Abstract
Why do members of some species live in groups while others are solitary? Group living (sociality) has often been studied from an evolutionary perspective, but less is known about the neurobiology of affiliation outside the realms of mating and parenting. Colonial species offer a valuable opportunity to study nonsexual affiliative behavior between adult peers. Meadow voles (Microtus pennsylvanicus) display environmentally induced variation in social behavior, maintaining exclusive territories in summer months, but living in social groups in winter. Research on peer relationships in female meadow voles demonstrates that these selective preferences are mediated differently than mate relationships in socially monogamous prairie voles, but are also impacted by oxytocin and HPA axis signaling. This review addresses day-length dependent variation in physiology and behavior, and presents the current understanding of the mechanisms supporting selective social relationships in meadow voles, with connections to lessons from other species.
Keywords: meadow vole, prairie vole, social behavior, sociality, group living, photoperiod, partner preference, oxytocin, estradiol, glucocorticoids
1. Introduction
All animals engage in social interactions, but in some species, the advantages of social behavior have led to group living (sociality) and/or selective affiliative relationships. There is striking variation in mammalian social behavior, from polar bears that only interact prosocially with adult conspecifics to mate, to bats living in colonies of thousands. Humans and other social primates live in groups ranging from families to societies. Same-sex social relationships among peers are a common feature of social species, and in many cases form the basis for group living. Despite inroads into the understanding of parent-offspring bonding and monogamy, relatively few studies have explored the factors involved in prosocial behavior outside the context of reproduction (reviewed in Anacker and Beery, 2013; Goodson et al., 2006; Tang-Martinez, 2003). Group-living species offer a valuable opportunity to study a different facet of affiliation: namely social relationships between adult members of a group. Here, I provide a brief overview of comparative approaches to the study of sociality in rodents, then focus on lessons from studies of seasonally social meadow voles. The shift between social and solitary living in this species allows for comparisons of social phenotypes within a single species, not confounded by variation in evolutionary history.
1.1. Sociality
Life in social groups carries costs and provides benefits, only some of which have been quantified in any given species. Some generally recognized benefits of group living include protection from predation, increased foraging efficiency, information exchange, access to mates, thermoregulatory benefits, and access to helpers for infant care. The most profound costs associated with sociality lie in competition for food, mates, and other limited resources. Other potential costs include disease transmission and increased susceptibility to predation (reviewed in (Lee, 1994; Krause et al., 2002). Despite these tradeoffs, sociality is widespread, with over 70 documented social species in 39 genera (Lacey and Sherman, 2007).
Sociality is not a uniform trait, and many attempts have been made to classify different types of social groups. Some classifications focus on the complexity and stability of relationships, distinguishing between gregarious species that form unstable associations and social species that form stable associations with complex rules related to kinship, recognition, and past interactions (Goodson, 2013; Lee, 1994; Lidicker and Patton, 1987). Many social groups are based on kinship and family structure in the absence of monogamy, especially same-sex groups comprised of mothers and non-dispersed female offspring (e.g. elephants, horses, lions, prairie dogs, and some human societies). Kinship is not required for social grouping, however, and when the benefits of sociality are high, unrelated individuals may come together either in loose aggregations, or to form specific and selective social groups.
Even closely related species vary markedly in the manner in which they are social. For example in prairie voles (Microtus ochrogaster), family units consisting of bonded breeding pairs, and non-dispersed offspring form the basis of social groups (Carter and Getz, 1993). In the laboratory, individuals exhibit selective preferences for both familiar mates and same-sex peers (Williams et al., 1992; DeVries et al., 1997a; Beery et al., 2018). In meadow voles, adult females mate promiscuously and maintain exclusive territories during the summer breeding season. In the Winter and Spring, however, they live in selective groups that also rely on preference for familiar individuals (described in detail below). Other rodents, such as degus, are social without exhibiting preferences for familiar peers (Shambaugh, Insel, and Beery, personal communication). Lab strains of mice and rats are highly inbred, but some studies shed insight on the behavior of their wild conspecifics. Wild Norway rats live in gregarious colonies, where social interactions may be beneficial for predator avoidance and under other stressful conditions (Macdonald et al., 1999). Mice can also be gregariously social, but exhibit distinct social and cognitive behaviors (Ellenbroek and Youn, 2016). Neither mice nor rats appear to form specific social preferences or bonds under normal circumstances (Beery et al., 2018; Schweinfurth et al., 2017), but form stable social hierarchies (Curley, 2016). Many other rodents provide opportunities to assess different aspects of social behavior. For reviews of mechanisms underlying mammalian sociality in a comparative context, see Anacker and Beery (2013) and Beery et al. (2016).
Sociality has evolved on numerous occasions, and the neurobiological pathways underlying it may share a common basis or differ in important ways. The variety of combinations of different social behavior patterns (for example, group living with or without monogamy, biparental care, or familiarity preferences) implies these separable behaviors must be subserved by different underlying circuitry. At the same time, mechanisms underlying specific behaviors show a surprising degree of conservation across broad taxonomic groups—for example oxytocin and related peptides are involved in muscle contractions and behaviors related to reproduction from C. elegans to mammals (Garrison et al., 2012; Althammer et al., 2018). A recent study found that several genes associated with variation in sociality in sweat bees have also been implicated in autism spectrum disorder in humans (Kocher et al., 2018). By taking advantage of natural variations in social behavior in rodents, as well as in other taxa, it should become possible to determine when such mechanisms represent species-specific approaches to sociality, and when they represent generalizable phenomena.
2. Meadow Voles: behavior
2.1. Meadow vole behavior in the wild
Voles have been the focus of population ecology studies for almost a century, based on intriguing boom and bust population cycles that remain incompletely accounted for to this day (DeVries et al., 1997b; Elton, 1924; Krebs, 1996; Krebs and Myers, 1974; Oli, 2003). Radiotelemetry and trapping studies revealed interesting species and season differences in space use and social behavior, ranging from territoriality in females only in meadow voles, to males only in taiga/yellow-cheek voles, to stable family groups in prairie voles (Getz et al., 1981; Madison, 1980; Ostfeld, 1985; Wolff and Lidicker Jr, 1980). These behavioral variations themselves became the focus of new investigations. Early studies on meadow voles identified a surprising number of predation threats, and suggested that behavior related to reproductive energetics might be an important determinant of population size and female territoriality in this species (Dale Madison, personal communication).
Meadow voles (figure 1A) are promiscuous breeders with multiple paternity within litters (Boonstra et al., 1993; Getz, 1972). Unlike other vole species, females are the more territorial sex, maintaining exclusive territories during the summer breeding season (Madison, 1980; Webster and Brooks, 1981; figure 1B). Voles remain active throughout the winter, and the formation of winter social groups likely evolved in part because of its thermoregulatory benefits, which have been demonstrated in voles and other rodents (Andrews et al., 1987; Andrews and Belknap, 1993; Gilbert et al., 2010; Kauffman et al., 2003). In fall and winter non-reproductive season, meadow vole territories collapse and females and males cohabit in groups (figure 1C; Madison et al., 1984).
Figure 1.
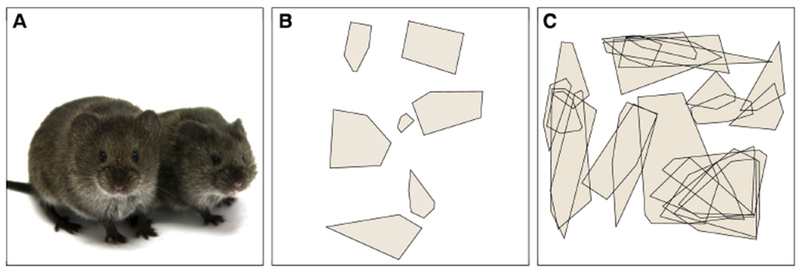
Meadow vole spatial ecology. A) Adult female meadow voles. B) In summer, females maintain exclusive territories, demonstrated by the non-overlapping home range perimeters of individual females on a sample day in July. C) Space use on a December day illustrates collapsed territories and existence of social groups including males and females. Telemetry data redrawn from Madison (1980) and Madison et al. (1984) and used with permission.
Winter social groups are ordinarily seeded by a female and her undispersed offspring, but predation on overwintering voles is substantial. By late December to early January, migration subsequent to predation leads to social groups that consist of unrelated adults. These mixed-sex groups consist of 3–10 voles that sleep in constellations of 2–5 (Madison and McShea, 1987). Addition of new members to these groups likely requires unusual social tolerance, which is not long lasting. Tests of dyadic interactions between field-caught voles indicate that males and females are tolerant of both nestmates and strangers during winter months when the gonads are regressed or not yet developed (McShea, 1990). By late winter and spring, group configurations become stable and no longer accept new group members (Madison et al., 1984; Madison and McShea, 1987). Females may continue to exhibit communal nesting for their first litter, particularly in female-female dyads, suggesting that same-sex affiliation may be particularly important for females. The frequency of this behavior decreases over the Spring and is absent by summer (Madison et al., 1984; McShea and Madison, 1984). Aggression towards strangers also increases in both sexes, concurrent with seasonal gonadal development (McShea, 1990). While both sexes exhibit some seasonal changes in social and aggressive behaviors, these variations are more extreme for females in both the field and laboratory (Boonstra et al. 1993, Beery et al. 2009).
Because meadow voles form selective social groups but mate promiscuously, this species allows for the study of selective peer affiliation that does not rely on substrates supporting reproductive pair-bonds.
2.2. Environmental influences on behavior in the laboratory
Laboratory studies have identified effects of photoperiod, temperature, food availability, and micronutrient availability on social and reproductive behaviors in meadow voles. For species that live in high latitudes, the most reliable cue signaling the time of year is day length/photoperiod (Prendergast et al., 2002; Paul et al., 2008). Housing in summer-like long day lengths (LDs) versus winter-like short day lengths (SDs) alters physiological traits in meadow voles including body mass, food intake, reproductive status, brain growth, and sex ratio of offspring (Dark et al., 1990, 1983; Gorman et al., 1994). Photoperiod also induces changes in social and anxiety behaviors in meadow voles in striking parallel to seasonal changes in field behaviors (described below). Laboratory research on seasonal changes in social behavior has thus centered on photoperiodic regulation (see table 1 for overview), although ecological signals of a milder winter—for example nutrients indicative of new plant growth—may provide an important signal for opportunistic reproduction outside the classic breeding season.
Table 1:
Laboratory variation in meadow vole physiology and behavior by day length.
| Endpoint | Finding | References |
|---|---|---|
| Olfactory preferences | LD females prefer male odor>own odor>female odor SD females prefer social odors (female odor>male odor>own odor) |
Ferkin and Zucker, 1991 |
| LD Males prefer female scents; no sex-specific preferences in SD | Ferkin and Gorman, 1992 | |
| Partner preferences | PP are formed in SD females in pairs and trios in the laboratory. |
Parker and Lee, 2003 Beery et al., 2008 Beery et al., 2009 Ondrasek et al., 2015 |
| SD females huddle more than LD females | Beery et al., 2008 | |
| Males form PP in both LD and SDs | Beery et al., 2009 | |
| Stranger interaction | SD females spend more time huddling with strangers in PPT SD females interact more during social interaction tests |
Lee et al. 2017 |
| Anxiety behavior | SD females spend more time in the open portion of a light/dark box | Ossenkopp et al. 2005 |
| Reproductive steroids | Estradiol/uterine mass is higher in LD housed females | Beery et al. 2008 |
| Estradiol and testosterone are higher in voles captured during the breeding season vs. the nonbreeding season | Galea and McEwen 1999 | |
| CORT secretion | Higher total CORT in LD (vs. SD) females | Anacker et al. 2016b |
| Higher free and total CORT in LD (vs. SD) males | Pyter et al. 2005 | |
| CRF1 receptor density | Higher in LD voles in the hippocampus | Beery et al. 2014 |
| CRF2 receptor density | Higher in SD voles in Cingulate cortex, hippocampus | |
| Oxytocin receptor density | Higher in SD voles in multiple regions including central amygdala, nucleus accumbens, and hippocampus |
Parker et al. 2001 Beery and Zucker 2010 |
| Brain growth | LD promote faster brain growth in male meadow voles | Dark et al. 1990 |
| Higher markers of neurogenesis in Fall vs. Summer | (Spritzer et al., 2017) |
2.2.1. Photoperiodic control of olfactory preferences
The olfactory preferences of female meadow voles change seasonally in the field, and this effect is recapitulated in response to photoperiod cues in the laboratory. Meadow vole females in summer (in the field) or housed in long day lengths (in the lab) prefer the scents of males over the scents of other females or their own scent, consistent with their reproductive and territorial state. In winter and in short day lengths, this preference reverses and females prefer the odors of other females to their own or male odors. Males showed no odor preferences in SDs (Ferkin and Gorman, 1992; Ferkin and Seamon, 1987; Ferkin and Zucker, 1991). These season and photoperiod changes are likely mediated at least in part by exposure to melatonin — a hormone that is secreted at night, and thus for longer durations during the winter — as melatonin treatment changed both the attractiveness of the odors produced by voles housed in LDs, and their preferences for other odors (Ferkin and Kile, 1996; Ferkin et al., 2007).
2.2.2. Photoperiodic control of affiliative behavior
In voles, social behavior is most often assessed using the partner preference test (PPT) (Williams et al., 1992) in which a focal vole is free to move throughout a three-chambered apparatus with stimulus voles — a familiar social partner/cagemate and an unfamiliar stranger — tethered in opposite chambers (figure 2A). The PPT allows the focal vole to come in direct contact with the tethered stimulus voles, permitting huddling. The three-hour duration of the test allows habituation to the test configuration and further promotes resting and social contact. This test was originally developed to assess opposite-sex mate preferences in prairie voles, but has since been extended to same-sex partner preferences in prairie voles (DeVries et al., 1997b; Beery et al., 2018) and meadow voles (Anacker et al., 2016a, 2016b; Beery et al., 2009, 2008; Beery and Zucker, 2010; Ondrasek et al., 2015; Parker and Lee, 2003), as well as other rodents (degus: Shambaugh, Insel and Beery personal communication; mice: Beery et al., 2018).
Figure 2.
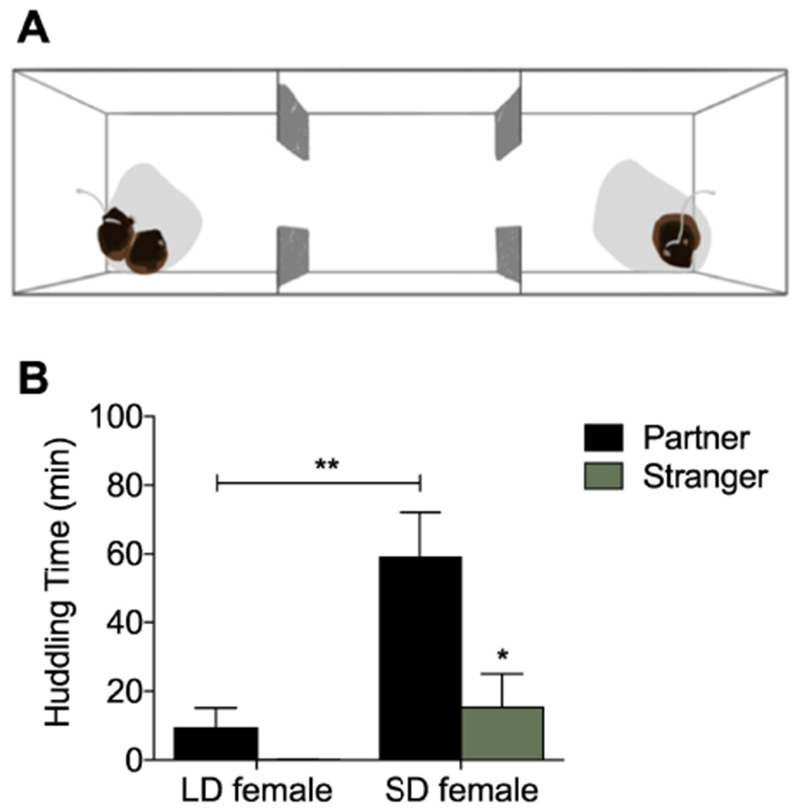
Social behavior variation in the lab. A) Partner preference test apparatus. To assess huddling time and selectivity of social behavior among peers, a same-sex cagemate is tethered on one end of the apparatus, and an unfamiliar same-sex vole is tethered at the opposite end. Tests are video-recorded for three hours and scored for time in each chamber, time huddling with each tethered subject, and activity. B) Variation in social huddling by day length. SD voles huddled more with their partners than LD voles. In this and other studies, SD meadow voles prefer familiar peers in PPTs, but still spend time huddling with strangers. Data excerpted from Beery et al. (2008).
Female meadow voles housed in short day-lengths in the laboratory form enduring social preferences for same-sex social partners (cage-mates) in PPTs, and these preferences persist after three weeks of separation (Parker and Lee, 2003). Preferences form within 24 hours of cohabitation with related or unrelated females, and preferences of equivalent magnitude can be formed between multiple cohoused individuals at the same time (Beery et al., 2009). In comparison to short-day housed individuals, long-day housed females huddled significantly less (Beery et al., 2008, figure 2B), mirroring seasonal differences in social behavior in the field. Males displayed intermediate levels of huddling, consistent with less dramatic shifts in field social behavior in males (Madison, 1980; Madison and McShea, 1987).
Long-day housed female meadow voles also sometimes prefer partners over strangers (Ondrasek et al., 2015, Goodwin et al. 2018). This may reflect particularly low tolerance of unfamiliar strangers by females in LDs. SD housed females prefer known social partners, but spent significantly longer in the cage of an unfamiliar stranger than do LD voles, and although overall huddling with strangers is low, it can be >80x longer than in LDs (Beery et al., 2008). SD females also appear to be responsive to increased incentive of a social group. While LD females prefer their partner to a trio of unrelated individuals, SD female meadow voles huddle with an unfamiliar group at a level equivalent to the partner (Ondrasek et al., 2015). In field settings, winter social groups maintain semi-flexible membership (Madison et al., 1984), which may result from increased interaction with, and tolerance of strangers.
2.2.3. Photoperiod and aggressive social interactions
Photoperiodic variation in affiliative behavior may build on day length-dependent changes in aggressive and anxiety behaviors. Field variation in aggression has been documented in male meadow voles, with more aggressive interactions between unfamiliar males during the breeding season, and more males first trapped with missing tails during this season (Turner and Iverson, 1973).
In social interaction tests of female meadow voles across day lengths, we found that SD-housed females interacted with novel conspecifics more than LD housed females, including both affiliative social contact and aggressive interactions (Lee et al., 2017). Increased social interaction between voles in winter may be further facilitated by social experience, as pair-housed SD meadow voles sniff, groom, and huddle with novel conspecifics more than individually-housed meadow voles (see 2.2.6). Increased interaction, particularly affiliative interaction, with novel voles is likely an important precursor to the formation of winter social groups.
2.2.4. Photoperiod and anxiety behaviors
Anxiety behavior may change with photoperiod because predation threats are somewhat mitigated in winter. Seasonal change in anxiety behavior may also contribute to changes in social tolerance and the capacity for affiliation with known individuals. In meadow voles, anxiety-like behavior has been assessed in terms of willingness to enter and spend time in bright, open arenas, presumed to be more threatening. Relative to LD meadow voles, voles housed in SDs spend more time in the light portion of a light-dark box—an apparatus in which subjects can spend time in a dark, sheltered space or explore a brightly lit and uncovered area. This effect was particularly large for females (Ossenkopp et al., 2005). A field study of male meadow voles found seasonal variation in open field exploration (Turner et al., 1983). Congruent with these findings, SD meadow vole females are more active in an open field, and spend more time in the center than do LD females (Reitz, 2014). Time spent investigating a novel conspecific in the social interaction test is classically used as a measure of anxiety (File and Seth, 2003), and SD meadow vole females are more interactive than LD females in this test (Lee et al., 2017).
2.2.5. Abiotic factors: food availability, food content and ambient temperature
Whereas photoperiod is the dominant cue for seasonal transitions in high latitude mammals, additional cues such as food availability, food content, and temperature may enhance responsiveness to varying conditions, potentially to great advantage. For this reason many seasonal rodent populations sustain a small number of photic non-responders, and photo-responsiveness may be enhanced by other conditions. For instance, male prairie voles exposed to short day lengths and low temperatures undergo complete gonadal regression, while males exposed to short day lengths alone exhibit a range of reproductive phenotypes (Kriegsfeld et al., 2000).
In meadow voles, low ambient temperature (10°C versus 21°C) enhanced huddling with an unfamiliar stranger vole in both long and short day lengths. Intriguingly this increased tolerance of a stranger occurred despite no significant increase in overall huddling levels (Ondrasek et al., 2015). Food restriction also leads to increased stranger huddling, but only in SD voles. This increase in stranger huddling occurred in addition to, rather than in place of, partner huddling. (Ondrasek et al., 2015).
Specific plant compounds have been associated with both inhibition and stimulation of reproduction in voles, altering the reproductive period under natural conditions. Compounds in senescent grasses such as paracoumaric acid and ferulic acid decrease female fertility in voles (Berger et al. 1987), whereas green vegetation and the compound 6-MBOA isolated from new growth increases fertility and fecundity (Berger et al., 1981; Sanders et al., 1981). This is also the case in meadow voles, in which seminal vesicle and testicular weights in males, and ovarian and uterine weights in females were larger in individuals injected with 6-MBOA than in matched controls (Cranford, 1983). Implants containing 6-MBOA have also been associated with increased female sex ratio in montane voles (Berger et al., 1987). The interaction between developmental photoperiod and plant nutrients has been tested in meadow voles, indicating that photoperiodic history and grains containing 6-MBOA interact to influence gonadal development. A potential role for 6-MBOA or vegetation type on seasonal social variation is therefore plausible, but has not been examined.
2.2.6. Biotic factors: social history
In addition to the physical environment, social history strongly impacts social behavior towards unfamiliar individuals: in 10 minute tests of social interaction with strangers in a neutral arena, female meadow voles housed alone exhibited more aggression and less affiliative social contact than pair-housed voles (Lee et al., 2017). Many important effects of social environment variables including weaning age, litter size, and extent of maternal care have been documented on later social behavior in voles and other rodents (e.g. Seitz, 1954; McGuire, 1988; Curley et al., 2009; Starr-Phillips and Beery, 2014).
3. Proximate factors influencing peer social behavior in meadow voles
3.1. Gonadal steroids
Seasonal changes in territory structure and social behavior coincide with changes in reproduction, and with circulating levels of the hormones that support capacity to reproduce (Galea and McEwen, 1999). The concentrations of gonadal steroids are thus natural candidates for the modulation of these behavioral changes. Multiple studies of day length and estradiol exposure in the laboratory demonstrate that gonadal steroids produce some but not all seasonal behavioral changes.
Consistent with seasonal differences in social behavior in the field, gonadally intact meadow voles housed in long day lengths show little huddling relative to voles housed in short day lengths. As expected, the same is true for ovariectomized, estradiol treated LD voles who experience a similar hormonal profile to intact LD voles. Ovariectomy without hormone replacement in LD females lowers estradiol exposure and uterine mass, but is insufficient to increase social huddling. In short day lengths, intact and ovariectomized females share a low estradiol profile and both groups huddle extensively; in contrast, ovariectomized/estradiol treated meadow voles exhibit reduced huddling (Beery et al., 2008). Thus, estradiol reduces social huddling in winter phenotype voles, but the absence of estradiol is insufficient to promote social huddling in summer day lengths.
Estradiol also plays an important role in the regulation of seasonal changes in olfactory preferences. In long day lengths, intact females prefer the odors of males, but this preference is eliminated by ovariectomy. In short day lengths, neither ovariectomy nor supplementation with estradiol alter olfactory preferences (Ferkin and Zucker, 1991). Together these studies indicate that changing levels of estradiol precipitate some but not all seasonal changes in social preference.
3.2. Stress, anxiety, and HPA Axis Regulation
Prior research has established important bidirectional links between anxiety and social behavior; for example, social contact can buffer stress responses in rodents, and social withdrawal is a symptom associated with long-term stress and PTSD (e.g. Williams and Eichelman, 1971; Kiyokawa et al., 2004; reviewed in Beery and Kaufer, 2015). In rats, increased anxiety behavior is associated with decreased social contact with a novel peer (Starr-Phillips and Beery, 2014), and social interaction testing with unfamiliar conspecifics is used as an assessment of both social and anxiety behaviors (File and Seth, 2003). We hypothesize that reduction in social anxiety and the ability to tolerate other individuals without becoming territorial or stressed may be a necessary permissive factor for sociality in winter months. As described in section 2.2.4, anxiety behaviors are lower in meadow voles housed in short (vs. long) photoperiods. Anxiety, hypothalamo-pituitary-adrenal (HPA) axis regulation, and social behavior are interconnected in many ways, and high levels of anxiety may prevent social behavior (Stowe et al., 2005; Hostetler and Ryabinin, 2013; Beery and Kaufer, 2015). Thus the HPA axis presents an interesting target for exploration of the mechanisms involved in permitting social tolerance and exploration in short day lengths. In meadow voles, photoperiod alters anxiety-like behaviors in addition to social behaviors, as detailed in section 2.2.4: females housed in short day lengths exhibit more exploratory behavior and less avoidance in classically anxiogenic situations.
We tested the effects of experience of stress on same-sex partner preferences in SD female meadow voles in new and established relationships. CORT was significantly elevated in response to a brief stress exposure (3 min forced swim test), and this stressor impaired the formation of a partner preference for a peer introduced immediately following the stressor. Partner preference in established partnerships was unaffected by stress exposure (Anacker et al., 2016b). Thus same-sex preference formation in meadow voles was altered in a similar fashion to opposite-sex preference formation in female prairie voles, in which females exposed to a stressor or CORT show reduced formation of partner preferences for a mate, unlike in males (DeVries et al., 1995, 1996).
Seasonal variation in HPA activity has been documented in several species (reviewed in (Romero, 2002). In meadow voles, factors influencing HPA axis signaling and CORT circulation in particular have been studied in both field and laboratory settings. In spring/summer populations of meadow voles, CORT relates to both population density and reproductive status. High population density is correlated with increased wounding in males and increased free and total CORT in both males and females (Boonstra and Boag, 1992). CORT in females is higher than in males during the breeding season in both field and laboratory, and highest in pregnant or lactating females (Boonstra and Boag, 1992; Galea and McEwen, 1999; Klein et al., 1997).
CORT levels vary with day length in females in the laboratory and likely in the field. Fecal glucocorticoid metabolites (reflecting free CORT) in female meadow voles were significantly higher in subjects housed in long day lengths (Anacker et al., 2016b) while in male meadow voles, long day lengths have been associated with lower free and total CORT (Pyter et al., 2005). In field samples, females meadow voles had higher (presumed total) CORT in summer than winter, but this difference was not significant (Galea and McEwen, 1999). Ongoing work is characterizing the relationship between seasonal changes in CORT, corticosterone binding globulin, and social behavior (K. Reitz, C. Freschlin, and A. Beery personal communication).
Increasing evidence suggests that the corticotropin-releasing factor system, encompassing multiple peptides and two receptor subtypes (CRF1 and CRF2), may be important in regulating social behaviors. CRF receptor density also alters negative feedback on CORT secretion and may contribute to seasonal changes in CORT regulation. In female meadow voles housed in LDs (vs. SDs), CRF1 receptor binding was greater, particularly in the hippocampus (Beery et al., 2014). CRF2 was greater in short day-lengths in the cingulate cortex and hippocampus. The hippocampus undergoes substantial seasonal decrease in size and cell count in many species including meadow voles (Galea and McEwen, 1999; Jacobs, 1996; Yaskin, 2011). Winter involution may play a role in changing CRF receptor densities, but concomitant increase and decrease in different receptor subtypes indicate that the change is not merely reflective of cell loss. Opposing changes in CRF1 and CRF2 receptor densities are particularly interesting because the actions of CRF2 often counter those of CRF1. Knockouts of CRF receptor subtypes 1 and 2 have opposite effects on anxiety behaviors (Bale and Vale, 2004) Thus, upregulation of CRF2 receptors with concomitant downregulation of CRF1 receptors in short day lengths is consistent with opposing roles of these receptors, and decreases in behavioral anxiety.
Individual differences in CRF receptor densities were correlated with the amount of time each individual spent huddling with stimulus voles, suggesting that CRF receptor density may be functionally related to pathways promoting huddling behavior. In particular, animals that huddled more showed more CRF1 receptor binding and less CRF2 receptor binding in subregions of the lateral septum, again highlighting the opposition between these systems (Beery et al., 2014). CRF production is known to increase with estradiol exposure, with estrogen response elements in the 5’ flanking region of the gene (Haas and George, 1989; Vamvakopoulos and Chrousos, 1993). Treatment with exogenous estradiol also had major effects on CRF subtype 1 and 2 receptor binding densities in multiple brain regions, suggesting that seasonal change in estradiol and CRF receptor regulation may be linked (Beery et al., 2014).
Together these findings suggest that season and photoperiod trigger changes in behavioral anxiety profile (see 2.2.4) and HPA axis regulation — including CRF receptor density and CORT circulation. Variation in these elements predicts individual differences in social behavior, indicating that non-sexual social behavior is shaped in important ways by these pathways.
3.3. Oxytocin
Across the animal kingdom, oxytocin (OT) and oxytocin-like neuropeptides mediate sexual and social behaviors from egg-laying to affiliation (Insel and Young, 2000). Since the initial discoveries of behavioral influences of OT and arginine vasopressin (AVP) in mammals, these neuropeptides have been implicated in several social behaviors, including individual recognition (Bielsky et al., 2005; Ferguson et al., 2001; Veenema et al., 2012), maternal attachment and aggression (Bosch and Neumann, 2012), and partner-preference formation (Johnson and Young, 2015). Oxytocin has also been the subject of studies of group living and social preferences among same-sex peers in birds and mammals (reviewed in Anacker and Beery, 2013; Goodson, 2012). Studies of the role of oxytocin in opposite-sex partner preferences laid the foundation for studies of same-sex social behavior in peers. Decades of work on this topic have been reviewed extensively elsewhere (Beery et al., 2016; Carter, 1998; Johnson and Young, 2015). Striking early findings included that oxytocin plays a critical role in formation of female preferences for a mate. Blockade of the OTR decreases time spent huddling with the mate, while infusion of OT into the brain hastens pair-bonding (Cho et al., 1999; Williams et al., 1992). Targeted infusions of OT or an OT antagonist directly to the nucleus accumbens are sufficient to induce or prevent pair-bond formation in females (Young et al., 2001), and infusions of AVP or a V1aR antagonist have corresponding effects when infused into the ventral pallidum of male prairie voles (Lim and Young, 2004; Winslow et al., 1993).
In female meadow voles, we have examined the effects of day length on oxytocin and oxytocin receptor production/distribution, relation of receptor density to behavior, effects of social manipulations on oxytocin, and effects of acute and chronic manipulations of neural oxytocin on social behaviors (see figure 3 for overview).
Figure 3.
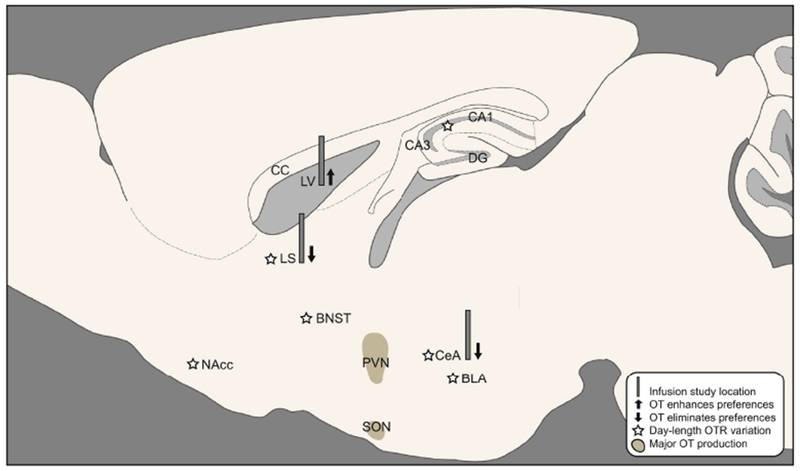
Oxytocin signaling pathways are implicated in meadow vole affiliative behavior. Stars indicate regions with significant day-length dependent variation in OTR density reported in one or more publications: CeA (Parker et al. 2001, Beery and Zucker 2010), LS and BLA (Parker et al. 2001), NAcc, BNST, Anterior hippocampus (Beery et al. 2014). In all cases, OTR density was higher in short day lengths. Cannulae indicate the location of OT infusion studies. Up arrows indicate that oxytocin infused to the lateral ventricle enhances partner preferences, while down arrows indicate elimination of partner preferences by oxytocin (Beery and Zucker 2010, Anacker et al. 2016, Christensen and Beery 2018. Major sites of oxytocin production (PVN, SON of the hypothalamus) are indicated with shading.
Regions in which oxytocin may act to influence social behaviors have been identified by receptor autoradiography. OTR distribution and density vary with day length in meadow voles (Beery and Zucker, 2010; Parker et al., 2001), with higher overall OTR expression in short day lengths. Oxytocin receptor density is associated with functional differences in social behavior at an individual level in meadow voles. Variation in OT receptor binding in meadow voles is correlated with huddling behavior, most notably in the lateral septum where more binding is associated with less huddling (Beery and Zucker, 2010). While oxytocin is typically thought of as enhancing prosocial behaviors, multiple converging lines of evidence suggest that the social effects of oxytocin are circuitry- and context-specific, at times enhancing agonistic behaviors (reviewed in Beery, 2015). Increased aggression may be related to the selectivity of oxytocin’s prosocial effects. For instance, formation of partner preferences for mates in prairie voles involves concomitant increases in aggression and aversion towards unfamiliar individuals (Getz et al., 1981; Gobrogge and Wang, 2011; Resendez and Aragona, 2013). In humans, one study found that oxytocin facilitates social behavior towards in-group members at the expense of an out-group (De Dreu et al., 2011). Oxytocin may thus play important roles in both prosocial and antisocial aspects of social selectivity.
Oxytocin administration influences social preferences and huddling behavior in female meadow voles. Infusion of oxytocin into the cerebral ventricles enhances preferences for a partner over a stranger, indicating a role for oxytocin in the specificity of huddling behavior. Interestingly, blockade of oxytocin receptors does not reduce preferences below the unmanipulated baseline, suggesting that oxytocin is not necessary for this level of preference and that other mechanisms also promote this social behavior (Beery and Zucker, 2010).
While chronic central administration of oxytocin enhances preferences, administration of oxytocin to specific brain regions during pairing can have the opposite effects, underscoring the complexity of oxytocin-social behavior interactions. For instance, oxytocin administration to the lateral septum completely eliminated selective partner preferences without reducing huddling time, acting principally via V1a receptors (Anacker et al., 2016a). Oxytocin infusion to the central nucleus of the amygdala similarly abolished same-sex partner preferences, acting via oxytocin receptors (Christensen and Beery, 2018). These studies underscore the different roles nonapeptides play on peer social behavior in multiple brain regions within the so-called social behavior network or social decision-making network (Goodson, 2005; Newman, 1999; O’Connell and Hofmann, 2012).
The region(s) in which icv OT infusion acts to enhance peer social preferences are thus currently unknown. The nucleus accumbens is a logical candidate, but prior studies have found that local infusions do not enhance opposite-sex mate preferences in meadow voles (in contrast to prairie voles), and accumbens OTR density is not correlated with same-sex peer huddling behavior (Ross et al., 2009; Beery and Zucker, 2010). Additional OT infusion studies in meadow voles—targeted to the prefrontal cortex, and perhaps nucleus accumbens—should prove useful in determining regions important for mediating peer social preferences.
3.4. Future directions in meadow voles
Social preferences may be driven by prosocial tendencies, including motivation to be with another individual, and by antisocial tendencies that keep individuals apart (e.g. territoriality, aggressiveness, and/or fear of unfamiliar individuals) (Hofmann et al., 2014). These alternative explanations for social behavior can be difficult to distinguish in most behavioral tests, as antisocial factors can nonetheless lead to selective social behavior—for instance one might be less afraid of a well-known individual. Research to date suggests SD-phenotype meadow voles prefer group members, but are more interactive with and tolerant of unfamiliar individuals than are LD voles. Assessment of the reward value of social contact, of motivation to work for different social stimuli, and of the role of dopamine in mediating peer relationships in meadow voles will contribute to our understanding of the underlying forces that lead to the specific social preferences that underlie peer relationships. In our initial work on this topic, it appears that peer relationships are not strongly reinforcing for female meadow voles, in that they do not condition place preferences for a cue associated with social housing (Goodwin et al., 2018). We are currently assessing the extent to which meadow and prairie voles will press a lever to gain access to a chamber housing a familiar or unfamiliar conspecific; preliminary findings suggest that female prairie voles work harder to access familiar (vs. unfamiliar) peers or mates, while male prairie voles work harder to access females (vs. males) of any familiarity (S. Lopez and A. Beery, personal communication).
Another important aspect of future inquiry will be the comparison of peer relationships in meadow voles and prairie voles. Lack of monogamy in meadow voles means that same-sex affiliative relationships are not maintained by the same mechanisms as monogamy. Prairie voles also exhibit peer partner preferences (Beery et al., 2018; DeVries et al., 1997b), and have been used to study social buffering, emotional contagion, social influences on drinking, and other social behavior topics (Anacker et al., 2011; Grippo et al., 2011; Lieberwirth and Wang, 2016; Burkett et al., 2016). While peer relationships are clearly of functional importance to prairie voles, it remains unknown whether they are mediated in a similar manner to mate relationships (e.g. requiring dopamine signaling in the nucleus accumbens), or whether they will be more similar to same-sex relationships in meadow voles, indicating common pathways across species. Comparisons of peer affiliation in meadow vs. prairie voles, and of peer vs. mate affiliation in prairie voles will therefore isolate the aspects of peer affiliation that generalize across species independent of mating system, or that vary in a species-specific manner.
In addition to these specific avenues, research on peer relationships in voles should improve our understanding of social behaviors involving social specificity, including differences in response to social peers and novel individuals, and much more.
4. Additional species, additional avenues
Sociality takes many forms, from temporary mating aggregations to stable societies. For example, one species may be considered “social” because it displays biparental care, social monogamy, and occasional cohabitation with additional adults (prairie voles). In another (gelada baboons), groups exist at multiple organizational scales from breeding groups to bands of groups, with additional, fluid levels of structure in between (Snyder-Mackler et al., 2012). The existence of diverse group types, and of distinct constellations of social behaviors within social species provides both opportunities and challenges. Evolution of different combinations of social behaviors suggests they are mediated by distinct underlying mechanisms that can be mixed and matched “cafeteria style” (Goodson, 2013), and this provides valuable opportunities to dissociate mechanisms underlying different behaviors. One challenge is that sociality encompasses many different group types, such that no species is representative of group living in a general sense. In order to understand factors underlying groups of multiple kinds, perspectives from multiple species and multiple research areas will be critical. Important work on mechanisms supporting the evolution of group living in particular has been conducted in a variety of non-human primates (e.g. Dunbar and Shultz, 2007), rodents (reviewed in Anacker and Beery, 2013; Beery et al., 2016), birds (Goodson, 2013; Goodson and Kingsbury, 2011; Wilson et al., 2016), fish (Gonzalez-Voyer and Kolm, 2010; Weitekamp and Hofmann, 2014), and insects (e.g. Shpigler et al., 2017; Kocher et al., 2018). Both surprising similarities and differences across species have been identified. Many other specific social behaviors (monogamy, paternal care, social hierarchy) have also been investigated.
As the field moves forward, one important goal will be to study the neurobiology of sociality in species for which adequate field data have been collected on social behavior and ecology (Taborsky et al., 2015), as in meadow voles. Comparisons of neural traits currently performed in two or three related species must be expanded to include multiple independently evolved origins of behaviors and consideration of phylogenetic signal (Garland Jr and Adolph, 1994; Hofmann et al., 2014). To date, a few genetic studies of social behaviors have used this approach, but the only neural trait compared across broad taxonomic groups is the relative volume of major brain regions (e.g. Bendesky et al., 2017; Hofmann et al., 2014; Turner et al., 2010). More detailed, phylogenetically informed analysis of the neural features of social and solitary species (e.g. OTR distribution across a phylogeny; A. Beery personal communication) and new laboratory techniques that allow manipulations of non-standard model organisms will play an important role in enhancing understanding of the neurobiology of diverse social behaviors.
Highlights:
Mechanisms underlying sociality and peer affiliation are relatively understudied
Meadow voles are seasonally social, allowing study of mechanisms supporting this transition
Long versus short day lengths induce differences in physiology and behavior
Roles of gonadal steroids, HPA axis, and oxytocin in vole sociality are reviewed
5. ACKNOWLEDGEMENTS
I am especially grateful to Irv Zucker for his unwavering support and mentorship throughout my career. Thanks also to many other advisors, collaborators, and researchers at UC Berkeley, UCSF, and at Smith College, and to Dale Madison for speaking with me about his early research interests. The most recent research described here was supported by the National Institute Of Mental Health of the National Institutes of Health under Award Number R15MH113085.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Althammer R, Jirikowski G, Grinevich Y, 2018. The oxytocin system of mice and men - Similarities and discrepancies of oxytocinergic modulation in rodents and primates. Peptides. https://doi.Org/10.1016/j.peptides.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Anacker AMJ, Beery AK, 2013. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci 7, 185 10.3389/fnbeh.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK, 2016a. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology 68, 156–162. https://doi.Org/10.1016/j.psyneuen.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Kaur S, Ryabinin AE, 2011. Prairie voles as a novel model of socially facilitated excessive drinking. Addict. Biol 16, 92–107. https://doi.Org/10.llll/j.1369-1600.2010.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, Beery AK, 2016b. Stress impairs new but not established relationships in seasonally social voles. Horm. Behav 79, 52–57. https://doi.Org/10.1016/j.yhbeh.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Andrews RV, Belknap RW, 1993. Season affects tolerance of cohabitation by deer mice. Physiol Behav 53, 617–20. [DOI] [PubMed] [Google Scholar]
- Andrews RV, Phillips D, Makihara D, 1987. Metabolic and thermoregulatory consequences of social behaviors between Microtus townsendii. Comp Biochem Physiol A 87, 345–8. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW, 2004. CRF and CRF Receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol 44, 525–557. 10.1146/annurev.pharmtox.44.101802.121410 [DOI] [PubMed] [Google Scholar]
- Beery AK, 2015. Antisocial oxytocin: complex effects on social behavior. Curr. Opin. Behav. Sci 6, 174–182. 10.1016/j.cobeha.2015.11.006 [DOI] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Front. Behav. Neurosci 12 10.3389/fnbeh.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Kamal Y, Sobrero R, Hayes LD, 2016. Comparative neurobiology and genetics of mammalian social behavior., in: Sociobiology of Caviomorph Rodents: An Integrated View. Wiley. [Google Scholar]
- Beery AK, Kaufer D, 2015. Stress, social behavior, and resilience: insights from rodents. Neurobiol. Stress 1, 116–127. 10.1016/j.ynstr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, Zucker I, 2008. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm. Behav 54, 153–159. 10.1016/j.yhbeh.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Routman DM, Zucker I, 2009. Same-sex social behavior in meadow voles: Multiple and rapid formation of attachments. Physiol. Behav 97, 52–57. 10.1016/j.physbeh.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, Grunberg DM, 2014. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Horm. Behav 66, 779–786. 10.1016/j.yhbeh.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–673. 10.1016/j.neuroscience.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Bendesky A, Kwon Y-M, Lassance J-M, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C, Hoekstra HE, 2017. The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439. 10.1038/nature22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PJ, Negus NC, Rowsemitt CN, 1987. Effect of 6-methoxybenzoxazolinone on sex ratio and breeding performance in Microtus montanus. Biol Reprod 36, 255–60. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Negus NC, Sanders EH, Gardner PD, 1981. Chemical triggering of reproduction in Microtus montanus. Science 214, 69–70. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-BB, Ren X, Terwilliger EE, Young LJ, 2005. The Via vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47, 503–13. 10.1016/j.neuron.2005.06.031 [DOI] [PubMed] [Google Scholar]
- Boonstra R, Boag PT, 1992. Spring declines in Microtus pennsylvanicus and the role of steroid hormones. J. Anim. Ecol 61, 339–352. [Google Scholar]
- Boonstra R, Xia X, Pavone L, 1993. Mating system of the meadow vole, Microtus penmylvcmicus. Behav. Ecol 4, 83–89. [Google Scholar]
- Bosch OJ, Neumann ID, 2012. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm. Behav 61, 293–303. 10.1016/j.yhbeh.2011.ll.002 [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal F.B.M. de, Young LJ, 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, 1998. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23, 779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, 1993. Monogamy and the prairie vole: battle of the sexes. Sci. Am 268, 100–106. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS, 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci 113, 1071. [DOI] [PubMed] [Google Scholar]
- Christensen JD, Beery AK, 2018. Oxytocin affects meadow vole social preferences differently by brain region and duration. Poster PS20055 bit. Congr. Neuroendocrinol. Tor. Can 10.6084/m9.figshare.7221428 [DOI] [Google Scholar]
- Cranford JA, 1983. Effect of 6-MBOAon Microtus pinetorum and Microtus pennsylvanicus of different ages.
- Curley JP, 2016. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biol. Lett 12 10.1098/rsbl.2016.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA, 2009. The Meaning of Weaning: Influence of the Weaning Period on Behavioral Development in Mice. Dev. Neurosci 31, 318–331. 10.1159/000216543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark J, Spears N, Whaling CS, Wade GN, Meyer JS, Zucker I, 1990. Long day lengths promote brain growth in meadow voles. Brain Res Dev Brain Res 53, 264–9. [DOI] [PubMed] [Google Scholar]
- Dark J, Zucker I, Wade GN, 1983. Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. Am J Physiol 245, R334–8. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ, 2011. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci U A 108, 1262–6. 10.1073/pnas.1015316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS, 1995. Modulation of pair bonding in female prairie voles {Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci U A 92, 7744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS, 1996. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl. Acad. Sci 93, 11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS, 1997a. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75, 295–301. [Google Scholar]
- DeVries AC, Taymans SE, Carter CS, 1997b. Social modulation of corticosteroid responses in male prairie voles. Ann. N. Y. Acad. Sci 807, 494–497. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S, 2007. Evolution in the social brain. Science 317, 1344–7. 10.1126/science.1145463 [DOI] [PubMed] [Google Scholar]
- Ellenbroek B, Youn J, 2016. Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech 9, 1079–1087. 10.1242/dmm.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton CS, 1924. Periodic Fluctuations in the Numbers of Animals: Their Causes and Effects. J. Exp. Biol 2, 119–163. [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ, 2001. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21, 8278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin MH, Gorman MR, 1992. Photoperiod and gonadal hormones influence odor preferences of the male meadow vole, Microtuspennsylvcmicus. Physiol Behav 51, 1087–91. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Kile JR, 1996. Melatonin treatment affects the attractiveness of the anogenital area scent in meadow voles (Microtus pennsylvanicus). Horm Behav 30, 227–35. 10.1006/hbeh.1996.0027 [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Leonard ST, Gilless JP, 2007. Exogenous melatonin administration affects self-grooming and conspecific odor preferences in long-photoperiod meadow voles (Microtus pennsylvanicus). Physiol. Behav 91, 255–263. 10.1016/j.physbeh.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin MH, Seamon JO, 1987. Odor preference and social behavior in meadow voles, Microtuspennsylvanicus’. seasonal differences. Can. J. Zool 65, 2931–2937. 10.1139/z87-445 [DOI] [Google Scholar]
- Ferkin MH, Zucker I, 1991. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fertil 92, 433–41. [DOI] [PubMed] [Google Scholar]
- File SE, Seth R, 2003. A review of 25 years of the social interaction test. Eur. J. Pharmacol 463, 35–53. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, 1999. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89, 955–64. [DOI] [PubMed] [Google Scholar]
- Garland T Jr, Adolph SC, 1994. Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol. Zool 67, 797–828. [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI, 2012. Oxytocin/Vasopressin-Related Peptides Have an Ancient Role in Reproductive Behavior. Science 338, 540–543. 10.1126/science.1226201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL, 1972. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. J. Mammal 53, 310–317. [Google Scholar]
- Getz LL, Carter CS, Gavish L, 1981. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol 8, 189–194. [Google Scholar]
- Gilbert C, McCafferty D, Le Maho Y, Martrette J-M, Giroud S, Blanc S, Ancel A, 2010. One for all and all for one: the energetic benefits of huddling in endotherms. Biol. Rev 85, 545–569. https://doi.org/10.llll/j.1469-185X.2009.00115.x [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Wang Z, 2011. Chapter 6 - Genetics of Aggression in Voles, in: Robert Huber DLB and R B. (Ed.), Advances in Genetics, Aggression. Academic Press, pp. 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Voyer A, Kolm N, 2010. Sex, Ecology and the Brain: Evolutionary Correlates of Brain Structure Volumes in Tanganyikan Cichlids. PLoS ONE 5, el4355 10.1371/journal.pone.0014355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, 2013. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 38, 465–478. 10.1016/j.psyneuen.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Goodson JL, 2012. Keeping Birds of a Feather Together. J. Neuroendocrinol 24, 525–526. [DOI] [PubMed] [Google Scholar]
- Goodson JL, 2005. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav 48, 11–22. 10.1016/j.yhbeh.2005.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y, 2006. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm. Behav 50, 223–236. 10.1016/j.yhbeh.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA, 2011. Nonapeptides and the Evolution of Social Group Sizes in Birds. Front. Neuroanat 5 10.3389/fnana.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, and Beery AK (2018). Comparative role of reward in long-term peer and mate relationships in voles. Horm Behav. doi: 10.1016/j.yhbeh.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Ferkin MH, Dark J, 1994. Melatonin influences sex-specific prenatal mortality in meadow voles. Biol Reprod 51, 873–878. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, Porges SW, 2011. 24-Hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosom. Med 73, 59–66. 10.1097/PSY.0b013e31820019e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DA, George SR, 1989. Estradiol or ovariectomy decreases CRF synthesis in hypothalamus. Brain Res. Bull 23, 215–218. 10.1016/0361-9230(89)90150-0 [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, Hurd PL, Lacey EA, Phelps SM, Solomon NG, Taborsky M, Young LJ, Rubenstein DR, 2014. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol 29, 581–589. 10.1016/j.tree.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE, 2013. The CRF system and social behavior: a review. Front. Neurosci 7 10.3389/fnins.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ, 2000. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol 10, 784–9. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, 1996. The economy of winter: phenotypic plasticity in behavior and brain structure. Biol. Bull 191, 92–100. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ, 2015. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci 3, 38–44. 10.1016/j.cobeha.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Paul MJ, Butler MP, Zucker I, 2003. Huddling, locomotor, and nest-building behaviors of furred and furless Siberian hamsters. Physiol Behav 79, 247–56. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y, 2004. Partner’s stress status influences social buffering effects in rats. Behav. Neurosci 118, 798–804. 10.1037/0735-7044.118.4.798 [DOI] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, Devries AC, Nelson RJ, 1997. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm. Behav 32, 30–39. 10.1006/hbeh.1997.1402 [DOI] [PubMed] [Google Scholar]
- Kocher SD, Mallarino R, Rubin BER, Yu DW, Hoekstra HE, Pierce NE, 2018. The genetic basis of a social polymorphism in halictid bees. Nat. Commun 9, 4338 10.1038/s41467-018-06824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Ruxton GD, Ruxton GD, 2002. Living in Groups. OUP Oxford. [Google Scholar]
- Krebs CJ, 1996. Population Cycles Revisited. J. Mammal 77, 8–24. 10.2307/1382705 [DOI] [Google Scholar]
- Krebs CJ, Myers JH, 1974. Population Cycles in Small Mammals. Adv. Ecol. Res 8, 267–399. 10.1016/S0065-2504(08)60280-9 [DOI] [Google Scholar]
- Kriegsfeld LJ, Trasy AG, Nelson RJ, 2000. Temperature and photoperiod interact to affect reproduction and GnRH synthesis in male prairie voles. J. Neuroendocrinol 12, 553–558. [DOI] [PubMed] [Google Scholar]
- Lacey EA, Sherman PW, 2007. The ecology of sociality in rodents, in: Wolff JO, Sherman PW. (Eds.), Rodent Societies: An Ecological & Evolutionary Perspective. University of Chicago Press, Chicago, pp. 243–254. [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2017. Comparative studies of affiliation, aggression, and reward in monogamous and promiscuous voles. Poster 15814 Soc. Neurosci. Wash. DC. [Google Scholar]
- Lee PC., 1994. Social structure and evolution, in: Slater PJB, Halliday T (Eds.), Behaviour and Evolution. Cambridge University Press, New York, NY, USA, pp. 266–303. [Google Scholar]
- Lidicker WZ, Patton JL, 1987. Patterns of dispersal and genetic structure in populations of small rodents, in: Chepko-Sade BD, Halpin ZT (Eds.), Mammalian Dispersal Patterns: The Effects of Social Structure on Population Genetics. University of Chicago Press, Chicago, pp. 144–161. [Google Scholar]
- Lieberwirth C, Wang Z, 2016. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr. Opin. Neurobiol., Systems neuroscience 40, 8–13. 10.1016/j.conb.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. 10.1016/j.neuroscience.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Macdonald D, Mathews F, Berdoy M, 1999. The behaviour and ecology of Rattus norvegicus’. from opportunism to kamikaze tendencies, in: Ecologically-Based Management of Rodent Pests. Australian Centre for International Agricultural Research, Canberra. [Google Scholar]
- Madison DM, 1980. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol 7, 65–71. [Google Scholar]
- Madison DM, FitzGerald RW, McShea WJ, 1984. Dynamics of social nesting in overwintering meadow voles {Microtuspennsylvanicus)’. possible consequences for population cycling. Behav. Ecol. Sociobiol 15, 9–17. [Google Scholar]
- Madison DM, McShea W, 1987. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Am. Zool 27, 899–908. [Google Scholar]
- McGuire B, 1988. Effects of Cross-Fostering on Parental Behavior of Meadow Voles (Microtus pennsylvanicus). J. Mammal 69, 332 10.2307/1381383 [DOI] [PubMed] [Google Scholar]
- McShea WJ, 1990. Social tolerance and proximate mechanisms of dispersal among winter groups of meadow voles, Microtus pennsylvanicus. Anim Behav 39, 346–351. [Google Scholar]
- McShea WJ, Madison DM, 1984. Communal nesting between reproductively active females in a spring population of Microtus pennsylvanicus. Can J Zool 62, 344–346. [Google Scholar]
- Newman SW, 1999. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. N. Y. Acad. Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2012. Evolution of a Vertebrate Social Decision-Making Network. Science 336, 1154–1157. 10.1126/science.1218889 [DOI] [PubMed] [Google Scholar]
- Oli MK, 2003. Population cycles of small rodents are caused by specialist predators: or are they? Trends Ecol. Evol 18, 105–107. [Google Scholar]
- Ondrasek NR, Wade A, Burkhard T, Hsu K, Nguyen T, Post J, Zucker I, 2015. Environmental modulation of same-sex affiliative behavior in female meadow voles (Microtuspennsylvanicus). Physiol. Behav 140, 118–126. 10.1016/j.physbeh.2014.12.021 [DOI] [PubMed] [Google Scholar]
- Ossenkopp K-PP, van Anders SM, Engeland CG, Kavaliers M, 2005. Influence of photoperiod and sex on locomotor behavior of meadow voles (.Microtus pennsylvanicus) in an automated light-dark “anxiety” test. Psychoneuroendocrinology 30, 869–79. 10.1016/j.psyneuen.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, 1985. Limiting resources and territoriality in microtine rodents. Am. Nat 1–15. [Google Scholar]
- Parker KJ, Lee TM, 2003. Female meadow voles (.Microtus penmylvcmicus) demonstrate same-sex partner preferences. J. Comp. Psychol 117, 283–9. 10.1037/0735-7036.117.3.283 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, Lee TM, 2001. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus). Behav. Neurosci 115, 1349–1356. 10.1037//0735-7044.115.6.1349 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ, 2008. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci 363, 341–61. 10.1098/rstb.2007.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I, 2002. Mammalian seasonal rhythms: behavior and neuroendocrine substrates, in: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R (Eds.), Hormones, Brain and Behavior. Academic Press, San Diego, pp. 93–156. [Google Scholar]
- Pyter LM, Weil ZM, Nelson RJ, 2005. Latitude affects photoperiod-induced changes in immune response in meadow voles (Microtuspenmylvcmicus ). Can. J. Zool 83, 1271–1278. 10.1139/z05-121 [DOI] [Google Scholar]
- Reitz K, 2014. Neuroendocrinology of stress and social behavior in female meadow voles. Theses Diss. Proj. http://scholarworks.smith.edu/theses/86 [Google Scholar]
- Resendez SL, Aragona BJ, 2013. Aversive motivation and the maintenance of monogamous pair bonding. Rev. Neurosci 24 10.1515/revneuro-2012-0068 [DOI] [PubMed] [Google Scholar]
- Romero LM, 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol 128, 1–24. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ, 2009. Variation in Oxytocin Receptor Density in the Nucleus Accumbens Has Differential Effects on Affiliative Behaviors in Monogamous and Polygamous Voles. J. Neurosci 29, 1312–1318. 10.1523/JNEUROSCI.5039-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EH, Gardner PD, Berger PJ, Negus NC, 1981. 6-methoxybenzoxazolinone: A Plant Derivative that Stimulates Reproduction in Microtus montanus. Science 214, 67–69. 10.1126/science.7025209 [DOI] [PubMed] [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, Taborsky M, 2017. Do female Norway rats form social bonds? Behav. Ecol. Sociobiol 71, 98. [Google Scholar]
- Seitz PFD, 1954. The effects of infantile experiences upon adult behavior in animal subjects: i. effects of litter size during infancy upon adult behavior in the rat. Am. J. Psychiatry 110, 916–927. 10.1176/ajp.110.12.916 [DOI] [PubMed] [Google Scholar]
- Shpigler HY, Saul MC, Corona E, Block L, Ahmed AC, Zhao SD, Robinson GE, 2017. Deep evolutionary conservation of autism-related genes. Proc. Natl. Acad. Sci 114, 9653–9658. 10.1073/pnas.1708127114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Beehner JC, Bergman TJ, 2012. Defining Higher Levels in the Multilevel Societies of Geladas (Theropithecus gelada). Int. J. Primatol 33, 1054–1068. 10.1007/sl0764-012-9584-5 [DOI] [Google Scholar]
- Spritzer MD, Panning AW, Engelman SM, Prince WT, Casler AE, Georgakas JE, Jaeger ECB, Nelson LR, Roy EA, Wagner BA, 2017. Seasonal and sex differences in cell proliferation, neurogenesis, and cell death within the dentate gyrus of adult wild-caught meadow voles. Neuroscience 360, 155–165. 10.1016/j.neuroscience.2017.07.046 [DOI] [PubMed] [Google Scholar]
- Starr-Phillips EJ, Beery AK, 2014. Natural variation in maternal care shapes adult social behavior in rats. Dev. Psychobiol 56, 1017–1026. 10.1002/dev.21182 [DOI] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z, 2005. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol. Behav 86, 369–78. 10.1016/j.physbeh.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Taborsky M, Hofmann HA, Beery AK, Blumstein DT, Hayes LD, Lacey EA, Martins EP, Phelps SM, Solomon NG, Rubenstein DR, 2015. Taxon matters: promoting integrative studies of social behavior: NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties. Trends Neurosci. 38, 189–191. 10.1016/j.tins.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Tang-Martinez Z, 2003. Emerging themes and future challenges: Forgotten rodents, neglected questions. J Mammal 84, 1212–1227. [Google Scholar]
- Turner BN, Iverson SL, 1973. The Annual Cycle of Aggression in Male Microtus Pennsylvanicus, and Its Relation to Population Parameters. Ecology 54, 967 10.2307/1935564 [DOI] [Google Scholar]
- Turner BN, Iverson SL, Severson KL, 1983. Seasonal changes in open-field behavior in wild male meadow voles (Microtus pennsylvanicus). Behav. Neural Biol 39, 60–77. [DOI] [PubMed] [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE, 2010. Monogamy evolves through multiple mechanisms: evidence from VlaR in deer mice. Mol. Biol. Evol 27, 1269–1278. 10.1093/molbev/msq013 [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP, 1993. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J. Clin. Invest 92, 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2012. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav 61, 50–56. 10.1016/j.yhbeh.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AB, Brooks RJ, 1981. Social behavior of Microtus pennsylvanicus in relation to seasonal changes in demography. J. Mammal 738–751. [Google Scholar]
- Weitekamp CA, Hofmann HA, 2014. Evolutionary themes in the neurobiology of social cognition. Curr. Opin. Neurobiol 28, 22–27. 10.1016/j.conb.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS, 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav 26, 339–49. [DOI] [PubMed] [Google Scholar]
- Williams RB, Eichelman B, 1971. Social Setting: Influence on the Physiological Response to Electric Shock in the Rat. Science 174, 613–614. 10.1126/science.174.4009.613 [DOI] [PubMed] [Google Scholar]
- Wilson LC, Goodson JL, Kingsbury MA, 2016. Seasonal Variation in Group Size Is Related to Seasonal Variation in Neuropeptide Receptor Density. Brain. Behav. Evol 10.1159/000448372 [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–8. 10.1038/365545a0 [DOI] [PubMed] [Google Scholar]
- Wolff JO, Lidicker WZ Jr, 1980. Population ecology of the taiga vole, Microtus xanthognathus, in interior Alaska. Can. J. Zool 58, 1800–1812. [Google Scholar]
- Yaskin VA, 2011. Seasonal changes in hippocampus size and spatial behavior in mammals and birds. Biol. Bull. Rev 1, 279–288. 10.1134/S2079086411030108 [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR, 2001. Cellular mechanisms of social attachment. Horm Behav 40, 133–8. 10.1006/hbeh.2001.1691 [DOI] [PubMed] [Google Scholar]


