Abstract
Introduction:
We report successful treatment of mesenteric diffuse large B-cell lymphoma (DLBCL) using localized involved site radiation therapy (ISRT), intensity modulated radiation therapy (IMRT), and daily CT-image guidance.
Methods:
Patients with mesenteric DLBCL treated with RT between 2011 and 2017 were reviewed. Clinical and treatment characteristics were analyzed for an association with local control (LC), progression free survival (PFS) and overall survival (OS).
Results:
Twenty-three patients were eligible. At diagnosis, the median age was 52 years (38–76), and 57% (n=13) had stage I/II DLBCL. All patients received frontline chemotherapy (ChT) (R-CHOP, n=19; dose-adjusted R-EPOCH, n=4) with median 6 cycles. Prior to RT, salvage ChT for refractory DLBCL was given to 43% (n=10) and autologous stem cell transplantation was administered in 13% (n=3). At the time of RT, PET-CT revealed five-point scale (5PS) of 1–3 (48%, n=11), 4 (9%, n=2), and 5 (44%, n=10). All patients received IMRT, daily CT imaging and ISRT. The median RT dose was 40 Gy (16.2–49.4). Relapse or progression occurred in 22% (n=5). At median follow-up of 37 months, the 3-year LC, PFS and OS rates were 80%, 75% and 96%, respectively. Among patients treated with RT after complete metabolic response to frontline ChT (n=8), 3-year PFS was 100%, compared to 61% for patients with history of chemorefractory DLBCL (n=15, p=0.055). Four of the five relapses occurred in patients with 5PS of 5 prior to RT (p=0.127).
Conclusion:
Mesenteric involvement of DLBCL can be successfully targeted with localized ISRT fields using IMRT and daily CT-image guidance.
Keywords: Involved site radiation therapy, five-point scale
Micro-abstract:
Lymph nodes in the mesentery are a common location for NHL, however there is often concern regarding the use of radiation therapy (RT) for patients with mesenteric involvement due to unpredictable target motion. We report the successful incorporation of RT for patients DLBCL and mesenteric involvement through the utilization of contemporary RT techniques including intensity modulated radiation therapy and daily low dose CT imaging for target localization.
Introduction:
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), and often has an aggressive clinical course [1]. Standard primary treatment for patients with DLBCL is immunochemotherapy with a doxorubicin-based regimen [2–5]. While this treatment is curative in many patients, refractory or relapsed disease occurs in 30–40% of cases [6, 7].
There is evidence that consolidative radiation therapy (RT) after chemotherapy for treatment of localized DLBCL can improve patient outcomes [7–14]. The RICOVER-60 NoRTh trial showed a survival benefit of post-R-CHOP radiation therapy for sites of bulky disease for any risk patients over 60 years of age [15]. A National Cancer Database analysis similarly showed improved OS for patients who received RT after chemotherapy for early-stage DLBCL [16]. The unpublished UNFOLDER study recently closed the experimental arm of R-CHOP without RT due to an unacceptably high rate of treatment failures [14].
In cases where relapse has already occurred, salvage chemotherapy and autologous stem-cell transplantation are often utilized to induce remission [17]. However, outcomes for certain patients are suboptimal and further progression is common [18]. In fact, for patients who fail second-line platinum therapy, response to subsequent chemotherapy regimens is limited, often less than 20% [19, 20]. Most relapses are at sites of initial disease, suggesting a role for radiation therapy [21]. Treatment with RT has been shown to improve outcomes in cases of relapsed or refractory disease [22].
Radiation therapy guidelines for the management of patients with non-Hodgkin lymphoma have evolved. Large extended and involved fields have given way to more limited RT target volumes where only sites of involvement are included with a limited margin for set up variability (involved site radiation therapy, ISRT), according to the International Lymphoma Radiation Oncology Group (ILROG) [23]. The ability to deliver effective modern RT with minimal toxicity is highly dependent on accurate target delineation as well as daily localization. However, for RT targets susceptible to unpredictable motion, such as mesenteric masses, there may be concern for missing the target when employing limited involved site radiation therapy fields. The use of daily cone-beam computed tomography (CT) has become routine when treating mobile masses such as lung tumors to ensure reproducibility of radiation treatment [24]. There have been reports of using cone beam CT to treat mobile abdominal masses, though this is not currently a common practice [25, 26].
Lymph nodes in the mesentery a common location for NHL, identified in 30–50% of all patients [27, 28]. Conventional 3-dimensional (3D)-radiation to the whole abdomen ensures that the tumor is included in the treatment volume, but this approach can cause undesirable side-effects such as nausea and diarrhea. Total dose delivery is also limited by potential toxicity to critical organs such as the kidneys [29, 30]. Due to the potential for unpredictable motion of mesenteric masses, there may be reluctance to administer localized ISRT for DLBCL with mesenteric nodal involvement. With daily CT image-guidance, however, the target can be localized prior to each treatment delivery Due to the fact that a soft tissue mass typically remains, even after a complete metabolic response, it is feasible to target only the site of initial involvement as it is visible on daily CT imaging. We report the successful treatment of patients with mesenteric DLBCL using ISRT, intensity modulated radiation therapy (IMRT) and daily CT image guidance.
Materials and Methods:
Patient identification and characteristics
After IRB approval, we reviewed all DLBCL patients with mesenteric involvement who were treated with radiation between 2011 and 2017 at MD Anderson Cancer Center. Patients were considered eligible for inclusion if they had biopsy-proven DLBCL and received RT for consolidation or definitive treatment targeting all sites of evident disease. The diagnosis of DLBCL was established by a hematopathologist using standard criteria and methods. Radiation therapy requirements were ISRT, IMRT, and daily CT image guidance. Demographics, tumor characteristics, and treatment details were extracted from the electronic medical record. Staging was performed according to the Ann-Arbor system. An initial fluorodeoxyglucose (FDG) positron emission tomography (PET) -CT scan was conducted for baseline disease assessment in most patients. Bulky disease was defined as a nodal mass or conglomerate measuring ≥ 7.5 cm in the maximum axial dimension on the initial diagnostic PET-CT, as per the RICOVER-60 trial [31].
Systemic therapy
All patients received frontline systemic therapy with either R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) or dose-adjusted R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin). Post-chemotherapy assessment was performed with PET-CT imaging according to the Lugano 5 point scale (5PS) [32]. Chemosensitivity was defined as having a 5PS score of 1–3 after frontline chemotherapy, whereas chemorefractory disease was defined as disease requiring more than one line of chemotherapy before RT (regardless of the 5PS score at the time of RT), or having a 5PS of 4–5 after one line of chemotherapy at the time of RT. The maximum axial dimension of the residual mesenteric mass just prior to RT was recorded.
Radiation treatment planning and delivery
Patients were treated free-breathing or using deep inspiration breath hold (DIBH) at the discretion of the treating physician. DIBH while, often utilized for treatment of the thorax, can also be considered for patients with disease presentations close to the diaphragm, where respiratory motion could be exaggerated. Indeed respiratory motion of abdominal organs can be over 2 cm, especially in the superior-inferior direction and this movement can be partially mitigated by DIBH[33, 34]. Patients were considered for breath hold as long as they could reliably perform the procedure. When utilized, DIBH was performed using a respiratory monitoring device and feedback system (Varian Real-time Position Management System, Palo Alto, CA). During CT simulation, three non-contrasted breath-hold CT scans were obtained for each patient to confirm consistency of the breath-hold. In the absence of therapy with DIBH, patients were treated free breathing with or without four dimensional (4D) CT simulation.
The clinical target volume (CTV) was created as per the ILROG involved site RT guidelines [7]. The entire residual CT mass was included along with the initial extent of the disease involving the mesentery itself. Regions where bulky masses were only pushing out toward the abdominal wall or down into the pelvis were not included, as these regressed (toward the mesentery) after systemic therapy. An internal clinical target volume (iCTV) was then created which included the location of the target on each of the breath hold scans or on all phases of the 4D CT scan. For patients treated free breathing that did not undergo 4D CT simulation, an iCTV was not created. The CT residual disease is intimately associated with the mesentery and motion of the Ct residual mass typically informs the location and therefore target for the microscopic CTV; however, it is certainly possible that there is independent motion of these two portions of the target. For patients where this is more of a concern given the overall size of the treatment volume, a larger iCTV can be applied at the discretion of the treating physician.
A 0.5- to 1.0-cm margin was added to the iCTV to create a planning target volume (PTV), at the discretion of the treating physician. For patients treated in free breathing without 4D CT simulation, a larger PTV margin of 1.0 to 1.5 cm was applied. Patients with a negative PET-CT at the time of RT received 30–36 Gy and patients with gross disease at the time of RT received intended prescription doses of 40–50 Gy. Some patients were treated with a boost whereby residual PET avid disease was treated to a higher total dose than the remaining PET negative CTV. IMRT plans were generated with the Pinnacle treatment planning system (Pinnacle, Philips Medical Systems, Fitchburg, WI) using 6-MV photon beams. Organs at risk (OAR), including the kidneys, liver and spinal cord were contoured on the planning CT scan. Plans were optimized to deliver the lowest possible dose to adjacent organs at risk (OARs).
Before each fraction of RT, CT-Image Guided Radiotherapy (IGRT) was performed using a high-speed in-room CT scanner (CT-on-Rails, GE Medical Systems, Milwaukee, WI) integrated with the Varian Exact Targeting System (Varian Medical Systems). In-house 3-dimensional CT alignment software was used to align daily CT images with the reference planning CT images [35]. The regions of interest (CTV and OARs), the prescription isodose line, and the 95% isodose line were overlaid on the daily-acquired CT images to evaluate target coverage. As all patients had a residual CT mass at time of RT, this mass was identified on each daily scan in the axial, coronal, and sagittal planes, and the CTV structure and prescription isodose line was confirmed to encompass the mass prior to treatment delivery. If a shift of >1.0 cm was required for CTV coverage, the treating physician reviewed the alignment and made appropriate shifts before treatment [36]. In these situations, the isodose lines were examined with respect to the normal tissues such as the kidneys. If the treating physician had concerns regarding increased dose to normal tissue with the isocenter shift, the patient was re-simulated and re-planned.
The interval between CT scan and treatment is typically about 10 minutes. Total treatment time varies, but is typically between 10–20 minutes depending upon plan complexity and breath hold requirements. During this time, patients must lie quietly in the treatment position and maintain NPO status to decrease respiratory and abdominal motion that could lead to motion of the target including intra-fractional motion.
Statistical Methods
Patient and tumor characteristics were compared using independent t-tests or Mann-Whitney U tests, Chi-square, and Fisher’s exact tests. Kaplan-Meier log-rank tests were used to analyze associations between clinical and treatment characteristics and local control (LC), progression-free survival (PFS) and overall survival (OS) with onset considered to be the first day of RT. Local failure was defined as recurrence or progression within the radiation field; distant failure was defined as relapse outside of the abdomen. PFS was defined as disease relapse, progression or death from any cause. OS was defined as death from any cause.
Results:
Patient and treatment characteristics
At time of diagnosis, the median age was 52 years (range 38–76), and 13 patients (57%) had stage I/II DLBCL (Table 1). The majority of patients had bulky disease at the time of initial diagnosis (87%, n=20, 2 patients did not have baseline PET-CT imaging available for review). All patients received frontline immunochemotherapy (R-CHOP, n=19; dose adjusted R-EPOCH, n=4) for a median of six cycles (range 3–8) (Table 2). Salvage chemotherapy for refractory DLBCL was given prior to radiation therapy in 10 patients (44%), with up to four chemotherapy regimens given. Autologous stem cell transplantation was administered in three patients (13%) before radiation. At the time of RT, PET-CT imaging revealed 5PS of 1–3 in eleven patients (48%), 5PS of 4 in two (9%) and 5PS of 5 in ten patients (44%). Most patients had a sizeable residual mass appreciated on CT imaging at the time of RT, with a median residual mass size of 6.1cm (range 2.1–17.3 cm). All patients received IMRT, daily CT imaging and ISRT. Approximately half (n=11, 47.8%) of patients were treated using the deep inspiration breath hold technique, while 12 (52.2%) were free breathing during treatment. Of the 12 patients that were free breathing, 4 patients underwent 4D CT simulation for treatment planning. The median shift required between the simulation and the first fraction was 0.85 cm (range 0.29–2.68).
Table 1:
Baseline Patient and Disease Characteristics
| All Patients (n=23) | Chemo-sensitive Patients (n=8) | Chemo-refractory Patients (n=15) | ||
|---|---|---|---|---|
| No. patients (%) median [range] | No. patients (%) median [range] | No. patients (%) median [range] | p-value | |
| Median age at diagnosis | 52 [38–76] | 50.5 [38–60] | 56 [39–76] | 0.213* |
| Gender | ||||
| Male | 17 (73.9%) | 6 (75.0%) | 11 (73.3%) | >0.999** |
| Female | 6 (26.1%) | 2 (25.0%) | 4 (26.7%) | |
| Stage at Diagnosis | ||||
| I | 1 (4.3%) | 0 (0.0%) | 1 (6.7%) | 0.805*** |
| II | 12 (52.2%) | 4 (50.0%) | 8 (53.3%) | |
| III | 5 (21.7%) | 2 (25.0%) | 3 (20.0%) | |
| IV | 5 (21.7%) | 2 (25.0%) | 3 (20.0%) | |
| Median LDHƗ | 670 [174–1200] | 730 [630–867] | 539 [174–1200] | 0.350* |
| ECOGƗƗ | ||||
| 0 | 11 (47.8%) | 5 (62.5%) | 6 (42.9%) | 0.329*** |
| 1 | 9 (39.1%) | 3 (37.5%) | 6 (42.9%) | |
| 2 | 2 (8.7%) | 0 (0.0%) | 2 (13.3%) | |
| Bulky disease at diagnosisƗƗƗ | 20 (87.0%) | 8 (100.0%) | 12 (92.3%) | >0.999** |
| Maximal disease diameter (cm) | 12.8 [7.2–20.1] | 11.9 [8.2–20.1] | 12.8 [7.2–16.1] | 0.860* |
| Double Hit | 1 (4.3%) | 0 (0.0%) | 1 (6.7%) | >0.999** |
| Transformed disease | 12 (52.2%) | 6 (75.0%) | 5 (33.3%) | 0.089** |
Mann-Whitney U Test
Fisher’s Exact Test
Maximum Likelihood Ratio
LDH only available for 6/8 chemo-sensitive cases and 11/15 chemo-refractory cases
ECOG only available for 14/15 patients with chemo-refractory disease
Maximum axial diameter of tumor available for 13/15 chemorefractory patients
Table 2:
Treatment Characteristics
| All Patients (n=23) | Chemo-sensitive Patients (n=8) | Chemo-refractory Patients (n=15) | ||
|---|---|---|---|---|
| No. patients (%) mean (st dev) median [range] | No. patients (%) mean (st dev) median [range] | No. patients (%) mean (st dev) median [range] | p-value | |
| First-line ChT | ||||
| R-CHOP | 19 (82.6%) | 6 (75.0%) | 13 (86.7%) | 0.589** |
| DA-R-EPOCH | 4 (17.4%) | 2 (25.0%) | 2 (13.3%) | |
| ChT regimens before RT | 1 [1–4] | 1 [1–1] | 2 [1–4] | 0.008* |
| SCT before RT | 3 (13.0%) | 0 (0.0%) | 3 (20.0%) | 0.526** |
| Residual mass (cm) | 6.1 [2.1–17.3] | 6.1 [3.5–11.1] | 6.2 [2.1–17.3] | 0.825* |
| 5PS Prior to RT | ||||
| 1–3 | 11 (47.8%) | 8 (100%) | 3 (20.0%) | <0.001*** |
| 4 | 2 (8.7%) | 0 (0.0%) | 2 (13.3%) | |
| 5 | 10 (43.5%) | 0 (0.0%) | 10 (66.7%) | |
| RT Dose (Gy) | 40.0 [16.2–49.4] | 30.6 [16.2–36.0] | 42 [30.6–49.4] | <0.001* |
| Dose >= 40 Gy | 13 (56.5%) | 0 (0.0%) | 13 (86.7%) | |
| Any Boost | 5 (21.7%) | 0 (0.0%) | 5 (33.3%) | 0.122** |
| DIBH utilized | 11 (47.8%) | 4 (50.0%) | 7 (46.2%) | >0.999** |
| Mean Right Kidney Dose (Gy) | 5.4 (3.7) | 4.6 (2.8) | 5.9 (4.2) | 0.441*** |
| Mean Left Kidney Dose (Gy) | 6.8 (3.8) | 5.2 (2.0) | 7.6 (4.4) | 0.088*** |
| Mean Liver Dose (Gy)Ɨ | 3.4 (2.4) | 3.5 (2.1) | 3.3 (2.6) | 0.894*** |
| Post-RT therapy | 2 (25%) | 7 (46.7%) | 0.400** | |
| Maintenance Rituxan | 2 (25%) | 0 (0.0%) | ||
| Allogeneic SCT | 0 (0.0%) | 4 (26.7%) | ||
| CAR-T cell therapy | 0 (0.0%) | 2 (13.3%) | ||
| Other salvage ChT | 0 (0.0%) | 1 (6.7%) | ||
Mann-Whitney U Test
Fisher’s Exact Test
Independent t-test
Mean Liver Dose only available for 16 patients total (5 chemo-sensitive and 11 chemo-refractory) Abbreviations: ChT, chemotherapy; R-CHOP, rituximab cyclophosphamide doxorubicin vincristine prednisone combination chemotherapy; DA-R-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab; RT = radiation therapy; SCT = stem cell transplant; CAR-T = Chimeric antigen receptor T-cell therapy; DIBH = deep inspiration breath hold;
The median radiation dose was 40 Gy (range, 16.2 – 49.4 Gy) administered in 20 fractions (range, 9–25) (Table 2). A boost was administered to 5 patients (22%). Among the 23 patients, 3 required re-planning due to the need for DIBH to reduce target motion that was noticed on daily CT imaging (n=1) and for adaptive planning for regressing tumors (n=2). For all patients, dose constraints were easily achieved for the liver and bilateral kidneys [37]. The mean dose to the left kidney, right kidney, and liver was 6.8 Gy (+/− 3.8), 5.2 Gy (+/− 3.7), and 3.4 Gy (+/− 2.4), respectively.
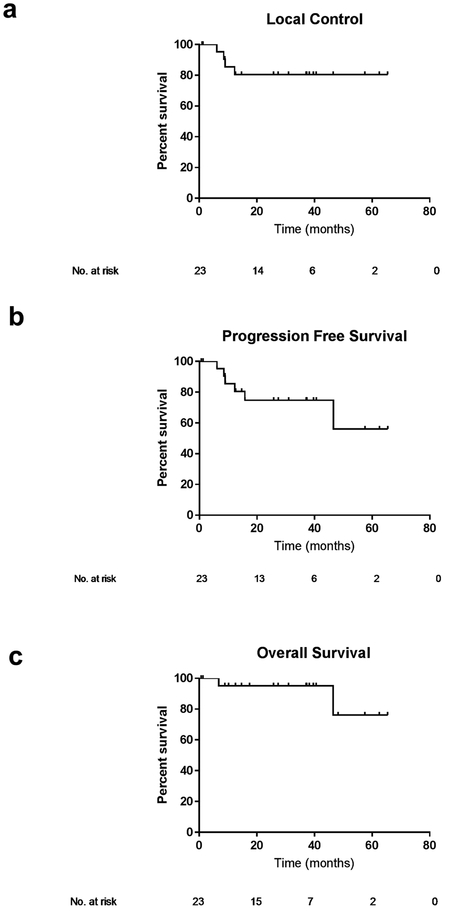
The median times to local failure, progression-free survival, and overall survival were not reached (Table 3). Median follow up for the entire cohort was 37 months (95% CI 25.1–29.6 months). The three-year local control rate for all patients was 80%, three-year progression-free survival was 75%, and overall survival was 95% (Figure 1a-c).
Table 3:
Treatment outcomes in chemo-sensitive and chemo-refractory patients
| All Patients (n=23) | Chemo-sensitive Patients (n=8) | Chemo-refractory Patients (n=15) | ||
|---|---|---|---|---|
| No. patients (%) Mean (std error) Median [95% CI] | No. patients (%) Mean (std error) Median [95% CI] | No. patients (%) mean (std error) Median [95% CI] | p-value | |
| Failure after Radiation | ||||
| Yes | 5 (21.7%) | 0 (0.0%) | 5 (33.3%) | 0.122* |
| No | 18 (78.3%) | 8 (100%) | 10 (66.7%) | |
| Recurrence | ||||
| Isolated local | 2 (8.7%) | 0 (0.0%) | 2 (13.3%) | 0.526* |
| Isolated distant | 1 (4.3%) | 0 (0.0%) | 1 (6.7%) | >0.999* |
| Combined local and distant | 2 (8.7%) | 0 (0.0%) | 2 (13.3%) | 0.526* |
| Median Follow up Time (months) | 37.3 [25.1–49.6] | 31.0 [14.1–47.9] | 37.3 [16.6–58.1] | 0.843 |
| 3-year Progression-Free Survival | 74.7 % (+/−9.9%) | 100.0% | 60.6 % (+/− 13.8%) | 0.055** |
| 3-year Local Control | 80.4 % (+/− 8.8%) | 100.0% | 69.2 % (+/− 12.8%) | 0.106** |
| 3-year Overall Survival | 95.2 % (+/−4.6%) | 100.0% | 92.3 % (+/−7.4%) | 0.356** |
Fisher’s Exact Test
Kaplan-Meier Log Rank test
Figure 1: Outcomes for the entire cohort.
Local control (a), progression-free survival (b) and overall survival (c) of all patients (n=23).
Chemo-sensitive Disease
Eight (34.8%) of the 23 patients were considered to have disease that was sensitive to the initial chemotherapy treatment course with 5PS of 1–3 after R-CHOP (n=6) or dose-adjusted R-EPOCH (n=2). All patients treated with consolidative RT after primary chemotherapy had bulky disease initially, with median maximum axial dimension of tumor on baseline PET imaging of 11.9 cm (range 8.2–20.1). After completion of frontline chemotherapy, and prior to initiation of RT, the median residual mesenteric mass size was 6.1 cm (range 3.4–11.1). Two patients received maintenance therapy after RT with rituximab. All patients with initially chemo-sensitive disease have remained free of any disease recurrence with a median follow-up of 31 months. Three-year LC, PFS, and OS in this cohort were all 100% (Figure 2a-c).
Figure 2: Outcomes according to disease response to chemotherapy.
Local control (a), progression-free survival (b) and overall survival (c) of patients with chemo-sensitive disease (n=8) compared to those with chemo-refractory disease (n=15).
Chemo-refractory Disease
Patients with chemo-refractory disease (n=15) were those who received salvage chemotherapy after frontline systemic treatment for refractory, relapsed or progressive disease regardless of the 5PS at the time of RT (n=10, 66.7%) or those who had persistent DLBCL with a 5PS of 4–5 at the time of RT after completion of frontline chemotherapy (n=5, 33.3%). These patients with chemo-refractory disease were treated with salvage RT. In this cohort, ten (66.7%) of the patients had 5PS of 5 on PET-CT scan just prior to RT, two (13.3%) had 5PS of 4, and 3 (20.0%) had 5PS of 1–3. There were no significant differences in baseline characteristics between chemo-sensitive and chemo-refractory patients (all p>0.05. Table 1). Patients treated with salvage radiation received significantly higher radiation doses than chemo-sensitive patients, with median dose of 42 Gy (range 30.6–49.4), compared to 30.6 Gy (range 16.2–36.0; p<0.001, Table 2). Figure 3 depicts a 66 year old patient with stage III DLBCL and a 13.7 cm mesenteric mass at diagnosis (Figure 3 a, b). After 6 cycles of R-CHOP, PET-CT imaging revealed a 5PS of 5 with persistent foci of intense uptake (Figure 3 c, d). The patient received IMRT to a dose of 42 Gy (Figure 3e, f) and had resolution of PET avid foci after RT (Figure 3g, h).
Figure 3. Salvage IMRT administered to a patient with 5 point scale (5PS) of 5 after 6 cycles of R-CHOP.
Baseline pre-therapy axial (a) and coronal images (b) in a 66 year old patient with stage III DLBCL and a 13.7 cm mesenteric mass denoted with arrows. After 6 cycles of R-CHOP, a 6.3 cm residual mass persisted with nodular foci of uptake (SUV of 13.2 and 12.4), including one focus of activity that was new since the interim pet-ct scan (c, d). The patient was treated with IMRT to a final dose of 42 Gy (e, f). Restaging PET-CT three months following completion of RT revealed complete resolution of the FDG avid foci in a decreased mesenteric mass of 5.5 cm (marked with arrows) (g, h).
Relapse or progression occurred in five (33.3%) of the chemo-refractory cases, at a median time of 9.0 months (range 6.1–15.8) after completion of radiation. Two patients experienced progression, two experienced relapse after achieving a complete response on PET-CT, and one had a residual avid mass in-field that has been decreasing in avidity after RT for 12 months without additional therapy. Failure occurred locally for two patients, distant in one patient, and combined local and distant in two patients. All patients with local failure received a radiation dose ≥ 40 Gy (range 40–49.4 Gy). Four of five failures occurred in patients with 5PS of 5 at the time of RT. The remaining failure occurred in a patient with previously chemo-refractory disease, who had received two lines of chemotherapy and stem cell transplantation, achieved a CR, and then received RT. This patient had a combined local and distant recurrence 12 months after completion of radiation. Among the 5 patients that experienced disease progression or relapse, no abdominal failures outside of the radiation field were observed.
Seven (47%) patients with chemo-refractory disease received additional therapy after RT, including allogeneic stem cell transplantation (n=4), chimeric antigen receptor (CAR) T cell therapy (n=2) and additional salvage chemotherapy (n=1). Of these seven patients that received additional treatment, three had no radiographic evidence of disease at the time of these therapies.
Three-year LC, PFS, and OS in this cohort of chemo-refractory patients was 69%, 61%, and 92% respectively (Figure 2a-c). There was a trend for inferior 3-year PFS for patients with chemo-refractory disease compared to those with chemo-sensitive DLBCL (p=0.055, Figure 2b).
In total, 2 deaths have occurred in the cohort. One was due to DLBCL. This patient had 5PS of 5 and history of chemo-refractory disease with progression 6 months after RT. The other death was secondary to myelodysplastic syndrome in a patient with a history of refractory DLBCL who underwent salvage chemotherapy, autologous SCT and radiation therapy, and who had no evidence of lymphoma at time of death.
Outcomes according to 5PS at the time of RT among all patients.
For patients with 5PS 1–3, there was a trend towards improved LC, PFS, and OS compared to patients with 5PS 4–5 (Figure 4a-c). In patients with 5PS of 5 just prior to RT (n=10), 3-year PFS was 56% compared to 91% for patients with 5PS of 1–4 (Figure 4e, p=0.137). These patients had multiple (median 3; range 1–4) chemotherapy regimens prior to RT for refractory DLBCL. The 3-year LC rate was 67% for patients with 5PS of 5 at the time of RT compared to 91% for those with 5PS of 1–4 (Figure 4d, p=0.143).
Figure 4: Outcomes according to 5 point scale (5PS).
Local control (a), progression-free survival (b), and overall survival (c) of all patients with 5PS 1–3 (n=11) compared to 5PS 4–5 (n=12). Local control (d), progression-free survival (e), and overall survival (f) of all patients with 5PS 1–4 (n=13) compared to 5PS 5 (n=10).
Toxicities
Grade 1–2 nausea/diarrhea occurred in 11 (48%) patients during treatment. There was no difference in toxicity incidence between patients with disease that was chemosensitive (n=3, 37.5%) or chemorefractory (n=7, 46.7%) (p=0.667). Two patients (8.7%) required a treatment break, one for intense abdominal pain with no cause determined, and the other due to severe gout. No patient with chemosensitive disease experienced grade 3 or higher toxicity, however two patients with chemorefractory disease (8.7%) experienced grade 3 late toxicities (p=0.526). One patient required surgical removal of a bleeding necrotic jejunal mass (RT dose, 47 Gy) and the other needed cauterization for radiation enteritis (RT dose, 40 Gy). Both patients had previously had SCTs; the patient with enteritis had a SCT before RT, and the patient with the jejunal mass had a SCT after RT treatment. The patient with the necrotic jejunal mass developed a fistula between the remaining residual mass and the jejunum. This was treated with resection of the residual mass (7cm) and an en bloc resection of the involved small bowel.
Discussion:
In this report, we describe the successful treatment of DLBCL patients with mesenteric involvement using localized ISRT fields, IMRT, and daily CT-guidance. Overall, the outcomes were quite favorable, with 79% of patients achieving disease free status at a median follow up time of roughly three years in this patient cohort with roughly 90% of patients having bulky disease at diagnosis. We found no relapses for patients who received consolidation radiation treatment after an initial complete response to frontline chemotherapy. Patients with chemo-refractory disease to either frontline or salvage treatments for whom poorer outcomes are expected, still had satisfactory local control rates of 69.2% and progression-free survival of around 60%.
Traditionally, patients with DLBCL are treated with immunochemotherapy with subsequent consideration for consolidation RT given the overall clinical picture and patient-specific risk factors. The benefits of RT in terms of LC and PFS have been demonstrated in multiple randomized and retrospective studies [8–13, 16] and seem to be greatest for patients with localized disease, bulky disease, or skeletal involvement [7, 14, 38]. The location within the abdomen allows mesenteric lymphomas to reach a large size before causing any clinical symptoms that lead to diagnosis [27]. Therefore, it is not uncommon for these patients to present with masses that, by size criteria, qualify as bulky. Given the benefit of RT for bulky disease in DLBCL, these patients should be considered for consolidation radiation therapy.
However, accurate delivery of radiation therapy necessitates localization of the target prior to treatment. This is often performed using daily imaging with x-rays that allows alignment of bony anatomy, used as a surrogate for alignment of the target tissues. However, this approach will not suffice for abdominal masses, as the motion of abdominal organs is independent of bony anatomy. CT-based imaging is commonly utilized for radiation treatment of gastrointestinal malignancies and can provide information regarding daily internal anatomy to inform treatment delivery. Although all abdominal organs demonstrate internal motion due to breathing and peristalsis, masses within the mesentery have been notorious for marked daily changes in location, which can complicate radiation treatment planning and delivery [25]. Given these technical challenges, many are reticent to refer for or to offer radiation therapy to disease in the mesentery, as there is some uncertainty as to whether a planned treatment is effectively delivered.
Advances in technology such as IMRT and CT-guidance have afforded radiation oncologists the ability to use smaller RT fields, even in circumstances in which target motion is a concern. IMRT allows physicians to target the tumor while sparing nearby organs at risk, simultaneously reducing toxicity as well as improving coverage of the target volume. Previous studies have exhibited IMRT to be feasible in treating gastric cancer [39, 40], and in fact have also demonstrated a reduction in toxicity with this approach [41]. However, the use of IMRT for a moving target is yet another obstacle. Using specialized software and CT imaging, physicians are able to provide sub-millimeter accuracy to localize and target a specific mass prior to each treatment [25, 35], making CT-guidance for treatment of a moving mesenteric target possible. In our study daily CT on rails imaging was utilized, which is known to have image quality comparable to a diagnostic CT scan. However in many centers, cone beam CT imaging is more readily available. While cone beam CT has been demonstrated to have inferior image quality, adjustments can be made to improve imaging features. This approach however is not ideal for patients with a large body habitus[42].
Even with these improvements in technology, there is still concern for motion that cannot be mitigated using pre-treatment daily CT imaging, such as intra-fractional motion. Keeping patients NPO and minimizing time between imaging and treatment decreases the likelihood that the target has been displaced. Given the uncertainties that cannot be fully addressed, it is important to apply appropriate ITV expansions using information from both diagnostic and simulation imaging. In addition, all patients in our series had a residual CT mass that could be visualized with daily CT imaging. Additional caution should be used when considering therapy for patients without a radiographic abnormality, as these targets will be more challenging to localize.
While our results were promising, we do not recommend treatment of patients with mesenteric disease without CT-based imaging guidance, as inter-fractional motion can be quite large. Indeed when treating gastric MALT lymphoma with smaller PTV margins of 5 – 10 mm, daily CT imaging (as opposed to imaging for bony alignment alone), is essential to assure accurate target coverage without compromise to organs at risk[36]. The median shift between simulation and the first fraction of treatment was 0.85cm. While this magnitude of variation in target location could typically be incorporated into a PTV margin, many patients have shifts that are much larger. There was one case in this series in which the shift was so significant than re-simulation using a breath hold technique was recommended. We have also previously reported on mobile mesenteric masses requiring adaptable treatment plans[25]. The need for adaptive planning is more common when the target lies was in close proximity to the kidneys or other normal structures. As it is impossible to predict whether a target will be highly mobile or fairly reproducible in location, daily CT-based image guidance is necessary. Two other patients in this series were re-simulated during treatment as the masses were noted to have responded substantially to radiation therapy. In this scenario, daily CT imaging provided an opportunity to monitor tumor response and the need for re-planning, thereby reducing the dose to nearly OARs.
Using these methods, we have shown that we have the technical capabilities to accurately treat DLBCL with mesenteric involvement, and outcomes are encouraging. In patients with previously chemo-responsive disease, the results are excellent, with no progressions or relapses in three years of median follow-up. Multiple studies have shown improved survival in patients treated with consolidative RT after chemotherapy, both before and after the addition of rituximab to the standard primary CHOP regimen [7–14]. Furthermore, prior studies have reported an association of bulky disease with worse outcomes for DLBCL patients [43]. In a previous study of 89 patients treated for DLBCL after primary immunochemotherapy, those with bulky disease (defined as > 5 cm) were approximately 7 times more likely to recur locally than those with smaller initial disease [44]. In our chemo-sensitive cohort, all patients had bulky disease at time of diagnosis (median 11.9 cm). Even after primary immunochemotherapy, the residual PET-CT negative masses were sizeable, with median maximum axial diameter of 6.1 cm. Our findings of 100% three-year progression free survival in this limited cohort are encouraging.
In the current study for patients with chemorefractory disease, RT resulted in disease control for 67% of patients. In the Collaborative trial in Relapsed Aggressive Lymphoma (CORAL) study, of the 145 DLBCL patients whose disease did not respond to second line platinum-based therapy, the overall response rate to 3rd line chemotherapy was 43%, with only 21% achieving a complete response [19]. Furthermore, the median overall survival from the time of second failure to death was only 5.9 months. Our study suggests that for patients with refractory DLBCL limited to one site, RT may be considered, as this cohort had encouraging results: 60% of patients with a 5PS of 5 remained free from progression after three-years.
When comparing differences in efficacy of radiation for patients with 5PS of 1–3 to those with 5PS of 4–5 prior to RT, we did not observe a statistically significant difference in PFS, LC, or OS. However, 80% of patients whose disease recurred after radiation had a 5PS of 5 prior to radiation. The lack of significance in treatment outcomes is likely due to small sample size. While gross disease at the time of RT may be associated with lower local control rates, it is also important to note that 60% of patients with 5PS of 5 remained free of disease, and 61% of patients with 5PS of 4–5 were disease free at the time of last follow up. This outcome is superior to those seen in previous studies [20, 45].
In the current study, 47% of patients with chemorefractory DLBCL received additional systemic therapy after completion of RT, including six patients that went on for aggressive therapies such as allogeneic stem cell transplantation and CAR-T cell therapy. These patients were not candidates for this therapy prior to RT. This highlights the utility of RT in permitting patients to qualify for additional aggressive and potentially curative therapies. In the CORAL study, patients whose disease responded to 3rd line systemic therapy and who underwent autologous stem cell transplantation had superior OS (p<0.0001). In patients with chemo-refractory DLBCL and mesenteric involvement, salvage RT may be considered as another tool to bridge to additional aggressive systemic therapies such as autologous stem cell transplantation or CAR T-cell therapy [20]. This approach has the potential to improve outcomes for this patient cohort where additional treatment options are desperately needed.
While salvage RT to refractory mesenteric DLBCL allowed some patients to undergo further systemic therapy, on the other hand, 20% of patients in the chemo-refractory cohort had salvage RT for a relapse sustained after autologous SCT. In this setting of localized relapses after autologous SCT, salvage RT represents an opportunity for a therapy with high overall response rates and, in some cases, durable local control. However, we did observe higher rates of grade 3 toxicity among the chemo-refractory cohort, possibly due to higher RT doses required, as well as a lower threshold for toxicity due to prior treatment with multiple courses of systemic therapy. This underscores the complexity and challenges of administering aggressive therapy in a vulnerable patient population.
Our study is not without limitations. In addition to its retrospective nature, the study has a limited patient number. However, we do demonstrate that patients with DLBCL involving the mesentery can be successfully treated with modern radiation therapy, including localized involved site radiation therapy fields, IMRT, and CT imaging. Daily image guided therapy with CT imaging enabled the accurate targeting of mesenteric disease despite the known mobility of the mesentery. We are encouraged by our results which demonstrate outcomes at least comparable to those expected for other similar-risk DLBCL patients. These data suggest that patients with DLBCL of the mesentery should be considered for radiation treatment at centers with the technologic capabilities to deliver CT-guided IMRT.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health National Cancer Institute, Cancer Center Support (Core) (grant CA 016672) to the University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
No actual or potential conflicts of interest exist.
References
- 1.Li Y, et al. , Racial differences in three major NHL subtypes: descriptive epidemiology. Cancer Epidemiol, 2015. 39(1): p. 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coiffier B, et al. , CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med, 2002. 346(4): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 3.Habermann TM, et al. , Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol, 2006. 24(19): p. 3121–7. [DOI] [PubMed] [Google Scholar]
- 4.Horvat M, et al. , Diffuse large B-cell lymphoma: 10 years’ real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone. Oncol Lett, 2018. 15(3): p. 3602–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, et al. , CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol, 2006. 7(5): p. 379–91. [DOI] [PubMed] [Google Scholar]
- 6.Lukenbill J and Hill B, Relapsed/refractory diffuse large B-cell lymphoma: review of the management of transplant-eligible patients. Leuk Lymphoma, 2015. 56(2): p. 293–300. [DOI] [PubMed] [Google Scholar]
- 7.Ng AK, et al. , Role of Radiation Therapy in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys, 2018. 100(3): p. 652–669. [DOI] [PubMed] [Google Scholar]
- 8.Philip T, et al. , Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med, 1995. 333(23): p. 1540–5. [DOI] [PubMed] [Google Scholar]
- 9.Aviles A, et al. , Adjuvant radiotherapy in patients with diffuse large B-cell lymphoma in advanced stage (III/IV) improves the outcome in the rituximab era. Hematology, 2018: p. 1–5. [DOI] [PubMed] [Google Scholar]
- 10.Miller TP, et al. , Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med, 1998. 339(1): p. 21–6. [DOI] [PubMed] [Google Scholar]
- 11.Phan J, et al. , Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol, 2010. 28(27): p. 4170–6. [DOI] [PubMed] [Google Scholar]
- 12.Horning SJ, et al. , Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol, 2004. 22(15): p. 3032–8. [DOI] [PubMed] [Google Scholar]
- 13.Kwon J, et al. , Additional survival benefit of involved-lesion radiation therapy after R-CHOP chemotherapy in limited stage diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys, 2015. 92(1): p. 91–8. [DOI] [PubMed] [Google Scholar]
- 14.Pinnix CC, Radiation Therapy for Diffuse Large B-Cell Lymphoma: Indications, Outcomes, and Controversies. Int J Radiat Oncol Biol Phys, 2016. 94(4): p. 641–4. [DOI] [PubMed] [Google Scholar]
- 15.Held G, et al. , Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol, 2014. 32(11): p. 1112–8. [DOI] [PubMed] [Google Scholar]
- 16.Vargo JA, et al. , Treatment Selection and Survival Outcomes in Early-Stage Diffuse Large B-Cell Lymphoma: Do We Still Need Consolidative Radiotherapy? J Clin Oncol, 2015. 33(32): p. 3710–7. [DOI] [PubMed] [Google Scholar]
- 17.Mundt AJ, Williams SF, and Hallahan D, High dose chemotherapy and stem cell rescue for aggressive non-Hodgkin’s lymphoma: pattern of failure and implications for involved-field radiotherapy. Int J Radiat Oncol Biol Phys, 1997. 39(3): p. 617–25. [DOI] [PubMed] [Google Scholar]
- 18.Haw R, et al. , Significance of a partial or slow response to front-line chemotherapy in the management of intermediate-grade or high-grade non-Hodgkin’s lymphoma: a literature review. J Clin Oncol, 1994. 12(5): p. 1074–84. [DOI] [PubMed] [Google Scholar]
- 19.Gisselbrecht C, et al. , Diffuse Large B-Cell Lymphoma (DLBCL) Patients Failing Second-Line RDHAP Or R-ICE Chemotherapy Included In The Coral Study. Blood, 2013. 122(21): p. 764-764. [Google Scholar]
- 20.Gisselbrecht C, et al. , Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol, 2010. 28(27): p. 4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhakal S, et al. , Patterns and Timing of Failure for Diffuse Large B-Cell Lymphoma After Initial Therapy in a Cohort Who Underwent Autologous Bone Marrow Transplantation for Relapse. Int J Radiat Oncol Biol Phys, 2016. 96(2): p. 372–378. [DOI] [PubMed] [Google Scholar]
- 22.Biswas T, et al. , Involved field radiation after autologous stem cell transplant for diffuse large B-cell lymphoma in the rituximab era. Int J Radiat Oncol Biol Phys, 2010. 77(1): p. 79–85. [DOI] [PubMed] [Google Scholar]
- 23.Illidge T, et al. , Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys, 2014. 89(1): p. 49–58. [DOI] [PubMed] [Google Scholar]
- 24.Dzyubak O, et al. , Evaluation of tumor localization in respiration motion-corrected cone-beam CT: prospective study in lung. Med Phys, 2014. 41(10): p. 101918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabaja B, et al. , Successful treatment of a free-moving abdominal mass with radiation therapy guided by cone-beam computed tomography: a case report. J Med Case Rep, 2010. 4: p. 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankine L, et al. , Cone-Beam Computed Tomography Internal Motion Tracking Should Be Used to Validate 4-Dimensional Computed Tomography for Abdominal Radiation Therapy Patients. Int J Radiat Oncol Biol Phys, 2016. 95(2): p. 818–26. [DOI] [PubMed] [Google Scholar]
- 27.Salemis NS, et al. , Diffuse large B cell lymphoma of the mesentery: an unusual presentation and review of the literature. J Gastrointest Cancer, 2009. 40(3–4): p. 79–82. [DOI] [PubMed] [Google Scholar]
- 28.Anis M and Irshad A, Imaging of abdominal lymphoma. Radiol Clin North Am, 2008. 46(2): p. 265–85, viii–ix. [DOI] [PubMed] [Google Scholar]
- 29.Goffinet DR, et al. , Abdominal irradiation in non-Hodgkin’s lymphomas. Cancer, 1976. 37(6): p. 2797–805. [DOI] [PubMed] [Google Scholar]
- 30.Le Bourgeois JP, et al. , Complications of total abdominal and spleen irradiation in patients with lymphomas. Recent Results Cancer Res, 1978. 65: p. 170–80. [DOI] [PubMed] [Google Scholar]
- 31.Pfreundschuh M, et al. , Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol, 2008. 9(2): p. 105–16. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, et al. , Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol, 2014. 32(27): p. 3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandner ED, et al. , Abdominal organ motion measured using 4D CT. Int J Radiat Oncol Biol Phys, 2006. 65(2): p. 554–60. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, et al. , Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: a dosimetric study. Radiat Oncol, 2012. 7: p. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Dong L, Court L, Wang H, Gillin M, Mohan R, TU-EE-A4–05: Validation of CT-Assisted Targeting (CAT) Software for Soft Tissue and Bony Target Localization. Med Phys, 2005. 32(6): p. 2106. [Google Scholar]
- 36.Wang H, et al. , Daily CT guidance improves target coverage during definitive radiation therapy for gastric MALT lymphoma. Pract Radiat Oncol, 2017. 7(6): p. e471–e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks LB, et al. , Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys, 2010. 76(3 Suppl): p. S10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Held G, et al. , Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol, 2013. 31(32): p. 4115–22. [DOI] [PubMed] [Google Scholar]
- 39.Badakhshi H, et al. , Image-guided intensity-modulated radiotherapy for patients with locally advanced gastric cancer: a clinical feasibility study. Gastric Cancer, 2014. 17(3): p. 537–41. [DOI] [PubMed] [Google Scholar]
- 40.Gene-Fu F, Liu RJ., Bair EB, Stanley L, Liauw MK, Clinical Outcomes for Gastric Cancer following Adjuvant Chemoradiation Utilizing Intensity Modulated versus Three-Dimensional Conformal Radiotherapy. PloS one, 2014. 9(1): p. e82642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trip AK, et al. , IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiother Oncol, 2014. 112(2): p. 289–94. [DOI] [PubMed] [Google Scholar]
- 42.Yang CC, et al. , Optimizing the target detectability of cone beam CT performed in image-guided radiation therapy for patients of different body sizes. J Appl Clin Med Phys, 2018. 19(3): p. 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfreundschuh M, et al. , Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol, 2008. 9(5): p. 435–44. [DOI] [PubMed] [Google Scholar]
- 44.Jegadeesh N, et al. , Predictors of local recurrence after rituximab-based chemotherapy alone in stage III and IV diffuse large B-cell lymphoma: guiding decisions for consolidative radiation. Int J Radiat Oncol Biol Phys, 2015. 92(1): p. 107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao W, et al. , Predictive value of [(1)(8)F]fluoro-2-deoxy-D-glucose positron emission tomography for clinical outcome in patients with relapsed/refractory diffuse large B-cell lymphoma prior to and after autologous stem cell transplant. Leuk Lymphoma, 2014. 55(2): p. 276–82. [DOI] [PubMed] [Google Scholar]