Abstract
We and others have shown increased risk of monoclonal gammopathy of undetermined significance (MGUS) in first-degree relatives of patients with multiple myeloma (MM). Whether familial risk of MGUS differs by the MM proband’s age at onset, tumor or clinical characteristics is unknown. MM and smoldering MM (SMM) cases (N=430) were recruited from the Mayo Clinic in Rochester, Minnesota between 2005-2015. First-degree relatives over age 40 provided serum samples for evaluation of MGUS (N=1179). Age and sex specific rates of MGUS among first-degree relatives were compared to a population-based sample. Cytogenetic subtypes were classified by Fluorescence in situ hybridization. MGUS was detected in 75 first-degree relatives for an age- and sex- adjusted prevalence of 5.8% (95% CI: 4.5-7.2). Prevalence of MGUS in first-degree relatives was 2.4 fold (95% CI: 1.9-2.9) greater than expected rates. Familial risk did not differ by proband’s age at diagnosis, gender, isotype, IgH translocation, or trisomy. This study confirms first-degree relatives of MM cases have a significantly higher risk of MGUS compared to the general population, regardless of age, gender, or tumor characteristics. In selected situations, such as multiple affected first-degree relatives, screening of first-degree relatives of MM cases could be considered for follow-up and prevention strategies.
INTRODUCTION
Multiple myeloma (MM) is a result of a malignant transformation of plasma cells, characterized by complex cytogenetic and molecular genetic aberrations.1-3 MM is preceded by the presence of an asymptomatic clonal plasma cell expansion, a condition referred to as monoclonal gammopathy of undetermined significance (MGUS), which progresses to symptomatic MM at a rate of 1% per year.4, 5 MGUS is the most common plasma cell proliferative disorder and is prevalent in 3% of the general population older than 50 years of age.4-8 The prevalence of MGUS increases from 1.7% in those 50 to 59 years of age to more than 5% in those older than 70 years.4-8 Men have a higher prevalence of MGUS than females, regardless of age.6, 8
Familial aggregation of MM9-22 and MGUS9, 14, 16, 23 has been reported in the literature, suggesting a shared genetic susceptibility. Familial aggregation of MM and MGUS with other B-cell malignancies, as well as solid tumors, has also been reported.19, 24-26 Studies that specifically investigated family history of MM demonstrate a 2- to 4-fold increased risk of MM among those with an affected first-degree relative.24, 25, 27 Until the recent studies by Vachon et al8 and Landgren et al,23 the risk of MGUS in family members of patients with MM and MGUS was largely not known. These studies demonstrated 2-3 fold increased risk of MGUS in first-degree relatives of MM or MGUS probands8, 23 and established family history of MM / MGUS as a risk factor for MGUS.
Familial MM / MGUS may represent a more aggressive disease, as hereditary diseases often have earlier onset and worse prognosis relative to sporadic disease.28, 29 MM is known to be a collection of cytogenetically distinct diseases,1, 30 with 40-50% of MM cases having a reciprocal translocation involving the IgH locus at chromosome 14q32 and most of the remaining patients having trisomies. In a small proportion of cases, both IgH abnormalities and trisomies are found in the same clone.31, 32 These primary MM cytogenetic subtypes are associated with differential prognosis.1, 2, 32, 33 For example, translocations t(4;14)(p16;q32) and t(14;16)(q32;q32) are associated with adverse prognosis.2, 33, 34 A more favorable prognosis occurs in patients with trisomies and translocation t(11;14)(q13;q32).2, 33, 34 As disease progresses, secondary aberrations develop and these further differentiate prognosis.2, 33 Familial aggregation may differ by tumor types, defined by specific translocations or trisomies present in MM.35 Contrary to our hypothesis that familial MM may be more aggressive, an earlier pilot study by our group evaluated the association of family history of MM or MGUS in first-degree relatives across cytogenetic subtypes and suggested that MM probands who had an affected first-degree relative with MGUS or MM (47%) were more likely to have trisomy compared to those without a family history (34%, P=0.13).35
Given these conflicting findings, our goal was to confirm familial aggregation of MGUS with MM and SMM, and to examine differences in risk of MGUS in first-degree relatives of MM / SMM probands by age at onset, tumor and clinical characteristics. Understanding risk of MGUS by family history status could impact risk stratification and screening recommendations for relatives of MM patients in the clinical setting.
MATERIALS AND METHODS
The Mayo Clinic Institutional Review Board approved this study design and conduct. Informed consent was obtained from all participants in this study, in accordance with the Declaration of Helsinki.36
Study probands
Pathologically confirmed MM and SMM patients were recruited at Mayo Clinic in Rochester, Minnesota between 2005 and 2015. Participants completed a health history and family history questionnaire, the latter which solicited the names and addresses of first-degree (siblings, children, parents) blood-related family members ages 40 years and older. Our sample included the population used in Vachon et al (2009)8 which previously recruited 205 probands and 619 first-degree relatives, referred to as Mayo 1. New recruitment efforts spanned through 2015 collected 225 additional probands and 560 first-degree relatives, referred to as Mayo 2. The total combine sample size for this study therefore included 430 myeloma probands (400 MM / 30 SMM) and 1179 first-degree relatives of the probands (Supplemental Table 1).
First-degree relatives
All first-degree relatives ages 40 years or older were mailed an invitation and consent form. Upon receipt of the completed consent form, a questionnaire, and blood kit were mailed to the participant. Serum samples were spun down after draw and returned to Mayo for processing within 24 hours via FedEx.
Serum protein electrophoresis
As described previously,8 all serum samples were processed and analyzed in an identical fashion and in the same laboratory (Mayo Clinic Protein Immunology Laboratory, Rochester, Minnesota) as the prior population-based study of MGUS in Olmsted County, Minnesota (reference population).4 Serum protein electrophoresis was performed on agarose gel (Helena Laboratories). The agarose strip was inspected by a technician and by 2 of the authors (RAK. and JAK.) who were blinded to participant characteristics, including family history. Any serum with a discrete band or thought to have a localized band was confirmed and typed by serum immunofixation (Hydrasys and Hydragel; Sebia). MGUS was defined in accordance with the standard definition used in the Olmsted County prevalence study.4 Comparisons of prevalence between first-degree relatives and the Olmsted County reference population were based on MGUS cases identified through this diagnostic strategy.
Cytoplasmic Immunoglobulin Fluorescence in situ Hybridization (cIgFISH) and cytogenetic subtypes
Cytogenetic subtypes were defined clinically using cIgFISH test.37, 38 Briefly, bone marrow aspirates were evaluated by immunofluorescent labeled antibody against cytoplasmic kappa and lambda immunoglobulin light chains to selectively identify the plasma cell population. The following fluorescence in situ hybridization (FISH) probes were evaluated, including: translocations of the immunoglobulin heavy chain gene region (IGH) using a break-apart IGH probe and a dual-fusion FISH (D-FISH) probe for the five common IGH partners (CCND1, CCND3, MAF, MAFB, FGFR3); Centromere probes (D3Z1, D7Z1, D9Z1, D15Z4, D17Z1) for copy number gain of chromosomes 3, 7, 9, 15 and 17; Locus-specific probe strategies for 17p deletion (D17Z1, TP53), 1q duplication (TP73, CKS1B) and monosomy 13/deletion 13q (RB1, LAMP1).
For each probe set, 50-100 plasma cells were evaluated if available, with a minimum of 25 plasma cells generally evaluated per FISH hybridization site. For D-FISH probes, at least 3 abnormal cells had to be identified to be considered positive. For trisomies, at least 5 cells with trisomy had to be identified to be considered positive. For monosomies and deletions, at least 5 cells with monosomy and at least 7 cells with deletion had to be identified to be considered positive. Less than 1% plasma cells in the bone marrow were considered insufficient numbers for FISH testing.
Cytogenetic subtypes were categorized into groups defined by IgH translocation (yes/no), trisomy (yes/no) and intermediate/ high-risk disease (yes/no): [t(4;14), t(14;16), t(14;20), and del17p vs. others]. These classifications were based on cytogenetic abnormalities in clinical course and prognosis.39
Statistical considerations
The statistical methods for calculating the prevalence rates and risk ratios were previously described.8 In summary, prevalence rates for MGUS were standardized to the US 2000 population by age group and sex. The same age-and-sex-specific expected rates of MGUS were used for each individual, derived from the Olmsted County reference population described in Kyle et al (2006).4 These expected rates were used as an offset in Poisson regression models to calculate risk ratios comparing categories of proband characteristics (age, gender, M-spike ≥1.5, heavy chain type, and cytogenetic classification). Although we had sufficient power for estimation of risk ratios within subgroups examined, we were limited in our comparisons across subgroups. The statistical power is dependent on the number of observed events. In our data, there were 75 first-degree relatives who were found to have MGUS. Thus, for a potential risk factor with an approximately 50% and 25% prevalence, the smallest relative risk for differences between groups we can detect with 80% power and alpha of 0.05 is between 1.9-2.1, respectively. We considered a Bonferroni corrected statistically significant p-value of 0.007 for the risk of MGUS in first degree relatives by MM proband characteristics.
We also performed survival analyses (Kaplan-Meier and log rank test) among MM cases, for overall survival (censored at 15 years follow-up) by family history of MGUS. Finally, for comparisons between proband and relative pairs by gender, age and heavy chain type, we used McNemar’s tests. For probands with more than one family member with MGUS, we randomly selected one relative.
Secondary analyses subset to MM probands diagnosed in the state of Minnesota only were also performed since Olmsted County is located in Minnesota and may more accurately represent the Minnesota cases.
Analyses were performed in SAS ® [Version 9.4 (SAS Institute, Cary NC)] and R version 3.2.3 (R Foundation for Statistical Computing, Vienna Austria) and code is available upon request.
RESULTS
Characteristics of MM / SMM probands and their first-degree relatives
A total of 430 probands (400=MM/30=SMM) and 1179 first-degree relatives were recruited and eligible for the study (Table 1). Probands were primarily Caucasian (97%), more likely to be male (56.0%) and between 50-79 years of age (N=369, 85.8%) (Table 1). First-degree relatives consisted of a larger proportion of females (58.7%) and siblings (62.8%) (Table 1). Of the 430 MM/SMM probands, 187 resided in Minnesota at the time of their diagnosis. When restricted to Minnesota diagnoses only, baseline characteristic distributions were similar (Supplemental Table 2). Characteristics of the 205 MM probands and 619 relatives from Mayo 18 also showed similar trends to that of the full sample (Supplemental Table 3).
Table 1.
Demographic characteristics of Myeloma (MM/SMM) probands and their first degree relatives
| Characteristics | Probands (n=430) |
First-degree relatives of probands (n=1179) |
|---|---|---|
| Sex | ||
| Male | 241 (56.0) | 487 (41.3) |
| Female | 189 (44.0) | 692 (58.7) |
| Age category (years) | ||
| <40 | 9 (2.1) | --- |
| 40-49 | 40 (9.3) | 317 (26.9) |
| 50-59 | 135 (31.4) | 280 (23.7) |
| 60-69 | 150 (34.9) | 292 (24.8) |
| 70-79 | 84 (19.5) | 181 (15.4) |
| 80+ | 12 (2.8) | 109 (9.2) |
| Relationship to proband | ||
| Parent | - | 139 (11.8) |
| Sibling | - | 741 (62.8) |
| Child | - | 299 (25.4) |
Abbreviations: MM, multiple myeloma; SMM, smoldering multiple myeloma; Values reported are n(%).
Age and gender distributions of MM probands who have a first-degree relative with MGUS (N=68 probands) versus MM probands whose first-degree relative did not have MGUS (N=362) were similar. The mean age of MM cases with a family history was 62.5 years (SD=9.9), and 61 years (SD=10.3) for those without (P=0.28). The proportion of males was also similar in both groups (55.9% and 56.1%, respectively, P=0.98).
Prevalence of MGUS in first-degree relatives of MM / SMM probands
Of 1179 first-degree relatives ages 40 years and older, 75 were diagnosed with MGUS (Table 2). The overall age and sex-adjusted prevalence of MGUS in first-degree relatives of MM probands was 5.8% overall, and 7.9% for ages 50+ years (Table 2). The prevalence of MGUS increased with age of first-degree relatives from 1.9% in ages 40-49 years to 13.8% in ages 80+ years (Table 2). Prevalence rates were consistent when previously published data was excluded8 (Supplemental Table 4). When restricting to Minnesota diagnoses only (N=187), the prevalence rates reflected similar trends but were even greater at older ages (Supplemental Table 5).
Table 2.
Prevalence of MGUS in first-degree relatives of Myeloma (MM / SMM) probands compared with Olmsted County residents
| First-degree relatives of probands | Olmsted County | |||||
|---|---|---|---|---|---|---|
| Age (years) | Total (n=1179) |
Total with MGUS (n=75) |
Prevalence (95% CI) |
Total (n=21463) |
Total with MGUS (n=694) |
Prevalence (95% CI) |
| 40-49 | 317 (26.9) | 6 (8.0) | 1.9 (0.7,4.1) | - | - | - |
| 50-59 | 280 (23.7) | 11 (14.7) | 3.9 (2.0,7.0) | 8373 (39.0) | 141 (20.3) | 1.7 (1.4,2.0) |
| 60-69 | 292 (24.8) | 21 (28.0) | 7.2 (4.5,11.0) | 6019 (28.0) | 178 (25.6) | 3.0 (2.5,3.4) |
| 70-79 | 181 (15.4) | 22 (29.3) | 12.2 (7.6,18.4) | 4508 (21.0) | 205 (29.5) | 4.6 (4.0,5.2) |
| 80+ | 109 (9.2) | 15 (20.0) | 13.8 (7.7,22.7) | 2563 (11.9) | 170 (24.5) | 6.6 (5.7,7.7) |
| Total, adjusted (Ages 40+) | 1179 (100) | 75 (100) | 5.8 (4.5,7.2) | - | - | - |
| Total, adjusted (Ages 50+) | 862 (73.1) | 69 (92.0) | 7.9 (6.0,9.8) | 21463 (100) | 694 (100) | 3.2 (3.0,3.5) |
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; SMM, smoldering multiple myeloma; 95% CI, 95% confidence interval. Values reported are n (%). Rates for MGUS determined by serum protein electrophoresis with immunofixation for confirmation. Adjusted=age and sex adjusted to 2000 US total population.
Risk of MGUS in first-degree relatives vs. Olmsted County population
MGUS prevalence rates in this study were substantially higher among first-degree relatives of MM / SMM probands compared to those seen in Olmsted County, across all age groups (Table 2; Figure 1). First-degree relatives of MM / SMM probands had a 2.4 fold increased risk of MGUS (95% CI: 1.9, 2.9) compared to the Olmsted County population. This was slightly higher among MM / SMM cases diagnosed in Minnesota (RR=2.8, 95% CI: 2.1, 3.8) (Table 3; Figure 3). Analysis of the families recruited in Mayo 18 also showed an increased risk in the overall sample (RR=2.2, 95% CI: 1.6, 3.1) and when restricted to Minnesota (RR=2.3, 95% CI: 1.4, 3.6).
Figure 1.
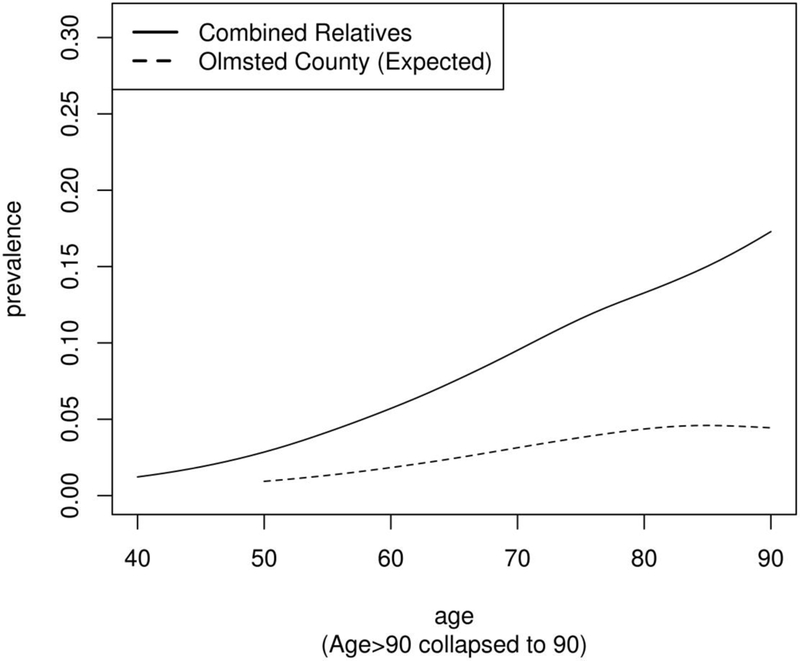
Prevalence of MGUS by age in first-degree relatives of patients with Myeloma (MM / SMM) compared to Olmsted County rates.
Table 3.
Risk of MGUS in first degree relatives by Myeloma (MM / SMM) proband characteristics
| Probands | |||||
|---|---|---|---|---|---|
| Proband characteristics | Total probands n (%) |
Total First- degree relatives N (%) |
Observed / Expected MGUS cases |
Risk ratio (95% CI)b |
p-valuea |
| Overall | 430 | 1179 | 2.4 (1.9,2.9) | ||
| Minnesota probands only | 187 (43) | 557 (47) | 2.8 (2.1,3.8) | ||
| Proband characteristics | |||||
| <55 years | 114 (27) | 305 (26) | 16 / 8.3 | 1.9 (1.2,3.1) | 0.29 |
| >=55 years | 316 (73) | 874 (74) | 59 / 22.8 | 2.6 (2.0,3.3) | |
| Female | 189 (44) | 524 (44) | 32 / 14.0 | 2.3 (1.0,4.3) | 0.67 |
| Male | 241 (56) | 655 (56) | 43 / 17.1 | 2.5 (1.4,1.9) | |
| M spike less than 1.5 g/dL | 115 (30) | 286 (27) | 12 / 7.2 | 1.7 (0.9,2.9) | 0.14 |
| M spike 1.5 g/dL or more | 267 (70) | 764 (73) | 54 / 20.1 | 2.7 (2.0,3.4) | |
| IgG | 274 (69) | 764 (69) | 50 / 20.1 | 2.5 (1.8,3.2) | 0.50 |
| IgA, IgM, light chain, or other | 126 (31) | 345 (31) | 19 / 9.2 | 2.1 (1.3,3.2) | |
| IgH=No | 205 (69) | 560 (68) | 36 / 14.6 | 2.5 (1.7,3.3) | 0.86 |
| IgH=Yes | 94 (31) | 259 (32) | 19 / 7.3 | 2.6 (1.6,4.0) | |
| Trisomies-No | 124 (41) | 328 (40) | 19 / 8.7 | 2.2 (1.4,3.3) | 0.43 |
| Trisomies-Yes | 175 (59) | 491 (60) | 36 / 13.2 | 2.7 (1.9,3.7) | |
| cHigh-Risk / Intermediate Mutations-No | 225 (75) | 627 (77) | 38 / 16.8 | 2.3 (1.6,3.0) | 0.20 |
| cHigh-Risk / Intermediate Mutations-Yes | 74 (25) | 192 (23) | 17 / 5.1 | 3.3 (2.0,5.2) | |
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; SMM, smoldering multiple myeloma; FDRs, first degree relatives; MN, Minnesota; 95% CI=95% confidence intervals. Values reported are n (%). Other=category consists of IgA, IgM, light chain, and other combined. Not all % add to 100%, due to missingness.
P-value for comparison of risk ratios between groups for each variable.
Ages 18-39 removed for total used for calculation of risk ratios (N=1179).
High-Risk / Intermediate Mutations=[(t(4;14), t(14;16), t(14;20), and del17p]
Differences in risk of MGUS in first-degree relative by MM / SMM proband characteristics were examined. First, comparisons were performed to test whether the increased risk of MGUS among first-degree relatives was unique to MM / SMM probands diagnosed at younger ages. Of the probands (N=430), 27% were diagnosed younger than age 55 years (Table 3). There was no evidence that risk of MGUS in first-degree relatives was higher in younger probands: >=55 years (RR=2.6, 95% CI: 2.0, 3.3) compared to those <55 years (RR=1.9, 95% CI: 1.2, 3.1) (P=0.29) (Table 3). Risk of MGUS in first-degree relatives was similar by gender of the MM / SMM proband (RRmales=2.5, 95% CI: 1.4, 1.9; RRfemales=2.3, 95% CI: 1.0, 4.3) (P=0.67) (Table 3). Of the 430 myeloma probands, 69% were IgG isotype and there was no evidence for difference between the risk of MGUS across the isotype categories (P=0.50) (Table 3). Results for size of M-protein were less clear. Probands with M-spikes >=1.5 g/dL or more had a higher risk of MGUS in first-degree relatives (RR=2.7, 95% CI: 2.0, 3.4), while first-degree relatives of probands with lower M-protein levels had a lower risk (g/dL) (RR=1.7, 95% CI: 0.9, 2.9) but the difference was not statistically significant (P=0.14) (Table 3).
Of the 430 probands, 70% (N=299) had clinical cytogenetic information available, including IgH translocations, trisomies, and high/intermediate risk status. Of these probands, 31% had IgH translocations, 59% were trisomies, and 25% were classified as high/intermediate risk (defined by presence of 4;14,14;16,14;20, or del 17p) with the majority (75%) defined as standard risk (Table 3). Risk of MGUS in first-degree relatives was similar for probands with IgH translocation (RR=2.6, 95% CI: 1.6, 4.0) and those without (RR=2.5, 95% CI: 1.7, 3.3) (P=0.86) (Table 3), as well as those with and without trisomies (RR=2.7,95% CI: 1.9, 3.7 and RR=2.2, 95% CI: 1.4, 3.3, respectively) (P=0.43) (Table 3). Risk of MGUS in first-degree relatives of probands with high-risk / intermediate cytogenetic risk (RR=3.3, 95% CI: 2.0, 5.2) was higher than the risk for relatives of MM probands without high-risk / intermediate mutational status (RR=2.3, 95% CI: 1.6, 3.0), but this difference was not statistically significant (P=0.20) (Table 3).
Characteristics of MM probands with a MGUS relative pair
Pairs of relatives were examined from the 68 families who had at least one first-degree relative with MGUS to compare characteristics of the MM proband and the MGUS relative. In families with two or more MGUS, one relative was randomly selected for the comparison. There was no evidence that the MM / MGUS relative pairs were more likely to have similar age at onset (P=0.44), gender (P=0.81) or heavy chain type (P=0.48).
Survival Analysis
Overall survival was compared for the 400 MM probands (excluding the SMM, n=30) by family history of MGUS. Kaplan-Meier curves demonstrate there was no difference in survival (censored at 15 years follow-up) between probands with a first-degree relative with MGUS (N=62) compared to those with a first-degree relative without MGUS (N=338) (HR= 0.93, 95% CI: 0.64, 1.35, P=0.7) (Figure 2). Analyses were similar among the subgroup of Minnesota only diagnoses (P=0.3) (data not shown).
Figure 2.
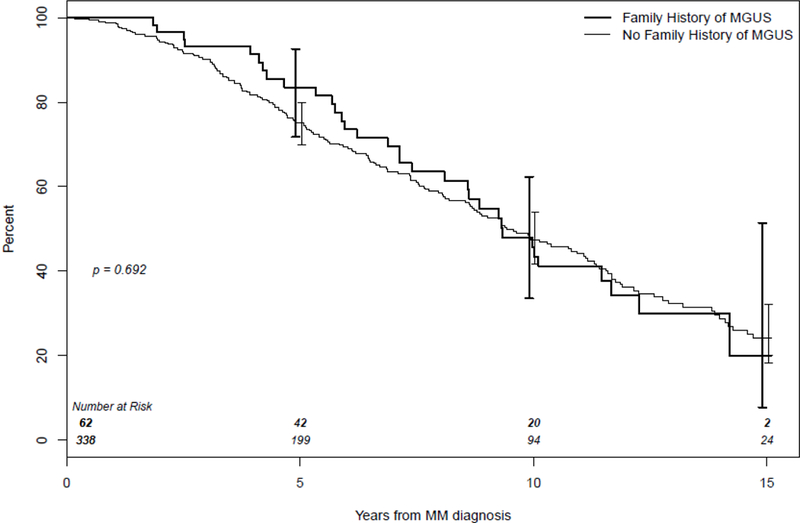
Kaplan-Meier curves for overall survival of Multiple Myeloma (MM) probands with a first-degree relative with Monoclonal gammopathy of undetermined significance (MGUS) (family history of MGUS) and MM probands with a first-degree relative without MGUS (no family history of MGUS).
DISCUSSION
This study confirms a 2- to 3- fold increase in the prevalence of MGUS among first-degree relatives of patients with MM / SMM compared to that of a general reference population (Olmsted County),4 using identical screening and diagnostic techniques. This is the largest study to examine risk of MGUS in first-degree relatives by clinical characteristics, including broad groups of cytogenetic subtypes of the MM / SMM probands. Even with the limited power for comparisons of risk by clinical characteristics, we see similar risks for gender, isotype, heavy chain translocation and trisomies, and higher risk ratios for later age at onset. Further studies should investigate whether familial risk of MGUS is higher among first-degree relatives of MM / SMM probands with intermediate / high risk cytogenetics, defined by (t(4;14), t(14;16), t(14;20), and del17p) and those with higher M-spike (>=1.5 g/dL or more), as our power was limited for these comparisons.
In the literature, there are few reports on whether familial MM and MGUS represent a more aggressive phenotype, in particular, defined by clinical characteristics and cytogenetics. Prior studies have estimated the risk of MM to be approximately 2.5 for those with a family history of MM.18, 40, 41 Some studies have reported a decrease in the age at MM diagnosis in successive generations,13, 40, 42, 43 suggestive of anticipation. Two prior studies suggested stronger associations of first-degree family history of MM and MM risk among MM cases diagnosed at younger ages.44, 45 A recent pooled case-control study from the International Multiple Myeloma Consortium reported an overall increased MM risk for an individual with a first-degree relative with MM (OR = 1.9, 95% CI: 1.3, 2.9); this was stronger in MM cases diagnosed before 55 years.46 VanValkenburg et al also reported that MM risk was associated with a family history of MM (OR= 3.8, 95% CI 1.8, 8.1), particularly among cases with early (≤60 years; OR= 4.6, 95% CI: 1.2, 17.3) verses late onset disease (>60 years; OR=3.4, 95% CI: 1.3, 9.0), although not statistically significant.44 Family history of hematologic malignancies and risk of MM by clinical features have also been studied previously.44 Relatives of patients with IgG / IgA MGUS had a 4.0-fold (95% CI: 1.7-9.2), 2.9-fold (95% CI: 1.7-4.9), and 20.0-fold (95% CI: 2.3-17.0) elevated risk of developing MGUS, MM, and lymphoplasmacytic lymphoma / Waldenstrom’s macroglobulinemia, respectively.23 Relatives of IgM MGUS patients had 5.0-fold (95% CI: 1.1-23.0) increased risk of chronic lymphocytic leukemia (CLL) and nonsignificant excess risk of MM and lymphoplasmacytic lymphoma / Waldenstrom’s macroglobulinemia.23 The results presented in the current study show no evidence for increased risk of MGUS in first-degree relatives with early age of MM proband or by isotypes. The inconsistency of results with the prior studies might suggest that differential genetic mechanisms are underlying aggregation of MM with MGUS compared to MM alone. Also, we were not able to comprehensively examine risk of other lymphoproliferative disorders, such as CLL and Waldenstrom’s macroglobulinemia, in our data.
The genetic basis underlying the inherited risk of developing MM is largely unknown. Genome-wide association and whole exome sequencing studies have identified common (N=17)47-52 and more recently, rare inherited susceptibility variants associated with increased risk of MM.53 Rare, truncating mutations in lysine-specific demethylase 1 (LSD1 / KDM1A), were found in one of 50 familial MM / MGUS kindreds examined, one early-onset case (<age 60 years) and 1.2% of MM patients unselected for family history. Additional somatic MM tumor analyses showed evidence of possible loss of heterozygosity (i.e.: 1p deletion) in some of the family members carrying the LSD1 / KDM1A truncating mutation, indicating both germline and somatic changes in familial MM.53 Further, of the common susceptibility variants, one was associated with increased risk of a specific MM subtype-Cyclin D translocation (t(11;14)(q13;q32)50 These results support an association of germline variation with somatic changes in the tumor.
Given that familial clustering of MM and MGUS in these families is likely due at least in part to shared germline variation, we hypothesized that we would see differential clustering by MM subtypes and clinical characteristics. Contrary to our hypothesis and findings of LSD1/KDM1A mutations among early onset cases, our results did not support increased familial risk by early age of onset. Further, we also did not see strong evidence for differences in familial MGUS by clinical MM subtypes or tumor characteristics. Our negative results may be due to the fact that familial MM / MGUS is most likely driven by rare variants segregating within a small number of families and analyses combining these families together increase heterogeneity. Future studies should aim to collect more detailed germline and tumor sequencing data. Given the array of clonal changes by MM subtypes, integrating germline-tumor will be useful. Further, our power was limited to conduct comparisons of familial clustering and evaluation by specific translocations, deletions and trisomies which may be the more relevant comparisons.
Survival analyses indicated no notable difference between overall MM survival in cases with and without a family history of MGUS. Few prior studies of survival by family history of MM or MGUS have been conducted with mixed findings. The largest to date showed a trend toward superior survival in MM patients with family history of lymphoproliferative disorders, compared to MM patients without family history among a study of N=13,947 MM patients.54 However, in an observational study comparing the prognosis of patients with lymphoma, leukemia, or MM from 55 multiple-case and 109 single-case families, patients from multiple-case families had an 8.3% poorer survival, however, no formal survival analyses for MM was conducted55. A strength of our study design is that none of the MM probands knew they had a family history of MGUS at diagnosis or had differential follow-up, eliminating a potential bias that relatives were screened because of a known family history. These results would be important to examine with larger samples sizes.
MGUS is a common condition and carries a lifelong increased risk of MM or other related malignancies4, 7. The confirmation of a significantly higher risk of MGUS in family members of MM / SMM has implications for clinical management of first-degree relatives of MM / SMM cases. In one study, MM patients with a prior history of MGUS had better survival than patients diagnosed with no antecedent MGUS history56. Similarly, a SEER-Medicare population analysis found that clinically recognized MGUS patients who are followed prior to their diagnosis of a monoclonal gammopathy-associated malignancy experience fewer major complications and have longer survival than those not followed57. Although these observations may be due to lead-time bias or other biological factors, it also raises the possibility that appropriate follow-up of MGUS may aid in the timely diagnosis of MM and prevent complications and prolong survival. This may be particularly important for first-degree relatives of patients with MM / SMM who are at significantly higher risk of MGUS and MM.
Gerkes et al proposed annual screening for MGUS, starting at age 40 years for individuals with more than one first degree relative, or those with one first-degree and at least one second-degree relative with MM58. However, to date, there is little evidence as to the benefit of screening and whether familial MGUS is more likely to progress to MM. Data from the iSTOP-MM trial in Iceland which is testing the value of screening for MGUS in the general population will be of value in this regard. As promising interventions to delay progression from MGUS to MM become available, first-degree relatives of any MM proband will be an important target population for early intervention. Despite the limitations of the data, in selected situations, such as multiple affected first-degree relatives, screening could be considered.
A limitation of our study is the primarily Caucasian population, including the MM probands, their relatives and the Olmsted County comparison population. The few studies performed in other racial/ethnic groups (including African American20, 44 and Chinese59 populations) also suggest an increased risk among relatives with a family history of MM. African American families with MM and MGUS in multiple generations have also been described11, 60. Another limitation is that information on other lymphoproliferative diseases in multiple generations was not assessed. Finally, our power for comparisons across clinical subgroups was limited, in particular for the analyses of M-protein size and intermediate/high risk subtype. Regardless of these limitations, this study is the largest of familial MGUS by clinical and tumor characteristics to date and one of the first to compare survival between MM cases with and without a family history of MM. Also, overall results were stronger among the subset of MM cases diagnosed in Minnesota, a population likely more comparable to the Olmsted County reference group.
Importantly, this study confirms the 2-3 fold increased risk of MGUS in first-degree relatives of MM probands regardless of age, gender or subtype of MM. Screening of first-degree relatives of MM cases could be considered in selected patients for follow-up and prevention strategies.
Supplementary Material
ACKNOWLEDGEMENTS
We would especially like to acknowledge the many MM / SMM patients and their families who contributed to this research. This work was supported in part by grants R01 CA107476, R01 CA168762, R25 CA092049, and P50 CA186781 (Mayo Clinic Myeloma SPORE) from the National Cancer Institute of the National Institutes of Health and the Mayo Clinic Cancer Center.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
References
- 1.Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J 2015; 5: e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet 2011; 204: 3–12. [DOI] [PubMed] [Google Scholar]
- 3.Munshi NFlsd1C, Avet-Loiseau H. Genomics in multiple myeloma. Clin Cancer Res 2011; 17: 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 5.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (mgus) consistently precedes multiple myeloma: A prospective study. Blood 2009; 113: 5412–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: A systematic review. Mayo Clin Proc 2010; 85: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346: 564–569. [DOI] [PubMed] [Google Scholar]
- 8.Vachon CM, Kyle RA, Therneau TM, Foreman BJ, Larson DR, Colby CL, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood 2009; 114: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson M, Hallberg B. Familial occurrence of hematologic malignancies and other diseases in multiple myeloma: A case-control study. Cancer Causes Control 1992; 3: 63–67. [DOI] [PubMed] [Google Scholar]
- 10.Judson IR, Wiltshaw E, Newland AC. Multiple myeloma in a pair of monozygotic twins: The first reported case. Br J Haematol 1985; 60: 551–554. [DOI] [PubMed] [Google Scholar]
- 11.Lynch HT, Ferrara K, Barlogie B, Coleman EA, Lynch JF, Weisenburger D, et al. Familial myeloma. N Engl J Med 2008; 359: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch HT, Sanger WG, Pirruccello S, Quinn-Laquer B, Weisenburger DD. Familial multiple myeloma: A family study and review of the literature. J Natl Cancer Inst 2001; 93: 1479–1483. [DOI] [PubMed] [Google Scholar]
- 13.Lynch HT, Watson P, Tarantolo S, Wiernik PH, Quinn-Laquer B, Isgur Bergsagel K, et al. Phenotypic heterogeneity in multiple myeloma families. J Clin Oncol 2005; 23: 685–693. [DOI] [PubMed] [Google Scholar]
- 14.Bizzaro N, Pasini P. Familial occurrence of multiple myeloma and monoclonal gammopathy of undetermined significance in 5 siblings. Haematologica 1990; 75: 58–63. [PubMed] [Google Scholar]
- 15.Zawadzki ZA, Aizawa Y, Kraj MA, Haradin AR, Fisher B. Familial immunopathies: Report of nine families and survey of literature. Cancer 1977; 40: 2094–2101. [DOI] [PubMed] [Google Scholar]
- 16.Ogmundsdottir HM, Einarsdottir HK, Steingrimsdottir H, Haraldsdottir V. Familial predisposition to monoclonal gammopathy of unknown significance, waldenstrom’s macroglobulinemia, and multiple myeloma. Clin Lymphoma Myeloma 2009; 9: 27–29. [DOI] [PubMed] [Google Scholar]
- 17.Ogmundsdottir HM, Haraldsdottirm V, Johannesson GM, Olafsdottir G, Bjarnadottir K, Sigvaldason H, et al. Familiality of benign and malignant paraproteinemias. A population-based cancer-registry study of multiple myeloma families. Haematologica 2005; 90: 66–71. [PubMed] [Google Scholar]
- 18.Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: A population-based case-control study. Int J Cancer 2006; 118: 3095–3098. [DOI] [PubMed] [Google Scholar]
- 19.Camp NJ, Werner TL, Cannon-Albright LA. Familial myeloma. N Engl J Med 2008; 359: 1734–1735; author reply 1735. [DOI] [PubMed] [Google Scholar]
- 20.Brown LM, Linet MS, Greenberg RS, Silverman DT, Hayes RB, Swanson GM, et al. Multiple myeloma and family history of cancer among blacks and whites in the u.S. Cancer 1999; 85: 2385–2390. [PubMed] [Google Scholar]
- 21.Bourguet CC, Grufferman S, Delzell E, DeLong ER, Cohen HJ. Multiple myeloma and family history of cancer. A case-control study. Cancer 1985; 56: 2133–2139. [DOI] [PubMed] [Google Scholar]
- 22.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 1994; 86: 1600–1608. [DOI] [PubMed] [Google Scholar]
- 23.Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in sweden. Blood 2009; 114: 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristinsson SY, Bjorkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 patients with multiple myeloma in sweden. Int J Cancer 2009; 125: 2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander DD, Mink PJ, Adami HO, Cole P, Mandel JS, Oken MM, et al. Multiple myeloma: A review of the epidemiologic literature. Int J Cancer 2007; 120 Suppl 12: 40–61. [DOI] [PubMed] [Google Scholar]
- 26.Frank C, Fallah M, Chen T, Mai EK, Sundquist J, Forsti A, et al. Search for familial clustering of multiple myeloma with any cancer. Leukemia 2016; 30: 627–632. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg AJ, Rajkumar SV, Vachon CM. Familial monoclonal gammopathy of undetermined significance and multiple myeloma: Epidemiology, risk factors, and biological characteristics. Blood 2012; 119: 5359–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans DG, Ingham SL. Reduced life expectancy seen in hereditary diseases which predispose to early-onset tumors. Appl Clin Genet 2013; 6: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilding A, Ingham SL, Lalloo F, Clancy T, Huson SM, Moran A, et al. Life expectancy in hereditary cancer predisposing diseases: An observational study. J Med Genet 2012; 49: 264–269. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg AJ, Rajkumar SV, Therneau TM, Singh PP, Dispenzieri A, Kumar SK. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia 2014; 28: 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Hieber M, Gutierrez ML, Perez-Andres M, Paiva B, Rasillo A, Tabernero MD, et al. Cytogenetic profiles in multiple myeloma and monoclonal gammopathy of undetermined significance: A study in highly purified aberrant plasma cells. Haematologica 2013; 98: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA, et al. Trisomies in multiple myeloma: Impact on survival in patients with high-risk cytogenetics. Blood 2012; 119: 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Wier S, Braggio E, Baker A, Ahmann G, Levy J, Carpten JD, et al. Hypodiploid multiple myeloma is characterized by more aggressive molecular markers than non-hyperdiploid multiple myeloma. Haematologica 2013; 98: 1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg AJ, Cousin M, Kumar S, Ketterling RP, Knudson RA, Larson D, et al. Differences in the distribution of cytogenetic subtypes between multiple myeloma patients with and without a family history of monoclonal gammopathy and multiple myeloma. Eur J Haematol 2013; 91: 193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. Jama 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 37.Dong H, Yang HS, Jagannath S, Stephenson CF, Brenholz P, Mazumder A, et al. Risk stratification of plasma cell neoplasm: Insights from plasma cell-specific cytoplasmic immunoglobulin fluorescence in situ hybridization (cig fish) vs. Conventional fish. Clin Lymphoma Myeloma Leuk 2012; 12: 366–374. [DOI] [PubMed] [Google Scholar]
- 38.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated mayo stratification of myeloma and risk-adapted therapy (msmart) consensus guidelines. Mayo Clin Proc 2009; 84: 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkumar SV, Kumar S. Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc 2016; 91: 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altieri A, Chen B, Bermejo JL, Castro F, Hemminki K. Familial risks and temporal incidence trends of multiple myeloma. Eur J Cancer 2006; 42: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 41.Hemminki K, Li X, Czene K. Familial risk of cancer: Data for clinical counseling and cancer genetics. Int J Cancer 2004; 108: 109–114. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande HA, Hu XP, Marino P, Jan NA, Wiernik PH. Anticipation in familial plasma cell dyscrasias. Br J Haematol 1998; 103: 696–703. [DOI] [PubMed] [Google Scholar]
- 43.Grosbois B, Jego P, Attal M, Payen C, Rapp MJ, Fuzibet JG, et al. Familial multiple myeloma: Report of fifteen families. Br J Haematol 1999; 105: 768–770. [DOI] [PubMed] [Google Scholar]
- 44.VanValkenburg ME, Pruitt GI, Brill IK, Costa L, Ehtsham M, Justement IT, et al. Family history of hematologic malignancies and risk of multiple myeloma: Differences by race and clinical features. Cancer Causes Control 2016; 27: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schinasi LH, Brown EE, Camp NJ, Wang SS, Hofmann JN, Chiu BC, et al. Multiple myeloma and family history of lymphohaematopoietic cancers: Results from the international multiple myeloma consortium. Br J Haematol 2016; 175: 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birmann BM, Andreotti G, De Roos AJ, Camp NJ, Chiu BCH, Spinelli JJ, et al. Young adult and usual adult body mass index and multiple myeloma risk: A pooled analysis in the international multiple myeloma consortium (immc). Cancer Epidemiol Biomarkers Prev 2017; 26: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell JS, Li N, Weinhold N, Forsti A, Ali M, van Duin M, et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma. Nat Commun 2016; 7: 12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan GJ, Johnson DC, Weinhold N, Goldschmidt H, Landgren O, Lynch HT, et al. Inherited genetic susceptibility to multiple myeloma. Leukemia 2014; 28: 518–524. [DOI] [PubMed] [Google Scholar]
- 49.Chubb D, Weinhold N, Broderick P, Chen B, Johnson DC, Forsti A, et al. Common variation at 3q26.2, 6p21.33, 17p11.2 and 22q13.1 influences multiple myeloma risk. Nat Genet 2013; 45: 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinhold N, Johnson DC, Chubb D, Chen B, Forsti A, Hosking FJ, et al. The ccnd1 c.870g>a polymorphism is a risk factor for t(11;14)(q13;q32) multiple myeloma. Nat Genet 2013; 45: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broderick P, Chubb D, Johnson DC, Weinhold N, Forsti A, Lloyd A, et al. Common variation at 3p22.1 and 7p15.3 influences multiple myeloma risk. Nat Genet 2011; 44: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaminathan B, Thorleifsson G, Joud M, Ali M, Johnsson E, Ajore R, et al. Variants in ell2 influencing immunoglobulin levels associate with multiple myeloma. Nat Commun 2015; 6: 7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scales M, Chubb D, Dobbins SE, Johnson DC, Li N, Sternberg MJ, et al. Search for rare protein altering variants influencing susceptibility to multiple myeloma. Oncotarget 2017; 8: 36203–36210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aradóttir K LS, Björkholm M, Goldin LR, Turesson I, Landgren O, Kristinsson SY. Family history of lymphoproliferative disease associated with a superior survival in multiple myeloma: A population-based study‥ In: EHA Learning Center. Aradóttir K. June 12 UoI, editor.(Abstract release date: May 21, 2015) [Google Scholar]
- 55.Rosenlof RC, Lemon HM, Rigby PG. Familial factors relating to prognosis of leukemia and lymphoma. Natl Cancer Inst Monogr 1971; 34: 283–289. [PubMed] [Google Scholar]
- 56.Sigurdardottir EE, Turesson I, Lund SH, Lindqvist EK, Mailankody S, Korde N, et al. The role of diagnosis and clinical follow-up of monoclonal gammopathy of undetermined significance on survival in multiple myeloma. JAMA Oncol 2015; 1: 168–174. [DOI] [PubMed] [Google Scholar]
- 57.Go RS, Gundrum JD, Neuner JM. Determining the clinical significance of monoclonal gammopathy of undetermined significance: A seer-medicare population analysis. Clin Lymphoma Myeloma Leuk 2015; 15: 177–186 e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerkes EH, de Jong MM, Sijmons RH, Vellenga E. Familial multiple myeloma: Report on two families and discussion of screening options. Hered Cancer Clin Pract 2007; 5: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Wang Y, Ji Z, Chen X, Pan Y, Gao G, et al. Risk factors for multiple myeloma: A hospital-based case-control study in northwest china. Cancer Epidemiol 2012; 36: 439–444. [DOI] [PubMed] [Google Scholar]
- 60.Jain M, Ascensao J, Schechter GP. Familial myeloma and monoclonal gammopathy: A report of eight african american families. Am J Hematol 2009; 84: 34–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


