Abstract
The objective of this study was to determine if enteral leucine or branched-chain amino acid (BCAA) supplementation increases muscle protein synthesis in neonates who consume less than their protein and energy requirements, and whether this increase is mediated via the upregulation of the mammalian target of rapamycin (mTOR) pathway or the decrease in muscle protein degradation signaling. Neonatal pigs were fed milk replacement diets containing reduced energy and protein (R), R supplemented with BCAA (RBCAA), R supplemented with leucine (RL), or complete protein and energy (CON) at 4-h intervals for 9 (n = 24) or 21 d (n = 22). On d 9 and 21, postprandial plasma amino acids and insulin were measured at intervals for 4 hr; muscle protein synthesis rate and activation of mTOR-related proteins were determined at 120 min post-feeding in muscle. For all parameters measured, the effects of diet were not different between d 9 or d 21. Compared to CON and R, plasma leucine and BCAA were higher (P ≤ 0.01) in RL- and RBCAA-fed pigs, respectively. Body weight gain, protein synthesis, and activation of S6 kinase (S6K1), 4E-binding protein (4EBP1), and eukaryotic initiation factor 4 complex (eIF4E·eIF4G) were decreased in RBCAA, RL, and R relative to CON (P < 0.01). RBCAA and RL upregulated (P ≤ 0.01) S6K1, 4EBP1 and eIF4E·eIF4G compared to R. In conclusion, when protein and energy are restricted, both leucine and BCAA supplementation increase mTOR activation but do not enhance skeletal muscle protein synthesis and muscle growth in neonatal pigs.
Introduction
Recent advances in the nutritional care of low-birth weight (LBW) infants have improved the outcome of these patients (Kumar et al. 2017). Nonetheless, LBW still represents the main cause of newborn deaths in the US (March of Dimes 2014). Nutritional deficiencies are a primary cause of growth faltering in LBW infants during the early weeks of life (Embleton et al. 2001; Rigo et al. 2002). Both nutrient intolerance and the risk of developing feeding-related pathologies have led to the implementation of feeding protocols which do not meet the infant’s nutrient requirements, and that often result in growth retardation with potential long-term adverse consequences (De Curtis and Rigo 2004; Berseth 2005). Infants that remain smaller than those born full-term at term equivalent age are at risk of continued growth failure (Ehrenkranz 2007), as well as developing type II diabetes (Whincup et al. 2008) and cardiovascascular disease (Lapillone et al. 2013; Atlantis et al. 2009). To increase lean mass accretion and minimize growth deficits in LBW infants, the use of diets above recommended protein intakes during the post-partum period has been proposed (Ziegler at al. 2002; Ehrenkranz 2007). However, feeding high protein formulas to neonates has been associated with increased levels of blood urea and ammonia (Hay 2008), suggesting that significant amounts of amino acids (AA) in the diet may be catabolized instead of being used for anabolic purposes (Hay 2008).
On the basis of studies in cells and rodents demonstrating anabolic effects of leucine (Leu) independent of its function as a substrate for protein synthesis, there has been recent interest in the potential use of supplemental Leu to improve overall nitrogen (N) retention and muscle growth. Previous studies in rodent models and humans have shown a stimulatory effect of Leu on protein synthesis in aged populations (Rieu et al. 2006) and post-exercise (Pasiakos et al. 2011). As such, Leu inclusion in the diet also may have the potential to improve the efficiency of dietary amino acid use for muscle protein synthesis and overall growth in LBW infants, while avoiding the generation of excessive amounts of N waste products. Previously, we showed that both oral and parenteral administration of Leu to neonatal pigs enhances muscle protein synthesis in the short-term by increasing the phosphorylation of the mechanistic target of rapamycin complex 1 (mTORC1) and its downstream effectors, namely ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor 4 (eIF4)-binding protein 1 (4EBP1; Escobar et al. 2005; Wilson et al. 2010; Torrazza et al. 2010; Suryawan et al. 2012; Figure 1). Further, when a Leu supplement was delivered as a pulse to an otherwise complete diet over a more extended period, rates of protein synthesis and lean mass accretion were increased (Boutry et al. 2016). Nonetheless, Leu supplementation did not improve muscle growth in neonatal pigs when energy and protein (Manjarin et al., 2016) intakes were restricted, despite an increase in mTOR pathway activation. This lack of efficacy on growth was associated with a decline in the post-prandial circulating valine (Val) and isoleucine (Ile) concentrations, which may have limited the protein synthetic activity in skeletal muscle (Manjarin et al., 2016). Consequently, in the present study we hypothesized that the efficiency of protein deposition in neonates would increase in response to supplementation of the diet with all BCAA when protein and energy intakes are restricted, and that this increase would be mediated through an increase in muscle protein synthesis via the upregulation of the mTORC1 pathway.
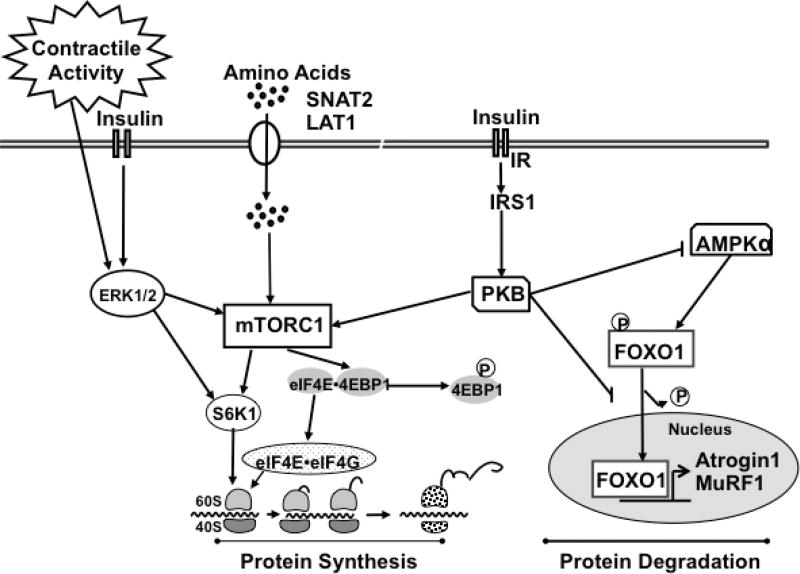
Fig. 1.
Schematic representation of protein synthesis and degradation pathways in skeletal muscle. Briefly, insulin signals by activating the insulin receptor (IR) leading to protein kinase B activation (PKB). Upon activation, mTORC1 phosphorylates its downstream effectors, ribosomal protein S6 kinase 1 (S6K1) and eIF4E-binding protein-1 (4EBP1). Phosphorylated 4E-BP1 releases eIF4E from the inactive eIF4E·4E-BP1 complex to form the active eIF4G·eIF4E complex that binds to mRNA and initiates translation. Amino acid signaling toward protein synthesis is less well understood; nonetheless, the consensus is that amino acids activate substrates downstream of mTOR, leading also to an increase in protein translation initiation via S6K1 and eIF4G· eIF4E activation. Extracellular signal-regulated kinases 1 and 2 (ERK1/2) also activate the mTOR pathway in response to insulin and contractile activity. In addition, insulin inactivates the transcription factors of the forkhead box O (FOXO) family and AMP-activated protein kinase α (AMPKα), which in turn downregulates the transcription of the E3 ubiquitin ligases muscle Atrophy F-Box/Atrogin-1 (MAFbx/Atrogin-1) and muscle RING-finger protein-1 (MuRF1), decreasing overall protein degradation.
Previously, we showed that the longissimus dorsi (LD) muscle, which contains primarily fast-twitch glycolytic fibers, undergoes a more rapid decline in protein synthetic activity during the early weeks of life compared with muscles that contain more slow oxidative fibers, such as the soleus and gastrocnemius (Davis et al. 1989; 2002). As such, the LD, soleus and gastrocnemius were assessed individually at 2 different developmental stages to account for potential interactions between Leu or BCAA content and fiber composition, and which could mask the effect of the diet on protein synthetic activity or mTOR activation pathway in skeletal muscle. Finally, the effects of BCAA supplementation and supplementation with Leu alone on muscle protein degradation signaling, insulin resistance, and amino acid transporter expression under energy and protein restriction conditions were assessed.
Materials and Methods
Animals and experimental design
The experimental design is depicted in Figure 2. Experiments were carried out in accordance with the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Research Council´s Guide for the Care and Use of Laboratory Animals (2011). Forty-six crossbred piglets (Sus scrofa, Yorkshire × Landrace × Hampshire × Duroc; Agriculture Headquarters, Texas Department of Criminal Justice, Huntsville, TX) were weaned when 2-d-old and 2.1 ± 0.2 kg body weight (BW) in 2 consecutive groups that were euthanized at 12 and 24 d of age, respectively. All animals were housed in individual stainless steel cages in temperature-controlled rooms (28°C), with additional zone heating provided if required. Pigs were bowl-fed ad libitum a commercial milk replacement diet (Soweena® Dry Fat 7–60™; Merrick Animal Nutrition, Middleton, WI, USA) until 4 d of age. At 3 d of age piglets were anesthetized with isoflurane (PPC, Richmond Hill, Ontario, CAN) after an overnight fast, a catheter was placed surgically in the left jugular vein using sterile technique (Davis et al. 1996). The catheter was flushed with sterile heparinized saline solution (100 IU·mL−1; APP, Lake Zurich, IL, USA) every 48 h, and maintained with a heparin lock. At the same time a gastric tube was inserted surgically for intra-gastric feeding. The day of the surgery was considered as d 0 of the study.
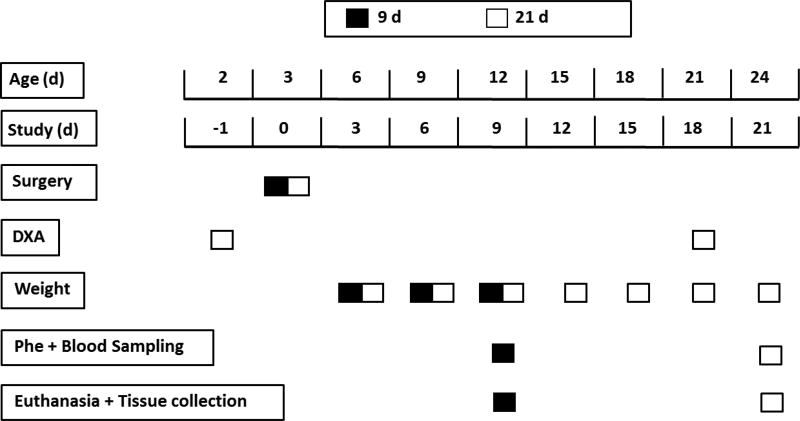
Fig. 2.
Outline of the experimental protocols. Four groups of 2-d-old piglets were assigned to 1 of 4 diets fed at 4-h intervals for 9 (n = 24) and 21 d (n = 22). Catheters were placed surgically in the left jugular vein and stomach on d 0 of the study. Quantitative assessment of fat and lean body composition was performed using dual-energy X-ray absorptiometry on d −1 and 18. Body weights were recorded every 3 d. Blood levels of circulating insulin, glucose, and free AA were measured on d 9 and 21 at 0, 30, 60, 90, 120, 180, and 240 min post-feeding. Protein synthesis rate was measured on d 9 and 21, followed by euthanasia and tissue collection.
On the day after surgery all piglets were randomly assigned to receive 1 of 4 liquid diets (Tables 1 and 2): 1) Control (CON; n = 6): 15 g CP and 215 kcal ME (kg body weight (BW)−1 · d−1), 2) Restricted + BCAA (RBCAA; n = 6): 10.5 g CP supplemented with 1.65 g BCAA and 156.2 kcal ME, 3) Restricted + Leu (RL; n = 6): 10.5 g CP supplemented with 1.19 g L-Leu and 154.8 kcal ME, and 4) Restricted (R; n = 6): 10.5 g CP and 150.7 kcal ME. The CON diet was formulated to exceed by 10% the nutrient requirements of 5–6 kg pigs including the indispensable AA requirements, according to NRC (2012), whereas RBCAA, RL and R diets were formulated to be 20% below CP and ME requirements, but to meet requirements for all other nutrients (Tables 1 and 2). The desired CP reductions were achieved by reducing both whey and casein in the diet. Dietary levels of Leu, Ile, and Val in RL and RBCAA diets were formulated to match or exceed those in the CON diet. In addition, alanine (Ala) was added to diet R to make the three restricted diets isonitrogenous. Protein to energy ratio and lactose content were kept constant across diets. Animals were fed 40 mL · kg BW−1 at 4-h intervals 6 times per day, with the milk replacement delivered over a 20 min period. Feeding was increased gradually to achieve full feeds at d 3 post-surgery.
Table 1.
Ingredient composition of experimental diets (%, as fed).
| Item | Control | Restricted + BCAA | Restricted + Leu | Restricted |
|---|---|---|---|---|
| Whey protein concentrate1 | 3.81 | 2.04 | 2.04 | 2.04 |
| Casein2 | 0.95 | 0.51 | 0.51 | 0.51 |
| Corn oil3 | 3.64 | 2.45 | 2.45 | 2.45 |
| Fat source2 | 3.64 | 2.45 | 2.45 | 2.45 |
| Lactose3 | 0.56 | 0.79 | 0.79 | 0.79 |
| Xanthan gum2 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix4 | 0.21 | 0.21 | 0.21 | 0.21 |
| Trace mineral premix2,5 | 0.90 | 0.90 | 0.90 | 0.90 |
| Amino Acids | ||||
| Lysine-HCl1 | 0.12 | 0.15 | 0.15 | 0.15 |
| DL-Methionine1 | 0.02 | 0.03 | 0.03 | 0.03 |
| Asparagine1 | 0.15 | 0.11 | 0.11 | 0.11 |
| Leucine1 | 0.00 | 0.56 | 0.56 | 0.06 |
| Isoleucine1 | 0.00 | 0.13 | 0.03 | 0.03 |
| Valine1 | 0.07 | 0.18 | 0.08 | 0.08 |
| Cysteine1 | 0.04 | 0.05 | 0.05 | 0.04 |
| Threonine1 | 0.05 | 0.07 | 0.07 | 0.07 |
| Histidine1 | 0.05 | 0.05 | 0.05 | 0.05 |
| Tryptophan1 | 0.01 | 0.02 | 0.02 | 0.02 |
| Phenylalanine1 | 0.11 | 0.10 | 0.10 | 0.10 |
| Tyrosine1 | 0.02 | 0.04 | 0.04 | 0.04 |
| Alanine1 | 0.19 | 0.00 | 0.15 | 0.47 |
| Glutamic acid1 | 0.41 | 0.29 | 0.29 | 0.29 |
| Aspartic acid1 | 0.28 | 0.20 | 0.20 | 0.20 |
| Glutamine1 | 0.20 | 0.14 | 0.14 | 0.14 |
| Glycine1 | 0.32 | 0.22 | 0.22 | 0.22 |
| Proline1 | 0.19 | 0.13 | 0.13 | 0.13 |
| Serine1 | 0.13 | 0.09 | 0.09 | 0.09 |
| Arginine1 | 0.07 | 0.07 | 0.07 | 0.07 |
| Water | 83.75 | 84.89 | 84.89 | 88.15 |
NutraBio®, Middlesex, NJ, USA
Dyets Inc., Bethlehem, PA, USA
Advanced Fat Pak 80; Milk Specialties, Eden Prairie, MN, USA
Dyets Inc., Bethlehem, PA, USA. Provided per kg premix: vitamin A, 4,409,171 IU; vitamin D-3, 661,376 IU; vitamin E, 17,637 IU; vitamin B-12, 15.4 mg; menadione, 1764 mg, riboflavin, 3307 mg; D-pantothenic acid, 11,023 mg; niacin, 19,841 mg; phytase, 200,000 FTU
Provided per kg premix: Fe, 110,000 mg; Zn, 110,000 mg; Mn, 26,400 mg; Cu, 11,000 mg; I, 200 mg; Se, 200 mg
Table 2.
Daily nutrient (g · kg BW−1 · d−1) and metabolizable energy (kcal · kg BW−1 · d−1) intake.
| Item | Control | Restricted + BCAA | Restricted + Leu | Restricted |
|---|---|---|---|---|
| DM | 29.57 | 22.66 | 22.53 | 22.11 |
| CP | 15.04 | 10.53 | 10.54 | 10.50 |
| ME | 215.00 | 156.21 | 154.83 | 150.57 |
| CHO | 3.00 | 3.00 | 3.00 | 3.00 |
| Amino Acids (SID)1 | ||||
| Arg | 0.42 | 0.30 | 0.30 | 0.30 |
| His | 0.32 | 0.23 | 0.23 | 0.23 |
| Ileu | 0.55 | 0.61 | 0.37 | 0.37 |
| Leu | 1.02 | 1.89 | 1.89 | 0.70 |
| Lys | 1.02 | 0.71 | 0.71 | 0.71 |
| Met | 0.26 | 0.19 | 0.19 | 0.19 |
| Cys | 0.29 | 0.21 | 0.21 | 0.21 |
| Phe | 0.60 | 0.42 | 0.42 | 0.42 |
| Tyr | 0.35 | 0.25 | 0.25 | 0.25 |
| Thr | 0.63 | 0.44 | 0.44 | 0.44 |
| Trp | 0.17 | 0.13 | 0.13 | 0.13 |
| Val | 0.69 | 0.72 | 0.48 | 0.48 |
| Ca | 0.09 | 0.06 | 0.06 | 0.06 |
| P | 0.10 | 0.06 | 0.06 | 0.06 |
SID (Standardized ileal digestible): values were calculated using the AA values and the standardized ileal digestibility values from NRC (2012)
Body weights (BW) were recorded every 3 d, and average daily gain (ADG) and feed efficiency were calculated for both d 0–9 and d 9–21 time periods. Animals in the first group (n = 24) were euthanized at d 9 of the study (12 d of age), and those in the second group (n = 24) at d 21 (24 d of age), using an injection of pentobarbital sodium (0.4 mL · kg BW−1; Schering-Plough, Union, NJ, USA). Tissue samples were obtained from the longissimus dorsi (LD), gastrocnemius, and soleus muscles, heart and liver. Tissue was weighed, frozen in liquid N and stored at −80°C until analyzed. Quantitative assessment of fat and lean body composition was performed only in animals euthanized on d 21 using dual-energy x-ray absorptiometry (DXA; Columbus et al. 2015) 1 d before surgery and on d 18 of the study. Animals were sedated with an intramuscular injection of telazol (2.2 mg · kg BW−1; Zoetis, Florham Park, NJ, USA,) and xylazine (1.1 mg · kg BW−1; Lloyd Laboratories, Shenandoah, IA, USA). Scans were performed using a Hologic Delphi-A scanner (Hologic, Bedford, MA, USA) and analyzed using the manufacturer’s software (version 11.2, Hologic, Inc., Waltham, MA, USA). Body composition data were adjusted according to Koo et al. (2004) to correct Hologic DXA Infant Scan Mode results to more accurately estimate piglet body composition.
Plasma insulin, glucose and amino acids
Blood was sampled from the jugular vein immediately before feeding and at 30, 60, 90, 120, 180, and 240 min post-feeding on d 9 (group 1) and d 21 (group 2). Samples were centrifuged at 12,000 × g for 2 min, and plasma was stored at −20°C. Insulin (µU · mL−1) was measured using a porcine insulin radioimmunoassay kit (Millipore, St. Charles, MO, USA) while glucose levels (mg · dL−1) were determined by the glucose oxidase method (Thermo Scientific, Waltham, MA, USA). Insulin resistance from basal (fasting) glucose and insulin concentrations was estimated using the homeostatic model assessment (HOMA), according to the formula: [fasting insulin (µU · mL−1) × fasting glucose (mg · dL−1)]/405. Plasma free AA concentrations were quantified by high-performance liquid chromatography (HPLC; PICO-TAG reverse-phase column; Waters, Mildford, MA, USA) using an analytical method based on deproteinization and derivatization of AA with phenylisothiocyanate (Burrin et al. 1995).
Protein synthesis rate
Ninety mins after feeding, animals were injected via the jugular vein catheter with 10 mL · kg BW−1 of a flooding dose of L-[4-3H]Phe (American Radiolabeled Chemicals, St. Louis, MO, USA) which provided 1.5 mmol Phe · kg BW−1 and 1 mCi of L-[4-3H] Phe · kg BW−1. Plasma samples were collected at 5, 15, and 30 min after injection and stored at −20°C for measurement of the free Phe specific radioactivity of the blood pool. Piglets were euthanized immediately after the last blood sample and tissues were collected. Free and protein-bound Phe in tissues were measured by HPLC using an anion exchange column (PA1 column; Dionex, Sunnyvale, CA, USA) followed by post-column derivatization, as previously described (Davis et al. 1999). Fractions were collected, and the radioactivity associated with the phenylalanine peak was measured by liquid scintillation counting (Tri-Carb 2500TR; Packard Instrument, Meriden, CT, USA).
The fractional rate of protein synthesis (KS: % of protein mass synthesized per d; % · d−1) for each tissue was calculated as:
where Sb (dpm · min−1) is the specific radioactivity of the protein-bound Phe, Sa (dpm · min−1) is the specific radioactivity of the tissue-free Phe calculated from the specific radioactivity at the time of the tissue collection corrected for the change over the labelling period from the linear regression of the blood specific radioactivity at 5, 15 and 30 min against time, and t is the labeling time in min of the specific tissue. Previous studies have demonstrated that, after a flooding dose of 3H-Phe is administered, the specific radioactivity of tissue-free Phe is in equilibrium with the aminoacyl-tRNA-specific radioactivity, and therefore the tissue-free Phe is a valid measure of the precursor pool-specific radioactivity (Davis et al. 1999).
Translational efficiency and capacity
To assess whether changes in fractional rate of protein synthesis are due to changes in ribosomal abundance and/or translational efficiency, total muscle protein was quantified using the Pierce BCA assay (Thermo Scientific, Rockford, IL, USA) (Lowry et al., 1951) and total muscle RNA content was quantified according to Munro and Fleck (1969). Ribosomal abundance, a measure of protein synthetic capacity (CS), was estimated as the total RNA-to-protein ratio (i.e., mg RNA · g protein−1), given that the majority of RNA in the tissue is ribosomal (Fiorotto et al. 2000). Ribosomal translational efficiency, namely protein synthetic efficiency (KRNA), was estimated as the total protein synthesized per total RNA (i.e., g protein · g RNA−1).
Western Blot analysis
Equal amounts of protein samples were electrophoretically separated on polyacrylamide gels (PAGE; C.B.S Scientific, Del Mar, CA, USA) and transferred to PVDF membranes (Pall Corporation, Pensacola, FL, USA). The membranes were incubated overnight with primary antibodies, followed by 1 h incubation with secondary antibody. Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System® (UVP, Upland, CA, USA). For normalization, immunoblots performed with anti-phosphospecific antibodies were exposed to stripping buffer (Pierce Biotechnology, Rockford, IL, USA) and reprobed with nonphosphospecific antibodies against total protein (Cell Signaling, Danvers, MA, USA), as previously described (Davis et al. 2000).
Primary antibodies used were against ribosomal protein S6 kinase (S6K1 Thr389 and total; Cell Signaling Technology; Beverly, MA, USA), eukaryotic initiation factor 4 (eIF4E)-binding protein-1 (4EBP1 Thr70 and total; Cell Signaling Technology), insulin receptor substrate 1 (IRS1 Ser1101 and total; Cell Signaling Technology), protein kinase B (PKB Ser308 and total; Cell Signaling Technology), Na+-coupled neutral AA transporter 2 (SNAT2; Aviva Systems Biology, San Diego, CA, USA), L-type AA transporter 1 (LAT1; Thermo Scientific, Rockford, IL, USA), the AMP-activated protein kinase alpha (AMPKα Thr172 and total; Cell Signaling Technology), muscle atrophy F-box/Atrogin-1 (ECM Biosciences, Versailles, KY, USA), muscle RING-finger protein 1 (MuRF1; R&D Systems, Minneapolis, MN, USA), forkhead box protein O1 (FOXO1 Ser256 and total; Cell Signaling Technology), and extracellular signal-regulated kinases 1 and 2 (ERK1/2 Thr202/Tyr204 and total; Cell Signaling Technology).
The eukaryotic initiation factor 4 complex (eIF4E·eIF4G) was immunoprecipitated using an anti-eIF4E monoclonal antibody obtained from aliquots of fresh tissue homogenates (gift of Dr. Leonard Jefferson, Pennsylvania State University, College of Medicine, Hershey, PA, USA). Briefly, muscle homogenates were processed as previously described (Suryawan et al., 2001) and immediately subjected to protein immunoblot analysis using rabbit anti-eIF4G (Bethyl Laboratories, Montgomery, TX, USA). Amounts of eIF4G were corrected by the eIF4E (Cell Signaling Technology) recovered from the immunoprecipitates.
Statistical analyses
Data was analyzed by a 3-way ANOVA using linear mixed models in SAS 9.2 (SAS Institute Inc., Cary, NC, USA), with diet (CON, RBCAA, RL, and R), muscle (LD, gastrocnemius, and soleus), day of study (d 9 and 21), and their interactions as fixed effects and initial body weight as covariate. A repeated measurement statement was included for parameters measured repeatedly post-feeding, with the structure of the covariance selected based on smallest Akaike information criterion. If an interaction was found to be not significant, it was removed from the model and the data reanalyzed. Normality of the residuals and presence of outliers were assessed in SAS. Non-normally distributed parameters were power transformed by a parameter φ whose optimal value was estimated using the maximum likelihood (ML) method (Piepho, 2009). P-values for pre-planned pairwise comparisons were calculated using Student’s t-tests. Data were presented as least square means ± SE. Significant effects were considered at P ≤ 0.05 and trends at P ≤ 0.1.
Results
Body weight, body composition, average daily gain, and tissue weights
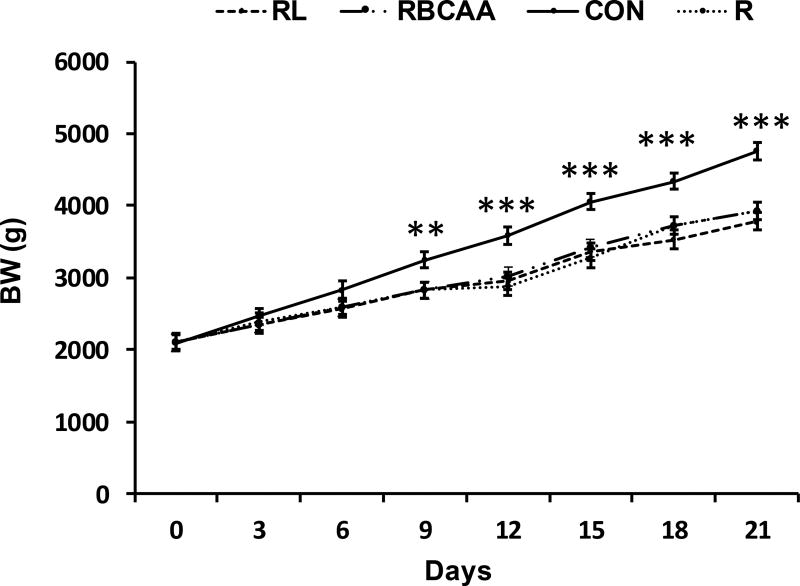
The effect of diet on BW, ADG, and feed efficiency during the first 9 d of study was not significantly different (P = 0.61) between animals euthanized on d 9 and d 21 (i.e., groups 1 and 2), so data for this time period were combined for analysis (n = 46). Values between d 9 and 21 of the study pertain only to animals euthanized on d 21 (n = 22). There were no differences in initial BW among the 4 groups (Figure 3). Piglet BW was higher for CON compared to RBCAA, RL, and R on d 9 (P ≤ 0.01), d 12 (P ≤ 0.001), d 15 (P ≤ 0.001), d 18 (P ≤ 0.001), and d 21 (P ≤ 0.001) of age. Average daily gain and feed efficiency were also higher in CON compared to RBCAA, RL, and R between 0–9 and 9–21 d intervals (P ≤ 0.001; Table 3). There were no differences in lean and fat body mass between animals prior to the study (Table 3). Total lean mass on d 18 and lean mass gain between d 0 and 18 was greater in CON compared to RBCAA, RL, and R (P ≤ 0.001), whereas total fat mass on d 18 did not differ among treatments (Table 3). Weight of the LD (P ≤ 0.0001) and liver (P ≤ 0.001) were lower in RBCAA, RL, and R groups than CON on d 9 and 21 and of the heart (P ≤ 0.01) on d 21 (Table 4). Compared to CON, gastrocnemius weight was lower in RL and RBCAA (P ≤ 0.05) and soleus weight was lower in RBCAA and R (P ≤ 0.05) on d 21. Weight increased significantly for all tissues between d 9 and 21 (P ≤ 0.05).
Fig. 3.
Body weight (BW) of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). Values are least square means ± SE. **P ≤ 0.01 ***P ≤ 0.001.
Table 3.
Average daily gain (ADG), feed efficiency (FE), and body composition of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + leucine (RL), or Restricted (R) diets between d 0 and 21 of the study.
| Item1,2 | CON | RBCAA | RL | R |
|---|---|---|---|---|
| ADG3, g·d−1 | ||||
| 0–9 | 128e ± 6.87 | 80.6f ± 7.17 | 79.4f ± 6.87 | 79.2f ± 7.17 |
| 9–21 | 132e ± 9.71 | 94.3f ± 10.6 | 95.8f ± 9.71 | 82.7f ± 10.6 |
| FE3, g BW·d−1 | ||||
| 0–9 | 0.22e ± 0.01 | 0.14f ± 0.01 | 0.14f ± 0.01 | 0.14f ± 0.01 |
| 9–21 | 0.25e ± 0.01 | 0.20f ± 0.01 | 0.20f ± 0.01 | 0.19f ± 0.01 |
| Lean mass4, g | ||||
| 0 | 1800 ± 145 | 1827 ± 159 | 1797 ± 145 | 1841 ± 158.9 |
| 18 | 3635e ± 145 | 2954f ± 159 | 2994f ± 145 | 2911f ± 158.9 |
| 0–18 | 1835e ± 127 | 1126f ± 139 | 1196f ± 139 | 1070f ± 126.5 |
| Fat mass4, g | ||||
| 0 | 328 ± 42 | 283 ± 46 | 304 ± 42 | 311 ± 45 |
| 18 | 552 ± 42 | 519 ± 46 | 440 ± 42 | 468 ± 45 |
| 0–18 | 224 ± 60 | 235 ± 65 | 177 ± 65 | 196 ± 72 |
Values are least square means ± SEM
Values with different superscripts are significantly different: cdP ≤ 0.01 efP ≤ 0.001
ADG and FE (G:F = grams of body weight per grams of feed) calculated between d 0–9 (n = 46) and d 9–21 (n = 22).
Lean and fat calculated only for second group of pigs (n=22)
Table 4.
Tissue weights (grams) of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + leucine (RL), or Restricted (R) diets for 9 and 21 d.
| Item1,2 | CON | RBCAA | RL | R |
|---|---|---|---|---|
| Day 9 | ||||
| n | 6 | 6 | 6 | 6 |
| LD | 50.9a ± 3.88 | 37.6b ± 3.88 | 37.1b ± 3.88 | 36.1b ± 3.88 |
| Gastrocnemius | 9.25 ± 1.63 | 8.33 ± 1.63 | 7.60 ± 1.63 | 8.46 ± 1.63 |
| Soleus | 3.40 ± 0.35 | 3.30 ± 0.35 | 2.80 ± 0.35 | 2.80 ± 0.35 |
| Liver | 115.9e ± 7.45 | 79.4f ± 7.45 | 85.4f ± 7.45 | 83.9f ± 7.45 |
| Heart | 18.07 ± 1.05 | 18.19 ± 1.05 | 18.46 ± 1.05 | 17.36 ± 1.05 |
| Day 21 | ||||
| n | 6 | 5 | 6 | 5 |
| LD | 62.9c ± 3.88 | 44.9d ± 4.26 | 44.3d ± 3.88 | 41.7d ± 4.26 |
| Gastrocnemius | 22.9a ± 1.63 | 15.9b ± 1.78 | 18.1b ± 1.63 | 19.4ab ± 1.78 |
| Soleus | 4.91a ± 0.35 | 3.53b ± 0.38 | 4.3a ± 0.35 | 3.12b ± 0.38 |
| Liver | 177.2e ± 7.45 | 128.0f ± 8.16 | 124.3f ± 7.45 | 113.1f ± 8.16 |
| Heart | 25.3c ± 1.05 | 20.9d ± 1.15 | 21.6d ± 1.05 | 20.6d ± 1.15 |
Values are least square means ± SEM
Values with different superscripts are significantly different: abP ≤ 0.05, cdP ≤ 0.01, efP ≤ 0.001
Insulin, glucose and free AA levels
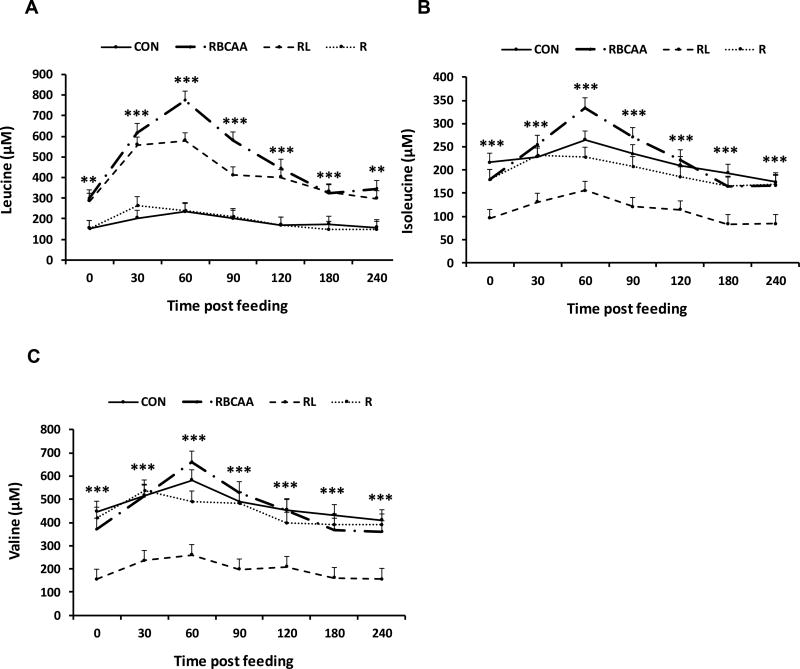
Fasting insulin levels were higher (P < 0.05) in CON compared to RBCAA, RL and R at both d 9 and d 21 (Table 5). Likewise, HOMA values were also higher (P < 0.05) in CON compared to RBCAA, RL and R on d 9 and 21 (Table 5). There were no differences in postprandial plasma levels of glucose and insulin among diets at either d 9 and 21 during 4h post feeding (P = 0.89 and 0.56 for insulin and glucose, respectively; data not shown). The effect of diet on postprandial levels of plasma AA was not different between animals euthanized on d 9 and d 21 (i.e., P-values for the interaction Diet×Time (after feeding)×Day was not significant; data are shown in Online Resource Table 1A), so only results pertaining to Diet×Time effect are discussed (Figure 4). Supplementation of Leu or RBCAA in the diet increased plasma Leu levels in both RL and RBCAA groups compared to CON and R between 0 and 240 min post-feeding (P ≤ 0.001; Figure 5A), whereas it decreased Ile and Val levels in RL between 0 and 240 min (P ≤ 0.001; Figure 5B and C). Plasma levels of proline, glutamate, aspartate and serine were higher (P ≤ 0.05) in CON compared to RBCAA, RL, and R at different times post-feeding. Data is included as online Supplemental Material along with the remainder of values for plasma EAA and NEAA (Online Resource Figure 1A–Q).
Table 5.
Postabsorptive insulin and glucose levels in d 9 and 21 piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + leucine (RL), or Restricted (R) diets between d 0 and 21 of the study. Blood was sampled 6 h after feeding. Homeostatic model assessment (HOMA) values were calculated according to the formula: [fasting insulin (µU · mL −1) × fasting glucose (mg · dL−1)]/405.
| Item1,2 | CON | RBCAA | RL | R |
|---|---|---|---|---|
| Insulin, µU·mL−1 | ||||
| d 9 | 3.83a ± 0.88 | 0.84b ± 0.96 | 1.66b ± 0.88 | 0.43b ± 0.88 |
| d 21 | 4.12a ± 0.88 | 2.56b ± 0.96 | 2.81b ± 0.88 | 1.62b ± 0.96 |
| Glucose, mg·dL−1 | ||||
| d 9 | 87.08 ± 7.45 | 99.90 ± 7.45 | 95.96 ± 7.45 | 81.59 ± 7.45 |
| d 21 | 95.19a ± 7.45 | 83.19ab ± 8.16 | 74.04b ± 7.45 | 73.56b ± 8.16 |
| HOMA | ||||
| d 9 | 0.77a ± 0.17 | 0.21b ± 0.19 | 0.36b ± 0.17 | 0.09b ± 0.17 |
| d 21 | 0.94a ± 0.17 | 0.56b ± 0.19 | 0.53b ± 0.17 | 0.31b ± 0.19 |
Values are least square means ± SEM
Values with different superscripts are significantly different: abP ≤ 0.05
Fig. 4.
Effect of diet on postprandial leucine, isoleucine, valine and branched-chain amino acid (BCAA) concentrations in plasma of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). Values are least square means ± SE. *P ≤ 0.05 **P ≤ 0.01 ***P ≤ 0.001.
Fig. 5.
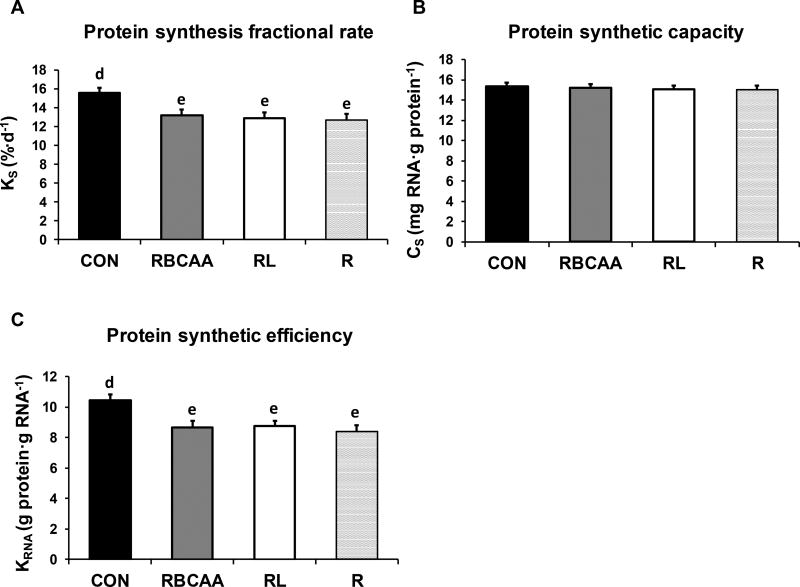
Fractional rates of protein synthesis (KS), protein synthetic capacity (CS) and protein synthetic efficiency (KRNA) in skeletal muscle of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). The interactions Diet×Muscle×Day, Diet×Day and Diet×Muscle were not significant, so only the effect of Diet is shown. Values are least square means ± SE; n = 11–12/group. defP ≤ 0.01.
Protein synthesis rate
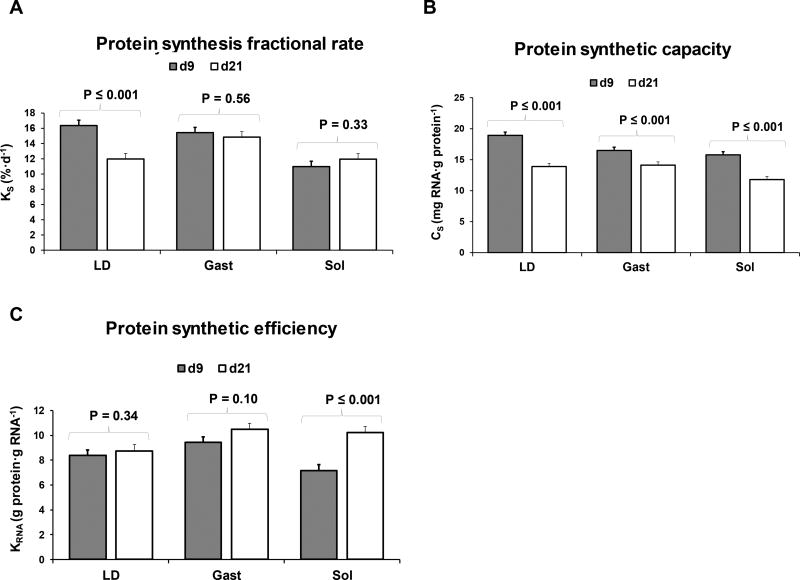
The interactions Diet×Muscle×Day, Diet×Day and Diet×Muscle for KS, CS, and KRNA parameters were all not significant (P-values are shown in Online Resource Table 1A), so only the effect of Diet and Muscle×Day of study are discussed (Figures 5 and 6). Fractional protein synthesis rate (KS) was greater in CON compared to RBCAA, RL, and R groups (P ≤ 0.01; Figure 5A). Likewise, KRNA was greater in CON compared to RBCAA, RL, and R (P ≤ 0.01; Figure 5C), while CS was not different among diets (Figure 5B). Between d 9 and 21, KS decreased in LD (P ≤ 0.001; Figure 6A), as well as CS (P ≤ 0.001; Figure 6B) but not KRNA (P = 0.34; Figure 6C). Conversely, KS did not change between d 9 and d 21 in either soleus (P = 0.33; Figure 6A) or gastrocnemius (P = 0.56; Figure 6A). CS decreased in both muscles (P ≤ 0.001; Figure 6B) while KRNA increased in soleus and tended to increase in gastrocnemius (P ≤ 0.001 and = 0.1, respectively; Figure 6C).
Fig. 6.
Fractional rates of protein synthesis (KS), protein synthetic capacity (CS), and protein synthetic efficiency (KRNA) in longissimus dorsi, gastrocnemius, and soleus of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). Values are least square means ± SE.
Protein signaling and amino acid transporter abundance
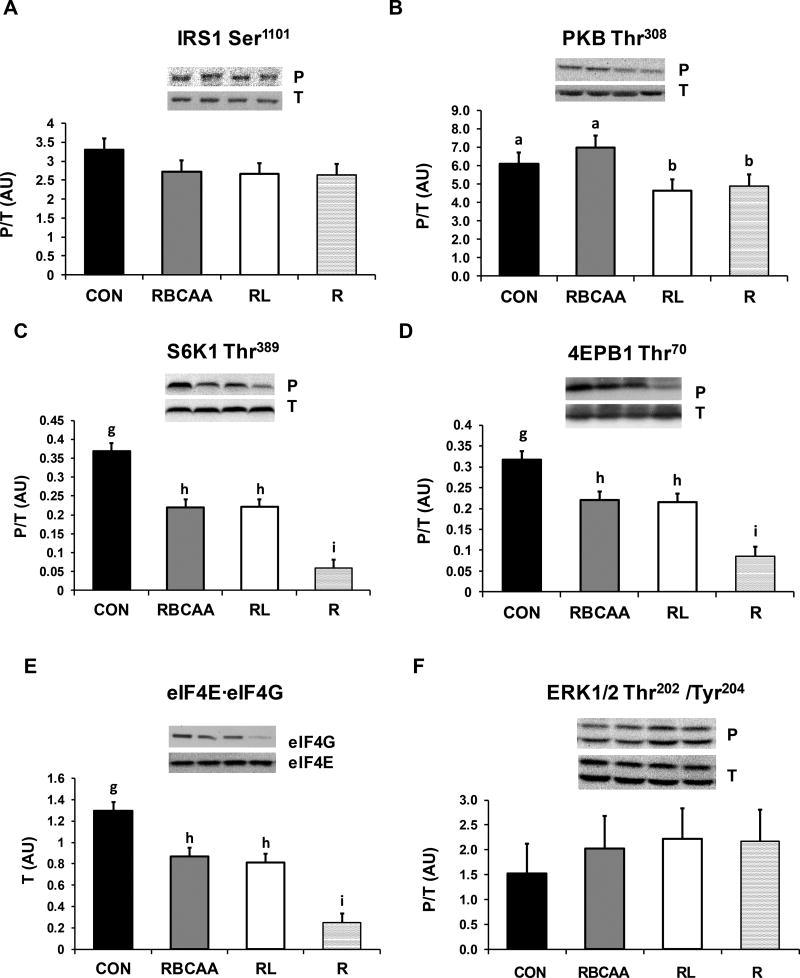
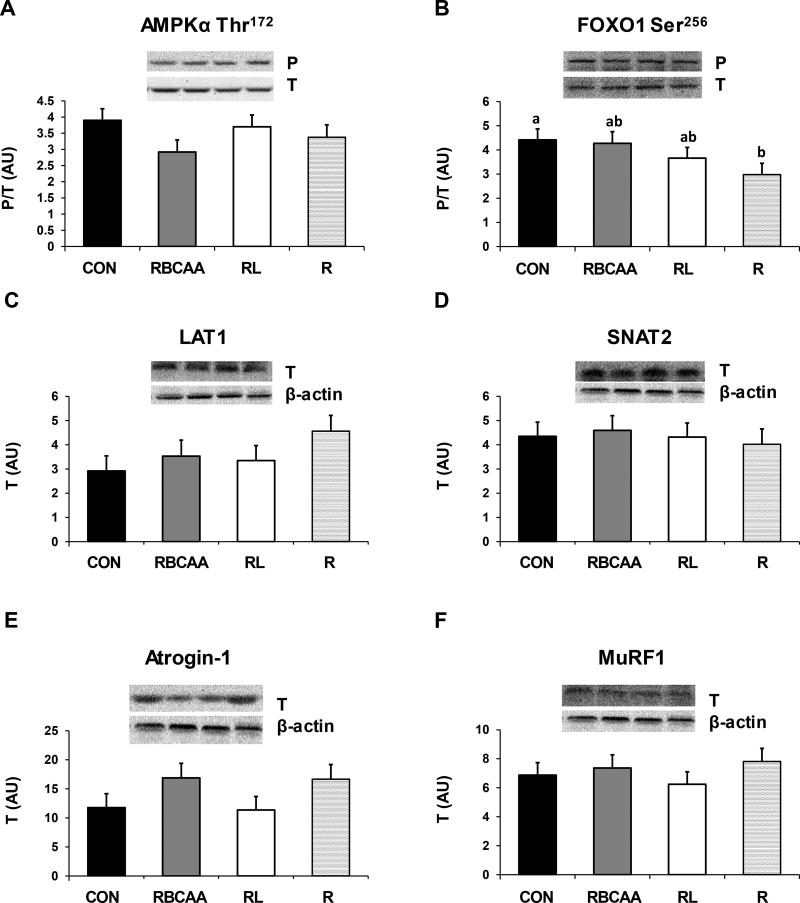
The interactions Diet×Muscle×Day, Diet×Day and Diet×Muscle for protein signaling and AA transporter abundances were all not significant (P-values are shown in Online Resource Table 1B), so only the effects of Diet and Muscle×Day of study are discussed. There were no differences in phosphorylation of IRS1 (Ser1101) among groups (Figure 7A). Phosphorylation of PKB (Thr308) was higher in both CON and RBCAA groups compared to RL and R (P ≤ 0.05; Figure 7B). Phosphorylation of S6K1 (Thr389) and 4EBP1 (Thr70) was higher in RL and RBCAA compared to R (P ≤ 0.01), and in CON compared to RBCAA and RL (P ≤ 0.01; Figure 7C and D). Likewise, the formation of the active eIF4E·eIF4G complex was higher in RL and RBCAA compared to R (P ≤ 0.05), and increased further in the CON group (P ≤ 0.05; Figure 7E). Phosphorylation of ERK1/2 (Thr202/Tyr204; Figure 7F) did not differ among groups. Phosphorylation of FOXO1 (Ser256) was higher in CON compared to R (P ≤ 0.05; Figure 8B) and was intermediate in RBCAA and RL groups. There were no differences among groups in the phosphorylation of AMPKα (Thr172) (Figure 8A) and in the protein abundance of LAT1, SNAT2, Atrogin1, and MuRF1 (Figures 8C–F).
Fig. 7.
Abundance of total (T) and phosphorylated (P) levels of IRS1, PKB, S6K1, 4EBP1, eIF4E·eIF4G, and ERK1/2 in skeletal muscle of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). The interactions Diet×Muscle×Day, Diet×Day and Diet×Muscle were not significant, so only the effect of Diet is shown. Values are least square means ± SE. abcP ≤ 0.05, defP ≤ 0.01
Fig. 8.
Abundance of total (T) and phosphorylated (P) levels of LAT1, SNAT2, AMPKα, FOXO1, MuRF1, and Atrogin-1 in skeletal muscle of piglets fed Control (CON), Restricted + BCAA (RBCAA), Restricted + Leu (RL), or Restricted (R) diets for 9 (n = 24) and 21 d (n = 22). The interactions Diet×Muscle×Day, Diet×Day and Diet×Muscle were not significant, so only the effect of Diet is shown. Values are least square means ± SE. abcP ≤ 0.05
Figures representing the developmental effects on muscle IRS1, PKB, S6K1, 4EBP1, AMPKα, FOXO1, and ERK1/2 phosphorylation, and eIF4E·eIF4G, LAT1, SNAT2, Atrogin-1, and MuRF1 abundance are included as online Supplemental Material (Online Resource Figures 2–3). Between d 9 and 21, phosphorylation of PKB (Thr308) decreased in all muscles (P ≤ 0.05), phosphorylation of S6K1 (Thr289) decreased in gastrocnemius (P ≤ 0.001) and tended to decrease in LD (P = 0.09), whereas phosphorylation of IRS-1 (Ser1101) and 4EBP1 (Thr70) and activation of eIF4E·eIF4G complex decreased in LD and gastrocnemius (P ≤ 0.01). Protein abundance of SNAT2 and atrogin-1 decreased in LD and soleus (P ≤ 0.05), whereas MurF1 decreased only in LD (P ≤ 0.0001). Phosphorylation of FOXO1 (Ser256) and ERK1/2 (Thr202/Tyr204) increased in LD and gastrocnemius from day 9 to 21 (P ≤ 0.001).
Discussion
Despite improvements in the nutritional management of LBW infants, their extrauterine growth frequently falters, in part due to the inadequate intake of protein during the early weeks of postnatal life (Embleton et al. 2001; Rigo et al. 2002; de Curtis and Rigo 2004). Although premature infants may be provided parenteral nutrition initially, the goal is to transition them to enteral feeding as soon as it can be tolerated (Berseth, 2001). However, the switch to oral feedings is limited by the concern of the infant´s ability to metabolize the nutrients offered and the risk of developing feeding-related pathologies (Berseth 2005) which often leads to dietary protein intakes that cannot support maximal growth (Embleton et al. 2001; Rigo et al. 2002). Using the neonatal pig as a model, our laboratory has demonstrated a dual effect of dietary Leu in promoting muscle protein accretion in newborns, by acting as an essential AA (EAA) in protein synthesis and also by modulating the intracellular signaling pathways that regulate translation initiation (Torrazza et al. 2010; Suryawan et al. 2012; Columbus et al., 2015; Boutry et al., 2016). Given that the clinical condition of LBW infants often precludes their consumption of full feeds over protracted lengths of time, in the present study we assessed the potential for using oral Leu supplementation alone, or in combination with Ile and Val, to enhance the lean growth of neonatal pigs when both protein and energy intakes are supotimal for an extended period.
Leu or BCAA supplementation does not improve growth in protein and energy-restricted neonates
Previously, we showed that when CP and ME intake were restricted by 30%, Leu supplementation increased mTOR activation in skeletal muscle of neonate pigs, but did not improve protein synthetic activity or muscle mass growth (Manjarin et al., 2016). In light of these findings, we hypothesized that leucine supplementation could enhance muscle protein synthesis if the reductions in dietary protein and energy intake were less severe. However, supplementation of Leu or BCAA when CP and ME intakes were restricted to only 20% below CP and ME requirements did not lead to an improvement in piglet body weight gain or muscle KS when compared to an unsupplemented restricted diet, despite an increase in the signaling pathways that promote translation initiation. These results suggest that substrate availability may be critical for Leu to increase protein synthesis, and support results from previous studies where dietary Leu did not maintain increased rates of muscle protein synthesis in adult humans beyond 3-h post-feeding when it was supplemented in a suboptimal single bolus of protein (Churchward-Venne et al. 2012). Similarly, long-term infusion of Leu failed to sustain increased rates of muscle protein synthesis in neonate pigs unless additional AA were administered concurrently (Wilson et al. 2010; Escobar et al. 2007). It its noteworthy that an increase in daily BCAA intake in adult humans undergoing 19 d caloric restriction increased body fat loss without inducing changes in lean mass (Mourier et al. 1997).
In assessing the metabolic effects of Leu or BCAA supplementation in a restricted diet, the negative effect of energy restriction on N retention in neonatal pigs cannot be discounted, as large amounts of dietary AA may have been catabolized for energy production instead of being used as substrate for protein synthesis. Data from LBW infants fed different energy intakes at the same protein level showed an increase in N utilization in parallel with higher energy intake (Duffy et al. 1981), whereas a positive relationship between energy intake and N retention levels was observed in young pigs (Campbell and Dunkin, 1983) and lambs (Black and Griffiths, 1975). Furthermore, long-term supplementation of pigs with Leu or BCAA in diets that were 30% CP-restricted, but with normal energy intake, resulted in an increase in mTOR pathway activation and muscle protein synthesis (Yin et al. 2010; Zhang et al., 2013; Columbus et al. 2015), as well as greater ADG (Yin et al. 2010; Zhang et al., 2013) in both neonatal and weaned pigs. As such, the chronic ME deficit induced by the restricted diet in the current study may have contributed to the lack of anabolic effect of Leu or BCAA supplementation on neonatal muscle protein synthesis, despite enhanced activation of the mTOR signaling pathway, as evidenced by increased S6K1 and 4EBP1 phosphoryation and eIF4E·eIF4G formation. Interestingly, phosphorylation of ERK1/2 and AMPKα did not differ among CON and restricted diets, even though activation of both proteins has been shown to increase under chronic caloric restriction conditions in skeletal muscle of monkeys and rats (Nadeau et al. 2006; Palacios et al. 2009). However, possible species- and age-specific regulation of AMPKα and ERK1/2, in addition to the shorter nature of our study, preclude further comparison among studies.
Ile and Val are not limiting for protein synthesis in Leu supplemented diets
A relevant observation in our previous work was that the post-prandial plasma levels of Val and Ile decreased in response to Leu supplementation, likely due to the Leu-induced upregulation of the activity of the branched chain-keto acid dehydrogenase enzymatic complex in BCAA oxidation (Tannous et al. 1963; Harper et al. 1970). This led us to speculate that both AA may have become rate-limiting for protein synthesis in neonatal pigs (Manjarin et al. 2016). However, we now have shown that BCAA supplementation does not enhance muscle protein synthesis compared to RL or R, despite preventing the decline in plasma Val and Ile concentrations. Interestingly, plasma Leu concentrations were greater in the BCAA compared RL group, despite Leu intake being matched. Given that Leu, Ile, and Val are all oxidized by the same enzymatic complex, it is possible that an increase in the amount of total enzymatic substrate may have lowered the rate of Leu oxidation, hence increasing Leu circulating levels. Alternatively, having higher Val and Ile competing for the AA transporters in the BCAA group may have decreased the amount of Leu taken up into the cells compared to RL. We cannot discount the possibility that AA other than Leu stimulate muscle protein synthesis through mTOR, and thus contribute to the higher protein synthesis rates in the CON group. For example, arginine and glutamine are known to increase S6K1 and 4EBP1 phosphorylation in pig trophoblasts (Kim et al. 2013) and enterocytes (Ban et al. 2004), whereas threonine and Ile activated the mTOR pathway in murine embryonic stem cells (Ryu and Han 2011) and skeletal muscle of rats (Anthony et al. 2000). Nonetheless, only proline was significantly decreased in the RBCAA and RL groups compared to CON, rendering unlikely that changes in muscle protein synthesis rate between diets were related to differences in EAA circulating levels in the present study.
Leu or BCAA supplementation may not ameliorate protein degradation signaling in skeletal muscle
Previous studies have identified the transcription factor FOXO1, as well as ubiquitin ligases Atrogin-1 and MuRF1, as key regulators of skeletal muscle proteolysis (Sandri et al. 2004; Stitt et al. 2004). In normal, growing myotubes and adult muscle fibers, the IGF-PI3K-AKT pathway suppresses the expression of Atrogin-1 and MuRF1 through phosphorylation of FOXO1, leading to its sequestration in the cytoplasm away from target genes (Sandri et al. 2004; Stitt et al. 2004). In addition, phosphorylation of AMPKα has also been shown to enhance proteolysis by increasing Atrogin-1 and MuRF1 expression through activation of FOXO1 (Krawiec et al. 2007). Phosphorylation of FOXO1 decreased in R but not RBCAA or RL groups compared to CON, which may indicate some reduction in protein degradation signaling by Leu and BCAA; however, the nuclear import of FOXO1 was not confirmed in the present study. In addition, expression of ubiquitin ligases and activation of AMPKα did not differ between diets, suggesting that the ubiquitin/proteasome pathway may be unchanged in the present condition. We cannot discard a possible decrease in protein degradation in response to BCAA and Leu supplementation via inhibition of the cellular autophagy systems. In this regard, previously we have observed decreased levels of microtubule-associated protein light 1 chain 3 subunit II (LC3-II), a protein which parallels the development of autophagosome (Wang et al. 2011; Kabeya et al. 2004), in response to Leu supplementation under septic (Hernandez-Garcia et al., 2016) and normal conditions (Boutry et al., 2013) in neonate pigs. Nonetheles, the autophagy-lysosomal degradation pathways were not assessed in the current study.
Leu or BCAA supplementation does not induce insulin resistance in skeletal muscle of neonate pigs
Studies in cultured myotubes and isolated rat skeletal muscles suggest that Leu and BCAA can impair insulin-mediated glucose uptake through a negative-feedback loop (Iwanaka et al. 2010; Tremblay et al. 2007), presumably mediated by mTOR-S6K1 phosphorylation and subsequent serine phosphorylation and inactivation of IRS-1 (Iwanaka et al. 2010; Tremblay et al. 2007). As such, we tested the hypothesis that the inclusion of Leu and BCAA in the restricted diets would increase insulin resistance through phosphorylation of IRS1 on Ser1011 (Tremblay et al. 2007), leading to a decrease in the phosphorylation of PKB and overall protein synthesis in the neonate pigs. However, IRS1 phosphorylation in muscle tissue did not differ between CON and restricted diets, and HOMA values in blood were lower in all groups compared to CON, providing further evidence that insulin signaling was not impaired in RBCAA- and RL-supplemented animals.
Supplementation of Leu or BCAA in the diet does not upregulate AA transporters abundance in skeletal muscle of neonate pigs
Beyond their classical function of transporting Leu, Ile, and Val across membranes, LAT1 and SNAT2 transporters have also been implicated in mTORC1 activation by sensing and signaling AA availability to the mTOR pathway (Hundal and Taylor, 2009). In addition, LAT1 and SNAT2 have been shown to increase their expression in skeletal muscle in response to higher EAA levels (Drummond et al. 2010). Although we cannot discard a regulatory effect of LAT1 and SNAT2 in the activation of mTOR signaling pathway in the present study, the increase in S6K1 and 4EBP1 phosphorylation and eIF4E·eIF4G formation in CON, Leu, and BCAA groups was not associated with an increase in the expression of these AA transporters in skeletal muscle.
Protein abundance and phosphorylation is developmentally regulated in skeletal muscle of neonate pigs
We have previously reported that feeding (Davis et al. 1996; 2000), insulin (Wray-Cahen et al. 1998), and Leu infusion (Escobar et al. 2007) can stimulate muscle protein synthesis to a lesser extent in older than in younger pigs. Similarly, results presented herein indicate that muscle protein synthesis rates and the activation of mTOR effectors decreased in LD of neonatal pigs between d 9 and 21, although no changes were observed in the gastrocnemius and soleus. Developmental differences in KS between muscles likely are attributable to different myofiber composition (Davis et al. 1989; 2002). Our previous study in rats showed that the gastrocnemius and soleus to be composed partially and entirely of slow oxidative fibers, respectively, whereas the extensor digitorum longus, a fast-twitch, glycolytic muscle like LD, undergoes a more rapid decline in protein synthetic activity during the early weeks of life (Davis et al. 1989). Similarly, the lower MuRF1 and Atrogin-1 abundance observed in LD of older pigs, along with the increase in phosphorylation of FOXO1, suggests a parallel developmental decrease in protein degradation rates in the skeletal muscle. Paradoxically, activation of ERK1/2, which also is upregulated by insulin (Gosmanov et al. 2002) and promotes mTOR activity and protein synthesis in skeletal muscle (Mccubrey et al. 2011), was augmented in LD and gastrocnemius between day 9 and 21 across all groups. Although speculative, this effect may be a consequence of the developmental increase in postprandial insulin levels observed in the study (data not shown), as well as an increase in muscle activity (Gosmanov et al. 2002) associated with piglet growth (Brown et al. 2015).
Consistent with previous findings in neonatal pigs (Davis et al. 1996) and rats (Davis et al. 1989), ribosomal abundance, estimated as the total RNA-to-protein ratio (i.e., mg RNA · g protein−1), decreased between d 9 and 21 resulting in a significant reduction of the muscle protein synthetic capacity (CS) in all 3 muscles. These data suggest that during the neonatal period, the age-related decline in KS occurs primarily as a result of a decrease in the muscle CS, whereas the feeding-induced stimulation of protein synthesis rate appears to be related to an increase in ribosomal translational efficiency (KRNA), estimated as the total protein synthesized per total RNA (i.e., g protein · g RNA−1). Interestingly, the steep decline in CS did not cause a decrease in the KS in soleus or gastrocnemius, as it was accounted for by the increase in KRNA in both muscles. These results are consistent with data from Davis et al (1989), which also indicated a precocious increase in KRNA in soleus in 16-d-old rats, resulting in higher KS protein synthetic activity compared to glycolytic muscles during the early weeks of life.
In conclusion, supplementation of Leu or all BCAA to a protein and energy restricted diet did not enhance fractional protein synthesis rates in skeletal muscle nor body weight or lean gain of neonatal pigs, even though it upregulated the activity of the signaling proteins involved in protein translation initiation. Further studies are needed to investigate whether long-term enteral Leu supplementation will enhance lean mass accretion in neonates when energy intake is less restricted.
Supplementary Material
Acknowledgments
The work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 (Davis) and AR-46308 (Fiorotto), National Institute of Child Health and Human Development HD-072891 and HD085573 (Davis), United States Department of Agriculture National Institute of Food and Agriculture grant 2013-67015-20438 (Davis), California State University Agricultural Research Institute grant 58982 (Manjarin), and by the USDA/ARS under Cooperative Agreement no. 6250-510000-055 (Davis). This work is a publication of the USDA, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- AA
amino acids
- AMPKα
AMP-activated protein kinase α
- AU
Arbitrary Units
- BCAA
branched-chain amino acids
- BW
body weight
- CON
control
- CP
crude protein
- CS
muscle protein synthetic capacity
- DXA
dual-energy x-ray absorptiometry
- EAA
essential amino acid
- eIF4E·eIF4G
eukaryotic initiation factor 4 complex
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- 4EBP1
eukaryotic initiation factor repressor 4E-binding protein 1
- FOXO1
forkhead box protein O1
- HPLC
high-performance liquid chromatography
- HOMA
homeostatic model assessment
- IRS1
insulin receptor substrate 1
- KRNA
protein synthesis efficiency
- KS
fractional rate of protein synthesis
- LAT
L-type amino acid transporter 1
- LBW
low birthweight
- LD
longissimus dorsi
- MAFbx
muscle Atrophy F-Box/Atrogin-1
- ME
metabolizable energy
- mTORC1
mammalian target of rapamycin complex 1
- MuRF1
muscle RING-finger protein-1
- NEAA
non-essential amino acids
- PKB/Akt
protein kinase B
- P/T
phosphorylated/total protein
- R
restricted
- RL
restricted supplemented with leucine
- RBCAA
restricted supplemented with BCAA
- S6K1
ribosomal protein S6 kinase 1
- SNAT
Na+-coupled neutral amino acid transporter 2
- TSC2
tuberous sclerosis protein 2
Footnotes
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
Experiments were carried out in accordance with the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Research Council´s Guide for the Care and Use of Laboratory Animals (2011).
Informed consent
Not applicable (no human subjects were used in this study).
References
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–1022. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and leucine regulate p70S6 kinase and 4E–BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–543. [PubMed] [Google Scholar]
- Berseth CL. Feeding methods for the preterm infant. Semin Neonatol. 2001;6:417–424. doi: 10.1053/siny.2001.0062. [DOI] [PubMed] [Google Scholar]
- Berseth CL. Feeding strategies and necrotizing enterocolitis. Curr Opin Pediatr. 2005;17:170–173. doi: 10.1097/01.mop.0000150566.50580.26. [DOI] [PubMed] [Google Scholar]
- Black JL, Griffiths DA. Effects of live weight and energy intake on nitrogen balance and total N requirement of lambs. Br J Nutr. 1975;33:399–413. doi: 10.1079/bjn19750044. [DOI] [PubMed] [Google Scholar]
- Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab. 2013;305:E620–631. doi: 10.1152/ajpendo.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry C, El-Kadi SW, Suryawan A, Steinhoff-Wagner J, Stoll B, Orellana RA, Nguyen HV, Kimball SR, Fiorotto ML, Davis TA. Pulsatile delivery of a leucine supplement during long-term continuous enteral feeding enhances lean growth in term neonatal pigs. Am J Physiol Endocrinol Metab. 2016;310:E699–E713. doi: 10.1152/ajpendo.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Klaffenbock M, Nevison IM, Lawrence AB. Evidence for litter differences in play behavior in pre-weaned pigs. Appl Anim Behav Sci. 2015;172:17–25. doi: 10.1016/j.applanim.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res. 1995;37:593–599. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Campbell RG, Dunkin AC. The effects of energy intake and dietary protein on nitrogen retention, growth performance, body composition and some aspects of energy metabolism of baby pigs. Br J Nutr. 1983;49:221–230. doi: 10.1079/bjn19830029. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus DA, Steinhoff-Wagner J, Suryawan A, Nguyen HV, Hernandez-Garcia A, Fiorotto ML, Davis TA. Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am J Physiol Endocrinol Metab. 2015;309:E601–610. doi: 10.1152/ajpendo.00089.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol. 1989;257:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol. 1996;270:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol. 1999;277:E103–E109. doi: 10.1152/ajpendo.1999.277.1.E103. [DOI] [PubMed] [Google Scholar]
- Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Pediatr. 2004;93:1563–1568. doi: 10.1080/08035250410022198. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy B, Gunn T, Collinge J, Pencharz P. The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (less than 1600 g) infants. Pediatr Res. 1981;15:1040–1044. doi: 10.1203/00006450-198107000-00013. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55. doi: 10.1053/j.semperi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? J Pediatr. 2001;107:270–273. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab. 2007;293:E1615–E1621. doi: 10.1152/ajpendo.00302.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorotto ML, Davis TA, Reeds PJ, Burrin DG. Nonnutritive factors in colostrum enhance myofibrillar protein synthesis in the newborn pig. Pediatr Res. 2000;48:1–7. doi: 10.1203/00006450-200010000-00015. [DOI] [PubMed] [Google Scholar]
- Gosmanov AR, Nodtredt NC, Brown R, Thomason DB. Exercise effects on muscle beta-adrenergic signaling for MAPK-dependent NKCC activity are rapid and persistent. J Appl Physiol. 1985;93:1457–1465. doi: 10.1152/japplphysiol.00440.2002. [DOI] [PubMed] [Google Scholar]
- Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion disproportionate amounts of amino acids. Physiol Rev. 1970;50:428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Hay WW. Strategies for feeding the preterm infant. Neonatology. 2008;94:245–254. doi: 10.1159/000151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-García AD, Columbus DA, Manjarín R, Nguyen HV, Suryawan A, Orellana RA, Davis TA. Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am J Physiol Endocrinol Metab. 2016;311:E791–E801. doi: 10.1152/ajpendo.00217.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaka N, Egawa T, Satoubu N, Karaike K, Ma X, Masuda S, Hayashi T. Leucine modulates contraction- and insulin-stimulated glucose transport and upstream signaling events in rat skeletal muscle. J Appl Physiol. 1985;108:274–282. doi: 10.1152/japplphysiol.00420.2009. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian holomogue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, Lewis SE, Anderson P, Goldspink DF. Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle Nerve. 1984;7:235–242. doi: 10.1002/mus.880070309. [DOI] [PubMed] [Google Scholar]
- Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. 2013;88:113–121. doi: 10.1095/biolreprod.112.105080. [DOI] [PubMed] [Google Scholar]
- Koo WW, Hockman EM, Hammami M. Dual energy X-ray absorptiometry measurements in small subjects: conditions affecting clinical measurements. J Am Coll Nutr. 2004;23(3):212–219. doi: 10.1080/07315724.2004.10719363. [DOI] [PubMed] [Google Scholar]
- Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab. 2007;292:E1555–E1567. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing nutrition in preterm low birth weight infants-consensus summary. Front Nutr. 2017;4:20. doi: 10.3389/fnut.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr. 2013;162(3 Suppl):S7–16. doi: 10.1016/j.jpeds.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manjarín R, Columbus DA, Suryawan A, Nguyen HV, Hernandez-García AD, Hoang NM, Fiorotto ML, Davis T. Leucine supplementation of a restricted protein and energy diet enhances mTORC1 activation but not muscle protein synthesis in neonatal pigs. Amino Acids. 2016;48:257–267. doi: 10.1007/s00726-015-2078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Accessed 6 October 2014];March of Dimes Foundation. http://www.marchofdimes.org/mission/prematurity-campaign.aspx.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Kempf CR, Chappell WH, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra M, Basecke J, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Cocco L, Martelli AM. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011;226:2762–2781. doi: 10.1002/jcp.22647. [DOI] [PubMed] [Google Scholar]
- Mourier A, Bigard AX, Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med. 1997;18:47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- Munro N, Fleck A. The determination of nucleic acids. Meth Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Nadeau KJ, Ehlers LB, Aguirre LE, Moore RL, Korinne NJ, Ortmeyer HK, Hansen BC, Reusch JEB, Draznin B. Exercise training and calorie restriction increase SREBP-1 expression in intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E90–E98. doi: 10.1152/ajpendo.00543.2005. [DOI] [PubMed] [Google Scholar]
- Nutrient requirements of swine. 11th rev ed. National Research Council. Washington (DC): National Academy Press; 2012. [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2011;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94:809–818. doi: 10.3945/ajcn.111.017061. [DOI] [PubMed] [Google Scholar]
- Piepho HP. Data transformation in statistical analysis of field trials with changing treatment variance. Agron J. 2009;101:865–869. [Google Scholar]
- Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo J, De Curtis M, Pieltain C. Nutritional assessment and body composition of preterm infants. Semin Neonatol. 2002;6:383–391. doi: 10.1053/siny.2001.0073. [DOI] [PubMed] [Google Scholar]
- Ryu JM, Han HJ. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J Biol Chem. 2011;286:23667–23678. doi: 10.1074/jbc.M110.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;281:E908–E915. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res. 2012;71:324–331. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous RE, Rogers QR, Harper AE. Effect of leucine-isoleucine and valine antagonism on the pattern of free amino acids in blood plasma and several tissues of the rat. Fed Proc. 1963;22:202–210. [Google Scholar]
- Torrazza RM, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr. 2010;140:2145–2152. doi: 10.3945/jn.110.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Brûlé S, Um SH, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ch, Wang Y, McNutt M, Zhu W. Autophagy process is associated with anti-neoplastic function. Acta Biochim Biophys Sin. 2011;43:425–432. doi: 10.1093/abbs/gmr028. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, et al. Birth weight and risk of type 2 diabetes: a systematic review. J Am Med Assoc. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr. 2010;140:264–270. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, Deng D, Tan BE, Liu ZQ, Wu G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. 2010;39:1477–1486. doi: 10.1007/s00726-010-0612-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qiao S, Ren M, Zeng X, Ma X, Wu Z, Thacker P, Wu G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids. 2013;45:1191–1205. doi: 10.1007/s00726-013-1577-y. [DOI] [PubMed] [Google Scholar]
- Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of very low birthweight infant. Clin Perinatol. 2002;29:225–244. doi: 10.1016/s0095-5108(02)00007-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.