Abstract
Doping with anabolic-androgenic steroids (AAS) is common among both male and female athletes and is a growing public health problem. Review of historical data of systematic state-sponsored doping programs implemented by the German Democratic Republic in elite female athletes and from clinical trials of testosterone administration in non-athlete women suggests that AAS have ergogenic effects in women. The use of AAS in female athletes has been associated with adverse effects that include acne, hirsutism, deepening of the voice and menstrual disturbances; life-threatening adverse effects such as cardiac arrhythmias and sudden death have also been reported. Therefore, detection of AAS abuse in female athletes is important to ensure fairness in competition; at the same time, the athletes should be educated regarding the adverse consequences of AAS use. Although administration of exogenous androgens have been associated with ergogenic effects, it remains unclear whether endogenous hyperandrogenism seen in some medical conditions such as disorders of sexual development (DSD), congenital adrenal hyperplasia and polycystic ovary syndrome, confers any competitive advantage. Well-designed studies are needed to determine the effects of endogenous hyperandrogenism on athletic performance in female athletes.
Keywords: Anabolic steroids, Testosterone, Hyperandrogenism, Muscle performance, Adverse effects
1. Introduction
Over the last several decades, use of anabolic-androgenic steroids (AAS) as performance-enhancing drugs among competitive athletes, recreational athletes, body-builders and those who desire to enhance their body image, has substantially increased. Estimates suggest that approximately 3–4 million Americans aged 13–50 years have used AAS; and within this group, approximately 1 million developed dependence on AAS (Pope et al., 2014). AAS represent a diverse class of synthetic derivatives of testosterone and are used primarily to increase muscle mass and muscle strength, and to improve physical performance. Not surprisingly, AAS are the most common class of ergogenic drugs used by athletes participating in competitive sports and a quarter of them develop AAS dependence (Hartgens and Kuipers, 2004). Although the abuse of AAS is substantially more common in men, its prevalence is also increasing in women with an estimated lifetime prevalence of 1.6% (Nieschlag and Vorona, 2015b). As a result, use of AAS has been officially banned from competitive sports for all athletes since the mid-1970s. Although there is abundant scientific literature describing the ergogenic effects of AAS among male athletes (Hartgens and Kuipers, 2004), data regarding their effects in female athletes are limited. Even more controversial is the issue of whether endogenous hyperandrogenism in athletes with disorders of sexual development (DSD), polycystic ovary syndrome and congenital adrenal hyperplasia provides any advantage in competitive sports.
In this paper, we review; i) physiology of androgen production in women; ii) ergogenic effects of AAS use in female athletes by providing a historical overview; iii) body composition and muscle performance among women with endogenous hyperandrogenism; iv) efficacy of testosterone administration on body composition and muscle performance in non-athlete androgen deficient women (HIV, hypopituitarism and surgical menopause); and lastly, v) discuss the potential adverse effects of AAS use in women.
2. Androgen physiology in women
The ovaries and the adrenal glands are the two major sources of androgens in women (Basaria and Dobs, 2006). Testosterone is produced directly from the ovaries and also via conversion of weaker androgens, androstenedione and dehydroepiandrosterone (DHEA), which are synthesized by the ovaries and the adrenal glands, respectively. In young premenopausal women, the ovary is responsible for approximately 25% of the testosterone production, while 75% is derived from the adrenal androgens. However, in postmenopausal women, the ovary becomes a major source of testosterone production and its contribution increases to 50% (Adashi, 1994). Although the climacteric ovary is atrophic and loses its capacity to synthesize estradiol and follicles, it continues to secrete androgens under the stimulation of postmenopausal gonadotropins (Sluijmer et al., 1995; Dowsett et al., 1980; Dennefors et al., 1980).
In ovulating women, serum levels of testosterone and androstenedione gradually increase during the follicular phase and peak during the pre-ovulatory phase (Judd and Yen, 1973; Sinha-Hikim et al., 1998). Serum androstenedione level further increases during the late luteal phase; while serum testosterone concentrations do not increase further (Judd and Yen, 1973; Sinha-Hikim et al., 1998). Based on normative data from the Framingham Heart Study, median serum total testosterone levels (measured by liquid chromatography tandem mass spectrometry) in healthy, cycling women age 19–45 years were 23.7 ng/dl in the follicular phase, 34.7 ng/dl in the ovulatory phase and 28.5 ng/dl in the luteal phase (Coviello et al., 2012).
Serum testosterone concentrations are lower in older postmenopausal women compared with young, menstruating women. However, unlike the cessation of ovarian production of estrogen and follicles at natural menopause, ovarian androgen production does not cease. In fact, the steepest decline in serum testosterone and DHEAS levels occur during the early reproductive years, between the ages of 20–40, and then plateauing during menopausal transition (Cappola et al., 2007; Davison et al., 2005). Indeed, average serum testosterone levels among women in their sixties is approximately 50% compared with women in their twenties (Cappola et al., 2007; Davison et al., 2005). Thus, the decline in androgens in women appears to be more of a function of aging rather than natural menopause (Basaria and Dobs, 2006; Davison et al., 2005). In contrast to natural menopause, surgical menopause is associated with a significant reduction in serum androgen levels. Indeed, population studies show a 50% decline in circulating serum testosterone levels in women who have undergone bilateral oophorectomy compared with naturally menopausal women (Judd et al., 1974; Laughlin et al., 2000).
3. Ergogenic effects of AAS use in female athletes
The use of AAS to increase lean mass and muscle strength is common in elite female sports; indeed, androgens are the most common class of agents used for doping by elite female athletes (Bermon, 2017). However; in contrast to male athletes in who there is substantial evidence showing ergogenic benefits of AAS use, the effects of AAS on physical performance in women have not been extensively studied (Lamb, 1984; Maravelias et al., 2005; Wu, 1997). Men abusing AAS have been reported to administer androgens that are 10–100 times the therapeutic dose that is prescribed to hypogonadal men in the clinics (Penatti et al., 2011; Trenton and Currier, 2005; Wu, 1997). Limited data suggest that some female athletes have reportedly taken AAS at doses that are similar to male athletes (Franke and Berendonk, 1997). Although there is a lack of objective data, small studies that interviewed female athletes report that some women perceived significant improvement in their muscle mass, muscle strength and athletic performance while taking AAS compared to the period when they were not using these agents (Strauss et al., 1985).
From 1965 to 1989, the German Democratic Republic (GDR) conducted a systematic doping program where they administered AAS to elite athletes competing in the Olympic Games (Fitch, 2008; Franke and Berendonk, 1997). Evidence regarding the performance enhancing effects of AAS in elite competitive sports comes from the release of several classified government documents after the unification of Germany in 1990. Review of scientific reports, secret doctoral theses and court documents from 1966 onwards revealed that hundreds of physicians and scientists were involved in doping research and were designated as “unofficial collaborators” of the Ministry for State Security. Some of these documents suggest that administration of AAS to female athletes was highly effective in enhancing their competitive performance, particularly in sporting events that required strength and speed. The reports suggested that administration of AAS in female athletes for four years improved shot-put distance by 4.5–5 m and discus throw distance by 11–20 m; while in racing events, athletes using AAS were 4–5 s and 7–10 s faster in the 400 m and 1500 m events, respectively. Indeed, the systematic administration of androgens in female German athletes was considered a success by the GDR doping program as it resulted in many victories in female sporting events. Some of these female athletes were given doses of nandrolone and testosterone esters that were even higher than the doses that were administered to male athletes participating in similar events. The document also suggests that the physicians who were employed by the GDR in the so-called “androgenic initiation” program were aware of the potential adverse effects of AAS in female athletes. Despite the virilizing side effects, many athletes continued to receive both oral and intramuscular AAS; but some intolerable side effects (acne, hirsutism, liver damage) led some athletes to discontinue AAS and withdraw from some of the events. The success observed among female athletes of the GDR formed the basis of AAS abuse in female competitive sports.
4. Athletic performance in women with endogenous hyperandrogenism
Although there is some evidence that the use of AAS by female athletes is associated with an improvement in physical performance (discussed above); the effects of elevated endogenous androgen concentrations on athletic performance among female athletes remains controversial. Limited studies have highlighted some association between endogenous androgens with athletic performance in elite female athletes. A recent report evaluated serum hormones and athletic performance in 1332 female athletes who were competing in the 2011 and 2013 IAAF World Championships; this cohort included athletes with endogenous hyperandrogenism and also those who were doping with AAS (Bermon, 2017). The report found that athletes with higher concentrations of serum testosterone showed competitive advantage over athletes with serum testosterone levels in the normal female range. Another recent report that compared endogenous serum androgen concentrations of Swedish women Olympic athletes with age-matched sedentary controls found that the athletes had significantly higher levels of precursor androgens (DHEA and 5-DIOL) which correlated positively with lean mass (Eklund et al., 2017). These reports suggest that elevated endogenous androgens might improve physical performance.
Limited studies have also evaluated women with conditions associated with increased endogenous androgen production such as congenital adrenal hyperplasia (CAH), polycystic ovary syndrome (PCOS), androgen insensitivity syndrome (AIS) and 5α-reductase deficiency (Wood and Stanton, 2012). In this section, we first provide a historical overview of elite female athletes with DSD who have participated in competitive sports. We follow this by discussion of body composition and muscle performance that have been studied among athletes with endogenous hyperandrogenism.
4.1. Historical overview
Disorders of sexual development (DSD) have attracted greater attention in the sports world since the 1936 Olympic Games in Berlin when concerns were raised about the masculine appearance of several female athletes (Padawer, 2016; Ritchie et al., 2008). To ensure that men were not attempting to compete as women, a mandatory gender verification was enforced, which continued until 1990 (Dickinson et al., 2002; Wood and Stanton, 2012). During its enforcement, gender verification testing identified several athletes with previously undiagnosed DSD, resulting in disqualifications from various competitions. One of the recent cases that has garnered the most spotlight involved a South African middle distance runner, Caster Semenya (Padawer, 2016; Xavier and McGill, 2012). Although she was cleared of any doping with AAS, Ms. Semenya was subjected to undergo gender verification testing after winning the 800-m race in the 2009 World Championships in Berlin as her masculine build raised questions about her sex. Although the results of her gender verification test were not made public, it is believed that she has a DSD that is responsible for elevated endogenous testosterone levels. The South African government filed a human rights complaint with the United Nations arguing that the gender verification testing of Ms. Semenya ordered by the International Association of Athletics Federations (IAAF) was discriminatory; this eventually led the IAAF to clear Ms. Semenya and allow her to resume competition in 2010. Ms. Semenya’s case, and other similar cases, prompted the IAAF and the International Olympic Committee (IOC) to review their policies regarding fairness in women’s sports and inclusion of female athletes with DSD in various sporting events. In April 2011, the IAAF and the IOC revised their policies to allow female athletes with DSDs or hyperandrogenism to participate in international competitions as long as their circulating serum testosterone levels are below the lower limit for men (<10 nmol/L); the exception to this rule included those women who are resistant to the effects of testosterone (due to inactivating mutations of the androgen receptor) or who undergo interventions to reduce serum testosterone levels (orchiectomy or hormone-suppressing drugs) below the male threshold (Sanchez et al., 2013; Sonksen et al., 2015; Xavier and McGill, 2012). These regulations were met with some skepticism as there is lack of concrete scientific evidence showing that endogenous hyperandrogenism in women significantly influences physical performance (Allen, 2016; Bermon et al., 2014; Healy et al., 2014; Sonksen et al., 2015). In July 2016, the Court of Arbitration for Sport ruled that the IAAF policy was not justified based on the current scientific evidence; however; the Court did acknowledge that naturally elevated endogenous serum testosterone levels may play some role in female athleticism. The court also ruled that requiring female athletes to undergo medical procedures to lower testosterone levels so that they can compete was ‘unjustifiably discriminatory’. As a result, a judging panel for the Court of Arbitration for Sport suspended the policy laid out by the IAAF until July 2017 in order to allow the IAAF sufficient time to provide scientific evidence on whether elevated endogenous serum testosterone levels confer a competitive advantage (Camporesi, 2016; Padawer, July 3, 2016).
5. Endogenous hyperandrogenism: impact on lean mass and muscle performance
Given the anabolic and ergogenic properties of testosterone, questions have been raised regarding whether athletes with DSD and other hyperandrogenic conditions (e.g. PCOS, CAH) have an unfair advantage over their normal counterparts. Recent data show a significantly higher prevalence (140 times) of hyperandrogenic 46XY DSD among female athletes compared to women in the general population (Bermon et al., 2014); interestingly, female athletes participating in long-distance endurance running had lower serum testosterone concentrations than those training for events requiring strength, power and speed, suggesting that the variability in endogenous androgen levels might determine the inclination of athletes towards certain sporting events. The IAAF groups DSDs into two categories; 1) “those that accord no advantage over other female athletes” (chromosomal abnormalities such as Turner’s Syndrome) and 2) “those that may accord some advantages” but are allowed to participate in competition (such as PCOS, CAH, partial AIS, 5α reductase deficiency). Unlike the ergogenic effects observed with exogenous AAS use, the benefits of higher endogenous androgen levels on competitive performance remain unclear.
In this section, we will review some common hyperandrogenic disorders and discuss the available evidence regarding body composition and muscle performance among these women.
5.1. Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among women of reproductive age and is characterized by clinical or biochemical hyperandrogenism, ovulatory dysfunction and polycystic ovaries on ultrasonography. Some studies suggest that PCOS is the most common reproductive endocrine disorder among female Olympic athletes; its prevalence even higher than hypothalamic amenorrhea that often results from caloric deficiency (Hagmar et al., 2009). Indeed, 15–31% of elite athletes have either clinical or biochemical profile that is suggestive of PCOS (Dadgostar et al., 2009; Eliakim et al., 2010); this exceeds estimated prevalence of 4–12% in the general population (Knochenhauer et al., 1998). Limited evidence from smaller studies have suggested that hyperandrogenism in women with PCOS might have beneficial effects on body composition and physical performance; indeed, higher circulating serum testosterone levels in these women have been associated with greater muscle mass independent of height (Douchi et al., 1999). In a case-control study of 80 women (40 PCOS and 40 controls), women with PCOS demonstrated greater muscle strength (assessed by 1-RM bench press and isometric handgrip strength tests) compared to the control group, which was independent of body composition (Kogure et al., 2015); furthermore, serum concentrations of testosterone were positively associated with increased muscle strength in the PCOS group. In a small cross-sectional study of female athletes with oligomenorrhea or amenorrhea, athletes with hyperandrogenism demonstrated greater lean mass and a higher maximal oxygen uptake (V02 max) and performance compared to women with normal androgen concentrations (Rickenlund et al., 2003). Although data are limited, these studies at least suggest that hyperandrogenism in women with PCOS could potentially impart some advantage to women competing in sporting events.
5.2. Congenital adrenal hyperplasia (CAH)
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders encompassing enzyme deficiencies in the adrenal steroidogenesis pathway that lead to impaired cortisol biosynthesis (Merke and Bornstein, 2005). The majority of the cases of CAH are due to 21-hydroxylase deficiency which is characterized by impaired cortisol and aldosterone production, and excessive production of androgens. Given that androgens promote lean mass and muscle strength, it is conceivable that women with CAH might have a competitive edge over their healthy counterparts. In a small observational study that compared the maximum voluntary muscle strength in 18 girls with CAH (age 4–12 years) with 78 healthy age-matched controls found that the girls with classic CAH demonstrated significantly greater muscle strength compared with girls of comparable age in the control group (Rodda et al., 1987). As data are lacking on muscle performance in adult women with CAH, no firm conclusions can be drawn from these data in children. However; the findings from these childhood studies do suggest that if this greater muscle strength is maintained during transition to adult-hood, this could possibly translate into improvements in physical performance. Another mechanism by which androgens may improve exercise capacity and endurance is by promoting erythropoiesis. Indeed, a recent study demonstrated a positive association between serum androgen levels and hematocrit in women with CAH (Karunasena et al., 2017). To the contrary, it has also been suggested that impairments in cortisol and epinephrine production by the adrenals and the resulting reduced glucose response to exercise in patients with classical CAH may limit physical performance (Eliakim and Nemet, 2010). Additionally, the medications that are used to treat CAH (in particular glucocorticoids) may also negatively impact physical performance due to their catabolic effects on skeletal muscle (Angoorani et al., 2012). Indeed, in a prospective study of adult patients with classic CAH who were on glucocorticoid replacement, both female and male patients demonstrated reduced muscle mass (Bechtold et al., 2014). Thus, it remains unclear whether CAH (and its treatment) positively or negatively impacts athletic performance.
5.3. Androgen insensitivity syndrome (AIS)
Loss of function mutations of the gene that encodes the androgen receptor result in androgen insensitivity syndrome (AIS) in 46, XY individuals. These patients have functional testes and normal testosterone production, but their androgen receptors are not responsive to circulating testosterone. Androgen insensitivity syndrome encompasses a clinical spectrum of partial to complete androgen insensitivity, varying from a completely female phenotype to a male phenotype with undervirilization or infertility. Patients with complete AIS are raised as girls and generally come to attention when they present with primary amenorrhea. Data suggest that the frequency of complete AIS among athletes is 1:421 compared to the incidence of 1:20,000 in the general population (Elsas et al., 2000). However, despite having circulating serum testosterone concentrations in the male range, patients with complete AIS are insensitive to the anabolic effects of androgens. Indeed, studies have shown that they have lower fat-free mass than male controls and their overall body composition is similar to XX female controls (Dati et al., 2009). Based on this evidence, one could conclude that athletes with complete AIS are unlikely to have superior athletic performance as their androgen receptors are mutated. To the contrary, it has been suggested that female athletes with partial AIS might have some competitive advantage over their normal counterparts as their androgen receptors are partially responsive to circulating androgens (Wood and Stanton, 2012). Studies show that women with partial AIS do exhibit more male-pattern behaviors compared with women without these conditions (Hines, 2011), at least suggesting that they are responsive to androgens. Well-designed studies comparing body composition and muscle performance in patients with AIS with that of age-matched control women might help answer these questions.
5.4. 5α-reductase deficiency
5α-reductase deficiency, a 46, XY DSD, is an autosomal recessive condition in which 46, XY patients with bilateral functioning testes and normal testosterone production have impairment in virilization during embryogenesis due to defective conversion of testosterone to dihydrotestosterone (DHT). Dihydrotestosterone binds to the androgen receptor with high affinity and is responsible for masculinization of the external genitalia; hence the external genitalia often are predominately female at birth in this condition; however, the range of undervirilization ranges from complete male with microphallus and hypospadias to completely female with either clitoromegaly or normal female genitalia (Sonksen et al., 2015; Wood and Stanton, 2012). Although the degree of virilization of the external genitalia is variable, normal masculinization of muscles is seen at puberty. Many patients with 5α-reductase deficiency are initially brought up as females, but many will switch to a male gender identity at puberty. However, some cases of previously undiagnosed 5α-reductase deficiency in elite young female athletes of reproductive age have been detected when they were found to have serum testosterone levels in the male range (520–640 ng/dl) during testing by antidoping authorities (Fenichel et al., 2013). As androgen receptors in this disorder are responsive to androgens, a female (XY) athlete with 5α-reductase deficiency and serum testosterone levels in the male range would, at least theoretically, be expected to have a competitive advantage in sporting events. Future studies are needed to systematically evaluate body composition and muscle strength in these patients and compare with controls.
6. Effects of exogenous testosterone administlration on body composition and muscle performance in women
Testosterone therapy has been widely promoted in women for the treatment of sexual dysfunction, and also for improving body composition, muscle strength, bone mineral density and cognition. It has been proposed that the testosterone dose-response relationship is different in women compared with men, and that clinically significant effects on androgen-dependent outcomes (such as muscle mass and strength) can be achieved at testosterone doses and concentrations that are substantially lower than those required to produce similar effects in men (Padero et al., 2002). In the United States (US), however, there is no FDA approved testosterone product for women due to lack of long-term safety data; even then, testosterone prescriptions are being given to otherwise-healthy women on an off-label basis.
As randomized trials of AAS use are not available (or possible) in female athletes, in this section, we summarize published data from some of the randomized controlled trials of testosterone therapy in specific populations of women (non-athletes) that have evaluated body composition and/or muscle performance.
6.1. Clinical trials of testosterone therapy in women with HIV and hypopituitarism
Some studies of testosterone replacement (in physiological doses) in women with androgen deficiency due to medical comorbidities have shown some beneficial effects on body composition. In a 6-month study of transdermal testosterone replacement (4 mg patch twice a week) in HIV-infected women with androgen deficiency and 10% weight loss, there was an increase in muscle mass and muscle strength which translated into some improvement in muscle function (Dolan et al., 2004). However; these findings have not been confirmed in other studies (Choi et al., 2005). Some clinical trials have evaluated the efficacy of testosterone replacement in patients with hypopituitarism; in a 12-month study of women with androgen deficiency due to hypopituitarism, testosterone administration (300 mg/day patch) was associated with a modest increase in fat free mass and thigh muscle area (Miller et al., 2006) (Fig. 1).
Fig. 1.
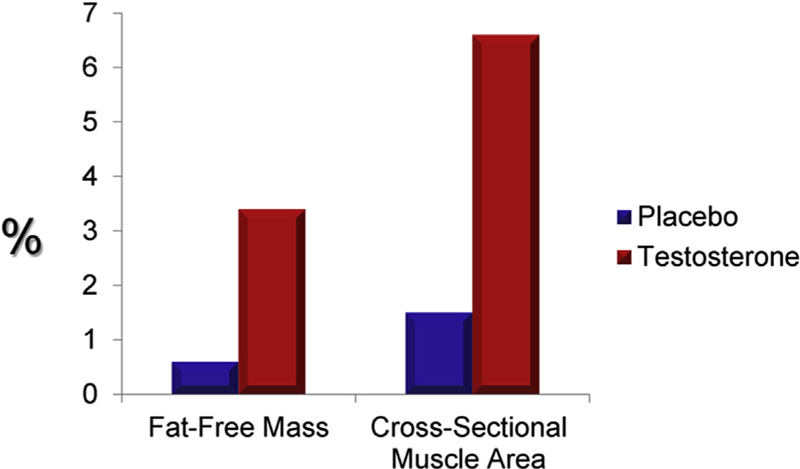
Change in % Fat Free Mass and Mid-Thigh Cross-sectional Muscle Area with Testosterone Replacement in Androgen-Deficient Women with Hypopituitarism. (Adapted from Miller et al.).
6.2. Clinical trials of testosterone therapy in healthy postmenopausal women
A few randomized trials of testosterone therapy have been conducted in postmenopausal women. In a 16-week double-blind randomized controlled trial of 40 postmenopausal women (natural and surgical) who were randomized to either oral 1.25 mg esterified estrogen ± 2.5 mg methyltestosterone or 1.25 mg esterified estrogen alone showed that women randomized to the combined estrogen-androgen group experienced a significant increase in lean mass and muscle strength as well reduction in total fat mass compared to estrogen-only group (Figs. 2 and 3) (Dobs et al., 2002). Data on the dose-response relationship of testosterone administration in women are limited. In one dose-response study of intramuscular testosterone administration, ranging from physiologic to supraphysiologic doses (Fig. 4) in 71 estrogen-treated hysterectomized postmenopausal women (ages 41–62 years), testosterone administration was associated with concentration-dependent gains in lean body mass (LBM), chest press power and loaded stair climbing power (Figs. 5–7) (Huang et al., 2015). However; significant improvements in these parameters were only observed at the highest dose of 25 mg/week that was associated with nadir serum testosterone concentrations of 211 ng/dl (supra-physiologic level for women). These dose-response relationships were maintained even after adjusting for baseline BMI. These data in healthy menopausal women suggest that significant gains in these outcomes require doses that result in serum testosterone concentrations in the low male range; this is likely to be detected by anti-doping agencies among female athletes doping with testosterone.
Fig. 2.
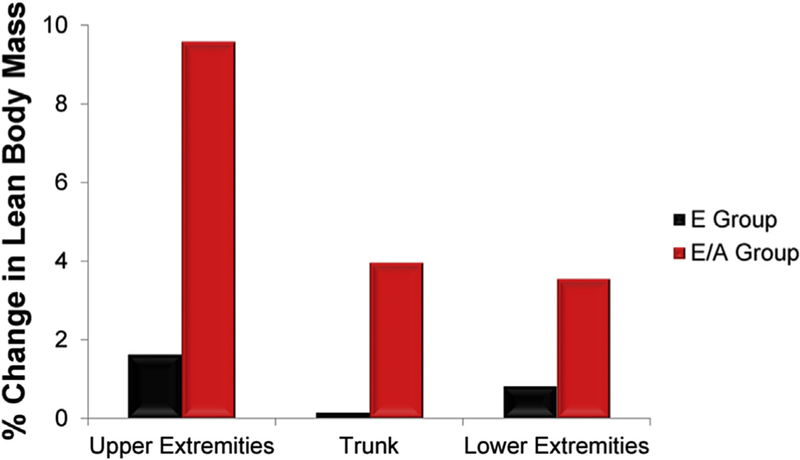
Effect of Oral Estrogen vs. Oral Estrogen-Androgen Replacement Therapy on % Lean Mass in Postmenopausal Women (Adapted from Dobs et al.).
Fig. 3.
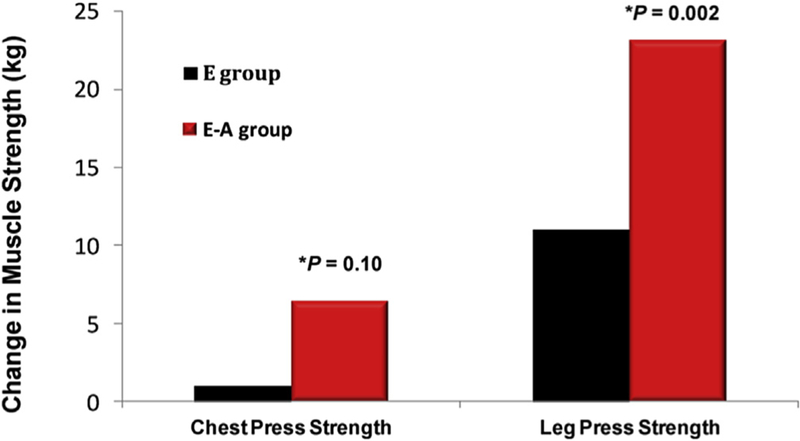
Effect of Oral Estrogen (E) vs. Oral Estrogen-Androgen (E–A) Replacement Therapy on Muscle Strength in Postmenopausal Women (Adapted from Dobs et al.).
Fig. 4.
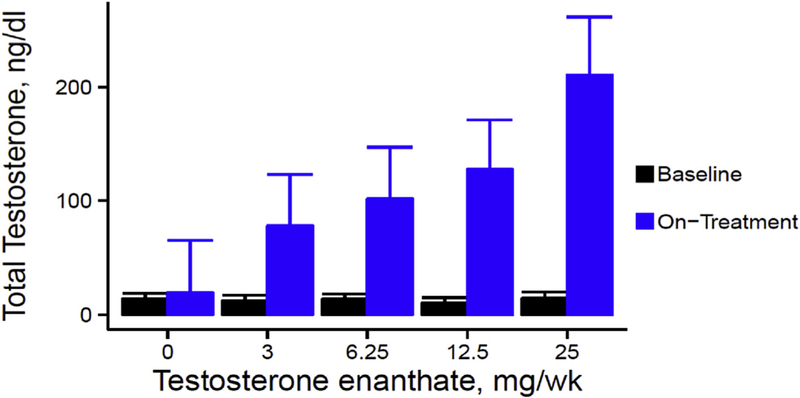
On-treatment Total Testosterone Concentrations in a dose-response study in postmenopausal women with androgen deficiency. Data represent mean and standard errors at baseline and on-treatment for each testosterone dose group (Adapted from Huang et al., 2014).
Fig. 5.
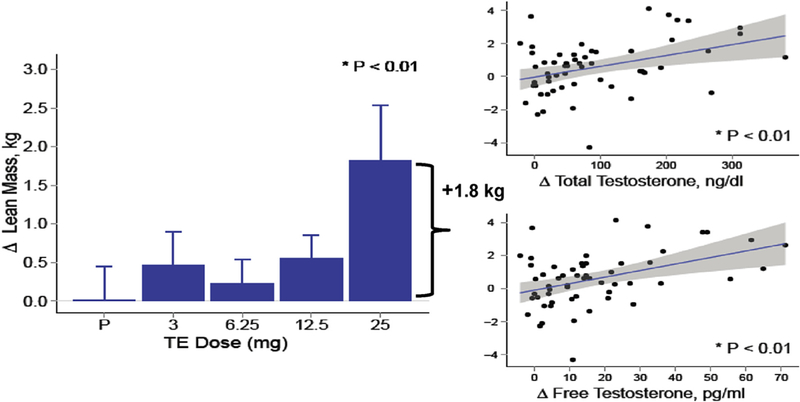
Lean Body Mass in a dose-response study in postmenopausal women with androgen deficiency. In the bar graphs on the left, data represent absolute mean (SE) changes from baseline for each treatment group. Scatter plots on the right display estimates and 95% CI for the generalized additive model of change in outcomes as function of free testosterone levels (Adapted from Huang et al., 2014).
Fig. 7.
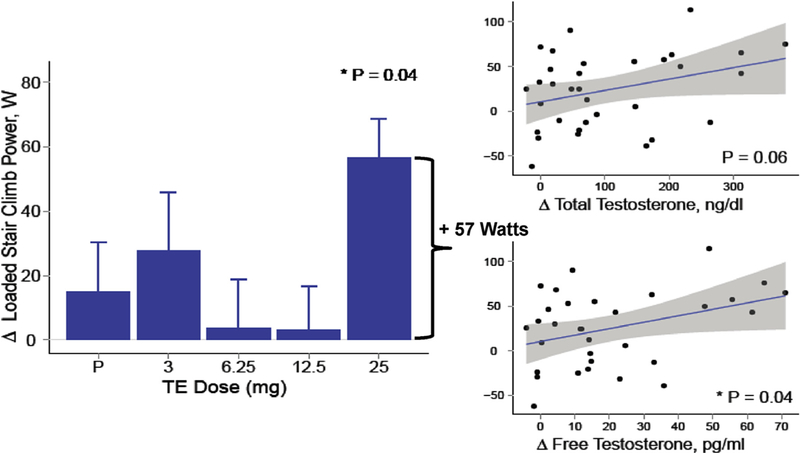
Loaded Stair-Climb Power in a dose-response study in postmenopausal women with androgen deficiency. In the bar graphs on the left, data represent absolute mean (SE) changes from baseline for each treatment group. Scatter plots on the right display estimates and 95% CI for the generalized additive model of change in outcomes as function of free testosterone levels (Adapted from Huang et al., 2014).
These data suggest that the use of AAS in supraphysiologic doses do result in ergogenic effects in non-athlete women; based on this, one could posit that they might result in enhancement of muscle performance in female athletes using AAS and justifies the ban proposed by the World Anti-Doping Agency.
7. Adverse effects of AAS in female athletes
The abuse of AAS among competitive athletes is growing at increasing rates worldwide and becoming a cause for concern among the medical professionals given the susceptibility to adverse effects. The most frequently reported adverse effects of AAS use among female athletes include hirsutism, alopecia, deepening of the voice, clitoromegaly, menstrual disturbances and aggression (Strauss et al., 1985). Previous studies in non-athlete women treated with supraphysiologic doses of androgens for a variety of medical conditions have reported voice changes that included pitch fluctuations and hoarseness (Baker, 1999; Damste, 1967; Talaat et al., 1987). These reports were recently confirmed in a 24-week dose-response trial of testosterone administration in post-menopausal women which showed lowering of the vocal pitch (assessed via functional voice testing) even in the absence of self-reported changes in voice by the participants (Huang et al., 2015). Interestingly, although the above-mentioned masculinizing side effects of AAS were reported to be undesirable by the majority of female athletes, they still considered AAS use acceptable due to their ergogenic effects (Strauss et al., 1985). In addition to masculinizing adverse effects, severe life-threatening adverse effects of AAS have also been reported among female athletes, including hepatic rupture, multi-organ failure and sudden death (Nieschlag and Vorona, 2015a; Povzun, 2016; Thiblin et al., 2009).
8. Conclusion
Anabolic-androgenic steroids are the most widely used performance enhancing drugs among athletes of both sexes and their illicit use has been increasing worldwide. The performance-enhancing effects of exogenous AAS in women have been demonstrated based on historical evidence from state-sponsored doping programs, from anectodal reports and from small dose-response clinical trials of testosterone administration in non-athlete women. Even though these drugs have been banned from sporting competitions, doping with AAS by female athletes continues despite the potential of serious adverse effects. These issues underscore the need for increased surveillance; and education and awareness among the medical community, sports community and lay public.
The issue of whether endogenous hyperandrogenism in female athletes due to certain medical disorders imparts a “competitive edge” in sporting events remains unresolved. The case of Ms. Semanya (and other athletes) has paved the way for important discussions regarding the privacy rights of female athletes as well as fairness in competition in sporting events. Although limited data in women with endogenous hyperandrogenism suggests some beneficial effects on lean mass and muscle strength, more research is needed to fully understand the impact of endogenous hyper-androgenism on athletic performance among elite female athletes.
Fig. 6.
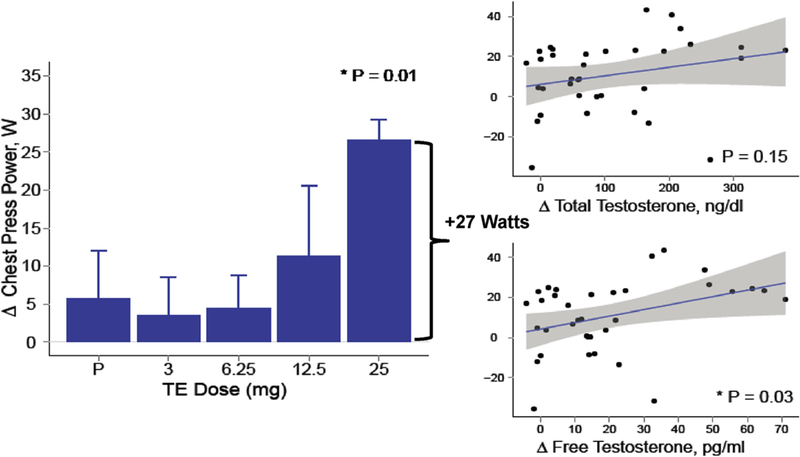
Chest Press Power in a dose-response study in postmenopausal women with androgen deficiency. In the bar graphs on the left, data represent absolute mean (SE) changes from baseline for each treatment group. Scatter plots on the right display estimates and 95% CI for the generalized additive model of change in outcomes as function of free testosterone levels (Adapted from Huang et al., 2014).
References
- Adashi EY, 1994. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil. Steril 62, 20–27. [DOI] [PubMed] [Google Scholar]
- Allen DB, 2016. Hormonal eligibility criteria for ‘includes females’ competition: a practical but problematic solution. Horm. Res. Paediatr 85, 278–282. [DOI] [PubMed] [Google Scholar]
- Angoorani H, Haratian Z, Halabchi F, 2012. Congenital adrenal hyperplasia in an elite female soccer player; what sports medicine clinicians should know about this? Asian J. Sports Med 3, 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, 1999. A report on alterations to the speaking and singing voices of four women following hormonal therapy with virilizing agents. J. voice Official J. Voice Found 13, 496–507. [DOI] [PubMed] [Google Scholar]
- Basaria S, Dobs AS, 2006. Clinical review: controversies regarding transdermal androgen therapy in postmenopausal women. J. Clin. Endocrinol. Metab 91, 4743–4752. [DOI] [PubMed] [Google Scholar]
- Bechtold S, Beyerlein A, Bonfig W, Dalla Pozza R, Putzker S, Otto R, Schmidt H, Schwarz HP, 2014. Sexual difference in bone geometry of adult patients with classical congenital adrenal hyperplasia: data using peripheral quantitative computed tomography. Horm. Res. Paediatr 82, 171–178. [DOI] [PubMed] [Google Scholar]
- Bermon S, 2017. June Androgens and athletic performance of elite female athletes. Curr Opin Endocrinol Diabetes Obes 24 (3), 246–251. [DOI] [PubMed] [Google Scholar]
- Bermon S, Garnier PY, Hirschberg AL, Robinson N, Giraud S, Nicoli R, Baume N, Saugy M, Fenichel P, Bruce SJ, et al. , 2014. Serum androgen levels in elite female athletes. J. Clin. Endocrinol. Metab 99, 4328–4335. [DOI] [PubMed] [Google Scholar]
- Camporesi S, 2016. Ethics of regulating competition for women with hyper-androgenism. Clin. Sports Med 35, 293–301. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, Zmuda JM, Harris T, Fried LP, 2007. Determinants of serum total and free testosterone levels in women over the age of 65 years. J. Clin. Endocrinol. Metab 92, 509–516. [DOI] [PubMed] [Google Scholar]
- Choi HH, Gray PB, Storer TW, Calof OM, Woodhouse L, Singh AB, Padero C, Mac RP, Sinha-Hikim I, Shen R, et al. , 2005. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J. Clin. Endocrinol. Metab 90, 1531–1541. [DOI] [PubMed] [Google Scholar]
- Coviello AD, KP N, Bhasin S, 2012. Reference Ranges for Circulating Testosterone in the Follicular and Luteal Phases of the Menstrual Cycle in a Healthy, Community-Based Sample of Women in the Framingham Heart Study [abstract]. Endocrine Reviews
- Dadgostar H, Razi M, Aleyasin A, Alenabi T, Dahaghin S, 2009. The relation between athletic sports and prevalence of amenorrhea and oligomenorrhea in Iranian female athletes. Sports Med. Arthrosc. Rehabil. Ther. Technol 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damste PH, 1967. Voice change in adult women caused by virilizing agents. J. Speech Hear. Disord 32, 126–132. [DOI] [PubMed] [Google Scholar]
- Dati E, Baroncelli GI, Mora S, Russo G, Baldinotti F, Parrini D, Erba P, Simi P, Bertelloni S, 2009. Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex. Dev 3, 188–193. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR, 2005. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab 90, 3847–3853. [DOI] [PubMed] [Google Scholar]
- Dennefors BL, Janson PO, Knutson F, Hamberger L, 1980. Steroid production and responsiveness to gonadotropin in isolated stromal tissue of human postmenopausal ovaries. Am. J. Obstet. Gynecol 136, 997–1002. [DOI] [PubMed] [Google Scholar]
- Dickinson BD, Genel M, Robinowitz CB, Turner PL, Woods GL, 2002. Gender verification of female Olympic athletes. Med. Sci. Sports Exerc 34, 1539–1542 discussion 1543. [DOI] [PubMed] [Google Scholar]
- Dobs AS, Nguyen T, Pace C, Roberts CP, 2002. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J. Clin. Endocrinol. Metab 87, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Dolan S, Wilkie S, Aliabadi N, Sullivan MP, Basgoz N, Davis B, Grinspoon S, 2004. Effects of testosterone administration in human immunodeficiency virus-infected women with low weight: a randomized placebo-controlled study. Arch. Intern Med 164, 897–904. [DOI] [PubMed] [Google Scholar]
- Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Nagata Y, 1999. Serum androgen levels and muscle mass in women with polycystic ovary syndrome. Obstet. Gynecol 94, 337–340. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cantwell B, Lal A, Jeffcoate SL, Harris AL, 1980. Suppression of postmenopausal ovarian steroidogenesis with the luteinizing hormone-releasing hormone agonist goserelin. J. Clin. Endocrinol. Metab 66, 672–677. [DOI] [PubMed] [Google Scholar]
- Eklund E, Berglund B, Labrie F, Carlstrom K, Ekstrom L, Hirschberg AL, 2017. June 23 Serum androgen profile and physical performance in women Olympic athletes. Br. J. Sports Med pii: bjsports-2017–097582. doi: 10.1136/bjsports-2017-097582, [Epub ahead of print]. [DOI] [PubMed]
- Eliakim A, Nemet D, 2010. Endogenous hyperandrogenism and exercise capacity lessons from the exercise-congenital adrenal hyperplasia model. J. Pediatr. Endocrinol. Metab 23, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Eliakim A, Marom N, Galitskaya L, Nemet D, 2010. Hyperandrogenism among elite adolescent female athletes. J. Pediatr. Endocrinol. Metab 23, 755–758. [DOI] [PubMed] [Google Scholar]
- Elsas LJ, Ljungqvist A, Ferguson-Smith MA, Simpson JL, Genel M, Carlson AS, Ferris E, de la Chapelle A, Ehrhardt AA, 2000. Gender verification of female athletes. Genet. Med 2 249–254. [DOI] [PubMed] [Google Scholar]
- Fenichel P, Paris F, Philibert P, Hieronimus S, Gaspari L, Kurzenne JY, Chevallier P, Bermon S, Chevalier N, Sultan C, 2013. Molecular diagnosis of 5alpha-reductase deficiency in 4 elite young female athletes through hormonal screening for hyperandrogenism. J. Clin. Endocrinol. Metab 98, E1055–E1059. [DOI] [PubMed] [Google Scholar]
- Fitch KD, 2008. Androgenic-anabolic steroids and the olympic games. Asian J. Androl 10, 384–390. [DOI] [PubMed] [Google Scholar]
- Franke WW, Berendonk B, 1997. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin. Chem 43, 1262–1279. [PubMed] [Google Scholar]
- Hagmar M, Berglund B, Brismar K, Hirschberg AL, 2009. Hyperandrogenism may explain reproductive dysfunction in olympic athletes. Med. Sci. Sports Exerc 41, 1241–1248. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H, 2004. Effects of androgenic-anabolic steroids in athletes. Sports Med 34, 513–554. [DOI] [PubMed] [Google Scholar]
- Healy ML, Gibney J, Pentecost C, Wheeler MJ, Sonksen PH, 2014. Endocrine profiles in 693 elite athletes in the postcompetition setting. Clin. Endocrinol. (Oxf) 81, 294–305. [DOI] [PubMed] [Google Scholar]
- Hines M, 2011. Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Front. Neuroendocrinol 32, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Basaria S, Travison TG, Ho MH, Davda M, Mazer NA, Miciek R, Knapp PE, Zhang A, Collins L, et al. , 2014. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause 21, 612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Pencina KM, Coady JA, Beleva YM, Bhasin S, Basaria S, 2015. Functional voice testing detects early changes in vocal pitch in women during testosterone administration. J. Clin. Endocrinol. Metab 100, 2254–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd HL, Lucas WE, Yen SS, 1974. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. Am. J. Obstet. Gynecol 118, 793–798. [DOI] [PubMed] [Google Scholar]
- Judd HL, Yen SS, 1973. Serum androstenedione and testosterone levels during the menstrual cycle. J. Clin. Endocrinol. Metab 36, 475–481. [DOI] [PubMed] [Google Scholar]
- Karunasena N, Han TS, Mallappa A, Elman M, Merke DP, Ross RJ, Daniel E, 2017. Androgens correlate with increased erythropoiesis in women with congenital adrenal hyperplasia. Clin. Endocrinol. (Oxf) 86, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R, 1998. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J. Clin. Endocrinol. Metab 83, 3078–3082. [DOI] [PubMed] [Google Scholar]
- Kogure GS, Silva RC, Picchi Ramos FK, Miranda-Furtado CL, Lara LA, Ferriani RA, Dos Reis RM, 2015. Women with polycystic ovary syndrome have greater muscle strength irrespective of body composition. Gynecol. Endocrinol 31, 237–242. [DOI] [PubMed] [Google Scholar]
- Lamb DR, 1984. Anabolic steroids in athletics: how well do they work and how dangerous are they? Am. J. Sports Med 12, 31–38. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D, 2000. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J. Clin. Endocrinol. Metab 85, 645–651. [DOI] [PubMed] [Google Scholar]
- Maravelias C, Dona A, Stefanidou M, Spiliopoulou C, 2005. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol. Lett 158, 167–175. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR, 2005. Congenital adrenal hyperplasia. Lancet 365, 2125–2136. [DOI] [PubMed] [Google Scholar]
- Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A, 2006. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab 91, 1683–1690. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Vorona E, 2015a. Doping with anabolic-androgenic steroids (AAS): adverse effects on non-reproductive organs and functions. Rev. Endocr. Metab. Disord 16, 199–211. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Vorona E, 2015b. Mechanisms in endocrinology: medical consequences of doping with anabolic-androgenic steroids: effects on reproductive functions. Eur. J. Endocrinol 173, R47–R58. [DOI] [PubMed] [Google Scholar]
- Padawer R, July, 3, 2016. Too Fast to Be Female The New York Times. [Google Scholar]
- Padero MC, Bhasin S, Friedman TC, 2002. Androgen supplementation in older women: too much hype, not enough data. J. Am. Geriatr. Soc 50, 1131–1140. [DOI] [PubMed] [Google Scholar]
- Penatti CA, Oberlander JG, Davis MC, Porter DM, Henderson LP, 2011. Chronic exposure to anabolic-androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology 61, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr., Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A, 2014. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am. J. Addict 23, 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povzun SA, 2016. Artificial illness as a result of non-medical use of anabolic-androgenic steroids: a case report and a review of literature. Arkh Patol 78, 48–53. [DOI] [PubMed] [Google Scholar]
- Rickenlund A, Carlstrom K, Ekblom B, Brismar TB, von Schoultz B, Hirschberg AL, 2003. Hyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performance. Fertil. Steril 79, 947–955. [DOI] [PubMed] [Google Scholar]
- Ritchie R, Reynard J, Lewis T, 2008. Intersex and the olympic games. J. R. Soc. Med 101, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda C, Jones DA, Round J, Grant DB, 1987. Muscle strength in girls with congenital adrenal hyperplasia. Acta Paediatr. Scand 76, 495–499. [DOI] [PubMed] [Google Scholar]
- Sanchez FJ, Martinez-Patino MJ, Vilain E, 2013. The new policy on hyper-androgenism in elite female athletes is not about “sex testing”. J. Sex. Res 50, 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, et al. , 1998. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J. Clin. Endocrinol. Metab 83, 1312–1318. [DOI] [PubMed] [Google Scholar]
- Sluijmer AV, Heineman MJ, De Jong FH, Evers JL, 1995. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J. Clin. Endocrinol. Metab 80, 2163–2167. [DOI] [PubMed] [Google Scholar]
- Sonksen P, Ferguson-Smith MA, Bavington LD, Holt RI, Cowan DA, Catlin DH, Kidd B, Davis G, Davis P, Edwards L, et al. , 2015. Medical and ethical concerns regarding women with hyperandrogenism and elite sport. J. Clin. Endocrinol. Metab 100, 825–827. [DOI] [PubMed] [Google Scholar]
- Strauss RH, Liggett MT, Lanese RR, 1985. Anabolic steroid use and perceived effects in ten weight-trained women athletes. JAMA 253, 2871–2873. [PubMed] [Google Scholar]
- Talaat M, Talaat AM, Kelada I, Angelo A, Elwany S, Thabet H, 1987. Histologic and histochemical study of effects of anabolic steroids on the female larynx. Ann. Otol., Rhinol., Laryngol 96, 468–471. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Mobini-Far H, Frisk M, 2009. Sudden unexpected death in a female fitness athlete, with a possible connection to the use of anabolic-androgenic steroids (AAS) and ephedrine. Forensic Sci. Int 184, e7–11. [DOI] [PubMed] [Google Scholar]
- Trenton AJ, Currier GW, 2005. Behavioural manifestations of anabolic steroid use. CNS Drugs 19, 571–595. [DOI] [PubMed] [Google Scholar]
- Wood RI, Stanton SJ, 2012. Testosterone and sport: current perspectives. Horm. Behav 61, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC, 1997. Endocrine aspects of anabolic steroids. Clin. Chem 43, 1289–1292. [PubMed] [Google Scholar]
- Xavier NA, McGill JB, 2012. Hyperandrogenism and intersex controversies in women’s olympics. J. Clin. Endocrinol. Metab 97, 3902–3907. [DOI] [PubMed] [Google Scholar]


