Abstract
Macrophages play a critical role in the establishment of a regulated inflammatory response following tissue injury. Following injury, CCR2+ monocytes are recruited from peripheral blood to wound tissue, and direct the initiation and resolution of inflammation that is essential for tissue repair. In pathologic states where chronic inflammation prevents healing, macrophages fail to transition to a reparative phenotype. Using a murine model of cutaneous wound healing, we found that CCR2-deficient mice (CCR2−/−) demonstrate significantly impaired wound healing at all time points post-injury. Flow cytometry analysis of wounds from CCR2−/− and wild type mice revealed a significant decrease in inflammatory, Ly6CHi recruited monocyte/macrophages in CCR2−/− wounds. We further show that wound macrophage inflammatory cytokine production is decreased in CCR2−/− wounds. Adoptive transfer of mT/mG monocyte/macrophages into CCR2+/+ and CCR2−/− mice demonstrated that labeled cells on days 2 and 4 traveled to wounds in both CCR2+/+ and CCR2−/− mice. Further, adoptive transfer of monocyte/macrophages from wild type mice restored normal healing, likely through a restored inflammatory response in the CCR2-deficient mice. Taken together, these data suggest that CCR2 plays a critical role in the recruitment and inflammatory response following injury, and that wound repair may be therapeutically manipulated through modulation of CCR2.
Keywords: Inflammation, Wound Healing, Macrophages, Recruitment, Chemokine Receptor
INTRODUCTION
Wound healing is dependent on a tightly regulated immune response to injury. The early inflammatory response, regulated mainly by recruited monocyte-derived macrophages (MoMΦs) from peripheral blood, is crucial for establishment of the healing cascade[1],[2]. These recruited MoMΦs are key players in establishment of this early inflammatory response, and their plasticity allows for the transition from the inflammatory to the regenerative phase of healing[3]. The early recruitment of monocytes to the wound is dependent on tracking through chemokine gradients via a variety of receptors and signaling molecules. For example, CCR2 is a chemokine receptor primarily found on MoMΦs and is an important for MoMΦ tracking from peripheral blood to injured tissues.
Recruited MoMΦs are responsible for both the initiation and resolution of inflammation due to their high plasticity[4]–[7]. Within the murine and human circulation, these essentially exist in one of two dominant phenotypes, termed “classical” and “non-classical” MoMΦs. These phenotypes are distinguished by unique patterns of chemokine receptor expression[8]. Classical MoMΦs, often characterized as Ly6CHi cells in mice, express high levels of the chemokine receptor CCR2, whereas non-classical MoMΦs express high levels of fractalkine receptor CX3CR1. This nomenclature was initially based on the macrophage response to monocyte chemoattractant protein-1 (MCP-1 or CCL2), and on their activation during the acute phase of inflammation[9],[10]. It is the CCR2+ Ly6CHi MoMΦs that respond to CCL2, the primary ligand for CCR2. The CCL2 chemokine functions to maintain homeostatic levels of monocytes in circulation as well as to direct the recruitment of MoMΦs to sites of tissue injury[11],[12]. Recruitment of Ly6CLo MoMΦs, however, has not been shown to be dependent on CCR2.
In addition to having distinct patterns of activation and recruitment, Ly6CHi and Ly6CLo cells demonstrate different functional characteristics. Upon entering injured tissue, Ly6CHi cells assume a pro-inflammatory role[13]. There, they begin to secrete pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6[13],[14]. Ly6CLo cells appear towards the end of the initial inflammatory phase, when they demonstrate anti-inflammatory characteristics and promote tissue remodeling, fibrosis, and wound healing through the production of TGF-β and IL-10[15]–[17]. In addition to these two populations of recruited monocytes, there is a cohort of tissue resident macrophages that are also designated as Ly6CLo cells. These tissue resident cells express the surface marker F4/80 (F4/80+), and have been found to be activated following tissue injury or inflammation, triggering their differentiation into macrophages[18]. Thus, it is unclear if Ly6CLo cells are recruited directly from circulation[18], are present at baseline levels in the tissues, or if they transition in the wound tissue to Ly6CLo cells from Ly6CHi cells; regardless, a clear phenotype shift from proinflammatory to anti-inflammatory has been documented post-injury in many tissues including liver, myocardium, and skeletal muscle[13],[18]–[21].
Deficiency of CCR2 can alter both monocyte homeostasis in the circulation and the recruitment of monocytes to sites of tissue injury. Mice deficient in CCR2 are known to have a deficiency in circulating Ly6CHi cells, as CCR2 is critical for mobilization of monocytes from the bone marrow during systemic inflammation[12]. Further, in liver tissues, depletion of CCR2+ MoMΦs at different stages of injury resulted in dysregulation of inflammation and altered tissue recovery[22]–[24]. While the role of CCR2 in healing of myocardium, liver, lungs, skeletal muscle, and other organs is well described, the literature regarding the role of CCR2 in cutaneous wound healing is less well-defined. It has been well-described that the phenotypic expressions exhibited by MoMΦs follow a clear temporal pattern in cutaneous wounds[25], but the relationship of CCR2 to different MoMΦ populations involved in tissue repair is less established. For example, Rodero et al found that CCR2-mediated MoMΦ recruitment occurs after excisional scalp injury in the murine model during the first four hours after initial tissue injury, and thus prior to the generation of granulation tissue[26]. Furthermore, studies by Crane et al and Willenborg et al investigate the role of CCR2 in cutaneous wound healing, however these studies examined F4/80+ cells and thus focus on tissue resident macrophages rather than recruited monocytes[20],[27]. The latter study also investigates wound healing at late tissue injury points, and focuses on the role of resident macrophages in the production of angiogenic mediators in tissue repair[27]. Given that CCR2 does play an essential role in MoMΦ recruitment and healing in other tissues, we hypothesized that CCR2 would impact recruited MoMΦs in cutaneous wound healing, and that deficiency of CCR2 would result in impaired wound healing through modulation of the inflammatory response.
In this study, we found that wound healing is impaired in CCR2-deficient mice, and that macrophages isolated from these mice demonstrated decreased inflammatory gene expression and protein levels. Furthermore, we demonstrated that early adoptive transfer of mT/mG tdTomato MoMΦs into CCR2+/+ and CCR2−/− mice results in significant tracking of monocytes to the site of injury both at days 2 and 4, and that transfer of wild type macrophages into CCR2-deficient mice after wounding was sufficient to rescue wound healing, likely through restoration of the inflammatory response. This is important, as the ability to modulate inflammation in wounds is translationally relevant given that chronic inflammation is often associated with pathologic wound healing. Our findings suggest that CCR2 plays an integral role in recruited monocyte/macrophage-mediated inflammation in wound healing and offer a therapeutic target for pathologic inflammation.
RESULTS
Wound healing is impaired in CCR2−/− mice.
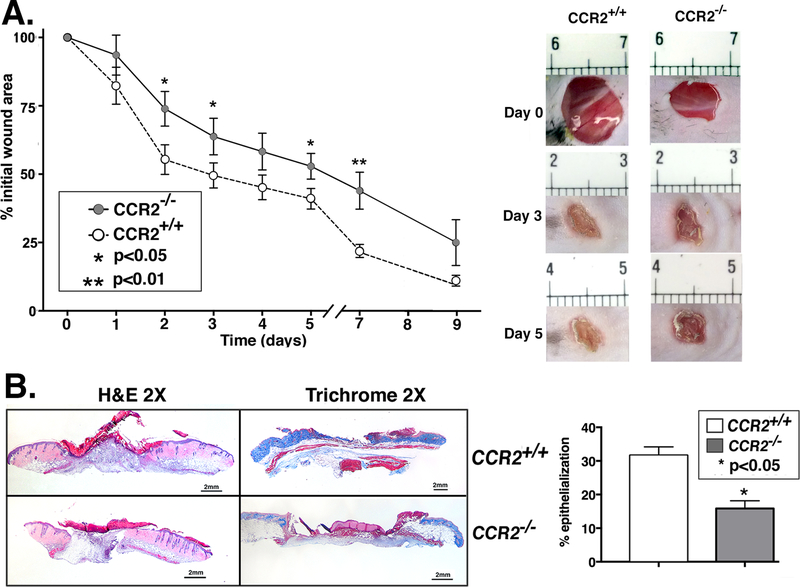
As has been shown in injured cardiac and hepatic tissues, CCR2’s role in the recruitment of MoMΦs is both necessary and time-dependent for proper healing[13],[24]. Consequently, we hypothesized that wound healing would be delayed or impaired in mice globally deficient in CCR2 (CCR2−/−). To investigate this, we wounded CCR2−/− mice and controls (CCR2+/+) with a 4mm punch biopsy, and measured wound area daily using NIH ImageJ software. As expected, CCR2−/− mice demonstrated significantly impaired wound healing at all time points compared with matched controls (Figure 1A). Representative images of mouse wounds are shown in Figure 1. In order to examine histologic changes during healing, day 3 whole wounds were paraffin-embedded and stained with H&E and Masson’s trichrome to examine epithelialization and collagen content, respectively. Figure 1B demonstrates both the impaired epithelialization and decreased collagen content in CCR2−/− mouse wounds compared with controls. Collectively, these findings suggest that a global lack of CCR2 impairs both the inflammatory and reparative phases of wound healing and that failure to establish the initial inflammatory phase in wound healing may lead to failure to progress through the subsequent phases of healing.
Figure 1. Wound healing is impaired in CCR2-deficient mice (CCR2−/−).
Wounds were created using 4 mm punch biopsies on the backs of CCR2−/− mice and matched controls (CCR2+/+). A: The change in wound area was recorded daily using ImageJ software (NIH) until complete healing was observed. Representative photographs of the wounds of CCR2−/− mice and controls on days 0, 3 and 5 post-injury are shown (*P < 0.05; **P < 0.01; n=14; data is representative of four independent experiments). B: Wounds were harvested on day 3, paraffin embedded and sectioned. 5 μM sections were stained with hematoxylin and eosin and with Masson’s Trichrome stain. Percent re-epithelialization was calculated by measuring distance traveled by epithelial tongues on both sides of wound divided by total distance for full re-epithelialization. Representative images are shown in 2X magnification (*P < 0.05; n=10; data is representative of two independent experiments). Statistical analysis was performed using a paired Student’s t-test. All data are expressed as mean ± SEM.
Loss of CCR2 in wound macrophages results in decreased inflammatory Ly6CHi monocyte/macrophages.
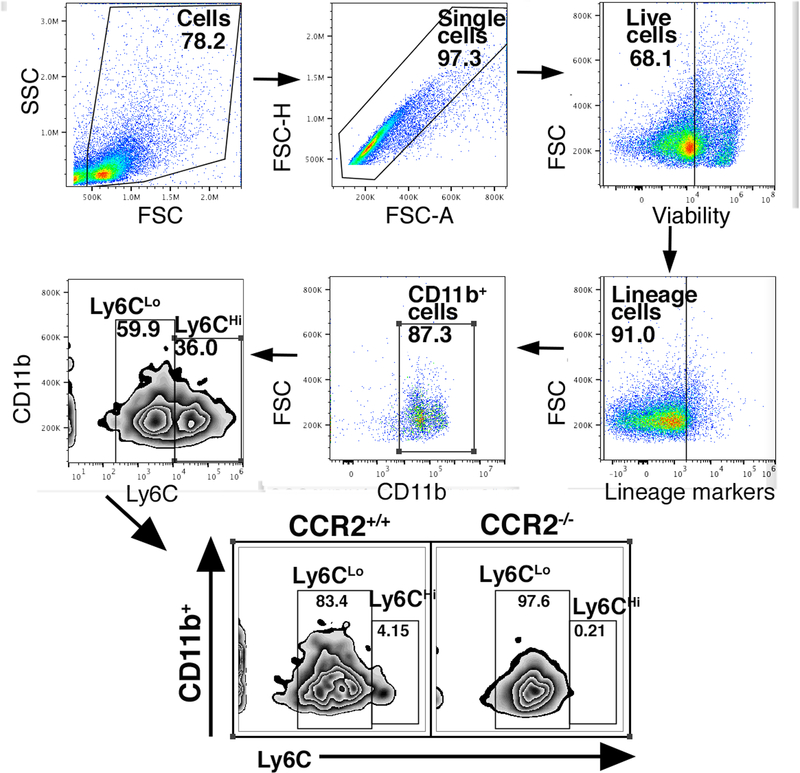
Since progression through the inflammatory phase of wound healing is largely directed by macrophage plasticity, we theorized that the impaired wound healing during this phase in CCR2-deficient mice was secondary to decreased influx of monocytes to the wound site and the failure of the initial inflammatory response that is necessary for progression through the healing cascade. Since CCR2 is expressed primarily on classically derived, pro-inflammatory macrophages, we hypothesized that inadequate early influx of these cells accounts for the impaired wound healing phenotype in CCR2-deficient mice. Work by our group and others has demonstrated that pro-inflammatory macrophages are important for the initiation of inflammation, and that inflammation is necessary for transition to the reparative phase of healing[28]. We have shown that absence of initial inflammation in wounds halts progression of the wound-healing cascade and prevents healing[29]. In order to examine this in our model, we isolated cutaneous wounds from CCR2−/− mice and matched controls during the early inflammatory phase, and analyzed these wounds by flow cytometry. We used a gating strategy selecting live, lineage− (CD3−, CD19− Ter119−, NK1.1−), non-neutrophil (Ly6G−), CD11b+ cells, as previously described (Figure 2A)[29],[30]. It has been previously documented by our group and others that Ly6CHi demonstrate a pro-inflammatory phenotype, whereas Ly6CLo cells demonstrate a reparative phenotype[8],[13],[31]–[33]. When we examined differences in Ly6CHi/Ly6CLo cells in the CCR2−/− and CCR2+/+ wounds, we found that there were significantly fewer Ly6CHi cells in the CCR2−/− cohort (0.21%) compared with controls (4.15%) (Figure 2A). These results suggest that fewer inflammatory Ly6CHi cells are present in CCR2-deficient wounds during the inflammatory phase.
Figure 2. Loss of CCR2 in Ly6CHi monocytes results in decreased inflammatory Ly6CHi monocyte/macrophages in wound tissue.
A: Wounds were harvested from CCR2−/− and matched controls on day 3 and processed for ex vivo intracellular flow cytometry. The gating strategy used for flow cytometry selected live, lineage- (CD3−, CD19−, Ter119−, NK1.1−), non-neutrophil (Ly6G−), CD11b+ cells as shown. Flow cytometry quantification of Ly6C revealed two populations of cells in both CCR2−/− and CCR2+/+ wounds, translating to Ly6CHi and Ly6CLo monocyte macrophages (CD3−, CD19−, Ter119−, NK1.1−, Ly6G−, CD11b+).(n=10; data is representative of two independent experiments).
To determine if the differences in the number of Ly6CHi cells recruited to the wound were due to differences in levels of the CCR2 ligand CCL2, we examined CCL2 expression in CCR2+/+ and CCR2−/− wounds. We did not find statistically significant differences in wound CCL2 between CCR2-deficient mice and controls (Supplemental Figure 1).
CCR2-deficient wound macrophages demonstrate decreased inflammation.
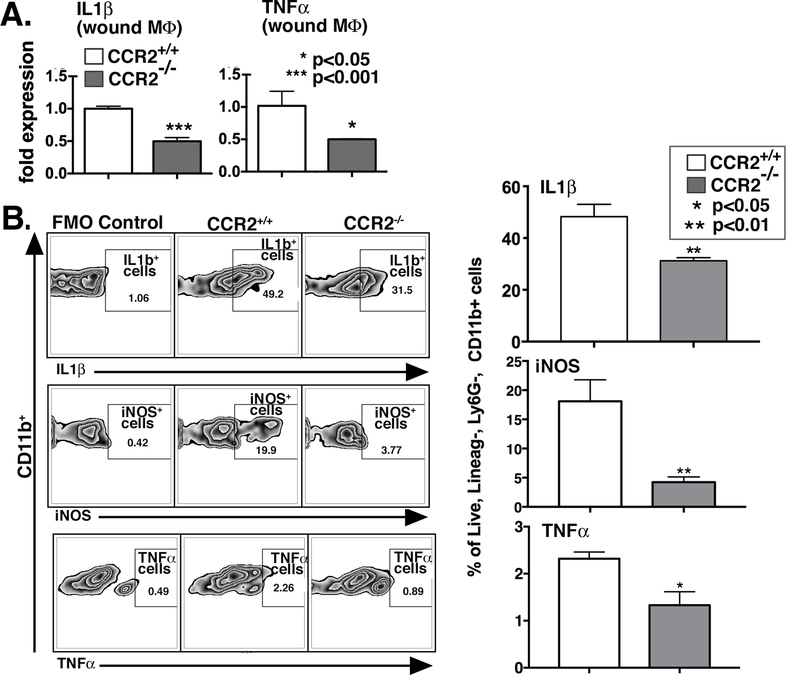
Macrophage phenotype is identified by cell surface receptors and cell function, often determined by intracellular mediator production[15]. In order to examine wound macrophage function, we isolated macrophages from cutaneous wounds of CCR2−/− and matched controls on post-injury day 3 and analyzed both inflammatory gene expression as well as cytokine production by intracellular flow cytometry. Day 3 is a key time point post-injury in the inflammatory phase when monocytes have had adequate time to enter the tissues, transform into macrophages, and assume a functional role[34],[35]. Previous studies suggest that recruited macrophage numbers are at their highest levels on day 3 postwounding[36]. Macrophages were isolated from wound tissue using magnetic-assisted cell sorting (MACS) to isolate CD19−, CD3−, Ly6G−, CD11b+ cells as previously described[37]. Gene expression of inflammatory cytokines critical for wound inflammation, including IL1β and TNF-α, was examined using quantitative PCR. As expected, we found significantly decreased expression of both IL1β (P<0.001) and TNF-α (P<0.05) in macrophages isolated from CCR2−/− wounds (Figure 3A).
Figure 3. Macrophages isolated from wounds of CCR2−/− mice display decreased inflammatory cytokine gene expression and protein levels.
A: Wound macrophages were isolated from CCR2−/− mice and matched controls on day 3 post-injury by MACS for CD11b+ (CD3−,CD19−, Ly6G−) cells. TNF-α and IL1β gene expression in isolated macrophages was measured by qPCR using 18s for normalization (*P<0.05; **P < 0.01; n=12, data is representative of two independent experiments). B: CCR2−/− and littermate control wounds harvested on day 3 were processed for ex vivo intracellular flow cytometry. Intracellular cytokine quantification by flow cytometry of IL1β, iNOS, and TNF-α in wound macrophages was examined (*P < 0.05, ** P < 0.01; n=10; data is representative of two independent experiments). Statistical analysis was performed using a paired Student’s t-test. All data are expressed as mean ± SEM.
In order to determine if gene expression patterns mirror cytokine production, we isolated wounds from CCR2−/− mice and matched controls and examined macrophages using a similar gating strategy as described in Figure 2A. Intracellular staining for inflammatory cytokines and mediators, IL1β, TNF-α and iNOS, was performed. We found that there was significantly decreased IL1β, TNF-α, and iNOS in the CCR2−/− wound macrophages (live, lineage−, Ly6G−, CD11b+ cells) compared with controls (Figure 3B). To determine whether changes in neutrophils are present in CCR2−/− versus controls, we isolated wounds on day 3 and performed flow cytometry interrogating the live, lineage−, Ly6G+ cell (neutrophil) population. We found no differences in the percentages of neutrophils in the CCR2−/− mice compared with controls, suggesting the differences in inflammatory profiles were not due to neutrophils (Supplemental Figure 2. Taken together, these results suggest that wound macrophages lacking CCR2 are less inflammatory, and early healing is impaired due to the lack of initial inflammation.
Myeloid-specific CCR2 is sufficient to rescue wound healing.
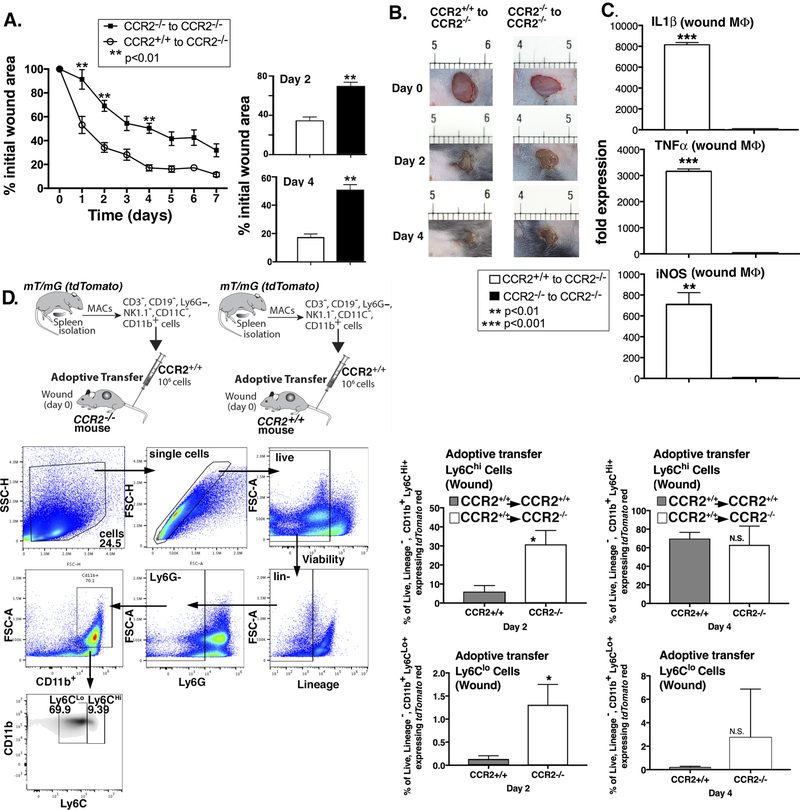
In order to determine if CCR2+ MoMΦs can restore healing in CCR2-deficient mice by restoring the initial inflammatory response, we performed an adoptive transfer using wild type and CCR2-deficient monocytes (CCR2+/+ and CCR2−/−) into CCR2−/− mice. We hypothesized that replacement of CCR2+/+ monocytes into CCR2−/− mice would improve wound healing. We extracted MoMΦs from CCR2+/+ and CCR2−/− spleens using Ficoll separation followed by MACS (CD3−, CD19−, Ly6G−, NK1.1−, CD11c−, CD11b+ cells). 1×106 cells (either CCR2+/+ or CCR2−/−) were administered via tail vein injection to CCR2−/− mice. Mice were then wounded and wound healing was monitored daily for 9 days. We found that wound healing was significantly improved in mice that received CCR2+/+ MoMΦs compared to mice that received CCR2-deficient MoMΦs (Figure 4A). Figure 4B shows representative images of mouse wound areas on days 0, 2, and 4 for comparison between the two groups, showing the improved wound healing in the CCR2-deficient mice that received wild type macrophages. In order to examine the mechanism for improved wound healing in the CCR2+/+ to CCR2−/− adoptive transfer, we examined gene expression in the MoMΦs from wounds of the adoptively transferred mice. This revealed significantly increased expression of IL1β, TNF-α, and iNOS in the CCR2-deficient mice that received wild type macrophages compared with those that did not (Figure 4C). This suggests that myeloid-specific CCR2 was sufficient to rescue wound healing in these mice, likely by restoring the initial inflammatory phase necessary for proper wound healing. To ensure that the macrophages we injected tracked to the wound and thus comprised a significant portion of the population we interrogated, we performed another adoptive transfer, injecting 1×106 CD3−, CD19−, Ly6G−, NK1.1−, CD11c−, CD11b+ cells from mT/mG mice (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) into CCR2+/+ and CCR2−/− mice. We examined wounds on day 2 and 4 and saw a significant number of labeled tdTomato+ (CD3−, CD19−, Ter119−, NK1.1−, Ly6G−, CD11b+) cells in both the CCR2−/− and CCR2+/+ mice. This percentage increased significantly from day 2 to 4 (Figure 4D). Further, when we examined Ly6CHi/Ly6CLo populations in wounds from the adoptively transferred mice, we found that CCR2−/− mice had a higher percentage of both Ly6CHi and Ly6CLo tdTomato+ (CD3−, CD19−, Ter119−, NK1.1−, Ly6G−, CD11b+) cells in their wounds, suggesting there were more recruited cells in this cohort. Taken together, these results suggest that there is increased recruitment of MoMΦs to CCR2−/− wounds and that improved wound healing in the CCR2-deficient mice that received wild type MoMΦs is likely due to the restoration of the initial inflammatory response.
Figure 4. Myeloid-specific CCR2 was sufficient to rescue wound healing in CCR2−/− mice.
CD3−, CD11c−, CD19−, Ly6G−, NK1.1−, CD11b+ single cell suspensions were isolated from CCR2−/− and CCR2+/+ spleens by MACS. 1 × 106 cells were injected intravenously via tail vein into wounded (day 0) CCR2−/− mice and wound closure was measured daily using ImageJ software (**P < 0.01; n=15; data is representative of two independent experiments). B: Representative images are shown from CCR2−/− and CCR2+/+ mice on days 0, 2, and 4. C: Wound macrophages were isolated from adoptively transferred CCR2+/+ to CCR2−/− and CCR2−/− to CCR2−/− mice on day 2 post-injury by MACS and gene expression (IL1β, iNOS, and TNF-α) in isolated macrophages was measured by qPCR using 18s for normalization (**P < 0.01; ***P<0.0001; n=12; data is representative of three independent experiments). D: 1×106 tdTomato-expressing CD3−, CD11c−, CD19−, Ly6G−, NK1.1−, CD11b+ single cell suspensions were injected via tail vein into wounded (day 0) CCR2−/− and control mice. Wounds were harvested on days 2 and 4 and single cell suspensions were processed for flow cytometry. Percentage of tdTomato+ (CD3−, CD19−, Ter119−, NK1.1−, Ly6G−, CD11b+) and subsequent stratification by Ly6C designation is shown (*P < 0.05; n=10; data is representative of two independent experiments). Statistical analysis was performed using a paired Student’s t-test. All data are expressed as mean ± SEM.
DISCUSSION
In this study, we investigated the role of CCR2 in MoMΦ recruitment and the initiation of the inflammatory phase of wound healing. We found that CCR2 is crucial for initial monocyte recruitment and for regulated wound healing. CCR2-deficient mice demonstrated significantly impaired wound healing, particularly during the early inflammatory phase, when recruitment of inflammatory cells is crucial for the initiation of the healing cascade and directs the subsequent progression of healing. Additionally, our results show that wound macrophages from CCR2-deficient mice display decreased expression of well-established proinflammatory mediators that are critical for normal tissue repair. Furthermore, when myeloid-specific CCR2 was replenished in the CCR2-deficient mice via adoptive transfer, wound healing was restored in these mice, suggesting that myeloid-specific CCR2 was both necessary and sufficient to rescue wound healing. In addition, upon analysis of wound macrophages isolated from CCR2−/− mice receiving wild type (CCR2+/+) MoMΦs, gene expression of key inflammatory mediators (IL1β, TNF-α, and iNOS) was restored. Taken together, these results suggest that CCR2 and recruited MoMΦs are necessary to mount the appropriate inflammatory response crucial for successful wound healing. Figure 5 depicts a summary diagram of CCR2-mediated recruitment.
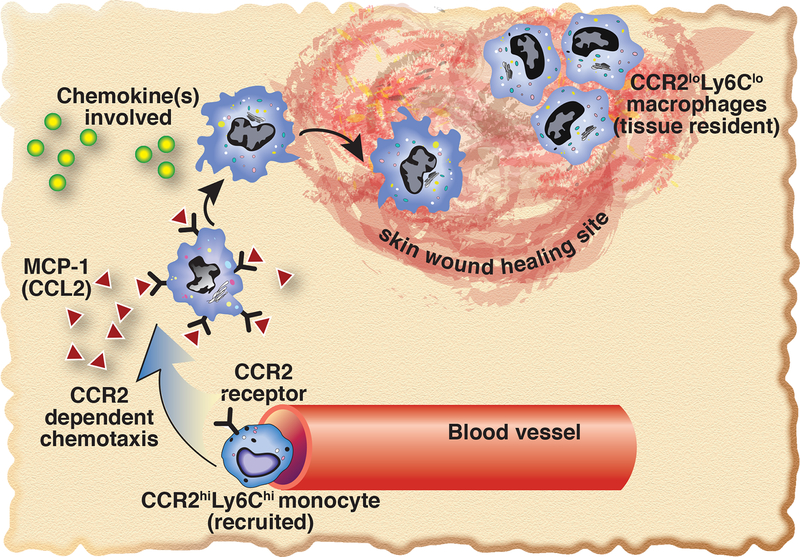
Figure 5. Schematic of CCR2-dependent Ly6CHi cell recruitment to peripheral wounds.
Upon initial tissue injury, CCL2, one of the primary ligands for CCR2, is increased in the wound. This ligand binds the CCR2 receptors that are present on Ly6CHi monocytes, recruiting these cells to the wound, allowing initiation of the macrophage-mediated inflammatory phase of wound healing.
The role of CCR2 in tissue repair has been investigated in liver and the myocardium[13],[24]. In the setting of liver injury, Dal-Secco et al found that fluorescent-labeled classical monocytes were recruited to the site of injury within the first 48 hours, forming a ring around the injury site[13]. Furthermore, CCR2-deficient mice had a significant reduction in recruitment of classical monocytes to the injury site. Similar findings were demonstrated in a murine model of cardiac injury, where an initial influx of CCR2+ monocytes occurred post-injury[24]. Interestingly, in a related study, Lavine et al revealed that blocking CCR2, and thus preventing recruitment to the tissue post-injury, improved cardiac remodeling[38].
While the role of CCR2 in tissue healing has been investigated in liver, cardiac tissues, skeletal muscle, and in healing of bone fractures, the majority of the studies examining the role of CCR2 in cutaneous wound healing revolve around tissue resident macrophage populations. For example, Willenborg et al used a similar model to our studies utilizing excisional punch biopsies; however, these authors examined F4/80+ resident macrophage inflammation and proangiogenic factors[27]. Interestingly, their results within the F4/80+ cell population mirrored the inflammatory profile of the recruited MoMΦs we examined in our study; that is, that CCR2 is necessary for the initial macrophage-mediated inflammatory response in skin wounds. They further concluded that it is these cells that give rise to a highly proangiogenic, VEGF-expressing macrophage subset[27]. A study by Crane et al also investigated the F4/80+ resident macrophage response to foreign body inflammation using subcutaneously implanted polyvinyl alcohol sponges[20]. In contrast, our work focuses on the effect of CCR2 on recruited circulating MoMΦ populations. We chose not to specifically target the F4/80+ resident macrophage population given that these macrophages are thought to populate the tissues prior to birth and are replaced through self-renewal, making their full characterization in granulation tissue lower yield[6],[32]. Furthermore, in one of our recent publications, we compared differences in Ly6C populations with and without inclusion of F4/80+ cells in our flow cytometry gates, and found no significant differences. This is most likely because following injury, the dominant MoMΦ cell populations are recruited from circulation, and thus, are initially not F4/80+.
To our knowledge, the only study in the literature to date regarding the role of CCR2 in circulating monocyte populations in cutaneous wound healing is by Rodero et al[26]. These authors developed a new model for real-time in vivo imaging of recruited monocyte populations after murine excisional scalp injury. They found a complete abrogation of monocyte infiltration in early wounds in CCR2-deficient mice. While these findings corroborate our findings, suggesting CCR2 is necessary for recruitment of circulating monocytes to the wound, their study only looked at the first four hours post-injury.
Our study is the first to examine the role of CCR2 in the initial inflammatory response of recruited circulating MoMΦs in wound healing. As expected, our flow cytometry results demonstrated decreased Ly6CHi cells in our CCR2−/− wounds; however, interestingly the CCR2−/− wounds demonstrated a significant increase in Ly6CLo cells. The reason for this increase in Ly6CLo cells is unknown; however, some studies suggest that Ly6CLo cells are derived from Ly6CHi cells after day 3 post-injury [13],[24],[38], while other studies suggest a separate wave of monocyte influx after day 3[39]. Thus, as the CCR2−/− mice expressed a predominance of Ly6CLo cells, yet were unable to recruit Ly6CHi cells, this suggests that a separate Ly6CLo influx may occur. Alternatively, and more likely, the Ly6CLo cells in this cohort may represent tissue resident macrophages, a population of cells that also expresses Ly6CLo within cutaneous wounds.
Although our study suggests that CCR2 is necessary for the initial inflammatory response, there are a few limitations that should be addressed. While we examined macrophage-mediated inflammation in wound healing, there are other factors that play a role in this complex process. Although monocytes appear to be the predominant cell expressing the CCR2 receptor, there has been recent evidence that CCR2 may be expressed on neutrophils in a murine model of rheumatoid arthritis[40]. Since neutrophils play a key role in the early stages of wound healing, it is possible that some of the healing deficits seen in our global CCR2−/− knockouts are mediated by neutrophils, although we did not detect differences in the neutrophil population. Additionally, since our studies focused specifically on recruited macrophage populations rather than tissue resident macrophages, it is possible that F4/80+ resident macrophages also contribute to the changes in healing seen in our experiments. Since the macrophage response to injury is complex, further studies are needed to determine the specific cells and genes that are critical for healing. It is clear that there are redundant pathways that contribute to inflammation post-injury.
To our knowledge, our study is the first to examine the role of CCR2 in recruited MoMΦs and inflammation in wound healing. These findings may allow for clinical translation via timed therapeutic manipulation of CCR2+ monocytes in both normal and pathologic wound healing. For example, non-healing type 2 diabetic wounds are characterized by an underlying dysregulation of the inflammatory phase of wound healing. Our lab and others have shown that diabetic mice demonstrate poor wound healing as a result of chronic, dysregulated macrophage-mediated inflammation in wounds. Interestingly, we have found that the primary ligand for CCR2, CCL2, is decreased early in diabetic wounds[41]. These findings suggest CCL2 as a possible target for the manipulation of the CCL2/CCR2 interaction to therapeutically alter the timing of macrophage-mediated inflammation in diabetic wounds to improve wound healing. Our findings show that CCR2 is crucial for the initial inflammatory response in tissues, and that the timing of MoMΦs subsets in the wound is integral for progression of the wound healing cascade.
MATERIALS AND METHODS
Mice.
All mice were maintained at the Biomedical Sciences and Research Building at the University of Michigan and all experiments were conducted under approval of the Institutional Animal Care and Use Committee (UCUCA), approval number PRO00005974. C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) and CCR2−/− (kind gift of Dr. Bethany Moore, University of Michigan) were obtained and maintained in breeding pairs by the University of Michigan Unit for Laboratory and Animal Medicine (ULAM). mT/mG mice (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) were purchased from Jackson Laboratory. All animals underwent procedures at 20 to 32 weeks of age with Institutional Animal Care and Use Committee approval.
Wound Healing Model.
Mice were anesthetized with intraperitoneal ketamine/xylazine mixture, dorsal hair was removed with Veet Hair Removal Cream, and dorsal skin was cleaned with sterile water. Full thickness 4mm punch biopsy wounds were then created at 2–4 locations on the mid-back (two wounds for wound monitoring, and four wounds for cell isolation, RNA, and protein experiments). Mice were allowed to recover on a heating pad and then were returned to the housing facility once ambulatory.
Assessment of Wound Healing.
An 8mp digital iPad camera was used to monitor wound healing over time. Internal scales were used in the photographs and wound area was calculated using NIH ImageJ Software (National Institute of Heath, Bethesda, MD). Initial wound size was calculated immediately after wounding, and wound closure was assessed over time as a percent of initial wound area.
Wound histology.
On day 3 post wounding, whole wounds were excised using a 6mm punch biopsy. Wound sections were fixed in 10% formalin overnight before embedding in paraffin. 5μM sections were stained with hematoxylin and eosin for evaluation of re-epithelialization and with Masson’s Trichrome stain for collagen deposition. Images were captured using Olympus BX43 microscope and Olympus cellSens Dimension software. Percent re-epithelialization was calculated by measuring distance traveled by epithelial tongues on both sides of wound divided by total distance needed for full re-epithelialization.
Wound Digestion.
Following sacrifice, wounds were collected from the backs of the mice post-mortem following CO2 asphyxiation using a 6mm wound biopsy. Sharp scissors were used to excise the full thickness dermis with a 1–2mm margin around the wound ensuring collection of granulation tissue and wounds were placed in RPMI. Wounds were then carefully minced with sharp scissors and digested by incubating in a 50 mg/ml Liberase TM (Roche) and 20U/ml DNaseI (Sigma-Aldrich) solution. Wound cell suspensions were then gently plunged and filtered through a 100µm filter to yield a single cell suspension. Cells were then either MACS sorted for CD11b+ cells for RNA studies or cultured ex-vivo for application of GolgiStop and subsequent staining for intracellular flow cytometry[37].
Magnetic-Assisted Cell Sorting (MACS) of Wound Macrophages.
CD11b+ cells were isolated from single cell suspensions as previously described[37]. Cell suspensions were incubated with FITC-labeled anti-CD19, anti-CD3, and anti-Ly6G magnetic beads (Biolegend) followed by anti-FITC microbeads (Miltenyi Biotec) and passed over magnetic columns. The eluent was then collected and incubated with anti-CD11b microbeads and passed through a second column to isolate CD11b+ cells. CD11b+ wound isolates were then collected and placed in Trizol® (Invitrogen).
Quantitative Polymerase Chain Reaction (qPCR).
After being placed in Trizol® RNA was isolated following the standard protocol using chloroform, isopropyl alcohol, and ethyl alcohol. cDNA was then created using either iScript® (BioRad) or Superscript III® Reverse Transcriptase (ThermoFisher Scientific). Quantitative PCR was performed using primers specific for CCL2, IL1β, TNF-α, and iNOS (Thermo Fisher Scientic). All genes were normalized to 18s rRNA (Thermo Fisher Scientic) and their expression was analyzed using the 2∆Ct method. Data was compiled in Microsoft Excel (Microsoft) and presented using Prism software (GraphPad).
Ex-Vivo Flow Culture.
For ex-vivo intracellular flow cytometry, single-cell wound suspensions were incubated in single-housed Teflon coated wells with RPMI + FBS + Glutamine + Pen/Strep + LPS for 90 minutes, followed by addition of GolgiStop (BD Biosciences) in a 1:2000 dilution to halt Golgi transport. After 120 minutes of incubation with GolgiStop, cells were aspirated from the Teflon coated wells and washed in PBS prior to staining for analytical flow cytometry.
Flow Cytometry.
Single cell suspensions were collected and washed two times with cold PBS and filtered into a 96-well plate for surface staining. Cells were initially stained with pacific orange LIVE/DEAD fixable viability dye (Thermofisher) and then washed two times with cold PBS. Cells were then resuspended in Flow Buffer (PBS, FBS, NaN3, and Hepes Buffer) and Fc-Receptors were blocked with anti-CD16/32 (Biolegend) prior to surface staining. Biotinylated monoclonal antibodies used for surface staining included: Anti-CD3, Anti-CD19, Anti-Ter-119, Anti-NK1.1, Anti-CD11b, Anti-Ly6G, and Anti-Ly6C (Biolegend). Following surface staining, cells were washed twice, and biotinylated antibodies were labeled with streptavidin APC-Cy7. Next, cells were either washed and acquired for surface-only flow cytometry, or were fixed with 2% formaldehyde and then washed/permeabilized with BD perm/wash buffer (BD Biosciences) for intra-cellular flow cytometry. After permeablilization, intra-cellular stains included: anti-IL1β (BD Biosciences), anti-TNF-α (Biolegend), anti-iNOS (affymetrix). After washing, samples were then acquired on a 3-Laser Novocyte Flow Cytometer (Acea Biosciences, Inc.). Data was analyzed using FlowJo software version 10.0 (Treestar, Inc.) and data was compiled using Prism software (GraphPad, Inc.). To verify gating and purity, all populations were routinely back-gated.
Adoptive transfer.
Two cohorts of CCR2−/− mice were wounded as described above. Spleens from wild type CCR2+/+ and CCR2−/− mice were harvested and CD3-, CD11c-, CD19-, Ly6G-, NK1.1- CD11b+ cells were isolated using microbeads and magnetic columns as described above. One million cells in 200 μL total volume were injected via tail vein into each mouse within two hours of wounding. One mouse cohort received wild type (CCR2+/+) CD11b+ cells while the other received CCR2−/− CD11b+ cells for control. Wound healing was monitored over time and wound area was calculated using NIH ImageJ Software (National Institute of Heath, Bethesda, MD). Initial wound size was calculated immediately after wounding, and wound closure was assessed over time as a percent of initial wound area. Another adoptive transfer was performed using mT/mG-labeled mice. Spleens from mT/mG CCR2+/+ mice were harvested and CD3−, CD11c−, CD19−, Ly6G−, NK1.1−, CD11b+ cells were isolated using microbeads and magnetic columns as described above. One million cells in 200 μL total volume were injected via tail vein into CCR2+/+ and CCR2−/− mice within two hours of wounding. Flow cytometry was performed on wounds on days 2 and 4 post-injury and analyzed for both recruitment of tdTomato+ (CD3−, CD19−, Ter119−, NK1.1−, Ly6G−, CD11b+) cells and Ly6CHi and Ly6CLo populations.
Statistical Analysis.
Data was analyzed using GraphPad Prism software version 6.0 and the paired student’s T-test was used to assess statistical significance. Unless otherwise specified, data is expressed as the mean +/− the standard error of the mean (SEM). A P-value of 0.05 or less was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Robin Kunkel for her assistance with the graphical illustrations.
This work was supported in part by NIH DK102357.
Abbreviations:
- MoMΦs
monocyte/macrophages
- CCR2
Chemokine Receptor 2
- CCL2
Chemokine Receptor Ligand 2, aka MCP-1
- MCP-1
Monocyte chemoattractant protein 1, aka CCL2
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell. Mol. Life Sci 2015; 72:4111–4126. Available at: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84944170532&doi=10.1007%2Fs00018-015-1995-y&partnerID=40&md5=78103723faf8f89982ce2a1fe580aa92 DOI: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin P Wound Healing--Aiming for Perfect Skin Regeneration. Science (80-. ) 1997; 276:75–81. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.276.5309.75DOI: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 2011; 117:5264–5272.DOI: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (80-. ) 2010; 330:841–5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3719181&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/20966214DOI: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med 2012; 209:1167–81. Available at: http://jem.rupress.org/content/209/6/1167.fullDOI: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012; 336:86–90. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22442384DOI: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 7.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Gomez Perdiguero E, Wieghofer P, et al. Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nat. Neurosci 2013; 16:273–280.DOI: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 8.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19:71–82.DOI: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 9.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler. Thromb. Vasc. Biol 2009; 29:1412–1418.DOI: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006; 211:609–618.DOI: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol 2006; 7:311–7. Available at: http://www.nature.com/doifinder/10.1038/ni1309%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/16462739DOI: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 12.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest 2007; 117:902–909.DOI: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CHY, Petri B, Ransohoff RM, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med 2015; 212:447–456. Available at: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84928254123&doi=10.1084%2Fjem.20141539&partnerID=40&md5=a192ee67c258cacb80452e6c5672ad55DOI: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol 1991; 3:65–70.DOI: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol 2005; 5:953–64. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16322748DOI: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23–35. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12511873%5Cnhttp://www.nature.com/nri/journal/v3/n1/pdf/nri978.pdfDOI: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549–555.DOI: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 18.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science (80-. ) 2007; 317:666–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17673663DOI: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 19.Arnold Ludovic,1 Adeline Henry,2 Françoise Poron,1 Yasmine Baba-Amer 1, van Nico Rooijen,3 Anne Plonquet,4 Romain K. Gherardi, and Bénédicte Chazaud 1, Arnold L, Henry A, Poron F, Baba-Amer Y, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007; 204:1057–1069. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17485518%5Cnhttp://www.ncbi.nlm.nih.gov/pmc/articles/PMC2118577/pdf/jem2041057.pdfDOI: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crane MJ, Daley JM, Van Houtte O, Brancato SK, Henry WL Jr., Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS One 2014; 9 Available at: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84899550476&doi=10.1371%2Fjournal.pone.0086660&partnerID=40&md5=3e71889f236bbe3ac83e96654a52eea6DOI: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilgendorf I, Gerhardt LMS, Tan TC, Winter C, Holderried TAW, Chousterman BG, Iwamoto Y, et al. Ly-6 chigh monocytes depend on nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res 2014; 114:1611–1622.DOI: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell C, Couton D, Couty J-P, Anson M, Crain A-M, Bizet V, Rénia L, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am. J. Pathol 2009; 174:1766–75. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2671265&tool=pmcentrez&rendertype=abstractDOI: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest 2005; 115:56–65.DOI: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014; 40:91–104.DOI: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albina JE, Mills CD, Henry WL, Caldwell MD. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J. Immunol 1990; 144:3877–3880. [PubMed] [Google Scholar]
- 26.Rodero MP, Licata F, Poupel L, Hamon P, Khosrotehrani K, Combadiere C, Boissonnas A. In vivo imaging reveals a pioneer wave of monocyte recruitment into mouse skin wounds. PLoS One 2014; 9DOI: 10.1371/journal.pone.0108212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willenborg S, Lucas T, Van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012; 120:613–625.DOI: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 28.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol 2010; 184:3964–77. Available at: http://www.jimmunol.org/content/184/7/3964.fullDOI: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 29.Kimball AS, Joshi A, Carson WF, Boniakowski AE, Schaller M, Allen R, Bermick J, et al. The histone methyltransferase mll1 directs macrophage-mediated inflammation in wound healing and is altered in a murine model of obesity and type 2 diabetes. Diabetes 2017; 66:2459–2471.DOI: 10.2337/db17-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimball AS, Joshi AD, Boniakowski AE, Schaller M, Chung J, Allen R, Bermick J, et al. Notch regulates macrophage-mediated inflammation in diabetic wound healing. Front. Immunol 2017; 8DOI: 10.3389/fimmu.2017.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013; 6:498–510. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3629381&tool=pmcentrez&rendertype=abstractDOI: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013; 38:79–91.DOI: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010; 10:453–460. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20467425DOI: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004; 22:812–822.DOI: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 2007; 15 Suppl 1:S18–26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17727462DOI: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 36.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol 1975; 78:71–100. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1915032&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza RE, Fang MM, Ennis WJ, Kohl TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013; 62:2579–2587.DOI: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. U. S. A 2014; 111:16029–34. Available at: http://www.pnas.org/content/111/45/16029.abstractDOI: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med 2007; 204:3037–47. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18025128%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2118517DOI: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talbot J, Bianchini FJ, Nascimento DC, Oliveira RDR, Souto FO, Pinto LG, Peres RS, et al. CCR2 expression in neutrophils plays a critical role in their migration into the joints in rheumatoid arthritis. Arthritis Rheumatol 2015; 67:1751–1759.DOI: 10.1002/art.39117. [DOI] [PubMed] [Google Scholar]
- 41.Kimball A, Schaller M, Joshi A, Davis F, denDekker A, Boniakowski A, Bermick J, et al. Ly6C<sup>Hi</sup> Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol 2018. Available at: http://atvb.ahajournals.org/content/early/2018/02/28/ATVBAHA.118.310703.abstract. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.