Abstract
Li–Fraumeni syndrome (LFS) is an autosomal dominant condition associated with a high risk of a broad range of childhood- and adult-onset cancers. LFS is related to germline mutations of the tumor-suppressor gene TP53. The most common reported leukemia associated with LFS is hypodiploid acute lymphoblastic leukemia, but myeloid malignancies including acute myeloid leukemia (AML), chronic myeloid leukemia, and myelodysplastic syndrome (MDS) are also reported, often in the setting of therapy-related disease. We reviewed the clinicopathologic characteristics including cytogenetics and molecular analysis for seven adult patients with LFS and hematologic malignancies evaluated at the Hereditary Hematologic Malignancy Clinic (HHMC) at MD Anderson Cancer Center. We present this LFS review series to increase awareness of LFS for the appropriate diagnosis of both patients and potentially affected relatives, as well as provide experience with patient outcomes in this difficult to treat population.
Keywords: leukemia, multiple lineage myelodysplasia
INTRODUCTION
Li–Fraumeni syndrome (LFS) is a rare autosomal dominant cancer predisposition syndrome caused by germline TP53 gene mutations, first described in 1969 by Li and Fraumeni (Li and Fraumeni 1969). Patients with this syndrome are at increased risk of developing multiple primary tumors including breast cancer, soft tissue sarcoma, brain tumors, osteosarcoma, and adrenocortical carcinoma (Gonzalez et al. 2009; Ruijs et al. 2010). Other cancers associated with LFS include ovarian, gastrointestinal, pancreatic, genitourinary, skin, renal, thyroid, prostate, and lung, as well as leukemia, lymphoma, and neuroblastoma (McBride et al. 2014). Incidence of leukemias in LFS is ∼4% (Bougeard et al. 2015; World Health Organization 2018), including hypodiploid acute lymphoblastic leukemia (ALL) and therapy-related myeloid disorders including acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) (Li et al. 1988; Law et al. 1991; Birch et al. 2001; Talwalkar et al. 2010).
Two published algorithms are utilized to identify patients at risk of LFS who would benefit from molecular testing, the classical LFS criteria and the Chompret criteria (Tinat et al. 2009; Bougeard et al. 2015), detailed in Table 1. Criteria include a personal history of sarcoma or other LFS spectrum tumors and a family history of cancers at a young age, indicating the importance of obtaining an accurate family history in the evaluation of cancer patients. When used alone, the classical LFS criteria have a lower sensitivity of 40% and the Chompret criteria have a sensitivity of 20% (Sorrell et al. 2013), but when combined, they have a sensitivity of 95% and a specificity of 52% in identifying patients with LFS (Ruijs et al. 2010).
Table 1.
Chompret and classical LFS criteria
| Classical LFS criteria (one of the following) | Proband with sarcoma diagnosed before age 45 yr |
| First-degree relative with any cancer before age 45 yr | |
| First- or second-degree relative with any cancer before age 45 yr or sarcoma at any age | |
| Chompret criteria (one of the following) | Proband with LFS tumors (sarcoma, premenopausal breast cancer, brain tumor, adrenocortical carcinoma, leukemia, or bronchoalveolar cancer), before age 46 yr |
| At least one first- or second-degree relative with an LFS tumor (except breast cancer if the proband has breast cancer, before age 56 yr or with multiple tumors) | |
| Proband with multiple tumors (except multiple breast tumors), two of which belong to LFS tumors and the first of which occurred before age 46 yr | |
| Proband diagnosed with adrenocortical carcinoma or choroid plexus tumor, irrespective of family history |
Age-related and sex-specific cancer risks with LFS were reported by Chompret et al. (2000). The overall risks of men to develop cancer by ages 16, 45, and 85 yr are 19%, 41%, and 73%, respectively, whereas the risks of women by ages 16, 45, and 85 yr are 12%, 84%, and 100%, respectively (Varley et al. 1997; Chompret et al. 2000). Truncating variants have been suggested to result in decreased penetrance and later age at onset compared to the more common missense mutations (Zerdoumi et al. 2013). Given the dramatic lifetime risk of cancer, individuals with a diagnosis of LFS are followed twice annually by the Li–Fraumeni Education and Early Detection (LEAD) cancer prevention clinic, with biannual physical exam, blood work, and imaging including whole-body magnetic resonance imaging (MRI) for early detection.
Despite the known association of LFS with hematologic malignancies, there is limited awareness of LFS in the adult malignant hematology community. In this case series, we report on the presentation, treatment, and patient outcome of seven adult patients with LFS and hematologic malignancy who were evaluated within the Hereditary Hematologic Malignancy Clinic (HHMC) at the University of Texas MD Anderson Cancer Center. This cohort includes patients who were diagnosed with de novo acute leukemia with a LFS diagnosis established during leukemia therapy, as well as patients with a history of solid tumors and known LFS diagnosis, who developed therapy-related AML or MDS during follow-up within the LEAD prevention clinic.
CLINICAL HISTORIES
Case 1
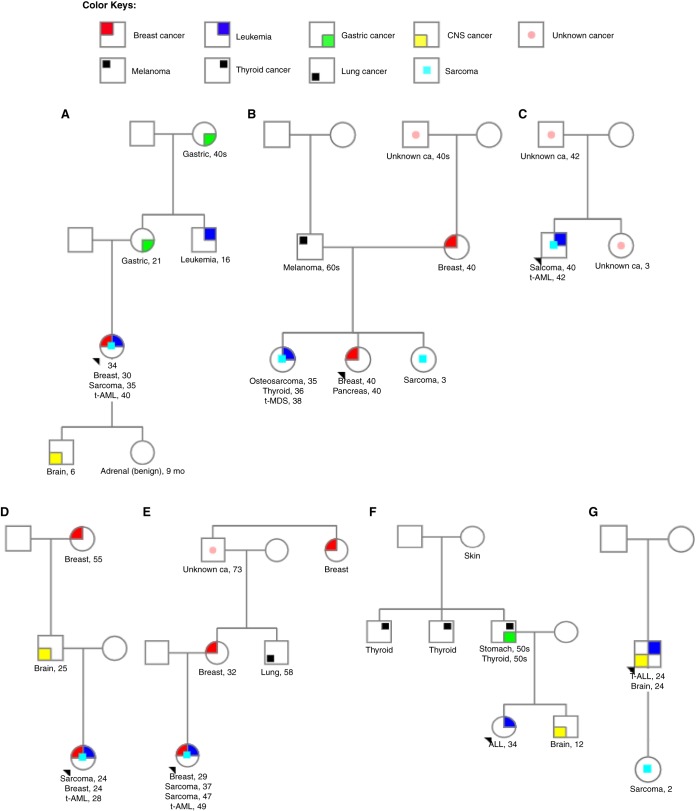
A 34-yr-old woman was referred to clinical cancer genetics because of a history of early-onset breast cancer. She was diagnosed with right breast invasive ductal carcinoma at age 30 and treated with chemotherapy and radiotherapy (Table 2). Her family history was significant for medulloblastoma in her son at age 6 yr, benign adrenal gland tumor in her daughter at age 9 mo, gastric cancer in her mother at age 21 yr, leukemia in her maternal uncle at age 16 yr, gastric cancer in her maternal grandmother when she was in her 40s, and throat cancer in her maternal great-aunt (grandmother's sister), as detailed in the pedigree in Figure 1A. The patient met Chompret criteria for LFS and underwent TP53 genetic testing, which revealed a pathogenic deletion of exons 10–11. After completion of breast cancer therapy, the patient was monitored within the LEAD program. At age 35 yr, she was diagnosed with a right thigh spindle cell sarcoma, which was treated with neoadjuvant chemotherapy + radiotherapy followed by surgical resection. Subsequently, she developed another sarcoma in her right axillary region, which was treated with chemotherapy followed by surgical resection. At age 40 yr, she was diagnosed with therapy-related AML (t-AML) with adverse cytogenetics (monosomal karyotype with extensive chromosomal abnormalities) and IDH2 mutation (Tables 3 and 4). The patient underwent induction chemotherapy with fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG). She attained a complete remission (CR) and after five courses, CR with negative MRD by multiparameter flow cytometry was confirmed and she was further consolidated with a haploidentical stem cell transplantation (SCT) with fludarabine, melphalan, and thiotepa (FMT) conditioning regimen from a tested and LFS-unaffected family member. The SCT was complicated by acute cutaneous graft versus host disease (GVHD), which resolved with topical steroids. Six months after transplantation, her AML relapsed (Table 5). She was then treated on a Phase 1 clinical trial of azacitidine with lirilumab without response, and she then elected to transition to hospice care after sequelae of neutropenic sepsis.
Table 2.
Treatment of solid tumors preceding hematologic malignancy
| Case no. | Age (years)/sex | Solid malignant neoplasms | Treatment received |
|---|---|---|---|
| 1 | 34/F | RT breast ductal carcinoma | Docetaxel + capecitabine × 4 courses (C), 5-fluorouracil, epirubicin, cyclophosphamide × 4 C, RT segmental mastectomy with axillary node dissection, XRT 5000 cGy in 25 fractions and tamoxifen |
| RT thigh spindle cell cancer | Adriamycin + ifosfamide × 6 C, XRT 5000 cGy in 25 fractions, and surgical resection | ||
| Sarcoma of RT axillary region | Gemcitabine + docetaxel × 5 C, and surgical resection | ||
| 2 | 32/F | LT maxillary sinus osteosarcoma | Doxorubicin + cisplatin × 4 C, and LT maxillectomy |
| Bilateral papillary thyroid cancer | Thyroidectomy with LT-sided paratracheal neck dissection | ||
| 3 | 42/M | Pleomorphic sarcoma of RT hip and gluteal region | Adriamycin + ifosfamide × 6 C, XRT 5000 cGy in 25 fractions, and radical resection of sarcoma |
| 4 | 28/F | RT breast pleomorphic spindle cell cancer | Adriamycin + ifosfamide × 6 C, XRT 5000 cGy in 25 fractions, and bilateral mastectomy |
| LT breast DCIS | |||
| 5 | 50/F | RT breast mixed ductal and lobular carcinoma | Modified radical mastectomy, 5-fluorouracil, doxorubicin, cyclophosphamide × 6 C, and prophylactic XRT to LT breast |
| High-grade osteosarcoma of chest wall | Adriamycin + ifosfamide and docetaxel + gemcitabine × 2 C, RT chest wall resection, and methotrexate × 8 C | ||
| 6 | 34/F | None | |
| 7 | 24/M | Astrocytoma | Surgical resection |
(RT) Right, (LT) left, (DCIS) ductal carcinoma in situ, (XRT) external beam radiation therapy, (C) cycle.
Figure 1.
(A) Pedigree charts of (A) case 1, (B) case 2, (C) case 3, (D) case 4, (E) case 5, (F) case 6, and (G) case 7.
Table 3.
Clinicopathologic characteristics of hematologic malignancy
| Case no. | Age (years) /sex | Cytogenetics at diagnosis | Germline TP53 mutation | Mutations in BM | Blood malignancy | Time to hematological malignancy diagnosis (from the treatment of primary cancer) (years) |
|---|---|---|---|---|---|---|
| 1 | 34/F | 44,X,add(X)(p22.1),−2,del(5)(q15q33),del(11)(p13),−12,−13,−13,−17,−17,+21,add(22)(p11.2),+3mar[6]/45,idem,+mar[9]/45,idem,+8[1]/89,XX,add(X)(p22.1)×2,−2,−2,+3,−4,del(5)(q15q33)×2,−10,del(11)(p13)×2,−12,−12,−13,−13,−13,−13,−14,−17,−17,−17,−17,+21,+21,+22,+22,add(22)(p11.2)×2, +7mar[1]/46,XX[3] | Del exons 10–11 | IDH2 | t-AML | 10 |
| 2 | 32/F | 45,X,der(X)t(X;3)(q22;q23),−3,add(5)(q22),der(6)t(3;6)(q13;q23),add(7)(q22),del(12)(p12),add(21)(p11.2),−22,+1∼3mar[cp20] | c.184G>T (p.E62*) and c.764T>C (p.I255T) | TP53a | t-MDS | 2 |
| 3 | 42/M | 44,XY,del(5)(q13),add(7)(q11.2),−11,−12,−17,−17,+r,+mar[18]/44,XY,del(5)(q13q33),add(7)(q11.2),−11,−12,−17,−17,+1∼2mar[cp2]/10 | c.800G>A (p.R267Q) and c.467G>A (p.R156H) | TET2, EGFR, TP53a | t-AML | 2 |
| 4 | 28/F | 44∼47,X,−X,del(9)(q13q22),−11,+17,der(17)add(17)(p11.2)add(17)(q11.2),der(17)add(17)(p11.2)hsr(11)(q23),+1∼2mar,2∼5dmin[cp20] | c.586C>T (p.R196*) | BCORL1, WT1, TP53a | t-AML | 4 |
| 5 | 50/F | 55∼58<2n>,XX,+X,+1,+8,+9,+10,+11,+11,+12,der(13;21)(q10;q10),i(13)(q10),+14,+19,+20,+20,+22[cp13]/46,XX,i(11)(p10)[1]/46,XX[6] | c.734G>A (p.R248Q) | BCOR, DNMT3A, TP53a | t-AML | 20 |
| 6 | 34/F | 62∼66,XX,−X,+1,−3,−4,−5,+6,−7,+8,−9,+12,−13,−13,−15,−16,−17,−17,+18,+18,add(20)(q13.2),+22[cp8]/46,XX[12] | c.325T>G (p.F109V) | JAK2, TP53a | ALL | 0 |
| 7 | 24/M | 41∼45,XY,−1,add(1)(p13),add(1)(p36.1),add(5)(q31),der(6)add(6)(p12)dup(6)(q23q23),+7,−12,−16,del(17)(p11.2),−19,+21,+1∼2mar[cp13] | c.524G>A (p.R175H) | NOTCH1, TP53a | Early precursor T-ALL | 0 |
(BM) Bone marrow, (RT) right, (t-AML) therapy-related acute myeloid leukemia, (LT) left, (t-MDS) therapy-related myelodysplastic syndrome, (DCIS) ductal carcinoma in situ, (T-ALL) T-cell acute lymphoblastic leukemia.
aGermline mutation.
Table 4.
Variants
| Gene | Chromosome | HGVS DNA reference | HGVS protein reference | Variant type | Predicted effect (substitution, deletion, etc.) | dbSNP/dbVar ID | Genotype (heterozygous/ homozygous) | ClinVar ID |
|---|---|---|---|---|---|---|---|---|
| TP53 | 17p13.1 | Unknown | Del exons 10-11 | Unknown | Unknown | Unknown | Unknown | Unknown |
| TP53 | 17p13.1 | NM_000546.5:c.184G > T | E62* | Single-nucleotide variant | Nonsense variant | Unknown | Unknown | SCV000882434 |
| TP53 | 17p13.1 | NM_000546.5:c.800G > A | R267Q | Single-nucleotide variant | Missense variant | rs587780075 | Heterozygous | 127823 |
| TP53 | 17p13.1 | NM_000546.5:c.467G > A | R156H | Single-nucleotide variant | Missense variant | rs371524413 | Heterozygous | 127811 |
| TP53 | 17p13.1 | NM_000546.5:c.586C > T | R196* | Single-nucleotide variant | Nonsense variant | rs397516435 | Heterozygous | 43589 |
| TP53 | 17p13.1 | NM_000546.5:c.743G > A | R248Q | Single-nucleotide variant | Missense variant | rs11540652 | Heterozygous | 12356 |
| TP53 | 17p13.1 | NM_000546.5:c.325T > G | F109V | Single-nucleotide variant | Missense variant | rs1057523496 | Heterozygous | 389644 |
| TP53 | 17p13.1 | NM_000546.5:c.524G > A | R175H | Single-nucleotide variant | Missense variant | rs28934578 | Heterozygous | 12374 |
Table 5.
Treatment outcomes of all seven patients
| Case no. | Age/sex | Hematological malignancy | Chemotherapy | Response | SCT | Status | Overall survivala |
|---|---|---|---|---|---|---|---|
| 1 | 34/F | t-AML | FLAG | CR/MRD− | + | Alive | 19 mo |
| AZA + lirilumab | NR | ||||||
| Venetoclax + LDAC | NR | ||||||
| 2 | 32/F | t-MDS | Decitabine | CR/MRD+, followed by NR | + | Dead | 7 mo |
| 3 | 42/M | t-AML | CLIA | CR/MRD | + | Dead | 9 mo |
| SGN-CD33A | NR | ||||||
| Decitabine | NR | ||||||
| 4 | 28/F | t-AML | Decitabine + fludarabine + cytarabine + venetoclax | NR | − | Dead | 5 mo |
| 5 | 50/F | t-AML | Decitabine | NR | − | Alive | 12 mo |
| FLAG | CR/MRD− | ||||||
| Decitabine + venetoclax | CRi/MRD+ | ||||||
| 6 | 34/F | ALL | Obinutuzumab + hyper-CVAD | CR/MRD− | + | Alive | 23 mo |
| 7 | 24/M | Early precursor T-ALL | Hyper-CVAD + asparaginase + bortezomib | CR/MRD− | + | Dead | 23 mo |
| Nelarabine | NR | ||||||
| LY3039478 (investigational drug) | NR | ||||||
| C2V2E | NR | ||||||
| Decitabine + FIA | NR |
(SCT) Stem cell transplantation, (t-AML) therapy-related acute myeloid leukemia, (FLAG) fludarabine, cytarabine, and granulocyte colony-stimulating factor, (AZA) 5-azacitidine, (LDAC) low-dose ara-cytarabine, (CLIA) cladribine, idarubicin, and ara-cytarabine, (t-MDS) therapy-related myelodysplastic syndrome, (T-ALL) T-cell acute lymphoblastic leukemia, (C2V2E) clofarabine, cyclophosphamide, vincristine, velcade, etoposide, (FIA) fludarabine, idarubicin, and ara-cytarabine, (CVAD) cyclophosphamide, vincristine, doxorubicin (adriamycin), dexamethasone, (CR) complete remission, (NR) no response, (Cri) complete remission with incomplete count recovery, (MRD) minimal residual disease (assessed by multiparameter flow cytometry).
aSurvival from the diagnosis of hematological malignancy.
Case 2
A 32-yr-old healthy woman was referred to clinical cancer genetics to undergo single-site TP53 genetic testing after a TP53 mutation was identified in her sister (proband). She was identified to have the familial TP53 nonsense mutation, c.184G>T (p.E62*). Her family history was significant for bilateral breast and pancreatic cancer in her sister at age 40 yr, sarcoma in another sister at age 3 yr, breast cancer in her mother at age 40 yr, and additional cancers in her maternal relatives (Fig. 1B). The patient was enrolled in the LEAD program for proactive surveillance and screening at age 32 yr. At 35 yr, she underwent bilateral prophylactic mastectomy. Seven months later, she reported painless palate swelling, imaging was performed that demonstrated a left maxillary sinus osteosarcoma, and it was treated with chemotherapy and left maxillectomy. Cancer staging for her osteosarcoma also diagnosed thyroid cancer and at 36 yr; she underwent thyroidectomy with left-sided paratracheal neck dissection for bilateral papillary thyroid cancer. Two years later, she was noted to have pancytopenia and was diagnosed with therapy-related MDS (t-MDS) with complex cytogenetics and dual TP53 mutations c.764T>C (p.I255T) and c.184G>T (p.E62*). She received three courses of decitabine (one 10-d induction cycle followed by two 5-d courses) and subsequently achieved clinical response with count recovery and persistent MRD as assessed by multiparameter flow cytometry. She proceeded to an allogenic matched unrelated donor SCT (MUD-SCT) with busulfan, fludarabine, and cyclophosphamide conditioning. Unfortunately, her disease relapsed 62 d post-SCT and she was restarted on treatment with 10 d of decitabine without regaining clinical response.
Case 3
A 42-yr-old man was evaluated by the HHMC because of a history of multiple cancers and therapy-related AML at a young age. His history included pleomorphic sarcoma of the right hip and gluteal area at age 40 yr, 2 yr prior to his AML diagnosis, which had been treated with chemotherapy, radiotherapy, and surgery. His family history was significant for a history of unknown cancer in his sister at age 3 yr and in his father at age 42 yr, as shown in Figure 1C. The patient met criteria for LFS evaluation, and TP53 germline analysis was performed on cultured skin fibroblasts, which revealed two alterations, c.800G>A (p.R267Q) and c.467G>A (p.R156H), both clinically classified as variants of uncertain significance (VUS) (Table 4). His AML was characterized by adverse cytogenetics (including deletions in 5q, 7q, and 11q) with mutations in EGFR and TET2 in addition to the two germline TP53 mutations. The patient underwent induction chemotherapy with cladribine, idarubicin, and ara-cytarabine (CLIA) and achieved a complete remission. He was given a course of consolidation chemotherapy with cladribine and cytarabine and remained in complete remission with positive MRD by multiparameter flow cytometry, followed by MUD allogeneic SCT. His disease relapsed 60 d post-SCT. He received salvage treatment with an investigational anti-CD33 monoclonal antibody with no response and then received decitabine for two monthly courses (5 d and then 10 d, respectively) with no evidence of response and overall stable disease. He later opted to transition home with comfort care.
Case 4
A 28-yr-old woman with LFS presented with therapy-related AML with complex cytogenetics and BCORL1, TP53, and WT1 mutations. She had a medical history of right breast pleomorphic spindle cell sarcoma and contralateral left breast ductal carcinoma in situ (DCIS), diagnosed at age 24 yr, after which genetic testing was performed and demonstrated a germline TP53 mutation c.586C>T (p.R196*) (Table 4). Her family history was significant for glioblastoma in her father at age 25 yr, breast cancer in her paternal grandmother at age 55 yr, and head and neck cancer in a paternal cousin at age 31 yr (Fig. 1D). The patient received induction chemotherapy with decitabine for 10 d followed by fludarabine and cytarabine for 3 d. Venetoclax was added after the initial induction cycle. She then received a second course of decitabine for 10 d with venetoclax. She received a total of three courses of this combination and achieved a partial remission, complicated by neutropenic fever and sepsis.
Case 5
A 50-yr-old woman was referred to clinical cancer genetics because of a history of multiple cancers: mixed ductal and lobular carcinoma of the right breast diagnosed at age 29 yr followed by postradiation high-grade osteosarcoma of the chest wall diagnosed at 37 yr. Her family history was significant for breast cancer in her mother at age 32 yr, lung cancer in maternal uncle at 58 yr, history of unknown cancer in her maternal grandfather at 73 yr, and breast cancer in her maternal great aunt (at a young age) as shown in Figure 1E. TP53 germline analysis was performed and revealed a c.734G>A (p.R248Q) pathogenic mutation (Table 4), confirming LFS. At the age of 49, she was diagnosed with t-AML with complex karyotype and mutations in BCOR, DNMT3A, and her known TP53 mutation. She was started on decitabine therapy, and she received four courses, two 10-d cycles followed by 5-d cycles. Her disease was persistent, and she then received FLAG re-induction, after which she achieved complete remission with positive MRD by multiparameter flow cytometry. She declined SCT and has received two additional courses of 5-d decitabine with the addition of venetoclax. She achieved complete remission with incomplete count recovery and is currently continued on decitabine with venetoclax treatment.
Case 6
A 34-yr-old woman with de novo hypodiploid ALL, a TP53 mutation at ∼50% variant allelic frequency, and a strong family history of cancers was referred to the HHMC for LFS evaluation. Her family history was significant for brain cancer in her brother at age 12 yr, gastric and thyroid cancers in her father in his 50s, thyroid cancer in her paternal uncle, and skin cancer (unknown details) in her paternal grandmother at young age (Fig. 1F). Germline TP53 testing on cultured skin fibroblasts identified a TP53 c.325T>G (p.F109V) mutation, initially classified as a VUS (Table 4). Family studies were initiated and the same variant was detected in the patient's father. Segregation data from other families were combined, and this variant was upgraded to a clinically actionable deleterious mutation by the clinical testing laboratory. She received induction chemotherapy with ofatumumab-HCVAD and subsequently achieved complete remission with negative MRD by multiparameter flow cytometry. She received three courses of consolidation with HCVAD, methotrexate, cytarabine, and rituximab followed by further consolidation with an allogeneic SCT with busulfan, fludarabine, and clofarabine conditioning from an HLA-identical and LFS-unaffected sibling. She experienced acute GVHD of the gastrointestinal tract and chronic skin GVHD, treated with systemic steroids. She is currently in remission 555 d post-SCT and continues to be followed in the LEAD clinic.
Case 7
A 24-yr-old man presented with de novo precursor T-cell ALL (T-ALL). During staging of his T-ALL, he was identified to have a synchronous asymptomatic brain tumor consistent with grade 2 astrocytoma. His family history was significant for rhabdomyosarcoma in his daughter at age 2 yr and lung cancer in his paternal great-grandfather in his 80s, as shown in Figure 1G. His T-ALL characteristics at the time of diagnosis included hypodiploid complex cytogenetics, and sequencing of the bone marrow demonstrated NOTCH1 and TP53 mutations. Based on the high clinical suspicion, evaluation for the germline versus somatic nature of this TP53 mutation was recommended, and he was referred to the HHMC. TP53 germline analysis confirmed the presence of a c.524G>A (p.R175H) mutation (Table 4) in cultured skin fibroblasts, consistent with LFS. He received induction with augmented HCVAD + asparaginase + bortezomib. He achieved complete remission with negative MRD (assessed by multiparameter flow cytometry) and then underwent MUD-SCT with a conditioning regimen of ATG, busulfan, and clofarabine. Unfortunately, his T-ALL relapsed 7 mo post-SCT. He was treated with HCVAD + bortezomib as salvage chemotherapy for two courses without response. He then received nelarabine without response, followed by an investigational Notch inhibitor, also without response. He then received clofarabine + cyclophosphamide + vincristine + bortezomib + etoposide followed by decitabine, fludarabine, idarubicin, and fludarabine (DAC-FIA) without response and ultimately transitioned home on hospice care.
RESULTS
LFS is a well-known cancer predisposition syndrome; however, the clinical awareness of LFS in adult hematology practices is minimal, and the expected outcomes of patients with LFS with various hematologic malignancies are not well described. We report on seven patients with LFS and hematologic malignancy, specifically two patients with hypodiploid ALL as their first presenting malignancy and five with therapy-related MDS or AML.
Of the patients with therapy-related myeloid malignancy, three patients had breast cancer (cases 1, 4, and 5), two had osteosarcoma (cases 2 and 5), and three had soft tissue sarcoma (cases 1, 3, and 5) preceding their hematologic malignancy. Additionally, one patient had thyroid cancer (case 2) and one had astrocytoma (case 7).
The treatments and outcomes of patients with hematological malignancies are listed in Table 2. The median time from last chemotherapy or radiation therapy to the development of therapy-related hematological malignancy was 2 yr (range: 0–20 yr).
Two patients (cases 6 and 7) had hypodiploid ALL; both received hyper-CVAD, achieved complete remission, and proceeded to SCT. Of the five patients with myeloid neoplasms, four patients (cases 1, 3, 4, and 5) had t-AML and one (case 2) had t-MDS. All patients with t-AML received intensive chemotherapy as induction or first salvage. The patient with t-MDS (case 2) received the hypomethylating agent decitabine for t-MDS, achieved complete remission, and proceeded to SCT. At last follow-up, two (cases 1 and 5) patients with myeloid malignancy and one with hypodiploid ALL remain alive.
All patients, except cases 4 and 5, underwent SCT. Of five patients who had SCT, three patients (cases 2, 3, and 7) received a MUD-SCT, case 1 had haploidentical SCT (donor was her son, tested negative for LFS), and case 6 had matched related donor SCT (donor was her sister, tested negative for LFS). Two patients (cases 2 and 3) with myeloid neoplasms (t-MDS and t-AML, respectively) went to SCT with MRD-positive disease (defined by multiparameter flow cytometry with sensitivity of 0.01%–0.1%) and relapsed on day 62 and 60 post-SCT, respectively. Three patients (cases 1, 6, and 7), including two (cases 6 and 7) with ALL and one (case 1) with t-AML, had MRD-negative disease prior to SCT, and only one patient (case 6) remains in remission. The cytogenetic and molecular characteristics of the hematological malignancies at the time of relapse were similar to the initial presentation in all cases.
Genetic anticipation has been frequently reported with inherited cancer syndromes including LFS (Trkova et al. 2002; Ariffin et al. 2014); we identified the occurrence of genetic anticipation in the pedigrees of all of our patients, with a decrease in the age of onset of cancer in successive generations, as shown in Figure 1A–G.
DISCUSSION
Patients with LFS have a significant lifetime risk of developing cancer (Hu et al. 2016; Mai et al. 2016; Asdahl et al. 2017). Although there are several published studies on LFS, the outcomes of patients with hematological malignancies remain poorly annotated. In this case series, we provide patient characteristics, including prior cancers and treatments received, leukemia diagnosis and treatments received, and outcomes in the largest case series of patients with LFS and hematologic malignancies to date.
TP53 mutations are frequently inherited, and family history remains a key criterion for the consideration of LFS. However, de novo mutations occur in ∼10%–20% of LFS cases (Chompret et al. 2000; Correa 2016); thus, consideration of LFS should not depend solely on a positive family history. Additionally, the phenotypic variability of cancers even within families that have the same mutation is described (Malkin 2011), and genetic anticipation, often observed in families with LFS, has been hypothesized to be due to telomere shortening (Tabori et al. 2007).
Although ALL is a common pediatric cancer, attention should be paid to patients with presumed somatic TP53 mutations identified on NGS panels and/or hypodiploid cytogenetics (defined as fewer than 45 chromosomes [Comeaux and Mullighan 2017]), or the phenomenon of masked hypodiploidy, where hypodiploid genome undergoes reduplication resulting in hyperdiploid karyotype (Carroll et al. 2009). In the setting of hypodiploid cytogenetics, >90% of patients will have a TP53 mutation, and prior work suggests about half of these patients may have germline TP53 mutations. Identification of these patients and timely recognition of potentially affected family members can identify appropriate sibling SCT donors and allow for augmented cancer screening programs.
Overall, although most patients with hematologic malignancy and LFS were able to obtain an initial response to induction therapy (either intensive cytarabine-based treatment or augmented decitabine), clinical responses were short, and outcomes were extremely poor. Somatic TP53 mutations, often associated with complex cytogenetic abnormalities, are well described in hematologic malignancies including MDS, AML, and ALL and are known to confer poor outcomes and treatment resistance (Bowen et al. 2009; Rücker et al. 2012; Kadia et al. 2016; Middeke et al. 2016). Kadia and colleagues identified younger patients with TP53-mutated AML had modestly improved survival with low-intensity chemotherapy compared to intensive chemotherapy (Kadia et al. 2016). Additionally, previous work has demonstrated the TP53-mutated lymphocytes in patients with LFS have an intrinsic resistance to conventional chemotherapeutic drugs (Pepper et al. 2003).
Five patients (cases 1–3, 6, and 7) had SCT, including one patient with ALL who continues in a durable remission more than 1 yr post-SCT. Despite allogeneic SCT in complete remission, including three of the five patients with MRD-negative status by flow cytometry at the time of complete remission, long-term disease control was not achieved with SCT. Future improved SCT regimens and post-SCT maintenance strategies are warranted. Studies have shown that surveillance programs might increase the survival chances in LFS patients (Villani et al. 2011; Etzold et al. 2015; Nandikolla et al. 2017). The National Comprehensive Cancer Network (NCCN) has guidelines for screening TP53 mutation carriers (Daly et al. 2017; Kratz et al. 2017), and whole-body MRI has been shown to be an effective screening tool for early detection of solid tumors (Table 6; Asdahl et al. 2017; Ballinger et al. 2017; Bojadzieva et al. 2017). The effectiveness of early detection of leukemia from screening tests such as biannual complete blood counts is less evident because of the sporadic nature of acute leukemias, and additional clinical research is needed.
Table 6.
Screening guidelines of adult patients in LEAD program
| Cancer | Exams and tests | Frequency |
|---|---|---|
| General cancer prevention | Complete physical exam with a focus on brain and thyroid | Every 6 mo |
| Adrenocortical tumor | Basic labs testsa Whole-body MRI |
Every year |
| Breast (begin screening at age 20–25 or 5–10 yr younger than first diagnosis of breast cancer in the family) | Clinical breast exam by a physician | Every 6 mo |
| Mammogram and MRI breast | Every year | |
| Brain | MRI brain | Every year |
| Colon (begin at age 25 or 5 yr before the earliest known colon cancer in the family) every 2–5 yr | Colonoscopy Esophagogastroduodenoscopy |
Every 2–5 yr |
| Leukemia/lymphoma | Basic laboratory testsa | Every year |
| Melanoma | Skin exam | Every year |
| Ovarian (begin at age 35) | Refer to a physician specialized in high-risk ovarian cancer screening | |
| Pancreas | Refer to a physician specialized in high-risk pancreatic cancer screening | |
| Sarcoma | Whole-body MRI | Every year |
aDifferential blood count and metabolic panel.
Therapy-related AML and t-MDS frequently occur in patients with LFS, likely related to their extensive cancer history and myelosuppressive prior treatments. Cytotoxic agents like alkylating agents and topoisomerase inhibitors, as well as radiation therapy, are known to be associated with t-MDS/t-AML (Godley and Larson 2008). Radiation exposure in patients with LFS should be avoided whenever possible because of the high risk of radiation-induced malignancies (Limacher et al. 2001; Cohen et al. 2005; Evans et al. 2006). Although curative therapy must be administered for the patient with LFS and a primary solid tumor, consideration to the augmented lifetime risk of t-MDS/AML is prudent.
At MD Anderson, the LEAD program enrolls patients with germline TP53 mutations (Lammens et al. 2010; Villani et al. 2016; Ross et al. 2017). Patients and their family members are screened per adult screening guidelines as shown in Table 6. The rationale behind these screening tests and exam is to detect cancer at an early age. For example, Case 2 was referred to LEAD for genetic testing after the patient's sister was found to have a TP53 mutation. She was subsequently diagnosed to have multiple cancers at early stages while being followed by the screening program. This highlights how attention to a strong personal and family history can raise suspicion for LFS and allow evaluation and surveillance programs, like LEAD, to lead to early intervention.
CONCLUSION
In this case series, we retrospectively reviewed the characteristics and outcomes of seven adult patients with LFS who developed hematologic malignancies. LFS increases the lifetime risk of cancer in many organ sites, and it is important to consider hypodiploid ALL as a possible presenting malignancy for individuals with LFS. In addition, therapy-related myeloid malignancies including MDS and AML are common in patients with LFS and portend a dismal prognosis with standard therapies and even allogenic SCT. Future TP53-independent leukemia treatment strategies and clinical trial participation are recommended. Screening and surveillance programs for inherited cancer predisposition syndromes like LFS are critical to increase awareness of patients and health-care providers, provide augmented cancer surveillance to improve patient outcomes, and also provide individualized and risk-based treatment decisions at all steps along the cancer pathway continuum.
METHODS
Patients with a diagnosis of LFS seen within the HHMC from 2014 to 2018 were included. Germline analysis for evaluation of TP53 status in patients with hematological malignancies included sequencing performed on cultured skin fibroblasts obtained from skin punch biopsy. Clinicopathologic characteristics of the hematologic malignancy including cytogenetics and molecular analysis, treatment, and clinical outcomes were reviewed. Clinical response was assessed using the International Working Group (IWG) criteria for AML and MDS (Cheson et al. 2003, 2006). Minimal residual disease (MRD) was evaluated by multiparameter flow cytometry with a sensitivity of 0.01%–0.1%.
ADDITIONAL INFORMATION
Data Deposition and Access
The variants have been deposited by external sources in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers: SCV000260755.5, SCV000218877.8, SCV000260299.5, SCV000253851.6, SCV000532251.3, SCV000261917.6, and SCV000882434.
Ethics Statement
Informed consent for all patients was obtained following institutional guidelines and in accordance with the Declaration of Helsinki and MDACC IRB; protocol PA14-0392.
Author Contributions
M.S., C.D.D., and S.A.B. designed the study, collected data, and wrote the manuscript. All the authors reviewed the manuscript and substantially contributed to the conception of the study and acquisition of the data and approved the final version to be published.
Funding
This work was supported in part by The University of Texas MD Anderson Cancer Center Support Grant (CA016672), the Charif Souki Cancer Research Fund, The University of Texas MD Anderson Cancer Center Leukemia Specialized Program of Research Excellence (SPORE grant P50 CA100632), and generous philanthropic contributions to MD Anderson's MDS/AML Moon Shot Program.
Competing Interest Statement
The authors have declared no competing interest.
Referees
Alla Keyzner
Anonymous
REFERENCES
- Ariffin H, Hainaut P, Puzio-Kuter A, Choong SS, Chan AS, Tolkunov D, Rajagopal G, Kang W, Lim LL, Krishnan S, et al. 2014. Whole-genome sequencing analysis of phenotypic heterogeneity and anticipation in Li–Fraumeni cancer predisposition syndrome. Proc Natl Acad Sci 111: 15497–15501. 10.1073/pnas.1417322111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asdahl PH, Ojha RP, Hasle H. 2017. Cancer screening in Li–Fraumeni syndrome. JAMA Oncol 3: 1645–1646. 10.1001/jamaoncol.2017.2459 [DOI] [PubMed] [Google Scholar]
- Ballinger ML, Best A, Mai PL, Khincha PP, Loud JT, Peters JA, Achatz MI, Chojniak R, Balieiro da Costa A, Santiago KM, et al. 2017. Baseline surveillance in Li–Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol 3: 1634–1639. 10.1001/jamaoncol.2017.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch JM, Alston RD, McNally RJ, Evans DG, Kelsey AM, Harris M, Eden OB, Varley JM. 2001. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 20: 4621–4628. 10.1038/sj.onc.1204621 [DOI] [PubMed] [Google Scholar]
- Bojadzieva J, Amini B, Day SF, Jackson TL, Thomas PS, Willis BJ, Throckmorton WR, Daw NC, Bevers TB, Strong LC. 2017. Whole body magnetic resonance imaging (WB-MRI) and brain MRI baseline surveillance in TP53 germline mutation carriers: experience from the Li–Fraumeni syndrome Education and Early Detection (LEAD) clinic. Fam Cancer 17: 287–294. 10.1007/s10689-017-0034-6 [DOI] [PubMed] [Google Scholar]
- Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M, Stoppa-Lyonnet D, Consolino E, Brugières L, et al. 2015. Revisiting Li–Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 33: 2345–2352. 10.1200/JCO.2014.59.5728. [DOI] [PubMed] [Google Scholar]
- Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, Gale RE, Hills R, Linch DC. 2009. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 23: 203–206. 10.1038/leu.2008.173 [DOI] [PubMed] [Google Scholar]
- Carroll AJ, Heerema NA, Gastier-Foster JM, Astbury C, Pyatt R, Reshmi SC, Borowitz MJ, Devidas M, Linda S, Loh ML, et al. 2009. Masked hypodiploidy: hypodiploid acute lymphoblastic leukemia (ALL) in children mimicking hyperdiploid ALL: a report from the Children's Oncology Group (COG) AALL03B1 study. Blood 114: 1580 10.1182/blood-2009-06-224220 [DOI] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, et al. 2003. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21: 4642–4649. 10.1200/JCO.2003.04.036 [DOI] [PubMed] [Google Scholar]
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, et al. 2006. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108: 419–425. 10.1182/blood-2005-10-4149 [DOI] [PubMed] [Google Scholar]
- Chompret A, Brugières L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, Hua D, Ligot L, Dondon MG, Bressac-de Paillerets B, et al. 2000. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 82: 1932–1937. 10.1054/bjoc.2000.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RJ, Curtis RE, Inskip PD, Fraumeni JF Jr. 2005. The risk of developing second cancers among survivors of childhood soft tissue sarcoma. Cancer 103: 2391–2396. 10.1002/cncr.21040 [DOI] [PubMed] [Google Scholar]
- Comeaux EQ, Mullighan CG. 2017. TP53 mutations in hypodiploid acute lymphoblastic leukemia. Cold Spring Harb Perspect Med 7: a026286 10.1101/cshperspect.a026286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa H. 2016. Li–Fraumeni syndrome. J Pediatr Genet 5: 84–88. 10.1055/s-0036-1579759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, et al. 2017. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, Version 2.2017. J Natl Compr Canc Netw 15: 9–20. 10.6004/jnccn.2017.0003 [DOI] [PubMed] [Google Scholar]
- Etzold A, Schröder JC, Bartsch O, Zechner U, Galetzka D. 2015. Further evidence for pathogenicity of the TP53 tetramerization domain mutation p.Arg342Pro in Li–Fraumeni syndrome. Fam Cancer 14: 161–165. 10.1007/s10689-014-9754-z [DOI] [PubMed] [Google Scholar]
- Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. 2006. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet 43: 289–294. 10.1136/jmg.2005.036319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley LA, Larson RA. 2008. Therapy-related myeloid leukemia. Semin Oncol 35: 418–429. 10.1053/j.seminoncol.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS, et al. 2009. Beyond Li–Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 27: 1250–1256. 10.1200/JCO.2008.16.6959 [DOI] [PubMed] [Google Scholar]
- Hu H, Liu J, Liao X, Zhang S, Li H, Lu R, Li X, Lin W, Liu M, Xia Z, et al. 2016. Genetic and functional analysis of a Li–Fraumeni syndrome family in China. Sci Rep 6: 20221 10.1038/srep20221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadia TM, Jain P, Ravandi F, Garcia-Manero G, Andreef M, Takahashi K, Borthakur G, Jabbour E, Konopleva M, Daver NG, et al. 2016. TP53 mutations in newly diagnosed acute myeloid leukemia: clinicomolecular characteristics, response to therapy, and outcomes. Cancer 122: 3484–3491. 10.1002/cncr.30203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Achatz MI, Brugières L, Frebourg T, Garber JE, Greer MC, Hansford JR, Janeway KA, Kohlmann WK, McGee R, et al. 2017. Cancer screening recommendations for individuals with Li–Fraumeni syndrome. Clin Cancer Res 23: e38–e45. 10.1158/1078-0432.CCR-17-0408 [DOI] [PubMed] [Google Scholar]
- Lammens CR, Bleiker EM, Aaronson NK, Wagner A, Sijmons RH, Ausems MG, Vriends AH, Ruijs MW, van Os TA, Spruijt L, et al. 2010. Regular surveillance for Li–Fraumeni syndrome: advice, adherence and perceived benefits. Fam Cancer 9: 647–654. 10.1007/s10689-010-9368-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JC, Strong LC, Chidambaram A, Ferrell RE. 1991. A germ line mutation in exon 5 of the p53 gene in an extended cancer family. Cancer Res 51: 6385–6387. [PubMed] [Google Scholar]
- Li FP, Fraumeni JF Jr. 1969. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med 71: 747–752. 10.7326/0003-4819-71-4-747 [DOI] [PubMed] [Google Scholar]
- Li FP, Fraumeni JF Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW. 1988. A cancer family syndrome in twenty-four kindreds. Cancer Res 48: 5358–5362. [PubMed] [Google Scholar]
- Limacher JM, Frebourg T, Natarajan-Ame TS, Bergerat JP. 2001. Two metachronous tumors in the radiotherapy fields of a patient with Li-Fraumeni syndrome syndrome. Int J Cancer 96: 238–242. [DOI] [PubMed] [Google Scholar]
- Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, Bremer RC, Rosenberg PS, Savage SA. 2016. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li–Fraumeni syndrome cohort. Cancer 122: 3673–3681. 10.1002/cncr.30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin D. 2011. Li–Fraumeni syndrome. Genes Cancer 2: 475–484. 10.1177/1947601911413466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KA, Ballinger ML, Killick E, Kirk J, Tattersall MH, Eeles RA, Thomas DM, Mitchell G. 2014. Li–Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol 11: 260–271. 10.1038/nrclinonc.2014.41 [DOI] [PubMed] [Google Scholar]
- Middeke JM, Herold S, Rücker-Braun E, Berdel WE, Stelljes M, Kaufmann M, Schäfer-Eckart K, Baldus CD, Stuhlmann R, Ho AD, et al. 2016. TP53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol 172: 914–922. 10.1111/bjh.13912 [DOI] [PubMed] [Google Scholar]
- Nandikolla AG, Venugopal S, Anampa J. 2017. Breast cancer in patients with Li–Fraumeni syndrome—a case-series study and review of literature. Breast Cancer 9: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C, Thomas A, Hoy T, Tighe J, Culligan D, Fegan C, Bentley P. 2003. Leukemic and non-leukemic lymphocytes from patients with Li–Fraumeni syndrome demonstrate loss of p53 function, Bcl-2 family dysregulation and intrinsic resistance to conventional chemotherapeutic drugs but not flavopiridol. Cell Cycle 2: 53–58. 10.4161/cc.2.1.249 [DOI] [PubMed] [Google Scholar]
- Ross J, Bojadzieva J, Peterson S, Noblin SJ, Yzquierdo R, Askins M, Strong L. 2017. The psychosocial effects of the Li–Fraumeni Education and Early Detection (LEAD) program on individuals with Li–Fraumeni syndrome. Genet Med 19: 1064–1070. 10.1038/gim.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, Habdank M, Kugler CM, Holzmann K, Gaidzik VI, et al. 2012. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119: 2114–2121. 10.1182/blood-2011-08-375758 [DOI] [PubMed] [Google Scholar]
- Ruijs MWG, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, Kluijt I, Sijmons RH, Aalfs CM, Wagner A, et al. 2010. TP53 germline mutation testing in 180 families suspected of Li–Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet 47: 421–428. 10.1136/jmg.2009.073429 [DOI] [PubMed] [Google Scholar]
- Sorrell AD, Espenschied CR, Culver JO, Weitzel JN. 2013. TP53 testing and Li–Fraumeni syndrome: current status of clinical applications and future directions. Mol Diagn Ther 17: 31–47. 10.1007/s40291-013-0020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabori U, Nanda S, Druker H, Lees J, Malkin D. 2007. Younger age of cancer initiation is associated with shorter telomere length in Li–Fraumeni syndrome. Cancer Res 67: 1415–1418. 10.1158/0008-5472.CAN-06-3682 [DOI] [PubMed] [Google Scholar]
- Talwalkar SS, Yin CC, Naeem RC, Hicks MJ, Strong LC, Abruzzo LV. 2010. Myelodysplastic syndromes arising in patients with germline TP53 mutation and Li–Fraumeni syndrome. Arch Pathol Lab Med 134: 1010–1015. [DOI] [PubMed] [Google Scholar]
- Tinat J, Bougeard G, Baert-Desurmont S, Vasseur S, Martin C, Bouvignies E, Caron O, Bressac-de Paillerets B, Berthet P, Dugast C, et al. 2009. 2009 version of the Chompret criteria for Li–Fraumeni syndrome. J Clin Oncol 27: e108–e109; author reply e110 10.1200/JCO.2009.22.7967 [DOI] [PubMed] [Google Scholar]
- Trkova M, Hladikova M, Kasal P, Goetz P, Sedlacek Z. 2002. Is there anticipation in the age at onset of cancer in families with Li–Fraumeni syndrome? J Hum Genet 47: 381–386. 10.1007/s100380200055 [DOI] [PubMed] [Google Scholar]
- Varley JM, Evans DG, Birch JM. 1997. Li–Fraumeni syndrome—a molecular and clinical review. Br J Cancer 76: 1–14. 10.1038/bjc.1997.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A, Tabori U, Schiffman J, Shlien A, Beyene J, Druker H, Novokmet A, Finlay J, Malkin D. 2011. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: a prospective observational study. Lancet Oncol 12: 559–567. 10.1016/S1470-2045(11)70119-X [DOI] [PubMed] [Google Scholar]
- Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, Gallinger B, Naumer A, Kohlmann W, Novokmet A, et al. 2016. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 17: 1295–1305. 10.1016/S1470-2045(16)30249-2 [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2018. Tumors associated with Tp53 germline mutations. IARC; http://p53.iarc.fr/TP53GermlineMutations.aspx [Google Scholar]
- Zerdoumi Y, Aury-Landas J, Bonaïti-Pellié C, Derambure C, Sesboüé R, Renaux-Petel M, Frebourg T, Bougeard G, Flaman JM. 2013. Drastic effect of germline TP53 missense mutations in Li–Fraumeni patients. Hum Mutat 34: 453–461. 10.1002/humu.22254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The variants have been deposited by external sources in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers: SCV000260755.5, SCV000218877.8, SCV000260299.5, SCV000253851.6, SCV000532251.3, SCV000261917.6, and SCV000882434.