Abstract
Purpose:
Cisplatin based chemotherapy regimens form the basis of systemic bladder cancer treatment, although they show limited response rates and efficacy. Recent molecular analysis of bladder cancer revealed a high incidence of mutations in chromatin regulatory genes, suggesting a therapeutic avenue for histone deacetylase inhibitors. We investigated the ability of the novel histone deacetylase inhibitor AR-42 to synergize with cisplatin in preclinical models of bladder cancer.
Materials and Methods:
We assessed the ability of the pan-histone deacetylase inhibitor AR-42 with and without cisplatin to destroy bladder cancer cells by survival and apoptosis assays in vitro, and by growth and differentiation in an in vivo xenograft model. We also assessed the response to the bladder cancer stem cell population by examining the effect of AR-42 on the CD44+CD49f+ population with and without cisplatin. Synergy was calculated using combination indexes.
Results:
The AR-42 and cisplatin combination synergistically destroyed bladder cancer cells via apoptosis and it influenced tumor growth and differentiation in vivo. When tested in the CD44+CD49f+ bladder cancer stem cell population, AR-42 showed greater efficacy with and without cisplatin.
Conclusions:
AR-42 may be an attractive novel histone deacetylase inhibitor with activity against bladder cancer. Its efficacy in bladder cancer stem cells and synergy with cisplatin warrant further clinical investigation. Our in vitro and animal model studies provide preclinical evidence that AR-42 may be administered in conjunction with cisplatin based chemotherapy to improve the treatment of bladder cancer in patients.
Keywords: urinary bladder neoplasms, histone deacetylase inhibitors, cisplatin, apoptosis, chromatin
Bladder cancer affects more than 54,000 men and 17,000 women in the United States annually, making it the fourth most common cancer in men and the ninth most common cancer in women.1 Primary chemotherapy is the mainstay of locally advanced and metastatic disease while neoadjuvant chemotherapy is indicated for muscle invasive urothelial carcinoma before radical cystectomy. Since its approval by the FDA (Food and Drug Administration) in 1978, platinum containing chemotherapeutic cis-diamminedichloroplatinum, or cisplatin, has formed the backbone of primary bladder cancer regimens in combination with methotrexate, vinblastine and doxorubicin or gemcitabine, in part through its ability to induce apoptosis.2 Limitations of cisplatin based therapy for bladder cancer reflect its limited response rate (40% to 50%) as well as its dose limiting nephrotoxicity and neurotoxicity.3
Histone acetylation and deacetylation have a critical role in chromatin formation and gene regulation. Histone acetylation relaxes the chromatin structure into transcriptionally active euchromatin while the opposing effect occurs upon deacetylation. HDAC inhibitors represent a class of compounds that disrupts the function of histone deacetylases, of which there are 4 classes and more than 11 sub-types. HDAC inhibitor function leads to the hyper-acetylation of histone as well as nonhistone proteins.4 Recently interest has focused on the antitumor ability of HDAC inhibitors to interfere with cancer cell proliferation through mechanisms such as cell cycle arrest, apoptosis and the induction of cellular differentiation.5 Currently the HDAC inhibitors vorinostat and romidepsin are approved as treatment of cutaneous T-cell lymphoma.6,7
Bladder cancer is an attractive disease for the use of HDAC inhibitors. Chromatin structure modulation may be a critical step in bladder cancer progression because increased expression of HDAC-1 and 2 is linked to high grade noninvasive urothelial carcinoma. Data from TCGA (The Cancer Genome Atlas) revealed that 76% of the bladder tumors analyzed had an inactivating mutation in at least 1 chromatin regulatory gene.8,9 In previous studies using VA, TSA and belinostat the bladder cancer cell lines were inhibited through cell cycle blockade, induction of apoptosis and reduced tumor growth in in vivo bladder cancer models.10–13 This suggests the possibility that HDAC inhibitors may synergize with the apoptotic effects of cisplatin and potentially increase clinical efficacy and the overall response rate.
AR-42 is a class I (HDAC 1, 2, 3 and 8) and class IIb (HDAC 6 and 10) HDAC inhibitor with activity against multiple cancer types, including chronic lymphocytic and acute myeloid leukemia, B-cell lymphoma, prostate and ovarian cancer, and human glioma cells.14 In fact AR-42 has the distinct ability to target leukemic stem cells while preserving normal hematopoietic stem and progenitor cells. Although it is a phenylbutyrate derivative, AR-42 shows increased activity even at sub μM concentrations.15 AR-42 is currently being evaluated in phase I/IIa clinical trials for hematological malignancies (unpublished data).
We provide essential preclinical data on the potential efficacy of AR-42 in bladder cancer. We hypothesized that the therapeutic ability of AR-42 against bladder cancer would be enhanced by synergy with cisplatin in vitro and in vivo.
MATERIALS AND METHODS
Bladder Cancer Cell Lines
The 2 human urothelial carcinoma cell lines SW780 (CRL-2169) and HT1376 (CRL-1472, ATCC®) were maintained as monolayer cultures in RPMI 1640 with L-glutamine supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin.
Flow Cytometry
Cells stained with anti-CD44 (559942/550989), anti-CD49f (555735) and isotype controls were analyzed on the LSR II flow cytometer (BD™). CD44+CD49f+ cells were sorted using the FACSAria™ cell sorter.
In Vitro Drug Viability Assay
SW780 and HT1376 cells cultured in 96-well plates at 5,000 per well were treated in triplicate for 48 hours with a titration of cisplatin and 1 of 4 HDAC inhibitors, including AR-42, NaB (303410–5G), VA (P4543–10G, Sigma-Aldrich®) and TSA (9950S, Cell Signaling Technology®). At 48 hours MTT was added. The preparations were incubated for 2 hours at 37C, dissolved in dimethyl sulfoxide and quantitated against a standard curve using an Infinite® M1000 Pro spectrophotometer.
On combination drug assays cisplatin was titrated with individual HDAC inhibitors at a constant ratio based on the IC50 concentration of each drug. The ratio of cisplatin to the HDAC inhibitors AR-42, NaB, VA and TSA was 5:1, 1:75, 1:75 and 80:1, respectively.
Time course combinations were performed using certain treatment regimens during 48 hours, including 1) cisplatin and AR-42 for 0 to 24 hours, 2) cisplatin and AR-42 for 24 to 48 hours, 3) cisplatin for 0 to 24 hours and AR-42 for 24 to 48 hours, and 4) AR-42 for 0 to 24 hours and cisplatin for 24 to 48 hours. All cells were washed and plated with fresh treatments or medium at the 24-hour mark.
In Vivo Tumor Model and Therapy
Total SW780 cells or a sorted CD44+CD49f+ fraction of SW780 cells were combined with human fetal bladder mesenchyma in a 1:10 ratio, mixed with Matrigel™ in a 1:1 ratio and subcutaneously implanted in NSG mice.16 At the onset of a palpable tumor on day 15 treatments were started in 1 of 4 groups, including 1) vehicle control, 2) 50 mg/kg AR-42 intraperitoneally 3 times per week, 3) 1.5 mg/kg cisplatin intraperitoneally weekly and 4) combined AR-42 and cisplatin. Tumor size was measured and tumor volume was estimated by multiplying width, length and depth by a factor of 0.4. The mice were sacrificed and the tumors were weighed and fixed in formalin.
Histology and Immunohistochemistry
Representative formalin fixed, paraffin embedded tissues were sectioned at 0.4 μm. Histology was assessed after hematoxylin and eosin staining. Immunohistochemistry was performed on sections that were deparaffinized and rehydrated, and then blocked for 1 hour in 5% bovine serum albumin and 5% goat serum in phosphate buffered saline. Sections were stained with CK5 at 1:5,000 (ab53121, Abcam®) and CK20 at 1:300 (M7019, Dako™) followed by incubation with biotinylated goat anti-rabbit or goat anti-mouse secondary antibodies at 1:750 using an avidin-biotin complex kit (Vector Laboratories, Burlingame, California). Sections were developed with streptavidin conjugated horseradish peroxidase and substrate, counterstained with hematoxylin, and dehydrated and mounted with Cytoseal™ 60. Images were assessed by light microscopy using an Axio Imager 2 (Carl Zeiss Microscopy, Thornwood, New York) and quantitated by the percent stained in deciles per high power field (400×).
Apoptosis Assay
To assay the apoptosis rate SW780 monolayer cells were treated with IC75 doses of 20 μM cisplatin and/or 5 μM AR-42 for 24 hours. Apoptosis was assessed by flow cytometry on the LSR II device using annexin V and PI staining with the Apoptosis Assay Kit (Biotium, Hayward, California) according to manufacturer instructions. We identified live cells by negative staining for annexin V and PI, early apoptotic cells by positive staining for annexin V but negative staining for PI, and late apoptotic cells by positive staining for annexin V and PI.
Statistics
One-way and 2-way ANOVA, and post hoc analysis were performed for group comparisons. Log transformations were done for tumor volume to improve normality. IC50 values of single drug treatments were calculated using CalcuSyn 2.1 (Biosoft®). The CI was calculated for each combination drug treatment at IC50, IC75 and IC90 points using CalcuSyn, version 2.1. CI less than 1.0 indicates synergistic interaction between drugs, values around 1.0 indicate an additive relationship and values greater than 1.0 reflect an antagonistic interaction.
RESULTS
Bladder Cancer Cell Susceptibility to HDAC Inhibition
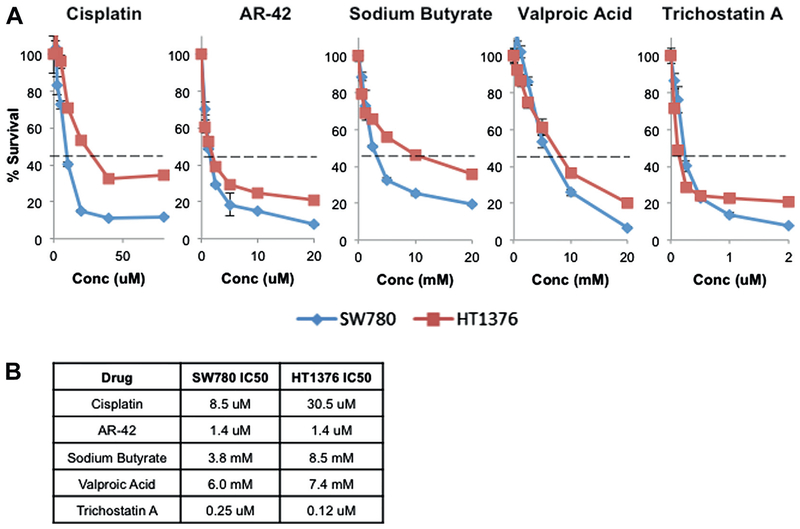
To test SW780 and HT1376 cell sensitivity to cisplatin and HDAC inhibition the cells were treated with a titration of cisplatin or one of the 4 HDAC inhibitors AR-42, NaB, VA or TSA. Cells were assessed for viability and IC50 was calculated (fig. 1, A). SW780 cells appeared more sensitive to all agents than HT1376 cells, which could have been due in part to the source of the cell lines. SW780 cells were derived from a low grade tumor while HT1376 cells were derived from a high grade tumor, potentially explaining the decreased drug sensitivity. The IC50 concentrations of NaB and VA showed significantly greater minimum effective doses than those of AR-42 and TSA, potentially limiting efficacy in vivo (fig. 1, B).
Figure 1.
Viability according to MTT incorporation in SW780 and HT1376 cells treated for 48 hours with titrated doses of single cisplatin and HDAC inhibitor drugs (A). Dashed horizontal lines indicate IC50 (Conc). Data represent mean of triplicate preparations and represent 3 independent experiments. Bars indicate SD. Calculated IC50 concentrations (B). uM, μM.
Synergy between Cisplatin and HDAC Inhibitors
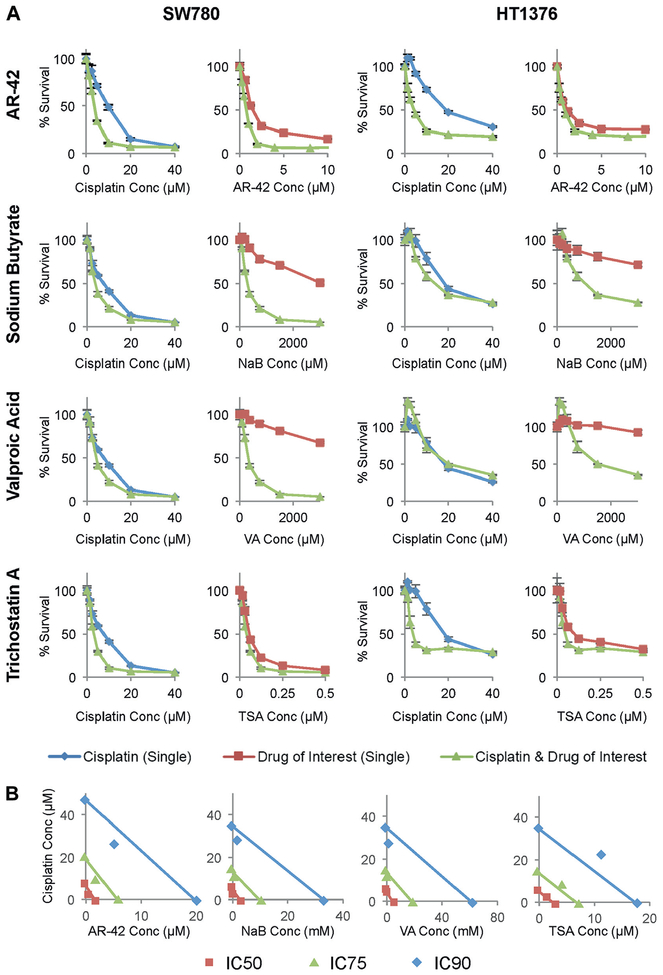
We next investigated combination treatment with cisplatin and each of the HDAC inhibitors to explore synergistic effects on cell viability (fig. 2, A). Cisplatin plus AR-42 and cisplatin plus NaB showed synergy at IC50 and IC90 dose levels as determined by combination index calculations and visualized in isobolograms (fig. 2, B). Cisplatin plus VA and cisplatin plus TSA showed synergy at IC50 but not at IC90 doses (see table).
Figure 2.
Cisplatin synergized with HDAC inhibitors, including novel broad-spectrum classes I and 2b HDAC inhibitor AR-42. Cisplatin was combined with AR-42, NaB, VA and TSA at ratio of 5:1, 1:75, 1:75 and 80:1, respectively, as determined by each IC50 (Conc) (A). Viability was measured by MTT incorporation in SW780 and HT1376 cells treated for 48 hours. Green curves represent combined therapies. Blue curves represent cisplatin alone. Red curves indicate HDAC inhibitor alone. Data represent mean of triplicate presentations and represent 3 independent experiments. Bars indicate SD. Isobolograms created with CalcuSyn show relationship of cisplatin and each HDAC inhibitor in SW780 cells (B). Combination data points on diagonal, lower left and upper right indicate additive, synergistic and antagonistic effects, respectively.
CI of cisplatin plus AR-42, NaB, VA or TSA in SW780 and HT1376 cells by IC
| Cisplatin Combination (dose) |
SW780 CI* | HT1376 CI* |
|---|---|---|
| AR-42: | ||
| IC50 | 0.581 | 0.376 |
| IC90 | 0.419 | 0.709 |
| NaB: | ||
| IC50 | 0.229 | 0.871 |
| IC90 | 0.629 | 0.988 |
| VA: | ||
| IC50 | 0.728 | 0.981 |
| IC90 | 1.021 | 5.566 |
| TSA: | ||
| IC50 | 0.685 | 0.764 |
| IC90 | 1.431 | 3.957 |
Value less than 1 indicates synergy.
Cancer Stem Cell Population More Sensitive to Combined Cisplatin and AR-42
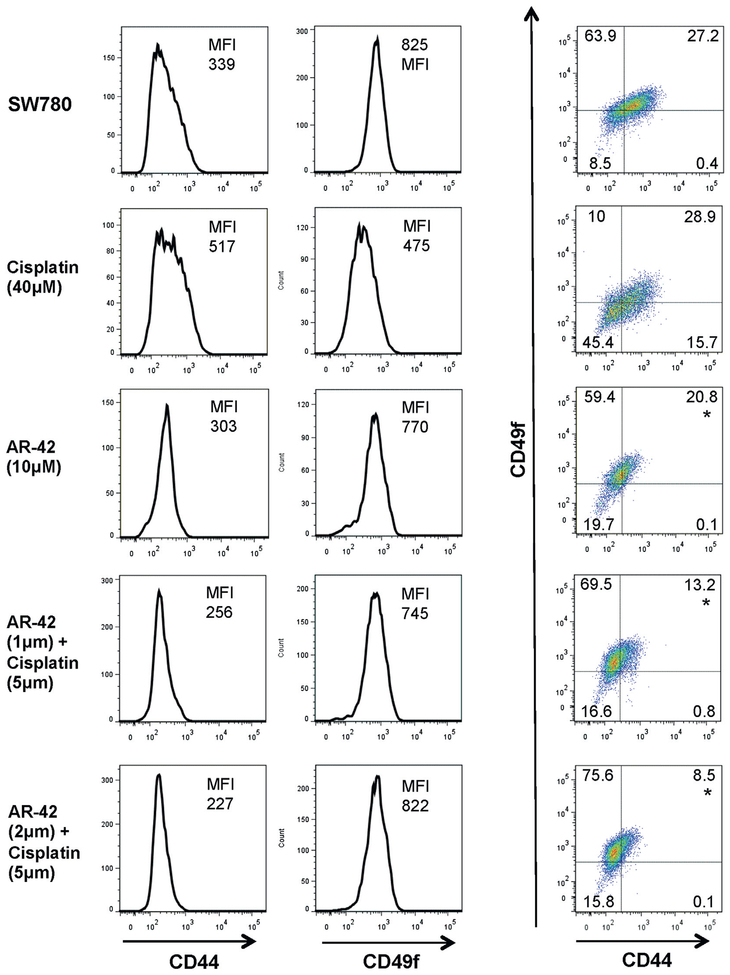
Cancer stem cells are more chemotherapy resistant.17 Because AR-42 had efficacy in leukemic stem cells,14 we assessed the response of AR-42 against bladder cancer stem cell populations. We analyzed SW780 cells by flow cytometry using the expression of CD44 and CD49f, which are surface markers characteristic of bladder cancer stem cells.18 This established that approximately a third of the SW780 monolayer populations were CD44+CD49f+. To examine whether AR-42 had higher affinity to target the bladder cancer stem cell population SW780 cells were treated with cisplatin, AR-42 and cisplatin plus AR-42 at IC75 concentrations. Flow cytometry was then done to examine the expression of stem cell surface markers in surviving populations. The percent of CD44+CD49f+ cells remained similar at 27% to 30% after cisplatin treatment but the percent of CD44+CD49f+ cells decreased significantly after treatment with AR-42 alone and combined with cisplatin (fig. 3). This suggests that compared to cisplatin alone AR-42 may increase the susceptibility of the bladder cancer stem cell population to treatment.
Figure 3.
Cisplatin and AR-42 combination decreased CD44+CD49f+ population. SW780 cells treated with indicated doses of cisplatin and AR-42 to achieve approximately IC75 doses were examined by flow cytometry to assess CD44 and CD49f expression in surviving population. Higher 2 μM AR-42 dose was combined with cisplatin to evaluate dose response. Data represent 3 independent experiments. MFI, median fluorescence intensity. Asterisk indicates CD44+CD49f+ cell group comparisons statistically significantly different (1-way ANOVA p <0.05).
In Vivo AR-42 Decreased Tumor Growth
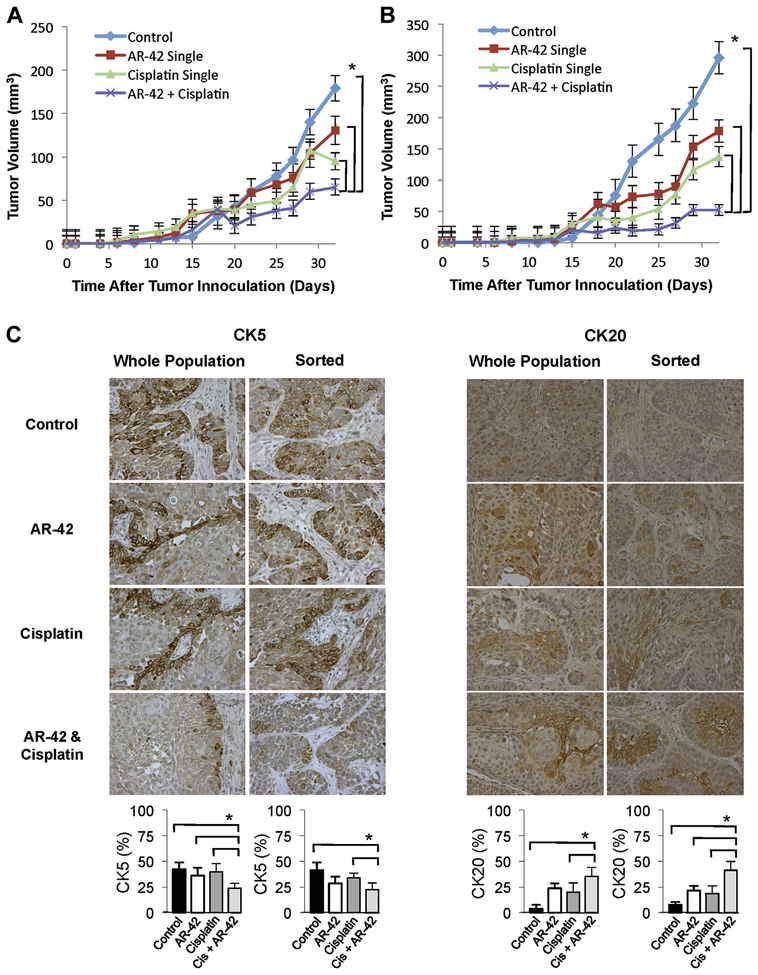
To examine the effects of cisplatin and AR-42 on bladder cancer in vivo growth and differentiation we treated NSG mice implanted with a subcutaneous xenograft consisting of SW780 cells combined with fetal bladder mesenchymal cells. The cisplatin plus AR-42 combination led to a decreased rate of tumor growth and reduced tumor size with a significant decrease in tumor volume after combined treatment vs treatment with AR-42 or cisplatin alone (fig. 4, A). To test the effects of treatment in the cancer stem cell population we sorted SW780 cells and implanted the same number of CD44+CD49f+ fraction cells with fetal bladder mesenchyma. Compared to tumors derived from wild-type SW780 cells the CD44+CD49f+ SW780 tumors grew larger but were more sensitive to AR-42 treatment. Cisplatin plus AR-42 significantly decreased tumor growth compared to treatment with AR-42 or cisplatin alone (fig. 4, B).
Figure 4.
In vivo combined cisplatin and AR-42 decreased tumor size relative to single treatment or untreated tumors in NSG mice implanted with unsorted (A) or CD44+CD49f+ enriched (B) SW780 cells mixed with fetal bladder mesenchymal cells in Matrigel suspension. Treatments began on day 15 after palpable tumor first presented. Curves indicate mean of 6 tumors. Bars indicate SEM. Asterisk indicates statistically significant (2-way ANOVA p <0.05). Representative unsorted and CD44+CD49f+ enriched tumors (C). CK5 and CK20 staining, reduced from ×400. Cis, cisplatin. Columns indicate mean of percent positive staining cells in 4 representative sections at 400× magnification. Bars indicate SD. Asterisk indicates statistically significant (1-way ANOVA p <0.05).
We examined tumor differentiation by staining with basal CK (CK5) and luminal CK (CK20). Changes in tumor differentiation significantly differed between cisplatin only and cisplatin plus AR-42 treatment in tumors derived from native SW780 cells and from the CD44+CD49f+ fraction of SW780 cells. Adding AR-42 decreased basal cell expression, as shown by CK5 staining, and increased luminal cell expression, as characterized by CK20 staining. This suggests that AR-42 treatment may lead to more tumor differentiation than that of untreated or cisplatin treated tumors (fig. 4, C).
Cisplatin and AR-42
Combination Enhanced Apoptosis.
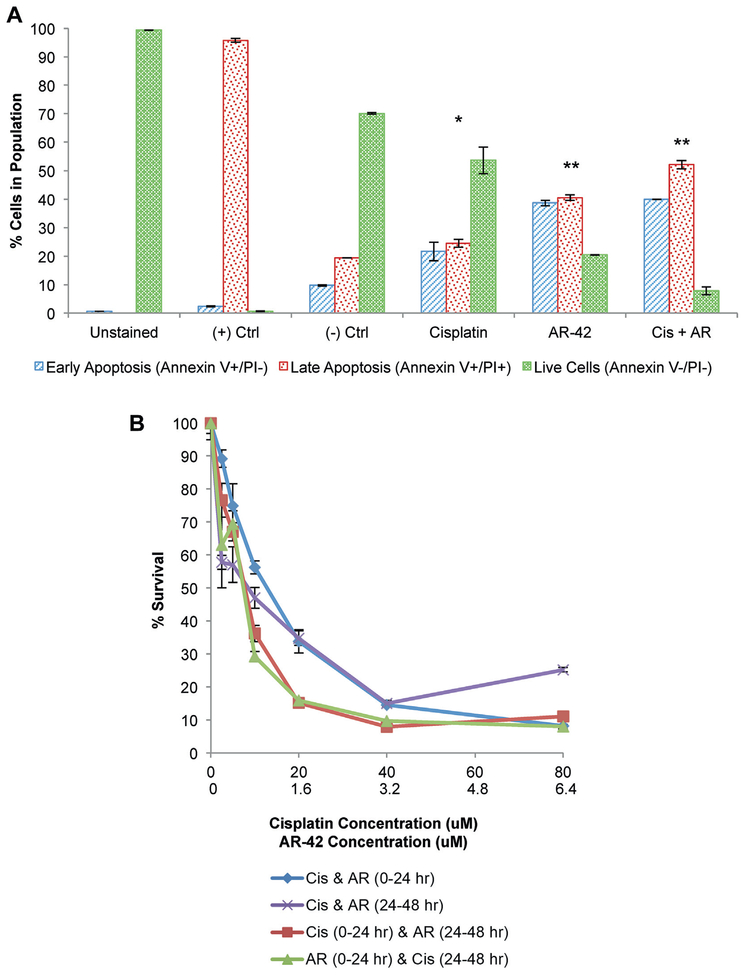
To evaluate the effects of combined cisplatin and AR-42 in apoptosis induction we examined apoptotic activity using a flow cytometry based assay. We found increased apoptosis in the combination treated population compared to that in cells treated with AR-42 or cisplatin alone (fig. 5, A).
Figure 5.
SW780 cells treated with cisplatin (Cis), AR-42 (AR) or cisplatin plus AR-42 for 24 hours showed enhanced apoptosis on annexin V and PI staining followed by flow cytometry (A). Annexin V+PI− cells were considered early apoptotic cells (blue bars). Annexin V+PI+ cells were considered late apoptotic cells (red bars). Live cells were unstained (green bars). Ctrl, control. Single asterisk indicates statistical significance between all populations and other treatment groups (1-way ANOVA p <0.05). Double asterisks indicate statistical significance between all populations and other treatment groups except early apoptotic cells between AR-42 and AR-42 plus cisplatin (1-way ANOVA p <0.05). SW780 cells were treated with varying cisplatin and AR-42 concentrations for 48 hours (B). Viability was assessed by MTT incorporation. Curves indicate mean of triplicate preparations and represent 2 independent experiments (not significant according to 1-way ANOVA p <0.05).
No Difference in Sequence.
Clinically the sequence of combination drug delivery may be important and affect the synergic effects. Thus, we performed in vitro studies to test differences in cell viability based on the sequence of cisplatin and AR-42 administration. During 48 hours concurrent administration in the first or second half of the treatment period yielded no difference in cell viability compared to treatments with 1 drug administered in the first 24 hours followed by the second drug in the next 24 hours (fig. 5, B). This finding suggests that the mechanism of synergy between cisplatin and AR-42 is not sequence dependent.
DISCUSSION
Bladder cancer treatments have remained essentially unchanged for decades with limited patient options. Cisplatin forms the basis of bladder cancer chemotherapy but it is limited by its efficacy and toxicity. However, recent understanding of its molecular subtypes, molecular targets and widespread development of novel immune and targeted therapies, such as those targeting the PD-1/PDL-1 axis, are encouraging and have made inroads in bladder cancer therapy.9,19,20
VA combined with cisplatin in bladder cancer cell lines previously demonstrated the synergistic effects of HDAC inhibitors.2,21 However, a phase II trial of VA in prostate cancer revealed significant neurotoxicity whether administered intravenously or orally.22 TSA remains in preclinical development and NaB requires a mM concentration to achieve a measurable response. Because AR-42 has shown efficacy for multiple cancers, including cancer stem cells in particular,14 we chose it as a promising HDAC inhibitor for analysis. To our knowledge we report for the first time that the novel HDAC inhibitor AR-42 synergizes with cisplatin against bladder cancer cells in vitro and in an in vivo tumor model.
Bladder cancer stem cells, which are marked by the surface markers CD44+ and CD49f+, represent a hierarchical organization of cells that can reconstitute all cell types of a specific tumor.18 Their resistance to chemotherapy may explain recurrence after latency periods. Studies suggest that more poorly differentiated tumors, which are marked by the basal surface markers CK14+CK5+ and the surface marker profile CD90+CD44+CD49f+, have a worse prognosis than less differentiated luminal subtypes that express luminal CK (CK20+) and the associated surface markers CD90−CD44−CD49f+.23 These subtypes were validated in large-scale analyses of patient expression patterns.9,19
The efficacy of HDAC inhibitors in stem cells is supported by recent evidence showing that HDAC-1 and 2 may be critical in embryonic stem cell self-renewal, in part by maintaining expression of the transcription factors Oct4, Nanog, Esrrb and Rex1.14,24 AR-42 previously showed efficacy for specifically targeting leukemic stem cells.14 Our results suggest that AR-42 has improved ability to destroy the CD44+CD49f+ population of bladder cancer cells compared to cisplatin. Treatment with combined AR-42 and cisplatin may lead to more differentiated and, therefore, less aggressive tumors.
Study limitations include the use of bladder cell lines as a surrogate for evaluating primary bladder cancer. The subcutaneous model may also not reflect the bladder microenvironment. However, we noted inconsistencies in generating orthotopic xenografts using human bladder cancer cell lines compared to our established murine models and the subcutaneous model facilitated the measurement of tumor growth. To our knowledge the potential targets of AR-42 to nonhistone proteins has not been addressed. An important future direction is to explore its mechanisms and expand the understanding of bladder cancer biology. Reports of HDAC inhibitors mediating autophagy and the potential inhibition of autophagy by cisplatin in melanoma cells must also be explored.25,26
CONCLUSIONS
In our objective to provide a preclinical analysis of the efficacy of AR-42 in bladder cancer we identified the ability of AR-42 to synergize with cisplatin to augment the destruction of bladder cancer cells in vitro and in vivo, and preferentially target the cancer stem cell population. This synergistic effect may not only improve cisplatin efficacy but also improve its overall response rate or allow for a lower cisplatin dose to be used. This remains a subject of future study. Whether these observations translate clinically to long-term durable responses must be tested in future clinical trials. In the future we may select the use and sequence of novel therapies for bladder cancer based on molecular and genetic markers.
ACKNOWLEDGMENTS
ARNO Therapeutics provided AR-42 through a Material Transfer Agreement.
Study received animal care and use committee approval.
Supported by the Broad Stem Cell Research Center Scholars in Translational Medicine, Perkins Foundation and STOP Cancer.
Abbreviations and Acronyms
- CI
combination index
- CK
cytokeratin
- HDAC
histone deacetylase
- IC50
percent of maximal inhibitory concentration
- NaB
sodium butyrate
- NSG
Nod-scid IL-2Rgammanull
- PI
propidium iodide
- TSA
trichostatin A
- VA
valproic acid
REFERENCES
- 1.Siegel R, Naishadham D and Jemal A: Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11. [DOI] [PubMed] [Google Scholar]
- 2.Von der Maase H, Hansen SW, Roberts JT et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068. [DOI] [PubMed] [Google Scholar]
- 3.Drayton RM and Catto JWF: Molecular mechanisms of cisplatin resistance in bladder cancer. Expert Rev Anticancer Ther 2012; 12: 271. [DOI] [PubMed] [Google Scholar]
- 4.Witt O, Deubzer HE, Milde T et al. : HDAC family: what are the cancer relevant targets? Cancer Lett 2009; 277: 8. [DOI] [PubMed] [Google Scholar]
- 5.Shankar S and Srivastava RK: Histone deacetylase inhibitors: mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv Exp Med Biol 2008; 615: 261. [DOI] [PubMed] [Google Scholar]
- 6.Prince HM and Dickinson M: Romidepsin for cutaneous T-cell lymphoma. Clin Cancer Res 2012; 18: 3509. [DOI] [PubMed] [Google Scholar]
- 7.Siegel D, Hussein M, Belani C et al. : Vorinostat in solid and hematologic malignancies. J Hematol Oncol 2009; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poyet C, Jentsch B, Hermanns T et al. : Expression of histone deacetylases 1, 2 and 3 in urothelial bladder cancer. BMC Clin Pathol 2014; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network: Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley MT, Yoon J, Yee H et al. : The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med 2007; 5: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallo S, Xi W, Hudak L et al. : HDAC inhibition delays cell cycle progression of human bladder cancer cells in vitro. Anticancer Drugs 2011; 22: 1002. [DOI] [PubMed] [Google Scholar]
- 12.Qu W, Kang YD, Zhou MS et al. : Experimental study on inhibitory effects of histone deacetylase inhibitor MS-275 and TSA on bladder cancer cells. Urol Oncol 2010; 28: 648. [DOI] [PubMed] [Google Scholar]
- 13.Ozawa A, Tanji N, Kikugawa T et al. : Inhibition of bladder tumour growth by histone deacetylase inhibitor. BJU Int 2010; 105: 1181. [DOI] [PubMed] [Google Scholar]
- 14.Guzman ML, Yang N, Sharma KK et al. : Selective activity of the histone deacetylase inhibitor AR-42 against leukemia stem cells: a novel potential strategy in acute myelogenous leukemia. Mol Cancer Ther 2014; 13: 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S, Symanowski J, Wong B et al. : A phase II clinical trial of oral valproic acid in patients with castration-resistant prostate cancers using an intensive biomarker sampling strategy. Transl Oncol 2008; 1: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peek EM, Li DR, Zhang H et al. : Stromal modulation of bladder cancer-initiating cells in a subcutaneous tumor model. Am J Cancer Res 2012; 2: 745. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang Z, Yu J et al. : Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett 2012; 322: 70. [DOI] [PubMed] [Google Scholar]
- 18.Chan KS, Espinosa I, Chao M et al. : Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009; 106: 14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Porten S, Kim S et al. : Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014; 25: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles T, Eder JP, Fine GD et al. : MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Jing Y, Ouyang S et al. : Inhibitory effect of valproic acid on bladder cancer in combination with chemotherapeutic agents in vitro and in vivo. Oncol Lett 2013; 6: 1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulp SK, Chen CS, Wang DS et al. : Antitumor effects of a novel phenylbutyrate-based histone deacetylase inhibitor, (S)-HDAC-42, in prostate cancer. Clin Cancer Res 2006; 12: 5199. [DOI] [PubMed] [Google Scholar]
- 23.Volkmer J-P, Sahoo D, Chin RK et al. : Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A 2012; 109: 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamaladdin S, Kelly RDW, O’Regan L et al. : Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc Natl Acad Sci U S A 2014; 111: 9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Bello B, Toscano M, Moretti D et al. : Cisplatin-induced apoptosis inhibits autophagy, which acts as a pro-survival mechanism in human melanoma cells. PLoS One 2013; 8: e57236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert T, Vanoli F, Chiolo I et al. : HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature 2011; 471: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]