Abstract
Post stroke cognitive impairment (PSCI) is an understudied, long-term complication of stroke, impacting nearly 30-40% of all stroke survivors. No cure is available once the cognitive deterioration manifests. To our knowledge, this is the first study to investigate the long-term effects of C21 treatment on the development of PSCI in aged animals. Treatments with C21 or vehicle were administered orally, 24 h post-stroke, and continued for 30 days. Outcome measures for sensorimotor and cognitive function were performed using a sequence of tests, all blindly conducted and assessed at baseline as well as at different time points post-stroke. Our findings demonstrate that the angiotensin receptor (AT2R) agonist C21 effectively prevents the development of PSCI in aged animals.
Keywords: Permanent stroke, post-stroke cognitive impairment, RAS modulation, AT2 receptor, Compound 21
1. INTRODUCTION
1.1. Post stroke cognitive impairment
Post stroke cognitive impairment (PSCI) is an understudied, long-term complication of stroke impacting nearly 30-40% of all stroke survivors [1]. Stroke increases the risk of cognitive impairment by at least 5-8 times [2]. This risk is further accelerated with aging in both men and women [3]. In fact, not only is advanced age the strongest risk factor for stroke throughout the lifespan [4], it is also the primary cause of cognitive decline worldwide [5], Although aging is unavoidable, PSCI can potentially be prevented and properly managed. Unfortunately, there are no FDA approved treatments for PSCI. With the aging of the population, the number of individuals with post-stroke cognitive deficits will continue to escalate [6], further increasing the urgency for treatment development in this area. In fact, The Stroke Progress Review Group at the National Institute of Neurological Disorders and Stroke identified PSCI a top research priority for the next 10 years [7]. Even with Alzheimer’s disease (AD), which is thought to be primarily caused by amyloid pathology, vascular dysfunction due to small vessel disease and stroke, may be of greater importance than amyloid itself in terms of influencing the disease course, especially in older individuals [8]. Likewise, stroke and vascular brain injury are now considered very early, and perhaps initial, pathogenic mechanism not only in PSCI but also in AD [9].
1.2. Pathophysiology
Until recently, it was thought that vascular dementia develops in a step-wise fashion due to repeated ischemic events, but recent data from a large, NIH-funded, epidemiologic trial proved that patients often experience a slowly progressive cognitive decline after a single-stroke lesion, in addition to an acute change [10, 11]. This continuous deterioration occurs even in the absence of any new stroke lesions [10]. Furthermore, with aging, synapse density is reduced and cortical neurons as well as axons are lost, leading to atrophy especially in the prefrontal cortical (PFC) and hippocampal systems. With vascular dysfunction, such age associated changes are aggravated. Moreover, vascular dysfunction is tightly linked to glial and neuronal dysfunction [12]. The resulting dysfunction and damage to neurons and glia will cause further endothelial cell atrophy and microvascular rarefaction owing to their mutual trophic support of vascular cells creating a vicious cycle [13]. The oxidative and pro-inflammatory environment induced by vascular dysfunction results in demyelination as hypoxia, inflammation and oxidative stress damage oligodendrocytes [14] and induce the development and progression of white matter lesions, all of which contribute to the initiation and progression of cognitive impairment [15]. Additionally, hypoperfusion and hypoxia, caused by vascular insufficiency facilitate amyloid β (Aβ) production by activating the APP cleavage enzyme β-secretase, necessary for Aβ production [14]. Aβ further sustains microglial activation and neuroinflammation [16]. It is also a potent vasoconstrictor that suppresses endothelium-dependent responses, reduces CBF and worsens cerebral hypoperfusion [14]. By reducing CBF and transvascular transport, Aβ reduces its own clearance leading to its accumulation and toxicity. This constitutes a feed-forward loop [14]. Therefore, despite the diversity of underlying brain pathology, the vascular alterations have similar mechanistic bases with hypoperfusion, oxidative stress and inflammation leading to endothelial damage, blood brain barrier (BBB) breakdown, demyelination and disruption of trophic coupling between neurovascular unit (NVU) components.
1.3. Renin-angiotensin system (RAS) modulation
Our group has shown that ARBs, by indirectly stimulating brain AT2Rs, are both vascular and neuroprotective after ischemic stroke [17]. Stimulation of the AT2R has emerged as a novel therapeutic strategy in vascular and CNS diseases by virtue of its anti-inflammatory, neuroprotective and tissue regenerating properties [18- 20]. Clinical trial evidence also supports that ARBs, when used for the treatment of hypertension, prevent cognitive and functional declines in older adults and lower the risk of dementia and AD [20]. These agents were also shown to be superior to other antihypertensive medications in preventing early declines in executive and overall cognitive function [21, 22], these agents also reduce BP, an AT1R effect which is undesirable in the acute stroke period [23]. Direct AT2R stimulation, on the other hand, would enable us to achieve the neurovascular benefits of ARBs without the risk of unintended and potentially dangerous, acute BP lowering. This is especially true with advanced age and chronic vascular disease, as these are associated with increased arterial stiffness as well as impairments in cerebrovascular autoregulation. This means that a fall in BP can lead to a disproportionate fall in cerebral perfusion. Long-term therapy with C21 may also be the ideal strategy for preventing PSCI in non hypertensive patients, in those where BP lowering should be avoided or in case of ARB allergies or intolerance. In fact, the direct AT2R agonist, C21, has been shown to support cognitive function in various animal models [15,17-19,25,27]. However, little is known about its effects on PSCI. The purpose of this investigation was to determine the impact of chronic AT2 receptor stimulation, with C21, on long-term cognitive function and development of PSCI in aged rats.
2. MATERIALS AND METHODS
2.1. Experimental Design
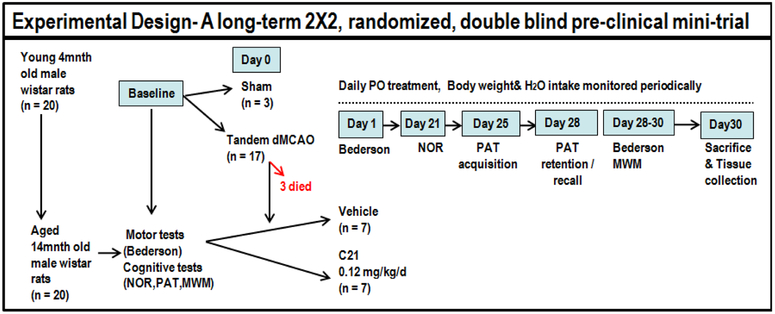
A total of 30 young adult, 4-months old, male Wistar rats, were obtained from Charles River Laboratories and singly housed with free access to food and water, on a 12:12 h light–dark cycle. These animals were kept for 10 months before starting experiments. Once aged (~14 months old, weight range 630–880g), all animals (with the exception of 3 which served as shams/unstroked control animals) were subjected to permanent focal ischemia. The stroked animals were randomly assigned to receive daily C21 or vehicle which was initiated the following day, 24 h after surgery, and continued for a total of 30 days (Fig.1). All experimental procedures and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Charlie Norwood Veterans Affairs Medical Center; Augusta, GA.
Fig. 1:
Schematic depiction of experimental design
2.2. Focal Cerebral Ischemia
Focal cerebral ischemia was produced by tandem distal middle cerebral artery occlusion (dMCAO). This is a permanent stroke model performed by craniectomy, followed by electrocoagulation of blood within the segment of the middle cerebral artery, distal to the inferior cerebral vein. This is accompanied by ventral mid-line incision, separation and “tandem” ligation of the ipsilateral common carotid artery (tandem dMCAO) with 4-0 silicon-coated nylon suture [24]. We chose this model because the suture occlusion model has not been validated in such large animals and the dMCAO allows direct observation of the occlusion and low mortality.
2.3. Treatment
Oral treatment with C21 (VicorePharma, GÖteborg, Sweden), or vehicle was initialed 24 h after stroke and continued for a total of 30 days [25]. Treatments were incorporated into the drinking water and adjusted, according to their daily intake, to provide them with 0.12 mg/kg/d of C21 an oral dose equivalent to 0.03 mg/kg/day IP (calculated using a bioavailability of 25%) [25. 26], This dose was based on our previous publications and shown to have superior cognitive benefits [17, 26, 27]. Treatments were continued for 30 days to combat potential ongoing inflammation [27]. All treatments were prepared and administered by a blinded investigator.
2.4. Time Window for Treatment
In this study, we initiated treatments 24 h after cerebral ischemia, so as to minimize the neuroprotective effects of C21 [28] and focus on the longer term progressive tissue damage and cognitive/neurobehavioral outcomes. Late administration of C21 allows us to assess the C21 effects on reparative mechanisms including modulation of inflammatory responses as we have reported in hypertensive animals [27].
2.5. Outcome Measures
2.5.1. Assessment of Functional Outcome
Since rodents are nocturnal animals, they exhibit the highest level of activity during the dark phase; all behavioral tests were and were conducted after 5PM, during the animals’ natural dark/active phase to ensure reliable results.
2.5.1.1. Body Weight
Weight monitoring gives an independent and unambiguous measure of an animal’s overall health and welfare [30]. For our studies, animals were weighed periodically before surgery and after tandem dMCAO until the day of sacrifice.
2.5.1.2. Neurobehavioral testing
Animals underwent neurobehavioral testing at baseline (before ischemic-insult) and again at different time points after the insult, allowing each individual animal to serve as its own control, hence presenting a more accurate determination of motor and cognitive abilities. The testing sequence, illustrated in Fig. 1, was selected based on the nature and purpose of each individual test and in accordance with the recommendation of a neurobehavioral specialist. In a way designed to minimize task interference - tasks with aversive components, specifically the Inhibitory Avoidance (IA) and Morris Water Maze (MWM), used for assessing spatial learning and long-term /reference memory, were conducted after assessing sensory-motor function (Bederson test) and non-aversive short-term/ working memory, Novel Object Recognition (NOR) test. All functional outcomes were assessed using validated, reproducible, blinded tests.
2.5.1.2.1. Sensorimotor testing
To assess sensorimotor functions, animals underwent the Bederson test, for which animals were assigned a score from 0-3. This score was based on the parameters: forelimb flexion; diminished resistance to lateral push; and contralateral circling. The animal was given one point for each parameter, with lower scores indicating better performance and a score of zero indicating complete absence of deficit [30, 31]. Animals swimming abilities were evaluated using the MWM (limb movement, swim speed and ability to climb onto the platform).
2.5.1.2.2. Cognitive testing
The NOR Test
The NOR test was performed to evaluate non-spatial working memory related to frontal-subcortical circuits [33]. This test, based on the spontaneous tendency of rodents to explore and interact with a novel object more than a familiar one, typically consists of 2 trials separated by a retention period and preceded by a habituation phase. The habituation phase (15min/d) was conducted on 2 separate days, before the start of the test, to allow animals to acclimate to their arena, which consisted of an empty standard size box. On the designated test day, animals were first subjected to an acquisition/sample trial, where the animal is presented with 2 identical (sample) objects and allowed to explore for 10min [32, 33]. Following sample object exposure, the animal was returned to its home cage for a 15min retention period. The 2nd preference/test trial (5min), which follows the retention period, was conducted in the same manner as the 1st trial, except that a new/novel object replaced one of the familiar/sample objects. This interval (delay) between sample and test trials was adjusted to 15min for selective testing of short-term working memory. The discrimination index (DI), which is the difference in exploration time for the objects divided by total time of exploration, and the recognition index (RI), which is the time of novel object exploration relative total time of exploration, were taken indicators of working memory [32-34].
Discrimination index (DI) = (TN- TF)/ (TN+ TF)
Recognition index (RI) = TN / (TN+ TF)
TF and TN are the times spent interacting with the familiar and novel object respectively.
The Inhibitory Avoidance (IA) Test
The IA Test (commonly referred to as Passive Avoidance Test) was used to assess aversive associative learning and related reference memory. During IA, the animal must actively inhibit the desire and withhold the response, to re-enter a dark chamber or explore a novel compartment, after it has experienced an aversive stimulus in that location [36]. For this test, one of the compartments in a Y-maze equipped with a metal floor is connected to an electric circuit box adjusted to deliver brief, moderate intensity electric shocks (3s duration, 1.5mA). For the acquisition trial, designed to condition against an animal's desire to enter a dark chamber or explore a novel compartment when exposed to an aversive stimulus, the shock compartment/arm was blocked and the animal introduced was into one of the “safe” arms and allowed l0mins to explore the 2 open arms. After l0mins, the door blocking the shock arm was opened allowing the animal to enter. Once the animal had fully entered the shock arm (base of the tail had crossed into the arm), its initial latency was recorded and it received a brief electric shock before being returned to its cage. After a 72h retention period, the test trial was conducted. This was performed in a manner similar to that of the acquisition trial except that the foot shock was omitted and all 3 arms were accessible to the animal from the start. The difference between training and test sessions, in latency for entering into the desired compartment (shock arm) was used as a measure of retention. This latency was recorded for up to 300s as an index of long-term aversive associative memory consolidation [35, 36]. Acquisition and retention trials were both performed before (pre) and again after (post) surgical procedure, in order to assess the effect of surgery/treatment by comparing the differences in transfer/step-through latency times between acquisition and retention trials at 2 time points (before and 4 weeks after surgery).
The Morris water maze (MWM) Test
The MWM, which is designed to assess learning, memory and cognitive flexibility, consisted of a large circular pool of water, 120 cm in diameter, 55cm height, filled to a depth of 35±1 cm with water at 25±2 °C. The circular pool was separated into quadrants designated northeast (NE), northwest (NW), southeast (SE) and south-west (SW), based on the 4 equally spaced cardinal points N (North), S (South), E (East), and W(West) around the edge of the pool. One of these quadrants contained a transparent escape platform, submerged 1.5 cm below the water surface and obscured from view. Visual extramaze cues were mounted to aid spatial navigation. The MWM was used to assess various aspects of learning and memory, in addition to motor performance [37,38].
MWM- Training/Learning Sessions
The initial training was a total of 7days and consisted of a single daily trial (120s) per day for 3 consecutive days. This was done to ensure all animals had adequate motor ability to complete the trials as well as allow appropriate acclimation to the test procedure. This was followed by 2 daily sessions, 4 trials each (60s/trial) for 2 consecutive days followed by a single daily 4 trial (60 s/trial) session for 2 more days. Animals were initially placed on the escape platform, located in a fixed position in the NE quadrant for 10s before the start of the first trial. Each trial consisted of releasing the rat into the water from 1 of the 4 starting locations and allowing it 120s to find the platform. If they did not reach the platform within 120s, they were gently guided to it, kept there for 10 s then removed. Trials were spaced 30s apart and the animals were held tightly in between trials. The starting location of each daily session was varied. All trials were recorded and video tracked by the computerized tracking system Etho-Vision XT 7 (Noldus, Leesburg, VA, USA). This automated system monitored animals’ swim patterns and calculated mean escape latency (seconds), total distance travelled to target (cm), and velocity to target (cm/s). Data from all training sessions were pooled for each individual animal, evaluated and compared between groups at the different time points [39].
MWM-Spatial Reference Memory Test
A standard place task/ probe test was used to assess spatial reference memory. This test was conducted on the final days of training. For this test, all procedures were kept the same as during training, except that the platform was removed and rats were allowed to swim for 60 s in an attempt to find it. Performance was evaluated by measuring time spent in the target quadrant/zones, proximity to the target location, and initial latency to the target zone [39].
MWM- Learning and Cognitive Flexibility/Reversal Training
Cognitive flexibility, which allows for flexible updating of representations in response to changing environmental contingencies, and “new” learning, was assessed by the reversal training/ reversal probe test. Such ability is critically dependent on pre-frontal cortical systems and strongly affected by the aging process across species. For reversal training, all parameters were kept the same, except that the platform was moved to a new location in the SE quadrant. Each rat was given a single daily session of 4 trials (120s each) per day for 2-3 consecutive days, to learn the new location. The reversal probe test was conducted similarly to the initial probe test, for which all procedures are kept the same as during training, except that the platform is removed and rats are allowed to swim for 60 s in an attempt to find it. Performance was evaluated by measuring time spent in the target and previous quadrants/zones, proximity to the target locations, and initial latency to the target and previous locations [39]
2.6. Animal Sacrifice and Tissue Collection
At day 30, animals were anesthetized with IP ketamine/xylazine and ten mins later, cerebrospinal fluid (CSF) and plasma was collected. The animals were then decapitated and their brains isolated for molecular analyses.
2.7. Protein Expression
Quantitative determination of Aβ1-42 concentrations, in cortical lysates, plasma and CSF, were carried out using a rat specific sandwich amyloid-β ELISA kit (Wako, USA) according to the manufacturer’s protocol [18], Protein expression of BDNF was achieved by ELISA (MBS00713 BDNF, Mybiosource) kit analyses.
2.8. Statistical Analysis
All statistical analyses were performed using SAS 9.4 and statistical significance was assessed using an alpha level of 0.05, unless otherwise noted. Descriptive statistics (means and standard deviations) for behavioral data measures in aged animals within group and at indicated measurement times were determined. Repeated measures mixed models or nonparametric repeated measures mixed models [40] were used to examine differences in behavioral outcomes for Bederson (nonparametric mixed model), NOR, PAT and MWM between the three groups over time. For each outcome, the mixed model contained fixed effects of group, time and the two-factor interaction between group and time. Animal nested within group was considered a random effect. The statistical test of interest is the F-test for the two-factor interaction between group and time. If this interaction was statistically significant, it indicated that the changes in the studied parameter over time are different in the three groups. A Bonferroni adjustment to the overall alpha level for the number of post hoc pair-wise comparisons performed within group between measurement times and within times between groups was used to control for the number of tests performed. To examine differences in weight over the 31 day period (from baseline/pre-surgery day 0) to the day of sacrifice (30 days following surgery) between groups, a quadratic growth curve model was utilized. This provided a better fit to the nonlinear changes in weight over time. Time (Day) was considered a continuous variable. The fixed effects in the model included group and random effects included the intercept, linear day term, and the quadratic day2 term. A compound symmetric variance co-variance structure provided the best model fit. Differences between groups for specific days (0, 1, 8, 10, and 30) were examined using the estimated least squares means from a repeated measures mixed model and using a Bonferroni adjustment to the overall alpha level for the number of comparisons made between groups. For the MWM reversal training and testing outcomes, as well as molecular parameters, one-way ANOVA was used to examine differences between the groups. A Tukey-Kramer multiple comparison test was used to examine post hoc pair-wise differences.
3. INTEGRATED RESULTS AND DISCUSSION SECTION
3.1. RAS modulator/ AT2R agonist C21 reduced weight loss and enhanced recovery post-stroke
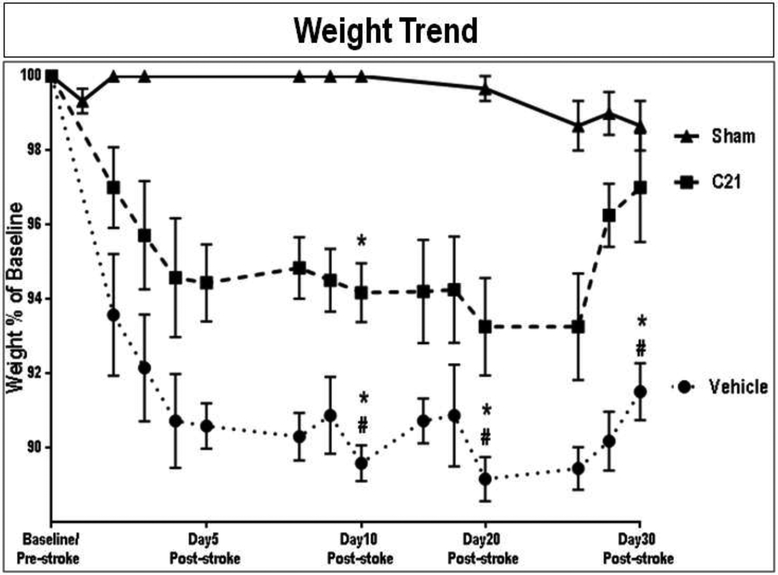
The weight trend represented as % of baseline (pre-surgical weight) varied for the different groups (Fig. 2), with the unstroked shams showing a significant preservation of their baseline weight throughout the study compared to that of stroked animals. The stroked animals showed marked weight loss at 24h post-stroke. These stroked animals continued to lose weight for the first few days post-stroke. In fact, the weight of the unstroked shams was significantly greater than that of stroked animals, compared to both C21 (P=0.0005) and vehicle (P<0.0001) treated groups, at days 8 and 10. Also, the relative weight of C21 treated animals was significantly greater than that of the vehicle treated group on both day 8 (P=0.0009) and day 10 (P=0.0008). Moreover, the C21 group showed complete recovery at 30 days post-stroke. This was not the case with the vehicle treated animals, which continued to display lower weight for the remainder of the study. In fact at Day 30, C21 (P=0.0002) and sham (P<0.0001) animals had significantly greater relative weights than vehicle treated animals, with no difference between sham and C21 at day 30 post-stroke. Therefore, C21, administered daily starting at 24 hours after tandem dMCAO significantly ameliorated weight loss at 8, 10 and 30 days post-stroke, compared to vehicle-treated controls.
Fig. 2: Effect of AT2R stimulation on the body weight of aged wistar animals post-stroke.
The weight trend is represented as % of baseline (pre-surgical weight). A compound symmetric variance co-variance structure provided the best model fit. Differences between groups for specific days (0, 1, 8, 10, and 30) were examined using the estimated least squares means from a repeated measures mixed model and using a Bonferroni adjustment to the overall alpha level for the number of comparisons made between groups (Group x Day x Day effect: F=3.92, P=0.0895. (n = 6-7 animals/group). Symbols and error bars indicate mean and SEM,
*indicate significantly different from sham
# indicate significantly different from treatment
3.2. All animals experienced complete motor recovery at 28 days post-tandem dMCAO
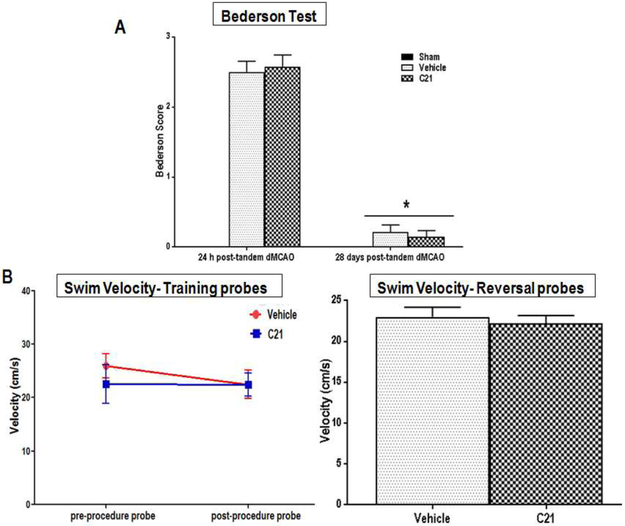
Aged animals subjected to tandem dMCAO (permanent occlusion) showed visible sensorimotor deficits within the first few hours of stroke. These deficits, marked with high Bederson scores, were most pronounced at 24 hr post-stroke and did not differ markedly between the different treatment groups at 28 days post-stroke (Fig. 3A) but were significantly higher than sham at 24h (P<0.0001). Indeed, both treatment groups showed a similar and significant sensorimotor recovery (P=0.0002 for C21 and P=0.0003 for vehicle) from 24h to 28 days post stroke, as evident by similarly low Bederson scores and comparable swimming abilities (Fig. 3B). The spontaneous motor recovery seen in our study is in agreement with that of other investigators employing a similar model (tandem dMCAO) in young untreated rats [41].
Fig. 3: Effect of AT2R stimulation on the sensorimotor function of aged wistar animals post-stroke.
Sensorimotor function in aged animals determined by (A) Bederson test of gait and motor coordination (Group × Time interaction: F(1,∞)=0.50, P<0.4776) and (B) Swim speeds on both initial and reversal tests of the Morris Water Maze (Probe Test F(1,12,.6)=4.53, p=0.0536; Reversal Test t(12)=−0.55, p=0.5949) as measures of swimming ability (a parameter of motor function) for the different treatment groups, (n = 7 animal s/group). Error bars indicate SEM,
3.3. RAS modulator/ AT2R agonist C21 resulted in preservation of non-spatial recognition and short-term working memory in aged rats post tandem dMCAO
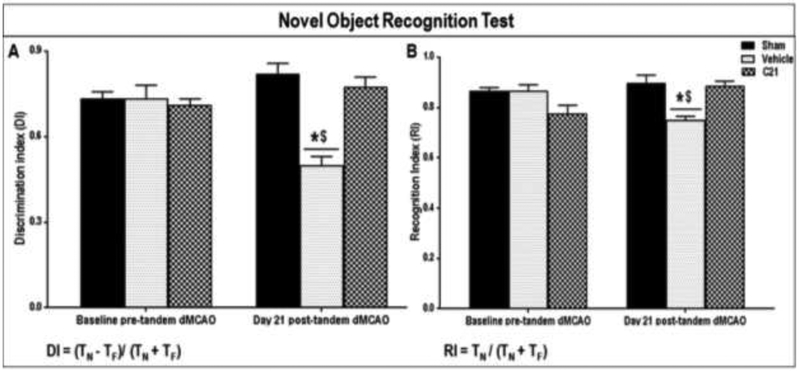
Although there were no statistically significant differences in discrimination index (DI) or recognition index (RI) between any of the groups at baseline, a statistically significant two-factor interaction between group and time was found for both DI (Fig. 4A) and RI (Fig. 4B), indicating that the changes for each outcome over time in the groups had a different pattern. Specifically, vehicle treated animals showed significant reductions in both DI and RI from baseline to 4 weeks post-stroke, such that their DI and RI at week 4 post-stroke were significantly lower than their baseline (P<0.000l) as well as to those of both sham and the C21 (P<.000l) groups at week 4 post-stroke. The sham and C21 treated animals; on the other hand, stayed relatively constant from baseline to 4 weeks. They showed no statistically significant differences in DI or RI between any of the different time points. A major advantage of this test is that it does not involve reference memory components (e.g. explicit rule learning), thus it can be considered a “pure” recognition memory test which reliably evaluates non-spatial working memory related to the frontal subcortical circuits. So, while sensitive to mPFC damage, performance is unaffected by lesions of the hippocampus and rodents with hippocampal damage can perform normally on the NOR test, making it a more selective index of mPFC function [41- 43], Another advantage is that this test does not involve positive reinforcements (e.g. food so doesn’t require food restriction) or negative reinforcements with strong aversive stimuli (e.g. electric shocks likely to produce stress and may influence performance) thus making it more comparable to the memory tests used in humans. This test, being quick and easy to implement, is therefore widely used for assessing cognitive impairment in pre-clinical research [34, 35, 44- 46]. The animals treated with C21 showed superior performance on the NOR test, used to evaluate non-spatial working memory, compared to vehicle treated animals. In fact, most of the C21 treated animals showed discrimination and recognition indices that were not much different from their original baseline pre-stroke values or from those of unstroked sham animals at 21 days post-stroke.
Fig. 4: Effect of AT2R stimulation on the non-spatial working memory of aged wistar animals post-stroke.
Effect of treatment on the (A) Discrimination Index (DI) (Group x Time interaction: F(2.16)=23.33, P<0.0001) and (B) Recognition Index (RI) (Group × Time interaction: F(2.16)=54.33, P=0.0003) of aged wistar rats, at 21 days post-stroke (n = 7 animals/group). Repeated measures ANOVA mixed models were used to examine differences in outcomes, between the groups over time. Bars and error bars indicate mean and SEM. Statistical significance is denoted by *P<0.001 for post hoc pair-wise comparisons from baseline and #P<0.001 for post hoc pair-wise comparisons “between groups” post-stoke, using the Bonferroni adjusted alpha.
3.4. RAS modulator/ AT2R agonist C21 resulted in preservation of reference memory and facilitated associative learning in aged rats post tandem dMCAO
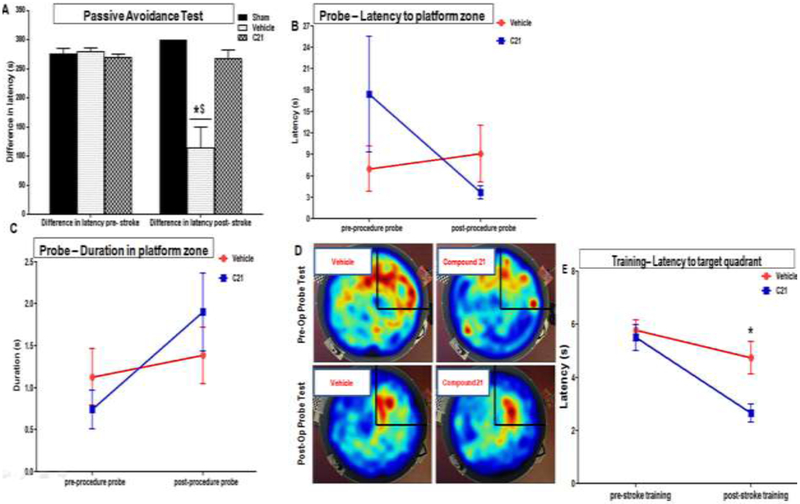
At baseline (before the surgical procedure), the PAT transfer/ step-through latency time significantly increased on the retention trial as compared to the acquisition trial for all the animals. These animals showed similarly high “differences” in the transfer/ step-through latency times between acquisition and retention trials at baseline (Fig. 5A). This not only indicates an intact reference memory, with retention of the aversive event, it also signifies effectual associative learning and recall of the connection between properties of the chamber and the foot shock. However, when this test was conducted again post-insult, only the sham and C21 treated animals showed the expected increase in step-through latency on the retention trial. In-fact the “differences” in the transfer/ step-through latency times between acquisition and retention trials post-stroke was no different from their baseline /pre-stroke values. This signifies a strong, stable long-term memory. On the other hand, the stroked, vehicle treated animals quickly entered the shock arm on the post-retention trial. These animals failed to show any meaningful increases in transfer/ step-through latency time on the retention trial. Their latency difference at 28 days poststroke was significantly lower than that of their baseline/pre-stroke values (P<0.0001)-effect of time- as well as significantly lower than that of their C21 (P<0.0001) and sham (P<0.0001) counterparts-effect of treatment. The protective effect of C21 treatment, on spatial reference memory, was confirmed with the MWM probe test. For this test, the statistically significant two-factor interaction between group and time existed for several parameters, indicating that the changes in such outcomes over time had a different pattern for the two treatment groups. While the aged vehicle treated rats, showed a significant increase (worsening) in latency to reach the target platform zone at 4 weeks post-stroke compared to their initial baseline values, the C21 treated animals actually showed decreases (improvements) in latency compared to their initial baseline values (Fig. 5B). These animals also tended to spend more time in the target zone (Fig. 5C & D). These results are consistent with those of the PAT and support the cognitive benefits of C21 which effectively preserved spatial, reference and long-term memory in aged animals post-stroke.
Fig. 5: Effect of AT2R stimulation on reference memory and associative learning in aged wistar animals post-stroke.
Reference memory and associative learning was assessed by the (A) Passive avoidance test (Group × Time interaction: F(2.16)=10.73, P<.0000l) and Morris Water Maze probe test in aged wistar rats. The platform was removed and rats were allowed to swim for 60 s in an attempt to find it. Performance was evaluated by measuring (B) initial latency to the target zone (Group×Time interaction: F(1,8.22)=4.26, p=0.0719) as well as (C) time spent in the target zones (GroupxTime interaction: F(1,4)=3.86, p=0.0695). (D) Heat maps illustrating relative time spent in the various locations during the probe tests both before (pre-op probe) as well as 4 weeks after stroke (post-op probe). The target (NE) quadrant is indicated with black lines. (E) Initial training latency to target quadrant (Group ×Time interaction: F(1,13.22)=4.25, p=0.0596). (n = 7 animals/treatment group) Symbols and error bars indicate mean and SEM, Repeated measures ANOVA mixed models were used to examine differences in outcomes, between the groups over time.
3.5. RAS modulator/ AT2R agonist C21 was associated with improved cognitive flexibility and reduced preservative behavior in aged rats post tandem dMCAO
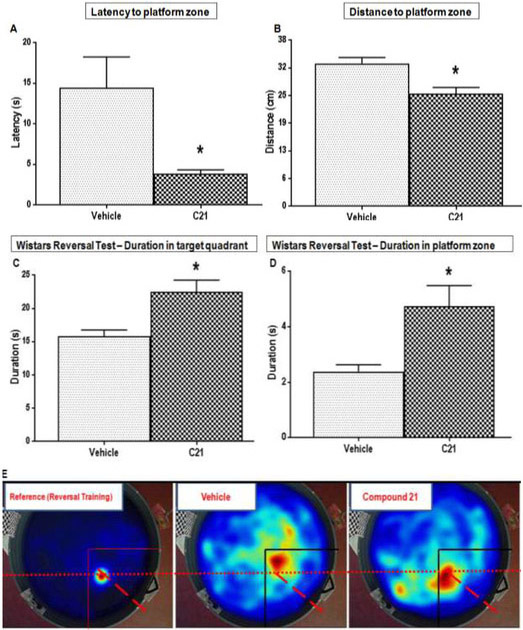
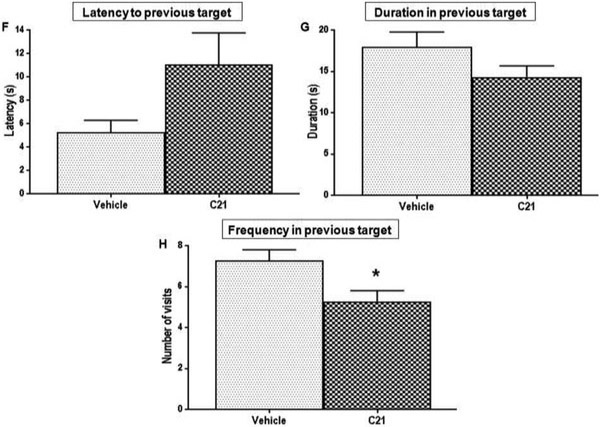
As stated in the previous section, all animals displayed similar learning patterns on the initial (NE platform location) training, a pattern was consistent at baseline and at 4 weeks poststroke. Although the C21 treated animals did show an initial latency to target quadrant that was significantly lower (better) than vehicle treated animals (P=0.0023) at 4 weeks post-stroke (Fig. 5E), the other initial training parameters did not differ significantly between the two groups at 4 weeks post-stroke. This was not the case with reversal training and testing, for which several outcomes showed statistically significant differences between the two treatment groups. C21 treated animals showed a significantly lower (better) latency to the new target (SE quadrant) platform zone, than those of vehicle treated animals (P=0.0169) at 4 weeks post-stroke (Fig. 6A). These animals also showed significantly lower mean distances to this new target zone (Fig. 6B) compared to vehicle treated animals (P=0.0046). This means their swim pattern was more focused, as they tended to swim closer to this target location while attempting to find it. Moreover, these C21 treated animals spent significantly more time, in the new target (SE) quadrant (P=0.0062) and zones (P=0.0198) compared to vehicle treated animals (Fig. 6C-E). This indicates that C21 treated animals maintained their ability to learn new information post-stroke and these animals were also able to effectively update information that had been previously learned before the stroke. On the other hand, animals treated with vehicle could not learn the new target location. In fact, the vehicle treated animals tended to display typical preservative behavior, which is characteristic of the poor cognitive flexibility often associated with VCI [39]. These animals had a lower latency to the previous quadrant (Fig. 6F). They also tended to spend more time in the previous target (NE) quadrant (Fig. 6G) and they visited this previous location much more frequently (P=0.0189) than animals treated with C21 (Fig. 6H). Vehicle treated animals committed more preservative errors, defined as visiting of the previous platform location, used during the initial reference memory training [39]. These deficits are attributed to difficulty with the updating of information in response to changing environmental contingencies. This may result from impaired consolidation or retrieval of brand new memories post-stroke, or with difficulty inhibiting an adaptive or previously learned response when it became no longer appropriate.
Fig. 6: Effect of AT2R stimulation on cognitive flexibility/ new learning in aged wistar animals post-stroke.
Cognitive flexibility and “new” learning was assessed by the reversal training/ test. Performance was evaluated by measuring (A) Latency to target zone (t(12)=−2.77, p=0.0169) (B) Distance to platform zone, which is how close they swim to target location while attempting to find it(t(12)=−3.47, p=0.0046), (C) Duration in new target (SE) quadrant (t(12)=3.31, p=0.0062) and (D) platform zone (t(12)=2.69, p=0.0198) which indicate effective consolidation and retention of the new memory (E) Heat maps illustrating relative time spent in the various locations during the reversal, the target (SE) quadrants is indicated with black lines. The (F) Latency to previous target (t(12)=l.99, p=0.0830), (G) Duration in previous (NE) target quadrant (t(12)=−1.60, p=0.1353) (H) Frequency to previous target (t(12)=−2.71, p=0.0189) are parameters indicating preservative behavior.(n = 7 animals/group, bars and error bars indicate mean and SEM, a one-way ANOVA or two-sample t-test were used to examine differences between the groups and a Tukey-Kramer multiple comparison test was used to examine post hoc pair-wise differences.
3. 6. RAS modulator/ AT2R Agonist C21 prevents cortical accumulation of Aβ1-42 in aged animals post-stroke
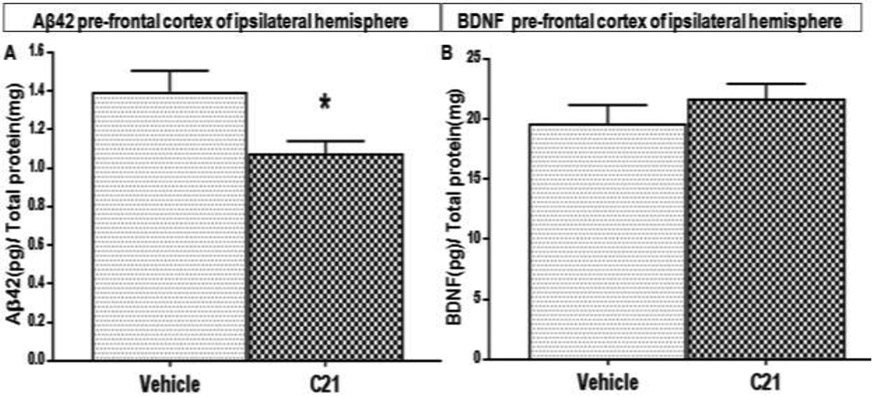
Compound 21 is probably exerting its positive effects on cognition by multiple mechanisms. Reducing inflammation and oxidative stress, as well as promoting cell survival through upregulation of endogenous growth factors, have all been reported and have been recently reviewed [47]. Although the primary endpoints in this investigation were behavioral, we looked for impact on molecules associated with learning and memory. Animals treated daily with C21, starting at 24 h after tandem dMC AO (permanent stroke), had markedly lower concentrations of amyloid beta (Aβ1-42) in their prefrontal cortex at 30 days post-stroke than those treated with vehicle (Fig. 7A). This is a novel finding, as we were the first to study the effect of C21on Aβ accumulation in aged animals after permanent middle cerebral artery occlusion. This reduction in brain Aβ1-42 may be the result of improved clearance and reduced synthesis or both. In fact we found that animals treated with C21 had relatively higher concentrations of Aβ1-42 in their plasma and CSF (data not shown), which may indicate improved clearance from brain parenchyma. While this difference did not reach statistical significance, it may become apparent with future studies using a larger sample size or a higher dose of C21. It may also indicate an additional mechanism of C21 involving reductions in the synthesis Aβ1-42.
Fig. 7: Effect of AT2R stimulation on Aβ1-42 and BDNF in concentrations in the pre-frontal cortex of aged wistar animals post-stroke.
Quantitative determinations of (A) Aβ1-42 and (B) BDNF concentrations in the pre-frontal cortical lysates of aged wistar rats at 30 days post-stroke, (n = 7 animals/group, Error bars indicate SEM, Statistical significance for post hoc comparisons using Tukey’s multiple comparison procedure are denoted by *P < 0.05)
3.7. Aged animals treated with AT2R agonist C21 had no change in BDNF expression in the prefrontal cortex at 30 days post-stroke
A growing body of evidence demonstrates that brain-derived neurotrophic factor (BDNF) plays a crucial role in learning and memory[46]. Moreover, we have previously demonstrated that the AT2R agonist, C21, increases BDNF expression after stroke in young Wistar rats and reduced neurologic deficits and infarct volume when administered after ischemia/ reperfusion [17]. In the current study, animals treated daily with C21, starting at 24 h after tandem dMCAO (permanent stroke), had no significant change in BDNF in their prefrontal cortex at 30 days post-stroke compared to those treated with vehicle (Fig. 7B). It is still possible that BDNF may have peaked during the initial phases post-stroke and was involved in the reparative effects of C21.
4. Conclusion-Significance of the proposed research and its relevance to population health:
At a time when stroke mortality is going down, the rate of stroke-related dementia almost doubled between 1990 and 2000[48]. Unlike sensorimotor deficits which are often maximal in the hours following stroke and tend to gradually improve, cognitive deficits increase over time, in both patients [49]and experimental animal models [50]. Currently, there is no established method/therapy for prevention of post-stroke cognitive impairment, apart from general vascular risk factor management. Moreover, no cure is available once the cognitive deterioration manifests. To our knowledge, this is the first study to investigate the long-term effects of oral treatment with C21 on the development of post-stroke cognitive impairment in aged normotensive animals. This permanent model of stroke was selected for several reasons. First, this model is associated with low overall mortality and higher long-term survival rates than other models, making it a preferred model in aged animals which are more susceptible to complications including increases in intracranial pressure (ICP) associated with edema and hemorrhagic transformation given their age and health condition [51]. Furthermore, according to the standards recommended by the Stroke Therapy Academic Industry Roundtable (STAIR), a permanent MCAO model should be studied first before proceeding to transient models [51]. Since we were the first to study the effect of longterm oral C21 treatment in aged rats, we opted for this model, which also allows better control over infarct size and location, in addition to consistency and good reproducibility of infarct, with visual confirmation of successful MCAO[51]. This thorough investigation included a comprehensive sequence of neurobehavioral tests assessing the various aspects of learning and memory in aged animals post-stroke. These tests were conducted both at baseline and at different stages during treatment.
The present study demonstrated that the AT2R agonist, C21, when started 24 h post-stroke and continued for 30 days, effectively prevented the development of PSCI in aged animals. Therefore, long-term therapy with C21 may be the best approach for preventing PSCI in various subsets of patients including non hypertensive or elderly patients, when BP lowering should be avoided or in case of ARB allergies or intolerance.
HIGHLIGHTS.
C21 reduced weight loss and enhanced recovery in aged rats post-stroke
C21 preserved non-spatial and short-term working memory in aged rats post-stroke
C21 preserved reference memory and facilitated associative learning post-stroke
C21 improved cognitive flexibility in aged rats post-stroke
C21 prevented cortical accumulation of Aβ1-42 in aged animals post-stroke
Acknowledgements
This study was supported by R21-NS088016, VA Merit Review (BX000891), RO1-NS063965 to Susan C Fagan and by VA Merit Review (BX000347), VA Research Career Scientist Award, and R01NS083559 to Adviye Ergul.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Doyle KP, Quach LN, Montse S, Axtell RC, Nguyen TV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS, B-Lymphocyte-Mediated Delayed Cognitive Impairment following Stroke, J Neurosc. 35 (2015)2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Delavaran H, Jönsson A-C, Lövkvist H, Iwarsson S, Elmståhl S, Norrving B, Lindgren A, Cognitive function in stroke survivors: A 10-year follow-up study, Acta Neurol. Scand. 136(2017) 187–194. [DOI] [PubMed] [Google Scholar]
- [3].Renjen PN, Gauba C, Chaudhari D, Cognitive Impairment After Stroke, Cureus. 7 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kalaria RN, Akinyemi R, Ihara M, Biochimica et Biophysica Acta Stroke injury, cognitive impairment and vascular dementia, BBA - Molecular Basis of Disease. 1862 (2016)915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hullinger R, Puglielli L, Molecular and cellular aspects of age-related cognitive decline and Alzheimer ’ s disease, Behavioural Brain Research. 322 (2017) 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Venna VR, Li J, Hammond MD, Mancini NS, Mccullough LD, Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke, European Journal of Neuroscience. 39 (2014) 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Füchtemeier M, Brinckmann MP, Foddis M, Kunz A, Po C, Curato C, Dirnagl U, Farr TD, Vascular Change and Opposing Effects of the Angiotensin Type 2 Receptor in a Mouse Model of Vascular Cognitive Impairment, J. Cereb. Blood Flow Metab. 35 (2015) 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prins ND, Scheltens P, White matter hyperintensities, cognitive impairment and dementia: an update., Nature Reviews. Neurology. 11 (2015) 157–165. [DOI] [PubMed] [Google Scholar]
- [9].Saavedra JM, Evidence to Consider Angiotensin II Receptor Blockers for the Treatment of Early Alzheimer’s Disease, Cell Mol Neurobiol. 36 (2016) 259–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wiesmann M, Kiliaan AJ, Claassen JA, Vascular aspects of cognitive impairment and dementia., J. Cereb. Blood Flow Metab. 33 (2013) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG, Trajectory of Cognitive Decline After Incident Stroke., JAMA. 314 (2015) 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zlokovic BV, Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders, Nat Rev Neuroscience. 12 (2011) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Madri JA, Modeling the neurovascular niche: implications for recovery from CNS injury., Journal of Physiology and Pharmacology : An Official Journal of the Polish Physiological Society. 60 (2009) 95–104. [PubMed] [Google Scholar]
- [14].Iadecola C, Dangerous Leaks: Blood-Brain Barrier Woes in the Aging Hippocampus, Neuron. 85 (2015)231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iwanami M, Mogi J, Tsukuda M, Wang K, Nakaoka X, Kan-no H, Chisaka H, Bai T, Shan H-Y, Kukida B-S, Horiuchi M, Direct angiotensin II type 2 receptor stimulation by compound 21 prevents vascular dementia, Journal of the American Society of Hypertension. 9(2015)250–256. [DOI] [PubMed] [Google Scholar]
- [16].Torika N, Asraf K, Danon A, Apte RN, Fleisher-Berkovich S, Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies, Plos One. 11 (2016) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alhusban A, Fouda AY, Pillai B, Ishrat T, Soliman S, Fagan SC, Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke., J Hypertens. 33(2015)170–180. [DOI] [PubMed] [Google Scholar]
- [18].Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, Min LJ, Steckelings UM, Unger T, Dahlof B, Horiuchi M, Direct stimulation of angiotensin II type 2 receptor enhances spatial memory, J Cereb Blood Flow Metab. 32 (2012) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Namsolleck P, Boato F, Schwengel K, Paulis L, Matho KS, Geurts N, Thöne-Reineke C, Lucht K, Seidel K, Hallberg A, Dahlöf B, Unger T, Hendrix S, Steckelings UM, AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression, Neurobiology of Disease. 51 (2013) 177–191. [DOI] [PubMed] [Google Scholar]
- [20].Yasar S, Xia J, Yao W, Furberg CD, Xue Q, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, Dekosky ST, Carlson MC, Antihypertensive drugs decrease risk of Alzheimer disease, Neurology. 81 (2013) 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rouch L, Cestac P, Hanon O, Cool C, Helmer C, Bouhanick B, Chamontin B, Dartigues JF, Vellas B, Andrieu S, Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms, CNS Drugs. 29 (2015) 113–130. [DOI] [PubMed] [Google Scholar]
- [22].Wharton DP, Felicia K Goldstein C, Zhao Liping, and Steenland IH, Levey Allan I., Rennin-Angiotensin-System Modulation may slow the Convension from Mild Cognitive Impairment to Alzheimer’s Disease, J Am Geriatr Soc. 63 (2015) 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jusufovic M, Sandset EC, Bath PMW, Karlson BW, Berge E, Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis., Int J Stroke. 10 (2015) 1–6. [DOI] [PubMed] [Google Scholar]
- [24].Yao H, Nabika T, Standards and pitfalls of focal ischemia models in spontaneously hypertensive rats: with a systematic review of recent articles., Journal of Translational Medicine. 10 (2012) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahmed HA, Ishrat T, Pillai B, Bunting KM, Patel A, Vazdarjanova A, Waller JL, Arbab AS, Ergul A, Fagan SC, Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals – A randomized double- blind pre-clinical study, Behavioural Brain Research. 346 (2018) 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam a K., Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M, Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist., J. Med. Chem. 47 (2004) 5995–6008. [DOI] [PubMed] [Google Scholar]
- [27].Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, Waller JL, Ergul A, Fagan SC, RAS modulation prevents progressive cognitive impairment after experimental stroke: A randomized, blinded preclinical trial. Journal of Neuroinflammation. 15 (2018) 1–16. doi: 10.1186/s12974-018-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ishrat T, Fouda AY, Pillai B, Eldahshan W, Ahmed H, Waller JL, Ergul A, Fagan SC. Dose-response, therapeutic time-window and tPA-combinatorial efficacy of compound 21: A randomized, blinded preclinical trial in a rat model of thromboembolic stroke. J Cerebr Blood Flow Metab (2018); March 14, ePub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Castillo J NM, and Dávalos A, Progression of ischaemic stroke and excitotoxic aminoacids., Lancet. 349 (1997) 79–83. [DOI] [PubMed] [Google Scholar]
- [30].Freret T, Bouet V, Improvements of the Stroke Model Guidelines – Animal body weight and long-term functional concerns, Journal of Experimental Stroke and Translational Medicine. 2 (2009) 28–31. doi: 10.6030/1939-067X-2.2.28. [DOI] [Google Scholar]
- [31].Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H, Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination, Stroke. 17 (1986)472–476. [DOI] [PubMed] [Google Scholar]
- [32].Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC, Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke., PloS One. 6(2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma J, Xiong JY, Hou WW, Yan HJ, Sun Y, Huang SW, Jin L, Wang Y, Hu WW, Chen Z, Protective Effect of Carnosine on Subcortical Ischemic Vascular Dementia in Mice, CNS Neuroscience and Therapeutics. 18 (2012) 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, Chambon P, Krezel W, Working memory deficits in retinoid X receptor gamma-deficient mice, Learn Mem. 12(2005)318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Antunes G, and Biala M, The novel object recognition memory: Neurobiology, test procedure, and its modifications, Cognitive Processing. 13 (2012) 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ, Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models, Frontiers in Genetics. 5 (2014) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Radahmadi M, Alaei H, Sharifi MR, Hosseini N, The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats, International Journal of Preventive Medicine. 4 (2013) 430–437. [PMC free article] [PubMed] [Google Scholar]
- [38].Bizon JL, Foster TC, Alexander GE, Glisky EL, Characterizing cognitive aging of working memory and executive function in animal models, Frontiers in Aging Neuroscience. 4 (2012) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vazdarjanova A, Bunting K, Muthusamy N, Bergson C, Calcyon upregulation in adolescence impairs response inhibition and working memory in adulthood., Molecular Psychiatry. 16 (2011) 672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brunner F, E., Domhof S and Langer, Nonparametric Analysis of Longitudinal Data in Factorial Designs. 1st Edition Wiley, New York, 2002. [Google Scholar]
- [41].Roof RL, Schielke GP, Ren X, Hall ED, A Comparison of Long-Term Functional Outcome After 2 Middle Cerebral Artery Occlusion Models in Rats, Stroke 32 (2001) 2648–2657. [DOI] [PubMed] [Google Scholar]
- [42].Oliveira AMM, Hawk JD, Abel T, Havekes R, Post-training reversible inactivation of the hippocampus enhances novel object recognition memory, Learning & Memory. 215 (2010) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Barker GRI, Warburton EC, When Is the Hippocampus Involved in Recognition Memory?, The Journal of Neuroscience. 31 (2011) 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T, Object recognition test in mice, Nature Protocols. 8 (2013) 2531–2537. [DOI] [PubMed] [Google Scholar]
- [45].Kouwenberg A, Martin GM, Skinner DM, Thorpe CM, Walsh CJ, Spontaneous Object Recognition in Animals : A Test of Episodic Memory, Advances in Object Recognition Systems. 2012 (2012) 25–40. [Google Scholar]
- [46].Bekinschtein P, Cammarota M, Medina JH, Neuropharmacology Invited review BDNF and memory processing, Neuropharmacology. 76 (2014) 677–683. [DOI] [PubMed] [Google Scholar]
- [47].Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: The renin angiotensin system, International Journal of Molecular Sciences. 19 (2018):876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A, Factors influencing the decline in stroke mortality a statement from the american heart association/american stroke association, Stroke. 45 (2014) 315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bohm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, Bath PMW, Yusuf S, Diener HC, Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke, European Heart Journal. 33 (2012) 2804–2812. [DOI] [PubMed] [Google Scholar]
- [50].Whitehead SN, Cheng G, Hachinski VC, Cechetto DF, Progressive increase in infarct size, neuroinflammation, and cognitive deficits in the presence of high levels of amyloid, Stroke. 38 (2007) 3245–3250. [DOI] [PubMed] [Google Scholar]
- [51].MacRae I, Preclinical stroke research - Advantages and disadvantages of the most common rodent models of focal ischaemia, British Journal of Pharmacology. 164 (2011) 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]