Abstract
A diagnostic criterion for drug addiction, persistent drug-craving continues to be the most treatment-resistant aspect of addiction that maintains the chronic, relapsing, nature of this disease. Despite the high prevalence of psychomotor stimulant addiction, there currently exists no FDA-approved medication for craving reduction. In good part, this reflects our lack of understanding of the neurobiological underpinnings of drug-craving. In humans, cue-elicited drug-craving is associated with the hyperexcitability of prefrontal cortical regions. Rodent models of cocaine addiction indicate that a history of excessive cocaine-taking impacts excitatory glutamate signaling within the prefrontal cortex to drive drug-seeking behavior during protracted withdrawal. This review summarizes evidence that the capacity of cocaine-associated cues to augment craving in highly drug-experienced rats relates to a withdrawal-dependent incubation of glutamate release within prelimbic cortex. We discuss how stimulation of mGlu1/5 receptors increases the activational state of both canonical and noncanonical intracellular signaling pathways and present a theoretical molecular model in which the activation of several kinase effectors, including protein kinase C, extracellular signal-regulated kinase and phosphoinositide 3-kinase (PI3K) might lead to receptor desensitization to account for persistent cocaine-craving during protracted withdrawal. Finally, this review discusses the potential for existing, FDA-approved, pharmacotherapeutic agents that target kinase function as a novel approach to craving intervention in cocaine addiction.
Keywords: cocaine, craving, ERK, extinction, glutamate, incubation, mGlu1, mGlu5, PKC, prefrontal cortex
1 |. INTRODUCTION
Persistent drug-craving continues to be the most treatment-resistant aspect of addiction that maintains the chronic, relapsing, nature of this disease. Although baseline craving (ie, craving in the absence of any overt exteroceptive stimuli) dissipates in a time-dependent manner during withdrawal from a drug (a.k.a., abstinence), the propensity for drug-associated cues and/or contexts to elicit drug-craving increases with the passage of time during withdrawal. This intensification of cue-elicited drug-seeking is observed in both humans suffering from substance use disorders and laboratory animal models of these disorders.1–4 The so-called incubation of cue-elicited drug-craving (hereafter referred to as “incubated craving”) was first described in cocaine-addicted humans,5 but has since been reported in humans addicted to nicotine,6 methamphetamine,7 heroin8 and alcohol.9 As a time-dependent increase in cue reactivity during withdrawal from drug could theoretically account for the chronic, relapsing nature of addiction, there has been a very concerted basic science research effort to understand the biobehavioral underpinnings of incubated craving.
This research effort was instigated by the seminal studies of Tran-Nguyen et al10 and Grimm et al11 using rat models of cocaine addiction, in which the presentation of cocaine-associated cues elicited greater “cocaine-craving” (operationally defined as conditioned operant responding or “drug-seeking behavior” in the absence of drug) during protracted withdrawal (ie, >30 days) than that observed in early (eg, 1 day) withdrawal. These basic behavioral findings regarding the incubation of cue-elicited cocaine-seeking have now been replicated over the past 19 years by many laboratories, using different animal species and procedural variations.1–3,12,13 Further, incubated craving has been now been modeled in animals with histories of methamphetamine,1–3,14–20 nicotine,21,22 alcohol23 and opioid24–28 self-administration. Typically, these incubation models involve a period of drug-taking in which rodents are trained to emit an operant response to receive access to drug (usually via an intravenous route of administration) and each drug delivery is signaled by the presentation of neutral cues (eg, tones and lights) that come to serve as conditioned reinforcers over the course of self-administration training. Following the drug-taking phase of the study, the animals undergo “forced abstinence” during which animals remain in the home cage away from drug and the drug-associated context/ cues for a designated period of time (the “withdrawal period” or “forced abstinence period”). Although there are procedural variations,1–3,12,13 testing for incubated craving typically involves a single re-exposure to the drug-associated context, during which time operant responding results in the presentation of the drug-associated stimuli, but no drug delivery (ie, testing is conducted under extinction conditions). It is in these latter regards that the “classic” incubation of craving model differs from another popular model of cue-elicited drug-seeking—the extinction-reinstatement model. In the typical extinction-reinstatement model, animals are re-exposed repeatedly to the drug-associated context (quite often in the absence of drug-paired discrete cues) for many days during the drug abstinence period and responding progressively declines over time as animals undergo extinction learning. Upon reaching a particular extinction criterion, responding is then re-invigorated by re-exposure to the discrete drug-associated cues, a stressor and/or the drug itself.29
Notably, incubated craving is not specific to drugs of abuse as it is observed also in rodent models of sucrose- or saccharin taking,30–36 suggesting that the neural processes underlying incubated craving may be shared across drug and nondrug reinforcers. Interestingly, at least in rat models of cocaine-seeking, incubated craving is accompanied by a withdrawal-related impairment in the ability of the cocaine-abstinent rats to learn to suppress cue-elicited drug-seeking behavior (a.k.a. extinction learning).37,38 This withdrawal-dependent impairment in extinction learning is reflected as perseverative drug-seeking behavior, even in the face of repeated re-exposure to drug-associated cues/contexts—a neurocognitive impairment theorized to contribute significantly to relapse vulnerability, particularly during late withdrawal when all signs of physical dependence have subsided.37
As highlighted previously,1–4,39–41 researching the mechanisms underpinning both incubated drug-craving and drug-related extinction learning is critical for understanding the neurobiological bases of the chronic, relapsing, nature of addiction. This review describes recent advances in our understanding of the role played by the excitatory amino acid neurotransmitter glutamate within the ventromedial aspect of the prefrontal cortex (vmPFC) in both of these craving-related phenomena and builds a hypothetical molecular model to account for incubated cocaine-craving, of relevance to the appropriate temporal targeting of cocaine addiction-related treatment strategies.
2 |. DRUG-CRAVING IS ASSOCIATED WITH ANOMALOUS PFC FUNCTION
The PFC commonly refers to the anterior pole of the mammalian brain and in humans.42 Comprised of functionally heterogeneous subregions, the PFC is considered, very generally, to be critical for purposive actions and reasoning, particularly in the integration of cortical and subcortical inputs to instigate, modify and inhibit goal-directed behavior. Generally, the major PFC functions include: planning, decision-making and executive attention, the latter of which is comprised of working memory (active memory), preparatory set (priming of sensory or motor regions for action) and inhibitory interference control (suppression of internal or external influences upon current action).43 Thus, damage to the PFC or PFC dysfunction reduces inhibition on sensory association cortices and the underlying reward and motor circuitry, permitting habitual or compulsive behaviors to pre-dominate. Imaging and behavioral studies in humans with substance use disorders indicate morphological or functional anomalies within a number of frontal cortical structures including the anterior cingulate, dorsolateral and orbitofrontal cortex. In addition, the medial aspects of the PFC (mPFC) exhibit both gross morphological anomalies and hypofunctionality,44 and the resultant deficits in executive functioning (including impairments in reversal learning and behavioral flexibility) are theorized to underpin the high levels of drug-craving, -seeking and -taking in this disorder.45,46 Supporting such theories, the severity of cue- or imagery-elicited drug-craving correlates with heightened activation of mPFC subregions in those with substance use disorders.44,47,48 Such findings have led to the testable hypothesis that drug-induced neuroadaptations within mPFC render the addict especially vulnerable to the influence of drug-associated cues over internal motivational states, cognition and behavior, to drive cue-induced craving and relapse.
Indices of abnormal mPFC activation are also observed within the context of the extinction-reinstatement animal model of relapse49 as well as within the incubation of cocaine-seeking model of craving.50–52 Although neuronal activity within the more dorsomedial aspect of the PFC (dmPFC; anterior cingulate and dorsal prelimbic cortex; see Figure 1) appears to be critical for drug-seeking behavior within the context of drug-, stress- and cue-primed reinstatement of drug-seeking,54,55 inactivation of the ventromedial aspect of the PFC (vmPFC; which in our rat studies includes the ventral prelimbic and infralimbic cortices in rat; see Figure 1) abolishes incubated cocaine-craving.50 Further, optogenetic data indicate a more critical role for the prelimbic cortical projections to the nucleus accumbens core in this regard.56 Moreover, incubated cocaine-craving in rats is not affected by pharmacological disruption of neuronal activity within the dmPFC.50 These results are consistent with the evidence that neurons within the prelimbic cortex, but not other PFC subregions, exhibit a cocaine withdrawal-dependent strengthening of their encoding of cocaine-associated stimuli,57 and that cue-elicited activation of prelimbic cortex (indexed by kinase activity) increases as a function of the time during cocaine abstinence.50,51 Such findings argue that withdrawal-dependent prelimbic hyperactivity invigorates cocaine-seeking in response to cue-elicited internal motivational states to drive incubated cocaine-seeking behavior during protracted withdrawal.
FIGURE 1.
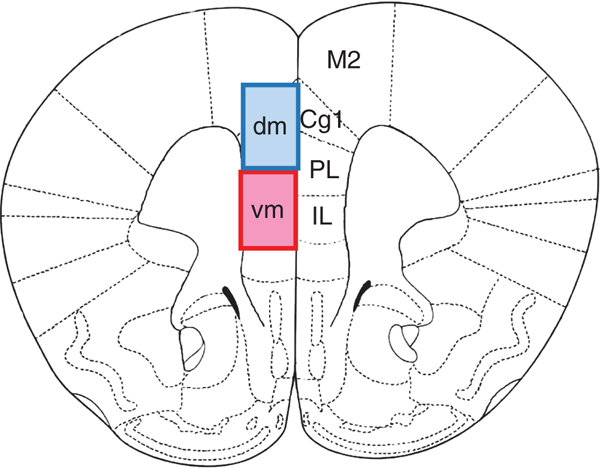
Schematic of the subregions and dorsal-ventral subdivision of the medial PFC discussed in this review. Coronal section through the medial PFC (mPFC) of the rat brain derived from the rat brain atlas of Paxinos and Watson,53 highlighting the location of the infralimbic (IL), prelimbic (PL), anterior cingulate cortex (Cg1) and secondary motor cortex (M2). In the rodent literature, the mPFC is sometimes subdivided along the ventral-dorsal plane into a dorsomedial subregion (dmPFC), which includes Cg1 and the more dorsal aspect of the PL, and a ventromedical subregions (vmPFC), which includes the more ventral aspect of the PL and the IL
3 |. THE GLUTAMATERGIC BASIS OF COCAINE-CRAVING
The principle excitatory neurotransmitter of the pyramidal efferents from the cortex is glutamate and, thus, glutamate is also the principle neurotransmitter of homo- and heterotopical cortical afferents to mPFC. Several thalamic nuclei, as well as the hippocampus, send dense glutamatergic projections to mPFC subregions and this neuro- anatomical organization well-positions glutamate as the prime driver of cue-elicited prelimbic cortex hyperexcitability in drug-craving individuals.58 Thus, we posited that time-dependent changes in glutamate-dependent activation of prelimbic cortex are likely involved in the incubation of drug-craving and perhaps also the extinction learning deficits that occur during protracted abstinence. To date, we have addressed this hypothesis within the context of incubated cocaine-seeking as discussed below.
Glutamate activates 3 subtypes of cation-gating inotropic receptors that include the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate (NMDA) and kainate receptors. In addition, glutamate stimulates 8 different subtypes of metabotropic glutamate (mGlu) receptors that differentially influence cellular function based on their synaptic localization and coupling to intracellular effectors.59 Although over a decade of basic science research has studied cocaine-induced dysregulation of corticostriatal glutamate transmission for behavioral sensitization, drug/cue/stress-induced relapse and incubation of cocaine-craving,13,60–62 there has been relatively little published research characterizing how abstinence from a history of cocaine-taking impacts biochemical indices of glutamate transmission within the mPFC proper. However, while some discrepancy exists in the literature, many studies have reported that repeated, experimenter-administered cocaine injections increase indices of mPFC glutamate, including extracellular glutamate content, glutamate receptor expression and the regulation of extracellular glutamate by Gαq/11-coupled mGlu1/5 and Gαi/o-coupled mGlu2/3 receptors.63–66 Importantly, when examined, the cocaine injection-induced changes in mPFC glutamate tend to manifest as a function of time following the removal of drug.65 Yet, how these glutamate-related biochemical adaptations arise, whether or not they are interrelated and how they influence psychomotor reactivity to cocaine-conditioned stimuli of relevance to cocaine-craving remain to be determined.
In contrast, the majority of studies employing animal models of intravenous cocaine self-administration indicate that a cocaine history blunts mPFC glutamate function, in a manner consistent with the reported hypofrontality in humans suffering from cocaine use disorder discussed above. For example, rats with a history of cocaine self-administration exhibit lowered extracellular glutamate content within the prelimbic cortex of the mPFC when assayed at 24 hours withdrawal and the magnitude of this reduction increases as a function of the amount of cocaine self-administered.67 Such data are consistent with the hypothesis that a cocaine-dependent decline in mPFC extracellular glutamate might drive “uncontrollable” cocaine-taking. However, the immunoblotting work to date reveals little overlap in the effects of cocaine-taking upon extracellular glutamate levels and the protein expression of Group 1 mGlu receptors and NMDA receptor (NMDAR) subunits.68 Further, although rats with an extensive cocaine-taking history exhibit an impairment in the capacity of the drug to lower extracellular glutamate within the prelimbic cortex in early withdrawal67 and a blunting of mGlu2/3-dependent long-term depression within neurons of prelimbic cortex also in early withdrawal,69 there is little evidence that cocaine self-administration alters the protein expression of mGlu2/ 3 receptors within vmPFC during either early or later withdrawal.70 Thus, although intact vmPFC function is required for extinction of both appetitive and aversive conditioning40 and mGlu1/5 receptor activation, in particular, is important for cocaine-related extinction learning,71 the large majority of animal studies examining for changes in mPFC glutamate receptor expression following a history of intravenous cocaine self-administration have yielded negative results.37,68,72,73
4 |. CUE-INDUCED GLUTAMATE ADAPTATIONS WITHIN vmPFC OF RELEVANCE TO INCUBATED COCAINE-CRAVING
It is notable, however, that in all of the abovementioned reports, rats were not re-exposed to the cocaine self-administration environment or to discrete cues associated with cocaine-taking/availability prior to tissue collection for protein assessment during withdrawal from cocaine. However, such re-exposure appears to be an important determinant of the manifestation of receptor protein changes, at least in the case of mGlu1/5 receptors. For example, a study by Ghasemzadeh et al72 failed to observed changes in vmPFC expression of mGlu1/5 when cocaine-experienced rats had undergone extensive extinction training over the course of 10 days following withdrawal from cocaine. Interestingly, a study employing a similar cocaine self-administration protocol, following which rats underwent only a single 2-hour extinction session in a test for incubated cocaine-craving, reported reduced mGlu1/5 expression within vmPFC, but only in rats re-exposed to cocaine-associated stimuli and allowed to engage in cue-reinforced responding during protracted (ie, 30 days) withdrawal.37 Moreover, a very strong inverse correlation exists between the magnitude of the cue-reinforced cocaine-seeking and vmPFC expression of mGlu1/5.51 In contrast, no changes in vmPFC mGlu1/5 expression were observed in rats with equivalent cocaine self-administration experience but left undisturbed in the home cage prior to tissue collection.37 Thus, for mGlu1/5 (and perhaps other glutamate receptors), the regulation of protein expression within vmPFC is complex, involving interactions between cocaine self-administration history, the duration of time following withdrawal from cocaine and the number of times animals are re-exposed to cocaine-associated stimuli and/or afforded the opportunity to engage in cue-reinforced behavior in the absence of the drug.
Despite the strong inverse relationship exists between vmPFC mGlu1/5 expression and the manifestation of cocaine-seeking,51 pharmacological manipulation of mGlu1/5 function within neither vmPFC nor dmPFC influences cue-reinforced responding when rats are tested immediately postinfusion for cue-reinforced responding during either early or later withdrawal from cocaine.37 These findings argue that mGlu1/5 function within vmPFC does not actively regulate cue-reinforced cocaine-seeking per se. However, when the same rats are assayed for cue-reinforced cocaine-seeking the day following microinfusion, prior mGlu1/5 inhibition blunts the extinction learning that is manifested by the rats in early withdrawal.37 The extinction impairment induced in cocaine-experienced rats by intra-vmPFC mGlu1/5 antagonist infusion during early withdrawal from cocaine appears to reflect interference with extinction memory consolidation as the carry-over effects of intra-vmPFC mGlu1/5 antagonist microinjection are comparable in rats infused immediately prior to or immediately following the initial test for cue-reinforced cocaine-seeking.37 Conversely, stimulating vmPFC mGlu1/5 function via a local agonist infusion facilitates extinction learning when rats in protracted withdrawal are tested for drug-seeking behavior the day following microinfusion.37 These neuropharmacological results demonstrate a necessary and active role for decreased vmPFC mGlu/5 receptor function in regulating the extinction impairment observed during protracted withdrawal from cocaine and are reminiscent of findings from the fear-conditioning literature.74 Together, such data argue an important role for intact vmPFC mGlu1/5 in the general mechanisms of inhibitory learning, although it remains to be determined whether or not the observed effects of vmPFC mGlu1/5 manipulations37,74 generalize to other addictive substances. Nevertheless, Group 1 mGlu receptor stimulation can augment inhibitory currents within PFC to a greater degree than excitatory currents,75 arguing that the time- and learning-dependent reduction in vmPFC mGlu1/5 function/expression reported in incubated rats37 may disinhibit glutamatergic output from the vmPFC to instigate a feedforward mechanism of neuronal hyperexcitability that promotes the saliency of drug-associated cues and high levels of cocaine-craving during protracted abstinence.
As reviewed previously,76 considerable in vivo microdialysis research supports a hyperexcitability of corticostriatal efferents upon cocaine re-exposure (a.k.a. priming), with data derived primarily from studies of cocaine-induced behavioral sensitization and the extinction-reinstatement model of relapse. In contrast, very few reports describe the glutamate cue/context reactivity of corticostriatal pathways in cocaine-experienced animals whose drug-seeking behavior has not been deliberately extinguished via repeated re-exposure to drug-associated stimuli.77–79 However, consistent with the notion that incubated cocaine-craving may reflect a disinhibition of glutamatergic output from the vmPFC, re-exposure to cocaine-associated cues following a brief (1-day) period of drug abstinence is sufficient to elicit a large increase in extracellular glutamate within nucleus accumbens subregions79 and re-exposure to cocaine-associated contexts facilitates cocaine-induced glutamate release within these subregions.77 In more direct support of a time-related dysregulation of extracellular glutamate within the vmPC proper, Shin et al80 examined how responding for neutral (saline-paired), sucrose- and cocaine-paired cues affected vmPFC glutamate levels during early vs later withdrawal (3 vs 30 days, respectively). Interestingly, only cocaine-experienced rats in later withdrawal (ie, rats exhibiting incubated cocaine-seeking) demonstrated a marked cue-elicited rise in vmPFC glutamate. These data provided the first evidence that the cue reactivity of glutamate terminals within the vmPFC incubates during protracted withdrawal. Further, a strong positive correlation existed between the magnitude of cue-induced drug-craving and the amount of glutamate release observed in the behaving rats.80 This correlation strengthens the notion that time-dependent neuroadaptations within glutamate efferents to the vmPFC and the activation of non-mGlu1/5 glutamate receptors might drive the cue-hyper-reactivity of cocaine-incubated rats. In support of this assertion, a recent study revealed that inhibition of vmPFC glutamate release, specifically within the prelimbic cortex, is sufficient to blunt incubated cocaine-seeking during protracted, but not early, withdrawal from cocaine.81 The questions remain as to the identities of the glutamatergic afferents to the prelimbic cortex that undergo plasticity during protracted withdrawal from cocaine and the specific postsynaptic glutamate receptors activated by this time-dependent incubation of cue-elicited glutamate release within vmPFC.
5 |. TARGETING PKCε ACTIVITY TO PREVENT m Glu1/5 DESENSITIZATION AND ASSOCIATED INHIBITORY LEARNING DEFICITS
As discussed above, although the relatively limited neuropharmacological data to date negate a role for reduced mGlu1/5 receptor activation within vmPFC as important for the manifestation of drug-seeking per se, the time-dependent downregulation of these receptors appears to impair inhibitory learning, which we theorize perpetuates high levels of drug-seeking behavior even during protracted withdrawal from cocaine.37 As such, it is important to try to understand the molecular underpinnings of the observed mGlu1/5 downregulation as a failure to learn to inhibit craving (and actions driven by that craving) upon re-exposure to drug-related stimuli obviously poses a massive barrier to addiction recovery.
mGlu1/5 receptors are well-described to undergo rapid desensitization upon stimulation.82,83 The fact that re-exposure to cocaine-associated cues during protracted withdrawal (ie, in rats exhibiting incubated responding) elicits a temporally coincident increase in extracellular glutamate80 and reduction in mGlu1/5 expression37 is suggestive of a causal relation. The rapid desensitization of mGlu1/5 involves receptor phosphorylation by either G-protein receptor kinases or protein kinase C (PKC) isozymes.82,83 In the case of PKC-dependent desensitization, phosphorylation of mGlu5 at Ser839 by the atypical (ie, calcium-independent) PKCε isozyme results in the uncoupling of the receptor from Gαq/11, at least when assayed in cultured cortical astrocytes.82 This result is very interesting based on the large body of literature implicating PKCε, and its activation by mGlu5 stimulation, in the neurobiology of drug addiction.84 Indeed, very recent studies by Miller et al51 revealed a positive correlation between incubated cocaine-craving and PKCε phosphorylation within vmPFC and in support of a potential link between PKCε activity and mGlu1/5 desensitization, PKCε phosphorylation (an index of enzyme priming) was inversely correlated with mGlu1/5 expression within the vmPFC of cocaine-experienced rats.51 Importantly, the transient inhibition of PKCε translocation within the vmPFC not only blunted the manifestation of incubated cocaine-craving in rats tested for incubated craving during protracted cocaine abstinence, but a residual inhibition of responding was observed on a second test conducted the next day.51 Of note, the inhibition of PKCε translocation within vmPFC did not affect drug-seeking behavior when assayed in early withdrawal from cocaine, which may reflect a floor effect upon kinase activity, as cocaine-experienced rats exhibit relatively low levels of phosphorylated PKCε within the vmPFC during early withdrawal from the drug.85
These recent results have led to one hypothetical model (Figure 1) in which incubated cocaine-craving involves a time-dependent increase in the cue reactivity of glutamate terminals within vmPFC—the mechanism(s) for which remain to be explored. This cue-hyper-reactivity, in turn, stimulates the activation of perisynaptic Group 1 mGlu receptors, instigating negative feedback loops involving PKCε activation and the uncoupling of mGlu1/5 from Gαq/11 (and perhaps also the recruitment of GRKs). Consequently, mGlu1/ 5 internalizes and is degraded, resulting in lower protein expression. As the “anti-craving” effects of PKCε inhibition are only apparent when rats are pretreated with the kinase inhibitor 20 minutes prior to the initial test for drug-craving and not immediately prior,51 the PKC-mediated G-protein uncoupling of mGlu1/5 is theorized to impair the acquisition of extinction learning during the initial cue re-exposure, with mGlu1/5 degradation theorized to compound the extinction deficit by impairing the consolidation of this learning.37 Consequently, cocaine-experienced individuals in protracted withdrawal have greater difficulty suppressing craving in response to both the acute and repeated re-exposure to drug-associated cues, and this extinction impairment ultimately renders these individuals highly susceptible to cue-elicited relapse even during protracted abstinence. This hypothetical model poses strategies aimed at either preventing mGlu1/5 desensitization within vmPFC as potentially effective approaches for curbing incubated craving in protracted withdrawal. Consistent with this hypothetical model, inhibiting incubated glutamate release within the vmPFC using mGlu2/3 agonists81 or inhibiting PKC priming51 both blunt incubated responding upon initial cue re-exposure, while pharmacologically stimulating mGlu1/5 activity during that initial cue re-exposure enables normal extinction learning to occur, thereby attenuating cue reactivity upon subsequent drug cue re-exposure.37
6 |. TARGETING ERK ACTIVITY TO PREVENT mGlu1/5 DESENSITIZATION AND ASSOCIATED INHIBITORY LEARNING DEFICITS?
In addition to PKC and various GRKs, mGlu1/5 can be phosphorylated also by a number of other kinases upon the activation of various neurotransmitter receptors, including the D1 dopamine receptor (D1R), the NMDAR and the tyrosine kinase B receptor (TrkB; receptor for brain-derived neurotropic factor or BDNF).86 Interestingly, D1R or TrkB stimulation induces mGlu5 phosphorylation at Thr1123 and Ser1126, which lie within the Homer binding domain of the receptor.86 As reviewed elsewhere,87 Homers constitute a family of scaffolding proteins that are encoded by 3 genes (Homer1–3) and members of this family are critical for mGlu1/5 trafficking and localization, as well as the coupling to intracellular effectors and regulation of various cation channels. During withdrawal from cocaine, the protein expression of the constitutively expressed Homer2 isoform is upregulated within vmPFC and the resultant imbalance between Homer1 and Homer2 isoforms within this region critically regulates cocaine-conditioned reward in mice and cocaine-primed reinstatement of cocaine-seeking in rats.88,89 Proline-directed kinases, including extracellular signal-regulated kinase (ERK), phosphorylate mGlu5 within the Homer binding domain,86 and the phosphorylation of mGlu5(Ser1126) recruits an additional scaffolding protein, Preso1, with the dynamic interaction between this protein triad augmenting the receptor affinity for Homers > 10-fold.86
We argue that the interactions between ERK and mGlu5 (and possibly also mGlu1) within vmPFC are possibly relevant to persistent cocaine-seeking in protracted withdrawal as incubated cocaine-craving is associated with increased ERK activity within vmPFC,50 and glutamate and BDNF release within the terminal regions of corticofugal projections contributes to the incubation of drug-craving.1–3,90,91 Although direct local inhibition of ERK within vmPFC does not alter the magnitude of incubated cocaine-seeking,50 the role for ERK and ERK-dependent phosphorylation of Group 1 mGlu receptors in the extinction resistance that accompanies incubated craving has not yet been explored. Thus, we propose another hypothetical model (Figure 2) relating time-dependent changes in cue-elicited BDNF and/or glutamate release within the vmPFC to the strengthening of mGlu1/5-Homer-ERK interactions facilitating mGlu1/5 desensitization by both homologous and heterologous mechanisms. More specifically, incubated glutamate release and/or the recruitment of BDNF/TrkB activation within the vmPFC can stimulate mitogen-activated protein kinase (MAPK) signaling either directly or indirectly through activation of a variety of second or third messenger systems, resulting in increased ERK activity.92,93 This hyperactivated ERK can then phosphorylate mGlu1/5 within the Homer binding domain via an interaction with Preso1,86 increasing both the affinity of the receptors for constitutively expressed Homer proteins86 and the probability of receptor desensitization.94 This probability of receptor desensitization is increased during protracted drug withdrawal secondary to the elevated expression of constitutively expressed Homer expression within vmPFC.95 Alternatively, it is conceivable that hyperphosphorylation of mGlu1/5 by ERK, PKCs and/or other kinases, including PI3K96 might instigate GRK/arrestin pathways for mGlu1/5 internalization and degradation, leading to neurocognitive dysfunction (Figure 3).
FIGURE 2.
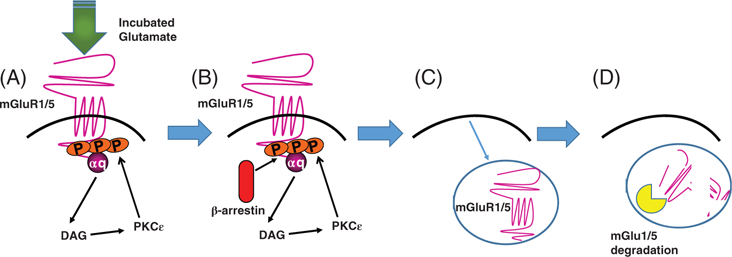
Hypothetical molecular model relating incubated glutamate release and canonical signaling through mGlu1/5 receptors in extinction resistance. A, The capacity of drug-associated stimuli to elicit glutamate release within the vmPFC incubates during protracted withdrawal, resulting in an overstimulation of postsynaptic mGlu1/5 receptors. B, mGlu1/5 stimulation results in the activation of various PKCs, of which the PKC isozyme is well characterized to phosphorylate the receptor, which recruits β-arrestin. C, β-Arrestin recruitment activates receptor internalization mechanisms, reducing cell surface expression and (D) receptor degradation. While insufficient to induce incubated craving, the reduction in functional mGlu1/5 is sufficient to induce a deficit in inhibitory learning and a failure to learn to suppress drug-craving and behaviors driven by that craving
FIGURE 3.
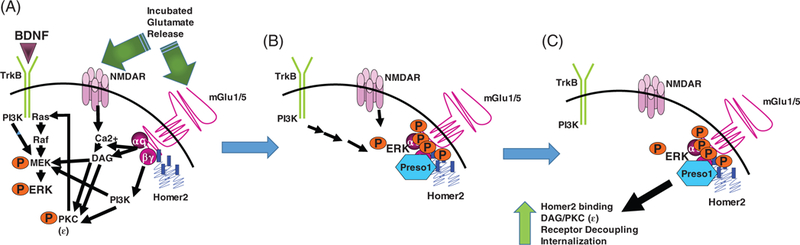
Hypothetical molecular model relating incubated ERK activation to mGlu1/5 receptor-dependent extinction resistance. (A, Time-dependent incubation of cue-elicited glutamate release, coupled with the recruitment of BDNF, activates a number of intracellular effectors that ultimately augment the activational state of ERK (in addition to or because of the stimulation of PKCs and PI3K). B, Activated ERK phosphorylates mGlu in the Homer binding domain and recruits Preso1, increasing Homer coupling to the receptor. As the Homer2 isoform is selectively overexpressed during protracted withdrawal, it is presumed that ERK/Preso1 interactions with mGlu receptors augment Homer2-mGlu1/5 interactions and shunt activated ERK away from the nucleus. C, The increased Homer2 binding to mGlu1/5 is predicted to facilitate canonical and noncanonical signaling through mGlu1/5, including the activation of PKC isozymes and PI3K. As illustrated in Figure 1, this kinase activation results in mGlu1/5 internalization leading to impaired extinction learning and memory and persistent cue-elicited drug craving
ERK activation also induces an increase in the transcription of the immediately early, truncated, Homer isoform Homer1a.97 Homer1a induction typically serves a dominant negative function, dismantling constitutive Homer protein scaffolding87 and receptor coupling to intracellular effectors. Homer1a is robustly induced by acute cocaine,98 raising the possibility that an ERK-dependent induction of Homer1a during early drug exposure serves normally to counteract any influence this kinase may have upon mGlu-Homer binding and receptor desensitization. However, the capacity of cocaine to induce Homer1a expression appears to develop tolerance with repeated drug exposure.98 Although the mechanism(s) involved in this tolerance are not known, one possibility may involve a shunting of ERK activity away from the nucleus by a drug-induced increase in ERK binding to Preso1.86 The ERK/Preso1-mediated strengthening of Homer scaffolding with mGlu1/5, coupled with the increased Homer2 expression89 and reduced Homer1a expression,99 are predicted to increase the activational state of canonical and noncanonical signaling pathways leading to rapid mGlu5 internalization/ degradation and extinction resistance.
7 |. CONCLUDING REMARKS AND FUTURE DIRECTIONS
Incubated cue-elicited drug-craving is a clinically relevant phenomenon observed across drugs of abuse that co-occurs, at least in an animal model of cocaine addiction, with deficits in the ability to learn to suppress conditioned drug-seeking behavior. Currently, no clinically approved pharmacotherapeutic exists for craving reduction in cocaine addiction. Based on the extant literature, this article presented hypothetical models relating a kinase-dependent desensitization of mGlu1/5 within the vmPFC to the time- and context-dependent preservative cocaine-craving during protracted withdrawal. From these models, a number of possible treatment strategies emerge that all have, at their core, the goal of maintaining normal mGlu1/5 function within vmPFC. These strategies include: reducing the excitability of glutamate terminals/glutamate release within vmPFC, which may be achieved via stimulation of autoreceptors81; augmenting mGlu1/5 function via treatment with positive allosteric modulators37; administration of TrkB, D1 or ionotopic glutamate receptor antagonists to prevent their induction by BNDF, dopamine and glutamate, respectively; treatment with small molecule inhibitors of mGlu1/5-Homer scaffolding; and inhibition of intracellular effectors known to participate in the desensitization of mGlu1/5 function. With respect to the latter, this can be achieved via the administration of selective or non-selective inhibitors of ERK, PKCs and/or PI3K,51,96 and it is notable that pharmaceutical agents targeting all 3 of these kinases, either alone or in combination, are either FDA-approved, or in Phase II/III clinical trials, as chemotherapies for treating a wide range of cancers, as well as wound healing and chronic pain.100–102 While these hypothetical models are based, in part, on circumstantial evidence derived exclusively from rodent models of incubated cocaine-seeking, they nevertheless provide a theoretical foundation upon which to base exploration of the molecular biology of incubated drug-craving and associated learning impairments of relevance to the design of effective treatment interventions for relapse prevention and addiction recovery.
Footnotes
Financial disclosure
The authors have no financial interests to disclose. No external funds were employed in the composition of this review article.
REFERENCES
- 1.Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addict Biol 2015;20:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Rubio FJ, Zeric T, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci 2015;35: 8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology 2015;40:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci 2011;34:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 1986;43:107–113. [DOI] [PubMed] [Google Scholar]
- 6.Bedi G, Preston KL, Epstein DH, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 2011;69:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One 2013;8:e68791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GB, Zhang XL, Zhao LY, et al. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology 2012;221:701–708. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Wu P, Xin X, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol 2014;20: 513–522. [DOI] [PubMed] [Google Scholar]
- 10.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 1998;19:48–59. [DOI] [PubMed] [Google Scholar]
- 11.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature 2001;412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 2016;224: 25–52. [DOI] [PubMed] [Google Scholar]
- 13.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 2016;17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM. Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol 2017; 22:977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caprioli D, Venniro M, Zhang M, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci 2017;37:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasnova IN, Marchant NJ, Ladenheim B, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 2014;39:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheyer AF, Loweth JA, Christian DT, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol Psychiatry 2016;80:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 2004;55:1082–1089. [DOI] [PubMed] [Google Scholar]
- 19.Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 2017;42:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Venniro M, Shaham Y. Translational research on incubation of cocaine craving. JAMA Psychiatry 2016;73:1115–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD. Role of central amygdala neuronal ensembles in incubation of nicotine craving. J Neurosci 2016;36:8612–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markou A, Li J, Tse K, Li X. Cue-induced nicotine-seeking behavior after withdrawal with or without extinction in rats. Addict Biol 2016. 10.1111/adb.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser SR, Deehan GA Jr, Knight CP, Toalston JE, McBride WJ, Rodd ZA. Parameters of context-induced ethanol (EtOH)-seeking in alcohol-preferring (P) rats: temporal analysis, effects of repeated deprivation, and EtOH priming injections. Alcohol Clin Exp Res 2016;40:2229–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci 2012;32: 11600–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YQ, Li FQ, Wang XY, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 2008;28:13248–13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalev U, Morales M, Hope BT, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 2001;156:98–107. [DOI] [PubMed] [Google Scholar]
- 27.Theberge FR, Li X, Kambhampati S, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 2013;73:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theberge FR, Pickens CL, Goldart E, et al. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology 2012; 224:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 2006;11:2–38. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama K, Barnes J, Grimm JW. Incubation of saccharin craving and within-session changes in responding for a cue previously associated with saccharin. Appetite 2014;72:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Counotte DS, Schiefer C, Shaham Y, O’Donnel P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology 2014;231:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimm JW, Barnes J, North K, Collins S, Weber R. A general method for evaluating incubation of sucrose craving in rats. J Vis Exp 2011; 4:e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav 2005;84:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology 2011;216:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm JW, Manaois M, Osincup D, Wells B, Buse C. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology 2007;194:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res 2007;181:292–296. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Shahar O, Sacramento AD, Miller BW, et al. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci 2013;33:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 2003;23:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res 2010;208:1–11. [DOI] [PubMed] [Google Scholar]
- 40.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 2009;16:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci 2013;34:689–695. [DOI] [PubMed] [Google Scholar]
- 42.Broadman K Neue Ergebnisse uber die vergliechende histologische Lokalisation der Grosshirnrinde mit besonderer Berücksichtigung des Stirnhirns. Anat Anz 1912;41(suppl):157–216. [Google Scholar]
- 43.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci 2000;1:59–65. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 2011;12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8:1481–1489. [DOI] [PubMed] [Google Scholar]
- 46.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 1999;146:373–390. [DOI] [PubMed] [Google Scholar]
- 47.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 1993;137:73–95. [PubMed] [Google Scholar]
- 48.Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Nat Acad Sci USA 1996; 93:12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct 2008;213:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 2009;56(suppl 1):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller BW, Wroten MG, Sacramento AD, et al. Incubation of cocaine-craving requires PKCε priming within vmPFC. Addict Biol 2017; 22:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 2000;20:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 7th ed. New York, NY: Academic Press; 2013. [Google Scholar]
- 54.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 2003;168:44–56. [DOI] [PubMed] [Google Scholar]
- 55.Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci 2010;3:101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma YY, Lee BR, Wang X, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse based remodeling of prefrontal cortex to accumbens projections. Neuron 2014;83:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine seeking before and following abstinence. Eur J Neurosci 2014;39:1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci 2006;26:8004–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szumlinski KK, Woodward JJ. Glutamate signaling in alcohol abuse and dependence. In: Noronha A, Cui C, Harris A, Crabbe JC, eds. Neurobiology of Alcohol Dependence Elsevier, San Diego, USA; 2013:173–206. [Google Scholar]
- 60.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 2015;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loweth JA, Scheyer AF, Milovanovic M, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 2014;17:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quintero GC. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr Dis Treat 2013;9:1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res 2007;1184: 295–305. [DOI] [PubMed] [Google Scholar]
- 64.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 2003;6:743–749. [DOI] [PubMed] [Google Scholar]
- 65.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 2011;63:348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timmer KM, Steketee JD. Group I metabotropic glutamate receptors in the medial prefrontal cortex: role in mesocorticolimbic glutamate release in cocaine sensitization. Synapse 2013;67:887–896. [DOI] [PubMed] [Google Scholar]
- 67.Ben-Shahar O, Szumlinski KK, Lominac KD, et al. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol 2012;17:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ben-Shahar O, Obara I, Ary AW, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 2009;63:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasanetz F, Lafourcade M, Deroche-Gamonet V, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry 2013;18:729–737. [DOI] [PubMed] [Google Scholar]
- 70.Szumlinski KK. Prefrontal cortex glutamate and cocaine craving during abstinence. In: Preedy VR, ed. The Neuroscience of Cocaine: Mechanisms and Treatment. Academic Press, London, UK; 2016:547–544. [Google Scholar]
- 71.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 2009;65:717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res 2011;1413:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry 2010;68:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 2011;21:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feedforward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol 2011;106:960–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 2011;63:348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology 2000;23: 335–344. [DOI] [PubMed] [Google Scholar]
- 78.Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci 2001;14:1843–1855. [DOI] [PubMed] [Google Scholar]
- 79.Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology 2010;211:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin CB, Serchia M, Shahin J, Ruppert-Mejor M, Szumlinski KK. Incubation of cue-elicited glutamate release within prefrontal cortex. Neuropharmacology 2016;102:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin CB, Templeton TJ, Chiu AS, et al. Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology 2017;128:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bradley SJ, Challiss RA. Defining protein kinase/phosphatase isoenzymic regulation of mGlu₅ receptor-stimulated phospholipase C and Ca2+ responses in astrocytes. Br J Pharmacol 2011;164:755–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gereau RW 4th, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron 1998;20:143–151. [DOI] [PubMed] [Google Scholar]
- 84.Olive MF, Newton PM. Protein kinase C isozymes as regulators of sensitivity to and self-administration of drugs of abuse-studies with genetically modified mice. Behav Pharmacol 2010;21:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller BW, Wroten MG, Sacramento AD, et al. Incubation of cocaine-craving requires PKCε priming within vmPFC. Addict Biol 2017; 22:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park JM, Hu JH, Milshteyn A, et al. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell 2013;154:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol 2007;8:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ary AW, Lominac KD, Wroten MG, et al. Imbalances in prefrontal cortex CC-Homer1 versus -Homer2 expression promote cocaine-seeking behavior. J Neurosci 2013;33:8101–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gould AT, Sacramento AD, Wroten MG, et al. Extended access to intravenous cocaine imbalances ventromedial prefrontal cortex Homer1 versus Homer2 expression: implications for relapse. Addict Biol 2015;20:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res 2015;279:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci 2011;34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cahill E, Salery M, Vanhoutte P, Caboche J. Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse. Front Pharmacol 2014;4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun WL, Quizon PM, Zhu J. Molecular mechanism: ERK signaling, drug addiction, and behavioral effects. Prog Mol Biol Transl Sci 2016;137:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur J Neurosci 2003;17: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 95.Gould AT, Sacramento AD, Wroten MG, et al. Extended access to intravenous cocaine imbalances ventromedial prefrontal cortex Homer1 versus Homer2 expression: implications for relapse. Addict Biol 2015;20:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szumlinski KK, Ary AW, Shin CB, et al. Cocaine-induced PI3K activation within ventromedial prefrontal cortex is critical for the expression of drug-seeking. Soc Neurosci Abstr 2017;79502. [Google Scholar]
- 97.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol 2008;75:112–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse 2009;63:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse 2009;63:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem 2016;109: 314–341. [DOI] [PubMed] [Google Scholar]
- 101.Jokinen E, Koivunen JP. MEK and PI3K inhibition in solid tumors: rationale and evidence to date. Ther Adv Med Oncol 2015;7: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Z, Wang Z, Liu X, Wang D. New development of inhibitors targeting the PI3K/AKT/mTOR pathway in personalized treatment of non-small-cell lung cancer. Anticancer Drugs 2015;26:1–14. [DOI] [PubMed] [Google Scholar]


