Abstract
The treatment of patients with relapsed and/or refractory multiple myeloma has improved considerably in the last 15 years, after the introduction of proteasome inhibitors and immunomodulatory drugs. The first clinical trials with new proteasome inhibitors have produced exciting results, particularly those comparing triplet regimens with standard doublet regimens, with a gain in progression-free survival accompanied by an acceptable safety profile and either similar or better health-related quality of life. New proteasome inhibitors hold the potential to fill unmet needs in multiple myeloma management regarding improvement of clinical outcomes, including delayed progression of disease in high-risk patients. This review summarizes the main pharmacological properties and clinical outcomes of these agents, and discusses their potential to change the whole multiple myeloma therapeutic landscape.
Keywords: Multiple myeloma, Proteasome inhibitors, Bortezomib, Ixazomib, Carfilzomib
Introduction
Multiple myeloma (MM) is a B-cell malignancy in which there is abnormal proliferation of neoplastic plasma cells that secrete monoclonal immunoglobulins.1 MM accounts for about 15% of hematopoietic neoplasms and 1% of cancers worldwide. In Europe and the United States, MM incidence has been estimated at 6.9 and 4–5 cases per 100,000 inhabitants, respectively.2, 3 Median age at the time of diagnosis is 66 years, with only 2% of patients aged <40 years on this occasion.1 In Latin America, few epidemiological data on the disease are available. In a recent report including 850 MM patients from Latin America, the median ages of the transplant-ineligible and transplant-eligible patients were 68.6 years and 55.0 years, respectively.4 In one of the largest Brazilian studies on MM, which included 1112 patients, the median age at presentation was 60.5 years.5 An increase in the incidence of MM is expected over the next few years, mainly due to the aging phenomenon of the population.2
MM results from a series of chromosomal alterations that accumulate during B cell maturation, partially as a result of the high level of DNA modification processes that take place in those cells (VDJ recombination, somatic hypermutation and IgH switch recombination) to generate antibody diversity. Therefore, as in other malignancies, MM is characterized by deregulation of the cell cycle, with increased cell survival and decreased apoptosis. Eventually, secondary molecular alterations will accumulate and will lead to the development of treatment resistance. Some of these secondary alterations affect signaling pathways such as RAS, PI3K, MAPK and NF-κB, among others.6, 7, 8
Chromosomal translocations and/or aneuploidy (mostly hyperdiploidy) are among the primary events associated with the precursor states of the disease, namely monoclonal gammopathy of undetermined significance (MGUS) and smouldering multiple myeloma (SMM). Translocations involving the IGH locus at chromosome 14 and specific oncogenes are detected in around 40% of patients. Hyperdiploidy is characterized by trisomies of some or all of chromosomes 3, 5, 7, 9, 11, 15 and 19 and is observed in more than 50% of patients. Although hyperdiploidy and IGH translocation may co-occur, that is not the rule.6, 7, 8 Germline variations may also contribute to the development of the disease, increasing its risk by 2–4 fold, as observed in familial cases.8 Inherited mutations, namely single-nucleotide polymorphisms (SNPS), have been detected in seven loci up to now: 2p23.3, 3p22.1, 3q26.2, 6p21.3, 7p15.3, 17p11.2, and 22q13.1.8
A wide range of secondary genetic events is also detected in MM patients, such as further translocations [t(4;14), t(14;16), t(14;20)], DNA losses and gains [del(1p), gain(1q), del(13q), del(17p13)], small mutations at KRAS, NRAS, FAM46C, DIS3 and TP53, and microRNA abnormalities and epigenetic alterations.6, 8 In general, hyperdiploidy is associated with better prognosis, and these patients are considered “low risk”. On the other hand, most of the other alterations have a worse prognosis and are considered “high risk”. The correlation between genetic alterations and prognosis has become progressively more challenging, as the molecular methods to detect them are continuously improving, with a trend to reveal individual rather than shared genetic profiles. For instance, trisomies 3 and 5 seem to cancel out the poor prognosis conferred by t(4;14).6
Until now, there is no cure for patients with MM, with the great majority of them developing resistance and/or relapse after the first-line therapy with new agents and/or high-dose therapy with autologous stem cell transplantation (ASCT), even those who achieved a complete response.1 The proteasome inhibitor (PI) bortezomib and the immunomodulators thalidomide and lenalidomide revolutionized MM treatment about 20 years ago, and up to now those drugs remain, along with dexamethasone, as the main regimen used in the upfront and refractory/relapsed settings.9 Nevertheless, the emergence of malignant plasma cell clones resistant to these agents, along with a need for drugs with a more convenient administration route and safer profile, led to the need for developing new drugs, such as new immunomodulatory drugs (IMiDs) and PIs, monoclonal antibodies, and histone deacetylase inhibitors (HDACs).9, 10, 11 New PIs have presented excellent results in clinical trials with relapsed and/or refractory MM patients and are the focus of this review.
Rationale for proteasome inhibition
Because the main function of plasma cells is antibody production, high amounts of proteins are secreted and released in serum as the underlying physiopathological mechanism in MM. As a consequence, the accumulation of misfolded or unfolded proteins in their endoplasmic reticulum (ER), a phenomenon known as ER stress, is more pronounced than in other types of cells. ER stress in turn activates a number of processes, including the ER-associated degradation (ERAD) pathway, characterized by ubiquitination and degradation of the misfolded proteins by the ubiquitin–proteasome system.12 Proteasome inhibition generates an accumulation of these proteins, with activation of pro- and anti-proliferative signals, disruption of cell cycle regulation, activation of apoptotic pathways and consequent cell death.13, 14
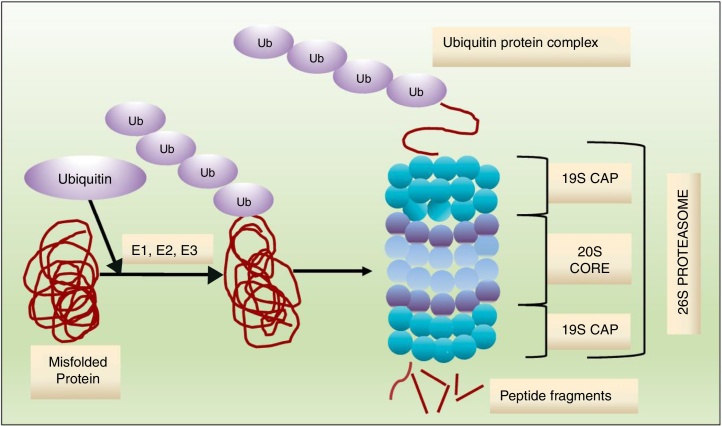
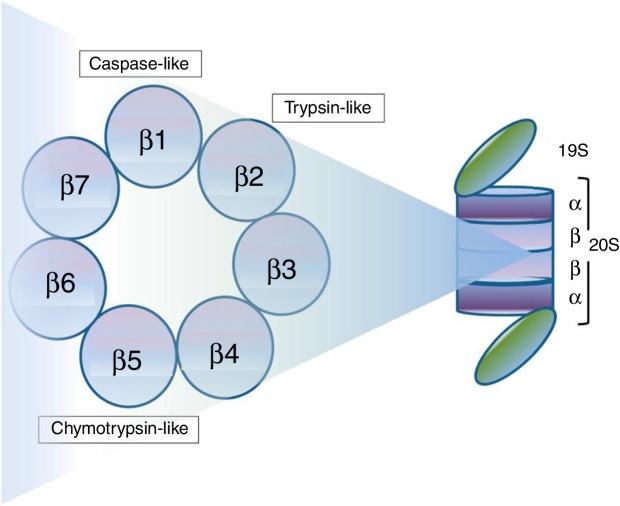
Protein degradation through the ubiquitin–proteasome system is ATP-dependent, and consists of a multistep enzymatic cascade (Figure 1), where ubiquitin is conjugated through a lysine residue at position 48. Proteins marked for protease degradation are polyubiquitinated by a set of enzymes: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligases (E3). Proteins carrying a polyubiquitinated chain are recognized by the 19S proteasome subunit of the 26S proteasome and degraded in small peptides by the 20S subunit.13, 14 As shown in Figure 2, the 19S regulatory particles “cover” both ends of the barrel-shaped 20S core. The four highly homologous rings that comprise the 20S core enclose a central catalytic chamber with active proteolytic sites. In turn, each ring contains seven α or β subunits, arranged one above the other in the order “α–β–β–α”. While the two outer rings (α) enclose a small aperture through which only the denatured polypeptide substrates can pass, the two central rings (β) contain multiple catalytic sites that work together in protein degradation. Each of these two rings has three proteolytic sites: one with caspase-like activity (C-L) located in the β1 subunit, one with trypsin-like activity (T-L) in the β2 subunit, and one with chymotrypsin-like activity (CT-L) in the β5 subunit.14 An alternative form of the 26S proteasome (also known as the constitutive proteasome), named immunoproteasome, is expressed abundantly in lymphoid and hematopoietic cells. The immunoproteasome contains three subunits homologous to the β1, β2 and β5 subunits of the 26S proteasome (named β1i, β2i and β5i, respectively) and is also targeted by PIs described in this review.13, 14, 15
Figure 1.
Ubiquitin–proteasome system. Ubiquitin ligases (E1, E2, E3) attach a chain of ubiquitins to lysine residues on the target protein to be degraded. In the proteasome, the ubiquitin chain is removed, and the target protein is unfolded and translocated to the interior of the proteasome, where it is degraded into peptide fragments by 3 threonine proteases.
Figure 2.
Structure of 26S proteasome. 26S proteasome comprises a 20S core flanked by regulatory particles (19S). The 20S core includes four heptameric rings of α or β subunits. The proteolytic sites with caspase-like, trypsin-like and chymotrypsin-like activity are located in the subunits β1, β2 and β5, respectively.
Source: Chhabra et al.14
The proteasome also regulates the intracellular levels of the anti-apoptotic protein NF-κB, which is constitutively present in the cytosol and inactivated by the IkB inhibitors. When phosphorylated, IkB is degraded by the 26S proteasome, allowing for the translocation of NF-κB to the nucleus. Proteasome inhibition increases the IkB availability in cytosol, inhibiting NF-κB and impairing one of the anti-apoptotic mechanisms of NF-κB-dependent tumor clones.16
Neoplastic cells usually have a higher level of proteasome activity when compared with normal cells and greater sensitivity to pro-apoptotic effects from proteasome inhibition, given their increased production and secretion of proteins. Therefore, the proteasome is considered a therapeutic target in neoplasias, and its inhibition is currently considered a cornerstone of myeloma treatment.13, 14, 17
In 2008, the publication of the VISTA study, which compared bortezomib plus melphalan–prednisone with melphalan–prednisone alone, showed the best results ever seen in patients not eligible for stem cell transplantation. The time to progression among patients in the bortezomib and in the control group was 24.0 and 16.6 months, respectively [hazard ratio (HR) = 0.48; p < 0.001], and 71% of the patients in the bortezomib group had a partial or better response, compared to 35% in the control group (p < 0.001).18 Recently, a meta-analysis of randomized controlled trials that evaluated the efficacy of induction regimens for transplant-eligible patients showed that the overall response rate (ORR) with bortezomib plus thalidomide plus dexamethasone was better than the ORR of the majority of other regimens.19 Moreover, the prognosis of patients with t(4;14) and other high-risk cytogenetic alterations significantly improved after the introduction of bortezomib into the clinical practice.20, 21 Nevertheless, an important drawback of bortezomib regimens is the induction of peripheral neuropathy, which has been the subject of several studies.21, 22
New proteasome inhibitors
PIs have different chemical structures, as well as distinct pharmacokinetic and pharmacodynamic characteristics (Table 1) and are in distinct phases of study. New PIs are contributing to the broadening of the clinical utility of this class of drugs in the MM treatment, as described below.
Table 1.
Principal chemical and pharmacological characteristics of 20S proteasome inhibitors in the treatment of multiple myeloma.
| Proteasome inhibitor | Chemical class | Binding kinetics | Route of administration | Half-life (min) | Main therapeutic targets | IC50 β5 (nM) |
Maximal proteasome inhibition at MTD (%) |
|---|---|---|---|---|---|---|---|
| Bortezomib | Boronate | Reversible | IV or SC | 110 | β5 > β1 > β2 | 7.9 ± 0.5 | 65–75 |
| Carfilzomib | Epoxyketone | Irreversible | IV | <30 | β5 > β2/β1 | <5 | >80 |
| Ixazomib | Boronate | Reversible | Oral | 18 | β5 > β1 | 3.4 | 73–99 |
| Oprozomib | Epoxyketone | Irreversible | Oral | 30–90 | β5 | 36/82 | >80 |
| Marizomib | β-Lactone | Irreversible | IV or oral | 10–15 | β5 > β2 > β1 | 3.5 ± 0.3 | 100 |
Carfilzomib
Carfilzomib is a tetrapeptide epoxyketone PI that binds irreversibly and with greater affinity than bortezomib to the β5 proteasome subunit and to the LMP7 (iβ5) immunoproteasome subunit. Because of its peptidic composition, carfilzomib can be degraded by endogenous proteases and plasma peptases, which reduces its efficiency; however, proteasome activity is reduced to less than 20% upon carfilzomib binding and its restoration only occurs by the synthesis of new individual subunits and their incorporation into a new proteasome. As an isolated agent, it is indicated in the treatment of patients with relapsed or refractory MM who received at least two previous therapies, including bortezomib and an IMiD.7, 13, 14, 16, 23, 24
In a phase 2 study with 266 relapsed MM patients, single-agent carfilzomib resulted in a progression-free survival (PFS) of 3.7 months and an OS of 15.6 months. Adverse events were manageable and consisted mainly of fatigue, anemia, nausea, and thrombocytopenia. Peripheral neuropathy was observed in 12.4% of patients. Of note, patients had a median of 5 previous lines of therapy, and 80% of them were either refractory or intolerant to both bortezomib and lenalidomide.25
In the open label phase 3 study ASPIRE study, 792 patients with relapsed MM were randomized to lenalidomide and dexamethasone with or without carfilzomib. The carfilzomib group presented a median PFS of 26.3 months versus 17.6 months in the control group (HR = 0.69; CI: 0.57–0.83; p = 0.0001), an overall response rate (ORR) of 87.1% versus 66.7% in the control group and a complete response rate (CR) of 31.8%, compared with 9.3% in the control group. Adverse events were similar in both groups, except for the increased incidence of cardiovascular events in the carfilzomib group (7.1% versus 3.9% in the control group), but patients in the carfilzomib arm had a better quality of life studied in the open label setting.26 This study led to FDA approval for an expanded indication of carfilzomib in combination with lenalidomide and dexamethasone in patients with refractory or relapsed MM who have received one to three lines of therapy. In Brazil, carfilzomib (56 mg/m2) in combination with dexamethasone has been approved by the Agência Nacional de Vigilância Sanitária (ANVISA) for the treatment of MM patients who have received from one to three previous treatments in combination with dexamethasone; as a monotherapy carfilzomib (27 mg/m2) has been approved for the treatment of refractory or relapsed MM patients who have received at least two previous therapies that were comprised of bortezomib and an IMiD.27
In the ENDEAVOR study, which directly compared carfilzomib with bortezomib in patients with relapsed MM, the carfilzomib group presented a median PFS of 18.7 months versus 9.4 in the bortezomib group (HR = 0.53; 95% CI: 0.44–0.65; p = 0.0001). Median OS was also superior in the carfilzomib arm (47.6 versus 40.0 months in the control arm; HR = 0.791; 95% CI 0.648–0.964; p = 0.010). Anemia, hypertension, thrombocytopenia and pneumonia represented the most frequent grade 3 or higher adverse events.28 Of note, subgroup analyses of the ENDEAVOR and the ASPIRE trials showed a significantly improved median PFS with carfilzomib over the control group in both the high cytogenetic-risk and in the standard cytogenetic-risk subgroups.26, 29
The FOCUS study was a randomized, phase 3, open-label study that compared carfilzomib monotherapy with low-dose corticosteroids and optional cyclophosphamide in relapsed and refractory MM. Median OS, the primary endpoint, was similar in both groups, namely 10.2 versus 10.0 months with carfilzomib and control, respectively (HR = 0.975; 95% CI 0.76–1.25; p = 0.4172). MM patients in this study were heavily pretreated, with a median of five regimens in both groups.30
The phase 3 CLARION trial compared carfilzomib with bortezomib combined with melphalan and prednisone in newly diagnosed, transplant ineligible MM patients. The study did not result in significant improvement in the median PFS in the carfilzomib group (22.3 months versus 22.1 months in the control group; HR = 0.91; 95% CI: 0.75–1.10; p = 0.159). OS data from this study are still immature. The overall safety profile was consistent with what has been previously observed in prior studies, with increased rates of cardiopulmonary and renal toxicities in the carfilzomib group and of peripheral neuropathy in the bortezomib group.31
Another phase 3 study in patients with relapsed and refractory MM, the ARROW trial, is currently ongoing to evaluate the efficacy and safety of carfilzomib administered once weekly 70 mg/m2 versus the standard twice-weekly 27 mg/m2 regimen, with dexamethasone in both arms.11 The former regimen was considered feasible, well tolerated and active based on the results of a phase 1/2 study (CHAMPION-1) with relapsed and/or refractory MM patients. In that study, median PFS was 12.6 months and the ORR was 77%, with fatigue (11%) and hypertension (7%) as the most common adverse events of grade ≥3.11, 32
Several studies, as well as a pooled safety analysis of the ASPIRE and the ENDEAVOR trials, have raised detected cardiac events, resulting from the administration of carfilzomib, in 5% to 12% of patients. In one of those reports, prior or underlying cardiovascular disease was likely associated with the cardiac events in at least some patients. The biological basis for those events, and whether they are specific to carfilzomib, is under investigation, with preliminary results suggesting that they are associated with carfilzomib33, 34 and maybe with bortezomib.35 Nevertheless, cardiac abnormalities seem to be reversible with the cessation of carfilzomib therapy and the administration of traditional heart failure treatments. Moreover, safe re-treatment with lower doses of carfilzomib has been suggested by at least two of the studies.34, 36, 37
Ixazomib
Ixazomib is an oral, boronic PI that potently, reversibly and selectively inhibits the proteasome, especially the CT-L (β5) proteolytic site of the 20S proteasome. Chemically, it appears as dipeptidyl boronic acid, which is rapidly hydrolyzed in water and converted into ixazomib, its active form. At high concentrations, it also inhibits C-L (β1) and T-L (β2) subunits and induces intracellular accumulation of ubiquitinated proteins. It has a half-life of dissociation with the 20S proteasome shorter than bortezomib (ixazomib: 18 min versus bortezomib: 110 min), with an improved pharmacokinetic and pharmacodynamic profile.7, 13, 14, 16, 23, 24 Of note, a preclinical study showed that ixazomib overcame resistance to bortezomib and triggered synergistic anti-MM activity with lenalidomide, dexamethasone or an HDAC.38
In the double blind, placebo-controlled phase 3 study TOURMALINE-MM1, 722 patients with refractory or relapsed MM previously treated with one to three lines of therapy were randomized to ixazomib in combination with lenalidomide and dexamethasone (ixazomib-Rd) or to a placebo in combination with lenalidomide and dexamethasone (placebo-Rd). A median PFS of 20.6 months was demonstrated in the ixazomib arm versus 14.7 in the placebo arm (HR = 0.74; 95% CI: 0.59–0.94; p = 0.01), as well as an ORR of 78.3% in the ixazomib group versus 71.5% in the placebo group (p = 0.04). Median OS was not reached in any arm. Of note, a subpopulation of patients with high-risk cytogenetics also benefited from the triplet regimen, showing a PFS of 21.4 months, compared to 9.7 months in the control arm (HR = 0.54; 95% CI: 0.32–0.92; p = 0.02).
The main adverse events with ixazomib were thrombocytopenia, fatigue, nausea and diarrhea, as observed with other PIs. The most common grade 3 and higher adverse event was neutropenia, but its frequency was similar in both groups. Peripheral neuropathy of any grade was observed in 27% of patients in the ixazomib arm versus 22% of those in the control arm, but only 2% of patients in each group presented grade 3 neuropathy. Health-related quality of life was similar in the two groups.39
Based on these results, ixazomib was approved by the FDA for use in combination with lenalidomide and dexamethasone in the treatment of MM patients who have received at least one prior therapy.
As a separate regional expansion of the TOURMALINE-MM1 study, the China Continuation study, which enrolled 115 patients, had identical an eligibility criteria and treatment scheme. Both PFS and OS were better in the ixazomib arm compared to the control arm, with HR of 0.598 (p = 0.035) and 0.419 (p = 0.001), respectively.40
In a subgroup analysis of the TOURMALINE-MM1 study, patient subgroups defined according to type and number of prior therapies were compared as to PFS.41 PFS was prolonged in the ixazomib arm, regardless of the type of prior therapy (HR 0.739 and 0.749 in PI-exposed and naive patients; HR 0.744 and 0.700 in IMiD-exposed and naive patients, respectively). Moreover, the results suggested that the PFS benefit with ixazomib (versus the control arm) in patients with 2 or 3 prior therapies or 1 prior therapy without transplant is greater than in patients with 1 prior therapy and transplant (HR of 0.58, 0.60 and 1.23, respectively).
Ixazomib is currently being investigated in the front-line setting (TOURMALINE MM2) and as a maintenance agent in two phase 3 trials, comparing it with a placebo in patients who achieved at least a partial response after ASCT (MM3) and in the non-transplant setting (MM4) in patients who achieved at least a partial response after induction therapy.10, 11, 42
Marizomib and oprozomib
Marizomib and oprozomib are still in the first phases of clinical development. Marizomib is an oral, non-peptidic, irreversible PI, with β-lactone in its structure, having been isolated from the marine actinomycete Salinispora tropica. It binds irreversibly to the three catalytic subunits of the 20S proteasome.7, 13, 14, 16, 24 Phase 1 studies demonstrated relatively low toxicities and no evidence of neuropathy or thrombocytopenia. In a dose escalation study with 15 patients with relapsed/refractory MM treated with marizomib in monotherapy, three patients resistant to bortezomib demonstrated at least partial response.43 Marizomib demonstrated synergy in vitro with several other anti-myeloma agents, including IMiDs and HDACs.44, 45
Oprozomib is an oral, irreversible PI derived from carfilzomib, which demonstrates carfilzomib-like potency in cytotoxicity assays. Similar to bortezomib and carfilzomib, it is highly selective for the CT-L (β5) subunit of the 20S proteasome.7, 13, 14, 16, 24 Two phase 1b/2 studies with oprozomib demonstrated a tolerable safety profile with low incidence of neuropathy. In one of them, 29 patients with relapsed/refractory MM were submitted to a dose escalation regimen of oprozomib/dexamethasone. Major challenges related to tolerability were gastrointestinal, such as diarrhea, as well as frequent nausea and vomiting. However, none of the patients in this study demonstrated worsening of the basal, or a new neuropathy.46, 47
Discussion
Treatment of relapsed or refractory MM is complex and based on cytogenetic and clinical peculiarities. Therapeutic regimens involving anthracyclic and alkylating antineoplastic agents have high toxicity and low adherence to treatment, especially in patients with advanced disease and/or presence of relevant comorbidities. Despite MM median overall survival (OS) of approximately five years and improvements in therapy results with the first PIs and IMiDs, the natural course of the disease remains in the form of relapse/refractoriness. Therefore, the search for new therapy options is an unquestionable clinical necessity.16
Based on the International Myeloma Working Group recommendations, biological relapse alone does not necessarily require immediate treatment, but symptomatic disease should eventually follow.48 At this time, the so-called backbone regimens, consisting of dexamethasone combined with either lenalidomide or bortezomib, have been traditionally used with relevant improvement in response and survival.45 Results from recent trials have shown that new agents may replace the usual ones used in backbone regimens or be added to them with significant benefit to the patients.49 The new IMiD pomalidomide, for instance, has been approved by the FDA and the EMEA for the treatment of patients with MM who have received two or more lines of therapy (with a PI and an IMiD) and experienced disease progression on or within 60 days of completion of the last therapy.45
Triple-agent therapy for MM consisting of a PI, an IMiD and dexamethasone has been the scope of several clinical trials that demonstrate better efficacy and a better safety profile of this regimen when compared with double-agent therapies (PI or IMiD plus dexamethasone) or regimens that comprise cytotoxic drugs. Triple-agent therapy also shows an interesting, although variable, safety profile according to the drugs used in each therapeutic regimen and the specific characteristics of the population included in each clinical trial (previous therapies, age, comorbidities, disease stage, previous autologous transplant, among others).16, 26, 50, 51, 52, 53, 54
In conclusion, new PIs are an important advance in relapsed and/or refractory MM treatment, increasing PFS, response rate and quality of life, even in subgroups of patients with poor prognosis. A number of ongoing studies aims to evaluate the efficacy and toxicity profiles of new therapy combinations involving new PIs and IMiDs and monoclonal antibodies (elotuzumab and daratumumab), already approved for the treatment of MM, among others, as a way to achieve a better chemosensitization of certain subpopulations of plasma cell clones, consequently overcoming their resistance to drugs.
Funding
This manuscript was developed with support of Takeda Pharmaceuticals.
Contribution
All authors have participated in the manuscript development.
Conflicts of interest
PYT is currently a Takeda's employee. ESM and RCF are currently an Evidências-Kantarhealth's employee. VTMH, EQC, RIB, AM, RJPM, JNS, JVP and RCF are advisors for Takeda, with no financial relationship. All authors declared no other conflicts of interest.
References
- 1.Rajkumar S.V., Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91(1):101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsenthaler C., Kane P., Gao W., Siegert R.J., Edmonds P.M., Schey S.A. Prevalence of symptoms in patients with multiple myeloma: a systematic review and meta-analysis. Eur J Haematol. 2016;97(5):416–429. doi: 10.1111/ejh.12790. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA: Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Hungria V.T., Maiolino A., Martinez G., Duarte G.O., Bittencourt R., Peters L. Observational study of multiple myeloma in Latin America. Ann Hematol. 2017;96(1):65–72. doi: 10.1007/s00277-016-2866-9. [DOI] [PubMed] [Google Scholar]
- 5.Hungria V.T., Maiolino A., Martinez G., Colleoni G.W., Coelho E.O., Rocha L. Confirmation of the utility of the International Staging System and identification of a unique pattern of disease in Brazilian patients with multiple myeloma. Haematologica. 2008;93(5):791–792. doi: 10.3324/haematol.11637. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S.K., Rajkumar V., Kyle R.A., van Duin M., Sonneveld P., Mateos M.V. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 7.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manier S., Salem K.Z., Park J., Landau D.A., Getz G., Ghobrial I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14(2):100–113. doi: 10.1038/nrclinonc.2016.122. [DOI] [PubMed] [Google Scholar]
- 9.Noonan K., Colson K. Immunomodulatory agents and proteasome inhibitors in the treatment of multiple myeloma. Semin Oncol Nurs. 2017;33(3):279–291. doi: 10.1016/j.soncn.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Brayer J., Baz R. The potential of ixazomib, a second-generation proteasome inhibitor, in the treatment of multiple myeloma. Ther Adv Hematol. 2017;8(7):209–220. doi: 10.1177/2040620717710171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larocca A., Mina R., Gay F., Bringhen S., Boccadoro M. Emerging drugs and combinations to treat multiple myeloma. Oncotarget. 2017;8(36):60656–60672. doi: 10.18632/oncotarget.19269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincenz L., Jager R., O’Dwyer M., Samali A. Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol Cancer Ther. 2013;12(6):831–843. doi: 10.1158/1535-7163.MCT-12-0782. [DOI] [PubMed] [Google Scholar]
- 13.Allegra A., Alonci A., Gerace D., Russo S., Innao V., Calabro L. New orally active proteasome inhibitors in multiple myeloma. Leuk Res. 2014;38(1):1–9. doi: 10.1016/j.leukres.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Chhabra S. Novel proteasome inhibitors and histone deacetylase inhibitors: progress in myeloma therapeutics. Pharmaceuticals (Basel) 2017;10(2) doi: 10.3390/ph10020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura H., Caturegli P., Takahashi M., Suzuki K. New insights into the function of the immunoproteasome in immune and nonimmune cells. J Immunol Res. 2015;2015:541984. doi: 10.1155/2015/541984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naymagon L., Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9(1):52. doi: 10.1186/s13045-016-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muchtar E., Gertz M.A., Magen H. A practical review on carfilzomib in multiple myeloma. Eur J Haematol. 2016;96(6):564–577. doi: 10.1111/ejh.12749. [DOI] [PubMed] [Google Scholar]
- 18.San Miguel J.F., Schlag R., Khuageva N.K., Dimopoulos M.A., Shpilberg O., Kropff M. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Z.H., Chen J.F., Li Y.X., Zhang R., Xiao L.F., Meng X.Y. Induction regimens for transplant-eligible patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials. Cancer Manag Res. 2017;9:287–298. doi: 10.2147/CMAR.S138932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavo M., Tacchetti P., Patriarca F., Petrucci M.T., Pantani L., Galli M. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 21.Sonneveld P., Goldschmidt H., Rosinol L., Blade J., Lahuerta J.J., Cavo M. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31(26):3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.E., Choi K., Han S., Lee J., Hong T., Park G.J. Bortezomib pharmacokinetics in tumor response and peripheral neuropathy in multiple myeloma patients receiving bortezomib-containing therapy. Anticancer Drugs. 2017;28(6):660–668. doi: 10.1097/CAD.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 23.Dou Q.P., Zonder J.A. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin–proteasome system. Curr Cancer Drug Targets. 2014;14(6):517–536. doi: 10.2174/1568009614666140804154511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubiczkova L., Pour L., Sedlarikova L., Hajek R., Sevcikova S. Proteasome inhibitors – molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014;18(6):947–961. doi: 10.1111/jcmm.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel D.S., Martin T., Wang M., Vij R., Jakubowiak A.J., Lonial S. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart A.K., Rajkumar S.V., Dimopoulos M.A., Masszi T., Spicka I., Oriol A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 27.FDA. 2015.
- 28.Dimopoulos M.A., Goldschmidt H., Niesvizky R., Joshua D., Chng W.J., Oriol A. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337. doi: 10.1016/S1470-2045(17)30578-8. [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos M.A., Moreau P., Palumbo A., Joshua D., Pour L., Hajek R. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 30.Hajek R., Masszi T., Petrucci M.T., Palumbo A., Rosinol L., Nagler A. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS) Leukemia. 2017;31(1):107–114. doi: 10.1038/leu.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facon T., Lee J.H., Moreau P., Niesvizky R., Dimopoulos M.A., Hajek R. Phase 3 study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) Clin Lymphoma Myeloma Leuk. 2017;17(1):e26–e27. [Google Scholar]
- 32.Berenson J.R., Cartmell A., Bessudo A., Lyons R.M., Harb W., Tzachanis D. CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127(26):3360–3368. doi: 10.1182/blood-2015-11-683854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornell R.F., Ky B., Weiss B.M., Du L., Carver J., Cohen A.D. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. 59th ASH annual meeting; Atlanta, GA; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grandin E.W., Ky B., Cornell R.F., Carver J., Lenihan D.J. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015;21(2):138–144. doi: 10.1016/j.cardfail.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Nowis D., Maczewski M., Mackiewicz U., Kujawa M., Ratajska A., Wieckowski M.R. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol. 2010;176(6):2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal A., Luthi J., Belohlavek M., Kortum K.M., Mookadam F., Mayo A. Carfilzomib and the cardiorenal system in myeloma: an endothelial effect? Blood Cancer J. 2016;6:e384. doi: 10.1038/bcj.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimopoulos M.A.R., Gavriatopoulou M., Psimenou M., Ziogas E., Eleutherakis-Papaiakovou D., Fotiou E.D. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449–454. doi: 10.1182/bloodadvances.2016003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kupperman E., Lee E.C., Cao Y., Bannerman B., Fitzgerald M., Berger A. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 39.Moreau P., Masszi T., Grzasko N., Bahlis N.J., Hansson M., Pour L. Oral ixazomib lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 40.Hou J., Jin J., Xu Y., Wu D., Ke X., Zhou D. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study. J Hematol Oncol. 2017;10(1):137. doi: 10.1186/s13045-017-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateos M.V., Masszi T., Grzasko N., Hansson M., Sandhu I., Pour L. Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1. Haematologica. 2017;102:1767–1775. doi: 10.3324/haematol.2017.170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson P.G., Kumar S., Laubach J.P., Paba-Prada C., Gupta N., Berg D. New developments in the management of relapsed/refractory multiple myeloma – the role of ixazomib. J Blood Med. 2017;8:107–121. doi: 10.2147/JBM.S102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson P.G., Spencer A., Cannell P., Harrison S.J., Catley L., Underhill C. Phase 1 clinical evaluation of twice-weekly marizomib (NPI-0052), a novel proteasome inhibitor, in patients with relapsed/refractory multiple myeloma (MM) Blood. 2011;118(21):302–312. [Google Scholar]
- 44.Das D.S., Ray A., Song Y., Richardson P., Trikha M., Chauhan D. Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD immunomodulatory drug pomalidomide. Br J Haematol. 2015;171(5):798–812. doi: 10.1111/bjh.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreau P., de Wit E. Recent progress in relapsed multiple myeloma therapy: implications for treatment decisions. Br J Haematol. 2017;179(2):198–218. doi: 10.1111/bjh.14780. [DOI] [PubMed] [Google Scholar]
- 46.Hari P.N., Shain K.H., Voorhees P.M., Gabrail N., Abidi M.H., Zonder J. Oprozomib and dexamethasone in patients with relapsed and/or refractory multiple myeloma: initial results from the dose escalation portion of a phase 1b/2, multicenter open-label study. Blood. 2014;124(21):53–3453. [Google Scholar]
- 47.Kaufman J.L., Siegel D.S., Vij R., Badros A., Neuman L., Wong H. Clinical profile of single-agent modified-release oprozomib tablets in patients (Pts) with hematologic malignancies: updated results from a multicenter, open-label dose escalation phase 1b/2 study. Blood. 2013;122(21):84–3184. [Google Scholar]
- 48.Laubach J., Garderet L., Mahindra A., Gahrton G., Caers J., Sezer O. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30(5):1005–1017. doi: 10.1038/leu.2015.356. [DOI] [PubMed] [Google Scholar]
- 49.Moreau P. How I treat myeloma with new agents. Blood. 2017;130(13):1507–1513. doi: 10.1182/blood-2017-05-743203. [DOI] [PubMed] [Google Scholar]
- 50.Cavo M., Tacchetti P., Patriarca F., Petrucci M.T., Pantani L., Galli M. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 51.Durie B.G., Hoering A., Abidi M.H., Rajkumar S.V., Epstein J., Kahanic S.P. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garderet L., Iacobelli S., Moreau P., Dib M., Lafon I., Niederwieser D. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30(20):2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 53.Moreau P., Touzeau C. Multiple myeloma: from front-line to relapsed therapies. Am Soc Clin Oncol Educ Book. 2015:e504–e511. doi: 10.14694/EdBook_AM.2015.35.e504. [DOI] [PubMed] [Google Scholar]
- 54.Rosinol L., Oriol A., Teruel A.I., Hernandez D., Lopez-Jimenez J., de la Rubia J. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 55.Molineaux S.M. Molecular pathways: targeting proteasomal protein degradation in cancer. Clin Cancer Res. 2012;18(1):15–20. doi: 10.1158/1078-0432.CCR-11-0853. [DOI] [PubMed] [Google Scholar]
- 56.Boccadoro M. 15th international myeloma workshop. 2015. Second-generation proteasome inhibition: what a difference a generation makes; p. e13. [Google Scholar]