanel P, Zaros C, et al. . 2016. Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011. Environ Int 97:56–67, PMID: 27788374, 10.1016/j.envint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Donaldson S, Odland JØ, Allard B. 2016. AMAP Assessment 2015: Human Health in the Arctic. Oslo, Norway:Arctic Monitoring and Assessment Program (AMAP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles-Smith CA, Silbergeld EK, Basu N, Bustamante P, Diaz-Barriga F, Hopkins WA, et al. . 2018. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47(2):170–197, PMID: 29388128, 10.1007/s13280-017-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel [European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM)]. 2012. Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J 10(12):2985, 10.2903/j.efsa.2012.2985. [DOI] [Google Scholar]
- Evers DC, Keane SE, Basu N, Buck D. 2016. Evaluating the effectiveness of the Minamata Convention on Mercury: principles and recommendations for next steps. Sci Total Environ 569–570:888–903, PMID: 27425440, 10.1016/j.scitotenv.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Fantom NJ, Serajuddin U. 2016. “The World Bank’s Classification of Countries by Income.” Policy Research Working Paper No. WPS7528. Washington, DC:World Bank Group. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). 2014. “State of World Fisheries and Aquaculture: Challenges and Opportunities.” Rome, Italy:FAO; http://www.fao.org/3/a-i3720e.pdf [accessed 30 November 2017]. [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). 2010. “Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption.” FAO Fisheries and Aquaculture Report No. 978. Rome, Italy:FAO/WHO; http://www.fao.org/docrep/014/ba0136e/ba0136e00.pdf [accessed 30 November 2017]. [Google Scholar]
- GEMS/Food Contaminants. 2018. Global Environment Monitoring System—Food Contamination Monitoring and Assessment Program. https://extranet.who.int/gemsfood/Default.aspx# [accessed 17 July 2018].
- Gibb H, O’Leary KG. 2014. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ Health Perspect 122(7):667–672, PMID: 24682486, 10.1289/ehp.1307864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JM, Chou HN, Gruninger SE, Franzblau A, Basu N. 2016. Exposures of dental professionals to elemental mercury and methylmercury. J Expo Sci Environ Epidemiol 26(1):78–85, PMID: 26329138, 10.1038/jes.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth E., III. 2010. Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ Res 110(3):226–236, PMID: 20116785, 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Ha E, Basu N, Bose-O’Reilly S, Dórea JG, McSorley E, Sakamoto M, et al. . 2017. Current progress on understanding the impact of mercury on human health. Environ Res 152:419–433, PMID: 27444821, 10.1016/j.envres.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Haines DA, Saravanabhavan G, Werry K, Khoury C. 2017. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int J Hyg Environ Health 220(2 Pt A):13–28, PMID: 27601095, 10.1016/j.ijheh.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2017. “Fourth Report on Human Biomonitoring of Environmental Chemicals in Canada.” Ottawa, ON, Canada:Health Canada. [Google Scholar]
- IPCS (International Program on Chemical Safety). 1990. “Methylmercury.” Environmental Health Criteria 101. Geneva, Switzerland:World Health Organization, International Program on Chemical Safety; http://www.inchem.org/documents/ehc/ehc/ehc101.htm [accessed 30 November 2017]. [Google Scholar]
- IPCS. 2003. “Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects.” Concise International Chemical Assessment Document No. 50. Geneva, Switzerland:World Health Organization; http://www.who.int/ipcs/publications/cicad/en/cicad50.pdf?ua=1 [accessed 10 August 2018]. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 2004. “Evaluation of Certain Food Additives and Contaminants. Sixty-first Report of the Joint FAO/WHO Expert Committee on Food Additives.” WHO Technical Report Series 922. Geneva, Switzerland:World Health Organization/Food and Agriculture Organization. [Google Scholar]
- JECFA. 2007a. Methylmercury (addendum). In: Safety Evaluation of Certain Food Additives and Contaminants. Prepared by the sixty-seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Food Additives Series 58. Geneva, Switzerland:World Health Organization, 269–315. http://www.inchem.org/documents/jecfa/jecmono/v58je01.pdf [accessed 10 August 2018]. [Google Scholar]
- JECFA. 2007b. “Methylmercury Evaluation of Certain Food Additives and Contaminants.” Sixty-seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 940). Geneva, Switzerland:World Health Organization/Food and Agriculture Organization; http://apps.who.int/iris/bitstream/10665/43592/1/WHO_TRS_940_eng.pdf [accessed 10 August 2018]. [Google Scholar]
- JECFA. 2011. “Safety Evaluation of Certain Contaminants in Food.” Prepared by the Seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Food Additives Series 63. Geneva, Switzerland:World Health Organization; http://www.inchem.org/documents/jecfa/jecmono/v63je01.pdf [accessed 10 August 2018]. [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. . 2012. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120(6):799–806, PMID: 22275730, 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk LE, Jørgensen JS, Nielsen F, Grandjean P. 2017. Public health benefits of hair-mercury analysis and dietary advice in lowering methylmercury exposure in pregnant women. Scand J Public Health 45(4):444–451, PMID: 28381203, 10.1177/1403494816689310. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Tomasallo C, Anderson H. 2011. Biomonitoring as an intervention against methylmercury exposure. Public Health Rep 126(4):568–574, PMID: 21800751, 10.1177/003335491112600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B, Gabrio T, Piechotowski I, Zöllner I, Schwenk M. 2007. Baden-Wuerttemberg Environmental Health Survey (BW-EHS) from 1996 to 2003: toxic metals in blood and urine of children. Int J Hyg Environ Health 210(3–4):357–371, PMID: 17353148, 10.1016/j.ijheh.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson HA, Chan LH, Mahaffey KR, Murray M, Sakamoto M, et al. . 2007. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio 36(1):3–11, PMID: 17408186, 10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2000. Toxicological Effects of Methylmercury. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- Nriagu J, Basu N, Charles S. 2012. Chapter 15: environmental justice: the mercury connection. In: Mercury in the Environment: Pattern and Process. Bank MS, ed. Berkeley, CA:University of California Press, 301–316. [Google Scholar]
- Obrist D, Kirk JL, Zhang L, Sunderland EM, Jiskra M, Selin NE. 2018. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio 47(2):116–140, PMID: 29388126, 10.1007/s13280-017-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHAT [U.S. National Toxicology Program (NTP) Office of Health Assessment and Translation (OHAT)]. 2015. OHAT Risk of Bias Tool for Human and Animal Studies. https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf [accessed 6 August 2018].
- Puklová V, Krsková A, Cerná M, Cejchanová M, Rehůrková I, Ruprich J, et al. . 2010. The mercury burden of the Czech population: an integrated approach. Int J Hyg Environ Health 213(4):243–251, PMID: 20417154, 10.1016/j.ijheh.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Windham-Myers L, Creswell JE. 2014. Rice methylmercury exposure and mitigation: a comprehensive review. Environ Res 133:407–423, PMID: 24972509, 10.1016/j.envres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JW, Kim BG, Kim YM, Kim RB, Chung JY, Lee KM, et al. . 2015. Trend of blood lead, mercury, and cadmium levels in Korean population: data analysis of the Korea National Health and Nutrition Examination Survey. Environ Monit Assess 187(3):146, PMID: 25716526, 10.1007/s10661-015-4348-2. [DOI] [PubMed] [Google Scholar]
- Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA. 2014. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92(4):254–269F, PMID: 24700993, 10.2471/BLT.12.116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Blum JD, Basu N, Rajaee M, Evers DC, Buck DG, et al. . 2015. Assessment of mercury exposure among small-scale gold miners using mercury stable isotopes. Environ Res 137:226–234, PMID: 25577187, 10.1016/j.envres.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Sherman L, Blum JD, Franzblau A, Basu N. 2013. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ Sci Technol 47(7):3403–3409, PMID: 23463943, 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Sioen I, De Henauw S, Van Camp J, Volatier JL, Leblanc JC. 2009. Comparison of the nutritional–toxicological conflict related to seafood consumption in different regions worldwide. Regul Toxicol Pharmacol 55(2):219–228, PMID: 19589366, 10.1016/j.yrtph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Stern AH, Smith AE. 2003. An assessment of the cord blood:maternal blood methylmercury ratio: implications for risk assessment. Environ Health Perspect 111(12):1465–1470, PMID: 12948885, 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, et al. . 2015. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr 101(3):530–537, PMID: 25733638, 10.3945/ajcn.114.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, DiGangi J, Evers DC, Petrlik J, Buck DG, Šamánek J, et al. . 2016. Economic implications of mercury exposure in the context of the global mercury treaty: hair mercury levels and estimated lost economic productivity in selected developing countries. J Environ Manage 183:229–235, PMID: 27594689, 10.1016/j.jenvman.2016.08.058. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 1997. Mercury Study Report to Congress. https://www.epa.gov/mercury/mercury-study-report-congress [accessed 30 November 2017].
- U.S. EPA. 2001. Chapter 4: risk assessment for methylmercury. In: “Water Quality Criterion for the Protection of Human Health: Methylmercury.” EPA-823-R-01-001. Washington, DC:U.S. EPA, Office of Science and Technology, Office of Water. [Google Scholar]
- UNEP (United Nations Environment Programme). 2012. Reducing Mercury Use in Artisanal and Small-Scale Gold Mining: A Practical Guide. http://wedocs.unep.org/handle/20.500.11822/11524 [accessed 3 August 2016].
- UNEP. 2016. Global Review of Mercury Monitoring Networks. http://www.mercuryconvention.org/Portals/11/documents/2016%20call%20for%20submissions/UNEP%20-%20Global%20Review%20of%20Mercury%20Monitoring%20Networks_Final.pdf [accessed 30 November 2017].
- UNEP. 2017. Minamata Convention Text and Annexes. http://mercuryconvention.org/Convention/Text/tabid/3426/language/en-US/Default.aspx [accessed 30 November 2017].
- UNEP/WHO. 2008. Guidance for Identifying Populations at Risk from Mercury Exposure. http://www.who.int/foodsafety/publications/chem/mercuryexposure.pdf [accessed 30 November 2017].
- Višnjevec AM, Kocman D, Horvat M. 2014. Human mercury exposure and effects in Europe. Environ Toxicol Chem 33(6):1259–1270, PMID: 24375779, 10.1002/etc.2482. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Bergström A, Carmichael A, Cordier S, Eggesbø M, et al. . 2012. European birth cohorts for environmental health research. Environ Health Perspect 120(1):29–37, PMID: 21878421, 10.1289/ehp.1103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2016. Environmental and occupational health hazards associated with artisanal and small-scale gold mining. Geneva, Switzerland:WHO; http://apps.who.int/iris/bitstream/handle/10665/247195/9789241510271-eng.pdf?sequence=1 [accessed 30 November 2017]. [Google Scholar]
- WHO-Europe (WHO Regional Office for Europe). 2015. Human Biomonitoring: Facts and Figures. Copenhagen, Denmark:WHO Regional Office for Europe; http://www.euro.who.int/__data/assets/pdf_file/0020/276311/Human-biomonitoring-facts-figures-en.pdf [accessed 30 November 2017]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.