Abstract
Background
The aim of this study was to assess the correlations of Twist expression with pathological and computed tomography (CT) characteristics and prognosis of non-small cell lung cancer (NSCLC).
Material/Methods
We enrolled 120 patients with lung cancer who underwent CT examination. The Twist protein expression level was detected in 120 cases of cancer tissues and a control group using immunohistochemical method. The survival curve was plotted using the Kaplan-Meier method and analyzed via log-rank test.
Results
The Twist expression was associated with tumor stage, differentiation degree, and presence or absence of lymph node metastasis, but had no correlations with sex, age, or histological type. Grade-3 bronchial involvement, pleural indentation, and hilar and mediastinal lymph node enlargement occurred more frequently in the high-expression Twist group compared with the low-expression Twist group. The overall survival rate of patients with Twist overexpression was significantly lower than that of patients with normal Twist expression. The mean survival time was 69.8 months in Twist protein expression-negative patients and 45.8 months in Twist protein expression-positive patients. Finally, the positive expression of Twist protein was significantly correlated with the long-term survival and prognosis of patients.
Conclusions
The Twist gene might be involved in the occurrence and development of NSCLC, which is correlated with patient prognosis.
MeSH Keywords: Lung Neoplasms; Pathological Conditions, Anatomical; Prognosis
Background
Lung cancer is a malignant tumor that causes millions of deaths each year worldwide. Non-small cell lung cancer (NSCLC) accounts for about 80% of all lung cancer cases, and around half of patients with stage I NSCLC die within 10 years after diagnosis [1,2]. Significant progress has been made in surgery, chemotherapy, radiotherapy, and new therapeutic strategies, but only 5–10% of NSCLC patients have long-term survival [3]. Treatment failure and poor prognosis of NSCLC are due to local and distant metastases [4].
Local invasion is regarded as the first step in malignancy, leading to distant metastasis. The epithelial-mesenchymal transition (EMT) plays a critical role in tumor invasion and metastasis. Currently, E-cadherin is considered as an inhibitor of tumor progression and invasion [5]. In fact, transcription inhibition has become accepted as a basic mechanism contributing to the inhibition of E-cadherin during tumor progression. As a transcription factor, Twist is believed to play an important role in tumor progression through inhibition of E-cadherin, with potential clinical significance [6,7]. The Twist-like proteins Twist-1 and Twist-2 (previously known as Dermo-1) belong to the basic helix-loop-helix (bHLH) family and are highly-conserved transcription factors characterized by targeting the basic deoxyribonucleic acid (DNA) binding domain of E-box sequence 59-CANNTG. In mammals, Twist-1 and Twist-2 are highly homologous in structure. The N-termini of Twist-1 and Twist-2 are more divergent, and they lack a glycine-rich region [8].
Even without transformation ability, Twist-1 and Twist-2 can bind to activated oncoprotein to promote EMT. Recently, Twist has been determined as the key regulatory factor for oncogene and cancer metastasis. Inhibiting Twist expression can suppress the metastasis of 4T1 cells from the breasts of BALB/c mice to the lungs. However, whether Twist could be used as a prognostic marker in lung cancer remains controversial. A previous study [9] demonstrated that Twist expression probably did not predict the survival of NSCLC, while another study [10] showed a correlation of Twist expression with prognosis. In addition, Hung et al. [11] showed that Twist is associated with shorter overall survival (OS), and its overexpression does not affect recurrence-free survival. The present retrospective study evaluated whether Twist could be used as a prognostic factor in NSCLC patients, and explored its correlations with pathological and CT characteristics.
Material and Methods
Clinical data
We collected data on 120 patients with lung cancer admitted in our hospital from January 1, 2008 to December 31, 2012. We also collected 120 samples of NSCLC tissues and 120 samples of corresponding non-tumor tissues, as well patient data (Table 1).
Table 1.
Clinical data of patients enrolled.
| Clinicopathological data | Case (n) | Ratio (%) | |
|---|---|---|---|
| Age (years old) | >60 | 50 | 41.7 |
| ≤60 | 70 | 58.3 | |
| Gender | Male | 81 | 67.5 |
| Female | 39 | 32.5 | |
| Pathological type | Squamous carcinoma | 63 | 52.5 |
| Adenocarcinoma | 57 | 47.5 | |
| Clinical stage | Stage I | 38 | 31.7 |
| Stage II | 34 | 28.3 | |
| Stage III | 47 | 39.2 | |
| Stage IV | 1 | 0.08 |
Inclusion criteria: (1) patients who fully understood this study and voluntarily signed the informed consent, (2) patients who were diagnosed with lung cancer via histology and/or cytology, (3) patients with definitely measurable lesions meeting the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), and (4) patients with an expected survival time of more than 12 weeks.
Exclusion criteria: (1) patients undergoing surgery or radiotherapy at 4 weeks before enrollment, (2) patients who previously underwent chemoradiotherapy, or (3) patients with the second primary malignant tumor detectable in clinic, or other malignant tumors (except for adequately treated skin basal cell carcinoma or cervical carcinoma in situ) in the last 5 years.
This study was approved by the Review Committee of the Affiliated Hospital of Qingdao University. Informed consent was obtained from all participants.
Immunohistochemistry
Paraffin-embedded specimens fixed in formalin were sliced into 4-mm sections, deparaffinized with xylene, and gradually re-hydrated in ethanol, followed by antigen retrieval in 0.01 moL/L citrate buffer (pH 6.0) under high pressure for 2 min. Immunostaining was conducted using the streptavidin-peroxidase (SP) method. The endogenous peroxidase activity was blocked using hydrogen peroxide (3%), and non-specific binding was reduced using normal goat serum followed by incubation of the sections with Twist (H-81) rabbit polyclonal antibody (diluted at 1: 100) (Santa Cruz, CA, USA) at 4°C overnight and subsequent peroxidase reaction using diaminobenzidine (DAB), counterstaining with hematoxylin, dehydration with ethanol, and fixation. All specimens were evaluated independently by 2 pathologists using the following criteria:
A total of 10 single fields of view were randomly selected from each glass slide. The scoring of Twist staining intensity was: 0 points (no signal), 1 point (weak), 2 points (moderate), and 3 points (strong). The scoring of percentage of positive cells was: 0 points (5%), 1 point (5–25%), 2 points (26–50%), and 3 points (>51%). Both scores of each field of view were multiplied to obtain the final score (0–9 points), and the average value of 10 fields of view was the final score of one specimen. Finally, scores were categorized as: negative (−) was 0 points, low expression (+) was 1 point, moderate expression (++) was 2–4 points, and high expression (+++) was 6–9 points. Scores of tumor specimens (++) and (+++) indicated overexpression, while the scores (−) and (+) indicated normal expression.
CT examination
All patients underwent a chest enhanced CT examination.
Imaging diagnosis was based on the criteria in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th edition). Images were analyzed by 2 senior radiologists (above deputy senior title), and the postoperative pathological staging results were used as the criterion standard.
Follow-up
Patients were comprehensively evaluated according to the patient’s imaging examination data and the alternation of chemotherapy regimens combined with the general conditions and complications of patients. OS was recorded via data query and telephone follow-up. Non-neoplastic death, loss to follow-up, and survival for more than 5 years were classified into censored data.
Statistical analysis
Statistical Product and Service Solutions (SPSS) software was used for the analysis of follow-up data in this study. The chi-square test was used to assess the correlation between Twist expression level and clinicopathological features of lung cancer patients. Survival curves were plotted using the Kaplan-Meier method and analyzed via log-rank test. All test methods were bilaterally distributed, and p<0.05 suggested that the difference was statistically significant.
Results
Twist expression in NSCLC
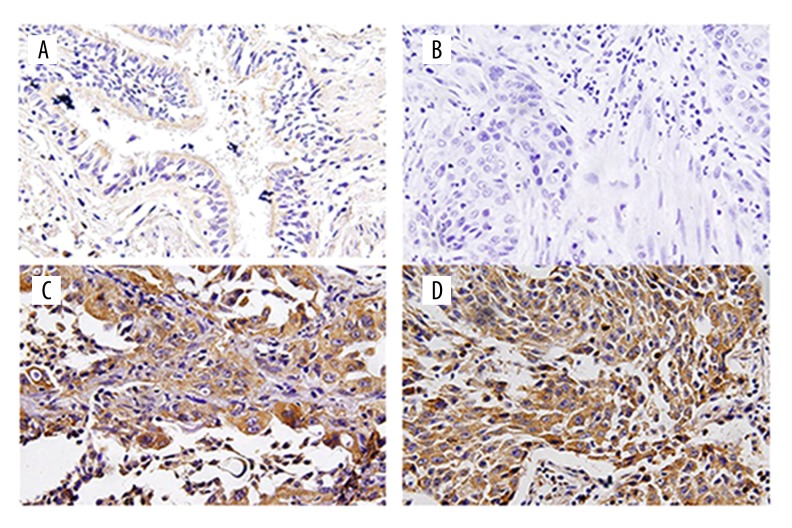
The Twist protein was mainly expressed in the cytoplasm, and immunohistochemical results revealed that the positive expression displayed brown yellow or dark brown staining (Figure 1). The Twist expression was significantly higher in cancer tissues than in the control group, and later stages were associated with higher expression level (p<0.05) (Table 2)
Figure 1.
(A) Twist is weakly expressed in normal tissues. (B) Twist is negatively expressed in lung cancer. (C) Twist is positively expressed in lung squamous carcinoma. (D) Twist is positively expressed in lung adenocarcinoma.
Table 2.
Twist expression in NSCLC group and control group.
| Twist protein expression | p | ||
|---|---|---|---|
| Low expression (n) | High expression (n) | ||
| Lung cancer in stage I–II | 32 | 40 | 0.023 |
| Lung cancer in stage III–IV | 10 | 38 | |
| Control group | 18 | 2 | |
Correlation between Twist expression and clinicopathological features
The correlation between Twist expression and clinicopathological features was analyzed and showed that the Twist expression was associated with tumor stage, differentiation degree, and presence or absence of lymph node metastasis (p<0.05), but had no correlations with the patient’s sex, age, or histological type. The ratio of Twist overexpression was significantly higher in patients with stage III–IV (56.25%) than in patients with stage I (21.05%) and stage II (32.35%, p<0.01). Twist overexpression rate was significantly higher in poorly-differentiated cancer tissues (66.67%) compared with well-differentiated (20.00%) and moderately-differentiated (26.79%) cancer tissues (p<0.01). The Twist overexpression rate in the lymph node metastasis group (48.58%) was also higher than that in the non-lymph node metastasis group (24.0%, p<0.01) (Table 3)
Table 3.
Correlation between Twist expression and clinicopathological features.
| Feature | Case (n) | Normal Twist expression | Twist overexpression | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 81 | 48 | 33 | 0.436 |
| Female | 39 | 26 | 13 | |
| Age | ||||
| >60 | 54 | 35 | 19 | 0.523 |
| ≤60 | 66 | 39 | 27 | |
| TNM stage | ||||
| I | 38 | 30 | 8 | |
| II | 34 | 23 | 11 | 0.003 |
| III–IV | 48 | 21 | 27 | |
| Differentiation degree | ||||
| Well differentiated | 25 | 20 | 5 | |
| Moderately differentiated | 56 | 41 | 15 | 0.000 |
| Poorly differentiated or no differentiation | 39 | 13 | 26 | |
| Histological type | ||||
| SCC | 63 | 38 | 25 | 0.750 |
| AC | 57 | 36 | 21 | |
| Lymph node metastasis | ||||
| N0 | 50 | 38 | 12 | 0.007 |
| N1, N2, N3 | 70 | 36 | 34 | |
Different CT manifestations
In the analysis of correlation between Twist expression intensity and CT characteristics, we found that grade-3 bronchial involvement, pleural indentation, and hilar and mediastinal lymph node enlargement were more frequent in the Twist high-expression group compared with the Twist low-expression group (p<0.05) (Table 4).
Table 4.
Correlation between Twist expression intensity and CT characteristics.
| CT characteristic | Twist expression intensity | p | ||
|---|---|---|---|---|
| (−)–(+) | (++) | (+++) | ||
| Lobulated sign | 32 | 23 | 44 | 0.26 |
| Spicule sign | 23 | 19 | 21 | 0.32 |
| Grade-3 bronchial involvement | 26 | 5 | 43 | 0.023 |
| Pleural indentation | 31 | 11 | 34 | 0.021 |
| Hilar and mediastinal lymph node enlargement | 29 | 9 | 47 | 0.002 |
Correlation between Twist expression and prognosis
To evaluate whether the Twist expression in NSCLC is correlated with patient prognosis, a Kaplan-Meier survival curve was plotted, revealing a significant correlation with the OS of patients (log-rank test, p<0.001). The OS of patients with Twist overexpression was obviously lower than that of patients with normal Twist expression. The mean survival time was 69.8 months in Twist protein expression-negative patients and 45.8 months in Twist protein expression-positive patients (p<0.01, Figure 2).
Figure 2.
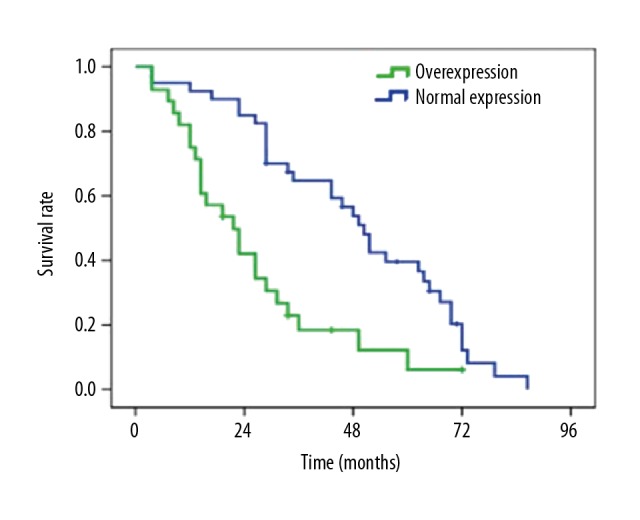
OS curve of lung cancer patients.
Twist protein expression was compared in patients who were followed up for ≥4 years, and it was found that the positive expression rate of Twist protein in patients with survival time ≥4 years was remarkably lower than in patients with survival time <4 years. The positive expression of Twist was significantly associated with long-term survival and prognosis (p<0.01, Table 5)
Table 5.
Correlation between Twist protein expression and 4-year survival rate of NSCLC patients.
| Survival time | Twist expression | χ2 | p | |
|---|---|---|---|---|
| Normal | Overexpression | |||
| >4 years | 63 | 17 | 6.497 | 0.001 |
| ≤4 years | 11 | 29 | ||
Discussion
NSCLC is a major type of lung cancer and a primary cause of cancer-related deaths worldwide. EMT is believed to play a critical role in the regulation of tumor invasion and metastasis. It has been found that Twist, a highly-conserved bHLH transcription factor, induces cancer progression through EMT. In addition, Twist expression is demonstrated to be associated worse survival of patients with cancer, including lung cancer [12].
During tumor progression, the re-activation of EMT-inducible transcription factors Twist-1 and Twist-2 stimulates production of cells with self-renewal ability, which is beneficial for the growth of the primary tumor and the occurrence of secondary tumors. Twist is also known as Twist-1 [13] and its overexpression in several epithelial cancer cell types is demonstrated to be correlated with poor prognosis, which is related to its ability to promote EMT [14,15]. New evidence suggests that Twist plays a role in oncoprotein, and its expression is positively correlated with metastasis and poor clinical prognosis of various human malignant tumors [16–19], including breast cancer, colorectal cancer, pancreatic ductal adenocarcinoma, and oral cancer.
Hui et al. proved that Twist overexpression is related to TNM stage, differentiation, and lymph node status in NSCLC patients, which is consistent with results of the present study, indicating that Twist is involved in the progression of NSCLC and acts as an independent biomarker for poor clinical prognosis [20].
Twist inhibits apoptosis of several human cancer cells, possibly through signaling pathways such as ARF/MDM2/p53, TNF-α, and IGF in osteoblasts and fibroblasts, but the mechanism of the Twist-related anti-apoptotic signaling pathway in lung cancer cells needs further study. A recent study revealed that Twist increases the activity of MMP9 in lung cancer H358 cells, and Twist-1 knockdown inhibits the invasion of lung cancer A549 and LTE cells, indicating that Twist-1 plays a critical role in cell invasion. Moreover, Twist also plays a role in embryogenesis and tumorigenesis, which is also involved in regulating cell invasion and metastasis. It was proved previously that Twist expression in NSCLC is significantly increased, which is related to the differentiation of NSCLC. In the present study, we found overexpression of Twist in 120 cases of NSCLC specimens, which was associated with advanced TNM stage, poor differentiation, lymph node metastasis, and lower survival rates of patients, which are consistent with conclusions in previous studies on primary NSCLC. Furthermore, it was found that Twist overexpression was correlated with the CT characteristics of a tumor, and the grade-3 bronchial involvement, pleural indentation, and hilar and mediastinal lymph node enlargement occurred more frequently in the Twist high-expression group compared with the Twist low-expression group. The above findings have not been reported in previous studies, and they provide a basis for early diagnosis of lung cancer. Due to the limited number of patients enrolled in the present study, large-cohort clinical studies are required to confirm the correlations of Twist expression with PET images to assess the diagnostic value of the combined examination of Twist expression with PET scan in lung cancer. However, if Twist expression is confirmed to be valuable for the diagnosis of lung cancer, the financial feasibility has to be considered in the future.
Conclusions
Our study findings suggest that Twist is closely correlated with the clinicopathological and CT characteristics and prognosis of NSCLC, suggesting that Twist expression can be used as a biomarker for predicting the prognosis of patients with NSCLC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ou SH, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: A population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110(7):1532–41. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 3.Haithcock BE, Stinchcombe TE, Socinski MA. Treatment of surgically resectable non-small-cell lung cancer in elderly patients. Clin Lung Cancer. 2009;10(6):405–9. doi: 10.3816/CLC.2009.n.076. [DOI] [PubMed] [Google Scholar]
- 4.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier W, Behrens J. Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 6.Peinado H, Cano A. New potential therapeutic targets to combat epithelial tumor invasion. Clin Transl Oncol. 2006;8(12):851–57. doi: 10.1007/s12094-006-0148-z. [DOI] [PubMed] [Google Scholar]
- 7.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle. 2008;7(14):2090–96. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 8.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 9.Miura N, Yano T, Shoji F, et al. Clinicopathological significance of Sip1-associated epithelial mesenchymal transition in non-small cell lung cancer progression. Anticancer Res. 2009;29(10):4099–106. [PubMed] [Google Scholar]
- 10.Lv T, Wang Q, Cromie M, et al. Twist1-mediated 4E-BP1 regulation through mTOR in non-small cell lung cancer. Oncotarget. 2015;6(32):33006–18. doi: 10.18632/oncotarget.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung JJ, Yang MH, Hsu HS, et al. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64(12):1082–89. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- 12.Wushou A, Hou J, Zhao YJ, et al. Twist-1 up-regulation in carcinoma correlates to poor survival. Int J Mol Sci. 2014;15(12):21621–30. doi: 10.3390/ijms151221621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanbabaei H, Teimoori A, Mohammadi M. The interplay between microRNAs and Twist1 transcription factor: A systematic review. Tumour Biol. 2016;37(6):7007–19. doi: 10.1007/s13277-016-4960-y. [DOI] [PubMed] [Google Scholar]
- 14.Ansieau S, Morel AP, Hinkal G, et al. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010;29(22):3173–84. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 15.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 16.Gong T, Xue Z, Tang S, et al. Nuclear expression of Twist promotes lymphatic metastasis in esophageal squamous cell carcinoma. Cancer Biol Ther. 2012;13(8):606–13. doi: 10.4161/cbt.19851. [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Lu S, Tian J, et al. TWIST expression in hypopharyngeal cancer and the mechanism of TWIST-induced promotion of metastasis. Oncol Rep. 2012;27(2):416–22. doi: 10.3892/or.2011.1481. [DOI] [PubMed] [Google Scholar]
- 18.Wushou A, Pan HY, Liu W, et al. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70(6):1473–79. doi: 10.1016/j.joms.2011.06.212. [DOI] [PubMed] [Google Scholar]
- 19.Ru GQ, Wang HJ, Xu WJ, et al. Upregulation of Twist in gastric carcinoma associated with tumor invasion and poor prognosis. Pathol Oncol Res. 2011;17(2):341–47. doi: 10.1007/s12253-010-9332-0. [DOI] [PubMed] [Google Scholar]
- 20.Hui L, Zhang S, Dong X, et al. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS One. 2013;8(4):e62171. doi: 10.1371/journal.pone.0062171. [DOI] [PMC free article] [PubMed] [Google Scholar]