Abstract
Background:
Vaccination of children with 13-valent pneumococcal conjugate vaccine (PCV13) led to declines in vaccine-type pneumococcal nasopharyngeal carriage among adults through indirect effects. In August 2014, PCV13 immunization of all U.S. adults ≥65 years of age was recommended. This study sought to define prevalence and serotype distribution of pneumococcal carriage among adults ≥65 years of age and to describe risk factors for colonization soon after introduction of PCV13 in adults.
Methods:
A cross-sectional survey of non-institutionalized U.S. adults ≥65 years of age was conducted in four states in 2015–2016. Demographic information, risk factors for disease, PCV13 vaccination history, and nasopharyngeal (NP) and oropharyngeal (OP) swabs were collected. NP and OP swabs were processed separately and pneumococcal isolates were serotyped by Quellung reaction. Antimicrobial susceptibility of pneumococcal isolates was performed. NP swabs also underwent real-time PCR for pneumococcal detection and serotyping.
Results:
Of 2989 participants, 45.3% (1354/2989) had been vaccinated with PCV13. Fifty-five (1.8%) carried pneumococcus (45 identified by culture and 10 by real-time PCR only) and PCV13 serotypes were found in eight (0.3%) participants. Almost half (22/45) of pneumococcal isolates were not susceptible to at least one of the antibiotics tested. Vaccine-type carriage among vaccinated and unvaccinated individuals was similar (0.2% vs. 0.1%, respectively). Respiratory symptoms were associated with higher odds of pneumococcal colonization (adjusted OR: 2.1; 95% CI = 1.1–3.8).
Conclusions:
Pneumococcal carriage among non-institutionalized adults ≥65 years of age was very low. Less than 0.5% of both vaccinated and unvaccinated individuals in our study carried vaccine-type serotypes. Over a decade of PCV vaccination of children likely led to indirect effects in adults. However, given the low vaccine-type carriage rates we observed in an already high PCV13 adult coverage setting, it is difficult to attribute our findings to the direct versus indirect effects of PCV13 on adult carriage.
Keywords: Streptococcus pneumonia, Pneumococcal conjugate vaccine, PCV13, Older adults, Pneumococcal carriage
1. Introduction
Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial pneumonia, sepsis, and meningitis, and an important cause of morbidity and mortality globally. Use of pneumococcal conjugate vaccines in the United States childhood immunization program since the year 2000 led to dramatic declines in invasive pneumococcal disease (IPD) in both children (direct effect and indirect effect from herd immunity) and adults (indirect effect) [1,2]. Despite these declines, burden of pneumococcal disease in adults ≥65 years of age continued to be high [3]. In 2010, the Advisory Committee on Immunization Practices (ACIP) recommended that 7-valent pneumococcal conjugate vaccine (PCV7) be replaced by 13-valent pneumococcal conjugate vaccine (PCV13) for children in the United States [4]. In 2013, three years after PCV13 replaced PCV7, incidence of IPD was almost three times higher in individuals ≥65 years of age (24.8 cases per 100,000) compared to children [5]. Similar to IPD, the incidence of pneumococcal community acquired pneumonia (CAP) was five times higher among adults ≥65 years of age than in younger adults in 2012 [3]. Approximately a quarter of IPD cases in persons ≥65 years of age in 2016 and 6% of CAP cases in adults ≥18 years in 2012 are caused by the serotypes included in PCV13 and are potentially vaccine preventable [6,7].
In August 2014, ACIP recommended routine use of PCV13 in adults ≥65 years of age [8]. This decision was based in part on the results of a large randomized placebo-controlled trial (CAPiTA trial) in the Netherlands that showed a PCV13 efficacy of 45% (95% CI: 14.2–65.3%) against vaccine-type (VT) non-bacteremic pneumococcal pneumonia, and an efficacy of 75% (95% CI: 41.4–90.8%) against VT IPD [9].
Pneumococcus is often carried in the upper respiratory tract. Although pneumococcal colonization is generally asymptomatic, carriage is considered to be a precursor of IPD [10]. Host factors, such as immune response, age, and underlying medical conditions, play a role in whether an individual develops disease following colonization [11]. Use of PCV13 in adults ≥65 years of age has the potential to impact pneumococcal colonization rates in this age group and therefore disease. However, colonization decreases with age [12,13] and the prevalence of pneumococcal colonization in older Americans after introduction of PCV13 in children in 2010 has not been well evaluated. In the United Kingdom, after PCV7 and prior to PCV13 introduction into the national infant immunization program, a pneumococcal carriage survey conducted in adults ≥ 65 years of age found a 2.2% pneumococcal carriage rate [14]. A study done in adults with HIV infection (19–66 years of age) in the United States from 2005 to 2007 (post PCV7 introduction) found 3.4% pneumococcal carriage [15,16]. In Alaska, immunization of children with PCV13 led to substantial declines in PCV13-type pneumococcal colonization among adults ≥45 years of age through indirect effects [16].
Given the high IPD and CAP incidence in the U.S. elderly population, we sought to define pneumococcal carriage prevalence and serotype distribution and to describe risk factors for pneumococcal colonization among adults ≥65 years of age in the United States before widespread use of PCV13 in this age group. These results can help to understand the potential impact of the ACIP recommendation for routine PCV13 use in U.S. adults ≥65 years of age on pneumococcal colonization rates.
2. Materials and methods
2.1. Study population and data collection
We conducted a cross-sectional survey of nasopharyngeal (NP) and oropharyngeal (OP) pneumococcal carriage among nonimmunosuppressed adults ≥65 years of age from July 13, 2015 through December 31, 2016 in four states participating in CDC’s Emerging Infections Program/Active Bacterial Core surveillance: Georgia, Maryland, New York and Tennessee. CDC and local institutional review boards approved the study. Written informed consent was obtained from study participants. We recruited adults from outpatient centers including clinical research units, geriatric or internal medicine clinics, community centers, assisted living facilities, low-income housing, retirement homes, and emergency departments. Participants were not eligible for enrollment if they had an underlying medical condition that met prior ACIP recommendations for PCV13 [17] or were residents of nursing homes or prisons. Participants without paired NP and OP specimens were excluded from analysis.
Study staff used standardized questionnaires to collect information on history of recent illness, chronic medical conditions, recent exposure to antimicrobials or healthcare settings, and pneumococcal vaccination history. After enrollment, study teams followed up with participants and their providers to obtain vaccination records. If the vaccination record could not be obtained from the participant, the study team contacted all healthcare providers from which the participant reported receiving care since 2014. Study teams made at least three attempts to contact providers to obtain vaccination histories for any pneumococcal vaccine (pneumococcal polysaccharide vaccine [PPSV23] or PCV13) received since August 2014. If receipt of either vaccine was incidentally found prior to August 2014, it was recorded. Verbal report of pneumococcal vaccination by the participant was not accepted as proof of vaccination. Participants were considered vaccinated with PCV13 if they had documentation of PCV13 receipt at least two weeks prior to study enrollment and unvaccinated if no documentation of PCV13 was found. Participants were considered to have missing vaccination status if providers could not be contacted after three attempts.
2.2. Specimen collection and processing
NP and OP specimens were collected and immediately placed in two separate vials with transport media containing 1.0 ml skim milk, tryptone, glucose, glycerol (STGG) transport medium. Inoculated STGG vials were kept at 4 °C within 4–5 h after collection and frozen at −70 °C until culture. Quality control of specimen collection was done using PCR for RNAseP on NP specimens to ensure specimens were appropriate for detection of pneumococci. Any participant with a NP specimen with RNAseP cycle threshold value greater than 35 or negative was excluded from analyses.
For pneumococcal isolation and identification, 200 μl of the STGG inoculated medium was transferred into 5.0 ml Todd Hewitt broth containing 0.5% yeast extract (THY) and 1 ml of rabbit serum which was then incubated at 37 °C in a CO2 incubator for 5–6 h. After incubation, 10 μl of cultured broth was streaked on tryptone soy II agar plates with 5% sheep blood (BAP) and incubated at 37 °C in CO2 for 18–24 h [12]. Alpha-hemolytic colonies resembling pneumococci were tested for susceptibility to optochin and bile solubility, and serotyped by Quellung reaction using CDC pneumococcal typing antisera [13].
Pneumococcal isolates were tested for susceptibility to commonly used antibiotics (Table 4) using the broth microdilution method. Isolates were classified as susceptible, intermediate, or resistant based on the non-parenteral breakpoints of the 2017 Clinical and Laboratory Standards Institute (CLSI) guidelines [18].
Table 4.
Antimicrobial resistance profiles for S. pneumoniae isolates (N = 45) detected by culture method.
| Susceptible | Intermediate | Resistant | |
|---|---|---|---|
| Amoxicillin | 42 (93.3%) | 2 (4.4%) | 1 (2.2%) |
| Cefotaxime | 44 (97.8%) | 1 (2.2%) | 0 |
| Ceftriaxone | 44 (97.8%) | 1 (2.2%) | 0 |
| Cefuroxime | 38 (84.4%) | 4 (8.9%) | 3 (6.7%) |
| Chloramphenicol | 45 (100%) | 0 | 0 |
| Ciprofloxacin | 45 (100%) | 0 | 0 |
| Clindamycin | 42 (93.3%) | 0 | 3 (6.7%) |
| Erythromycin | 30 (66.7%) | 0 | 15 (33.3%) |
| Levofloxacin | 45 (100%) | 0 | 0 |
| Linozelid | 45 (100%) | 0 | 0 |
| Meropenem | 42 (93.3%) | 1 (2.2%) | 2 (4.4%) |
| Penicillin | 32 (71.1%) | 10 (22.2%) | 3 (6.7%) |
| Quinupristin/Dalfopristin | 45 (100%) | 0 | 0 |
| Rifampin | 45 (100%) | 0 | 0 |
| Tetracycline | 40 (89.9%) | 0 | 5 (11.1%) |
| Trimethoprim-sulfaethoxazole | 39 (86.7%) | 4 (8.9%) | 2 (4.4%) |
| Vancomycin | 45 (100%) | 0 | 0 |
NP swabs also underwent real-time PCR targeting lytA for detection of pneumococcal DNA [19]. DNA extraction was performed by manually transferring 200 μl of the STGG inoculated NP samples into 1.5 ml cryotubes containing 100 μl of Tris-EDTA buffer with 0.04 g/ml lysozyme and 75 U/ml mutanolysin (Sigma Chemical Co., USA). The mixture was incubated for 1 h at 37 °C followed by addition of 20 ml of proteinase K. After mixing briefly with a vortex, 400 μl of lysis buffer (Qiagen, USA) was added. After the lysis step, the extraction process followed the manufacturer’s procedures using the NucliSENS®EasyMAG®automated nucleic acid extraction system (Biomerieux, USA). Bacterial DNA extracts were eluted in 100 μl of elution buffer and stored at −20 °C until PCR testing. PCR for OP specimens was not performed due[C0] to potential for false-positive results (detection of serotype-specific pneumococcal homolog genes of non-pneumococcal streptococci) [19,20]. Multiplex PCR serotype assay for 37 serotypes (including VT serotypes) was performed on all lytA positive NP specimens [21].
2.3. Sample size and analysis
The initial sample size calculation was performed assuming this survey would be used as a baseline to measure the impact of the PCV13 recommendation on the pneumococcal carriage rate in adults. However, after preliminary data analysis found PCV13 coverage to be very high, the sample size calculation was modified, using a point prevalence estimate of 0.5% VT pneumococcal carriage with an upper/lower bound of ±0.25%. Based on this modified calculation, the desired sample size for our study was 3049 participants across all sites.
Statistical analysis was performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC). The primary outcome was the proportion of adults ≥65 years of age colonized with pneumococcus and VT serotypes. Pneumococcal and VT carriage prevalence was stratified by PCV13 vaccination status. Additional secondary outcomes included the proportion of pneumococci carried by adults that are resistant to commonly used antibiotics.
To identify factors associated with pneumococcal colonization and receipt of PCV13, we performed univariable and multivariable logistic regression. The multivariable logistic regression model was constructed using backward selection. Variables with a P value <0.20 in the univariable analyses were included as candidates in the multivariable logistic regression model. A two-sided P value of < 0.05 was considered statistically significant.
3. Results
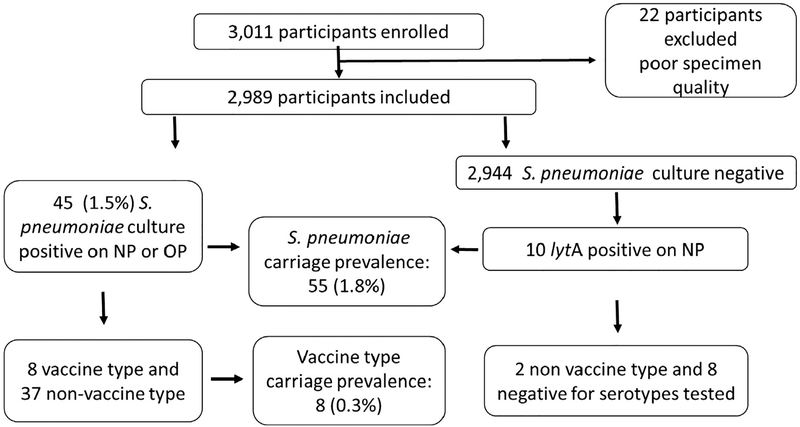
We enrolled 3011 adults; 2989 (99.3%) had good specimen quality and were included in the analysis (Fig. 1). Median age was 74 years (range 65–102 years), 1927 (64.5%) were female, and 2179 (72.9%) were white (Table 1). Most participants reported living alone or with one other person. Few participants (<1%) reported any children under five living in the same household. Approximately 7% of participants reported smoking, and 48% reported having at least one chronic medical condition. Less than 1% of enrolled participants reported having pneumonia or influenza in the month prior to enrollment, and 6% of participants reported taking any antibiotic in the prior 2 weeks. Most participants reported having both Medicare and private supplemental health insurance.
Fig. 1.
Participant flow diagram.
Table 1.
Characteristics of study participants enrolled across four US states sites, 2015–2016.
| Study Site1 | GA (n = 1106) | MD (n = 543) | NY (n = 769) | TN (n = 571) | All sites (n = 2989) |
|---|---|---|---|---|---|
| Age in years (range) | 74.2 (65–98) median: 73 | 75.1 (65–100) median: 74 | 78.6 (65–102) median: 78 | 74.1 (65–97) median: 73 | 75.5 (65–102) median: 74 |
| Race | |||||
| White | 685 (61.9%) | 429 (79.0%) | 664 (85.4%) | 401 (70.2%) | 2179 (72.9%) |
| Black | 363 (32.8%) | 98 (18.1%) | 95 (12.4%) | 157 (27.5%) | 713 (23.9%) |
| Multi-racial or other | 46 (4.2%) | 13 (2.4%) | 9 (1.2%) | 7 (1.2%) | 75 (2.5%) |
| Hispanic | 31 (2.8%) | 6 (1.1%) | 6 (0.8%) | 6 (1.1%) | 49 (1.6%) |
| Female | 635 (57.4%) | 350 (64.5%) | 567 (73.7%) | 375 (65.7%) | 1927 (64.5%) |
| Self-reported residence type | |||||
| Private residence | 676 (61.1%) | 513 (94.5%) | 588 (76.5%) | 348 (61.0%) | 2125 (71.1%) |
| Retirement home | 421 (38.1%) | 10 (1.8%) | 174 (22.6%) | 222 (38.9%) | 827 (27.7%) |
| Other | 9 (0.8%) | 20 (3.7%) | 7 (0.9%) | 1 (0.03%) | 37 (1.2%) |
| Insurance type | |||||
| Private only | 32 (2.9%) | 24 (4.4%) | 7 (0.9%) | 42 (7.4%) | 105 (3.5%) |
| Medicare/Medicaid only | 150 (13.6%) | 55 (10.1%) | 84 (10.9%) | 141 (24.7%) | 430 (14.4%) |
| Medicare and Private | 757 (68.4%) | 434 (79.9%) | 661 (86.0%) | 348 (61.0%) | 2200 (73.6%) |
| Military | 159 (14.4%) | 22 (4.1%) | 14 (1.8%) | 39 (6.8%) | 234 (7.8%) |
| Other/Uninsured/Unknown | 8 (0.7%) | 8 (1.5%) | 3 (0.4%) | 1 (0.2%) | 21 (0.7%) |
| No. of household members | 1.6 (1–9) median: 1 | 1.8 (1–10) median: 2 | 1.5 (1–6) median: 1 | 1.6 (1–7) median: 1 | 1.6 (1–10) median: 1 |
| Children aged < 5 years living in house | 9 (0.8%) | 8 (1.5%) | 1 (0.1%) | 4 (0.7%) | 22 (0.7%) |
| Smoker | 86 (7.8%) | 27 (5.0%) | 40 (5.2%) | 48 (8.4%) | 201 (6.7%) |
| Live with smoker | 51 (4.6%) | 22 (4.1%) | 24 (3.1%) | 18 (3.2%) | 115 (3.9%) |
| Adult day care | 9 (0.8%) | 39 (7.2%) | 1 (0.1%) | 0 | 49 (1.6%) |
| Any chronic conditions | 503 (45.5%) | 204 (37.6%) | 395 (51.4%) | 340 (59.5%) | 1442 (48.2%) |
| Asthma or COPD2 | 156 (14.1%) | 84 (15.5%) | 112 (14.6%) | 83 (14.5%) | 435 (14.5%) |
| Stroke | 76 (6.9%) | 45 (8.3%) | 63 (8.2%) | 80 (14.0%) | 264 (8.8%) |
| Diabetes | 285 (25.8%) | 90 (16.6%) | 151 (19.6%) | 101 (17.7%) | 627 (21.0%) |
| Cirrhosis/Liver failure | 9 (0.8%) | 0 | 0 | 2 (0.4%) | 11 (0.4%) |
| Heart failure or CVD3 | 154 (14.1%) | 52 (9.6%) | 197 (25.6%) | 259 (45.4%) | 662 (22.1%) |
| Chronic kidney disease (not on dialysis) | 36 (3.3%) | 16 (2.9%) | 24 (3.1%) | 13 (2.3%) | 89 (3.0%) |
| Any Illness symptom in prior 2 weeks4 | 515 (46.6%) | 252 (46.4%) | 426 (55.4%) | 70 (12.3%) | 1263 (42.3%) |
| Cough prior 2 weeks | 294 (26.6%) | 114 (21.0%) | 196 (25.5%) | 33 (5.8%) | 637 (21.3%) |
| Runny nose prior 2 weeks | 402 (36.4%) | 198 (36.5%) | 334 (43.4%) | 53 (9.3%) | 987 (33.0%) |
| Any antibiotics prior 2 weeks | 67 (6.1%) | 29 (5.3%) | 55 (7.2%) | 33 (5.8%) | 184 (6.2%) |
| Received PCV13 vaccine | 430 (38.9%) | 284 (52.3%) | 410 (53.3%) | 230 (40.3%) | 1354 (45.3%) |
| Unknown PCV13 vaccine status | 78 (7.0%) | 28 (5.1%) | 9 (1.2%) | 54 (9.5%) | 169 (5.7%) |
| Received PPSV23 vaccine | 608 (58.4%) | 239 (45.9%) | 552 (72.6%) | 252 (48.7%) | 1651 (58.2%) |
GA: Georgia; MD: Maryland; NY: New York; TN: Tennessee.
COPD: chronic obstructive pulmonary disease.
CVD: cardiovascular disease.
symptoms include cough, fever, runny nose, or sore throat.
3.1. Vaccination history
Vaccination history was obtained for 94% of participants. Across all sites, 45% of study participants had documented receipt of PCV13 (range: 39% in Georgia to 53% in New York) (Table 1). However, if we restrict our data to participants with Medicare/Medicaid but no additional supplemental insurance, the PCV13 vaccination rate was 34.7%. Non-white participants and those living in southern states (Georgia and Tennessee) were less likely to be vaccinated with PCV13 (Table 5). PPSV23 vaccination was documented for 52% of participants in the medical records for the period reviewed by study teams.
Table 5.
Factors associated with vaccination with 13-valent pneumococcal conjugate vaccine.
| Unadjusted1 | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower CI | Upper CI | P value | aOR | Lower CI | Upper CI | P value | |
| Black or other race | 0.64 | 0.54 | 0.76 | <0.0001 | 0.70 | 0.58 | 0.84 | 0.0001 |
| Female | 1.07 | 0.92 | 1.25 | 0.39 | ||||
| Age | 1.02 | 1.01 | 1.03 | 0.002 | ||||
| Private or military insurance | 1.54 | 1.24 | 1.91 | 0.0001 | 1.41 | 1.13 | 1.77 | 0.003 |
| Any healthcare exposure since August 20142 | 2.68 | 1.49 | 5.12 | 0.002 | 2.23 | 1.20 | 4.17 | 0.01 |
| Any respiratory symptom in prior 2 weeks | 1.24 | 1.07 | 1.44 | 0.01 | ||||
| Any chronic condition | 1.39 | 1.20 | 1.62 | <0.0001 | 1.49 | 1.27 | 1.74 | <0.0001 |
| Smoker | 0.77 | 0.57 | 1.04 | 0.09 | ||||
| Study site (referent group: New York) | ||||||||
| Georgia | 0.61 | 0.51 | 0.74 | <0.0001 | 0.69 | 0.56 | 0.83 | 0.0002 |
| Maryland | 1.05 | 0.84 | 1.31 | 0.67 | ||||
| Tennessee | 0.68 | 0.55 | 0.86 | 0.0009 | 0.73 | 0.58 | 0.92 | 0.008 |
variables with P < 0.20 were included in the logistic regression model.
PCV13 recommendation for adults 65 years of age and older was made in August of 2014; thus, healthcare exposures from that time forward provided an opportunity for individuals to be vaccinated.
3.2. Laboratory results
Of the 2989 adults included in the analysis, 45 (1.5%) had pneumococcus isolated from either an OP or NP specimen (35 NP only, 4 OP only, and 6 from both). Ten were culture negative but had pneumococcus detected by PCR, resulting in an overall pneumococcal carriage rate of 1.8% (Fig. 1). Among the 55 participants with pneumococcus detected, 56 serotypes were identified (Table 2). Two serotypes were detected in one participant (one by both Quel-lung reaction and PCR [serotype 35F] and one by PCR only [serotype 14]). Eight of the 55 participants were colonized with a pneumococcal serotype found in PCV13, indicating an overall VT carriage rate of 0.3%. The VT pneumococcal carriage rate was similar in vaccinated and unvaccinated participants (Table 3).
Table 2.
Pneumococcal serotypes detected in 55 participants.
| Serotype | N = 56* | |
|---|---|---|
| PCV13 vaccine type | 19F | 4 |
| 19A | 2 | |
| 3 | 1 | |
| 14* | 1 | |
| Non-vaccine type | 11A | 6 |
| 23A | 4 | |
| 23 B | 4 | |
| 9N | 3 | |
| 15A/15F | 3 | |
| 15C | 3 | |
| 35F* | 3 | |
| 7C | 2 | |
| 16F | 2 | |
| 33F/33A/37 | 2 | |
| 6C | 1 | |
| 10A | 1 | |
| 21 | 1 | |
| 28A | 1 | |
| 29 | 1 | |
| 31 | 1 | |
| 34 | 1 | |
| 35B | 1 | |
| Negative** | 8 |
One specimen tested positive by culture isolation and PCR for both for serotype 35F and by PCR only for serotype 14.
Negative for all 37 serogroup/serotypes encompassed in the detection by real time PCR: 1, 2, 3, 4, 5, 6A/6B, 6C/6D, 7F/7A, 9V/9A, 11A/11D, 12F/12A/12B/44/46, 14, 15F/15A, 16F, 18A/18B/18C/18D, 19A, 19F, 22F/22A, 23A, 23F, 33F/33C/37.
Table 3.
Pneumococcal carriage among adults ≥65 years of age who were vaccinated and not vaccinated with PCV13.
| Vaccinated (N = 1354) | Non-Vaccinated (N = 1466) | |
|---|---|---|
| Overall pneumococcal carriage, n (%)* | 24 (1.8%) | 23 (1.6%) |
| Vaccine type pneumococcal carriage, n (%)** | 3 (0.2%) | 2 (0.1%) |
6 participants colonized with pneumococcus excluded due to unknown vaccination status.
2 participants colonized with pneumococcus excluded due to unknown vaccination status.
Of the 45 pneumococcal isolates, 22 (48.9%) were either intermediate or resistant to one or more antibiotic. Non-susceptibility was most common to erythromycin and penicillin (Table 4). Carriage of non-susceptible strains was similar in those who were vaccinated with PCV13 and those who were unvaccinated (0.4% [6/1354] vs. 0.8% [12/1478]; p = 0.22).
3.3. Factors associated with pneumococcal colonization and with PCV13 vaccination
In univariate analysis, factors associated with pneumococcal colonization were younger age, study site, and respiratory symptoms in the prior two weeks. However, in the multivariable model, only respiratory symptoms remained associated with pneumococcal colonization (adjusted OR: 2.1; 95% CI = 1.1–3.8).
In multivariable analysis, individuals with any exposure to healthcare settings since August 2014 (aOR: 2.23; CI 1.20–4.17), having supplemental health insurance (aOR: 1.41; CI (1.13–1.77), and any chronic medical conditions (aOR: 1.49; CI 1.27–1.74) were more likely to be vaccinated with PCV13 (Table 5). Participants who were black or other race (aOR: 0.70; CI 0.58–0.84), from Georgia (aOR: 0.69; CI 0.56–0.83), or from Tennessee (aOR: 0.73; CI 0.58–0.92) were less likely to be vaccinated with PCV13.
4. Discussion
Following PCV13 introduction in adults ≥65 years of age, carriage of pneumococcus was rare among those who were not nursing homes residents or did not have severe immunosuppressing conditions. Only presence of respiratory symptoms was associated with carriage, which is consistent with other studies [12,22,23]. Our study enrolled a large sample size across four U.S. states, which allowed for a greater opportunity to detect VT serotypes. PCV13 serotypes accounted for 14% of those with pneumococcus detected and an overall 0.3% carriage rate.
Indirect effects from years of pediatric vaccination likely contributed to the very low carriage rate. Consecutive carriage surveys among children in Georgia pre- and post- PCV13 introduction into the national childhood immunization schedule showed a dramatic decline in VT pneumococcal colonization from 29% in 2010 to 3% in 2013, following PCV13 introduction [24]. Introduction of pneumococcal conjugate vaccines into the routine infant immunization schedule has been shown to decrease VT carriage in adults in the United States and in Kenya [16,25]. It is also possible that direct effects of PCV13 vaccination contributed to the observed low VT carriage rates, given the PCV13 coverage of 45.3% among study participants. Therefore, it is not possible to determine the individual contributions of direct effect versus indirect effects of PCV13 vaccination in this study.
The prevalence of overall and VT pneumococcal carriage in our study was similar to what the United Kingdom reported in the same age group (2.2% and 0.5%, respectively) after PCV7 introduction and before PCV13 introduction into their national infant immunization program [14]. However, more recent studies show higher carriage prevalence. A study among older adults with influenza-like illness found carriage rates as high as 36% [26]. A recent longitudinal study among U.S. adults ≥65 years of age found a monthly pneumococcal carriage prevalence ranging from 0 to 17% [27]. Different laboratory methods have been utilized for detection of pneumococci in adults making comparison between studies difficult. Most studies showing high colonization rates in adults include real-time PCR targeting lytA on either OP swabs or saliva, increasing detection rate by at least five fold. It has been demonstrated that non-pneumococcal streptococci (e.g., S. mitis, S. oralis, S. infantis) carry homolog genes of pneumococci, which could lead to false-positive detection and PCR serotyping results [20,28]. This is more problematic with OP or saliva specimens because of the diversity of streptococci that can be found in the oropharynx [29]. Due to this diversity, a lytA detection from an OP or saliva specimen may not be from the same organisms DNA as the DNA from which the serotype was detected, and most published studies do not report Ct values to allow for comparison. In general, a reported Ct value from the serotyping should not be lower than that of the lytA due to the fact that the methodology for serotyping uses multiplex assays which require more DNA copies in order to detect while the lytaA assay is a singleplex requiring less copies for detection. Therefore, it is possible that pneumococcal carriage surveys relying on PCR of oral specimens may have overestimated pneumococcal carriage rates [14], even though molecular methods may be better suited to detecting lower density colonization which is commonly found in the adult population when compared to culture. Also, less than 1% of our adult participants had children living in the same household, and the median number of household members was one. It is well known that children are the main reservoir of S. pneumoniae, and that residing in a crowded household is a risk factor for pneumococcal colonization [30]; none of those were common among our study population.
Among the eight participants with VT pneumococcal carriage, the serotypes isolated (19F, 19A and 3) are also the most common vaccine serotypes causing IPD among adults ≥65 years of age in the United States [31]. Antimicrobial susceptibility profiles of the 45 pneumococcal isolates were similar to what is reported for IPD cases in the United States, with high prevalence of erythromycin and tetracycline resistance. We observed a high prevalence of penicillin non-susceptible isolates (28.9%) using the non-parenteral CLSI breakpoint for resistant isolates. However, if we had used the non-meningitis parenteral breakpoint, the prevalence of non-susceptible isolates would decrease to 2.2%, which is similar to that of isolates from IPD cases in the United States (4.3% of 2597 invasive pneumococcal isolates) [32] and from healthy children 6–59 months of age in Georgia (3.0% of 99 NP carriage isolates) [24].
Our study provides insights on factors associated with pneumococcal carriage and with PCV13 receipt among study participants. For pneumococcal carriage, we found an association of respiratory symptoms in the past 2 weeks, which is not surprising given that the risk of pneumococcal acquisition increases following acute respiratory illness with influenza or parainfluenzae [33]. PCV13 receipt was associated with supplementary health insurance, health care exposures since the ACIP PCV13 recommendation in August 2014, and chronic medical conditions. Participants with chronic medical conditions and supplemental insurance likely had higher numbers of healthcare encounters, providing more opportunities to receive PCV13. When compared to vaccination coverage estimates using Centers for Medicare & Medicaid Services data, our study showed a higher rate of PCV13 vaccination among older Americans (45.3% vs 31.5%) [34]. However, we also found lower rates of vaccine coverage among non-white individuals when compared to white individuals, which is consistent with other reports [35].
There are some limitations to our study. First, vaccination with PCV13 was quite high and colonization was very low at the time of enrollment, precluding us from obtaining a true baseline carriage estimate prior to PCV13 introduction in adults. High vaccine coverage could have contributed to the low observed colonization rates; however, when we stratified overall and VT pneumococcal carriage by PCV13 vaccination status, the observed rates were similar. Second, we did not enroll participants with severe immunosuppressing conditions or residents of nursing homes, which does not allow us to make inferences about pneumococcal colonization rates in these populations. Based on a number of factors, it is possible that pneumococcal carriage rates are higher in nursing home residents than what we observed in adults in the community [36,37]. Third, even though we included both NP and OP swabs to measure the pneumococcal colonization rate in adults, we relied solely on a culture-based method for OP specimens, given that current molecular methods for pneumococcal detection may lead to false-positive results [20,28]. Therefore, it is possible that we underestimated pneumococcal colonization. Finally, our findings might not be generalizable to the entire U.S. population ≥65 years of age. Compared to the 2015 American Community Survey from the U.S. Census Bureau [38], the median age of our study participants and the percentage with health insurance were similar to those of the U.S. population. However, we enrolled a higher percentage of African Americans and females and a far smaller percentage of Hispanics compared to the U.S. population.
5. Conclusion
This is the largest pneumococcal carriage survey done among adults ≥65 years of age in the United States. Pneumococcal colonization of the upper respiratory tract among older Americans was rare after over a decade of PCV use in children and after PCV13 introduction in older adults. Both vaccinated and unvaccinated participants in our study had a carriage rate of less than 0.5% for VT pneumococcal serotypes. Many years of PCV vaccination in pediatric populations likely led to significant indirect effects in adults. However, recent PCV13 recommendation for all U.S. adults ≥65 years of age may have also contributed to the low observed rates.
Acknowledgements
We thank the individuals who participated in this study and study team members including: Diane Kober, Sarah Witter, Cynthia Whitney, Bernard Beall, Laura McKnight, Gail Hughett, Kathleen Shutt, Nicole Bond.
Funding source
This work was funded by the Emerging Infections Program (EIP) Cooperative Agreement between the four EIP sites and the Centers for Disease Control and Prevention.
Financial disclosure
Dr. Harrison has served as a consultant to Merck, GSK, and Sanofi Pasteur. Dr Rouphael has received funds from Merck, Pfizer and Sanofi Pasteur to conduct clinical studies at Emory. Dr. Talbot has received research funding from Sanofi Pasteur and serves as an advisor for Seqirus. All other authors have no financial disclosures relevant to this article.
Footnotes
Potential conflict of interest
The authors have no potential conflicts to disclose.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201(1):32–41. [DOI] [PubMed] [Google Scholar]
- [2].Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015;15(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med 2015;373 (5):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children – Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep 2010;59(9):258–61. [PubMed] [Google Scholar]
- [5].CDC. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae; 2014. [Google Scholar]
- [6].CDC. Pneumococcal Disease Surveillance and Reporting. Figure 3 2013; www.cdc.gov/pneumococcal/surveillance.html [accessed 4/1/2018,2018].
- [7].Wunderink RG, Self WH, Anderson EJ, et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis : Off Publ Infect Dis Soc Am 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63 (37):822–5. [PMC free article] [PubMed] [Google Scholar]
- [9].Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372 (12):1114–25. [DOI] [PubMed] [Google Scholar]
- [10].Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vacc 2012;11(7):841–55. [DOI] [PubMed] [Google Scholar]
- [11].Brooks LRK, Mias GI. Streptococcus pneumoniae’s virulence and host immunity: aging, diagnostics, and prevention. Front Immunol 2018;9:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J 2008;27(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Regev-Yochay G, Raz M, Dagan R, et al. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis : Off Publ Infect Dis Soc Am 2004;38(5):632–9. [DOI] [PubMed] [Google Scholar]
- [14].Hamaluba M, Kandasamy R, Ndimah S, et al. A cross-sectional observational study of pneumococcal carriage in children, their parents, and older adults following the introduction of the 7-valent pneumococcal conjugate vaccine. Medicine (Baltimore) 2015;94(1):e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Onwubiko C, Swiatlo E, McDaniel LS. Cross-sectional study of nasopharyngeal carriage of Streptococcus pneumoniae in human immunodeficiency virus-infected adults in the conjugate vaccine era. J Clin Microbiol 2008;46 (11):3621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bruce MG, Singleton R, Bulkow L, et al. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine 2015;33(38):4813–9. [DOI] [PubMed] [Google Scholar]
- [17].CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61(40):816–9. [PubMed] [Google Scholar]
- [18].Institute CaLS. Performance standards for antimicrobial susceptibility testing 28th ed. In. Zone diameter and MIC breakpoints for Streptococcus pneumoniae. Wayne, Pennsylvania; 2018. [Google Scholar]
- [19].Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007;45(8):2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carvalho Mda G, Pimenta FC, Moura I, et al. Non-pneumococcal mitis-group streptococci confound detection of pneumococcal capsular serotype-specific loci in upper respiratory tract. PeerJ 2013;1:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pimenta FC, Roundtree A, Soysal A, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 2013;51(2):647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Donkor ES, Annan JA, Badoe EV, Dayie NT, Labi AK, Slotved HC. Pneumococcal carriage among HIV infected children in Accra, Ghana. BMC Infect Dis 2017;17 (1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Feola TD, Bonville CA, Cibula DA, et al. Nasopharyngeal pneumococcal carriage rates among HIV-infected adults following widespread pediatric use of conjugate pneumococcal vaccine-13. Hum Vacc Immunother 2016;12 (9):2441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Desai AP, Sharma D, Crispell EK, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J 2015;34(11):1168–74. [DOI] [PubMed] [Google Scholar]
- [25].Kim GB L, Conklin L, Odoyo A, Carvalho M, Odiembo H, Pimenta F. et al. Impact of 10-valent pneumococcal conjugate vaccine (PCV10) on pneumococcal colonization in children <5 years and HIV-infected Parents–Kenya, 2009–2013 In: International Symposium on Pneumococci and Pneumococcal Diseases; 2016; Glasgow, Scotland. [Google Scholar]
- [26].Krone CL, Wyllie AL, van Beek J, et al. Carriage of Streptococcus pneumoniae in aged adults with influenza-like-illness. PLoS One 2015;10(3):e0119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Branche AR, Yang H, Java J, et al. Effect of prior vaccination on carriage rates of Streptococcus pneumoniae in older adults: a longitudinal surveillance study. Vaccine 2018;36(29):4304–10. [DOI] [PubMed] [Google Scholar]
- [28].Simoes AS, Tavares DA, Rolo D, et al. lytA-based identification methods can misidentify Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2016;85 (2):141–8. [DOI] [PubMed] [Google Scholar]
- [29].Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol 2010;192(19):5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reisman J, Rudolph K, Bruden D, Hurlburt D, Bruce MG, Hennessy T. Risk factors for pneumococcal colonization of the nasopharynx in Alaska Native adults and children. J Pediatr Infect Dis Soc 2014;3(2):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Surveillance ABC. Emerging Infections Program Network Unpublished data (2016) Atlanta, GA: US Department of Health and Human Services, CDC; 2013. [Google Scholar]
- [32].CDC. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae; 2015. [Google Scholar]
- [33].Grijalva CG, Griffin MR, Edwards KM, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis : Off Publ Infect Dis Soc Am 2014;58 (10):1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA. Pneumococcal vaccination among medicare beneficiaries occurring after the advisory committee on immunization practices recommendation for routine use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults aged >/=65 years. MMWR Morb Mortal Wkly Rep 2017;66(27):728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations – United States, 2015. Morb Mortal Wkly Rep Surveill Summaries (Washington, DC : 2002) 2017;66(11):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].From the Centers for Disease Control and Prevention. Outbreak of pneumococcal pneumonia among unvaccinated residents of a nursing home–New Jersey, April 2001. JAMA. 2001;286(13):1570–71. [PubMed] [Google Scholar]
- [37].Dumyati G, Stone ND, Nace DA, Crnich CJ, Jump RL. Challenges and strategies for prevention of multidrug-resistant organism transmission in nursing homes. Current Infect Dis Rep 2017;19(4):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bureau USC. American Community Survey. In: Bureau USC; ed2015.