Abstract
Binge alcohol-drinking elicits symptoms of negative affect such as anxiety upon cessation, which is a source of negative reinforcement for perpetuating this pattern of alcohol abuse. Binge-induced anxiety during early (24 h) withdrawal is associated with increased expression of metabotropic glutamate receptor 5 (mGlu5) within the nucleus accumbens shell (AcbSh) of adult male mice, but was unchanged in anxiety-resilient adolescents. Herein, we determined the role of mGlu5 signaling in withdrawal-induced anxiety via pharmacological manipulation using the mGlu5 negative allosteric modulator MTEP and the positive allosteric modulator CDPPB. Adult (PND 56) and adolescent (PND 28) male C57BL/6J mice binge-drank for 14 days under 3-bottle-choice procedures for 2 h/day; control animals drank water only. Approximately 24 h following the final alcohol presentation, animals were treated with 30 mg/kg IP MTEP, CDPPB, or vehicle and then tested, thirty minutes later, for behavioral signs of anxiety. Vehicle-treated binge-drinking adults exhibited hyperanxiety in all paradigms, while vehicle-treated binge-drinking adolescents did not exhibit withdrawal-induced anxiety. In adults, 30 mg/kg MTEP decreased alcohol-induced anxiety across paradigms, while 3 mg/kg MTEP was anxiolytic in adult water controls. CDPPB was modestly anxiogenic in both alcohol- and water-drinking mice. Adolescent animals showed minimal response to either CDPPB or MTEP, suggesting that anxiety in adolescence may be mGlu5-independent. These results demonstrate a causal role for mGlu5 in withdrawal-induced anxiety in adults and suggest age-related differences in the behavioral pharmacology of the negative reinforcing properties of alcohol.
Keywords: Binge-drinking, Adolescence, Group 1 metabotropic glutamate receptors, Anxiety, Alcoholism
1. Introduction
Both clinical and preclinical studies consistently report that chronic binge alcohol-drinking is associated with symptoms of negative affect and dysphoria during periods of abstinence. Binge-drinking is defined as a pattern of consumption sufficient to elevate blood alcohol concentrations (BAC) to ≥80 mg/dl, which relates to approximately 4–5 drinks in a 2-h period (NIAAA, 2004). Frequent binge-drinkers typically develop tolerance to the hedonic rewarding properties of alcohol, leading to an escalation of intake in order to reach a desired level of subjective intoxication. However, elevated consumption coupled with frequent bouts of intoxication/withdrawal exacerbates the severity and duration of subsequent withdrawal symptoms (Ballenger and Post, 1978; Becker and Hale, 1993; Carrington et al., 1984). Over time, withdrawal-induced negative affect fuels the transition to addiction by shifting the primary motivation for drinking from positive to negative reinforcement in order to alleviate this aversive state during periods of abstinence.
While both adults and adolescents engage in binge-drinking, it is especially prevalent amongst adolescents (CDCP, 2014). In fact, over 90% of alcohol consumed by underage drinkers is in the form of binge-drinking episodes (NIAAA, 2017). Adolescents typically consume larger quantities of alcohol than adults, yet adolescents are reportedly less susceptible to the negative consequences of acute intoxication (e.g., locomotor incoordination and sedation) and adolescents also experience fewer ‘hangover’-like symptoms such as withdrawal-induced anxiety and dysphoria (Doremus et al., 2003; Spear and Varlinskaya, 2005; Varlinskaya and Spear, 2004; White et al., 2002). Recent work in our laboratory has successfully recapitulated these age-related differences in withdrawal-induced negative affect using a mouse model of voluntary binge-drinking. We have shown that adult alcohol-drinking mice exhibit increased behavioral indices of anxiety during early (24 h) withdrawal (Lee et al., 2016). This elevated anxiety coincides with increased expression of metabotropic glutamate receptor 5 (mGlu5) within the nucleus accumbens shell (AcbSh) during acute withdrawal, as indicated by western blotting (Cozzoli et al., 2012; Obara et al., 2009; Lee et al., 2016, 2017b). In contrast, adolescent drinkers were resilient to both withdrawal-induced hyperanxiety and increased mGlu5 expression within the AcbSh during early withdrawal (Lee et al., 2016, 2017b).
mGlu5 signaling is known to play a significant role in alcohol abuse, as systemic administration of mGlu5 antagonist reduces alcohol reinforcement and voluntary consumption (Hodge et al., 2006; Lominac et al., 2006; McMillen et al., 2005; Schroeder et al., 2005). Our lab has previously shown increased mGlu5 expression within both the AcbSh (Cozzoli et al., 2009, 2012; Lee et al., 2016; Obara et al., 2009; Szumlinski et al., 2008) and the CEA (Cozzoli et al., 2014; Lee et al., 2017b; Obara et al., 2009) during acute alcohol withdrawal in adult animals and intracranial administration of mGlu5 antagonist within the AcbSh (Cozzoli et al., 2009, 2012; Gass and Olive, 2009) and CEA (Cozzoli et al., 2014) reduces alcohol consumption. Both the CEA and AcbSh are components of the extended amygdala, and drug-induced dysregulation within this circuitry is known to mediate many of the negative affective consequences of drug abuse (reviewed in Gilpin et al., 2015; Koob, 2003).
Given the evidence of alcohol-induced upregulation of mGlu5 within brain regions implicated in anxiety, we hypothesized that this could be a causal mechanism involved in withdrawal-induced anxiety. Additionally, an age-dependent insensitivity to alcohol-induced upregulation of mGlu5 signaling in adolescent drinkers could constitute a neurobiological basis for their resilience to withdrawal-induced hyperanxiety. Indeed, glutamatergic dysregulation is implicated in the etiology of both addiction (reviewed in Cleva and Olive, 2012; Holmes et al., 2013; Kalivas et al., 2009; Tsai et al., 1995) and anxiety (reviewed in Bergink et al., 2004; Simon and Gorman, 2006; Swanson et al., 2005). Not only do mGlu5 receptor antagonists attenuate behavioral measures of both drug seeking and anxiety in animal models (e.g., Backstrom et al., 2004; Cozzoli et al., 2009, 2012, 2014; Klodzinska et al., 2004; Kumar et al., 2013; Lou et al., 2014; Sinclair et al., 2012), but they have also shown anxiolytic efficacy in human clinical trials (Pecknold et al., 1982; Porter et al., 2005) and mGlu5 antagonism is thought to contribute to the therapeutic efficacy of the alcoholism medication Acamprosate (De Witte et al., 2005; Harris et al., 2002; Mann et al., 2008). Thus, a mutual basis of glutamatergic dysfunction could contribute to the high comorbidity between addiction and affective disorders.
In the present study, we assessed the functional significance of mGlu5 signaling in withdrawal-induced anxiety in adult and adolescent binge-drinking mice using the mGlu5 negative allosteric modulator 3-[(2-Methyl-1,3-thiazol-4- yl)ethynyl]pyridine (MTEP) and the positive allosteric modulator 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), which have high potency and selectivity (Busse et al., 2004; Lindsley et al., 2004). Additionally, allosteric modulators are of particular interest for their pharmacotherapeutic potential due to their ‘self-limiting’ activity (Epping-Jordan et al., 2007). In contrast to direct competitive antagonists/agonists, allosteric modulators have no intrinsic activity, but instead enhance or suppress the activity of the receptor in the presence of a ligand (Conn et al., 2009).
We predicted that MTEP treatment would reduce early withdrawal-induced anxiety in adult drinkers, while treatment with the CDPPB should exacerbate alcohol-induced mGlu5 hyperactivation and increase anxiety. In adolescent alcohol-drinking mice, we hypothesized that increasing mGlu5 signaling with CDPPB would elicit a hyperanxious, adult-like phenotype during early withdrawal. This would suggest that withdrawal-induced anxiety is mediated by a common underlying mechanism in both adult and adolescent bingers, and a resistance to alcohol-induced neuroadaptations of mGlu5 could underlie the resilience to withdrawal-induced negative affect seen in adolescent drinkers. Based on the evidence supporting the anxiolytic properties of mGlu5 antagonism in both humans and laboratory animals (e.g., Kotlinska and Bochenski, 2008; Kumar et al., 2013; Varty et al., 2005), we also anticipated an anxiolytic effect of MTEP treatment in alcohol-naïve animals, although to a lesser extent than hyperanxious adult mice in alcohol withdrawal.
2. Materials and methods
The binge-drinking and behavioral testing procedures employed herein were nearly identical to those used in previous studies in our lab (Lee et al., 2015, 2016, 2017b) and are summarized briefly below. All procedures were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 2014) and approved by the IACUC of the University of California, Santa Barbara.
2.1. Subjects
The animals used in this study were male C57BL/6 mice that were either PND 28 (adolescents) or PND 56 (adults) at the onset of drinking, to maintain consistency with our previous experiments. C57BL/6 mice are commonly used in alcohol studies due to their propensity to consume alcohol (Hwa et al., 2011; Le et al., 1994). Animals were housed in a climate-controlled vivarium under a reverse light/dark cycle (lights off at 10am) in groups of 4 per cage. Animals were identified using small animal ear tags (Stoelting, Wood Dale, IL). Food and water were available ad libitum, except during the 2-h alcohol-drinking period. The study consisted of 2 age groups (adults and adolescents), 2 drinking groups (alcohol or water), and 3 treatment groups (MTEP, CDPPB, or vehicle); n = 11/group.
2.2. Drinking-in-the-dark (DID) procedures
Half of the animals from each age group were subjected to 14 consecutive days of binge-drinking under 3-bottle DID procedures. Control animals received a single water bottle only. Alcohol-access was restricted to 14 days in order to correspond to the estimated duration of early-mid adolescence in mice (Spear, 2000) and to maintain consistency across age groups. Each day prior to the drinking period, animals were separated into individual drinking cages and allowed to acclimate for approximately 45 min. Animals were then given concurrent access to 10, 20, and 40% (v/v) unsweetened ethanol solutions for 2 h, beginning 3 h into the circadian dark cycle- the time of peak daily fluid intake (Rhodes et al., 2005). At the conclusion of the drinking period, animals were returned to their original group cages. The amount of alcohol consumed each day was calculated by bottle weight immediately before and after the drinking period and expressed as a function of the animal’s body weight (in kg).
2.2.1. Blood alcohol sampling
Submandibular blood samples were collected from all alcohol-drinking animals on day 11 of drinking, immediately upon conclusion of the 2-h drinking period. The scheduling of the blood sampling was selected to ensure that the animals’ intakes had stabilized, while also allowing ample time for recovery prior to behavioral testing. BACs were determined using an Analox alcohol analyzer (model AM1, Analox Instruments USA, Lunenburg, MA).
2.3. Drugs
The initial study used a high 30 mg/kg dose of both MTEP (Sigma Aldrich; St. Louis, MO) and CDPPB (NIMH C-918; Bethesda, MD) dissolved in 90% sterile water: 10% Tween-80 (Sigma Aldrich; St. Louis, MO), injection vol = 0.01 ml/g. This dose was selected from the high end of the dose-range typically reported to be behaviorally effective in the literature. For example, anxiolytic effects of MTEP have been reported at 20 mg/kg (Klodzinska et al., 2004) and 30 mg/kg achieves 100% receptor occupancy (Busse et al., 2004), while a 30 mg/kg dose of CDPPB reverses amphetamine-induced prepulse inhibition deficits (Kinney et al., 2005). Based on the results obtained at the 30 mg/kg MTEP dose, an additional follow-up replicate of animals was treated with a low 3 mg/kg dose of MTEP in order to establish a dose-response relationship, as conducted previously in studies by the Szumlinski laboratory (Cozzoli et al., 2014, 2009). Animals from each drinking group were sub-divided into their drug or vehicle treatment groups and were injected intraperitoneally at 30 min prior to the onset of behavioral testing for emotionality.
2.4. Behavioral testing
Drug administration and subsequent behavioral testing commenced approximately 24 h following the final alcohol presentation (Fig. 1) and occurred during the circadian dark phase.
Fig. 1. Procedural time-line of the present study.
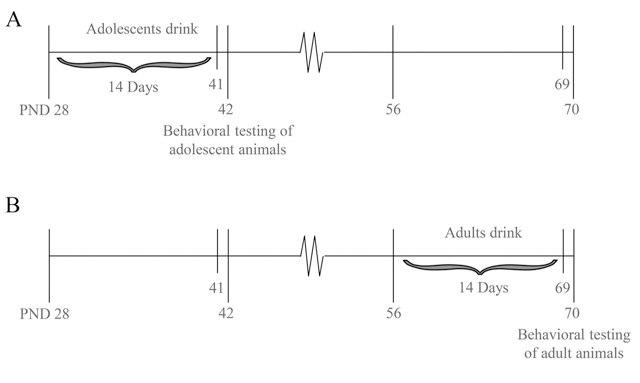
Beginning on PND 28 (adolescents; Panel A) or PND 56 (adults; Panel B), mice were presented with 10, 20 and 40% alcohol (v/v) for 14 consecutive days. Twenty-four hours later, the mice were pretreated with 3 or 30 mg/kg MTEP or 30 mg/ kg CDPPB and then tested for anxiety-like behavior in a behavioral test battery.
2.4.1. Light-dark box
The light/dark shuttle box test was used to anxiety-like behaviors (Bourin and Hascoet, 2003; Crawley, 1985). Animals were placed into a polycarbonate box measuring 46 cm long × 24 cm high × 22 cm wide containing 2 distinct environments; half of the box was white and uncovered and the other half black and covered, separated by a central divider with an opening. Animals started on the dark side and the latency to enter the light side, number of light-side entries, and total time spent in the light side of the shuttle box were recorded during a 15-min trial using Any-maze™ tracking software (Stoelting Co., Wood Dale, IL). This apparatus was also used to assess general locomotor activity by measuring the total distance traveled during the trial.
2.4.2. Marble-Burying
The marble-burying test was used to measure anxiety-induced defensive burying (Nicolas et al., 2006). In our paradigm, 10 square glass pieces (2.5 cm2 × 1.25 cm tall) were placed in the animals’ home cage, 5 at each end. The total number of marbles 75+% buried at the end of the 20-min trial was recorded. Trials were video recorded and later scored by an experimenter who was blinded to the experimental design with a stopwatch for the latency to begin burying marbles and the total time spent burying.
2.4.3. Porsolt forced swim test
Each animal was placed into an 11-cm diameter cylindrical container and the latency to first exhibit immobility (defined as no horizontal or vertical displacement of the animal’s center of gravity for ≥5 s), total time spent immobile, and the numbers of immobile episodes were monitored throughout the entire 6-min trial period using AnyMaze™ tracking software (Stoelting Co., Wood Dale, IL, USA).
2.5. Statistical analyses
A repeated measures ANOVA was used to analyze intake data for all alcohol-drinking animals to determine age differences in alcohol consumption across the 14-day drinking period. Pearson’s correlational analysis was conducted to determine the relationship between alcohol intake and resulting BACs from blood samples collected on day 11 of drinking. A repeated measures ANOVA was also performed on adults and adolescents separately to ensure there were no intake differences between treatment groups. Adult and adolescent behavioral data were analyzed independently using two-way ANOVAs with Tukey’s post hoc tests for significant results. As the 30 mg/kg and 3 mg/kg MTEP doses were conducted on separate cohorts of animals, all behavioral data were normalized and expressed as a percent of the control average for vehicle-treated water drinkers within each age group. A priori planned comparisons were conducted between alcohol and water vehicle-treated animals in order to assess the effects of alcohol withdrawal, independent of treatment. In order to assess the interaction between age and treatment in alcohol-experienced animals, adolescent behavioral data were then normalized and expressed as a percentage of the control average for adult vehicle-treated water drinkers, then analyzed via two-way ANOVA. Similarly, a priori planned comparisons were conducted between adults and adolescents vehicle-treated animals in order to compare the effects of alcohol withdrawal across age, independent of treatment. All calculations and analyses were performed using SPSS v.21 statistical software (IBM, 2012); α = 0.05 for all tests.
3. Results
3.1. Alcohol intake
Adults consumed an average of 4.15 ± 0.17 g/kg and adolescents an average of 6.10 ± 0.32 g/kg across the 14-day drinking period. The repeated measures ANOVA revealed a significant age effect showing that adolescents consumed significantly more alcohol, overall, compared to adults [F(1.76) = 28.83, p < 0.001]. On day 11 of drinking, adults consumed an average of 4.23 ± 0.18 g/kg with a resulting BAC of 80.91 ± 5.94 mg/dl and adolescents consumed an average of 5.16 ± 0.22 g/kg with a resulting BAC of 88.84 ± 5.85 g/kg (Fig. 2A). Collapsed across age, intake was significantly correlated with BAC (r= 0.504, p < 0.001; Fig. 2B). Within each age group, there were no significant differences in alcohol intake amongst the animals slated to receive vehicle, MTEP or CDPPB [Adults: F(3.38) = 0.84, p= 0.47; adolescents: F(3.38) = 0.48, p= 0.70]
Fig. 2. Age-related differences in alcohol intake and BACs.
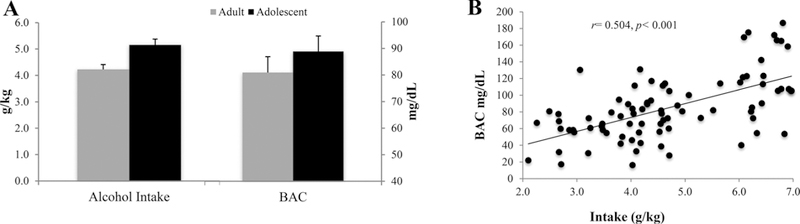
Blood was collected on drinking day 11 for BAC analysis. (A) Adults consumed an average of 4.23 ± 0.18 g/kg with a resulting BAC of 80.91 ± 5.94 mg/dL and adolescents consumed an average of 5.16 ± 0.22 g/kg with a resulting BAC of 88.84 ± 5.85 g/kg. Data represent mean + SEM. (B) Collapsed across age, alcohol intake was significantly correlated with BAC on day 11.
3.2. The effects of systemic CDPPB and MTEP upon behavior in adult-onset binge-drinking mice
3.2.1. Light-dark box
There was a significant drinking X treatment interaction in the number of light-side entries [F(3.73) = 9.45, p < 0.001; Fig. 3A]. In vehicle-treated animals specifically, alcohol-drinking mice made fewer light-side entries than water controls (p= 0.024). The 30 mg/kg MTEP treatment increased light-side entries, compared to vehicle in alcohol mice only (p < 0.001), while the lower 3 mg/kg dose increased entries in water-drinking mice only (p= 0.019). CDPPB treatment decreased light-side entries in both water and alcohol mice, compared to their vehicle-treated control group (p < 0.001 and p= 0.011, respectively). There was a significant drinking X treatment interaction in the time spent on the light side [F(3.74) = 11.24, p < 0.001; Fig. 3B]. Vehicle-treated alcohol animals spent less time on the light side, compared to water controls (p= 0.024). In alcohol-drinking mice, 30 mg/kg MTEP treatment increased light-side time, while CDPPB treatment decreased entries versus vehicle treatment (p= 0.033 and p= 0.029, respectively). In water controls, both 30 mg/kg MTEP and CDPPB treatment decreased the time spent on the light side compared to vehicle (p’s < 0.001), while 3 mg/kg MTEP increased light-side time (p= 0.045). A significant treatment effect [F(3.72) = 6.67, p < 0.001; Fig. 3C] revealed a longer latency to first light-side entry in CDPPB-treated animals overall, compared to vehicle, as well as both 3 and 30 mg/kg MTEP (p < 0.001, p= 0.003, and p= 0.001, respectively). Visual inspection of the data suggested that this main effect was driven primarily by a significant increase in the latency exhibited by CDPPB-treated water controls (p < 0.001). There were no effects of alcohol or treatment upon the distance traveled by the animals in the light-side of the apparatus (p’s > 0.1; Fig. 3D).
Fig. 3. The effects of MTEP and CDPPB upon the behavior of adult mice in the light-dark box test.
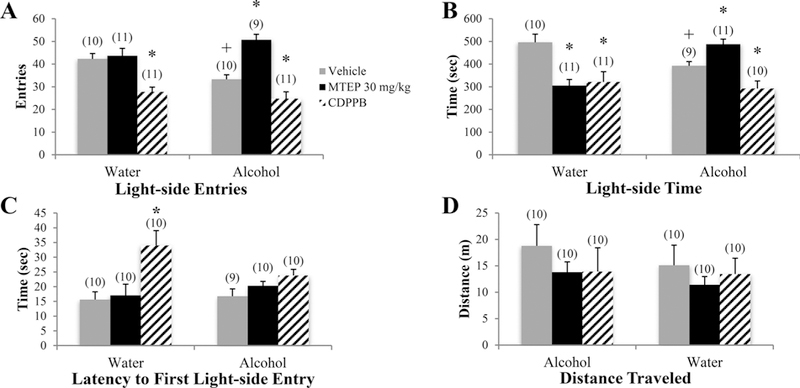
(A) There was a significant drinking X treatment interaction in the number of light-side entries. Vehicle-treated alcohol drinkers made fewer light-side entries compared to water drinkers. 30 mg/kg MTEP treatment increased light-side entries compared to vehicle in alcohol drinkers only, while 3 mg/kg MTEP increased entries in water drinkers only. CDPPB treatment decreased light-side entries in both water and alcohol drinkers compared to vehicle. (B) There was a significant drinking X treatment interaction in the time spent on the light side. Vehicle-treated alcohol drinkers spent less time on the light side compared to vehicle-treated water drinkers. In alcohol drinkers, 30 mg/kg MTEP treatment increased light-side entries while CDPPB treatment decreased entries compared to vehicle treatment. In water drinkers, both MTEP and CDPPB treatment decreased time spent on the light side, while 3 mg/kg MTEP increased light-side time. (C) A significant treatment effect showed a longer latency to first light-side entry in CDPPB-treated animals compared to vehicle. Visual inspection of the data shows that this main effect is driven primarily by a significant increase in CDPPB-treated water drinkers. (D) There were no effects of alcohol or treatment on distance traveled. *p < 0.05 vs. vehicle treatment within same drinking group, +p < 0.05 vs. vehicle-treated water drinkers. Data represent mean + SEM of the number of animals indicated in parentheses.
3.2.2. Marble-Burying
There was a significant drinking X treatment interaction in the number of marbles buried [F(3.74) = 3.02, p= 0.035; Fig. 4A]. Among vehicle-treated animals, alcohol mice buried more marbles compared to water controls (p= 0.044). Treatment with 30 mg/kg MTEP decreased the number of marbles buried in both water and alcohol mice (p= 0.038 and p < 0.001, respectively). Treatment with 3 mg/kg MTEP decreased the number of marbles buried in water-drinking animals only (p= 0.04). A significant drinking effect showed that overall, alcohol mice spent more time burying compared to water controls [F (1.74) = 4.63, p= 0.035; Fig. 4B]. In vehicle-treated animals, there was a strong trend towards more time spent burying in alcohol mice, compared to water controls (p= 0.052). Additionally, a significant treatment effect [F(3.74) = 7.16, p < 0.001] revealed decreased time spent burying in 30 mg/kg MTEP-treated animals, relative to vehicle, 3 mg/kg MTEP, and CDPPB (p’s < 0.001). There was a significant drinking effect on the latency to start burying [F(1.68) = 4.15, p= 0.046; Fig. 4C], indicating a shorter latency in alcohol mice overall compared to water controls. In vehicle-treated animals specifically, alcohol-drinking mice had a shorter latency to start burying, compared to water controls (p= 0.014) and 30 mg/kg MTEP significantly increased latency in alcohol mice compared to vehicle (p= 0.002).
Fig. 4. The effects of 30 mg/kg MTEP and CDPPB upon the behavior of adult mice in the marble-burying test. Marble burying, CDPPB, and high-dose MTEP in adult drinkers.
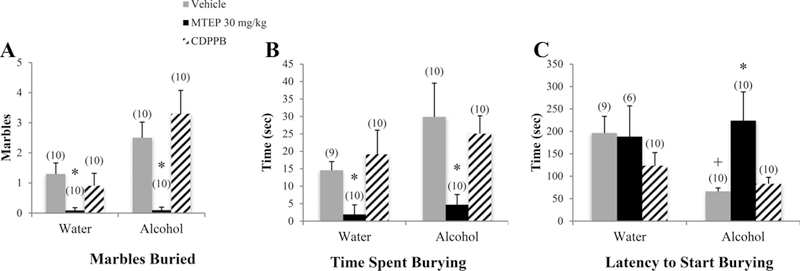
(A) There was a significant drinking X treatment interaction in the number of marbles buried. MTEP treatment decreased the number of marbles buried in both water and alcohol drinkers. There was also a trend toward more burying in vehicle-treated alcohol drinkers compared to water drinkers. (B) A significant treatment effect showed decreased burying in MTEP-treated animals compared to vehicle. There was also a trend toward more time spent burying in vehicle-treated alcohol drinkers compared to water drinkers. (C) There was a trend toward a treatment effect in latency to start burying, driven primarily by a significant increase in MTEP-treated alcohol drinkers compared to vehicle. Also, vehicle-treated alcohol drinkers had a shorter latency to start burying compared to water drinkers. The small sample size in MTEP-treated water drinkers was due to 4 animals that did not engage in any burying and therefore had no latency data. *p < 0.05 vs. vehicle treatment within same drinking group, +p < 0.05 vs. vehicle-treated water drinkers. Data represent mean + SEM of the number of animals indicated in parentheses.
3.2.3. Porsolt forced swim test
There was a significant drinking X treatment interaction in the time spent immobile [F(3.70) = 8.26, p < 0.001; Fig. 5A]. Among vehicle-treated animals, alcohol mice spent less time immobile in the FST, compared to water controls (p= 0.045). In alcohol mice specifically, 30 mg/kg MTEP-treated animals spent more time immobile compared to vehicle treatment (p= 0.017), while CDPPB-treated animals spent less time immobile (p= 0.046). A significant drinking effect showed that alcohol mice exhibited fewer immobile episodes overall versus water controls [F(1.75) = 6.34, p= 0.014; Fig. 5B]. Among vehicle-treated animals, alcohol mice exhibited fewer immobile episodes, compared to water drinkers (p= 0.030). In water control animals specifically, CDPPB treatment reduced immobile episodes, relative to vehicle treatment (p= 0.016). A significant drinking effect also showed that alcohol mice exhibited a longer latency to first immobility overall, compared to water drinkers [F(1, 70) = 12.56, p= 0.001; Fig. 5C], which was driven primarily by a significantly longer latency in CDPPB-treated alcohol mice compared to vehicle (p= 0.009). There was no significant difference in latency among vehicle-treated animals (p > 0.05).
Fig. 5. The effects of 30 mg/kg MTEP and CDPPB upon the behavior of adult mice in the FST.
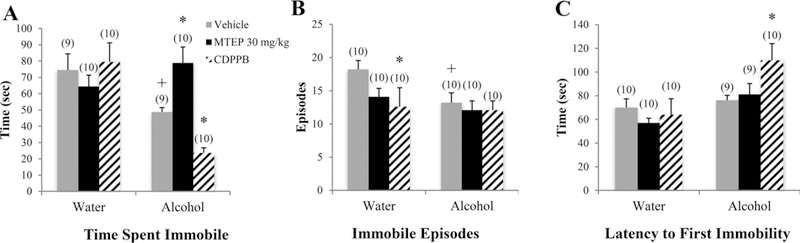
(A) There was a significant drinking X treatment interaction in the time spent immobile. In vehicle-treated animals, alcohol drinkers spent less time immobile compared to water drinkers. In alcohol drinkers, MTEP-treated animals spent more time immobile compared to vehicle treatment while CDPPB-treated animals spent less. (B) In vehicle-treated animals, alcohol drinkers had fewer immobile episodes compared to water drinkers. In water drinkers, CDPPB treatment reduced immobile episodes compared to vehicle treatment. There was also a trend toward reduced immobile episodes in MTEP-treated water drinkers. (C) Alcohol drinkers had a longer latency to first immobility compared to water drinkers; however, this difference was driven primarily by a significant increase in CDPPB-treated alcohol drinkers compared to vehicle treatment. *p < 0.05 vs. vehicle treatment within same drinking group, +p < 0.05 vs. vehicle-treated water drinkers. Data represent mean + SEM of the number of animals indicated in parentheses.
3.3. The effects of systemic CDPPB and MTEP upon behavior in adolescent-onset binge-drinking mice
3.3.1. Light-dark box
There was a significant drinking effect showing that alcohol mice made fewer light-side entries overall compared to water controls [F (1.75) = 14.00, p < 0.001; Fig. 6A]. Among vehicle-treated animals, alcohol mice made fewer light-side entries compared to water drinkers (p= 0.018). In alcohol mice, 30 mg/kg MTEP-treated animals made more light-side entries, compared to vehicle treatment (p= 0.001). In water controls, both 3 and 30 mg/kg MTEP-treated animals showed a trend toward increased light-side entries compared to vehicle (p= 0.057 and p= 0.068, respectively). There was a significant drinking X treatment interaction in the time spent on the light side [F (3.76) = 3.84, p= 0.013; Fig. 6B]. Among vehicle-treated animals, alcohol mice spent less time on the light side compared to water control animals (p= 0.012). In water controls, 3 mg/kg MTEP-treated animals spent more time on the light side, relative to vehicle treatment (p= 0.002). In alcohol mice, 30 mg/kg MTEP-treated animals spent more time on the light side compared to vehicle treatment (p= 0.013). There was also a significant drinking X treatment interaction in the latency to first light-side entry [F(3.72) = 3.37, p= 0.023; Fig. 6C]. Among vehicle-treated animals specifically, alcohol mice had a longer latency to first light-side entry compared to water drinkers (p= 0.007). In alcohol mice specifically, 30 mg/kg MTEP-treated animals had a shorter latency to enter compared to vehicle treatment (p= 0.003), with a similar trend in 3 mg/kg MTEP-treated animals (p= 0.065). Among water controls, 3 mg/kg MTEP-treated animals had a shorter latency compared to vehicle treatment (p= 0.027). There were no significant group differences in distance traveled (p > 0.05; Fig. 6D).
Fig. 6. The effects of 30 mg/kg MTEP and CDPPB upon the behavior of adolescent mice in the light-dark box test.
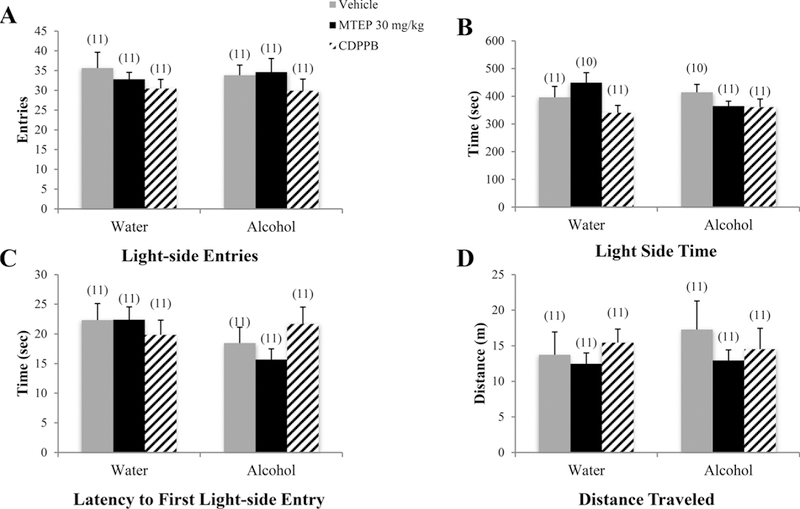
There were no group differences in any of the dependent variables measured. Data represent mean + SEM of the number of animals indicated in parentheses.
3.3.2. Marble-Burying
A significant drinking effect indicated that alcohol mice buried more marbles overall compared to water controls [F(1.77) = 9.35, p= 0.003; Fig. 7A]. Among vehicle-treated animals specifically, alcohol mice buried more marbles than water controls (p= 0.018). In alcohol mice, 30 mg/kg MTEP-treated animals buried fewer marbles compared to vehicle-treated animals (p= 0.041). There was a significant treatment effect in the total time spent burying [F (3.76) = 16.35, p < 0.001; Fig. 7B]. Overall, 30 mg/kg MTEP-treated animals spent less time burying while CDPPB-treated animals spent more, compared to vehicle (p= 0.002 and p= 0.001, respectively). The increase in burying in CDPPB-treated animals was significant in both water and alcohol drinkers (p= 0.008 and p= 0.022, respectively); however, the reduction in burying in 30 mg/kg MTEP-treated animals was significant in alcohol drinkers (p= 0.008) but trending in water drinkers (p= 0.061). A significant drinking effect showed that alcohol drinkers had a shorter latency to begin burying compared to water drinkers [F(1.66) = 7.75, p= 0.007; Fig. 7C]. Among vehicle-treated animals specifically, alcohol mice had a shorter latency compared to water controls (p= 0.032). There was also a significant treatment effect [F(3.66) = 6.18, p= 0.001] showing that 30 mg/kg MTEP-treated animals had a longer latency overall compared to vehicle (p= 0.006). Within alcohol mice specifically, 30 mg/kg MTEP-treated animals had a longer latency compared to vehicle (p= 0.028).
Fig. 7. The effects of 30 mg/kg MTEP and CDPPB upon the behavior of adolescent mice in the marble-burying test.
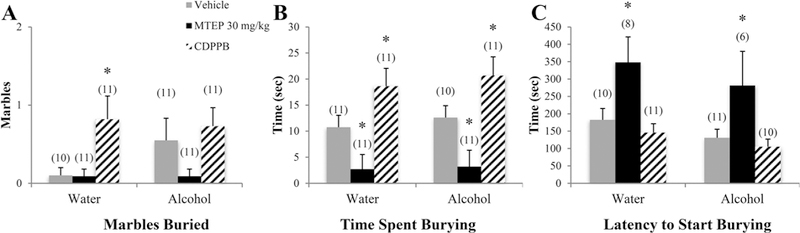
(A) A main treatment effect showed that CDPPB-treated animals buried more marbles overall compared to MTEP treatment, independent of drinking group. This group difference is primarily driven by a significant increase in CDPPB-treated water drinkers specifically. (B) In both water and alcohol drinkers, CDPPB-treated animals buried more marbles compared to vehicle-treated animals while MTEP-treated animals buried less. (C) MTEP treatment increased latency to start burying overall compared to vehicle and CDPPB treatment in both water and alcohol drinkers. *p < 0.05 vs. vehicle treatment within same drinking group. Data represent mean + SEM of the number of animals indicated in parentheses.
3.3.3. Porsolt forced swim test
There was a significant drinking effect on total time spent immobile in the FST showing that alcohol mice spent more time immobile overall compared to water controls [F(1.72) = 6.49, p= 0.013,; Fig. 8A]. Among vehicle-treated animals specifically, alcohol mice spent more time immobile compared to water controls (p= 0.035). There was also a significant treatment effect [F(3.72) = 5.06, p= 0.003], which showed that CDPPB-treated animals spent less time immobile overall compared to both vehicle and 30 mg/kg MTEP treatment (p= 0.001 and p= 0.006, respectively). There was a significant drinking X treatment interaction in the number of immobile episodes [F(3.75) = 3.47, p= 0.02; Fig. 8B]. Among alcohol-drinking mice, CDPPB-treated animals had fewer immobile episodes compared to vehicle treatment (p= 0.003). However, there was no significant effect of alcohol among vehicle-treated animals. There was a significant treatment effect in the latency to first immobility [F(3.74) = 6.28, p= 0.001; Fig. 8C]. Overall, CDPPB-treated animals had a longer latency to first immobility compared to vehicle and both 3 and 30 mg/kg (p= 0.001, p < 0.001, and p= 0.002, respectively). Among alcohol mice specifically, CDPPB-treated animals had a significantly longer latency compared to vehicle (p= 0.001). In vehicle-treated animals, there was a trend toward shorter latency in alcohol mice compared to water controls (p= 0.067).
Fig. 8. The effects of 30 mg/kg MTEP and CDPPB upon the behavior of adolescent mice in the FST.
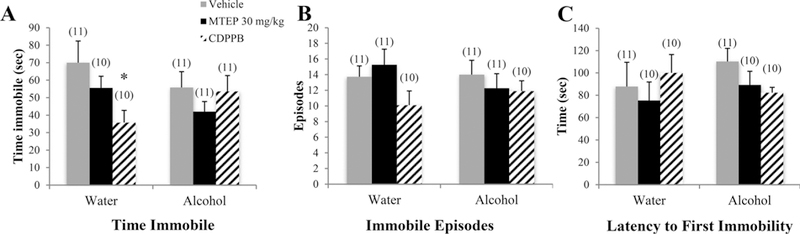
(A) There was a trend toward a drinking X treatment interaction in the total time spent immobile, attributable primarily to a significant decrease in CDPPB-treated water drinkers compared to vehicle treatment. There were no group differences in (B) the number of immobile episodes or (C) the latency to first immobility. *p < 0.05 vs. vehicle treatment within same drinking group. Data represent mean + SEM of the number of animals indicated in parentheses.
3.4. Comparison of the effects of systemic CDPPB and MTEP upon behavior in adult and adolescent animals
Results are summarized in Table 1.
Table 1.
In order to directly compare the magnitude of the effects of systemic treatment with MTEP and CDPPB upon alcohol withdrawal-induced anxiety between adult- and adolescent-onset binge-drinking mice, the data were normalized to the average of the adult-water-vehicle controls and are presented here as mean ± SEM. vs. adult (i.e., an age difference; see Sect. 3.4).
| Test | Dependent measure | Vehicle |
3 mg/kg MTEP |
30 mg/kg MTEP |
CDPPB |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Adolescent | Adult | Adolescent | Adult | Adolescent | Adult | Adolescent | ||||||
| Light-dark box | Light-side entries | 78.72 ± 4.77 | 58.58 ± 3.19* | 77.07 ± 4.68 | 69.03 ± 6.06 | 119.78 ± 2.92 | 95.27 ± 6.75* | 58.46 ± 7.28 | 60.39 ± 3.82 | ||||
| Time spent on light side (sec) | 79.07 ± 3.76 | 75.19 ± 8.94 | 91.11 ± 7.59 | 87.57 ± 5.52 | 98.34 ± 2.45 | 98.12 ± 4.63 | 58.87 ± 6.78 | 72.43 ± 6.17 | |||||
| Latency to first light-side entry (sec) | 107.55 ± 15.93 | 163.25 ± 14.43* | 135.19 ± 21.14 | 123.46 ± 23.25 | 130.06 ± 9.68 | 100.52 ± 11.62 | 152.50 ± 13.30 | 139.04 ± 18.10 | |||||
| Marble burying | Marbles buried | 192.31 ± 40.13 | 90.91 ± 37.14* | 130.77 ± 30.44 | 92.31 ± 37.68 | 7.69 ± 14.01 | 20.98 ± 14.99 | 253.85 ± 59.64 | 97.90 ± 29.50* | ||||
| Time spent burying (sec) | 205.16 ± 66.92 | 86.64 ± 15.73* | 192.53 ± 36.62 | 74.87 ± 19.45 | 32.23 ± 6.52 | 22.05 ± 7.87 | 172.48 ± 35.25 | 142.05 ± 25.84 | |||||
| Latency to begin burying (sec) | 33.81 ± 3.96 | 66.71 ± 12.67 | 50.18 ± 8.19 | 73.73 ± 13.06 | 114.27 ± 32.74 | 143.63 ± 50.11 | 42.48 ± 7.25 | 43.51 ± 3.83 | |||||
| Forced swim test | Immobile episodes | 72.53 ± 8.18 | 121.41 ± 11.91* | 65.93 ± 7.05 | 119.78 ± 6.59* | 66.43 ± 7.61 | 98.90 ± 11.49* | 66.42 ± 7.60 | 45.45 ± 4.92 | ||||
| Time spent immobile (sec) | 65.39 ± 3.66 | 105.55 ± 13.99* | 58.72 ± 8.16 | 82.37 ± 17.76 | 105.94 ± 13.14 | 89.73 ± 9.99 | 31.79 ± 4.11 | 49.86 ± 4.37 | |||||
| Latency to first immobility (sec) | 108.78 ± 5.95 | 67.54 ± 12.53* | 120.49 ± 10.44 | 62.97 ± 7.58* | 115.93 ± 12.92 | 98.93 ± 15.63 | 157.05 ± 20.05 | 180.59 ± 18.54 | |||||
p < 0.05
3.4.1. Light-dark box
There was a significant age X treatment interaction in the number of light-side entries [F(3.72) = 2.74, p= 0.049]. Among vehicle-treated animals, adolescents exhibited fewer light-side entries overall compared to adults (p= 0.011). Among 30 mg/kg MTEP-treated animals, adults exhibited more light-side entries than adolescents (p= 0.002). There was a significant treatment effect on the total time spent on the light side [F(3.74) = 17.83, p < 0.001]. Vehicle-treated animals spent less time on the light side compared to 30 mg/kg MTEP (p= 0.001), with a similar trend for 3 mg/kg MTEP (p= 0.05). CDPPB-treated animals spent less time on the light side compared to both 3 and 30 mg/kg MTEP-treated animals (p’s < 0.001). Although the age X treatment interaction did not quite reach significance [F(3.80) = 2.45, p= 0.071], adolescent vehicle-treated animals had a longer latency to first light-side entry compared to adult vehicle-treated animals (p= 0.027).
3.4.2. Marble-Burying
There was a significant age X treatment interaction in the number of marbles buried [F(3.74) = 2.75, p = 0.048]. Among vehicle-treated animals, adults buried more marbles than adolescents (p= 0.043). Among CDPPB-treated animals, adult mice buried more marbles compared to adolescents (p = 0.002). There was a significant age effect showing that adults spent more time marble burying compared to adolescents [F(1.76) = 9.58, p= 0.003]. Among vehicle-treated animals, adults buried more marbles than adolescents (p= 0.012). There was also a significant treatment effect [F(2.58) = 16.42, p < 0.001] on the time spent burying. Overall, 30 mg/kg MTEP-treated animals spent less time burying compared to both MTEP3 and CDPPB (p= 0.001 and p < 0.001, respectively). There was a significant treatment effect in the latency to begin burying [F(3.69) = 7.38, p < 0.001]. 30 mg/kg MTEP-treated animals had a longer latency compared to vehicle, 3 mg/ kg MTEP, and CDPPB (p < 0.001, p= 0.002 and p < 0.001, respectively).
3.4.3. Porsolt forced swim test
There was an age X treatment interaction in the number of immobile episodes in the FST [F(3.75) = 8.64, p < 0.001]. Among vehicle-treated animals, adolescents had more immobile episodes than adults (p < 0.001). In both 3 and 30 mg/kg MTEP-treated animals, adolescents exhibited more immobile episodes than adults (p < 0.001 and p = 0.007, respectively). In CDPPB-treated animals, there was a negative trend towards fewer immobile episodes in adolescents compared to adults (p = 0.07). There was an age X treatment interaction in the time spent immobile in the FST [F(3.70) = 2.74, p = 0.049]. Among vehicle-treated animals, adolescents spent more time immobile than adults (p= 0.009). There was an age X treatment interaction in the latency to first immobility [F(3.70) = 2.74, p = 0.027]. Among vehicle-treated animals, adolescents had a shorter latency than adults (p= 0.043). In 3 mg/kg MTEP-treated animals, adolescents had a shorter latency than adults (p= 0.004).
4. Discussion
Consistent with a recent study by our group (Lee et al., 2017a), our 2-week, 3-bottle, binge drinking protocol was sufficient to elicit high voluntary alcohol consumption, with both adult and adolescent animals drinking at ‘binge’ levels (BACs ≥80 mg/dl). Also consistent with the literature (Doremus et al., 2005; Spear and Varlinskaya, 2005; SAMHSA, 2008; Vetter et al., 2007), including prior work from our group (Lee et al., 2016, 2017b), adolescent animals consumed significantly higher quantities of alcohol compared to adult drinkers across the 14-day drinking period. As observed in our previous work (Lee et al., 2016), despite the high alcohol intake, adolescent mice were resilient to withdrawal-induced behavioral dysfunction during early withdrawal, while adult animals exhibited behavioral signs of hyper-anxiety. Importantly, the results of the present study extend prior evidence for an age-related difference in the up-regulation of mGlu5 expression by a prior binge-drinking history (Lee et al., 2016) by demonstrating a causal role for mGlu5 signaling in the manifestation of withdrawal-induced anxiety in adult, but not adolescent, binge drinkers.
4.1. MGlu5 activity is necessary for alcohol withdrawal-induced anxiety in adult-onset binge-drinkers
A 2-week binge-drinking experience is sufficient to increase anxiety–like behavior in adult mice during early (24 h) alcohol withdrawal across various paradigms, including the FST, light-dark box and marble-burying tests (Lee et al., 2017b; present study) and the manifestation of this hyper-anxious state is associated with elevated mGlu5 expression, at least within the AchSh (Lee et al., 2016). Herein, MTEP pretreatment dose-dependently reduced withdrawal-induced anxiety in adult-onset binge drinkers, as indicated by increased light-side time and entries, reduced marble-burying and increased latency to start burying, as well as increased time spent immobile in the FST. In all cases, the 30 mg/kg MTEP dose lowered the expression of anxiety-like behavior to the level of water controls, indicating a reversal of the alcohol-withdrawn hyper-anxious phenotype.
Although immobility in the FST is traditionally interpreted as reflecting behavioral despair (Porsolt et al., 1977; Porsolt et al., 2001), alcohol-withdrawn adult mice consistently exhibit reduced immobility in this assay (Lee et al., 2015, 2016, 2017a,b). We demonstrated previously that this behavioral reaction can be reversed by anxiolytic pretreatment (including 3 mg/kg MTEP), arguing that it reflects an anxiety-like response to the swim stressor (Lee et al., 2017a). However, the 3 mg/kg MTEP dose was ineffective at attenuating any signs of anxiety-like behavior expressed by the alcohol-withdrawn adults in the present study, including those expressed during the FST. One possible explanation for the discrepancy in FST findings between our reports may relate to the timing of MTEP pretreatment as FST testing occurred approximately 1.5 h post-injection in the present study (as FST was the last assay in the battery), while FST testing occurred 30 min post-injection in our earlier work (FST was the only assay) (Lee et al., 2017a). Thus, the possibility exists that the anxiolytic effectiveness of MTEP in the FST may have dissipated over the course of the longer pretreatment interval or may have been occluded by the prior behavioral testing of the mice in the present study. This being said, the 3 mg/kg MTEP dose was also ineffective at reducing withdrawal-induced anxiety-like behavior in the light-dark box and the marble-burying assays that preceded FST testing in the adult mice of the present study.
While the precise factor(s) contributing to the differential sensitivity of the alcohol-withdrawn adult mice to low-dose MTEP between our prior and present work remain to be fully elucidated, the present results nevertheless indicate that a higher MTEP dose effectively reduces hyper-anxiety expressed by alcohol-withdrawn adult mice across several assays. Thus, it would appear from the present results that early alcohol withdrawal produces a right-ward shift in the dose-response function for MTEP-induced anxiolysis − an interpretation consistent with the fact that a prior 2-week history of binge-drinking up-regulates mGlu5 expression within certain extended amygdala structures (Lee et al., 2016, 2017b). Pending replication, these findings may have important ramifications for the therapeutic application of MTEP, or related mGlu5 NAMs, for the treatment of the negative reinforcing properties of alcohol that drive excessive intake.
4.2. MTEP-induced anxiolysis exhibits an inverted U-shaped dose-response function in alcohol-naïve adult mice
The 3 mg/kg MTEP dose was robustly anxiogenic in adult water drinkers, while 30 mg/kg MTEP was a less effective anxiolytic in these animals. Indeed, the anxiolytic effects of certain group 1 mGlu antagonists are reported to exhibit an inverted U-shape dose-response curve in alcohol-naïve animals (Belozertseva et al., 2007; Busse et al., 2004; Koltunowska et al., 2013; Varty et al., 2005). Thus, the lower efficacy of the 30 mg/kg MTEP dose in water controls argues that this dose lies on the descending limb of the “therapeutic”/anxiolytic dose-response function in alcohol-naïve animals. The fact that the 30 mg/kg dose exerted anxiolytic effects in alcohol-withdrawn adults supports the notion that early withdrawal from adult-onset binge-drinking shifts the dose-response function for MTEP-induced anxiolysis to the right of alcohol-naïve animals and future work should examine more directly how withdrawal-induced changes in MTEP sensitivity relate to receptor expression and function within brain regions regulating emotional reactivity.
4.3. MGlu5 activity is not necessary for the manifestation of anxiety in adolescent mice
The expression of mGlu5 isoforms is developmentally regulated at both the mRNA and protein levels, with levels declining significantly throughout the brain from the juvenile to the adult phases of development (e.g., Romano et al., 1996, 2002). In our own hands, alcohol-naïve adolescent B6 mice tend toward higher basal mGlu5 monomer expression within the AcbSh than adults (Lee et al., 2016), although adolescent-onset binge-drinking does not upregulate mGlu5 expression within extended amygdala structures in early withdrawal as it does in adult mice (Lee et al., 2016, 2017b). Given the latter observation, it was not surprising that MTEP exerted comparable effects upon the anxiety-related behavior expressed by alcohol-naïve and −experienced adolescents. However, compared to adult animals, neither dose of MTEP was particularly effective at altering anxiety-like behavior in adolescent animals, irrespective of their alcohol experience. Even the lower 3 mg/ kg MTEP dose (which is an effective anxiolytic in alcohol-naïve adult mice) only increased in light-side time in adolescent animals. The relatively weak effect of MTEP in adolescent vs. adult mice argues that while mGlu5 expression may be higher in younger versus older animals, receptor signaling may be less efficient in younger animals and thereby occlude the detection of an MTEP effect. Alternatively, the possibility exists that the biomolecular mediators of anxiety vary with development and that anxiety is independent of mGlu5 signaling in adolescent animals. Unfortunately, the limited number of MTEP and CDPPB doses employed in this study precludes any firm conclusions in either regard. However, given that affective disorders typically manifest during adolescence/young adulthood, the present behavioral data raise the possibility of age-dependent differences in the anxiolytic effectiveness of MTEP of potential relevance for treating anxiety disorders during the adolescent phase of development.
4.4. MGlu5 activity is not sufficient for alcohol withdrawal-Induced anxiety in either adult-or adolescent-onset binge-drinkers
mGlu5 receptor expression is up-regulated within extended amygdala structures by a prior history of voluntary alcohol intake (Cozzoli et al., 2009, 2012, 2014; Lee et al., 2016; Obara et al., 2009; Szumlinski et al., 2008) but this effect depends upon the age of drinking-onset (Lee et al., 2016, 2017b). Thus, we hypothesized that if mGlu5 hyperactivity is sufficient to drive alcohol withdrawal-induced negative affect, then treatment with a positive allosteric modulator should augment anxiety expressed by both alcohol-naïve and −experienced mice, possibility irrespective of age. Indeed, CDPPB was modestly anxiogenic in both adult and adolescent animals. In adults, CDPPB decreased light-side time and entries, irrespective of alcohol experience, and also decreased immobility across various factors in the FST, although no CDPPB effects were observed in the marble burying test. In adolescents, CDPPB pretreatment increased the total time spent marble-burying, irrespective of alcohol experience, and increased the number of marbles buried and reduced time immobile in water controls. Taken together, these data do not argue strongly in favor of a major role for mGlu5 stimulation in regulating basal anxiety or for a binge alcohol-induced increase in mGlu5 signaling (Lee et al., 2016, 2017b) as the sole mediator of withdrawal-induced anxiety. Furthering this idea, the anxiety exhibited by adult alcohol-naïve animals treated with CDPPB was still less than that exhibited by alcohol-drinking controls infused with vehicle. Not-withstanding a full dose-response analysis of CDPPB’s anxiolytic effects, it would appear from our behavioral pharmacological data that increased mGlu5 expression/function is necessary, but not sufficient, to mediate withdrawal-induced anxiety in mice. It would be important in future work to more fully characterize the dose-response function for CDPPB-induced anxiogenesis, particularly as it relates to alcohol-induced changes in mGlu5 expression/function within neurocircuits regulating emotional reactivity.
5. Conclusions
We have shown previously that binge alcohol-drinking during adulthood results in mGlu5 upregulation. The present study suggests that this upregulation is causally related to the manifestation of anxiety-like behaviors during early withdrawal in a mouse model of binge-drinking as pharmacologically inhibiting mGlu5 signaling results in a dose-dependent reduction in anxiety-like behaviors. In contrast, adolescent mice with a history of binge-drinking are resistant to both alcohol-induced mGlu5 upregulation and withdrawal-induced anxiety, despite consuming larger quantities of alcohol. Allosteric modulation of mGlu5 signaling produces minimal effects in adolescent animals, independent of prior alcohol experience. These results point to the existence of age-specific underlying mechanisms mediating the ontogeny of anxiety and alcohol withdrawal-induced negative affect. As it relates to the human conditions, this study highlights the importance of age-specific considerations in the pharmacotherapeutic treatment of both anxiety and substance abuse disorders in the clinical population.
Acknowledgments
Role of source funding
Funding provided by NIAAA grant AA024044 to KKS and a Graduate Opportunity fellowship from the UCSB Graduate Division to KML.
Footnotes
Conflict of interest
None.
References
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R, 2004. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29, 921–928. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM, 1978. Kindling as a model for alcohol withdrawal syndromes. Br. J. Psychiatry 133, 1–14. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL, 1993. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal kindling. Alcohol. Clin. Exp. Res 17, 94–98. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY, 2007. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol 17, 172–179. [DOI] [PubMed] [Google Scholar]
- Bergink V, van Megen HJGM, Westenberg HGM, 2004. Glutamate and anxiety. Eur. Neuropsychopharmacol 14, 175–183. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, 2003. The mouse light/dark box test. Eur. J. Pharmacol 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND, 2004. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29, 1971–1979. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Fact Sheets - Underage Drinking http://www.cdc.gov/alcohol/fact-sheets/underage-drinking.htm. [Google Scholar]
- Carrington CD, Ellinwood EH Jr., Krishnan RR, 1984. Effects of single and repeated alcohol withdrawal on kindling. Biol. Psychiatry 19, 525–537. [PubMed] [Google Scholar]
- Cleva RM, Olive MF, 2012. mGlu receptors and drug addiction. Wiley Interdiscip. Rev. Membr. Transp. Signal 1, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW, 2009. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK, 2009. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci 29, 8655–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK, 2012. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol. Clin. Exp. Res 36, 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK, 2014. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdale. Neuropsychopharmacology 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, 1985. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev 9, 37–44. [DOI] [PubMed] [Google Scholar]
- De Witte P, Littleton J, Parot P, Koob G, 2005. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs 19, 517–537. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP, 2003. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol. Biochem. Behav 75, 411–418. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP, 2005. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol. Clin. Exp. Res 29, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan M, Le Poul E, Rocher JP, 2007. Allosteric modulation: a novel approach to drug discovery. Innov. Pharm. Technol 24, 22–26. [Google Scholar]
- Gass JT, Olive MF, 2009. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl.) 204, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M, 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM, 2002. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD: suggesting a novel site of action at metabotropic glutamate receptors. Alcohol. Clin. Exp. Res 26, 1779–1793. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP, 2006. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl.) 183, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH, 2013. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl.) 229, 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA, 2011. Persistent escalation of alcohol drinking in C57BL/6: J mice with intermittent access to 20% ethanol. Alcohol. Clin. Exp. Res 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H, 2009. Glutamate transmission in addiction. Neuropharmacology 56 (Suppl. 1), 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL Jr., 2005. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther 313, 199–206. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Tatarczynska E, Chojnacka-Wojcik E, Nowak G, Cosford ND, Pilc A, 2004. Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABA(A) signaling. Neuropharmacology 47, 342–350. [DOI] [PubMed] [Google Scholar]
- Koltunowska D, Gibula-Bruzda E, Kotlinska JH, 2013. The influence of ionotropic and metabotropic glutamate receptor ligands on anxiety-like effect of amphetamine withdrawal in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 242–249. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2003. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. Neuropsychopharmacol 13, 442–452. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M, 2008. The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur. J. Pharmacol 598, 57–63. [DOI] [PubMed] [Google Scholar]
- Kumar J, Hapidin H, Bee YT, Ismail Z, 2013. Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav. Brain Funct 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Ko J, Chow S, Quan B, 1994. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol. Biochem. Behav 47, 375–378. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK, 2015. Binge alcohol drinking elicits persistent negative affect in mice. Behav. Brain Res 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK, 2016. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front. Cell. Neurosci 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Bocz MD, Szumlinski KK, 2017a. Anxiolytic effects of buspirone and MTEP in the porsolt forced swim test. Chronic Stress 10.1177/2470547017712985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Solton NR, Szumlinski KK, 2017b. Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not, adult mice. Front. Psychol 8, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O’Brien J, Lemaire A, Williams W, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR, 2004. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1, 3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem 47, 5825–5828. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK, 2006. Behavioral and neurochemical interactionsbetween Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Dep 85, 142–156. [DOI] [PubMed] [Google Scholar]
- Lou ZZ, Chen LH, Liu HF, Ruan LM, Zhou WH, 2014. Blockade of mGluR5 in the nucleus accumbens shell but not core attenuates heroin seeking behavior in rats. Acta Pharmacol. Sin 35, 1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J, 2008. Acamprosate: recent findings and future research directions. Alcohol. Clin. Exp. Res 32, 1105–1110. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL, 2005. Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol 40, 494–497. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2004. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter, pp. 3.
- National Institute on Alcohol Abuse and Alcoholism, 2017. Underage Drinking.
- Nicolas LB, Kolb Y, Prinssen EP, 2006. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol 547, 106–115. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK, 2009. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res 33, 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T, 1982. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol 2, 129–133. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M, 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther 229, 327–336. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S, 2001. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci 8, 10A. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V, Malherbe P, 2005. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther 315, 711–721. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Romano C, van den Pol AN, O’Malley KL, 1996. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA splice variants, and regional distribution. J. Comp. Neurol 367, 403–412. [DOI] [PubMed] [Google Scholar]
- Romano C, Smout S, Miller JK, O’Malley KL, 2002. Developmental regulation of metabotropic glutamate receptor 5b protein in rodent brain. Neuroscience 111, 693–698. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2008. The National Survey on Drug Use and Health Report — Quantity and Frequency of Alcohol Use Among Underage Drinkers Office of Applied Statistics, Rockville, MD. [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW, 2005. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl.) 179, 262–270. [DOI] [PubMed] [Google Scholar]
- Simon AB, Gorman JM, 2006. Advances in the treatment of anxiety: targeting glutamate. NeuroRx 3, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT, 2012. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol. Biochem. Behav 101, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI, 2005. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev. Alcohol 17, 143–159. [PubMed] [Google Scholar]
- Spear LP, 2000. Modeling adolescent development and alcohol use in animals. Alcohol Res. Health 24, 115–123. [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD, 2005. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov 4, 131–U134. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE, 2008. Accumbens Homer2over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology 33, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Gastfriend DR, Coyle JT, 1995. The glutamatergic basis of human alcoholism. Am. J. Psychiatry 152, 332–340. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2004. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol. Clin. Exp. Res 28, 40–50. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R, 2005. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl.) 179, 207–217. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP, 2007. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol. Clin. Exp. Res 31, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS, 2002. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol. Biochem. Behav 73, 673–677. [DOI] [PubMed] [Google Scholar]


