Abstract
Background:
All human islets used in research and for the clinical treatment of diabetes are subject to ischemic damage during pancreas procurement, preservation, and islet isolation. A major factor influencing islet function is exposure of pancreata to cold ischemia during unavoidable windows of preservation by static cold storage (SCS). Improved preservation methods may prevent this functional deterioration. In the present study, we investigated whether pancreas preservation by gaseous oxygen perfusion (persufflation) better preserved islet function versus SCS.
Methods:
Human pancreata were preserved by SCS or by persufflation in combination with SCS. Islets were subsequently isolated, and preparations in each group matched for SCS or total preservation time were compared using dynamic glucose stimulated insulin secretion as a measure of β cell function and RNA sequencing to elucidate transcriptomic changes.
Results:
Persufflated pancreata had reduced SCS time, which resulted in islets with higher glucose stimulated insulin secretion compared to islets from SCS only pancreata. RNA sequencing of islets from persufflated pancreata identified reduced inflammatory and greater metabolic gene expression, consistent with expectations of reducing cold ischemic exposure. Portions of these transcriptional responses were not associated with time spent in SCS and were attributable to pancreatic reoxygenation. Furthermore, persufflation extended the total preservation time by 50% without any detectable decline in islet function or viability.
Conclusions:
These data demonstrate that pancreas preservation by persufflation rather than SCS prior to islet isolation reduces inflammatory responses and promotes metabolic pathways in human islets, which results in improved β cell function.
Introduction:
Quality human islets are essential for emerging clinical therapeutics for type 1 diabetes.1,2 Human islets are obtained from deceased donor pancreata, which experience unavoidable periods of preservation during procurement, transport, and islet isolation. The most widely used method of pancreas preservation is static cold storage (SCS), where the organ is placed in preservation solution and maintained between 4–8°C.3 Reports indicate that SCS is insufficient to maintain oxygenation in larger (human, porcine) pancreata.3,4 An alternative to SCS is gaseous oxygen perfusion (persufflation)5, which improves pancreatic oxygenation, viability, structural integrity, and ATP content.6–8 A proposed mechanism for these improvements is improved oxygenation (reduced cold ischemia), which has a demonstrable impact on β cell function.9,10 Previous research has shown persistent changes in islets exposed to hypoxia that result in poorer transplant outcomes.11
Given that persufflation improves the preservation of intact pancreata, we hypothesize that islets isolated from persufflated pancreata will have more robust insulin secretion due to transcriptional adaptations to cold ischemia that negatively impact β cell function. Despite previous research, which indicated that enhanced SCS (ie, the incorporation of a perfluorocarbon with a higher oxygen solubility) may be sufficient to maintain a larger fraction of the volume of rodent pancreata due to small size, evidence from a study using rodent islets indicated pancreatic persufflation improved insulin secretion and viability, and morphology.3,4,12 Given the beneficial effects even in smaller pancreata, in the present study, we evaluate the transcriptomes and function of human islets isolated from pancreata preserved by persufflation (SCS+PSF) and islets from pancreata preserved by SCS.
Material and Methods
Persufflation and Islet Culture
Twenty-four human islet preparations were obtained from deceased donors with informed consent from the Integrated Islet Distribution Program, the University of California – San Francisco, the McGill University Health Centre, and the University of Arizona. Islets were isolated from pancreata preserved by static cold storage (SCS, n=11) or by SCS followed by persufflation (SCS+PSF, n=13). Treatment groups were not randomized within an institution but SCS+PSF pancreata were collected from institutions capable of organ persufflation and preparations selected for our experimental cohorts were based on comparable length of SCS total preservation time (TPT). TPT was the sum of SCS time and persufflation (PSF) time. In the SCS group, TPT represents only duration of SCS.
Persufflation was performed as described previously.6,8 Briefly, pancreata were procured from donors with the spleen and duodenum left intact to minimize gas leaks upon persufflation. All persufflation procedures were conducted under sterile conditions. Following procurement, the superior mesenteric and splenic arteries of the pancreas were fitted with appropriately sized, sterile cannulae and secured with surgical silk. Pancreata were attached to a persufflator via sterile silicone tubing connected to the adapters, which directly supplied the pancreatic vasculature with 40% humidified oxygen (Giner, Inc., Auburndale, MA). Leaks were identified and ligated with surgical silk and portal-venous outflow was observed to ensure that oxygen was dispersed throughout the organ. Pancreata were then submerged in Belzer University of Wisconsin cold preservation solution (Preservation Solutions, Inc., Elkhorn, WI) and maintained at 4°C for the duration of preservation.
Islets were cultured in GRex vessels (Wilson Wolf Corporation, St. Paul, MN) containing CMRL1066 media (Mediatech, Inc., Manassas, VA) with 0.5% Human Serum Albumin (Grifols), 10 units/mL heparin (SAGENT, Schaumburg, IL)), and 1% Penicillin/Streptomyocin (GE Healthcare Life Sciences, Logan, UT). Islets were cultured at ambient temperature and O2 supplemented with 5% CO2 prior to assessments, which were conducted at 37°C.
Characterization of Islet Purity and Viability
Islet preparation purity was determined at each isolation center by positive dithizone (DTZ) staining. Islet viability was measured by oxygen consumption rate normalized to DNA (OCR/DNA) as reported previously.13 Briefly, islets were placed in stirred, sealed titanium chambers whose oxygen partial pressure was continuously monitored (Instech Laboratories, Plymouth Meeting, PA). Following measurements of oxygen consumption, islets were carefully retrieved, and their DNA content was measured using a Quant-iT™ PicoGreen dsDNA kit according to manufacturer’s instructions (Life Technologies, Carlsbad, CA). A linear region of the measured pO2 versus time plot was identified and the slope was calculated and normalized to chamber DNA content, yielding OCD/DNA.
While fluorescein diacetate/propidium iodide staining is currently the standard assessment for islet viability, it is subjective and cannot detect cell death until the late stages of apoptosis.14,15 OCR/DNA is a more objective, sensitive assay which is also predictive of diabetes reversal, and it was therefore employed in this study as a correlate for islet viability.15–18
RNAseq Data Analysis
The RNAseq cohort was comprised of islets with similar TPT (n=4 SCS, n=5 SCS+PSF; Table 1).19 From each preparation, RNA was isolated using an RNeasy Mini Kit (Qaigen) from 3000 islet equivalents (IE). Samples with RNA quality (RIN score) score >7.0 as assessed by the Agilent Bioanalyzer were prepared using the Illumina TruSeq RNA Sample Prep Kit (Illumina Inc., San Diego, CA) and sequenced on the Illumina HiSeq2500. Reads were trimmed with Trimmomatic and assessed for quality by FASTQC (Babraham Bioinformatics, Cambridge, UK).20
Table 1.
Shown above are characteristics for the 24 individual islet preparations used in the present study. BMI= body mass index. TPT=total preservation time. CIT=cold ischemia time. OCR/DNA=oxygen consumption rate normalized to DNA. Purity for islet preparations was determined at each isolation center by positive dithizone (DTZ) staining and percentage calculated as DTZ positive cells Assessments column denotes what experiments for this manuscript each donor islet preparation was included in.
| ID | Treatment | Age (Years) | Sex | BMI | Purity (%) | TPT (Hours) | CIT (Hours) | OCR/DNA (nmol O2/min*mg DNA) | Assessments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SCS | 44 | F | 36.7 | 85 | 9.9 | 9.9 | 179.1 | RNAseq |
| 2 | SCS | 44 | F | 23 | 80 | 12.2 | 12.2 | 109.1 | RNAseq |
| 3 | SCS | 58 | M | 26.8 | 90 | 6.1 | 6.1 | 182.3 | PCR, RNAseq, GSIS |
| 4 | SCS | 61 | F | 31.1 | 90 | 5.6 | 5.6 | 124.6 | PCR, RNAseq |
| 5 | SCS | 20 | M | 41 | 90 | 9.8 | 9.8 | 172.4 | PCR, GSIS |
| 6 | SCS | 39 | F | 26.9 | 95 | 13.6 | 13.6 | 91.0 | PCR |
| 7 | SCS | 23 | M | 22 | 90 | 14.7 | 14.7 | 89.5 | PCR |
| 8 | SCS | 28 | M | 28 | 93 | 10.3 | 10.3 | 103.9 | GSIS |
| 9 | SCS | 30 | M | 29.1 | 95 | 9.6 | 9.6 | 166.7 | GSIS |
| 10 | SCS | 36 | M | 33.8 | 95 | 8.0 | 8.0 | 163.1 | GSIS |
| 11 | SCS | 40 | M | 35.4 | 90 | 8.3 | 8.3 | 191.2 | GSIS |
| 12 | SCS+PSF | 58 | M | 26.0 | 95 | 13.8 | 6.8 | 121.7 | PCR, RNAseq |
| 13 | SCS+PSF | 45 | M | 27.6 | 95 | 16.3 | 6.3 | 104.2 | PCR, RNAseq, GSIS |
| 14 | SCS+PSF | 64 | F | 28.6 | 75 | 11.8 | 1.3 | 105.6 | RNAseq, GSIS |
| 15 | SCS+PSF | 53 | M | 29.3 | 81 | 8.8 | 1.5 | 167.6 | RNAseq, GSIS |
| 16 | SCS+PSF | 54 | M | 40.3 | 95 | 13.1 | 5.5 | 107.7 | RNAseq, GSIS |
| 17 | SCS+PSF | 34 | M | 37.7 | 90 | 16.3 | 13.0 | 132.7 | PCR |
| 18 | SCS+PSF | 35 | M | 24.8 | 95 | 17.1 | 14.0 | 161.2 | PCR |
| 19 | SCS+PSF | 33 | F | 31.1 | 87 | 12.8 | 2.0 | 174.2 | GSIS |
| 20 | SCS+PSF | 58 | M | 25.8 | 85 | 8.0 | 2.4 | 168.6 | GSIS |
| 21 | SCS+PSF | 56 | F | 41.9 | 75 | 9.4 | 6.3 | 154.4 | GSIS |
| 22 | SCS+PSF | 42 | M | 34.2 | 85 | 11.0 | 8.0 | 147.5 | GSIS |
| 23 | SCS+PSF | 17 | M | 27.7 | 93 | 7.4 | 4.1 | 161.4 | GSIS |
| 24 | SCS+PSF | 44 | M | 26.5 | 98 | 16.3 | 12.5 | 139.5 | GSIS |
Transcriptome and differential expression analysis were conducted using TopHat and Cufflinks programs.21 Paired-end reads were aligned to the reference genome (iGenomes, Homo sapien, Ensembl GRCh37) with Bowtie2 (v2.1.0) and TopHat (v2.0.9). The number of paired reads for each sample ranged from 30–60 million. Samples mapped at a rate of 95 ± 1% with 92 ± 1% concordant pair alignments. Cufflinks (v2.1.1) was used to assemble the aligned reads based on Ensembl build GRCh37 with annotation only option.21 Islets contain secretory cells with several genes, including insulin, account for a large fraction of the total transcripts. To capture low frequency genes and increase robustness of differential expression testing, the assembly parameters used were; -N/upper-quartile-norm and –-max-bundle-frags was kept at 1 million. A secondary analysis was performed to capture abundant transcripts by increasing the maximum number of reads allowed for a locus (--max-bundle-frags) to 25 million. CuffDiff was used to determine differential expression using default parameters and –b/frag-bias-correct.21 Normalized gene expression is presented as fragments per kilobase exon per million reads mapped (FPKM). Genes were differentially expressed if P<0.05 with a false discovery rate set to 5%. CummeRbund (Bioconductor) R package was used to visualize CuffDiff results.
Functional enrichment of differentially expressed genes was determined with gene ontology (GO) terms and pathways using KOBAS (v3.0).22 Pathways and GO terms were significant if the corrected P<0.05 following a Fisher’s Exact Test with Benjamini Hochberg correction.
Quantitative PCR
RNA was extracted from islets matched for TPT (n=4 SCS+PSF islets and n=5 SCS islets) that included subjects independent of the RNAseq analysis. RNA was reverse transcribed into cDNA with iScript then amplified with SsoAdvanced™ SYBR®Green (Biorad). mRNA concentrations were measured with a custom PrimePCR array containing validated human probes for 45 genes. The analysis was performed in duplicate on a CFX Connect PCR detection system (Biorad) with 40 cycles of 95°C for 10s and 60°C for 45s following a 2 minute incubation at 95°C. Specificity was determined with melt curve after product amplification. The C(t) values were normalized using the geometric mean of 3 reference genes; ACTB, HPRT1, and TBP. Fold change was calculated with the 2−ΔΔCT method.23
Insulin Secretion
Glucose stimulated insulin secretion (GSIS) was assessed using a Peri-4.2 perifusion system (Biorep Technologies Inc., Miami Lakes, FL) using a cohort of SCS+PSF islets matched for TPT and a separate cohort of SCS+PSF islets matched for time spent in SCS. Triplicate samples of 100 IE were exposed to 2.8 mM glucose for 20 minutes, then 16.7 mM glucose for 40 minutes. Glucose solutions were made in Krebs-Ringer Bicarbonate buffer saturated with 95% O2 and 5% CO2. Perfusate insulin content was determined by ELISA (ALPCO, Salem, NH) and normalized to DNA.19 Stimulation index was calculated by dividing stimulated AUC by normalized basal AUC. Normalized basal AUC was calculated by multiplying the average basal insulin secretion rate by the stimulated time interval. First and second phase AUC were calculated separately and normalized to basal AUC.
Statistical Analysis
Data were compared with mixed procedures in SAS (Cary, NC) and presented as means ± SEM (Prism v7.03 GraphPad Software, La Jolla, CA). Nonparametric tests were applied to data not normally distributed. Significance was defined at P < 0.05.
Results
Donor Islet Characteristics
Average donor age (42 ± 3 years) BMI (30.6 ± 1.2) were not different between SCS (n=11) and SCS+PSF (n=13; Table 1) and for all experimental subcohorts. Sex effect was not evaluated; all groups included males and females. Islet purity was not different between SCS and SCS+PSF groups (Table 1; 90.3 ± 1.4 and 88.3 ± 2.2 respectively). Islet viability was not different between SCS and SCS+PSF groups (Table 1, 143.0 ± 11.9 and 142.0 ± 7.1 nmol O2/min*mg DNA respectively).
Differential Gene Expression in Islets Isolated from Persufflated Pancreata
RNAseq detected 19,129 transcripts. Gene expression patterns show a treatment effect as SCS+PSF islets and SCS islets clustered more closely to their respective treatments (Figure S1). The most abundant genes were insulin (INS), insulin like growth factor 2 (IGF2), and the INS-IGF2 read-through transcript variant. Differential expression was not analyzed for these genes or 4 abundant protein-coding genes: glucagon, pancreatic enzyme elongation factor 1 complex, transthyretin, and pancreatic carboxypeptidase A1.
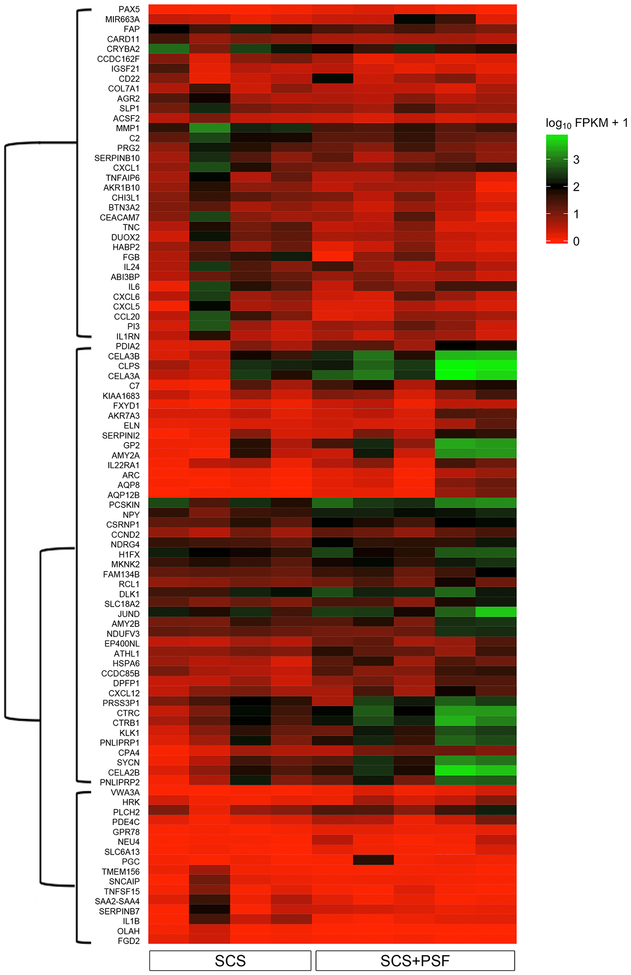
One hundred transcripts (0.5% of total) were differentially expressed in SCS+PSF islets versus SCS islets (Figure S2). No differential splicing or promoter usage was found between experimental groups. Thus, the 100 genes differentially expressed in SCS+PSF islets are not a result of specific transcript variants. A heatmap of gene expression depicts subsets with higher or lower expression levels in SCS+PSF islets versus SCS islets (Figure 1).
Figure 1: Heatmap of genes differentially expressed in SCS+PSF versus SCS islets.
Expression is presented as log2 FPKM with a pseudo-addition of 1 to avoid visual bias from genes with FPKM<1. Genes are clustered by Jensen-Shannon distance of FPKM to identify expression trends. There are 3 primary gene clusters denoted by a dendrogram along the y axis. Genes in the top branch of the map are lower in SCS+PSF islets compared to SCS while genes in the middle branch have higher expression in SCS+PSF islets compared to SCS. Genes in the bottom branch have low expression in both SCS+PSF and SCS but are significantly higher in SCS+PSF. The heatmap excludes noncoding and pseudo genes (<5% of total differentially expressed genes).
The most down-regulated genes (largest negative fold change) in SCS+PSF compared to SCS islets (SCS FPKM>5) included: chemokine ligand 20 (CCL20, log2FC=-5.4), interleukin 1, beta (IL1B, log2FC=-4.9), and chemokine ligand 5 (CXCL5, log2FC=-4.4). Genes downstream of IL1β were also lower; matrix metallopeptidase 1 (MMP1) and caspase recruitment domain family member 11 (CARD11). Functional analysis of genes lower in SCS+PSF islets identified inflammation. Of the 11 significantly enriched pathways, 9 (82%) involved immune processes (Table 2). Similarly, the most significant (P<1×10−4) GO functions seen in down-regulated genes were cytokine activity and inflammatory response.
Table 2: Pathways Enriched in Differentially Expressed Genes.
The 41 genes with negative fold change in islets isolated from persufflated pancreata compared to static cold storage pancreata were annotated to canonical pathways. Significantly enriched pathways (P<0.05) were determined using KOBAS to search public databases (Reactome & KEGG). P values presented were determined by Fisher’s exact test. The same procedure was performed on the 59 genes with positive fold change in islets isolated from persufflated pancreata compared to static cold storage pancreata.
| Pathways Enriched in Downregulated Genes | |
| Pathways | P Value |
| Chemokine receptors bind chemokines | 9.4×10−4 |
| IL23-mediated signaling events | 1.4×10−3 |
| Cytokine-cytokine receptor interaction | 1.8×10−3 |
| Signal transduction through IL1R | 1.9×10−3 |
| TNF signaling pathway | 7.8×10−3 |
| Plasminogen activating cascade | 8.6×10−3 |
| Peptide ligand-binding receptors | 1.5×10−2 |
| G alpha (i) signalling events | 2.8×10−2 |
| Signaling by Interleukins | 3.7×10−2 |
| NOD-like receptor signaling pathway | 4.2×10−2 |
| Interleukin-1 signaling | 5.0×10−2 |
| Pathways Enriched in Upregulated Genes | |
| Pathways | P Value |
| Pancreatic secretion | 8.0×10−7 |
| Protein digestion and absorption | 7.2×10−4 |
| Lipid digestion, mobilization, and transport | 1.2×10−2 |
| Digestion of dietary carbohydrate | 2.0×10−2 |
| Na+/Cl− dependent transporters | 5.1×10−2 |
| Fat digestion and absorption | 5.3×10−2 |
| Neurotransmitter Release Cycle | 5.4×10−2 |
The most up-regulated genes (largest positive fold change) in SCS+PSF islets (SCS+PSF FPKM>100) are enriched for cellular metabolism, pancreatic secretion, and ion transport (Table 2). Up-regulated genes coded for prosurvival proteins such as Jun D Proto-Oncogene (JUND, log2FC=3.2), and proteins essential for β cell granule exocytosis including glycoprotein zymogen granule member 2 (GP2, log2FC=6.0) and pancreas-specific protein disulfide isomerase (PDIA2, log2FC=3.7).24,25 Intercellular neurotransmitter release cycle and Na+/Cl− dependent transporters were pathways enriched, however the genes within these pathways are known to function in β cells; solute (monoamine) carrier family 18 member 2 (VMAT2) and solute (neurotransmitter) carrier family 6, member 13 (GAT2). Consistent with previous research which demonstrated that persufflation raised the ATP/Pi ratio in pancreata versus those preserved by traditional methods, members of the FXYD-domain containing ion transport regulators, FXYD1 and FXYD7, which regulate the ATP/ADP ratio and membrane potential within cells, were higher in SCS+PSF islets.26
RTqPCR Validates RNAseq and Effects Specific to Persufflation
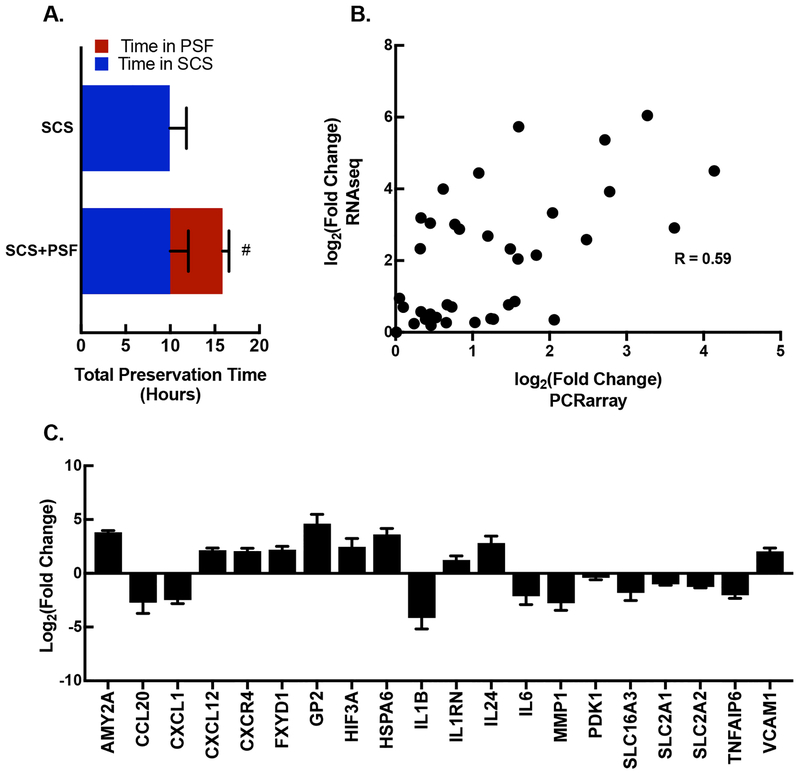
To confirm that changes in immune and metabolic genes were attributable to persufflation, an expanded cohort of SCS+PSF and SCS islets were analyzed by RTqPCR (individual sample characteristics presented in Table 1). SCS+PSF and SCS were exposed to the same cold ischemia (SCS) samples were included based on time spent in SCS This cohort contained SCS+PSF samples with similar time was matched for time spent in SCS and had longer TPT (Figure 2A). The fold changes of 42 genes from RTqPCR correlated (R=0.59, P<0.01) with fold changes generated by RNAseq (Figure 2B). Of the 26 targets selected based on RNAseq results, 20 genes (77%, P<0.05) were altered in SCS+PSF islets compared to SCS islets (Figure 2C).
Figure 2: RTqPCR validation of RNAseq results for metabolic and immunologic genes.
A. The average (±SEM) total organ preservation time (hours) is presented for islets isolated from SCS+PSF and SCS pancreata. These cohorts were matched for similar time in SCS. Experimental groups are presented on y axis (n=4 for SCS+PSF and n=5 for SCS). Bar area in blue denotes time in SCS, and the red area of the bar denotes time in PSF. #P<0.05 denotes difference in total preservation time. B. Absolute values of log2 transformed fold changes for 42 individual genes are plotted for RNAseq and RTqPCR (PCRarray) results. There is a significant (P<0.001) correlation between the 2 methods: slope of 0.59 with a 95% confidence interval of 0.328 to 0.763. C. PCR array identified 20 genes with immune and metabolic properties that were significantly different in PSF+SCS islets compared to SCS islets (log2 transformed; P<0.05). The genes are: AMY2A, Amylase Alpha 2A; CCL20, Chemokine Ligand 20; CXCL1, Chemokine Ligand 1; CXCL12, Chemokine Ligand 12; CXCR4, Chemokine Receptor 4; FXDY1, Phospholemman; GP2, Glycoprotein 2; HIF3A, Hypoxia Inducible Factor 3A; HSPA6, Heat Shock Protein A6; IL1B, Interleukin 1B; IL1RN, Interleukin 1 Receptor Antagonist; IL24, Interleukin 24; IL6, Interleukin 6; MMP1, Matrix Metallopeptidase 1; PDK1, Pyruvate Dehydrogenase Kinase 1; SLC16A3, Monocarboxylic Acid Transporter 4; SLC2A1, Glucose transporter 1; TNFAIP6, TNF Alpha Induced Protein 6; VCAM1, Vascular Cell Adhesion Molecule 1.
Insulin Secretion is Enhanced in Islets from Persufflated Pancreata
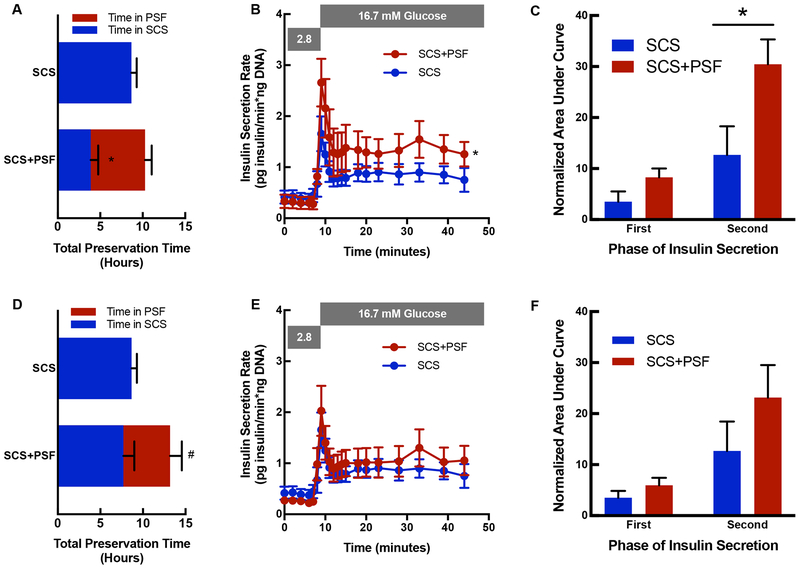
Functional assessments of SCS+PSF and SCS islets are shown for a cohort matched for TPT (Figure 3A–C) and for a cohort matched for SCS time (Figure 3D–F). When TPT was matched between SCS+PSF and SCS groups, PSF improved insulin secretion (P<0.05, Figure 3B), demonstrated by stimulation indices of 7.3 ± 2.1 and 2.5 ± 0.4 respectively, and attributable to higher second phase insulin secretion (P<0.01, Figure 3C). When SCS time was matched, TPT was extended by 52 ± 9% in the SCS+PSF group (P=0.01, Figure 3D) and there was no difference in GSIS (Figure 3E–F).
Figure 3: Function of SCS+PSF and SCS islets.
A. Total organ preservation time (hours) is presented for islets isolated from a cohort matched for total preservation time. Experimental groups are presented on y axis (n=8 for SCS+PSF and n=6 for SCS). Bar area in blue denotes time in SCS, and red area denotes time in PSF. *P<0.05 denotes difference between time in SCS (blue). B. GSIS was determined in islets isolated from SCS (blue) and SCS+PSF (red) pancreata that were matched for total preservation time. The insulin secretion rate (Y axis) by time (minutes, x axis) is presented. SCS+PSF islets have higher insulin secretion than SCS islets (*, P<0.05). C. First and Second Phase AUC are presented for islets isolated from a cohort matched for duration of total preservation time. AUC is presented on the y axis and insulin secretion phase is presented on the x axis for SCS islets (blue, n=6) and SCS+PSF islets (red, n=8). *P<0.05 denotes difference between SCS and SCS+PSF second phase insulin secretion. D. Total organ preservation time (hours) is presented for islets isolated from a cohort matched for duration of SCS (blue portion). Experimental groups are presented on y axis (n=5 for SCS+PSF and n=6 for SCS) and the red portion of the bar in SCS+PSF indicates PSF. #P<0.05 denotes difference between total preservation time. E. GSIS is presented for SCS and SCS+PSF islets for the SCS matched cohort and no difference in responsiveness were identified. F. First and Second Phase AUC are presented for islets isolated from a cohort matched for duration of SCS. AUC is presented on the y axis and insulin secretion phase is presented on the x axis for SCS islets (blue, n=6) and SCS+PSF islets (red, n=5).
Discussion
This study demonstrates that pancreas preservation by gaseous oxygen perfusion (persufflation) versus the current clinical standard of SCS reduces inflammatory gene expression and improves insulin secretion in isolated human islets. Despite widespread use, SCS of the pancreas does not prevent ischemic damage in islets, and extended periods of SCS lowers the probability of a successful islet isolation, lowers yields, increases cell death, and limits the therapeutic potential for transplantation.1,7,27–30 Given that logistical constraints lead to unavoidable windows of organ storage, improved preservation methods are required. Accordingly, we demonstrate that persufflation lowers expression levels of inflammatory genes compared to SCS only islets and extends the preservation time for pancreata without additional loss of GSIS or viability.
Deficits associated with preservation by SCS are attributable to hypoxia.1,7,10,11,27,28,31 Islets respond to hypoxia in part by hypoxia inducible factor 1 alpha (HIF1α).28,32 HIF1α mRNA expression was not different in the present study. However, activation of HIF1α through posttranslational stabilization upregulates proinflammatory cytokine genes.31,37 Additionally, the expression of IL1β, a known stabilizer of HIF1α, an inhibitor of oxidative metabolism, and an inhibitor of insulin secretion was lower in SCS+PSF islets.33–35 IL1β may therefore serve as a critical link between oxygen-sensing and insulin secretion. Furthermore, we demonstrate in 2 independent analyses that persufflation reduces proinflammatory cytokines. In addition to IL1β, the proinflammatory cytokines, IL6, CXCL1 and CCL20 were lower in SCS+PSF islets. These have clinical significance as they have been associated with poor islet transplant outcomes for diabetic therapeutics. Additionally, in rodent and human islets, hypoxic exposure in the pretransplant period initiates an inflammatory response, thereby linking hypoxia to proinflammatory cytokines that are known inhibitors of β cell function.11,36 By supplying oxygen via the organ vasculature, persufflation inherently limits the hypoxia experienced during preservation. However, we observed differences in gene expression between test groups which were matched for duration of SCS. These changes include enhanced transcription of adaptive metabolic response genes such as hypoxia inducible factor 3 alpha (HIF3A) and pyruvate dehydrogenase kinase (PDK1), as well as decreased expression of the inflammatory factors IL6, CXCL1, and CCL20. These data indicate that regulation of gene expression in islets is partially attributed to persufflation itself. For example, HIF3A expression was lower in SCS+PSF islets. HIF3α antagonizes HIF1α-regulated genes, which suggests a mechanism by which persufflation abates or reverses ischemic damage that occurred during SCS.37 Furthermore, when time spent in SCS was matched between groups, persufflation extended TPT by approximately 50% with no detectable loss of viability or function. The functional benefit was more pronounced in a cohort with matched TPT because the SCS duration was shorter for islets from persufflated pancreata. GSIS was significantly improved in SCS+PSF islets, consistent with previous literature that shows that the duration of SCS is correlated with insulin secretion responsiveness.10,27 These findings underscore the importance of starting organ persufflation early in the preservation process in order to prevent functional deterioration. The data also show that deterioration in insulin secretion due to SCS can be halted with organ persufflation.
To our knowledge, this study represents the first characterization of human islets from pancreata preserved using persufflation, and demonstrates that persufflation enhances insulin secretion and prevents harmful proinflammatory signaling. Anti-inflammatory interventions have improved islet transplant outcomes in animal models.38–40 Therefore, reduced inflammation in SCS+PSF islets may prove to minimize curative islet doses and improve transplant outcomes. The potential to extend TPT while maintaining function has significant logistical advantages for β cell replacement strategies and islet transplantation into diabetic patients.7,12 Finally, the ability to study human islets with minimal hypoxic exposure has value in our collective understanding of human islet physiology, and for the development of technologies to enable their clinical use.
Supplementary Material
Acknowledgments
We would like to acknowledge the members of the Dr. Papas and Dr. Limesand laboratories as well as our collaborators at Sanofi-Aventis; Paul August, Peter Strop, and Vasudeo Badarinarayana. We would like to thank Giner, Inc. for contributing to the development of the persufflator, and Dr. Linda Templeman and Simon Stone for their technical assistance on the operation of the persufflator. We would like to thank Biorep Technologies Inc. for generously donating the perifusion apparatus used in the present study. We would also like to thank the University of Arizona Genetics Core and the University of Arizona Biocomputing Facility for their assistance with sequencing and analysis. We would like to acknowledge the centers that supplied human islets including; the Integrated Islet Distribution Program (IIDP), the University of Minnesota and the University of California - San Francisco, the McGill University Health Centre. A portion of raw sequencing data for control SCS islets was included from a previously published cohort.19
Funding
This work was supported by the National Institutes of Health grants NIH/SBIR 5R44DK070400-04 (K.K.P., Co-Principal Investigator with Dr. Linda Templeman, Giner Inc.) and R01DK084842 (S.W.L., Principal Investigator) and NIH/NIDDK DP3 grant 1DP3DK106933-01 (K.K.P., Principal Investigator).
Abbreviations:
- SCS
static cold storage
- SCS+PSF
static cold storage + persufflation, the organ preservation technique of partial or full persufflation with oxygen gas
- TPT
total preservation time
- IE
islet equivalents
- FPKM
fragments per kilobase of exon per million fragments mapped
- FC
fold change
- GO
gene ontology
- OCR/DNA
oxygen consumption rate normalized to DNA concentration
- GSIS
glucose stimulated insulin secretion
Footnotes
Duality of Interest
Klearchos K. Papas, PhD served as Consultant/Chair SAB Giner Inc, is a co-inventor on pending patents related to persufflation with University of Minnesota and Giner Inc., and was the PI or co-PI on the NIH/NIDDK grants that partially funded the work. He is no longer affiliated with Giner Inc. “The remaining authors declare no conflicts of interest”.
Guarantee
K.K.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni RN, Stewart AF. Summary of the keystone islet workshop (april 2014): The increasing demand for human islet availability in diabetes research. Diabetes. 2014;63(12):3979–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwanaga Y, Sutherland DE, Harmon JV, Papas KK. Pancreas preservation for pancreas and islet transplantation. Curr Opin Organ Transplant. 2008;13(2):135–141. [DOI] [PubMed] [Google Scholar]
- 4.Papas KK, Hering BJ, Guenther L, Rappel MJ, Colton CK, Avgoustiniatos ES. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37(8):3501–3504. [DOI] [PubMed] [Google Scholar]
- 5.Burns BD, Robson JG, Smith GK. The survival of mammalian tissues perfused with intravascular gas mixtures of oxygen and carbon dioxide. Can J Biochem Physiol. 1958;36(5):499–504. [PubMed] [Google Scholar]
- 6.Suszynski TM, Rizzari MD, Scott WE 3rd, Tempelman LA, Taylor MJ, Papas KK. Persufflation (or gaseous oxygen perfusion) as a method of organ preservation. Cryobiology. 2012;64(3):125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott WE 3rd, Weegman BP, Ferrer-Fabrega J, et al. Pancreas oxygen persufflation increases ATP levels as shown by nuclear magnetic resonance. Transplant Proc. 2010;42(6):2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott WE 3rd, O’Brien TD, Ferrer-Fabrega J, et al. Persufflation improves pancreas preservation when compared with the two-layer method. Transplant Proc. 2010;42(6):2016–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, Torre MA, Karrison T, Thistlethwaite JR. The correlation between donor characteristics and the success of human islet isolation. Transplantation. 1994;57(6):954–958. [DOI] [PubMed] [Google Scholar]
- 10.Lakey JR, Rajotte RV, Warnock GL, Kneteman NM. Human pancreas preservation prior to islet isolation. cold ischemic tolerance. Transplantation. 1995;59(5):689–694. [DOI] [PubMed] [Google Scholar]
- 11.Cantley J, Walters SN, Jung MH, et al. A pre-existent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant. 2013;22(11):2147–2159. [DOI] [PubMed] [Google Scholar]
- 12.Reddy MS, Carter N, Cunningham A, Shaw J, Talbot D. Portal venous oxygen persufflation of the donation after cardiac death pancreas in a rat model is superior to static cold storage and hypothermic machine perfusion. Transpl Int. 2014;27(6):634–639. [DOI] [PubMed] [Google Scholar]
- 13.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98(5):1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14(6):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colton CK, Papas KK, Pisania A, et al. Characterization of islet preparations. Cell Transplant. 2007;85–133. DOI: 10.1016/B978-012369415-7/50007-7. [DOI] [Google Scholar]
- 16.Papas KK, Bellin MD, Sutherland DE, et al. Islet oxygen consumption rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS One. 2015;10(8):e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitzmann JP, O’Gorman D, Kin T, et al. Islet oxygen consumption rate dose predicts insulin independence for first clinical islet allotransplants. Transplant Proc. 2014;46(6):1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KE, Kelly AC, Min CG, et al. Acute ischemia induced by high-density culture increases cytokine expression and diminishes the function and viability of highly purified human islets of langerhans. Transplantation. 2017;101(11):2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, Roberts A, Goff L, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and cufflinks. Nat Protoc. 2012;7(3):562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie C, Mao X, Huang J, et al. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39(Web Server issue):W316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 24.Fu XM, Zhu BT. Human pancreas-specific protein disulfide-isomerase (PDIp) can function as a chaperone independently of its enzymatic activity by forming stable complexes with denatured substrate proteins. Biochem J. 2010;429(1):157–169. [DOI] [PubMed] [Google Scholar]
- 25.Laurent G, Solari F, Mateescu B, et al. Oxidative stress contributes to aging by enhancing pancreatic angiogenesis and insulin signaling. Cell Metab. 2008;7(2):113–124. [DOI] [PubMed] [Google Scholar]
- 26.Nita II, Hershfinkel M, Lewis EC, Sekler I. A crosstalk between na(+) channels, na(+)/K(+) pump and mitochondrial na(+) transporters controls glucose-dependent cytosolic and mitochondrial na(+) signals. Cell Calcium. 2015;57(2):69–75. [DOI] [PubMed] [Google Scholar]
- 27.Lakey JR, Warnock GL, Rajotte RV, et al. Factors in cadaveric donors that affect recovery of human islets of langerhans. Transplant Proc. 1995;27(6):3265. [PubMed] [Google Scholar]
- 28.Lakey JR, Warnock GL, Ao Z, Rajotte RV. Bulk cryopreservation of isolated islets of langerhans. Cell Transplant. 1996;5(3):395–404. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro AM. Islet transplantation in type 1 diabetes: Ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9(4):385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. [DOI] [PubMed] [Google Scholar]
- 31.de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–875. [PubMed] [Google Scholar]
- 32.Cantley J, Grey ST, Maxwell PH, Withers DJ. The hypoxia response pathway and beta-cell function. Diabetes Obes Metab. 2010;12 Suppl 2:159–167. [DOI] [PubMed] [Google Scholar]
- 33.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17(14):2115–2117. [DOI] [PubMed] [Google Scholar]
- 34.Sandler S, Andersson A, Hellerstrom C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987;121(4):1424–1431. [DOI] [PubMed] [Google Scholar]
- 35.Ohara-Imaizumi M, Cardozo AK, Kikuta T, Eizirik DL, Nagamatsu S. The cytokine interleukin-1beta reduces the docking and fusion of insulin granules in pancreatic beta-cells, preferentially decreasing the first phase of exocytosis. J Biol Chem. 2004;279(40):41271–41274. [DOI] [PubMed] [Google Scholar]
- 36.Kelly AC, Smith KE, McCarthy FM, et al. Transcriptome profiles of human pancreatic islets following transient ischemia identify inflammatory and cell death responses. Endocrine Abstracts. 2015;38:P185. [Google Scholar]
- 37.Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koulmanda M, Bhasin M, Fan Z, et al. Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci U S A. 2012;109(38):15443–15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koulmanda M, Sampathkumar RS, Bhasin M, et al. Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant. 2014;14(7):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.